Open Access Article
Open Access ArticleNon-metal boron atoms on a CuB12 monolayer as efficient catalytic sites for urea production†
Changyan
Zhu
 a,
Chaoxia
Wen
a,
Miao
Wang
a,
Min
Zhang
a,
Chaoxia
Wen
a,
Miao
Wang
a,
Min
Zhang
 *a,
Yun
Geng
*a,
Yun
Geng
 a and
Zhongmin
Su
a and
Zhongmin
Su
 *ab
*ab
aInstitute of Functional Material Chemistry, Faculty of Chemistry, National & Local United Engineering Laboratory for Power Batteries, Northeast Normal University, Changchun 130024, China. E-mail: mzhang@nenu.edu.cn
bSchool of Chemistry and Environmental Engineering, Changchun University of Science and Technology, Changchun 130022, China
First published on 24th December 2021
Abstract
An electrocatalytic C–N coupling reaction to convert CO2 and N2 into urea under mild conditions has been proposed to be a promising alternative experimentally, but the development of highly stable, low-cost and high-performance non-metal catalytic sites remains rare and challenging. Herein, a global-minimum CuB12 monolayer with superior stability has been identified based on first-principles computations, and the most significant finding is that the CuB12 monolayer possesses the best catalytic activity among the reported urea catalysts thermodynamically and kinetically. All possible reaction pathways to form urea (NH2CONH2) starting from the CO2 molecule and N2 molecule, including the CO2 pathway, OCOH pathway, CO pathway, NCON pathway and mixed pathway, as well as the kinetic energy barriers of six possible C–N coupling reactions are systematically investigated. Non-metal B atoms at the midpoint of the edges of the squares act as excellent catalytic sites with a limiting potential of urea production of 0.23 V through the CO2 pathway and OCOH pathway and the lowest kinetic energy barrier of C–N bond formation (0.54 eV) through the reaction *CO + *NHNH → *NHCONH. Therefore, this study not only identifies the first non-metal B catalytic sites for urea formation, but also perfects the reaction mechanism to convert CO2 and N2 into urea, which could provide great guiding significance to explore other high-performance urea catalysts.
Introduction
Electrocatalytic technology is considered to be the most promising strategy to replace energy-inefficient traditional industrial processes and achieve environmentally friendly approaches under ambient conditions.1–5 In the last 20 years, electrocatalytic technology has been widely applied in various fields, including electrocatalytic CO2 reduction reactions (CO2RR) to alleviate the greenhouse effect,6–8 electrocatalytic N2 reduction reactions (NRR) to reduce energy consumption in the Haber–Bosch process,9–11 electrocatalytic oxygen reduction and evolution reactions (ORR/OER) to promote the industrial applications of metal–air batteries,12–14 and electrocatalytic hydrogen evolution reaction (HER) to obtain nonpolluting and zero emission energy resources.15–17 In particular, the exploration of superior electrocatalysts for a lower-barrier C–C coupling reaction and highly effective activation of inert N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bonds has always been of great concern and has made great progress until now. The faradaic efficiency has increased to 79 ± 2% for the electrosynthesis of the C2 product on boron-doped copper catalysts through tuning the ratio of Cuδ+ to Cu0 active sites.18 A high faradaic efficiency of 15.4 ± 1% toward the C3 product (n-propanol) has also been achieved on a double sulfur vacancy-rich CuS catalyst at −1.05 V versus the reversible hydrogen electrode, and the partial current density reaches up to 9.9 mA cm−2 at −0.85 V in flow cells, which is the best reported electrochemical CO2 reduction toward n-propanol.19 A faradaic efficiency of 56.55% and an NH3 yield rate of 7.48 μg h−1 mg−1 starting from the N2 molecule have been realized on a single-atom dispersed Fe–N–C catalyst under ambient conditions.20
N triple bonds has always been of great concern and has made great progress until now. The faradaic efficiency has increased to 79 ± 2% for the electrosynthesis of the C2 product on boron-doped copper catalysts through tuning the ratio of Cuδ+ to Cu0 active sites.18 A high faradaic efficiency of 15.4 ± 1% toward the C3 product (n-propanol) has also been achieved on a double sulfur vacancy-rich CuS catalyst at −1.05 V versus the reversible hydrogen electrode, and the partial current density reaches up to 9.9 mA cm−2 at −0.85 V in flow cells, which is the best reported electrochemical CO2 reduction toward n-propanol.19 A faradaic efficiency of 56.55% and an NH3 yield rate of 7.48 μg h−1 mg−1 starting from the N2 molecule have been realized on a single-atom dispersed Fe–N–C catalyst under ambient conditions.20
Inspired by the C–C coupling reaction and the activation of the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond, electrocatalytic C–N bond formation to convert CO2 molecules and N2 molecules into urea (NH2CONH2) under ambient conditions has been proposed to be a promising alternative to harsh industrial processes.21 Urea is regarded as one of the most important nitrogen fertilizers with a high nitrogen content (46%),22 while its industrial synthesis requires very large energy consumption under harsh reaction conditions (350–550 °C and 150–350 bar) and emits a massive amount of the green-house gas CO2.23–26 Therefore, the exploration of high-performance catalysts for urea production is a significant challenge and has attracted tremendous attention since 2020. In 2020, Chen et al. have successfully realized electrochemical urea synthesis with a formation rate of 3.36 mmol g−1 h−1 and a faradaic efficiency of 8.92% at −0.4 V versus the reversible hydrogen electrode via PdCu alloy nanoparticles on TiO2 nanosheets.21 In 2021, Yuan et al. have achieved a higher urea formation rate and faradaic efficiency at the same applied potential via Mott–Schottky Bi–BiVO4 heterostructures (5.91 mmol g−1 h−1 and 12.55%) and BiFeO3/BiVO4 heterojunctions (4.94 mmol g−1 h−1 and 17.18%), respectively.27,28 Meanwhile, Meng et al. reported that the urea faradaic efficiency increased to 23.26% at −0.79 V versus the reversible hydrogen electrode on oxygen vacancy-rich ZnO porous nanosheets by using CO2 and nitrite contaminants as precursors.29 Recently, Zhu et al. have proposed three MBene (Mo2B2, Ti2B2 and Cr2B2) two-dimensional (2D) materials as excellent electrocatalysts for urea formation with limiting potentials ranging from −0.49 eV to −0.65 eV by means of DFT methods.30 It is noted that urea is formed all on the metal sites in previous reports. The non-metal B atom possesses both empty and occupied p orbitals, which results in unique advantages to efficiently adsorb and activate the inert N2 molecule by accepting the lone-pair electrons of N2 and donating electrons to the anti-bonding orbitals of N2 to weaken the N
N triple bond, electrocatalytic C–N bond formation to convert CO2 molecules and N2 molecules into urea (NH2CONH2) under ambient conditions has been proposed to be a promising alternative to harsh industrial processes.21 Urea is regarded as one of the most important nitrogen fertilizers with a high nitrogen content (46%),22 while its industrial synthesis requires very large energy consumption under harsh reaction conditions (350–550 °C and 150–350 bar) and emits a massive amount of the green-house gas CO2.23–26 Therefore, the exploration of high-performance catalysts for urea production is a significant challenge and has attracted tremendous attention since 2020. In 2020, Chen et al. have successfully realized electrochemical urea synthesis with a formation rate of 3.36 mmol g−1 h−1 and a faradaic efficiency of 8.92% at −0.4 V versus the reversible hydrogen electrode via PdCu alloy nanoparticles on TiO2 nanosheets.21 In 2021, Yuan et al. have achieved a higher urea formation rate and faradaic efficiency at the same applied potential via Mott–Schottky Bi–BiVO4 heterostructures (5.91 mmol g−1 h−1 and 12.55%) and BiFeO3/BiVO4 heterojunctions (4.94 mmol g−1 h−1 and 17.18%), respectively.27,28 Meanwhile, Meng et al. reported that the urea faradaic efficiency increased to 23.26% at −0.79 V versus the reversible hydrogen electrode on oxygen vacancy-rich ZnO porous nanosheets by using CO2 and nitrite contaminants as precursors.29 Recently, Zhu et al. have proposed three MBene (Mo2B2, Ti2B2 and Cr2B2) two-dimensional (2D) materials as excellent electrocatalysts for urea formation with limiting potentials ranging from −0.49 eV to −0.65 eV by means of DFT methods.30 It is noted that urea is formed all on the metal sites in previous reports. The non-metal B atom possesses both empty and occupied p orbitals, which results in unique advantages to efficiently adsorb and activate the inert N2 molecule by accepting the lone-pair electrons of N2 and donating electrons to the anti-bonding orbitals of N2 to weaken the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond.31–33 Moreover, the electron-deficient B atom can also adsorb and activate the inert CO2 by providing electrons to CO2 and breaking the inherent π bond.34–36 Therefore, the non-metal boron atom possesses unique advantages to efficiently adsorb and activate the CO2 molecule and N2 molecule, simultaneously, which is a prerequisite to synthesize urea by the C–N coupling reaction. Further considering the B atom's intrinsic merits of low cost, environmental friendliness and long durability,37–39 the development of non-metal B catalytic sites for urea production possesses great scientific significance.
