Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly effective and chemoselective hydrodeoxygenation of aromatic alcohols†
Caiyun
Xu
a,
Haihong
Wu
 *a,
Zhanrong
Zhang
*a,
Zhanrong
Zhang
 *b,
Bingxiao
Zheng
a,
Jianxin
Zhai
a,
Kaili
Zhang
a,
Wei
Wu
a,
Xuelei
Mei
a,
Mingyuan
He
*a and
Buxing
Han
*b,
Bingxiao
Zheng
a,
Jianxin
Zhai
a,
Kaili
Zhang
a,
Wei
Wu
a,
Xuelei
Mei
a,
Mingyuan
He
*a and
Buxing
Han
 *ab
*ab
aShanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai 200062, China. E-mail: hhwu@chem.ecnu.edu.cn; hemingyuan@126.com; hanbx@iccas.ac.cn
bBeijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid and Interface and Thermodynamics, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: zhangzhanrong@iccas.ac.cn; hanbx@iccas.ac.cn
First published on 19th January 2022
Abstract
Effective hydrodeoxygenation (HDO) of aromatic alcohols is very attractive in both conventional organic synthesis and upgrading of biomass-derived molecules, but the selectivity of this reaction is usually low because of the competitive hydrogenation of the unsaturated aromatic ring and the hydroxyl group. The high activity of noble metal-based catalysts often leads to undesired side reactions (e.g., saturation of the aromatic ring) and excessive hydrogen consumption. Non-noble metal-based catalysts suffer from unsatisfied activity and selectivity and often require harsh reaction conditions. Herein, for the first time, we report chemoselective HDO of various aromatic alcohols with excellent selectivity, using porous carbon–nitrogen hybrid material-supported Co catalysts. The C–OH bonds were selectively cleaved while leaving the aromatic moiety intact, and in most cases the yields of targeted compounds reached above 99% and the catalyst could be readily recycled. Nitrogen doping on the carbon skeleton of the catalyst support (C–N matrix) significantly improved the yield of the targeted product. The presence of large pores and a high surface area also improved the catalyst efficiency. This work opens the way for efficient and selective HDO reactions of aromatic alcohols using non-noble metal catalysts.
Introduction
Biomass represents a green and sustainable carbon resource.1 In comparison with other energy sources, biomass is renewable, abundant and widely distributed worldwide.2,3 Thus, making innovative use of biomass to replace non-renewable fossil reserves for the production of valuable chemical products has attracted significant attention.4–9 In this regard, chemical transformation and upgrading of biomass-derived molecules into useful chemical and fuel products are highly important.10–14 Owing to the high oxygen content of biomass and its chemical derivatives, the applications of biorefinery products are often limited. For example, bio-oil produced by rapid pyrolysis of biomass is a valuable second-generation renewable energy and resource carrier.15,16 However, it generally contains a large number of oxygen-containing compounds, which are thermodynamically unstable. The many different self- and/or cross-polymerization reactions between ketones, aldehydes, acids and alcohols that are contained in bio-oil significantly hinder the incorporation of bio-oil as the feedstock into existing refinery equipment.17 To address this problem, many hydrodeoxygenation (HDO) approaches have been developed for the upgrading of biomass-derived molecules.18 HDO refers to the transformation of a hydroxyl group into the corresponding alkyl group, which constitutes a powerful synthetic tool for the synthesis of complex natural products, bioactive molecules and petrochemicals.19,20 In recent years, chemoselective HDO has not only received extensive attention in organic synthesis, but also has been proved to be an effective way for the upgrading of biomass-derived molecules. Previous studies have also demonstrated that selective HDO is an effective method to improve the stability of bio-oil. To date, researchers have developed many methods for chemoselective HDO of oxygen-containing molecules, such as alcohols, aldehydes, ketones, amides and nitrogen-containing compounds.21–23 Among them, alcohols are widely used as versatile precursors for the production of other organic molecules in the chemical industry. The selective HDO of alcohols is one of the most fundamental transformations in organic chemistry and plays a crucial role in the total synthesis of complex natural products bearing multifunctional groups.24,25 In terms of biomass upgrading, selective HDO of aromatic alcohols via catalytic hydrogenolysis of C–OH bonds is very important, to produce valuable chemicals from lignin which is the only natural renewable aromatic biomass resource. Herein, one of the key challenges is the precise control of selectivity.26 The selectivity of HDO of aromatic alcohol substrates is regulated by the competitive hydrogenation of the unsaturated aromatic ring and the hydroxyl group. During the reaction, aromatic alcohols are readily adsorbed on the metal surface of catalysts. Thermodynamically, conformation between the benzene ring and the metal surface tends to be flat. Thus, the π-electron interaction promotes the hydrogenation of the unsaturated aromatic ring, which renders the selectivity of HDO not satisfactory.27Indeed, hydrogenolysis of C–OH bonds has been investigated using both noble and non-noble metal-based catalysts.28–30 Noble metal-based catalysts (e.g., Ru,31,32 Au33 and Pd-based catalysts34,35) often exhibit higher activity towards hydrogenation and HDO reactions than their non-noble metal-based counterparts. However, their high activity often inevitably leads to undesired side reactions (e.g., saturation of the aromatic ring) and high hydrogen consumption, which often renders the reaction selectivity not satisfactory. In comparison, less attention has been paid to chemoselective HDO of aromatic alcohols using non-noble metal-based catalysts.36–38 The lower activity of non-noble metal catalysts often leads to a high reaction temperature and pressure, which in turn probably deactivates the catalyst. Moreover, a limited range of substrates and unclear active sites under these conditions limit their practical applicability.39 In this respect, development of effective, robust, non-noble metal-based heterogeneous catalysts which could be readily separated and recycled is very important and attractive.
