Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Lipase-catalyzed esterification in water enabled by nanomicelles. Applications to 1-pot multi-step sequences†
Vani
Singhania
 a,
Margery
Cortes-Clerget
a,
Margery
Cortes-Clerget
 a,
Jade
Dussart-Gautheret
a,
Jade
Dussart-Gautheret
 a,
Bhornrawin
Akkachairin
ab,
Julie
Yu
a,
Bhornrawin
Akkachairin
ab,
Julie
Yu
 a,
Nnamdi
Akporji
a,
Nnamdi
Akporji
 a,
Fabrice
Gallou
a,
Fabrice
Gallou
 c and
Bruce H.
Lipshutz
c and
Bruce H.
Lipshutz
 *a
*a
aDepartment of Chemistry and Biochemistry, University of California, Santa Barbara, CA 93106, USA. E-mail: lipshutz@chem.ucsb.edu
bProgram on Chemical Biology, Chulabhorn Graduate Institute, Center of Excellence on Environmental Health and Toxicology (EHT), Ministry of Education, 54 Kamphaeng Phet 6, Laksi, Bangkok 10210, Thailand
cNovartis Pharma AG, CH-4057 Basel, Switzerland
First published on 27th December 2021
Abstract
Esterification in an aqueous micellar medium is catalyzed by a commercially available lipase in the absence of any co-factors. The presence of only 2 wt% designer surfactant, TPGS-750-M, assists in a 100% selective enzymatic process in which only primary alcohols participate (in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with carboxylic acid). An unexpected finding is also disclosed where the simple additive, PhCF3 (1 equiv. vs. substrate), appears to significantly extend the scope of usable acid/alcohol combinations. Taken together, several chemo- and bio-catalyzed 1-pot, multi-step reactions can now be performed in water.
1 ratio with carboxylic acid). An unexpected finding is also disclosed where the simple additive, PhCF3 (1 equiv. vs. substrate), appears to significantly extend the scope of usable acid/alcohol combinations. Taken together, several chemo- and bio-catalyzed 1-pot, multi-step reactions can now be performed in water.
Introduction
Textbook and related chemical methods continue to discuss the fundamental preparation of esters from acids and alcohols, typically performed in organic solvents, that require removal of water.1 Alternatively, biocatalytic processes, while offering mild conditions, high selectivity, and, in general, environmental friendliness, are typically used in aqueous buffered media.2–6 Hence, esterification via enzymatic catalysis in water involving lipase, esterase, etc., where water is the by-product, seems counterintuitive.7–10 Special conditions (e.g., the presence of an organic solvent, prior acid activation, membrane-bound or solid supports, etc.) are required to address this issue.11,12 Only earlier this year did a report appear, outside the food industry,7,13 describing an enzyme-catalyzed ester synthesis in aqueous alcoholic media.14Given the importance of the ester linkage in so many industrial products, it seems remarkable that such a functional group has yet to be constructed via a straightforward biocatalytic-based esterification; that such green technology indicative of the options available to synthetic chemists in water, in the absence of any organic solvent, and in particular, dipolar aprotic choices including DMSO,15 has yet to be described. We now report that use of an economical, commercially available lipase can, indeed, catalyze esterification in water as the reaction medium, and do so using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio of acid and alcohol. Moreover, these take place in the absence of any co-factor. The key to successful levels of conversion is the presence of the designer surfactant TPGS-750-M (DL-α-tocopherol methoxypolyethylene glycol succinate) in the reaction medium.16 Even further levels of conversion can be anticipated by the presence of a simple additive (1 equiv.), as disclosed herein. Applications of this new esterification, together with chemocatalysis, leads to unprecedented tandem, 1-pot processes illustrative of the potential for organic synthesis to be conducted in sequential fashion, all done in water.
1 stoichiometric ratio of acid and alcohol. Moreover, these take place in the absence of any co-factor. The key to successful levels of conversion is the presence of the designer surfactant TPGS-750-M (DL-α-tocopherol methoxypolyethylene glycol succinate) in the reaction medium.16 Even further levels of conversion can be anticipated by the presence of a simple additive (1 equiv.), as disclosed herein. Applications of this new esterification, together with chemocatalysis, leads to unprecedented tandem, 1-pot processes illustrative of the potential for organic synthesis to be conducted in sequential fashion, all done in water.
