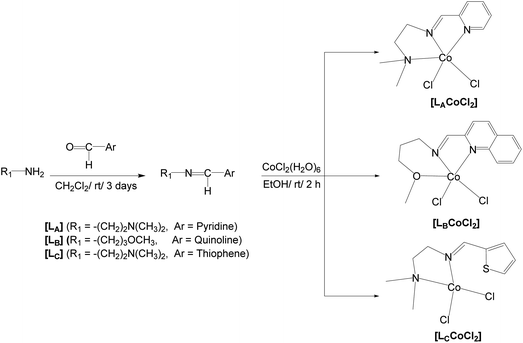
Open Access Article
Open Access ArticleCatalytic performance of tridentate versus bidentate Co(II) complexes supported by Schiff base ligands in vinyl addition polymerization of norbornene†
Kyeonghun Kim‡
 a,
Saira Nayab‡ab,
Yerim Choa,
Hyewon Jungd,
Hyeonuk Yeo
a,
Saira Nayab‡ab,
Yerim Choa,
Hyewon Jungd,
Hyeonuk Yeo c,
Hyosun Lee
c,
Hyosun Lee *a and
Sang-Ho Lee
*a and
Sang-Ho Lee *d
*d
aDepartment of Chemistry and Green-Nano Materials Research Center, Kyungpook National University, Daegu, 41566, Republic of Korea. E-mail: hyosunlee@knu.ac.kr
bDepartment of Chemistry, Shaheed Benazir Bhutto University, Sheringal Dir (Upper) 18000, Khyber Pakhtunkhwa, Islamic Republic of Pakistan
cDepartment of Chemistry Education and Department of Pharmacy, Kyungpook National University, Daegu, 41566, Republic of Korea
dCenter for Advanced Specialty Chemicals, Korea Research Institute of Chemical Technology, Ulsan 44412, Republic of Korea. E-mail: slee@krict.re.kr
First published on 21st December 2022
Abstract
A series of Co(II) complexes supported by Schiff base ligands, LA–LC, where LA, LB, and LC are (E)-3-methoxy-N-(quinolin-2-ylmethylene)propan-1-amine, (E)-N1,N1-dimethyl-N2-(pyridin-2-ylmethylene)ethane-1,2-diamine, and (E)-N1,N1-dimethyl-N2-(thiophen-2-ylmethylene)ethane-1,2-diamine, respectively, were designed and synthesized. Structural studies revealed a distorted trigonal bipyramidal geometry for [LBCoCl2] and a distorted tetrahedral geometry for [LCCoCl2]. After activation with modified methyl aluminoxane (MMAO), all the Co(II) complexes catalyzed the polymerization of norbornene (NB) to yield vinyl-type polynorbornenes (PNBs) with activities of up to 4.69 × 104 gPNB mol Co−1 h−1 at 25 °C. High-molecular-weight (Mn of up to 1.71 × 105 g mol−1) soluble PNBs with moderate molecular-weight distributions (MWD) were obtained. The activity of the Co(II)/MMAO catalytic system is influenced by the steric hindrance and electronic properties of the ligands.
Introduction
Polynorbornene (PNB), which is synthesized via vinyl addition polymerization, has received considerable attention because of its fascinating properties that stem from its saturated bicyclic backbone.1 The NB skeleton confers a high thermal stability, low dielectric constant, and high optical transparency, which are attractive characteristics for applications in microelectronics and optics.1–4 Both early and late transition metal complexes have been reported as active catalysts for the vinyl addition polymerization of NB.1,5–11 Recently, promising catalytic activities have also been reported for late transition metal catalysts, such as Pd- and Ni-based catalysts.12–15The choice of ancillary ligand significantly affects the catalytic performance of the metal-based initiators in a polymerization reaction.16–19 Imine-derived ligands are considered privileged because of their structural flexibility, fine tunability, and selectivity toward metal atoms that enable the construction of attractive geometries, ranging from N,N′-bidentate to N,N′,X-tridentate and N,N′,N,X′-tetradentate.20,21 For instance, a variety of imine-derived Pd(II) modified methyl aluminoxane (Pd(II)/MMAO) systems have displayed high activities toward NB polymerization with effective control of the resultant PNB properties.4,20,22 Numerous studies on NB polymerization conducted with Co(II) complexes have reported moderate activities.23–26 The Wang group studied a 2-((3,5-dimethyl-1H-pyrazol-1-yl)methyl)pyridine}CoCl2 system that afforded only 31% yield and PNB with an Mn value of 6.05 × 103 g mol−1.27 Similarly, a 2,6-bis[1-(2,5-ditertbutylphenylimino)ethyl] pyridine-derived Co(II) system exhibited an activity of 11.4 × 103 gPNB mol Co−1 h−1 at 30 °C after 12 h.28 The Frederic group studied a CoCl2 and pyridine bis(imine)Co(II)/MAO system for NB polymerization that resulted in high-molecular-weight PNB (Mn = 4.2 × 105 g mol−1).29 The Sato group studied Co(II) complexes activated with d-MAO, which resulted in 99% yield within 3 h in chlorobenzene at room temperature and yielded low-molecular-weight PNB (3.2 × 104 g mol−1).30 However, the aforementioned complexes were only synthesized in 5% yield when MAO was used for activation.
Recently, we reported a square pyramidal Co(II) complex with an iminomethylpyridine-derived Schiff base ligand (LA) as an effective catalyst for syndio-enriched poly(methyl methacrylate) MMA polymerization with an activity of up to 4.48 × 104 g PMMA mol Co−1 h−1 and synthesized high-molecular-weight PMMA (11.1 × 105 g mol−1).31 The same complex, with the N,N,N-coordination mode, exhibited promising results when utilized in the vinyl addition polymerization of NB, which prompted us to explore Co(II) complexes bearing N,N,O-tridentate (LB) and N,N-bidentate (LC) imine ligands with diverse geometries for NB polymerization (Scheme 1). In this work, we present a set of Co(II) complexes with trigonal bipyramidal and tetrahedral geometries for LB and LC, respectively, and estimate their catalytic performance in the vinyl addition polymerization of NB. Activation with MMAO resulted in excellent activity, and a Co(II)/MMAO system affording 99% yield in the conversion of NB to PNB within 2 h at 25 °C is reported for the first time. Additionally, the effects of ligand architecture, polymerization temperature, and time on the NB polymerization performance of the studied Co(II) complexes were also investigated.