Metal organic frameworks (MOFs) can be used as a precursor for the preparation of nanoporous carbon-based materials for different applications, such as catalysis, drug delivery, adsorption and separation.40–44 Their large internal surface area, high porosity and mild synthesis conditions make them ideal starting materials for preparation of catalyst supports. Among them, zeolitic imidazolate framework-67 (ZIF-67), a sodalite-type MOF produced by the regular arrangement of Co2+ and 2-methylimidazole (2-MeI) is a chemically and thermally stable MOF that has attracted significant attention. Owing to its high amount of active cobalt sites, ZIF-67 is a promising candidate for the synthesis of Co-based catalysts.45–49 Direct reduction of ZIF-67 in H2 at a high temperature can produce a porous nitrogen-doped carbon supported Co catalyst. During reduction, the Co ions in ZIF-67 are transformed into metallic Co, while the 2-MeI organic linkers become the N-doped carbon support. In this process, ZIF-67 plays a parallel role as both the template and precursor to produce the catalyst.
Herein, for the first time, we report the efficient and chemoselective HDO of aromatic alcohols, using ZIF-67-derived catalysts consisting of Co particles embedded in a porous carbon–nitrogen hybrid material (Co@CN) as the catalyst. Specifically, we synthesized several Co@CN catalysts including Co@CN-500, Co@CN-600 and Co@CN-700, obtained by reducing ZIF-67 at 500, 600 and 700 °C, respectively. In addition, we also synthesized a Co@CN-ph catalyst in a similar way to that of Co@CN-700, by using 1,10-phenanthroline as the organic ligand instead of 2-MeI in the ZIF precursor. The C–OH bonds of both primary and secondary aromatic alcohols are selectively cleaved using Co@CN-700, while the aromatic functionality remains intact. The corresponding aromatic compounds are produced in excellent yields. This efficient chemical transformation could be achieved under mild reaction conditions (120 °C, 2 MPa H2). Notably, Co@CN-700 is mesoporous and exhibits a high surface area, and the Co NPs are homogeneously distributed on the carbon–nitrogen hybrid support. These properties lead to the excellent catalytic properties. The catalyst is also magnetically-separable and stable during recycling, making it a very promising catalyst for chemoselective HDO reactions of different types of aromatic alcohols.