Results and discussion
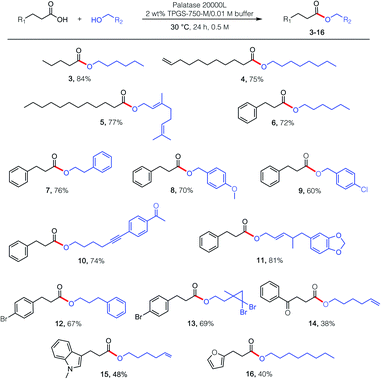
Esterification reactions were tested using four commercially available lipases: those from Candida rugosa, Rhizopus niveus, Rhizomucor miehei, and Burkholderia cepacia, with Rhizomucor miehei providing the best results (Table 1).17 For optimization purposes, lipase-catalyzed esterification between partners valeric acid (1) and n-hexanol (2) was initially examined. While keeping the concentration of valeric acid at either 0.25 M or 0.50 M, varied amounts of hexanol were added at both global reaction concentrations and temperatures. At 0.25 M (entries 1–3), increasing the quantity of hexanol introduced to the reaction mixture maintained at 40 °C improved the extent of product conversion. Increasing the concentration to 0.50 M (entries 4–6), unfortunately afforded no observed improvement. However, lowering the temperature to 30 °C led to essentially full conversion, thereby allowing for use of the ideal ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (entry 8). Neither further reduction in temperature to rt (entry 7) nor increase in reaction temperature to 50 °C (entry 9) gave comparable levels of conversion relative to those observed at 30 °C.
1 (entry 8). Neither further reduction in temperature to rt (entry 7) nor increase in reaction temperature to 50 °C (entry 9) gave comparable levels of conversion relative to those observed at 30 °C.
Using these optimized conditions (i.e., 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acid
1 acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alcohol, 30 °C), several esterification reactions were examined, catalyzed by lipase derived from Rhizomucor miehei (Scheme 1; commercially available; see the ESI†). Given an enzyme's typical preference for certain structural features associated with reaction partners, as witnessed with carboxylic acids, somewhat greater flexibility was observed in terms of the alcohol that participated in the esterification process. While products 3–16 reflect these enzymatic requirements, they are solely representative of the possibilities for lipases, perhaps in general, to effect esterification in water. Unexpectedly, while the presence of 2 wt% TPGS-750-M (i.e., 20 mg mL−1 of water) in the buffered aqueous medium proved beneficial in most cases, increasing the amount to either 4 or 6 wt%, unlike that observed with KRED,18 led to no further benefit. While these esterifications in water seem unexpected, the key to success may be the presence of the micelles. Hence, the water-insoluble product esters likely locate within their inner cores, which feature a purely hydrophobic pocket. Thus, opportunities for competitive hydrolysis by water are negated due to this “reservoir” effect.18
alcohol, 30 °C), several esterification reactions were examined, catalyzed by lipase derived from Rhizomucor miehei (Scheme 1; commercially available; see the ESI†). Given an enzyme's typical preference for certain structural features associated with reaction partners, as witnessed with carboxylic acids, somewhat greater flexibility was observed in terms of the alcohol that participated in the esterification process. While products 3–16 reflect these enzymatic requirements, they are solely representative of the possibilities for lipases, perhaps in general, to effect esterification in water. Unexpectedly, while the presence of 2 wt% TPGS-750-M (i.e., 20 mg mL−1 of water) in the buffered aqueous medium proved beneficial in most cases, increasing the amount to either 4 or 6 wt%, unlike that observed with KRED,18 led to no further benefit. While these esterifications in water seem unexpected, the key to success may be the presence of the micelles. Hence, the water-insoluble product esters likely locate within their inner cores, which feature a purely hydrophobic pocket. Thus, opportunities for competitive hydrolysis by water are negated due to this “reservoir” effect.18
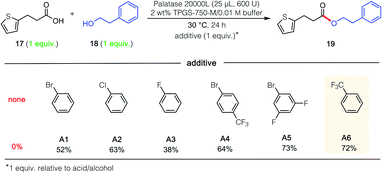
In attempts to extend these lipase-catalyzed esterification reactions to a broader range of heteroaromatic ring-containing partners (i.e., beyond those in 15 and 16; Scheme 1), educt 3-(2-thiophenyl)propionic acid (17; Fig. 1), the analog of 3-phenylpropionic acid (Scheme 1, R1 = Ph) was examined, along with phenethyl alcohol (18), which had successfully led to product 7. In the event, and notwithstanding the thiophene's identical distance from the reactive carboxyl site, esterification was completely shut down (Fig. 1; 0% yield). Unexpectedly, therefore, when acid 17 was admixed with either the phenyl- or p-bromophenylpropionic acid (see 20 in Scheme 2) and the same alcohol (18), the major ester formed contained the thiophene subunit, product 19! This finding initiated a search for an additive that, ultimately, enabled esterification of 17 to 19 in 72% that was otherwise completely inhibited (notwithstanding the presence of the surfactant). This phenomenon is unlikely to be a simple solvent effect, since common solvents like methylene chloride (no conversion), hexanes (25% yield), cyclohexane (29% yield) and toluene (50% yield) were comparatively ineffective.19 Also as shown in Fig. 1, among the additives investigated (A1–A6), trifluoromethylbenzene (PhCF3; A6) was selected given its effectiveness, commercial availability, non-chlorinated status, and attractive economics.
