Open Access Article
Open Access ArticlePaired electrolysis enabled annulation for the quinolyl-modification of bioactive molecules†
Shiqi
You
 a,
Mengyao
Ruan
a,
Cuifen
Lu
a,
Mengyao
Ruan
a,
Cuifen
Lu
 a,
Li
Liu
a,
Li
Liu
 a,
Yue
Weng
a,
Guichun
Yang
a,
Shengchun
Wang
b,
Hesham
Alhumade
c,
Aiwen
Lei
a,
Yue
Weng
a,
Guichun
Yang
a,
Shengchun
Wang
b,
Hesham
Alhumade
c,
Aiwen
Lei
 *bc and
Meng
Gao
*bc and
Meng
Gao
 *a
*a
aCollege of Chemistry and Chemical Engineering, Ministry of Education Key Laboratory for the Synthesis and Application of Organic Functional Molecules, Hubei University, Wuhan, 430062, P. R. China. E-mail: drmenggao@163.com
bCollege of Chemistry and Molecular Sciences and the Institute for Advanced Studies (IAS), Wuhan University, Wuhan 430072, Hubei, P. R. China. E-mail: aiwenlei@whu.edu.cn
cDepartment of Chemical and Materials Engineering, Faculty of Engineering, Center of Research Excellence in Renewable Energy and Power Systems, King Abdulaziz University, Jeddah 21589, Saudi Arabia
First published on 7th February 2022
Abstract
A paired electrolysis enabled cascade annulation that enables the efficient synthesis of highly functionalized quinoline-substituted bioactive molecules from readily available starting materials is reported. Using this methodology, two goals, namely, the direct synthesis of quinolines and the introduction of quinoline moieties to bioactive molecules, can be simultaneously achieved in one simple operation. The use of electroreduction for the activation of isatin, together with the further anodic oxidation of KI to catalytically result in a cascade annulation, highlight the unique possibilities associated with electrochemical activation methods. This transformation can tolerate a wide range of functional groups and can also be used as a functionalization tactic in pharmaceutical research as well as other areas.
Introduction
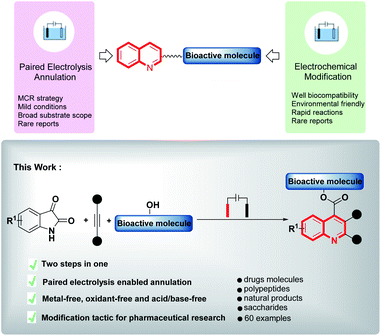
The quinoline moiety is the central core of a wide variety of naturally occurring and manmade compounds, many of which exhibit extraordinary biological and pharmaceutical properties (e.g. antimalarial, antifungal, antibacterial, antiprotozoal, antitubercular, anticancer, antitumor, anti-HIV, antiproliferative, antiinflammatory, antioxidant, DNA binding and antihypertensive and antiviral).1 Alternatively, quinolines provide frameworks for industrial uses including organic light-emitting diodes (OLEDs) and photovoltaic cells, as well as solvents for terpenes and resins.2 Consequently, a variety of novel and expeditious approaches for the preparation of a diverse range of substituted quinolines have been developed.3 The quinoline scaffold has even been described as a “privileged structure”, since members of this structural class are able to interact with different types of bioactive targets and have attracted the interest of investigators in a broad spectrum of sciences from chemistry and biology to medicine.4 Hence, introducing quinoline derivatives into bioactive molecules in the late-stage functionalization of natural products and drugs under mild conditions is of equal importance to the direct synthesis of quinolines in the field of chemical biology and pharmacology. Achieving these two goals in one simple operation would have great appeal.Organic electrosynthesis, as an environmentally friendly synthetic strategy has witnessed a renaissance.5 Compared to traditional methods that often proceed at strong oxidative or reductive conditions with elevated temperature or pressure, electrochemical reactions are usually carried out under milder conditions by precisely varying the applied electrode potential. Therefore, electrosynthesis is usually compatible with highly functionalized substrates and has displayed great potential in both synthetic and bioconjugation chemistry.6 Many efforts have been made to develop efficient electrochemical reactions for the synthesis of various heterocyclic structures, in which electrochemical multicomponent reactions have emerged as a versatile and efficient tool for the construction of heterocycles, and has attracted considerable attention.7 However, the vast majority of these methodologies have focused on electrogenerated based Michael addition reactions, a strategy that requires substrates bearing an acidic methylene hydrogen, e.g. malononitrile or malonate, but examples of the synthesis of bioactive molecule-bearing heterocycles using these multi-component reaction methodologies are rare. Herein, we introduce a ‘one-stone, two-birds’ strategy for accessing valuable quinolines using a paired electrolysis enabled multi-component reaction approach and the simultaneous late-stage functionalization of various bioactive molecules (Scheme 1). We started from commercially available isatins, alkynes, and hydroxy-containing bioactive molecules, and various quinoline-substituted target molecules were isolated in moderate to excellent yields. The traditional routes to quinolines started from isatins usually suffer from harsh conditions and poor tolerance of functional groups, the use of electroreduction for the activation of isatin, together with the further anodic oxidation of KI to catalytically accomplish the intermolecular cascade annulation under mild pH neutral conditions, highlight the unique possibilities associated with electrochemical activation methods.