Results and discussion
Initially, Co-ZIF synthesized at room temperature exhibits high crystallinity and has a uniform polyhedral structure with a smooth surface and diameters between 300 and 500 nm, as revealed by its SEM (Fig. 1a) and high-resolution TEM images (Fig. 1b). The original ZIF-67 shows a representative XRD pattern due to its well-organized framework, which is in good consistency with previous work (Fig. 1c). Then the synthesized Co-ZIF is reduced at 500, 600 and 700 °C, in an hydrogen/argon atmosphere. After reduction, only the diffraction peaks of metallic Co and graphitic carbon are present in the XRD patterns, indicating the successful transformation of Co-ZIF into Co@CN (Fig. 1d). With the increase of the reduction temperature, the diffraction peaks of Co become more dominant, due to increased crystallinity of Co particles.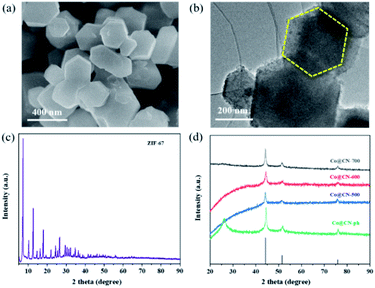 | ||
| Fig. 1 (a) SEM image of Co-ZIF, (b) HR-TEM image of Co-ZIF, (c) XRD pattern of Co-ZIF, (d) XRD patterns of Co@CN-500, Co@CN-600, Co@CN-700 and Co@CN-ph. | ||
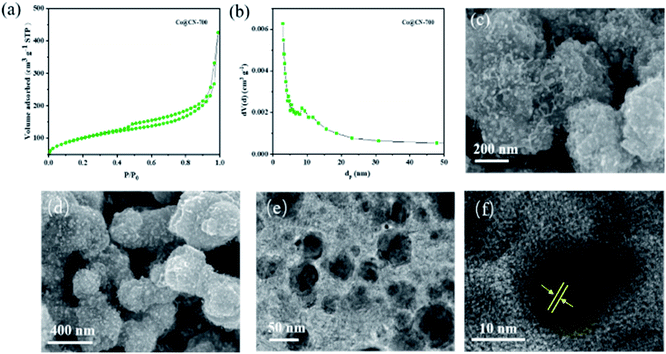
The N2 adsorption–desorption isotherm and BJH pore size distribution of Co@CN-700 indicates that it exhibits a mesoporous structure (Fig. 2). When P/P0 < 0.2, the adsorption speed of the catalyst is fast. When P/P0 is between 0.4 and 0.8, the catalyst has obvious hysteresis, indicating the presence of mesopores. When P/P0 is close to 1, the isotherm does not tend to be flat but adsorbs upwards, suggesting the presence of large pores. Notably, the BET surface area of Co@CN catalysts increased from 48.9 m2 g−1 to 111.9 m2 g−1 with the increase of the reduction temperature from 500 °C to 700 °C. For more structural details, please refer to the ESI (Fig. S1 and Table S1†). SEM images of Co@CN-700 (Fig. 2c and d) revealed that after reducing Co-ZIF, the polyhedron morphology of ZIF crystals changed into rough and spongy-type particles. The Co nanoparticles are uniformly dispersed in the Co@CN-700 catalyst and the interplanar distance of the Co nanoparticles for the lattice fringe is around 0.12 nm, as shown in the HR-TEM image of the Co@CN-700 catalyst (Fig. 2f). More morphological details are shown in the ESI (Fig. S2†). ICP-OES analysis indicates that the Co content in Co@CN-700 is about 38 wt% (ESI, Table S2†).
XPS characterization of synthesized catalysts was further conducted to investigate their chemical structures and bonding states (Fig. 3). The high-resolution Co 2p3/2 XPS spectra were deconvoluted into three distinct peaks centered at 778.1 eV, 780.0 eV and 783.0 eV, which are assigned to Co0, CoxOy and Co-Nx, respectively. The intensity of the characteristic peaks of CoxOy (ca. 780.0 eV) and Co-Nx (ca. 783.0 eV) decreased with the increase of the reduction temperature from 500 to 700 °C. This indicates that CoxOy and Co-Nx are reduced to Co0 during reduction. The characteristic peak of Co0 is the most dominant in the XPS spectrum of Co@CN-700. In contrary, the characteristic peak of CoxOy in the XPS spectrum of Co@CN-ph is the most significant, while the Co-Nx and Co0 diffraction peaks are not shown. Hence, the presence of Co-Nx could potentially promote the formation of Co0 (Fig. 3a). The N 1s spectrum was deconvoluted into three sub-peaks, corresponding to pyridinic-N at 398.4 eV, pyrrolic-N at 400.3 eV and graphitic-N at 401.4 eV (Fig. 3b). The intensity of graphite-N slightly increased with the increase of the reduction temperature from 500 °C to 700 °C, because of its higher thermal stability. No graphitic-N was observed from the XPS spectrum of Co@CN-ph.