Experimental section
Materials
CoCl2·6H2O (Sigma-Aldrich; 98%), 2-pyridinecarboxaldehyde (Sigma-Aldrich; 99%), 2-thiophencaboxaldehyde (Sigma-Aldrich; 99%), 3-metholxypropylamine (Sigma-Aldrich; 99%), bicyclo[2.2.1]hept-2-ene(Sigma-Aldrich; 99%), magnesium sulphate (MgSO4) (Sigma-Aldrich; ≥ 99.5%), 2-quinolinecarbxaldehyde (TCI; 96%), and N,N′-dimethylethylenediamine (TCI; 98%) were used as received. Anhydrous solvents such as methylene chloride (CH2Cl2), ethanol (EtOH), n-hexane (n-Hex), diethyl ether (Et2O), 1,2-dichloroethane (C2H4Cl2), chlorobenzene, and toluene were purchased from Aldrich Chemicals Corp. and degassed before use. Modified methylaluminoxane (MMAO) was purchased from Lake Materials as 7.10 wt% aluminum in methylcyclohexane solution.Preparation of Schiff base ligands
(E)-N1,N1-Dimethyl-N2-(pyridin-2-ylmethylene)ethane-1,2-diamine (LA), (E)-3-methoxy-N-(quinolin-2-ylmethylene)propan-1-amine (LB), and (E)-N1,N1-dimethyl-N2-(thiophen-2-ylmethylene)ethane-1,2-diamine (LC), were synthesized according to literature procedures.31–33Synthesis of (E)-N1,N1-dimethyl-N2-(pyridin-2-ylmethylene)ethane-1,2-diamine cobalt(II) chloride, [LACoCl2]
LACoCl2 was synthesized according to the literature.31Synthesis of (E)-3-methoxy-N-(quinolin-2-ylmethylene)propan-1-amine cobalt(II) chloride, [LBCoCl2]
A solution of LB (0.228 g, 1.00 mmol) in anhydrous ethanol (10.0 mL) was added to a solution of CoCl2·6H2O (0.238 g, 1.00 mmol) in ethanol (10.0 mL) at room temperature. Precipitation of green solid occurred while stirring the reaction mixture for 12 h. The precipitate was filtered and washed with cold ethanol (50.0 mL × 2). The solid was dried under a vacuum at 60 °C (0.26 g, 72%). X-ray quality crystals of [LBCoCl2] were obtained by the layering of hexane on the CH2Cl2 solution of [LBCoCl2]. mp (°C): 191. Analysis calculated for C14H16Cl2N2OPd (%): anal. calcd for C14H16Cl2CoN2O: C, 46.95; H, 4.50; N, 7.82. Found: C, 47.13; H, 4.53; N, 7.88. FT-IR (solid (neat); cm−1): ν(sp2 C–H) 3016; ν(sp3 C–H) 2883 m; ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)imine 1639 m; ν(C
N)imine 1639 m; ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)quinoline 1554 m; ν(C
N)quinoline 1554 m; ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)quinoline 1456 m; ν(C–O) 1090 m; ν(M–N) 564 m.
C)quinoline 1456 m; ν(C–O) 1090 m; ν(M–N) 564 m.
Synthesis of (E)-N1,N1-dimethyl-N2-(thiophen-2-ylmethylene)ethane-1,2-diamine cobalt(II) chloride, [LCCoCl2]
A method analogous to that used to prepare [LBCoCl2] was adopted for the synthesis of [LCCoCl2]; in this case, LC (0.364 g, 2.00 mmol) and CoCl2·6H2O (0.476 g, 2.00 mmol) used to yield the final product (0.46 g, 75%). X-ray quality crystals of [LCCoCl2] were obtained by layering hexane on the CH2Cl2 solution of [LCCoCl2]. mp (°C): 200. Anal. calcd for C9H14Cl2CoN2S: C, 34.63; H, 4.52; N, 8.98; S, 10.27. Found: C, 34.66; H, 4.54; N, 8.87; S, 10.45. FT-IR (solid (neat); cm−1): ν(sp2 C–H) 2976; ν(sp3 C–H) 2901 m; ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)imine 1653 s; ν(C
N)imine 1653 s; ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)thiophene 1454 m; ν(C–S) 857 m; ν(M–N) 588 m.
C)thiophene 1454 m; ν(C–S) 857 m; ν(M–N) 588 m.
General polymerization procedure
The polymerization was conducted by applying the Schlenk technique under argon in the flame-dried flask. In the general polymerization procedure, the flask was charged with 15 mmol of NB and 15 μmol of catalyst in 20 mL of solvent (chlorobenzene, toluene, or dichloroethane). The reaction mixture was stirred for 5 min at prescribed temperatures, followed by the addition of MMAO (3.10 mL of 7.10 wt% in methylcyclohexane) under a dry argon atmosphere. The flask was sealed and stirred for the prescribed time. After the prescribed time, the reaction was terminated by the addition of acidic ethanol (200 mL; HCl 1%). The resulting precipitated polymer was collected by filtration, washed with ethanol, and dried overnight under vacuum at 80 °C and characterized by SEC.Measurements
The NMR spectra of ligands were recorded on a Bruker Avance III (500 MHz for 1H NMR, 125 MHz for 13C NMR) spectrometer. The chemical shifts are recorded in ppm units (δ) using SiMe4 as an internal standard; coupling constants (J) are reported in Hertz (Hz). The Fourier-transform infrared (FT-IR) spectra of synthesized ligands and their corresponding Co(II) complexes were recorded on Bruker FT/IR-Alpha (neat) and data were reported in reverse centimeter (cm−1). Elemental analysis (C, H, N) of the synthesized Co(II) complexes were performed on an elemental analyzer (EA 1108; Carlo-Erba, Milan, Italy). Melting points of synthesized Co(II) complexes, [LnCoCl2] (Ln = LA–LC), were determined with an IA9100 (Electrothermal) instrument. 1H NMR spectrum of PNB demonstrating the absence of double bonds was recorded using Avance III-500 MHz spectrometer, confirming the vinyl-type polymerization of NB. The FT-IR of the resultant PNB was determined using Bruker FT/IR-Alpha (neat), and the representative FT-IR spectra are given in ESI.† The number-average molecular weights (Mn), weight-average molecular weights (Mw), and polydispersity indices (PDI = Mw/Mn) of the obtained PNB samples were determined by size exclusion chromatography (SEC, ACQUITY APC XT, Waters Corp., USA) using chloroform as the eluent at 0.6 mL min−1, with the column temperature set to 40 °C. Mn, Mw, and PDI values were calculated against polystyrene standards.X-ray crystallographic studies