 | ||
| Scheme 1 Paired electrolysis enabled annulation to construct quinoline-substituted bioactive molecules. | ||
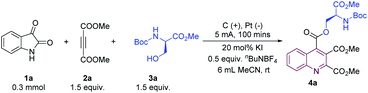
Initially, isatin (1a), dimethyl but-2-ynedioate (2a) and a serine residue (3a) were used as model substrates for the electroreduction-induced cascade annulation. To our delight, the desired quinoline product (4a) was obtained in 85% yield under a constant current of 5 mA with 20 mol% KI in a simple undivided cell (Table 1, entry 1). KI was found to be required for this cascade annulation to proceed and, in its absence, there was no reaction (Table 1, entry 2). Replacing CH3CN with DCM did not favor the annulation (Table 1, entry 3). The effect of electrode materials was also explored. Using a carbon rod as the cathode decreased product yield, to some extent (Table 1, entry 4). A good yield was obtained with KI as electrolyte (Table 1, entry 5). Additionally, control experiments indicated that no product was produced in the absence of electric current (Table 1, entry 6). More detailed information can be found in the ESI.†
| Entry | Variation from standard conditionsa | Yield (%) |
|---|---|---|
| a Standard conditions: carbon rod anode, platinum plate cathode (15 mm × 15 mm × 0.3 mm), constant current = 5 mA, 1a (0.3 mmol), 2a (1.5 equiv.), 3a (1.5 equiv.) nBu4NBF4 (0.5 equiv.), KI (0.2 equiv.), 6 mL MeCN, undivided cell, 100 min. Isolated yields were shown. | ||
| 1 | Standard conditions | 85 |
| 2 | Without KI | N.D. |
| 3 | CH2Cl2 as the solvent | Trace |
| 4 | Carbon as cathode | 70 |
| 5 | I2 instead of KI | N.D. |
| 6 | KI as electrolyte | 89 |
| 7 | Without electric current | N.R. |
Remarkably, this protocol also provided direct access to the late-stage derivatization of valuable drugs and natural products (Scheme 2). Tetrahydrofurfuryl alcohol, perilla alcohol, cinnamyl alcohol and isoborneol, which are important food and flavoring additives, also participated in the annulation reaction with moderate to good yields, respectively (4aj–4am). Additionally, menthol, geraniol and phytol could also be readily converted into the corresponding products in 42–70% yields (4an–4ap). Saccharides are important types of biomolecules for which late-stage functionalization would be highly desirable. Interestingly, this reaction can be used for the synthesis of various quinoline-substituted saccharide derivatives, which highlights the potential of this method for bioconjugation reactions (4aq–4as). Meanwhile, when various drug molecules, such as bucetin (an analgesic-antipyretic drug), ospemifene (an estrogen receptor modulator) metronidazole (an antibiotic and anti-protozoan drug), picaridin (a commonly used insect repellent), dehydroepiandrosterone (a steroid agent) and testosterone were treated with isatin (1a) and dimethyl but-2-ynedioate (2a), the desired functionalized products 4at (75%), 4au (67%), 4av (60%), 4aw (73%), 4ax (52%) and 4ay (54%) were obtained, respectively. To further explore the utilities of this electrochemical cascade annulation for the synthesis of quinoline-substituted bioconjugates, gram scale reactions were performed for three kinds of transformations (4av, 4ak and 4al). In these cases, the reaction remained selective and good yields were obtained.