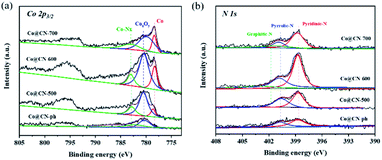 | ||
| Fig. 3 Deconvoluted XPS core-level spectra of Co@CN catalysts. (a) Co 2p core level peaks, and (b) N 1s core level peaks. | ||
Subsequently, we started our investigation using 1-phenylethanol as a model compound under a hydrogen atmosphere. Using ethanol as the solvent, the control experiment indicated that the reaction could not proceed at 120 °C in the absence of the catalyst (Table 1, entry 1). Commercial Co3O4 and Zn@CN synthesized in a similar way to that of Co@CN as catalysts could not catalyze the reaction as well (Table 1, entry 4 and 5). In contrast, the Co@CN catalysts synthesized by ZIF-67 at different temperatures including Co@CN-500, Co@CN-600 and Co@CN-700 could significantly promote the reaction (Table 1, entries 8–10). Co@CN-700 showed the best catalytic performance and the C–OH bond of 1-phenylethanol was effectively and selectively cleaved, affording ethylbenzene with a yield of up to 99.7% (Table 1, entry 10). Using other common solvents such as THF and cyclohexane instead of ethanol did not significantly affect the yields of the targeted product, although slightly lower yields of ethylbenzene were obtained (Table 1, entry 11 and 12). Activated carbon supported Co nanoparticles (Co@AC) and Co@CN-ph synthesized in a similar way to that of Co@CN-700 could also promote the reaction, and the yields of ethylbenzene reached 90.8% (Table 1, entry 6) and 71.8% (Table 1, entry 7), respectively. These catalysts were less effective in comparison with Co@CN-700. The abovementioned experimental results clearly suggest that the structure of the N-doped carbon skeleton had a significant effect on the reactivity of the HDO reaction. This is primarily because the incorporation of nitrogen atoms (i.e., formation of the carbon–nitrogen hybrid) changed the chemical, electrical and functional properties of the carbon matrix, thereby affecting the catalytic activity of the loaded metal NPs. This effect is also verified by the XPS characterization that the Co-Nx peak is very obvious in Co@CN materials, but almost missing in the spectrum of Co@CN-ph. The strong interaction between nitrogen atoms and Co nanoparticles not only stabilized the Co particles, but also improved the reducing ability of the catalyst, enabling the alcohol groups to be efficiently converted into the alkyl group. For more details on optimization of reaction conditions, please refer to the ESI (Fig. S3†).
| Entry | Catalyst | Solvent | H2 (MPa) | Temp. (°C) | Yield (%) |
|---|---|---|---|---|---|
| a Without catalyst. b ZIF-67 was calcined in air at 700 °C. c 1,10-Phenanthroline was used instead of MeI as the ligand for synthesizing the catalyst precursor. d ZIF-67 was reduced at 500 °C in 10% H2/Ar. e ZIF-67 was reduced at 600 °C in 10% H2/Ar. f ZIF-67 was reduced at 700 °C in 10% H2/Ar. Reaction conditions: 1-phenylethanol (1 mmol), catalyst (40 mg), solvent (2 mL), reaction time: 8 h. The GC yields were obtained using dodecane as an internal standard. | |||||
| 1 | Blanka | Ethanol | 2 | 120 | n.r. |
| 2 | ZIF-67 | Ethanol | 2 | 120 | n.r. |
| 3 | ZIF-67 (air)b | Ethanol | 2 | 120 | n.r. |
| 4 | Co3O4 | Ethanol | 2 | 120 | n.r. |
| 5 | Zn@CN | Ethanol | 2 | 120 | n.r. |
| 6 | Co@AC | Ethanol | 2 | 120 | 90.8 |
| 7 | Co@CN-phc | Ethanol | 2 | 120 | 71.8 |
| 8 | Co@CN-500d | Ethanol | 2 | 120 | 90.4 |
| 9 | Co@CN-600e | Ethanol | 2 | 120 | 97.2 |
| 10 | Co@CN-700f | Ethanol | 2 | 120 | 99.7 |
| 11 | Co@CN-700 | THF | 2 | 120 | 96.7 |
| 12 | Co@CN-700 | Cyclohexane | 2 | 120 | 93.2 |
| 13 | Co@CN-700 | Ethanol | 2 | 100 | 45.1 |
| 14 | Co@CN-700 | Ethanol | 1 | 120 | 92.5 |
| 15 | Co@CN-700 (20 mg) | Ethanol | 2 | 120 | 56.2 |
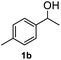
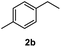
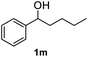
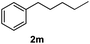
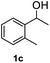
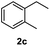
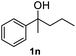
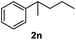
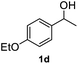
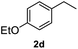
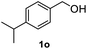
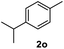
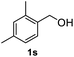
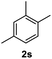
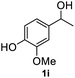
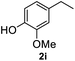
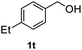
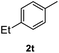
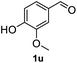
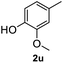