The X-ray-quality single crystal was coated with paratone-N oil and the diffraction data was measured with different levels of synchrotron radiation: the crystal of [LBCoCl2] with synchrotron radiation λ = 0.650 Å at 293(2) K and [LCCoCl2] with synchrotron radiation λ = 0.630 Å at 220(2) K. All crystals on an ADSC Quantum-210 detector at 2D SMC with silicon (111) double crystal monochromator (DCM) at the Pohang Accelerator Laboratory, South Korea. The PAL BL2D-SMDC program34 was used for data collection (detector distance is 63 mm, omega scan; Δω = 3°, exposure time is 1 s per frame) and HKL3000sm (Ver. 703r)35 was used for cell refinement, reduction, and absorption correction. Structures were solved by direct method and refined by full-matrix least-squares refinement using the SHELXL-2018 (ref. 36) computer program. The positions of all non-hydrogen atoms were refined with anisotropic displacement factors. All hydrogen atoms were placed using a riding model, and their positions were constrained relative to their parent atoms using the appropriate HFIX command in SHELXL-2014. X-ray crystallography with PLS-II 2D-SMC beamline was supported by MSIP and POSTECH. Data collection and integration were performed with SMART and SAINT-Plus software packages.37 Semiempirical absorption corrections based on equivalent reflections were applied by SADABS.38 Structures were solved by direct methods and refined using a full-matrix least-squares method on F2 using SHELXTL.39 All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were added to their geometrically ideal positions. Crystallographic refinements and structural data are summarized in Table S1.†Results and discussion
Design of Schiff base ligands and coordination patterns of Co(II) complexes
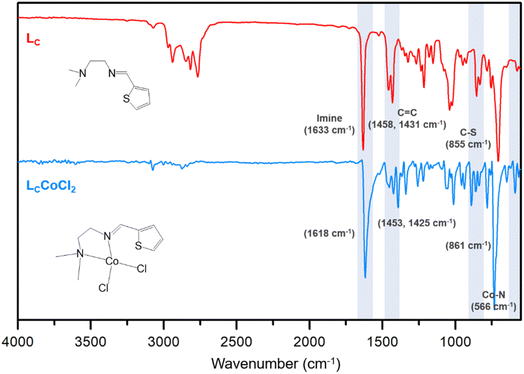
To promote catalytic activity by leveraging the structural differences of Co(II) complexes, Schiff base imine ligands for the Co(II) complex were prepared via condensation of various amines with the corresponding aromatic aldehydes, as illustrated in Scheme 1. The obtained ligands were purified by vacuum distillation, and their chemical structures were successfully confirmed by nuclear magnetic resonance (NMR) (Fig. S1–S6†) and Fourier transform infrared (FT-IR) spectroscopic analyses (Fig. 1, S7 and S8†).Ligands bearing nitrogen with different hybridizations are easily ligated to the metal center. In this regard, Co(II) readily accepts Schiff base ligands (Ln) upon mixing with CoCl2·6H2O to form monomeric complexes [LnCoCl2] (Ln = LA–LC) in high yields (72–75%). The chemical structures of the obtained Co(II) complexes were confirmed using spectroscopic techniques (Fig. 1 and S7–S8†). For example, a comparison of the FT-IR spectra of the ligands (Ln = LA–LC) with those of the complexes [LnCoCl2] (Ln = LA–LC) revealed a slight shift of the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) peak to a lower frequency, indicating that complexation was achieved through the bonding of imine nitrogen and the M(II) center.31,32,40 For instance, the C
N) peak to a lower frequency, indicating that complexation was achieved through the bonding of imine nitrogen and the M(II) center.31,32,40 For instance, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N band appeared at 1633 cm−1 for LC, whereas it appeared at 1618 cm−1 for [LCCoCl2]. The C
N band appeared at 1633 cm−1 for LC, whereas it appeared at 1618 cm−1 for [LCCoCl2]. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond became weaker upon chelation because of the inductive effect resulting from the sharing of the lone electron pair on the imine nitrogen with the M(II) center.41 The ligation of ligands to metal ions through the nitrogen atom was also confirmed by the presence of new bands at 608, 565, and 566 cm−1 assigned to ν(M–N).40,42,43 The vibration peak observed in the 1453–1425 cm−1 region is assigned to symmetrical and asymmetrical ν(C
N bond became weaker upon chelation because of the inductive effect resulting from the sharing of the lone electron pair on the imine nitrogen with the M(II) center.41 The ligation of ligands to metal ions through the nitrogen atom was also confirmed by the presence of new bands at 608, 565, and 566 cm−1 assigned to ν(M–N).40,42,43 The vibration peak observed in the 1453–1425 cm−1 region is assigned to symmetrical and asymmetrical ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) stretching vibrations of the thiophene ring.44,45 In the present study, ν(C–O) and (C–S) stretching vibrations were observed at 1040 and 861 cm−1, respectively, in the FT-IR spectra of Co(II) complexes.31,45–47
C) stretching vibrations of the thiophene ring.44,45 In the present study, ν(C–O) and (C–S) stretching vibrations were observed at 1040 and 861 cm−1, respectively, in the FT-IR spectra of Co(II) complexes.31,45–47
These complexes were stable under atmospheric conditions and could be stored for months at room temperature without deterioration. The purity of the complexes [LnCoCl2] (Ln = LA–LC) was verified by performing composition analysis (%) of the C, H, and N constituents, and good correlations between the calculated and observed values were found (Fig. S9†).