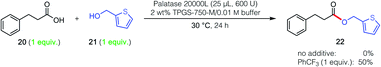 | ||
| Scheme 2 Impact of PhCF3 on inverted substrate pair (i.e., vs. reaction pair in Fig. 1). | ||
The unpredictable yet very intriguing impact of the additive on the inverted combination of reaction partners (i.e., phenylpropanoic acid, 20 and 2-thiophenemethanol, 21) was also studied. In the event, similar results were found where, in the absence of additive, no conversion to ester 22 was observed. However, when PhCF3 (1 equiv.) was in the aqueous reaction medium, a remarkable 50% yield was obtained (Scheme 2).
Further studies were carried out on the effect induced by this additive (PhCF3) on the lipase derived from Rhizomucor miehei,20 focusing on several control reactions (Table 2). The highest yield was observed in the presence of one equivalent of additive (entry 3); using half this amount dramatically lowered the extent of conversion (entry 2). Using PhCF3 in excess (entry 4) led to a decrease in yield to 57%. Neither the micellar medium alone (entry 1) nor just the aqueous buffer (entry 5) is capable of mediating the intended reaction, a strong indication that acid 17 by itself, as observed previously (vide supra), is not an acceptable reaction partner for this lipase-mediated esterification.
| Entry | Solventa | Additiveb (equiv.) | Yieldc (%) |
|---|---|---|---|
| a 1 mL of solvent used for 0.5 M global concentration. b One equiv. of this additive represents ca. 6% of the total reaction volume. c Isolated yield of 19. | |||
| 1 | 2 wt% TPGS-750-M/0.01 M buffer | — | 0 |
| 2 | 2 wt% TPGS-750-M/0.01 M buffer | PhCF3 (0.5) | 14 |
| 3 | 2 wt% TPGS-750-M/0.01 M buffer | PhCF 3 (1) | 72 |
| 4 | 2 wt% TPGS-750-M/0.01 M buffer | PhCF3 (2) | 57 |
| 5 | 0.01 M buffer (no surfactant) | PhCF3 (1) | 0 |
The remarkable impact of the combination of an additive A1–A6, together with the buffer and surfactant in the pot, was further investigated on other substrates 19, and 23–28 (Fig. 2). Thus, in addition to the initial discovery involving esterification to product 19, the isolated yield using PhCF3 of esters 23 and 27 increased from 0 to 36% and from 0 to 50%, respectively. And while more modest enhancements in yields were observed for products 24–26 and 28, the overall net positive trend using PhCF3 is clear.