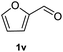
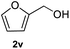
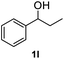
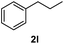
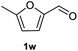
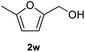
After optimization of reaction conditions, we further explored the substrate scope for the HDO reaction over Co@CN-700. The C–OH bonds of a diverse range of secondary and primary alcohols were selectively and effectively cleaved, affording the corresponding aromatic hydrocarbons in excellent yields (Table 2). Specifically, phenylethanol derivatives with various electron-donating or electron-withdrawing substituent groups on the aryl ring (1b–1k) can be effectively converted into dehydroxylated products with excellent yields (87.8–99%). Among them, several substrates (e.g., 1f, 1g, 1i, and 1q) which contain hydroxyl and methoxy groups on the benzene ring can be obtained from lignin transformation. Hence, the designed selective HDO catalyst is also very suitable for further upgrading of biomass-derived molecules. Moreover, we have also tested several bio-based aldehydes that could be directly derived from lignin and cellulose (e.g., 1u, 1v, and 1w), and their corresponding products were generated in excellent yields. Secondary alcohols with various carbon chain lengths (1l–1n) are also suitable substrates, and the corresponding selective HDO products were generated in excellent yields. Moreover, the designed catalyst is effective for selective HDO of monohydric alcohol substrates with excellent selectivity (1o–1t). For comparison, we also tested ethanol and other aliphatic alcohol substrates with various chain lengths including n-butanol, n-pentanol, n-hexanol and n-octanol, and these aliphatic alcohols cannot be converted under the same conditions using Co@CN-700. The hydroxyl groups in these substrates are intact after the reaction (Fig. S4†). Hence, the solvent is stable under reaction conditions and the presence of aromatic functionality (i.e., aryl group) is crucial for the HDO reaction to proceed.
| Substrate | Product | Yield | Substrate | Product | Yield |
|---|---|---|---|---|---|
| a Unless otherwise noted, the reaction was carried out with 1 mmol substrate, 2 mL ethanol, and 40 mg catalyst (Co@CN-700). Reaction temperature: 120 °C, reaction time: 8 h, and H2 pressure: 2 MPa. | |||||
|
|
91.7% |
|
|
>99% |
|
|
>99% |
|
|
>99% |
|
|
>99% |
|
|
91.8% |
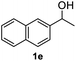
|
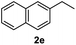
|
87.8% |
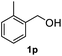
|
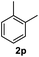
|
>99% |
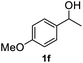
|
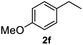
|
>99% |
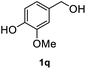
|
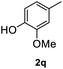
|
>99% |
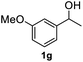
|
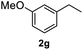
|
>99% |
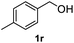
|
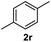
|
>99% |
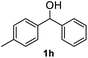
|
|
>99% |
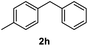
|
|
>99% |
|
|
>99% |
|
|
>99% |
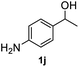
|
|
94.1% |
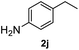
|
|
>99% |
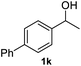
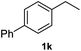
|
|
93.1% |
|
|
>99% |
|
|
>99% |
|
|
90.3% |
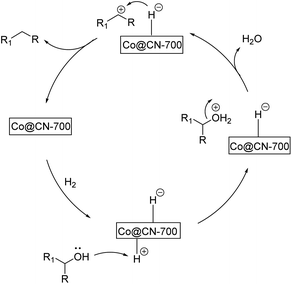
Based on the above-mentioned experimental results, we propose the following reaction pathway for the selective HDO reaction (Fig. 4). First, both aromatic alcohol substrates and H2 molecules are adsorbed onto the catalyst. The catalyst promotes the hetero-cracking of H2 molecules to generate H+ and H−. Then, H+ combines with the hydroxyl group of the alcohol substrate and a water molecule is released. The resultant benzyl cations (Ph-CH2+ or Ph-CH+-R) can be stabilized by aromatic rings. This effect is more obvious for substrates with the electron-donating groups on the benzene ring. Finally, H− is attached to the benzyl cation to generate the final aromatic alkane product. As abovementioned, the XPS characterization results indicate that CoxOy was primarily transformed into Co0 in the Co@CN-700 catalyst. The presence of Co0 represents that the active sites of the catalyst are crucial to promote the chemoselective HDO reaction. More importantly, the interaction between nitrogen atoms and Co NPs (Co-Nx coordination) significantly improves the catalytic performance of Co@CN-700.