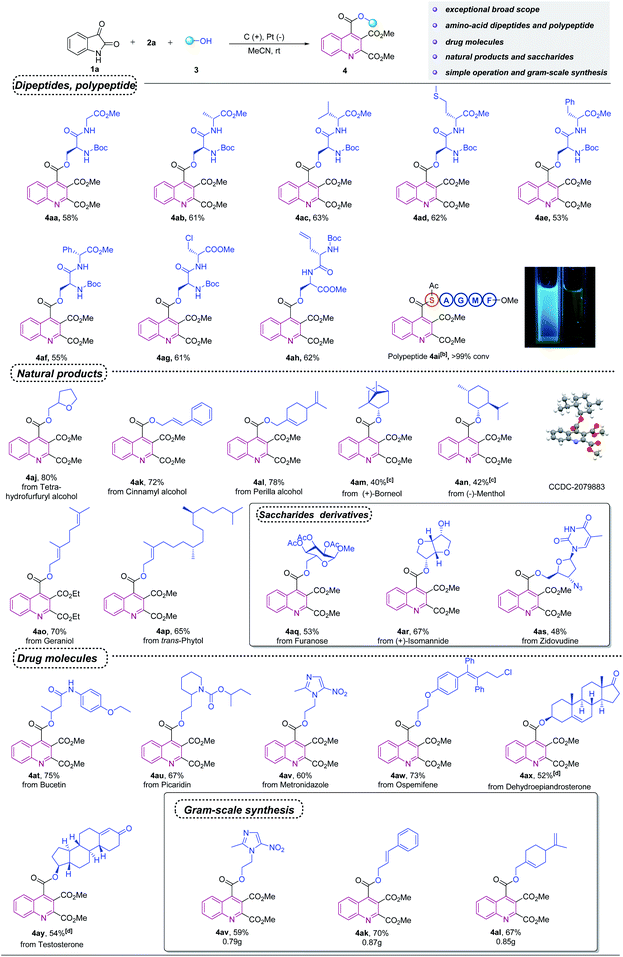 | ||
| Scheme 2 Scope of bioactive moleculesa. aStandard condition as shown in Table 1. bReaction conditions: graphite rod anode, platinum plate cathode, constant current = 5 mA, polypeptides 3ai (5 mg), 1a (10 mg), 2a (12 μL), nBu4NBF4 (5 mg), KI (2 mg), CH3CN (0.75 mL), room temperature, 30 min. Conversion of products are shown, as determined by HPLC. c4.0 equiv. of isoborneol and menthol were used. d3.0 equiv. of DHEA and testosterone were used. | ||
Results and discussion
We next explored the generality of the protocol for a wide range of isatin derivatives (Scheme 3). The effect of substituents on the benzene ring of the isatin molecule was first scrutinized. Molecules with electron-donating groups and halogens at different positions reacted smoothly to yield the corresponding products with satisfactory efficiencies (4ba–4bi). Trifluoromethyl and nitro groups as electron-withdrawing groups were also amenable (4bj and 4bk). The scope of the reaction with respect to alkynes was also explored. The installation of the bulky tert-butyl group in the ester moiety had no effect on the progress of the reaction, and the corresponding product 4bm was produced in 60% yield. Other different electron-withdrawing groups, such as, acyl and trifluoromethyl groups, all afforded the products in moderate to good yields (4bn–4bp).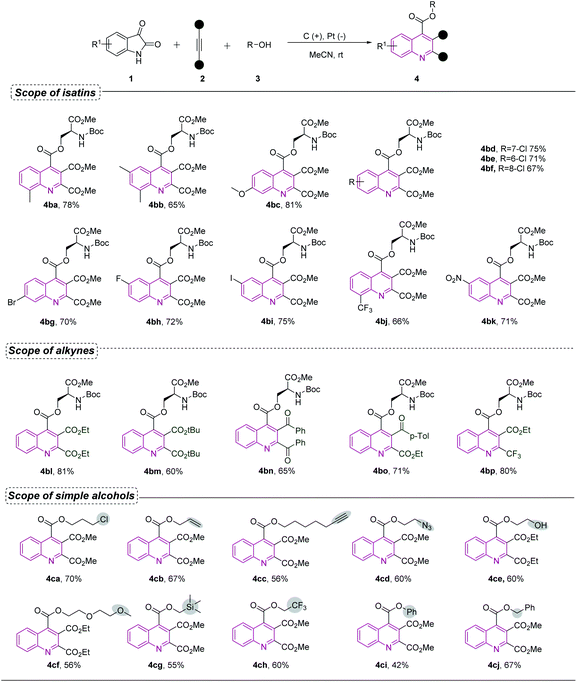 | ||
| Scheme 3 Substrate scope for isatins, alkynes and selected simple alcoholsa. aStandard condition as shown in Table 1. | ||
Further exploration revealed that the cascade annulation was amenable to simple alcohols (Scheme 3). The reaction also proceeded effectively with various alcohols. Several typical functional groups such as chloro, alkenyl, alkynyl, azido and trimethylsilyloxy groups were well tolerated under the standard conditions (4ca–4cd and 4cg). Owing to importance of the fluorine atom in drug molecules, we attempted to introduce fluorine atoms into products. To our delight, good yields of the targeted molecules, containing a trifluoromethyl group (4ch) were successfully obtained under the electrochemical conditions. Alkoxy alcohols were well tolerated, affording the corresponding products (4cf). When a molecule containing a glycol group was used as a substrate, one