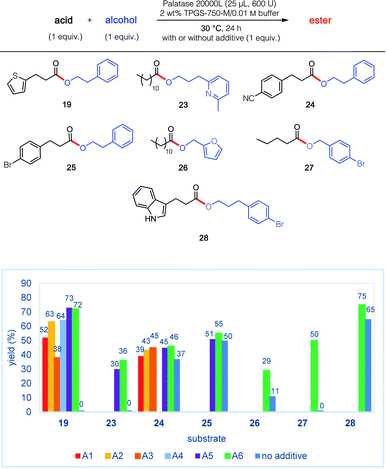 | ||
| Fig. 2 Impact of various additives (see Fig. 1) on lipase-catalysed esterification reactions. | ||
The manner in which PhCF3 influences enzymatic esterification may be due to a positive allosteric regulation, altering the enzymatic cavity. Typically, however, this phenomenon relies on far more functionalized molecules;21 indeed, the overall dramatic effect mediated by such a simple additive as PhCF3 appears to be unprecedented, perhaps suggesting that enzymatic modification leading to greater substrate tolerance may not require the types of modulators in current use. Another explanation may be the direct alteration of the entrance to the enzymatic pocket, thereby adding a variable element of “promiscuity” to the level of acceptance associated with its “natural” structural features.22 Yet another explanation is that this additive may be providing a hydrophobic layer that alters the extent of lid opening at the enzymatic site.23 While a more definitive analysis as to which role is operative awaits further scrutiny, these observations may be indicative of future discoveries perhaps applicable to other enzymatic arrays, thereby augmenting the already huge potential of bio-catalytic processes in organic synthesis.
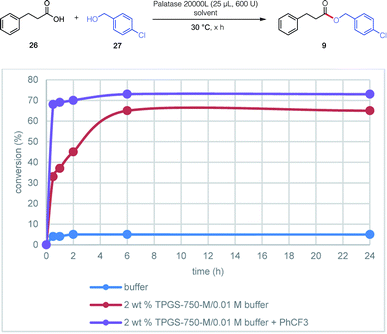
Contributions by the surfactant and additive could be individually evaluated based on the “reservoir effect” first discovered in enzymatic ketone reductions (KREDs).18 This phenomenon was also observed in the lipase-catalyzed esterification between 3-phenylpropanoic acid 20 and (4-chlorophenyl)methanol 29 leading to ester 9 (Fig. 3). Thus, while esterification in only buffer did not exceed 5% after a 24 hour period (reflecting commonly observed enzymatic inhibition), the presence of only 2 wt% TPGS-750-M increased the level of conversion to 65%. The same reaction with added PhCF3 (A6, 1 equiv.) further raised the conversion to 73%. Noteworthy, however, was the dramatic increase in reaction rate, reaching 70% after only two hours.
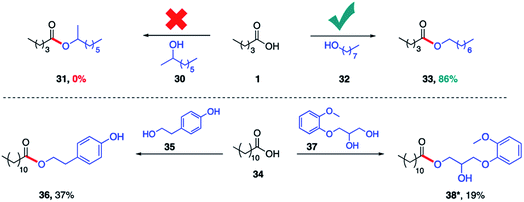
The same lipase (Rhizomucor miehei) was also found to selectively catalyze esterification with primary alcohols to the complete exclusion of secondary alcohols. Hence, while valeric acid (1) was converted in the presence of 1-octanol (32) to product 33 (86%), identical treatment with 2-octanol (30) afforded none of the corresponding ester 31 (Scheme 3). This specificity prevails even when the alcohol functional groups are present within the same molecule. Thus, irrespective of the connectivity of the alcohol on a sp2 carbon (phenol, 35) or an sp3 carbon (secondary alcohol, 37), the primary alcohol is esterified exclusively. The same selective outcome favoring esterification of a primary alcohol is observed in the presence of PhCF3 (1 equiv.).
With water being the common denominator associated with chemo- and bio-catalysis, virtually unlimited opportunities are available for combining each in 1-pot sequences. The benefits to be realized from such a synthetic strategy include not only minimizing workups and thereby, waste creation, but also time invested (“time economy”),24 and processing (“pot economy”).25 Importantly, while maintaining global concentrations that typically range between 0.25 and 1 M,18 the sequence of reactions involving chemo- or bio-catalysis can be varied (vide infra).
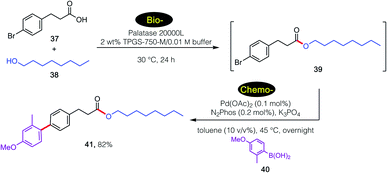
For example, a sequence involving bio- followed by chemo-catalysis is shown in Scheme 4. Lipase-catalyzed esterification of 39 with 40 leads to product 41. Without isolation, and given the presence of the aryl bromide being amenable to a ppm Pd-catalyzed Suzuki–Miyaura coupling26 involving arylboronic acid 42, final product 43 is isolated in 82% overall yield. It is important to note the lack of competing hydrolysis of the intermediate ester after the increase of pH upon addition of base, presumably reflecting the preferred location of newly formed ester 41 within the lipophilic inner micellar cores, rather than in the basic aqueous medium.