The Co@CN-700 catalyst could also be readily recycled under standard conditions. In total, five cycles of the HDO reaction of 1-phenylethanol were carried out at 120 °C (Fig. 5). The spent catalyst could be readily separated from the reaction mixture using an external magnetic field after each reaction (Fig. S5†). The recovered catalyst was washed, dried at 80 °C and reused in the next reaction. The catalyst is still very effective after five runs of the reaction, and the conversion and yield of 1-phenylethanol were almost the same as those of the first run (Fig. 5). In addition, from the detailed morphology and XRD characterization of the recovered Co@CN-700 catalyst, there is no obvious change in its features such as the morphology (Fig. S6 and S7†). XPS characterization of the fresh and recovered Co@CN-700 catalyst indicated that different cobalt species did not change obviously after the reaction (Fig. S8†). The characteristic peak of Co0 was still the most dominant in the XPS spectrum of the recovered catalyst, which further proves the excellent recyclability of the synthesized Co@CN-700 catalyst. This also verified that in the case of nitrogen doping, the oxidation resistance of Co0 has been improved. As a result, the Co@CN-700 catalyst showed excellent heterogeneous catalytic performance in terms of selectivity, stability and recyclability.
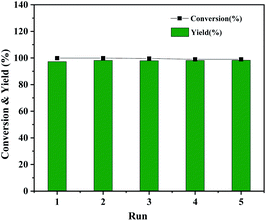 | ||
| Fig. 5 Recyclability test of Co@CN-700. Reaction conditions were the same as those shown in Table 1, entry 1. | ||
Conclusions
In summary, the robust Co-ZIF derived Co@CN-700 catalyst can effectively promote the chemoselective HDO reaction of various aromatic alcohols in ethanol and H2, under relatively mild conditions (120 °C, 2 MPa H2, 8 h). The C–OH bonds of a range of primary and secondary aromatic alcohols were selectively cleaved, while leaving the aromatic functionality intact. Notably, nitrogen atom doping on the carbon skeleton can interfere with the electrical neutrality of adjacent carbon atoms, and this effect leads to imbalance in the charge state, which could enhance the efficiency of the HDO reaction.48,50 The interaction/coordination between nitrogen atoms and Co NPs (Co-Nx) is crucial to achieve excellent catalytic efficiency and selectivity. In addition, the presence of large pores and a high surface area also improves the catalytic performance of Co@CN-700. More active sites (i.e., Co0) are exposed in this catalyst and thus significantly promote the reaction. During the HDO reaction, water was generated as the only by-product and the catalyst can also be readily recovered and recycled. This robust and cheap catalyst has significant potential application in biofuel and biorefinery processes.Data availability
The authors declare that all data supporting the findings of this study are available within the paper [and its ESI†].Author contributions
C. Y. X., H. H. W., Z. R. Z., M. Y. H. and B. X. H. conceived the project, and designed and implemented the experiments. C. Y. X., H. H. W., Z. R. Z., M. Y. H. and B. X. H. analyzed the data and wrote the manuscript. All the other authors discussed the results and helped in data analysis.Conflicts of interest
The authors declare no competing interests.Acknowledgements
The work was supported by the National Key Research and Development Program of China (2017YFA0403103), the National Natural Science Foundation of China (21972146), the Chinese Academy of Sciences (QYZDY-SSW-SLH013), and Youth Innovation Promotion Association of CAS (2017043).Notes and references
- M. J. Mulvihill, E. S. Beach, J. B. Zimmerman and P. T. Anastas, Annu. Rev. Energy, 2011, 36, 271–293 Search PubMed.
- L. Zhou, Z. Liu, Y. Bai, T. Lu, X. Yang and J. Xu, J. Energy Chem., 2016, 25, 141–145 CrossRef.
- C. H. Lam, A. J. Bloomfield and P. T. Anastas, Green Chem., 2017, 19, 1958–1968 RSC.
- W. T. Ding, H. Li, R. Zong, J. Y. Jiang and X. F. Tang, ACS Sustainable Chem. Eng., 2021, 9, 3498–3508 CrossRef CAS.
- J. Ma, S. Shi, X. Jia, F. Xia, H. Ma, J. Gao and J. Xu, J. Energy Chem., 2019, 36, 74–86 CrossRef.