hydroxyl group is retained, providing the opportunity for further derivatization (4ce). Phenol and benzyl alcohol could also be readily converted into the corresponding products in acceptable yields (4ci and 4cj). Unfortunately, the electron rich alkynes and terminal alkynes, such as phenylacetylene, 1,2-diphenylethyne and methyl propiolate were not suitable reaction partners for this approach, and the substrates containing amine group were incompatible with this transformation (for more details, see ESI†).
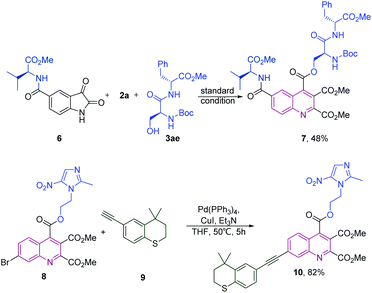
We next explored the utilities of this electrochemical cascade annulation for the synthesis of quinoline-substituted bioconjugates. Parthenolide (PTL, shown in Scheme 4a), a naturally occurring sesquiterpene lactone derived from medicinal plants, drew extensive scientific research due to its potency to induce the apoptosis of cancer stem cells (CSCs). Studies have found that PTL, cotreated with other bioactive molecules, showed a broad spectrum of cancer inhibition, including leukemia, lymphoma, bone cancer, and breast cancer.8 Therefore, the modification of PTL with quinoline scaffolds would be necessary and meaningful to improve their pharmacological properties. To our delight, a series of PTL derivatives were easily obtained under our electrochemical conditions. The in vitro antiproliferative activity of synthesized compounds was also evaluated in three human cancer cell lines using the CCK-8 assay, including HCT116 (colorectal carcinoma), U87MG (glioblastoma) and MGC803 (gastric cancer). It could be seen in the table that all synthesized compounds showed moderate to significant potency toward these cancer cell lines, which highlighted the potential of this method to pharmaceutical research (Scheme 4b). Furthermore, some highly functionalized quinoline-substituted bioconjugates could also be further linked with more biomolecules by using operationally simple techniques (Scheme 5). Initially, we treated the valine residue substituted isatin 6 with dipeptides under the standard conditions, quinoline derivatives connected to two different amino acid residues or dipeptides such as 7 was obtained in 48% yields. A bromine functional group in quinolines can be used in Sonogashira reactions with drug molecules to afford the product 10.