Structural analyses of obtained Co(II) complexes depending on the Schiff base imine ligands by X-ray crystallographic study
Single-crystal X-ray diffraction studies were performed to determine the geometries of the Co(II) complexes. ORTEP diagrams for [LBCoCl2] and [LCCoCl2] are shown in Fig. 2, and the selected bond lengths and angles are listed in Table S2.†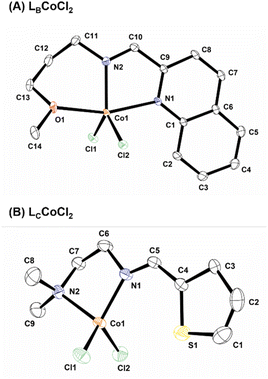 | ||
| Fig. 2 An ORTEP diagram of (A) [LBCoCl2] and (B) [LCCoCl2] with thermal ellipsoids at 50% probability. All hydrogen atoms have been omitted for clarity. | ||
Five-coordinate [LACoCl2] had a square pyramidal geometry around the Co(II) metal center, whereas the coordination environment around the Co(II) center with five-coordinate [LBCoCl2] was distorted trigonal bipyramidal and was obtained via coordination with the nitrogen atoms of the quinoline and azomethine moieties and the oxygen atom of the methoxy functionality of LB. The same is evident from a comparison of the geometric parameter (τ5) for the five-coordinate complexes; complexes [LACoCl2], and [LBCoCl2] coordinated with N,N,N- and N,N,O-tridentate ligands, respectively, and adopted square pyramidal and trigonal bipyramidal geometries (Table 1).31 The τ5 parameter is one and zero for perfect trigonal bipyramidal and perfect square pyramidal, respectively.48,49 Based on the τ5 values, the geometry of [LBCoCl2] is more distorted than that of [LACoCl2].
| Complexes | Geometry | τ5 | τ4 | References |
|---|---|---|---|---|
| a [bpma] = 4-bromo-N-((pyridin-2-yl)methylene)benzenamine.b [mpme] = (E)-2-morpholino-N-(pyridin-2-ylmethylene)ethanamine.c [dppd] = (E)-N1,N1-dimethyl-N3-(pyridin-2-ylmethylene)propane-1,3-diamine.d [pmha] = (E)-N-(pyridin-2-ylmethylene)hexan-1-amine. | ||||
| Trigonal bipyramidal (D3h) | Trigonal bipyramidal | 1.00 | 48 and 49 | |
| [LACoCl2] | Square pyramidal | 0.10 | 31 | |
| [LBCoCl2] | Trigonal bipyramidal | 0.62 | This work | |
| [(bpma)Co(μ–Cl)Cl]2a | Trigonal bipyramidal | 0.776 | 43 | |
| [(mpme)CoCl2]b | Square pyramidal | 0.414 | 31 | |
| [(dppd)CoCl2]c | Square pyramidal | 0.025 | 31 | |
| Square pyramidal (C4v) | Square pyramidal | 0.00 | 48 and 49 | |
| Square planar (D4h) | Square planar | 0.00 | 50 | |
| [LCCoCl2] | Tetrahedral | 0.88 | This work | |
| [(pmha)PdCl2]d | Square planar | 0.07 | 32 | |
| Tetrahedral (Td) | Tetrahedral | 1.00 | 50 | |
The Co–Nquinoline and Co–Nimine bond lengths in [LBCoCl2] were 2.1827(12) and 2.0509(12) Å, respectively. The fact that the Co–Nquinoline bond is longer than the Co–Nimine bond is probably due to the difference in basicity between the quinoline and imine nitrogen atoms. These structural parameters agree well with the finding that the imine M(II) complexes have a trigonal bipyramidal structure.43 Additionally, oxygen is also coordinated with the Co(II) center with a bond length of 2.212(12) Å, which is in contrast with the pyridine-derived Co(II) complexes previously studied.31 The average bond length of Co–Cl was 2.2723 Å, which was within the usual range for Co(II) complexes.28 The double imine (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) bond distances of 1.2798(17) and 1.278(3) Å for [LBCoCl2] and [LCCoCl2], respectively, agree well with the reported values for Co(II) complexes.30 The C(9)–C(10) and C(4)–C(5) bond distances of 1.4739(19) and 1.442(4) Å, respectively, for [LBCoCl2] and [LCCoCl2], respectively, also fall within the usual range, which is indicative of delocalized π-electrons.43 The Nimine–Co–Nquinoline (107.14(4)°) bond angle for the five-membered ring was comparable to, albeit slightly larger than, that reported for the Co(II) complex. The Noxygen–Co–Nimine and Clterminal–Co–Clterminal bond angles in [LBCoCl2] were 118.68(3)° and 11.11(1)°, respectively.30
C) bond distances of 1.2798(17) and 1.278(3) Å for [LBCoCl2] and [LCCoCl2], respectively, agree well with the reported values for Co(II) complexes.30 The C(9)–C(10) and C(4)–C(5) bond distances of 1.4739(19) and 1.442(4) Å, respectively, for [LBCoCl2] and [LCCoCl2], respectively, also fall within the usual range, which is indicative of delocalized π-electrons.43 The Nimine–Co–Nquinoline (107.14(4)°) bond angle for the five-membered ring was comparable to, albeit slightly larger than, that reported for the Co(II) complex. The Noxygen–Co–Nimine and Clterminal–Co–Clterminal bond angles in [LBCoCl2] were 118.68(3)° and 11.11(1)°, respectively.30
The geometry of [LCCoCl2] can be best described as distorted tetrahedral and is obtained via coordination with the N,N-bidentate ligand, and two chloro ligands. The bond lengths of Co–Nimine and Co–Namine are 2.040(2) and 2.078(2) Å, respectively. As shown in Table 1, the average Co–Cl and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C distances of 2.2239(9) and 1.278(3) Å, respectively, are within the acceptable range, respectively.31 The τ4 value is proposed as a simple metric for quantitatively evaluating the geometry of four-coordinated complexes (Table 1).50 Complexes with an ideal square planar geometry are characterized by a τ4 value of zero, whereas a τ4 value of one is characteristic of ideal tetrahedral geometry. The τ4 parameters of [LCCoCl2] were compared in the reported work and found to be distorted tetrahedral.31,50 Additionally, the angle between the five-membered N,N′ chelating ring and thiophene ring in [LCCoCl2] was 17.04(3)°.
C distances of 2.2239(9) and 1.278(3) Å, respectively, are within the acceptable range, respectively.31 The τ4 value is proposed as a simple metric for quantitatively evaluating the geometry of four-coordinated complexes (Table 1).50 Complexes with an ideal square planar geometry are characterized by a τ4 value of zero, whereas a τ4 value of one is characteristic of ideal tetrahedral geometry. The τ4 parameters of [LCCoCl2] were compared in the reported work and found to be distorted tetrahedral.31,50 Additionally, the angle between the five-membered N,N′ chelating ring and thiophene ring in [LCCoCl2] was 17.04(3)°.