- W. Anutrasakda, K. Eiamsantipaisarn, D. Jiraroj, A. Phasuk, T. Tuntulani, H. Liu and D. Tungasmita, Catalysts, 2019, 9(1), 92 CrossRef.
- J. Li, S. Zhang, B. Gao, A. Yang, Z. Wang, Y. Xia and H. Liu, Fuel, 2016, 166, 196–202 CrossRef CAS.
- J. Li, L. Liu, Y. Liu, M. Li, Y. Zhu, H. Liu, Y. Kou, J. Zhang, Y. Han and D. Ma, Energy Environ. Sci., 2014, 7, 393–398 RSC.
- Z. Zhang, J. Song and B. Han, Chem. Rev., 2017, 117, 6834–6880 CrossRef CAS PubMed.
- Q. Bu, H. Lei, A. H. Zacher, L. Wang, S. Ren, J. Liang, Y. Wei, Y. Liu, J. Tang, Q. Zhang and R. Ruan, Bioresour. Technol., 2012, 124, 470–477 CrossRef CAS PubMed.
- Y. Jing, Y. Guo, Q. Xia, X. Liu and Y. Wang, Chem, 2019, 5, 2520–2546 CAS.
- Y. Liu, Y. Zhu and C. Li, Front. Chem. Sci. Eng., 2017, 12, 3–8 CrossRef.
- M. Hu, W. Wu and H. Jiang, ChemSusChem, 2019, 12, 2911–2935 CrossRef CAS PubMed.
- R. Lu, F. Lu, X. Si, H. Jiang, Q. Huang, W. Yu, X. Kong and J. Xu, ChemSusChem, 2018, 11, 1621–1627 CrossRef CAS PubMed.
- F. Cherubini, Energy Convers. Manage., 2010, 51, 1412–1421 CrossRef CAS.
- X. Pei, Y. Deng, Y. Li, Y. Huang, K. Yuan, J. F. Lee, T. S. Chan, J. Zhou, A. Lei and L. Zhang, Nanoscale, 2018, 10, 14719–14725 RSC.
- G. Moos, M. Emondts, A. Bordet and W. Leitner, Angew. Chem., Int. Ed., 2020, 59, 11977–11983 CrossRef CAS PubMed.
- Y. Jing, Y. Wang, S. Furukawa, J. Xia, C. Sun, M. J. Hulsey, H. Wang, Y. Guo, X. Liu and N. Yan, Angew. Chem., Int. Ed., 2021, 60, 5527–5535 CrossRef CAS PubMed.
- M. Liu, Z. Zhang, H. Liu, T. Wu and B. Han, Chem. Commun., 2020, 56, 7120–7123 RSC.
- K. A. Rogers and Y. Zheng, ChemSusChem, 2016, 9, 1750–1772 CrossRef CAS PubMed.
- Z. Dong, J. Yuan, Y. Xiao, P. Mao and W. Wang, J. Org. Chem., 2018, 83, 11067–11073 CrossRef CAS PubMed.
- Y. Y. Mei, S. P. Zhang, H. Wang, S. X. Jing, T. Hou and S. S. Pang, Energy Fuels, 2020, 34, 3422–3428 CrossRef CAS.
- H. Duan, J. Dong, X. Gu, Y. K. Peng, W. Chen, T. Issariyakul, W. K. Myers, M. J. Li, N. Yi, A. F. R. Kilpatrick, Y. Wang, X. Zheng, S. Ji, Q. Wang, J. Feng, D. Chen, Y. Li, J. C. Buffet, H. Liu, S. C. E. Tsang and D. O'Hare, Nat. Commun., 2017, 8, 591 CrossRef PubMed.
- A. Córdova, S. Afewerki and C. Palo-Nieto, Synthesis, 2020, 52, 2330–2336 CrossRef.
- C. H. Ko, S. H. Park, J. K. Jeon, D. J. Suh, K. E. Jeong and Y. K. Park, Korean J. Chem. Eng., 2012, 29, 1657–1665 CrossRef CAS.
- J. L. Huang, X. J. Dai and C. J. Li, Eur. J. Org. Chem., 2013, 2013, 6496–6500 CrossRef CAS.
- Q. Chen, H. Z. Kang, X. Liu, K. Jiang, Y. F. Bi, Y. M. Zhou, M. Y. Wang, M. Zhang, L. Liu and E. H. Xing, ChemCatChem, 2020, 12, 1948–1952 CrossRef CAS.
- J. J. Ibrahim, C. B. Reddy, X. L. Fang and Y. Yang, Eur. J. Org. Chem., 2020, 2020, 4429–4432 CrossRef CAS.