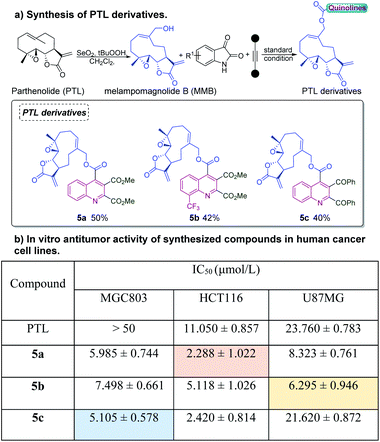 | ||
| Scheme 4 (a) Synthesis of PTL derivatives. (b) In vitro antitumor activity of PTL derivatives in human cancer cell lines. | ||
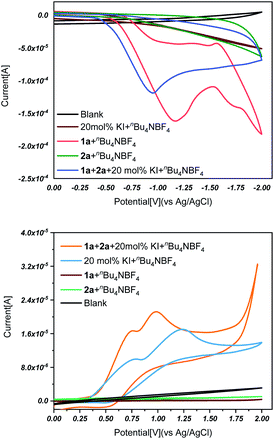
With the method being established, efforts were then made to understand the reaction mechanism. We initiated our study by conducting cyclic voltammetry (CV) experiments. As shown in Fig. 1, using KI as the electrolyte (Fig. 1), the cyclic voltammograms showed irreversible reduction waves for isatin (1a). This result indicates that isatin is reduced by the cathode with the generation of a radical-anion intermediate. On the other side, the potassium iodide produced a dual oxidation peak at 0.82 V and 1.22 V versus Ag/Ag+, respectively. No other oxidation peak was observed under these chemical conditions. These results indicate that in this electrochemical transformation, both isatin (1a) and KI are simultaneously reduced/oxidized with the generation of reactive intermediates.
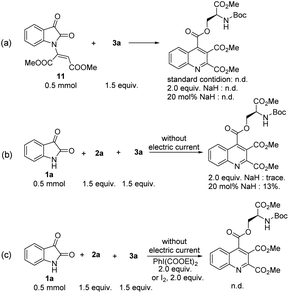
To collect more mechanistic information concerning this transformation. Some mechanistic experiments were performed. No product was detected when dimethyl 2-(2,3-dioxoindolin-1-yl) maleate 11 was used as a substrate (Scheme 6a), indicating that 11 is not a reaction intermediate. In addition, when 2.0 equivalents of NaH were used to instead of an electric current, the reaction mixture was complicated and the corresponding product was produced in trace yield, however, catalytic amount of NaH indeed led to the desired product with low yield (Scheme 6b). This indicates that alkoxide ions are not likely the mainly active species in this transformation, however, we cannot exclude the possibility that the ring-opening step involves nucleophilic attack of electrogenerated alkoxide ions. In this case, the KI would act as the anodic sacrificial agents. Moreover, when molecular iodine and a hypervalent iodine reagent were employed as the oxidant, the desired product was not obtained (Scheme 6c).
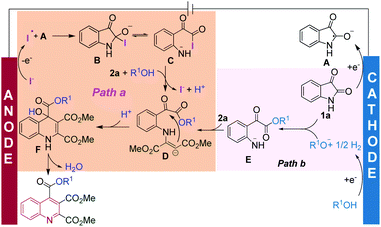
Based on the above studies, a proposed mechanism for this electrochemical cascade annulation is shown in Scheme 7. In the first step, 1a is reduced at the cathode to generate the isatin radical anion A. At the same time, the anodic oxidation of KI generates an iodine radical. Radical–radical cross coupling between the isatin radical anion A and iodine radical affords intermediate B. After a ring-opening reaction, nucleophilic substitution and addition generates intermediate D, with the regeneration of the iodine anion to restart the catalytic cycle (Path a). In the meanwhile, we cannot exclude the possibility that the alcohol may also be reduced at the cathode to generate alkoxide ions, and then reacts with the isatin to furnish the ring-opening intermediate E. In this case, the KI would act as the anodic sacrificial agents (Path b). Finally, intramolecular cyclization then generates intermediate F with excellent regioselectivity, and dehydration gives the corresponding quinoline product.