N triple bond.31–33 Moreover, the electron-deficient B atom can also adsorb and activate the inert CO2 by providing electrons to CO2 and breaking the inherent π bond.34–36 Therefore, the non-metal boron atom possesses unique advantages to efficiently adsorb and activate the CO2 molecule and N2 molecule, simultaneously, which is a prerequisite to synthesize urea by the C–N coupling reaction. Further considering the B atom's intrinsic merits of low cost, environmental friendliness and long durability,37–39 the development of non-metal B catalytic sites for urea production possesses great scientific significance.
In this work, the entirely planar CuB12 monolayer with superior stability has been identified to be a global-minimum configuration. Most importantly, the non-metal B atoms at the midpoint of the edge of the square are confirmed to be excellent catalytic sites on the CuB12 monolayer with a limiting potential of urea production of 0.23 V through the CO2 pathway and OCOH pathway and the lowest kinetic energy barrier of C–N bond formation (0.54 eV) through the reaction *CO + *NHNH → *NHCONH, which presents the best catalytic activity thermodynamically and kinetically among the reported urea catalysts. Furthermore, the competitive CH3OH and CH4 products can be significantly suppressed. In addition, all possible reaction pathways starting from the CO2 molecule and N2 molecule for urea production, including the CO2 pathway, OCOH pathway, CO pathway, NCON pathway and mixed pathway, are plotted and investigated in detail.
Results and discussion
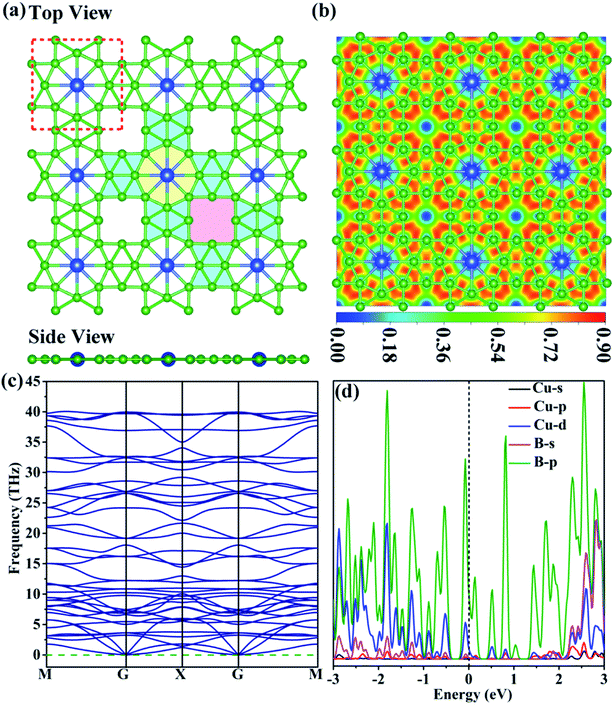
Structure, stability and electronic properties of the CuB12 monolayer
The entirely planar CuB12 monolayer with the space group of P4/MMM is obtained and confirmed to be the global-minimum structure after a comprehensive search combined with first-principles calculation. The unit cell of the CuB12 monolayer consists of one Cu atom and twelve B atoms in a square shape with the optimized lattice parameters of 6.18 Å (Fig. 1a and Table S2†). In the CuB12 monolayer, the B8-unit (blue-green colored parts in Fig. 1a) is formed by eight B atoms via multi-center multi-electron bonds, which is consistent with that in the predicted α-FeB6 monolayer and is regarded as a subunit.40 The entire planar periodic framework can be considered to be an extended framework of the B8-unit along a and b directions by sharing the vertex. Meanwhile, two different eight-membered rings are constructed, including a round eight-membered ring with one embedded Cu atom (yellow colored part in Fig. 1a) and a square eight-membered ring with no anchored metal atom (pink colored part in Fig. 1a). Hence, each Cu atom binds with eight adjacent B atoms to form a planar hyper-coordinate moiety with 2.13 and 2.22 Å Cu–B bond lengths, and the Cu atom donates electrons to the adjacent B atoms to stabilize the electron-deficient boron framework. This CuB12 monolayer develops planar hyper-coordinate 2D materials.41–44 The computed electron transfer from the Cu atom to the boron framework is 0.46|e| based on the Bader charge,45 which is also further supported by its electron location function (ELF) map46 in the (001) direction (Fig. 1b). Moreover, the localized electron density over 0.75 among B atoms and uniform electron density around 0.50 in the whole ELF map suggest that strong bonding among B atoms exists and the delocalized electrons are distributed in the whole monolayer framework, which guarantees the superior stability of the CuB12 monolayer. Remarkably, the square eight-membered ring can still exist in a steady state without any metal atom, which can be attributed to the multi-center multi-electron bonds with an ultrahigh electron density of 0.90 in the square eight-membered ring. The Bader charge analysis indicates that each B atom at the midpoint of the edges of the squares possesses +0.30|e|, which is expected to be potential catalytic sites.To evaluate the relative stability of the predicted CuB12 monolayer, its thermodynamic, kinetic, thermal and mechanical stabilities are all examined. The cohesive energy of the CuB12 monolayer is first computed to inspect the thermodynamic stability and to evaluate the binding strength of the connected framework, which is defined as Ecoh = (ECu + 12EB − ECuB12)/13, in which ECu, EB and ECuB12 are the energies of a single Cu atom, a single B atom, and the CuB12 monolayer, respectively. The calculated cohesive energy of the CuB12 monolayer (5.71 eV per atom) is comparable to those of the predicted α-FeB6 monolayer with the same B8-unit (5.79 eV per atom),40 the predicted ScB12 monolayer with the same stoichiometry (5.94 eV per atom),47 the predicted Ni2B5 monolayer (5.82 eV per atom),48 and the experimentally available borophenes (5.90 eV per atom for triangular-borophene,49 5.95 eV per atom for β12-borophene,50 and 5.96 eV per atom for χ3-borophene50). Such cohesive energy indicates that the CuB12 monolayer has a strongly bonded network and excellent thermodynamic stability. The kinetic stability of the CuB12 monolayer is then confirmed by its phonon spectrum with no imaginary phonon modes (Fig. 1c). Its highest frequency of 1334 cm−1 (40 THz) is comparable to those of the α-FeB6 monolayer (1316 cm−1),40 ScB12 monolayer (1290 cm−1),47 and AlB6 nanosheet (1150 cm−1).51 The phonon densities of states (PDOSs) of the CuB12 monolayer presents the highest frequency corresponding to the B–B interactions, indicating robust B–B bonds in the B8-unit (Fig. S1†). These data suggest the good kinetic stability of the CuB12 monolayer. The outstanding thermal stability of the CuB12 monolayer is also confirmed by AIMD simulations, because the CuB12 monolayer can well maintain its original configuration with the B8-unit up to 1200 K at the end of 10 ps MD simulation (Fig. S2†). Moreover, an explicit solvent environment is also evaluated to verify the stability of the CuB12 monolayer under aqueous conditions. 112 H2O molecules are present on the 3 × 3 supercell surface, which corresponds to the number of H2O in 6 layers of pristine ice. The integral structure of the CuB12 monolayer at 300 K at the end of 5 ps AIMD simulations can be well kept under aqueous conditions (Fig. S3†), suggesting its stability in the explicit solvent environment. The mechanical stability of the CuB12 monolayer is examined by using computed elastic constants (C11 = 194.93 N m−1, C22 = 215.99 N m−1, C12 = C21 = 46.59 N m−1 and C44 = 19.87 N m−1). These data meet the Born criteria: C11C22 – C122 > 0 and C66 > 0,52 indicating that the CuB12 monolayer possesses good mechanical stability. Finally, the interlayer strengths in the complete overlap stacking (AA) and the crossing overlap stacking (AB) bilayers are both examined (Fig. S4†). The computed interlayer energies suggest that the AB bilayer is more favorable in energy with a relatively higher interaction energy of 38.4 meV per atom and a relatively shorter interlayer distance of 3.19 Å, which is weaker than the corresponding value of the graphene bilayer (141 meV per atom at a distance of 3.08 Å).53 The reason for the AB bilayers possessing a stronger interaction energy is that the electron repulsion between the upper-layer Cu atom and the vacant site of the eight-membered ring in the sub-layer is weaker than that in the AA bilayers with the electrostatic repulsion between two Cu atoms in the upper layer and the sub-layer. In addition, the ELF plot also indicates that there is no obvious electron location between the layers. As mentioned above, the CuB12 monolayer satisfies all stable conditions to be a promising 2D material, which provides a prerequisite for the wide utilization of a catalyst.