- J. M. Herrmann and B. Konig, Eur. J. Org. Chem., 2013, 2013, 7017–7027 CrossRef CAS.
- Z. Sun, B. Fridrich, A. de Santi, S. Elangovan and K. Barta, Chem. Rev., 2018, 118, 614–678 CrossRef CAS PubMed.
- X. M. Yang, Y. Liang, Y. Y. Cheng, W. Song, X. F. Wang, Z. C. Wang and J. S. Qiu, Catal. Commun., 2014, 47, 28–31 CrossRef CAS.
- N. Kalutharage and C. S. Yi, J. Am. Chem. Soc., 2015, 137, 11105–11114 CrossRef CAS PubMed.
- H. Nishikawa, D. Kawamoto, Y. Yamamoto, T. Ishida, H. Ohashi, T. Akita, T. Honma, H. Oji, Y. Kobayashi, A. Hamasaki, T. Yokoyama and M. Tokunaga, J. Catal., 2013, 307, 254–264 CrossRef CAS.
- S. G. Wang, P. Zhou, L. Jiang, Z. H. Zhang, K. J. Deng, Y. H. Zhang, Y. X. Zhao, J. L. Li, S. Bottle and H. Y. Zhu, J. Catal., 2018, 368, 207–216 CrossRef CAS.
- X. Pei, Y. Deng, B. Duan, T.-S. Chan, J.-F. Lee, A. Lei and L. Zhang, Nano Res., 2018, 11, 3145–3153 CrossRef CAS.
- M. Besson, P. Gallezot and C. Pinel, Chem. Rev., 2014, 114, 1827–1870 CrossRef CAS PubMed.
- N. Antil, A. Kumar, N. Akhtar, R. Newar, W. Begum and K. Manna, Inorg. Chem., 2021, 60, 9029–9039 CrossRef CAS PubMed.
- P. Zhang, N. Chen, D. Chen, S. Yang, X. Liu, L. Wang, P. Wu, N. Phillip, G. Yang and S. Dai, ChemCatChem, 2018, 10, 3299–3304 CrossRef CAS.
- S. Sitthisa, T. Pham, T. Prasomsri, T. Sooknoi, R. G. Mallinson and D. E. Resasco, J. Catal., 2011, 280, 17–27 CrossRef CAS.
- H. F. Wang, L. Chen, H. Pang, S. Kaskel and Q. Xu, Chem. Soc. Rev., 2020, 49, 1414–1448 RSC.
- Y. Gu, Y. N. Wu, L. Li, W. Chen, F. Li and S. Kitagawa, Angew. Chem., Int. Ed., 2017, 56, 15658–15662 CrossRef CAS PubMed.
- H. He, R. Li, Z. Yang, L. Chai, L. Jin, S. I. Alhassan, L. Ren, H. Wang and L. Huang, Catal. Today, 2021, 375, 10–29 CrossRef CAS.
- J. G. Lee, S. Yoon, E. Yang, J. H. Lee, K. Song, H. R. Moon and K. An, J. Catal., 2020, 392, 302–312 CrossRef CAS.
- W. Gong, Y. Lin, C. Chen, M. Al-Mamun, H. S. Lu, G. Wang, H. Zhang and H. Zhao, Adv. Mater., 2019, 31, e1808341 CrossRef PubMed.
- G. H. Zhong, D. X. Liu and J. Y. Zhang, J. Mater. Chem. A, 2018, 6, 1887–1899 RSC.
- U. I. Kramm, I. Herrmann-Geppert, J. Behrends, K. Lips, S. Fiechter and P. Bogdanoff, J. Am. Chem. Soc., 2016, 138, 635–640 CrossRef CAS PubMed.
- L. Jiang, P. Zhou, C. Liao, Z. Zhang and S. Jin, ChemSusChem, 2018, 11, 959–964 CrossRef CAS PubMed.
- V. Ranaware, D. Verma, R. Insyani, A. Riaz, S. M. Kim and J. Kim, Green Chem., 2019, 21, 1021–1042 RSC.
- W. X. Hou, Y. Huang and X. Liu, Catal. Lett., 2020, 150, 3017–3022 CrossRef CAS.
- Z. Wei, J. Wang, S. Mao, D. Su, H. Jin, Y. Wang, F. Xu, H. Li and Y. Wang, ACS Catal., 2015, 5, 4783–4789 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc06430d |
| This journal is © The Royal Society of Chemistry 2022 |