Conclusions
In summary, we report on a paired electrolysis enabled cascade annulation that enables the efficient synthesis of highly functionalized quinoline-substituted bioactive molecules from readily available starting materials. Merging electrochemical organic synthesis with bioconjugation chemistry, two goals, namely, the direct synthesis of quinolines and introducing quinolines to biomolecules, can be simultaneously achieved in one simple operation. This transformation can tolerate a wide range of biomolecules, e.g. serine residues, polypeptides, saccharides, drugs and natural products, and can also be used as a functionalization tactic for pharmaceutical research and other areas. We anticipate that advances in electrochemically induced bioconjugation will lead to an expanding library of interdisciplinary methodologies.Data availability
All the data have been included in the ESI.†Author contributions
M. Gao, A. Lei conceived the project. S. You, M. Ruan, C. Lu, L, Liu, Y. Weng, G. Yang, S. Wang and H. Alhumade performed the experiments, analyzed the data, and discussed the results. M. Gao and S. You wrote the manuscript, Supplementary Information and contributed other related materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21702081 and 22031008). Science Foundation of Wuhan (2020010601012192).Notes and references
- (a) V. R. Solomon and H. Lee, Curr. Med. Chem., 2011, 18, 1488–1508 CrossRef CAS; (b) P. Williams, A. Sorribas and M.-J. R. Howes, Nat. Prod. Rep., 2011, 28, 48–77 RSC; (c) B. D. Bax, P. F. Chan, D. S. Eggleston, A. Fosberry, D. R. Gentry, F. Gorrec, I. Giordano, M. M. Hann, A. Hennessy, M. Hibbs, J. Huang, E. Jones, J. Jones, K. K. Brown, C. J. Lewis, E. W. May, M. R. Saunders, O. Singh, C. E. Spitzfaden, C. Shen, A. Shillings, A. J. Theobald, A. Wohlkonig, N. D. Pearson and M. N. Gwynn, Nature, 2010, 466, 935–940 CrossRef PubMed; (d) K. Andries, P. Verhasselt, J. Guillemont, H. W. H. Göhlmann, J.-M. Neefs, H. Winkler, J. Van Gestel, P. Timmerman, M. Zhu, E. Lee, P. Williams, D. de Chaffoy, E. Huitric, S. Hoffner, E. Cambau, C. Truffot-Pernot, N. Lounis and V. Jarlier, Science, 2005, 307, 223 CrossRef CAS PubMed; (e) J. K. Natarajan, J. N. Alumasa, K. Yearick, K. A. Ekoue-Kovi, L. B. Casabianca, A. C. de Dios, C. Wolf and P. D. Roepe, J. Med. Chem., 2008, 51, 3466–3479 CrossRef CAS PubMed; (f) A.-M. Lord, M. F. Mahon, M. D. Lloyd and M. D. Threadgill, J. Med. Chem., 2009, 52, 868–877 CrossRef CAS PubMed; (g) S. Andrews, S. J. Burgess, D. Skaalrud, J. X. Kelly and D. H. Peyton, J. Med. Chem., 2010, 53, 916–919 CrossRef CAS PubMed; (h) M. Rouffet, C. A. F. de Oliveira, Y. Udi, A. Agrawal, I. Sagi, J. A. McCammon and S. M. Cohen, J. Am. Chem. Soc., 2010, 132, 8232–8233 CrossRef CAS PubMed.
- (a) S. A. Jenekhe, L. Lu and M. M. Alam, Macromolecules, 2001, 34, 7315–7324 CrossRef CAS; (b) A. K. Agrawal and S. A. Jenekhe, Macromolecules, 1991, 24, 6806–6808 CrossRef CAS.
- (a) B. B. Touré and D. G. Hall, Chem. Rev., 2009, 109, 4439–4486 CrossRef; (b) L. Li, Z. Chen, X. Zhang and Y. Jia, Chem. Rev., 2018, 118, 3752–3832 CrossRef CAS PubMed; (c) L. Li and W. D. Jones, J. Am. Chem. Soc., 2007, 129, 10707–10713 CrossRef CAS PubMed; (d) X. Zhang, X. Song, H. Li, S. Zhang, X. Chen, X. Yu and W. Wang, Angew. Chem., Int. Ed., 2012, 51, 7282–7286 CrossRef CAS PubMed; (e) Y. Wang, C. Chen, J. Peng and M. Li, Angew. Chem., Int. Ed., 2013, 52, 5323–5327 CrossRef CAS; (f) Y. Matsubara, S. Hirakawa, Y. Yamaguchi and Z.-i. Yoshida, Angew. Chem., Int. Ed., 2011, 50, 7670–7673 CrossRef CAS PubMed; (g) K. P. Kawahara, W. Matsuoka, H. Ito and K. Itami, Angew. Chem., Int. Ed., 2020, 59, 6383–6388 CrossRef CAS PubMed; (h) H. Jiang, X. An, K. Tong, T. Zheng, Y. Zhang and S. Yu, Angew. Chem., Int. Ed., 2015, 54, 4055–4059 CrossRef CAS PubMed; (i) X. Ji, H. Huang, Y. Li, H. Chen and H. Jiang, Angew. Chem., Int. Ed., 2012, 51, 7292–7296 CrossRef CAS PubMed; (j) Y. Liu, Y. Hu, Z. Cao, X. Zhan, W. Luo, Q. Liu and C. Guo, Adv. Synth. Catal., 2018, 360, 2691–2695 CrossRef CAS; (k) Z.-Y. Gu, T.-H. Zhu, J.-J. Cao, X.-P. Xu, S.-Y. Wang and S.-J. Ji, ACS Catal., 2014, 4, 49–52 CrossRef CAS; (l) X. Zhang, X. Ma, W. Qiu, J. Evans and W. Zhang, Green Chem., 2019, 21, 349–354 RSC; (m) Z. Zhang, J. Tan and Z. Wang, Org. Lett., 2008, 10, 173–175 CrossRef CAS PubMed; (n) X. Xu, R. Sun, S. Zhang, X. Zhang and W. Yi, Org. Lett., 2018, 20, 1893–1897 CrossRef CAS PubMed; (o) B.-Q. Wang, C.-H. Zhang, X.-X. Tian, J. Lin and S.-J. Yan, Org. Lett., 2018, 20, 660–663 CrossRef CAS PubMed; (p) S. San Jang, Y. H. Kim and S. W. Youn, Org. Lett., 2020, 22, 9151–9157 CrossRef CAS PubMed; (q) S.-F. Jiang, C. Xu, Z.-W. Zhou, Q. Zhang, X.-H. Wen, F.-C. Jia and A.-X. Wu, Org. Lett., 2018, 20, 4231–4234 CrossRef CAS PubMed; (r) C. Gronnier, G. Boissonnat and F. Gagosz, Org. Lett., 2013, 15, 4234–4237 CrossRef CAS PubMed; (s) R. Yi, X. Li and B. Wan, Org. Chem. Front., 2018, 5, 3488–3493 RSC; (t) B. Ali, M. Khalid, S. Asim, M. Usman Khan, Z. Iqbal, A. Hussain, R. Hussain, S. Ahmed, A. Ali, A. Hussain, M. Imran, M. A. Assiri, M. Fayyaz ur Rehman, C. Wang and C. Lu, Molecules, 2021, 26, 2760 CrossRef CAS PubMed; (u) M. S. A. Mehedi and J. J. Tepe, J. Org. Chem., 2020, 85, 6741–6746 CrossRef CAS PubMed; (v) C. P. Jones, K. W. Anderson and S. L. Buchwald, J. Org. Chem., 2007, 72, 7968–7973 CrossRef CAS PubMed; (w) K. Mal, S. Chatterjee, A. Bhaumik and C. Mukhopadhyay, ChemistrySelect, 2019, 4, 1776–1784 CrossRef CAS; (x) A. A. Afkham, J. Mokhtari, A. J. Haghighi and I. Yavari, ChemistrySelect, 2018, 3, 9159–9161 CrossRef CAS; (y) M. J. Campbell and F. D. Toste, Chem. Sci., 2011, 2, 1369–1378 RSC; (z) H. Sajjadi-Ghotbabadi, S. Javanshir and F. Rostami-Charati, Catal. Lett., 2016, 146, 338–344 CrossRef CAS; (a a) L. He, H. Li, H. Neumann, M. Beller and X.-F. Wu, Angew. Chem., Int. Ed., 2014, 53, 1420–1424 CrossRef CAS PubMed; (a b) I. Yavari, S. Seyfi and Z. Hossaini, Tetrahedron Lett., 2010, 51, 2193–2194 CrossRef CAS.
- (a) J. P. Michael, Nat. Prod. Rep., 2008, 25, 166–187 RSC; (b) A. Weyesa and E. Mulugeta, RSC Adv., 2020, 10, 20784–20793 RSC; (c) G. A. Ramann and B. J. Cowen, Molecules, 2016, 21, 986 CrossRef; (d) N. A. Harry, S. M. Ujwaldev and G. Anilkumar, Org. Biomol. Chem., 2020, 18, 9775–9790 RSC.
- (a) M. Yan, Y. Kawamata and P. S. Baran, Chem. Rev., 2017, 117, 13230–13319 CrossRef CAS; (b) S. Tang, Y. Liu and A. Lei, Chem, 2018, 4, 27–45 CrossRef CAS; (c) M. D. Kärkäs, Chem. Soc. Rev., 2018, 47, 5786–5865 RSC; (d) P. Gandeepan, L. H. Finger, T. H. Meyer and L. Ackermann, Chem. Soc. Rev., 2020, 49, 4254–4272 RSC; (e) P. Xiong and H.-C. Xu, Acc. Chem. Res., 2019, 52, 3339–3350 CrossRef CAS PubMed; (f) J. C. Siu, N. Fu and S. Lin, Acc. Chem. Res., 2020, 53, 547–560 CrossRef CAS PubMed; (g) C. Ma, P. Fang and T.-S. Mei, ACS Catal., 2018, 8, 7179–7189 CrossRef CAS.
- (a) Y. Weng, C. Song, C.-W. Chiang and A. Lei, Chem. Commun., 2020, 3, 171 CrossRef CAS; (b) D. Alvarez-Dorta, C. Thobie-Gautier, M. Croyal, M. Bouzelha, M. Mével, D. Deniaud, M. Boujtita and S. G. Gouin, J. Am. Chem. Soc., 2018, 140, 17120–17126 CrossRef CAS; (c) C. Song, K. Liu, Z. Wang, B. Ding, S. Wang, Y. Weng, C.-W. Chiang and A. Lei, Chem. Sci., 2019, 10, 7982–7987 RSC; (d) A. D. Pagar, M. D. Patil, D. T. Flood, T. H. Yoo, P. E. Dawson and H. Yun, Chem. Rev., 2021, 121, 6173–6245 CrossRef CAS PubMed.
- (a) Y. Jiang, K. Xu and C. Zeng, Chem. Rev., 2018, 118, 4485–4540 CrossRef CAS PubMed; (b) L. Fotouhi, M. M. Heravi, V. Zadsirjan and P. A. Atoi, Chem. Rec., 2018, 18, 1633–1657 CrossRef CAS; (c) M. N. Elinson, A. I. Ilovaisky, V. M. Merkulova, D. V. Demchuk, P. A. Belyakov, Y. N. Ogibin and G. I. Nikishin, Electrochim. Acta, 2008, 53, 8346–8350 CrossRef CAS; (d) M. N. Elinson, A. I. Ilovaisky, A. S. Dorofeev, V. M. Merkulova, N. O. Stepanov, F. M. Miloserdov, Y. N. Ogibin and G. I. Nikishin, Tetrahedron, 2007, 63, 10543–10548 CrossRef CAS; (e) I. Chiarotto, L. Mattiello and M. Feroci, Acc. Chem. Res., 2019, 52, 3297–3308 CrossRef CAS PubMed; (f) P. Antico, V. Capaccio, A. DiMola, A. Massa and L. Palombi, Adv. Synth. Catal., 2012, 354, 1717–1724 CrossRef CAS; (g) M. N. Elinson, A. S. Dorofeev, S. K. Feducovich, S. V. Gorbunov, R. F. Nasybullin, F. M. Miloserdov and G. I. Nikishin, Eur. J. Org. Chem., 2006, 2006, 4335–4339 CrossRef; (h) M. N. Elinson, A. S. Dorofeev, F. M. Miloserdov, A. I. Ilovaisky, S. K. Feducovich, P. A. Belyakov and G. I. Nikishin, Adv. Synth. Catal., 2008, 350, 591–601 CrossRef CAS; (i) A. Upadhyay, L. K. Sharma, V. K. Singh and R. K. P. Singh, Tetrahedron Lett., 2016, 57, 5599–5604 CrossRef CAS.
- (a) R. R. A. Freund, P. Gobrecht, D. Fischer and H. D. Arndt, Nat. Prod. Rep., 2020, 37, 541–565 RSC; (b) M. Sztiller-Sikorska and M. Czyz, Pharmaceuticals, 2020, 13, 194 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2079883. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc06757e |
| This journal is © The Royal Society of Chemistry 2022 |