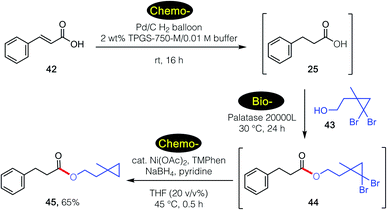
These tandem, mixed bio- and chemo-catalysis sequences are not limited to two steps. As shown in Scheme 5, catalytic hydrogenation of cinnamic acid 44 in water in the presence of the Pd/C27 led to intermediate acid 20. Subsequent lipase-catalyzed esterification with alcohol 45 gave gem-dibromocyclopropane 46, which was then reduced by nickel nanoparticles28 to give product 47 in 65% overall isolated yield, all done, sequentially, in water.
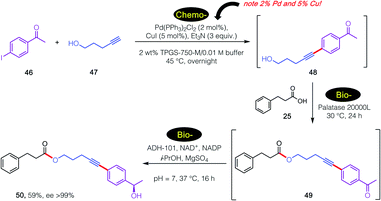
Further extending the options for this approach to chemoenzymatic catalysis and, importantly, substantiating enzymatic compatibility, another 3-step sequence was carried out as illustrated in Scheme 6. In this case, an initial, traditional Pd-catalyzed Sonogashira coupling was performed29 specifically as a test of enzymatic compatibility with such high, indeed unsustainable amounts of both Pd and Cu.30 Thus, and notwithstanding these levels of metal present in the aqueous medium, initial product alcohol 50 was successfully subjected (after adjustment to pH = 2) to lipase-catalyzed esterification.31 The 1-pot reaction mixture containing ester 51 was subsequently adjusted to pH 7, after which addition of an alcohol dehydrogenase (ADH-101)18 provided secondary nonracemic alcohol 52 in 59% overall isolated yield (ee >99%).
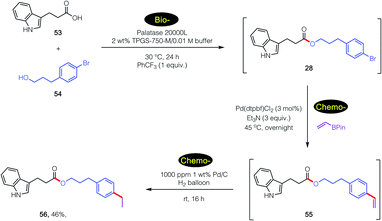
An even more complex carboxylic acid containing an indole (53) was also amenable to a tandem, 1-pot process (Scheme 7). An initial biocatalytic esterification to 28, which benefited from the presence of PhCF3 (vide supra; Fig. 2), was followed (without isolation) by two consecutive chemocatalysis steps: a Pd-catalyzed Suzuki–Miyaura vinylation to 55, and thence olefin reduction27 to arrive at ester 56. It is worthy of note that the first, selective enzymatic step takes place without protecting group chemistry (of the indole), in further alignment with the 12 Principles of Green Chemistry.32,33
Conclusions
An economically attractive, commercially available lipase has been identified shown to effectively catalyze esterification reactions “in water”, requiring an ideal 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of acid and alcohol. The enzymatic process is not only tolerant of the nanomicelles present in the aqueous medium, derived from TPGS-750-M, but is made all the more effective in terms of reaction conversion as well as avoidance of competitive hydrolysis by their presence. Also discussed is the unusual and unexpected finding that by adding PhCF3 (1 equiv. relative to substrate) to the reaction medium the extent of conversion can be further advanced. Alteration of the enzymatic pocket using such an uncharacteristically simple additive may present exciting opportunities for extending enzymatic tolerance of additional structural features associated with a broader range of substrates of interest. Unprecedented 1-pot sequences described herein suggest that chemo- and bio-catalysis can now be used in variable combinations within the same aqueous medium, making this approach to synthesis especially attractive in terms of time and pot economy, and perhaps most noteworthy, environmental friendliness.