It is known that the activity of the catalyst is essentially governed by its electronic properties. Hence, the projected density of states (PDOS) of the CuB12 monolayer is computed to preliminarily evaluate its potentiality as a catalyst for urea production. It is clear that the CuB12 monolayer is intrinsically metallic due to no gap at the Fermi level using the PBE functional (Fig. 1d). The conducting nature is mainly originated from the p orbitals of B atoms due to the highest contribution near the Fermi level. Moreover, the co-existence of the occupied and unoccupied p-orbitals near the Fermi level can regulate the moderate “acceptation-donation” interaction between catalytic sites and reaction intermediates.54,55 The outstanding electronic conductivity and the moderate “acceptation-donation” interaction are both beneficial for its application as an electrocatalyst.
Reaction mechanism of electrocatalytic urea production
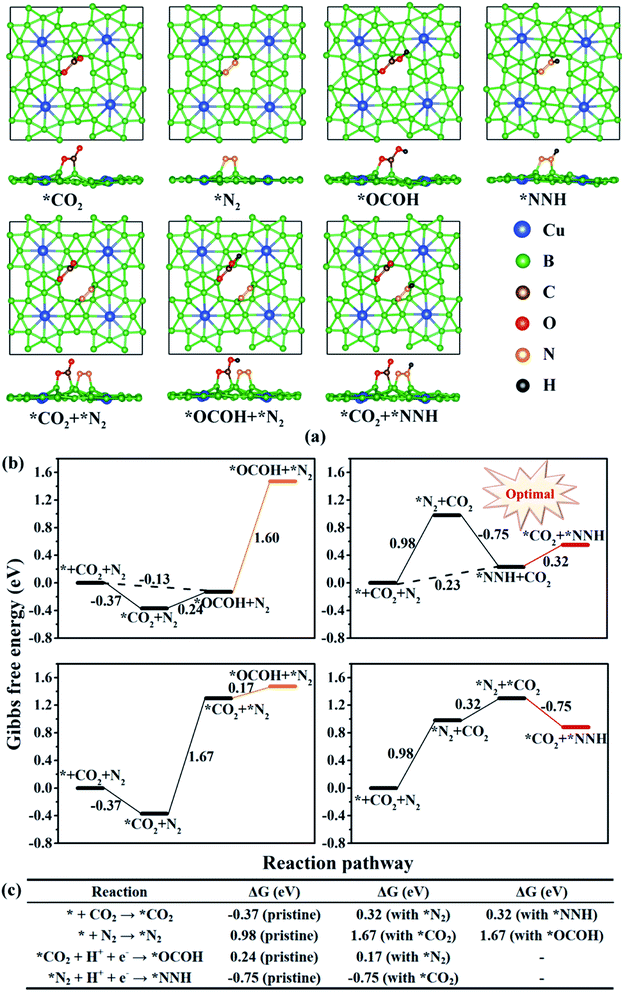
The reaction mechanism for electrochemical urea production starting from CO2 and N2 has been proposed in 2020, in which six consecutive protonation and reduction processes are involved.21 In the proposed reaction pathway, the adsorption of the *OCOH intermediate and the subsequent reduction to *CO are the first two protonation pathways, and they are also significant to further react for urea production. Subsequently, the C–N coupling reaction of *CO and *N2 to form a *NCON intermediate is a kinetically determining factor in synthesizing urea. The *NCON intermediate is continuously hydrogenated to *NCONH, *NHCONH/*NCONH2, *NHCONH2, and *NH2CONH2, and then urea is finally released. This reaction pathway is named the NCON pathway in our work (marked in purple in Fig. 2a). However, is the NCON pathway the only reaction pathway for urea formation? To better explain the issue and complement the reaction mechanism of urea production, we review previous reports about C–N bonding formation and urea formation. Two valuable pieces of evidence are noticed: (1) the reduction of adsorbed *N2 to *NNH with accessible Gibbs free energy change values (ΔG <0.75 eV) on most catalyst surfaces;27,28,30 (2) the C–N bonding formation originated from *CO and *NH2 on Te-doped Pd nanocrystals.56 These results imply that it is also possible to form a C–N bond through *CO and *N2Hx intermediates. Therefore, we propose another three potential reaction pathways for electrochemical urea production. According to the CO2 reduction step, they are named the CO2 pathway marked in red, the OCOH pathway marked in blue and the CO pathway marked in orange, respectively (Fig. 2a). The potential reaction intermediates on the CuB12 monolayer in the aforementioned four reaction pathways are all optimized (side view in Fig. 2b and top view in S5†). Their detailed information, including total energy, zero-potential correction energy and entropy contribution energy, is also given in Table S3.†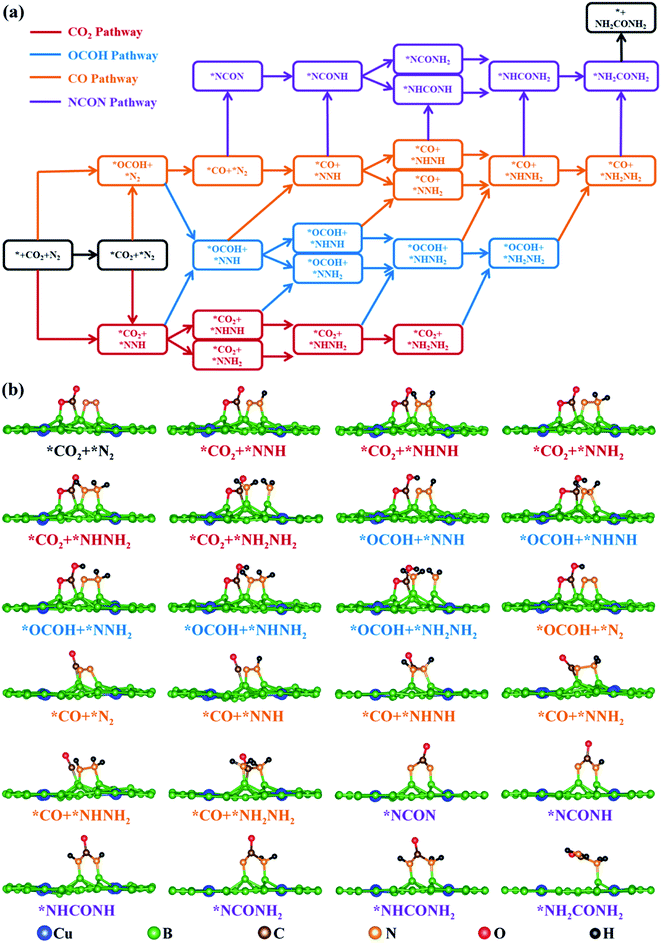 | ||
| Fig. 2 (a) Schematic depiction of all possible mechanisms for urea production. (b) Side view of all optimized possible reaction intermediates for urea production on the CuB12 monolayer. | ||
In these four reaction pathways, the effective adsorption of *CO2 and *N2 on the catalyst surface is a prerequisite for electrochemical urea production. Moreover, *CO2 and the first hydrogenated *NNH, or the first hydrogenated *OCOH and *N2 as initial reactants are also feasible for urea production, because the inert CO2 molecule and N2 molecule can be directly activated and reduced to *OCOH and *NNH by the surface *H. In the CO2 pathway, the first four proton-coupled electron transfer steps convert the N2 molecule to a *NH2NH2 intermediate, and the following two proton-coupled electron transfer steps reduce *CO2 to a *CO intermediate. The coupling reaction between the *CO intermediate and *NH2NH2 intermediate promotes the C–N bonding formation and the *NH2CONH2 product formation. In the OCOH pathway, the adsorbed *CO2 is preferentially hydrogenated to a *OCOH intermediate. The formed *OCOH intermediate remains until the inert N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond is completely broken and converted into a *NH2NH2 intermediate. After that, the *OCOH intermediate is further reduced to a *CO intermediate and simultaneously one H2O molecule is released. In the CO pathway, the hydrogenation process of the N2 molecule has just begun after the adsorbed *CO2 is continuously reduced to a *CO intermediate. Specifically, the C–N coupling reaction can occur in any elementary reaction in this CO pathway, including *CO + *N2 → *NCON, *CO + *NNH → *NCONH, *CO + *NNH2 → *NCONH2, *CO + *NHNH → *NHCONH, *CO + *NHNH2 → *NHCONH2, and *CO + *NH2NH2 → *NH2CONH2. Moreover, many mixed pathways can be feasible in Fig. 2a, such as * + CO2 + N2 → *NNH + CO2 → *CO2 + *NNH → *CO2 + *NHNH → *OCOH + *NHNH → *CO + *NHNH → *NHCONH → *NHCONH2 → *NH2CONH2 → * + NH2CONH2, * + CO2 + N2 → *NNH + CO2 → *CO2 + *NNH → *OCOH + *NNH → *OCOH + *NNH2 → *CO + *NNH2 → *NCONH2 → *NHCONH2 → *NH2CONH2 → * + NH2CONH2, * + CO2 + N2 → *NNH + CO2 → *CO2 + *NNH → *CO2 + *NHNH → *OCOH + *NHNH → *OCOH + *NHNH2 → *CO + *NHNH2→ *NHCONH2 → *NH2CONH2 → * + NH2CONH2 and so on. The schematic depiction presents greater possibilities and more reaction pathways for C–N bonding formation and urea production.