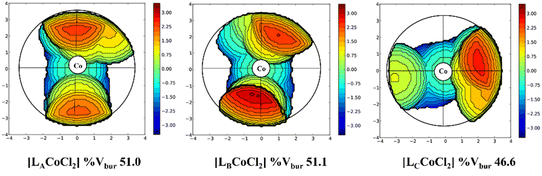
The ligand topologies around the Co(II) center were visualized and calculated using the SambVca 2.1 program.51 The steric maps showed that [LACoCl2] with a pyridine moiety exhibited a crowded environment around the Co(II) center with a Vbur value of 51.0%, whereas [LBCoCl2] with a quinoline-derived ligand showed a Vbur value of 51.1%. [LCCoCl2] exhibited the least crowded environment with Vbur 46.6%. Topographic steric maps of [[LnCoCl2] (Ln = LA–LC)] illustrating the steric bulk of the attached ligands around the metal center are shown in Fig. 3.
The catalytic activity of Co(II) complexes for the vinyl addition polymerization of NB
To compare the catalytic reactivity for NB polymerization, three Co(II) complexes were examined as catalysts in conjunction with MMAO as a co-catalyst (Fig. 4 and Table 2). The two Co(II) complexes, [LBCoCl2] and [LCCoCl2], proved to be highly active and produced PNB with a higher yield than [LACoCl2] at a [NB]/[Al]/[Co] ratio of 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500
500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
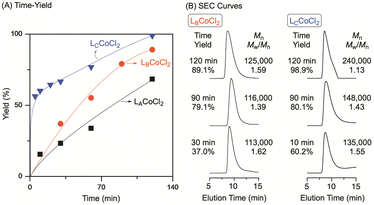
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in chlorobenzene at 25 °C. However, [LCCoCl2] clearly showed faster propagation in the early stages of polymerization and afforded a narrower molecular weight distribution (MWD) with a higher molecular weight of PNB. The obtained PNB was further analyzed by NMR for structural analysis (Fig. S10 and S11†), and the FT-IR spectra of PNB showed no signals at 1620–1680, 966, and 760 cm−1, indicating that NB was polymerized by vinyl addition (Fig. S12†).32,52
1 in chlorobenzene at 25 °C. However, [LCCoCl2] clearly showed faster propagation in the early stages of polymerization and afforded a narrower molecular weight distribution (MWD) with a higher molecular weight of PNB. The obtained PNB was further analyzed by NMR for structural analysis (Fig. S10 and S11†), and the FT-IR spectra of PNB showed no signals at 1620–1680, 966, and 760 cm−1, indicating that NB was polymerized by vinyl addition (Fig. S12†).32,52
| Entry | Catalysta | Temp. (°C) | Yieldb (%) | Activityc (g mol Cat−1 h−1)×104 | Mnd (g mol−1) | Mw/Mnd |
|---|---|---|---|---|---|---|
| a [NB]0/[MMAO]0/[Co(II) catalyst]0 = 650 mM/325 mM/0.65 mM in chlorobenzene 20 mL at 25 °C for 2 h.b Yield is defined as (a mass of dried polymer recovered)/(a mass of monomer used).c Activity is a gPNB mol Co−1 h−1.d Measured via SEC calibrated with PS standards in chloroform (40 °C, flow rate 1.0 mL min−1). | ||||||
| 1 | [LACoCl2] | 0 | 82.5 | 4.09 | 125![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.59 |
| 2 | [LACoCl2] | 25 | 72.7 | 3.44 | 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.65 |
| 3 | [LACoCl2] | 40 | 59.0 | 2.79 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.08 |
| 4 | [LBCoCl2] | 0 | 85.2 | 4.04 | 181![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.39 |
| 5 | [LBCoCl2] | 25 | 89.1 | 4.22 | 125![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.59 |
| 6 | [LBCoCl2] | 40 | 67.9 | 3.22 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.79 |
| 7 | [LCCoCl2] | 0 | 86.4 | 4.09 | 121![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.63 |
| 8 | [LCCoCl2] | 25 | 98.9 | 4.69 | 240![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.13 |
| 9 | [LCCoCl2] | 40 | 59.0 | 2.79 | 177![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.29 |
A comparison of catalyst efficiencies showed that the [LCCoCl2] complex had the highest catalytic activity (4.69 × 104 gPNB mol Co−1 h−1) at 25 °C. Under identical experimental conditions, the activities of the Co(II) complexes decreased in the following order: [LCCoCl2] > [LBCoCl2] > [LACoCl2] (Table 2). From the polymerization results, the effect of the catalyst structure on NB polymerization is apparent. For instance, Co(II) complexes bearing N,N-bidentate ligands exhibited higher activities than those bearing N,N,N- and N,N,O-tridentate ligands. These results agree with previous reports, where sterically demanding substituents around the metal center negatively affected NB polymerization. The presence of bulky substituents hinders the facile coordination of the bulky NB monomer with the active site, resulting in a decrease in activities.53–55 Similarly, the difference in the activities of [LACoCl2] and [LBCoCl2], which have tridentate ligands and almost the same buried volumes (Fig. 3), is apparent (Table 2); the quinoline moiety-bearing catalyst exhibited higher activities than the Co(II) catalyst complexes bearing pyridine-based ligands. These results agree well with our recent reports on Pd(II) complexes bearing amino-methylpyridine and amino-methylquinoline derivatives.53
Co(II) coordinates with the nitrogen atoms of the ligand via a lone pair of electrons. The ease of availability of these lone pairs controls the net positive charge on the metal center, i.e., the higher the basicity of nitrogen and better the electron availability, the stronger will be the Co–N bond, as is evident from the Co–Nquinoline (2.1827(12) Å) and Co–Npyridine (2.1610(1) Å) bond lengths. This scenario can result in a slightly less electropositive metal center for monomer addition. The quinoline ring, which is less basic than pyridine, could exert a more diverse steric and electronic environment on the reactive center than the pyridine ring, providing a more electropositive Pd(II) center. Generally, the transition metal complexes with ligands which made the metal center more electron deficient exhibited higher activities as the olefin could easily coordinate to the electron-deficient metal center.56 On the other hand, bulky substituted ligands attached to the transition metal complexes are of great benefit to the polymerization, because the bulky moieties can either protecting the metal center and manipulating β-H elimination is inhibited in the polymerization process. These results indicated the apparent but irregular influences of the steric and electronic effects, originating from the collaborations of basicity and steric bulk of ligand substituents. This phenomenon has been supported by recent work by Shi and Jin group, demonstrating the steric hindrance and electronic effect of on the catalytic properties of Pd and Ni complexes.56,57 Thus, not only the steric hindrance but also the electronic properties of ligands have a significant influence on catalytic performance.