1 ratio of acid and alcohol. The enzymatic process is not only tolerant of the nanomicelles present in the aqueous medium, derived from TPGS-750-M, but is made all the more effective in terms of reaction conversion as well as avoidance of competitive hydrolysis by their presence. Also discussed is the unusual and unexpected finding that by adding PhCF3 (1 equiv. relative to substrate) to the reaction medium the extent of conversion can be further advanced. Alteration of the enzymatic pocket using such an uncharacteristically simple additive may present exciting opportunities for extending enzymatic tolerance of additional structural features associated with a broader range of substrates of interest. Unprecedented 1-pot sequences described herein suggest that chemo- and bio-catalysis can now be used in variable combinations within the same aqueous medium, making this approach to synthesis especially attractive in terms of time and pot economy, and perhaps most noteworthy, environmental friendliness.
Author contributions
All authors contributed equally to the preparation of this ms.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by Novartis and the NSF (CHE 18-56406).Notes and references
- J. Otera and J. Nishikido, Esterification: Methods, Reactions, and Applications, Wiley-VCH, New York, 2009, DOI:10.1002/9783527627622.
- R. O. M. A. de Souza, L. S. M. Miranda and U. T. A. Bornscheuer, Chem.–Eur. J., 2017, 23, 12040–12063 CrossRef CAS PubMed.
- S. Wu, R. Snajdrova, J. C. Moore, K. Baldenius and U. T. Bornscheuer, Angew. Chem., Int. Ed., 2021, 60, 88–119 CrossRef CAS PubMed.
- S. C. Hammer, A. M. Knight and F. H. Arnold, Curr. Opin. Green Sustain. Chem., 2017, 7, 23–30 CrossRef.
- F. H. Arnold, Angew. Chem., Int. Ed., 2018, 57, 4143–4148 CrossRef CAS PubMed.
- R. A. Sheldon and J. M. Woodley, Chem. Rev., 2018, 118, 801–838 CrossRef CAS PubMed.
- A. G. A. Sá, A. C. de Meneses, P. H. H. de Araújo and D. de Oliveira, Trends Food Sci. Technol., 2017, 69, 95–105 CrossRef.
- S. K. Karmee, Appl. Microbiol. Biotechnol., 2009, 81, 1013–1022 CrossRef CAS PubMed.
- J. Müller, M. A. Sowa, B. Fredrich, H. Brundiek and U. T. Bornscheuer, ChemBioChem, 2015, 16, 1791–1796 CrossRef PubMed.
- M. W. Larsen, D. F. Zielinska, M. Martinelle, A. Hidalgo, L. J. Jensen, U. T. Bornscheuer and K. Hult, ChemBioChem, 2010, 11, 796–801 CrossRef PubMed.
- N. N. Gandhi, N. S. Patil, S. B. Sawant, J. B. Joshi, P. P. Wangikar and D. Mukesh, Catal. Rev., 2000, 42, 439–480 CrossRef CAS.
- L. Mestrom, J. G. R. Claessen and U. Hanefeld, ChemCatChem, 2019, 11, 2004–2010 CrossRef CAS.
- J. Sun and S. Liu, J. Food Biochem., 2015, 39, 11–18 CrossRef CAS.
- P. Pongpamorn, C. Kiattisewee, N. Kittipanukul, J. Jaroensuk, D. Trisrivirat, S. Maenpuen and P. Chaiyen, Angew. Chem., Int. Ed., 2021, 133, 5813–5817 CrossRef . In this recent report, use of a carboxylic acid reductase (CAR) catalyzes esterification in an aqueous alcoholic medium. Both Mg2+ and ATP are required, and is imidazole, to generate the esters shown in modest yields..
- (a) C. An, M. H. Shaw, A. Tharp, D. Verma, H. Li, H. Wang and X. Wang, Org. Lett., 2020, 22, 8320–8325 CrossRef CAS PubMed; (b) S. Bojarra, D. Reichert, M. Grote, A. G. Baraibar, A. Dennig, B. Nidetzky, C. Mügge and R. Kourist, ChemCatChem, 2018, 10, 1192–1201 CrossRef CAS.
- B. H. Lipshutz, S. Ghorai, A. R. Abela, R. Moser, T. Nishikata, C. Duplais, A. Krasovskiy, R. D. Gaston and R. C. Gadwood, J. Org. Chem., 2011, 76, 4379–4391 CrossRef CAS PubMed . See also: D. Goswami, Appl. Biochem. Biotechnol., 2020, 191, 744–762 CrossRef PubMed.