N triple bond is completely broken and converted into a *NH2NH2 intermediate. After that, the *OCOH intermediate is further reduced to a *CO intermediate and simultaneously one H2O molecule is released. In the CO pathway, the hydrogenation process of the N2 molecule has just begun after the adsorbed *CO2 is continuously reduced to a *CO intermediate. Specifically, the C–N coupling reaction can occur in any elementary reaction in this CO pathway, including *CO + *N2 → *NCON, *CO + *NNH → *NCONH, *CO + *NNH2 → *NCONH2, *CO + *NHNH → *NHCONH, *CO + *NHNH2 → *NHCONH2, and *CO + *NH2NH2 → *NH2CONH2. Moreover, many mixed pathways can be feasible in Fig. 2a, such as * + CO2 + N2 → *NNH + CO2 → *CO2 + *NNH → *CO2 + *NHNH → *OCOH + *NHNH → *CO + *NHNH → *NHCONH → *NHCONH2 → *NH2CONH2 → * + NH2CONH2, * + CO2 + N2 → *NNH + CO2 → *CO2 + *NNH → *OCOH + *NNH → *OCOH + *NNH2 → *CO + *NNH2 → *NCONH2 → *NHCONH2 → *NH2CONH2 → * + NH2CONH2, * + CO2 + N2 → *NNH + CO2 → *CO2 + *NNH → *CO2 + *NHNH → *OCOH + *NHNH → *OCOH + *NHNH2 → *CO + *NHNH2→ *NHCONH2 → *NH2CONH2 → * + NH2CONH2 and so on. The schematic depiction presents greater possibilities and more reaction pathways for C–N bonding formation and urea production.
Catalytic activity of the CuB12 monolayer toward urea production
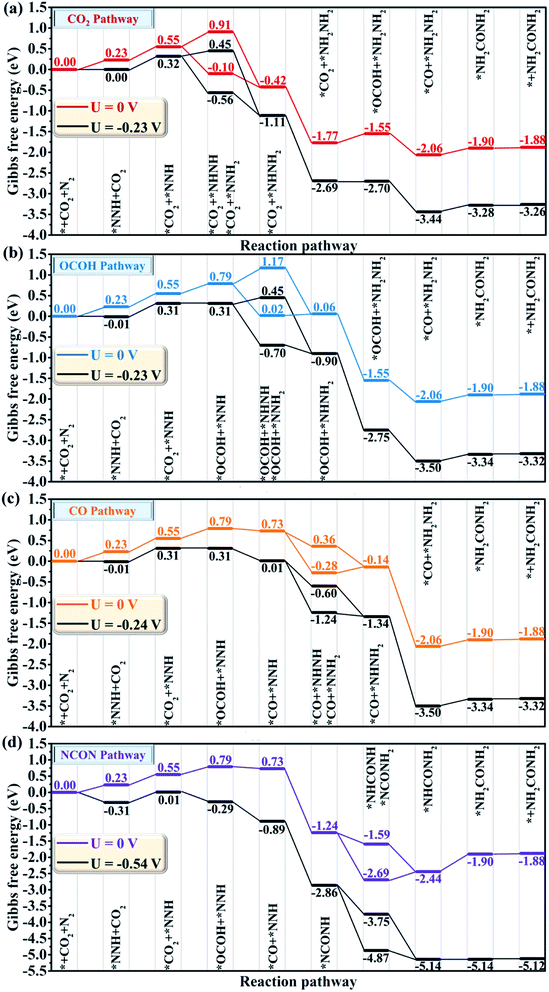
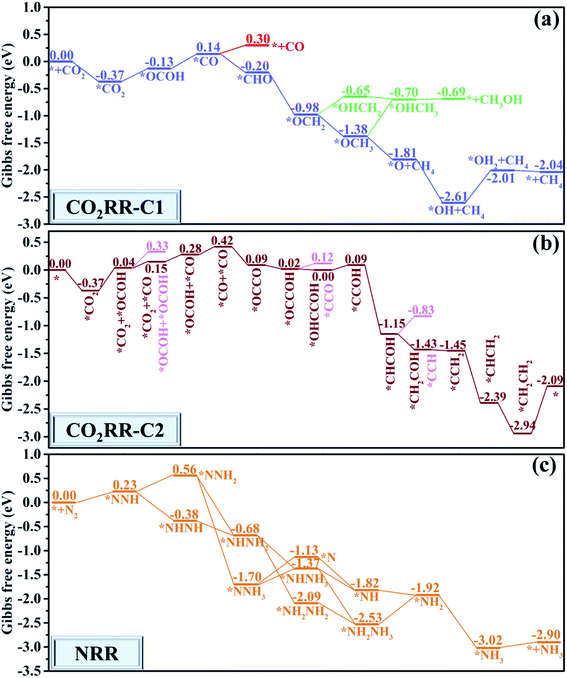
After complementing the reaction mechanism for electrochemical urea production starting from CO2 and N2, the catalytic activity and the optimal reaction process are further evaluated on the CuB12 monolayer. The optimal catalytic sites and the initial adsorption configurations of *CO2 and *N2 are first screened according to the ΔG values. Herein, thirteen possible configurations of the CO2 molecule and N2 molecule adsorbed on the CuB12 monolayer are considered (Fig. S6 and S7†). The computed ΔG values indicate that CO2 and N2 are favorably adsorbed on the midpoint-B atoms of squares (Fig. 3a, *CO2 and *N2). The adsorption of *CO2 is exothermic with a downhill energy of −0.37 eV, while the adsorption of *N2 is endothermic with an uphill energy of 0.98 eV (Fig. 3b). Nevertheless, the C–O and N–N bond lengths are both still elongated compared to the free CO2 and N2 molecules (from 1.17 Å to 1.22 Å and 1.38 Å for the C–O bond and from 1.16 Å to 1.24 Å for the N–N bond), and the linear structure of the free CO2 molecule is greatly bent with the O–C–O angle being 121.09°. It is noted that some catalysts possessing very weak interaction with CO2 and N2 can still covert them to the corresponding CO, CH4 and NH3, which can be interpreted by the effective adsorption of the first hydrogenated *OCOH and *NNH intermediates.21,57–64 Therefore, *OCOH and *NNH are also considered to be the initial C-based reactant and N-based reactant (Fig. 3a, *OCOH and *NNH). Interestingly, the computed ΔG values are both small, that is −0.13 eV and 0.23 eV for the reaction * + CO2 + H+ + e− → *OCOH and * + N2 + H+ + e− → *NNH, respectively (Fig. 3b). As a result, four possible reaction pathways are proposed to form the first hydrogenated intermediate for urea production (Fig. 3b), including * + CO2 + N2 + H+ + e− → *OCOH + N2 → *OCOH + *N2, * + CO2 + N2 + H+ + e− → *NNH + CO2 → *CO2 + *NNH, * + CO2 + N2 → *CO2 + N2 → *CO2 + *N2 + H+ + e− → *OCOH + *N2, and * + CO2 + N2 → *N2 + CO2 → *CO2 + *N2 + H+ + e− → *CO2 + *NNH. Among them, the * + CO2 + N2 + H+ + e− → *NNH + *CO2 + *NNH reaction is regarded as the optimal reaction pathway with the lowest ΔG value of 0.23 eV, and the *CO2 + *NNH intermediate is regarded as the optimal initial reactant for urea production. Moreover, the ΔG value is the same or slightly lower compared with the elementary reaction *CO2 + H+ + e− → *OCOH and *N2 + H+ + e− → *NNH when another N2 molecule or CO2 molecule emerged in the adjacent catalytic sites (Fig. 3c). Hence, it can be deduced that the adsorbed *N2 or *CO2 can facilitate the hydrogenation process of the adjacent sites, which is consistent with previous reports.21,27,28 However, the adsorption of the CO2 molecule and N2 molecule is more difficult when the adjacent catalytic site is occupied by another molecule or intermediate, and the adsorption free energy increases by ∼0.60 eV (Fig. 3c). It is speculated that the delocalized electrons and the multi-center multi-electron bonds are destroyed somewhat in the squares after the first molecule or intermediate is adsorbed, which results in an increased ΔG value for the following adsorption process. Importantly, the concentration, the ratio and the cycling period/interval of reactants CO2 and N2 must be optimized to maximize the production of urea and to suppress the competitive side reaction as mentioned for the PdCu alloy nanoparticles on TiO2 nanosheets.21The whole reaction process for urea production beginning with the first hydrogenation intermediate of *CO2 + *NNH through the CO2 pathway, the OCOH pathway, the CO pathway, the NCON pathway and the mixed pathway on the CuB12 monolayer is systematically investigated and analyzed (Fig. 4 and S8†). In the CO2 and OCOH pathways, the first hydrogenation process (* + N2 + H+ + e− → *NNH) is its potential determining step for urea production. The corresponding ΔG value for this step is 0.23 eV, which decreases to the lowest value among all previous reports (0.78 eV for the PdCu surface,21 0.48 eV for Mott–Schottky Bi–BiVO4 heterostructures,27 0.54 eV for BiFeO3/BiVO4 heterojunctions,28 0.49 eV for Mo2B2,30 0.65 eV for Ti2B2,30 and 0.52 eV for Cr2B2 (ref. 30)). In the CO pathway, the *OCOH + *NNH intermediate formation from the *CO2 + *NNH intermediate is its potential determining step, and the computed ΔG value is 0.24 eV. In the aforementioned three pathways, the formation of urea is very straightforward after the formation of the first hydrogenation intermediate for carbon-based (*OCOH) and nitrogen-based (*NNH) reactants. In the NCON and mixed pathways, the potential determining step is the final hydrogenation step from *NHCONH2 to *NH2CONH2 with a positive ΔG value of 0.54 eV. Therefore, the maximum limiting potential for different reaction pathways on the CuB12 monolayer is only 0.54 V, indicating superior electrocatalytic activity toward urea formation. Moreover, the formed urea molecule on the CuB12 monolayer can be easily released due to the negligible desorption free energy value (0.02 eV).