To investigate the optimal reaction parameters for the polymerization of NB using the [LnCoCl2]/MMAO system, polymerization was performed under various conditions, such as different [Al]/[Co] ratios, temperatures, polymerization times, and solvents (Table 2, Fig. S13 and Table S3–S5†). The polymer yield and catalytic activity depended on the reaction conditions, and polymerization at 25 °C with a [NB]/[Al]/[Co] ratio of 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500
500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was found to be the most effective. The co-catalyst MMAO plays an important role in forming an active species, i.e., maintaining active catalyst precursors as well as forming catalytically active cation–anion ion pairs.57,58 In the absence of MAO, the chloride complexes were inactive for NB polymerization due to the lack of a cationic M+–C center for the initiation and propagation steps of polymerization.57 The catalytic activity of [LBCoCl2] sharply increased from 0.3 to 3.48 × 104 gPNB mol Co−1 h−1 on increasing the [Al]/[Co] ratio from 100 to 400 (Table S3†). The highest catalytic activity (4.22 × 104 gPNB mol Co−1 h−1) was achieved when the ratio of [Al]/[Co] was increased to 500 (Table S3,† entry 5). Excess MMAO is desirable for polymerization, which might be due to the scavenging of impurities and regeneration of the active species deactivated by transformation/elimination in the system.58–60 However, a further increase in the MMAO concentration had a negative effect on NB polymerization. Although the polymerization mechanism is no obvious at this point, however the polymerization should start with the formation of a metal alkyl complex as the catalytically active species generated by the reaction of metal complex with MAO. The norbornene monomer occupies the coordination site, and the norbornene would insert into the M–C bond; a repetition of the coordination insertion steps leads to polymer chain growth. Generally, the catalytic species with decrease electron density on the N atom, leading to rapider insertion of monomer.61,62
1 was found to be the most effective. The co-catalyst MMAO plays an important role in forming an active species, i.e., maintaining active catalyst precursors as well as forming catalytically active cation–anion ion pairs.57,58 In the absence of MAO, the chloride complexes were inactive for NB polymerization due to the lack of a cationic M+–C center for the initiation and propagation steps of polymerization.57 The catalytic activity of [LBCoCl2] sharply increased from 0.3 to 3.48 × 104 gPNB mol Co−1 h−1 on increasing the [Al]/[Co] ratio from 100 to 400 (Table S3†). The highest catalytic activity (4.22 × 104 gPNB mol Co−1 h−1) was achieved when the ratio of [Al]/[Co] was increased to 500 (Table S3,† entry 5). Excess MMAO is desirable for polymerization, which might be due to the scavenging of impurities and regeneration of the active species deactivated by transformation/elimination in the system.58–60 However, a further increase in the MMAO concentration had a negative effect on NB polymerization. Although the polymerization mechanism is no obvious at this point, however the polymerization should start with the formation of a metal alkyl complex as the catalytically active species generated by the reaction of metal complex with MAO. The norbornene monomer occupies the coordination site, and the norbornene would insert into the M–C bond; a repetition of the coordination insertion steps leads to polymer chain growth. Generally, the catalytic species with decrease electron density on the N atom, leading to rapider insertion of monomer.61,62
Temperature is another crucial parameter for controlling the NB polymerization progress, in addition to the use of MMAO. When NB polymerization was conducted at 0 °C using [LCCoCl2], PNB was obtained in 86.4% yield (Table 2, entry 7). The PNB yield and activity increased appreciably, with the yield increasing from 86.4% to 98.9% and the activity for [LCCoCl2] increasing from 4.09× 104 gPNB mol Co−1 h−1 to 4.69 × 104 gPNB mol Co−1 h−1, when the polymerization temperature was increased up to 25 °C (Table 2, entry 8). However, when the temperature exceeded 40 °C, a decrease in the catalytic activity compared to that at 25 °C was observed, indicating the importance of a suitable polymerization temperature for this Co(II)/MMAO system. No clear trends in the Mn values and MWD with decreasing polymerization temperature were observed for the [LnCoCl2]/MMAO system (Table 2). It is not difficult to realize that the rate of monomer consumption and the resultant PNB yield increased with polymerization time, while the catalytic activity decreased because the rate of catalysis was slowed down due to the viscosity of the converted PNB (Table S3†).63 For example, in the case of [LCCoCl2], the activity dropped from 64.1× 104 gPNB mol Co−1 h−1 at 5 min to 4.69× 104 gPNB mol Co−1 h−1 at 120 min (Table S4,† entries 1 and 7).
The polarity of the solvent also has a significant effect on the activity of the [LnCoCl2]/MMAO (Ln = LA–LC) system for PNB synthesis. An increase in the relative polarity of the solvent from toluene (polarity 0.099) to chlorobenzene (0.188) resulted in a significant increase in the PNB yield at 25 °C. A further increase in the polarity of the solvent, i.e., using 1,2-dichloroethane (0.327) as the polymerization solvent, had a detrimental effect on the catalytic performance of the [LnCoCl2]/MMAO (Ln = LA–LC) system. Thus, the appropriate choice of polymerization solvent with optimal polarity is crucial for controlling NB polymerization (Table S5†). Polymerization in toluene was carried out at 80 °C and yields of 65–70% were obtained, indicating that the Co(II) catalysts had reasonable thermal stability.