- R. C. Rodrigues and R. Fernandez-Lafuente, J. Mol. Catal. B: Enzym., 2010, 64, 1–22 CrossRef CAS.
- (a) M. Cortes-Clerget, N. Akporji, J. Zhou, F. Gao, P. Guo, M. Parmentier, F. Gallou, J. Y. Berthon and B. H. Lipshutz, Nat. Commun., 2019, 10, 2169 CrossRef PubMed; (b) N. Akporji, V. Singhania, J. Dussart-Gautheret, F. Gallou and B. H. Lipshutz, Chem. Commun., 2021, 57, 11847–11850 RSC.
- P. Villo, O. Dalla-Santa, Z. Szabó and H. Lundberg, J. Org. Chem., 2020, 85, 6959–6969 CrossRef CAS PubMed.
- The additive, PhCF3, was not nearly as effective upon evaluation in the presence of other lipases used in this study (Candida rugosa, Rhizopus niveus, and Burkholderia cepacia).
- (a) M. A. Cuendet, H. Weinstein and M. V. LeVine, J. Chem. Theory Comput., 2016, 12, 5758–5767 CrossRef CAS PubMed; (b) D. E. Koshland, G. Némethy and D. Filmer, Biochem, 1966, 5, 365–385 CrossRef CAS PubMed; (c) M. Jacque, J. Wyman and J. Changeux, J. Mol. Biol., 1965, 12, 88–118 CrossRef.
- A. G. Sandstrom, Y. Wikmark, K. Engstrom, J. Nyhlen and J. E. Backvall, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 78–83 CrossRef PubMed.
- (a) F. Khan, D. Lan, R. Durrani, W. Huan, Z. Zhao and Y. Wang, Front. Bioeng. Biotechnol., 2017, 5, 16 Search PubMed; (b) A. M. Brzozowski, U. Derewendat, Z. S. Derewendat, D. M. Lawson, J. P. Turkenburg, F. Bjorkling, B. Huge-Jensen, S. A. Patkar and L. Thim, Nature, 1991, 351, 491–494 CrossRef CAS PubMed; (c) A. Roussel, S. Amara, A. Nyyssölä, E. Mateos-Diaz, S. Blangy, H. Kontkanen, A. Westerholm-Parvinen, F. Carrière and C. Cambillau, J. Mol. Biol., 2014, 426, 3757–3772 CrossRef CAS PubMed.
- Y. Hayashi, J. Org. Chem., 2021, 86, 1–23 CrossRef CAS PubMed.
- Y. Hayashi, Chem. Sci., 2016, 7, 866–880 RSC.
- N. Akporji, R. R. Thakore, M. Cortes-Clerget, J. Andersen, E. Landstrom, D. H. Aue, F. Gallou and B. H. Lipshutz, Chem. Sci., 2020, 11, 5205–5212 RSC.
- B. S. Takale, R. R. Thakore, E. S. Gao, F. Gallou and B. H. Lipshutz, Green Chem., 2020, 22, 6055–6061 RSC.
- A. B. Wood, M. Cortes-Clerget, J. R. A. Kincaid, B. Akkachairin, V. Singhania, F. Gallou and B. H. Lipshutz, Angew. Chem., Int. Ed., 2020, 132, 17740–17746 CrossRef.
- R. Chinchilla and C. Najera, Chem. Soc. Rev., 2011, 40, 5084–5121 RSC.
- National Research Council (US) Chemical Sciences Roundtable, The Role of the Chemical Sciences in Finding Alternatives to Critical Resources: A Workshop Summary, Replacing Critical Materials with Abundant Materials, National Academies Press (US), Washington (DC), 2012, vol. 4 Search PubMed.
- We have no experimental proof at this time as to why enzymatic denaturation has yet to be seen independent of the amounts and nature of the metals present in the medium. Nonetheless, we suggest that the explanation may be attributed to the likelihood that the catalysts are predominantly localized in and around the micellar array; hence, as far as the enzyme is concerned, it may not be exposed to the high levels of potentially denaturing metals present, which is a textbook phenomenon.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, 1998 Search PubMed.
- R. A. Sheldon, D. Brady and M. L. Bode, Chem. Sci., 2020, 11, 2587–2605 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc05660c |
| This journal is © The Royal Society of Chemistry 2022 |