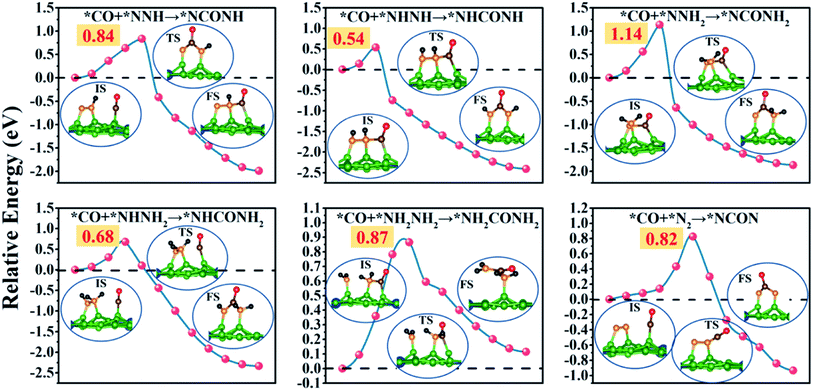
Remarkably, the C–N coupling reactions through the simultaneously adsorbed *CO and various *N2Hy intermediates (*NNH, *NHNH, *NNH2, *NHNH2, and *NH2NH2) are all thermodynamically exothermic, expect for *CO + NH2NH2 → *NH2CONH2 with a slightly positive ΔG value of 0.11 eV. In particular, the C–N coupling products *NCONH, *NHCONH, *NCONH2, and *NHCONH2 are lower by ∼2.0 eV in energy than the corresponding C–N coupling reactants *CO + *NNH, *CO + *NHNH, *CO + *NNH2, and *CO + *NHNH2. Thermodynamic evaluation demonstrates that the C–N bond formation is feasible through various pathways. Their kinetic energy barriers are also investigated to further evaluate the feasibility of forming the urea molecule (Fig. 5). The computed kinetic energy barrier is 0.84 eV for *NCONH, 0.54 eV for *NHCONH, 1.14 eV for *NCONH2, 0.68 eV for *NHCONH2 and 0.87 eV for *NH2CONH2, respectively. Among them, the lowest kinetic energy barrier is 0.54 eV for the reaction *CO + NHNH → *NHCONH, which is also lower compared with those in previous reports (0.79 eV for the PdCu surface,21 0.58 eV for Mo2B2,30 0.81 eV for Ti2B2,30 and 0.71 eV for Cr2B2 (ref. 30)), indicating that the C–N bond coupling on the CuB12 monolayer is kinetically feasible. Moreover, the computed kinetic energy barriers for *CO and *N2Hy are comparable to or even lower than that of the direct coupling reaction between *CO and *N2 (the reaction *CO + NNH2 → *NCONH2 with a 1.14 eV kinetic energy barrier is excepted), which indicates that the formation of *NCON species from *CO and *N2 is not the sole reaction pathway for urea production. Therefore, it is of significance to complement the reaction mechanism and investigate the optimal formation pathway among all the potential schemes for urea production.
Catalytic selectivity of the CuB12 monolayer toward urea production
Besides the outstanding stability and activity, the catalytic selectivity toward urea formation is another intrinsic characteristic that influences the faradaic efficiency. The computed ΔG value for the H atom adsorbed on three different types of B atoms (from −0.27 eV to 0.14 eV in Fig. S9†) is comparable to that for the first hydrogenation step (−0.13 eV for * + CO2 + H+ + e− → *OCOH and 0.23 eV * + N2 + H+ + e− → *NNH). The HER can be efficiently suppressed experimentally by adjusting the electrolyte pH under neutral conditions. It is thus possible to maximize the urea production with real experimental adjustments and suppress the influence of the HER through applied potentials.61,65 Meanwhile, the adsorbed *H can serve as a proton source to interact with other reaction intermediates via the Langmuir–Hinshelwood mechanism.30,66,67The catalytic selectivity toward urea production compared to the competitive CO2RR to the C1 product is assessed. As shown in Fig. 6a and S10,† three possible reduction products (CO, CH3OH and CH4) are considered in the CO2RR side reaction. Their desorption free energies are 0.16 eV for CO, 0.01 eV for CH3OH, and −0.03 eV for CH4, respectively, indicating that these possible reduction products can be easily released. Moreover, another possible intermediate *OCHO for the first hydrogenation step is not considered due to the larger kinetic energy barrier starting from the adsorbed *CO2. For the 2e− reduction product CO, the first hydrogenation process (*CO2 + H+ + e− → *OCOH) is its potential determining step with a ΔG value of 0.23 eV. The formed CO molecule can further react with the *N2Hy intermediates via the Eley–Rideal mechanism, which is also beneficial for urea formation.30,66,67 For the 6e− reduction product CH3OH, the reaction *OCH2 + H+ + e− → *OHCH2 is the potential determining step with a ΔG value of 0.33 eV. Remarkably, the *OCH2 intermediate is attacked by a proton-electron (H+/e−) pair to form the *OCH3 intermediate with a negative ΔG value of −0.40 eV, or to form the *OHCH2 intermediate with a positive ΔG value of 0.33 eV. To further assess the product distribution of CH3OH and CH4, the Boltzmann distribution formula exp[−(ΔG)/(kBT)] is employed based on the Gibbs free energy difference.68 The computed CH3OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH4 molar ratio is 1
CH4 molar ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.19 × 1012 at ambient temperature, indicating a strong selectivity toward the CH4 product on the CuB12 monolayer. Therefore, the 6e− reduction product CH3OH is not the major competitive product. For the 8e− reduction product CH4, the reaction *O + H+ + e− → *OH is its potential determining step with a ΔG value of 0.60 eV, which is larger than the maximum ΔG value (0.54 eV) for urea production. These data suggest that the formation of CH4 can be greatly suppressed on the CuB12 monolayer. Moreover, the further reduction of *CO should be prohibited on the CuB12 monolayer, which can improve the selectivity toward urea production. To better confirm the excellent catalytic selectivity toward urea production, the energy barriers of CO desorption and its further reduction to a *CHO intermediate are also computed (Fig. S11–S14†). It is obvious that *CO is preferred to be released due to a relatively lower kinetic energy barrier (0.84 eV) than that for the further reduction to a *CHO intermediate (1.76 eV). Therefore, the CO molecule is kinetically the final reduction product, which is also favorable to synthesize urea.
2.19 × 1012 at ambient temperature, indicating a strong selectivity toward the CH4 product on the CuB12 monolayer. Therefore, the 6e− reduction product CH3OH is not the major competitive product. For the 8e− reduction product CH4, the reaction *O + H+ + e− → *OH is its potential determining step with a ΔG value of 0.60 eV, which is larger than the maximum ΔG value (0.54 eV) for urea production. These data suggest that the formation of CH4 can be greatly suppressed on the CuB12 monolayer. Moreover, the further reduction of *CO should be prohibited on the CuB12 monolayer, which can improve the selectivity toward urea production. To better confirm the excellent catalytic selectivity toward urea production, the energy barriers of CO desorption and its further reduction to a *CHO intermediate are also computed (Fig. S11–S14†). It is obvious that *CO is preferred to be released due to a relatively lower kinetic energy barrier (0.84 eV) than that for the further reduction to a *CHO intermediate (1.76 eV). Therefore, the CO molecule is kinetically the final reduction product, which is also favorable to synthesize urea.
The catalytic selectivity toward urea production compared to the competitive CO2RR to the C2 product is also assessed, in which the whole reaction pathways and the corresponding intermediates are investigated (Fig. 6b and S15†). As shown in Fig. 6b, the adsorption and hydrogenation of the second CO2 molecule to generate the *CO2 + *OCOH intermediate is uphill by 0.41 eV in the Gibbs free energy diagram, which is the potential limiting step to form the C2 product. The formed *CO2 + *OCOH intermediate is preferred to be hydrogenated to the *CO2 + *CO intermediate through releasing one water molecule rather than the *OCOH + *OCOH intermediate due to the relatively lower ΔG value (0.11 eV for *CO2 + *CO and 0.29 eV for *OCOH + *OCOH). Afterward, the *CO2 + *CO intermediate can be continuously attacked by two H+/e− pairs to generate the *OCOH + *CO intermediate and *CO + *CO intermediate with a positive ΔG value of 0.13 eV and 0.14 eV, respectively. The C–C coupling reaction of the *CO + *CO intermediate to obtain the *OCCO intermediate is an exothermic process with a downhill energy of 0.33 eV. Two O atoms of the *OCCO intermediate are attacked by another two H+/e− pairs to form the *OCCOH intermediate and *OHCCOH intermediate with a negative ΔG value. The next hydrogenation step of the *OHCCOH intermediate can generate the *CCOH intermediate followed by the generation of one H2O molecule, and the computed ΔG value for this step is 0.09 eV. Subsequently, the *CCOH intermediate can be continuously reduced to *CHCOH, *CHCOH, *CH2COH, *CCH2, *CHCH2 and *CH2CH2 intermediates by five H+/e− pairs with no energy demand. Finally, a free CH2CH2 molecule can be generated after overcoming a thermodynamic barrier of 0.85 eV. According to the above discussion, two CO2 molecules can be finally converted to CH2CH2 on the CuB12 monolayer. The corresponding potential limiting step is the first hydrogenation step (*CO2 + CO2 + H+ + e− → *CO2 + *OCOH) with a maximum ΔG value of 0.41 eV, which is still higher than the optimal ΔG value of the reaction * + N2 + H+ + e− → *NNH (0.23 eV) to produce urea. Encouragingly, the competitive reaction of CO2 reduction to CH2CH2 can be greatly suppressed on the CuB12 monolayer.
The selectivity of the CuB12 monolayer toward urea production compared with the NRR competitive reaction is also evaluated, where all possible reaction mechanisms and intermediates are considered (Fig. 6c and S16†). The optimal reaction pathway for NH3 formation is N2 → *NNH → *NHNH → *NHNH2 → *NHNH3 → *NH → *NH2 → *NH3 → NH3. During this procedure, the generation of the first hydrogenation intermediate *NNH possesses a maximum ΔG value of 0.23 eV. Unfortunately, the potential determining step and the limiting potential for the byproduct NH3 and the targeted urea are both identical. From another point of view, the NH3 product is exactly the right reactant to synthesize urea in industrial processes, so it can probably promote the urea formation by other approaches.
Conclusions
In summary, the entirely planar CuB12 monolayer with superior stability is identified to be a global-minimum configuration after a comprehensive search combined with first-principles calculation. The B atoms at the midpoint of the edges of the squares are firstly confirmed to be distinguished non-metal catalytic sites, which provide an electrocatalyst to convert the CO2 molecule and N2 molecule into urea. On the CuB12 monolayer, all possible reaction pathways starting from the CO2 molecule and N2 molecule for urea production, including the CO2 pathway, OCOH pathway, CO pathway, NCON pathway and feasible mixed pathways, are systematically investigated, which enrich the recognition of urea formation. Most importantly, the limiting potential of urea production through the CO2 pathway and OCOH pathway is 0.23 V, which is much lower than the values of reported urea catalysts. The minimal kinetic energy barrier is 0.54 eV through *CO and *NHNH coupling reactions among six possible C–N coupling reactions accompanied by the hydrogenation of N2, which is 0.28 eV lower than that through *CO and *N2 coupling reactions. Furthermore, the competitive CH3OH and CH4 products can be suppressed in the urea formation process. This study will stimulate more experimentalist and theorist efforts to apply the perfected urea formation pathways and C–N formation pathways to explore other high-performance urea catalysts.Methods
Global-minimum structure prediction
The global-minimum structure prediction of the CuB12 monolayer was performed by the particle-swarm optimization (PSO) method within the evolutionary scheme as implemented in the CALYPSO code,69 which can efficiently search for ground or metastable structures just depending on the input chemical compositions. Both planar and puckered structures are considered during its structure search. The population size and the number of generations are set to be 50 and 30, respectively. Unit cells containing 1 and 2 formula units (f.u.) are considered.DFT computations
All first principles calculations were performed by spin-polarized density functional theory (DFT)70via the Vienna ab initio simulation package (VASP).71 The ion-electron interactions were expressed by the projector augmented wave (PAW) pseudopotential.72 The electronic exchange-correlation effects were described with the generalized gradient approximation of the Perdew–Burke–Ernzerhof (PBE) functional with the generalized gradient approximation (GGA).73 The solvation effects were simulated using the linearized implicit Poisson–Boltzmann solvation model as implemented in the VASP (VASP-sol), where the dielectric constant for water was considered to be 80.74,75 Grimme's semiempirical DFT-D3 approach was chosen for the van der Waals (vdW) interaction between reaction intermediates and the CuB12 monolayer.76,77 A vacuum distance of 20 Å along the z direction was used to minimize interaction between periodic images, and a 2 × 2 supercell was employed to investigate the catalytic activity of the CuB12 monolayer. The lane-wave cutoff energy was set to be 500 eV in all computations, and the Monkhorst–Pack k-point mesh resolution in reciprocal space was set to be 2π × 0.025 Å−1 for all structures. The structures were optimized with an energy convergence of 10−5 eV and a force convergence of 10−2 eV Å−1 for each atom, respectively.Stability evaluation
The phonon dispersion spectra were computed using the finite displacement method with the PHONOPY program.78 The ab initio molecular dynamics (AIMD) simulations were performed in the NVT ensemble with a time step of 1.0 fs for 10 ps with a 3 × 3 supercell. Different temperatures (T = 300 K, 600 K, 900 K, 1200 K, 1500 K) were controlled using the Nosé–Hoover method.79Free energy computations
The Gibbs free energy diagram of each elementary step in the electrochemical synthesis of urea was obtained by using the computational hydrogen electrode (CHE) model proposed by Nørskov et al.80,81 The transition states and kinetic barriers for the C–N coupling reaction were identified by the climbing-image nudged elastic band (CI-NEB) method.82 The computational details of global-minimum structure prediction and Gibbs free energy can be found in the ESI.†Data availability
All the data supporting this article have been included in the main text and the ESI.†Author contributions
M. Z. designed the research, and C. Y. Z., C. X. W. and M. W. demonstrated the initial idea and collected all the data. C. Y. Z. wrote the paper. M. Z., Y. G. and Z. M. S revised the paper and all authors commented on it.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (21673036 and 21771035), the Education Department of Jilin Prov. China (JJKH20211284KJ) and the Fundamental Research Funds for the Central Universities (2412018ZD006). Most computations were carried out on TianHe-2 at the LvLiang Cloud Computing Center of China. We acknowledge the support of the computational resources from the Institute of Theoretical Chemistry, Jilin University.References
- Z. Seh, J. Kibsgaard, C. Dickens, I. Chorkendorff, J. Nørskov and T. Jaramillo, Science, 2017, 355, eaad4998 CrossRef PubMed.
- H. Jin, C. Guo, X. Liu, J. Liu, A. Vasileff, Y. Jiao, Y. Zheng and S. Qiao, Chem. Rev., 2018, 118, 6337–6408 CrossRef CAS PubMed.
- Z. Li, S. Ji, Y. Liu, X. Cao, S. Tian, Y. Chen, Z. Niu and Y. Li, Chem. Rev., 2020, 120, 623–682 CrossRef CAS PubMed.
- H. Zhuo, X. Zhang, J. Liang, Q. Yu, H. Xiao and J. Li, Chem. Rev., 2020, 120, 12315–12341 CrossRef CAS PubMed.
- Z. Zeng, L. Gan, H. Yang, X. Su, J. Gao, W. Liu, H. Matsumoto, J. Gong, J. Zhang, W. Cai, Z. Zhang, Y. Yan, B. Liu and P. Chen, Nat. Commun., 2021, 12, 4088 CrossRef CAS PubMed.
- L. Wang, W. Chen, D. Zhang, Y. Du, R. Amal, S. Qiao, J. Wu and Z. Yin, Chem. Soc. Rev., 2019, 48, 5310–5349 RSC.
- X. Qiu, H. Zhu, J. Huang, P. Liao and X. Chen, J. Am. Chem. Soc., 2021, 143, 7242–7246 CrossRef CAS PubMed.
- C. Morales-Guio, E. Cave, S. Nitopi, J. Feaster, L. Wang, K. Kuhl, A. Jackson, N. Johnson, D. Abram, T. Hatsukade, C. Hahn and T. Jaramillo, Nat. Catal., 2018, 1, 764–771 CrossRef CAS.
- J. Choi, B. Suryanto, D. Wang, H. Du, R. Hodgetts, F. Ferrero Vallana, D. MacFarlane and A. Simonov, Nat. Commun., 2020, 11, 5546 CrossRef CAS PubMed.
- B. Suryanto, K. Matuszek, J. Choi, R. Hodgetts, H. Du, J. Bakker, C. Kang, P. Cherepanov, A. Simonov and D. MacFarlane, Science, 2021, 372, 1187–1191 CrossRef CAS PubMed.
- Y. Gu, B. Xi, W. Tian, H. Zhang, Q. Fu and S. Xiong, Adv. Mater., 2021, 33, 2100429 CrossRef CAS PubMed.
- D. Yu, Y. Ma, F. Hu, C. Lin, L. Li, H. Chen, X. Han and S. Peng, Adv. Energy Mater., 2021, 11, 2101242 CrossRef CAS.
- B. Hu, A. Huang, X. Zhang, Z. Chen, R. Tu, W. Zhu, Z. Zhuang, C. Chen, Q. Peng and Y. Li, Nano Res., 2021, 13, 1–7 Search PubMed.
- M. Tong, F. Sun, Y. Xie, Y. Wang, Y. Yang, C. Tian, L. Wang and H. Fu, Angew. Chem., Int. Ed., 2021, 60, 14005–14012 CrossRef CAS PubMed.
- K. Zhou, Z. Wang, C. Han, X. Ke, C. Wang, Y. Jin, Q. Zhang, J. Liu, H. Wang and H. Yan, Nat. Commun., 2021, 12, 3783 CrossRef CAS PubMed.
- Y. Wu, X. Li, Y. Wei, Z. Fu, W. Wei, X. Wu, Q. Zhu and Q. Xu, Adv. Mater., 2021, 33, 2006965 CrossRef CAS PubMed.
- Y. Wei, R. A. Soomro, X. Xie and B. Xu, J. Energy Chem., 2021, 55, 244–255 CrossRef.
- Y. Zhou, F. Che, M. Liu, C. Zou, Z. Liang, P. De Luna, H. Yuan, J. Li, Z. Wang, H. Xie, H. Li, P. Chen, E. Bladt, R. Quintero-Bermudez, T. Sham, S. Bals, J. Hofkens, D. Sinton, G. Chen and E. Sargent, Nat. Chem., 2018, 10, 974–980 CrossRef CAS PubMed.
- C. Peng, G. Luo, J. Zhang, M. Chen, Z. Wang, T. Sham, L. Zhang, Y. Li and G. Zheng, Nat. Commun., 2021, 12, 1580 CrossRef CAS PubMed.
- M. Wang, S. Liu, T. Qian, J. Liu, J. Zhou, H. Ji, J. Xiong, J. Zhong and C. Yan, Nat. Commun., 2019, 10, 341 CrossRef CAS PubMed.
- C. Chen, X. Zhu, X. Wen, Y. Zhou, L. Zhou, H. Li, L. Tao, Q. Li, S. Du, T. Liu, D. Yan, C. Xie, Y. Zou, Y. Wang, R. Chen, J. Huo, Y. Li, J. Cheng, H. Su, X. Zhao, W. Cheng, Q. Liu, H. Lin, J. Luo, J. Chen, M. Dong, K. Cheng, C. Li and S. Wang, Nat. Chem., 2020, 12, 717–724 CrossRef CAS PubMed.
- B. Comer, P. Fuentes, C. Dimkpa, Y. Liu, C. Fernandez, P. Arora, M. Realff, U. Singh, M. Hatzell and A. Medford, Joule, 2019, 3, 1578–1605 CrossRef CAS.
- F. Barzagli, F. Mani and M. Peruzzini, Green Chem., 2011, 13, 1267–1274 RSC.
- M. Kitano, Y. Inoue, Y. Yamazaki, F. Hayashi, S. Kanbara, S. Matsuishi, T. Yokoyama, S. Kim, M. Hara and H. Hosono, Nat. Chem., 2012, 4, 934–940 CrossRef CAS PubMed.
- M. Kitano, S. Kanbara, Y. Inoue, N. Kuganathan, P. Sushko, T. Yokoyama, M. Hara and H. Hosono, Nat. Commun., 2015, 6, 6731 CrossRef CAS PubMed.
- D. Kayan and F. Köleli, Appl. Catal., B, 2016, 181, 88–93 CrossRef CAS.
- M. Yuan, J. Chen, Y. Bai, Z. Liu, J. Zhang, T. Zhao, Q. Wang, S. Li, H. He and G. Zhang, Angew. Chem., Int. Ed., 2021, 60, 10910–10918 CrossRef CAS PubMed.
- M. Yuan, J. Chen, Y. Bai, Z. Liu, J. Zhang, T. Zhao, Q. Shi, S. Li, X. Wang and G. Zhang, Chem. Sci., 2021, 12, 6048–6058 RSC.
- N. Meng, Y. Huang, Y. Liu, Y. Yu and B. Zhang, Cell Rep. Phys. Sci., 2021, 2, 100378 CrossRef CAS.
- X. Zhu, X. Zhou, Y. Jing and Y. Li, Nat. Commun., 2021, 12, 4080 CrossRef CAS PubMed.
- C. Ling, X. Niu, Q. Li, A. Du and J. Wang, J. Am. Chem. Soc., 2018, 140, 14161–14168 CrossRef CAS PubMed.
- X. Zhang, T. Wu, H. Wang, R. Zhao, H. Chen, T. Wang, P. Wei, Y. Luo, Y. Zhang and X. Sun, ACS Catal., 2019, 9, 4609–4615 CrossRef CAS.
- X. Lv, W. Wei, F. Li, B. Huang and Y. Dai, Nano Lett., 2019, 19, 6391–6399 CrossRef CAS PubMed.
- T. He, K. Reuter and A. Du, J. Mater. Chem. A, 2020, 8, 599–606 RSC.
- G. Qin, Q. Cui, A. Du and Q. Sun, ChemCatChem, 2020, 12, 1483–1490 CrossRef CAS.
- Z. Feng, Y. Tang, Y. Ma, Y. Li, Y. Dai, H. Ding, G. Su and X. Dai, Appl. Surf. Sci., 2021, 538, 148145 CrossRef CAS.
- G. Welch, R. Juan, J. Masuda and D. Stephan, Science, 2006, 314, 1124–1126 CrossRef CAS PubMed.
- L. Dai, Y. Xue, L. Qu, H. Choi and J. Baek, Chem. Rev., 2015, 115, 4823–4892 CrossRef CAS PubMed.
- Y. Xu, M. Kraft and R. Xu, Chem. Soc. Rev., 2016, 45, 3039–3052 RSC.
- H. Zhang, Y. Li, J. Hou, K. Tu and Z. Chen, J. Am. Chem. Soc., 2016, 138, 5644–5651 CrossRef CAS PubMed.
- R. Islas, T. Heine, K. Ito, P. v. R. Schleyer and G. Merino, J. Am. Chem. Soc., 2007, 129, 14767–14774 CrossRef CAS PubMed.
- L. Yang, E. Ganz, Z. Chen, Z. Wang and P. v. R. Schleyer, Angew. Chem., Int. Ed., 2015, 54, 9468–9501 CrossRef CAS PubMed.
- Y. Wang, Y. Li and Z. Chen, Acc. Chem. Res., 2020, 53, 887–895 CrossRef CAS PubMed.
- T. Heine and G. Merino, Angew. Chem., Int. Ed., 2012, 51, 4275–4276 CrossRef CAS PubMed.
- W. Tang, E. Sanville and G. Henkelman, J. Phys.: Condens. Matter, 2009, 21, 084204 CrossRef CAS PubMed.
- A. Savin, R. Nesper, S. Wengert and T. Fässler, Angew. Chem., Int. Ed., 1997, 36, 1808–1832 CrossRef CAS.
- C. Zhu, H. Chen, C. Wang, M. Zhang, Y. Geng, Q. Li and Z. Su, J. Phys. Chem. C, 2020, 124, 23221–23229 CrossRef CAS.
- C. Zhu, C. Wang, M. Zhang, H. Chen, Y. Geng and Z. Su, ACS Appl. Mater. Interfaces, 2021, 13, 3845–3855 CrossRef CAS PubMed.
- A. Mannix, X. Zhou, B. Kiraly, J. Wood, D. Alducin, B. Myers, X. Liu, B. Fisher, U. Santiago, J. Guest, M. Yacaman, A. Ponce, A. Oganov, M. Hersam and N. Guisinger, Science, 2015, 350, 1513–1516 CrossRef CAS.
- B. Feng, J. Zhang, Q. Zhong, W. Li, S. Li, H. Li, P. Cheng, S. Meng, L. Chen and K. Wu, Nat. Chem., 2016, 8, 563–568 CrossRef CAS PubMed.
- B. Song, Y. Zhou, H. Yang, J. Liao, L. Yang, X. Yang and E. Ganz, J. Am. Chem. Soc., 2019, 141, 3630–3640 CrossRef CAS PubMed.
- M. Born and K. Huang, Dynamical theory of crystal lattices; Clarendon press, 1954 Search PubMed.
- R. W. G. Wyckoff, Crystal Structures, Interscience Publishers, New York, 2nd edn, 1963, vol. 1, p. 7 Search PubMed.
- X. Guo, J. Gu, S. Lin, S. Zhang, Z. Chen and S. Huang, J. Am. Chem. Soc., 2020, 142, 5709–5721 CrossRef CAS PubMed.
- C. Zhu, C. Wen, C. Wang, M. Zhang, Y. Geng and Z. Su, J. Mater. Chem. A, 2021, 9, 10855–10868 RSC.
- Y. Feng, H. Yang, Y. Zhang, X. Huang, L. Li, T. Cheng and Q. Shao, Nano Lett., 2020, 20, 8282–8289 CrossRef CAS PubMed.
- X. Zheng, Y. Ji, J. Tang, J. Wang, B. Liu, H. Steinrück, K. Lim, Y. Li, M. Toney, K. Chan and Y. Cui, Nat. Catal., 2019, 2, 55–61 CrossRef CAS.
- M. Shi, D. Bao, B. Wulan, Y. Li, Y. Zhang, J. Yan and Q. Jiang, Adv. Mater., 2017, 29, 1606550 CrossRef PubMed.
- D. Bao, Q. Zhang, F. Meng, H. Zhong, M. Shi, Y. Zhang, J. Yan, Q. Jiang and X. Zhang, Adv. Mater., 2017, 29, 1604799 CrossRef PubMed.
- S. Li, D. Bao, M. Shi, B. Wulan, J. Yan and Q. Jiang, Adv. Mater., 2017, 29, 170000 Search PubMed.
- J. Wang, L. Yu, L. Hu, G. Chen, H. Xin and X. Feng, Nat. Commun., 2018, 9, 1795 CrossRef PubMed.
- M. Shi, D. Bao, S. Li, B. Wulan, J. Yan and Q. Jiang, Adv. Energy Mater., 2018, 8, 1800124 CrossRef.
- C. Ling, Y. Zhang, Q. Li, X. Bai, L. Shi and J. Wang, J. Am. Chem. Soc., 2019, 141, 18264–18270 CrossRef CAS PubMed.
- J. Montoya, C. Tsai, A. Vojvodic and J. Nørskov, ChemSusChem, 2015, 8, 2180–2186 CrossRef CAS PubMed.
- L. Hu, A. Khaniya, J. Wang, G. Chen, W. Kaden and X. Feng, ACS Catal., 2018, 8, 9312–9319 CrossRef CAS.
- T. Cheng, H. Xiao and W. Goddard, J. Am. Chem. Soc., 2016, 138, 13802–13805 CrossRef CAS PubMed.
- E. Skúlason, T. Bligaard, S. Gudmundsdóttir, F. Studt, J. Rossmeisl, F. Abild-Pedersen, T. Vegge, H. Jónsson and J. Nørskov, Phys. Chem. Chem. Phys., 2012, 14, 1235–1245 RSC.
- Y. Tian, C. Zhu, L. Yan, J. Zhao and Z. Su, J. Mater. Chem. A, 2019, 7, 15341–15346 RSC.
- Y. Wang, J. Lv, L. Zhu and Y. Ma, Comput. Phys. Commun., 2012, 183, 2063–2070 CrossRef CAS.
- D. Singh and J. Ashkenazi, Phys. Rev. B, 1992, 46, 11570 CrossRef.
- B. Barbiellini, M. Puska, T. Korhonen, A. Harju, T. Torsti and R. Nieminen, Phys. Rev. B, 1996, 53, 16201 CrossRef CAS PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B, 1999, 59, 1758 CrossRef CAS.
- J. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- M. Fishman, H. Zhuang, K. Mathew, W. Dirschka and R. Hennig, Phys. Rev. B, 2013, 87, 245402 CrossRef.
- K. Mathew, R. Sundararaman, K. Letchworth-Weaver, T. Arias and R. Hennig, J. Chem. Phys., 2014, 140, 084106 CrossRef.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef.
- A. Togo and I. Tanaka, Scr. Mater., 2015, 108, 1–5 CrossRef CAS.
- G. Martyna, M. Klein and M. Tuckerman, J. Chem. Phys., 1992, 97, 2635–2643 CrossRef.
- J. Nørskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J. Kitchin, T. Bligaard and H. Jonsson, J. Phys. Chem. B, 2004, 108, 17886–17892 CrossRef.
- J. Rossmeisl and A. Logadottir, J. Chem. Phys., 2005, 319, 178–184 CAS.
- G. Mills and H. Jónsson, Phys. Rev. Lett., 1994, 72, 1124 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc04845g |
| This journal is © The Royal Society of Chemistry 2022 |