The Co(II) system reported herein exhibited a higher yield (98% within 2 h) with a lower co-catalyst concentration ([Al]/[Co] ratio of 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 25 °C than a recently reported Pd(II) complex bearing tridentate quinoline-based ligands (76% yield within 5 min with a Al/Pd ratio of 5000
1) at 25 °C than a recently reported Pd(II) complex bearing tridentate quinoline-based ligands (76% yield within 5 min with a Al/Pd ratio of 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), although the activity of our system was slightly lower (Table 2).64 Similarly, compared to the [LnCoCl2]/MMAO system reported here, a recently studied cationic acetylacetonate bis(secondary amine) Pd(II) complex displayed a higher activity (1.3 × 105 gPNB mol Pd−1 h−1) but a lower yield (56% in 4 h) for vinyl-type NB polymerization in the presence of 25 eq. BF3·OEt2 as the co-initiator.65
1), although the activity of our system was slightly lower (Table 2).64 Similarly, compared to the [LnCoCl2]/MMAO system reported here, a recently studied cationic acetylacetonate bis(secondary amine) Pd(II) complex displayed a higher activity (1.3 × 105 gPNB mol Pd−1 h−1) but a lower yield (56% in 4 h) for vinyl-type NB polymerization in the presence of 25 eq. BF3·OEt2 as the co-initiator.65
Conclusions
A series of Co(II) complexes based on N,N,N- and N,N,O-tridentate and N,N-bidentate Schiff base ligands were studied. The geometries around the Co(II) center in [LBCoCl2] and [LCCoCl2] can be described as distorted trigonal bipyramidal and distorted tetrahedral, respectively. To optimize the polymerization of NB by the [LnCoCl2]/MMAO system, various polymerization conditions were investigated. Upon activation with MMAO, the Co(II) complexes served as promising catalysts for the vinyl-type polymerization of NB. Catalytic activities of up to 4.69 × 104 gPNB mol Co−1 h−1 at 25 °C were demonstrated by [LnCoCl2]/MMAO in chlorobenzene. This system represents the first example of a Co(II)/MMAO system yielding 99% yield at 25 °C. It has been demonstrated that the steric hindrance and electronic properties of ligand architectures influence their catalytic properties.Author contributions
K. Kim and S. Nayab contributed equally to this work. The manuscript was written through the contributions of all authors. All authors have given approval for the final version of the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This research was supported by the National Research Foundation (NRF) of Republic of Korea, funded by the Ministry of Education, Science, and Technology (MEST) (Grant No. 2019R1A2C1088654). This work was also supported by the Technology Innovation Program (TIP # 20011123, Development of Cyclic Olefin Polymer (COP) with High Heat Resistance and High Transmittance) funded by the Korea Evaluation Institute of Industrial Technology (KEIT) and the Ministry of Trade, Industry & Energy (MOTIE, Republic of Korea).Notes and references
- F. Blank and C. Janiak, Coord. Chem. Rev., 2009, 253, 827–861 CrossRef CAS.
- I. V. Nazarov, E. V. Bermesheva, K. V. Potapov, Z. B. Khesina, M. M. Il’in, E. K. Melnikova and M. V. Bermeshev, Mendeleev Commun., 2021, 31, 690–692 CrossRef CAS.
- K. H. Park, R. J. Twieg, R. Ravikiran, L. F. Rhodes, R. A. Shick, D. Yankelevich and A. Knoesen, Macromolecules, 2004, 37, 5163–5178 CrossRef CAS.
- T. Zheng, H. Liao, J. Gao, L. Zhong, H. Gao and Q. Wu, Polym. Chem., 2018, 9, 3088–3097 RSC.
- C. Janiak and P. G. Lassahn, J. Mol. Catal. A: Chem., 2001, 166, 193–209 CrossRef CAS.
- W. Kaminsky and A. Laban, Appl. Catal., A, 2001, 222, 47–61 CrossRef CAS.
- L. Boggioni, H. Harakawa, S. Losio, K. Nomura and I. Tritto, Polym. Chem., 2021, 12, 4372–4383 RSC.
- Y. Li, L. Jiang, L. Wang, H. Gao, F. Zhu and Q. Wu, Appl. Organomet. Chem., 2006, 20, 181–186 CrossRef CAS.
- B. L. Goodall, W. Risse and J. P. Mathew, WO1995014048A1, 1996.
- D. A. Barnes, G. M. Benedikt, B. L. Goodall, S. S. Huang, H. A. Kalamarides, S. Lenhard, L. H. McIntosh, K. T. Selvy, R. A. Shick and L. F. Rhodes, Macromolecules, 2003, 36, 2623–2632 CrossRef CAS.
- C. Chen, Nat. Rev. Chem., 2018, 2, 6–14 CrossRef CAS.
- J. Dong, D. Yang and B. Wang, Eur. J. Inorg. Chem., 2021, 2021, 4661–4668 CrossRef CAS.
- D. Yang, Y. Tang, H. Song and B. Wang, Organometallics, 2016, 35, 1392–1398 CrossRef CAS.
- X. Wang, B. Dong, Q. Yang, H. Liu, Y. Hu and X. Zhang, Inorg. Chem., 2021, 60, 2347–2361 CrossRef CAS PubMed.
- S. Das, V. Subramaniyan and G. Mani, Inorg. Chem., 2019, 58, 3444–3456 CrossRef CAS PubMed.
- M. C. Sacchi, M. Sonzogni, S. Losio, F. Forlini, P. Locatelli, I. Tritto and M. Licchelli, Macromol. Chem. Phys., 2001, 202, 2052–2058 CrossRef CAS.
- Z. Chen and M. Brookhart, Acc. Chem. Res., 2018, 51, 1831–1839 CrossRef CAS PubMed.
- W. H. le Roux, M. Matthews, A. Lederer, A. J. van Reenen and R. Malgas-Enus, J. Catal., 2022, 405, 571–587 CrossRef CAS.
- F. Wang and C. Chen, Polym. Chem., 2019, 10, 2354–2369 RSC.
- M. Li, X. Shu, Z. Cai and M. S. Eisen, Organometallics, 2018, 37, 1172–1180 CrossRef CAS.
- M. Li, X. Shu, Z. Cai and M. S. Eisen, Polym. Chem., 2019, 10, 2741–2748 RSC.
- J. Long, H. Gao, F. Liu, K. Song, H. Hu, L. Zhang, F. Zhu and Q. Wu, Inorg. Chim. Acta, 2009, 362, 3035–3042 CrossRef CAS.
- G. Leone, A. Boglia, A. C. Boccia, S. T. Scafati, F. Bertini and G. Ricci, Macromolecules, 2009, 42, 9231–9237 CrossRef CAS.
- F. P. Alt and W. Heitz, Macromol. Chem. Phys., 1998, 199, 1951–1956 CrossRef CAS.
- M. C. Sacchi, M. Sonzogni, S. Losio, F. Forlini, P. Locatelli, I. Tritto and M. Licchelli, Macromol. Chem. Phys., 2001, 202, 2052–2058 CrossRef CAS.
- F. Pelascini, F. Peruch, P. J. Lutz, M. Wesolek and J. Kress, Macromol. Rapid Commun., 2003, 24, 768–771 CrossRef CAS.
- D. Zhang, Q. Yue, J. Wang, G. Shigeng and L. Weng, Inorg. Chem. Commun., 2009, 12, 1193–1196 CrossRef CAS.
- L. L. Benade, S. O. Ojwach, C. Obuah, I. A. Guzei and J. Darkwa, Polyhedron, 2011, 30, 2878–2883 CrossRef CAS.
- J. Chen, Y. Huang, Z. Li, Z. Zhang, C. Wei, T. Lan and W. Zhang, J. Mol. Catal. A: Chem., 2006, 259, 133–141 CrossRef CAS.
- Y. Sato, Y. Nakayama and H. Yasuda, J. Organomet. Chem., 2004, 689, 744–750 CrossRef CAS.
- J. Lee, K. Kim, H. Lee and S. Nayab, Polyhedron, 2021, 196, 115003 CrossRef CAS.
- K. Kim, S. Nayab, A. Rim Jeong, Y. Cho, H. Yeo and H. Lee, Inorg. Chim. Acta, 2022, 539, 121025 CrossRef CAS.
- C. Anderson, M. Crespo, M. Font-Bardía, A. Klein and X. Solans, J. Organomet. Chem., 2000, 601, 22–33 CrossRef CAS.
- J. W. Shin, K. Eom and D. Moon, J. Synchrotron Radiat., 2016, 23, 369–373 CrossRef PubMed.
- Z. Otwinowski and W. Minor, Methods Enzymol., 1997, 307–326 CAS.
- G. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- SMART and SAINT-Plus v 6.22, Bruker AXS Inc., Madison, Wisconsin, USA, 2000 Search PubMed.
- G. M. Sheldrick, SADABS v 2.03, University of Göttingen, Germany, 2002 Search PubMed.
- SHELXTL v 6.10, Bruker AXS Inc, Madison, Wisconsin, USA, 2000 Search PubMed.
- S. Park, J. K. Lee, H. Lee, S. Nayab and J. W. Shin, Appl. Organomet. Chem., 2019, 33(4), e4797 CrossRef.
- X.-L. Yang, G.-Q. Zhong and L. Wu, J. Chem., 2013, 2013, 1–5 Search PubMed.
- S. H. Ahn, S.-I. Choi, M. J. Jung, S. Nayab and H. Lee, J. Mol. Struct., 2016, 1113, 24–31 CrossRef CAS.
- S. Park, J. Lee, H. Lee, A. R. Jeong, K. S. Min and S. Nayab, Appl. Organomet. Chem., 2018, 33, e4766 CrossRef.
- Y. Mei-Rong, S. Yu and X. Yong-Jin, SpringerPlus, 2014, 3, 701 CrossRef PubMed.
- S. Kulandaivalu, Z. Zainal and Y. Sulaiman, Int. J. Polym. Sci., 2016, 2016, 1–12 CrossRef.
- M. Vairalakshmi, R. Princess, B. K. Rani and S. J. Raja, J. Chil. Chem. Soc., 2018, 63, 3844–3849 CrossRef CAS.
- S. Chandra and R. Kumar, Transition Met. Chem., 2004, 29, 269–275 CrossRef CAS.
- A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn and G. C. Verschoor, J. Chem. Soc., Dalton Trans., 1984, 1349–1356 RSC.
- D. C. Crans, M. L. Tarlton and C. C. McLauchlan, Eur. J. Inorg. Chem., 2014, 2014, 4450–4468 CrossRef CAS.
- L. Yang, D. R. Powell and R. P. Houser, Dalton Trans., 2007, 955–964 RSC.
- L. Falivene, R. Credendino, A. Poater, A. Petta, L. Serra, R. Oliva, V. Scarano and L. Cavallo, Organometallics, 2016, 35, 2286–2293 CrossRef CAS.
- A. R. Jeong, S. Nayab, E. Kim, H. Yeo and H. Lee, J. Mol. Struct., 2022, 1264, 133238 CrossRef CAS.
- H. Yuan, H. Liu, F. You and X. Shi, Inorg. Chem. Commun., 2020, 119, 108139 CrossRef CAS.
- J. Deng, H. Gao, F. Zhu and Q. Wu, Organometallics, 2013, 32, 4507–4515 CrossRef CAS.
- V. Appukuttan, J. H. Kim, C. S. Ha and I. Kim, Korean J. Chem. Eng., 2008, 25, 423–425 CrossRef CAS.
- Z.-J. Yao and G.-X. Jin, Coord. Chem. Rev., 2013, 257, 2522–2535 CrossRef CAS.
- F. You, H. Liu, G. Luo and X. Shi, Dalton Trans., 2019, 48, 12219–12227 RSC.
- E. Y.-X. Chen and T. J. Marks, Chem. Rev., 2000, 100, 1391–1434 CrossRef CAS PubMed.
- W. Kaminsky, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 3911–3921 CrossRef CAS.
- S. D. Ittel, L. K. Johnson and M. Brookhart, Chem. Rev., 2000, 100, 1169–1204 CrossRef CAS PubMed.
- D. H. Lee, J. H. Lee, G. H. Eom, H. G. Koo, C. Kim and I. M. Lee, Bull. Korean Chem. Soc., 2011, 32, 1884–1890 CrossRef CAS.
- Y. M. Xu, K. Li, Y. Wang, W. Deng and Z. J. Yao, Polymers, 2017, 9, 105 CrossRef PubMed.
- H. Liu, H. Yuan and X. Shi, Dalton Trans., 2019, 48, 609–617 RSC.
- H. Liu, H. Yuan and X. Shi, Inorg. Chem. Commun., 2020, 119, 108139 CrossRef.
- H. Liu, H. Yuan and X. Shi, J. Mol. Struct., 2017, 1133, 411–421 CrossRef.
Footnotes |
| † X-Ray crystallography with PLS-II 2D-SMC beamline was supported by MSIP and POSTECH. CCDC 2178180 and 2178181 contain the supplementary crystallographic data for [LBCoCl2] and POSTECH [LCCoCl2]. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ra07241f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |