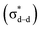Open Access Article
Open Access ArticleEvolution from superatomic Au24Ag20 monomers into molecular-like Au43Ag38 dimeric nanoclusters†
Jiayu
Xu‡
a,
Lin
Xiong‡
b,
Xiao
Cai
a,
Shisi
Tang
a,
Ancheng
Tang
a,
Xu
Liu
 a,
Yong
Pei
a,
Yong
Pei
 *b and
Yan
Zhu
*b and
Yan
Zhu
 *a
*a
aSchool of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093, China. E-mail: zhuyan@nju.edu.cn
bDepartment of Chemistry, Key Laboratory of Environmentally Friendly Chemistry and Applications of Ministry of Education, Xiangtan University, Xiangtan 411105, China. E-mail: ypnku78@gmail.com
First published on 17th February 2022
Abstract
Hierarchical assembly of nanoparticles has been attracting wide interest, as advanced functionalities can be achieved. However, the ability to manipulate structural evolution of artificial nanoparticles into assemblies with atomic precision has been largely unsuccessful. Here we report the evolution from monomeric Au24Au20 into dimeric Au43Ag38 nanoclusters: Au43Ag38 inherits the kernel frameworks from parent Au24Ag20 but exhibits distinct surface motifs; Au24Ag20 is racemic, while Au43Ag38 is mesomeric. Importantly, the evolution from monomers to dimers opens up exciting opportunities exploring currently unknown properties of monomeric and dimeric alloy nanoclusters. The Au24Ag20 clusters show superatomic electronic configurations, while Au43Ag38 clusters have molecular-like characteristics. Furthermore, monomeric Au24Ag20 catalysts readily outperform dimeric Au43Ag38 catalysts in the catalytic reduction of CO2.
Introduction
Nanoparticle assembly in nanoscience and nanotechnology is important because of the exceptional properties produced that can serve for fundamental investigations and new applications.1–9 Despite the impressive advances in the assembly of artificial nanoparticles, the precise assembly of nanoparticles with atomic-scale manipulation remains a great challenge. This hinders researchers from creating and optimizing the functionality of nanomaterials. The key requirement for achieving such assemblies is the formation of truly atomically defined particles. Metal nanoclusters with absolutely precise formulae and atomic structures have provided access to currently challenging issues regarding conventional nanoparticles.In a series of seminal studies, gold or silver nanoclusters with icosahedral structures have been demonstrated to be basic building blocks forming the hierarchical assembly of nanoclusters with elegant structures. For example, Au38(SR)24 with a double-icosahedral Au23 kernel is assembled from two icosahedral Au13 units in a coplanar manner.10,11 [Au25(PPh3)10(SR)5Cl2]2+ is evolved from two Au13 icosahedra sharing one vertex and [Au37(PPh3)10(SR)10X2]+ is formed by three Au13 icosahedra sharing vertices in a linear form.12,13 Recently Ag61(dpa)27(SbF6)4 was found to contain four linear vertex-sharing Ag13 icosahedra.14 Similarly, the cores of both Au2Ag42(SAdm)27(BPh4) and [Au2Ag48(S-tBu)20(Dppm)6Br11]Br(BPh4)2 are composed of two icosahedral Ag13 units.15 Besides icosahedral M2Au36(PET)24 was reported from the structural fusion of [HMAu8(PPh3)8]+ and [MAu24(PET)18]−.16 Ag2Au50(SR)36 was synthesized from two Au25(SR)18 units assembled with two Ag atoms in a hand-in-hand mode.17 These studies are exciting in the rational design and fabrication of tailored structures and reveal that the perfect nanocluster assembly deserves more efforts to tailor the functionality on an atom-by-atom basis.18–21
In this work, we successfully synthesized monomeric Au24Ag20(C12H13)24Cl2 and Au24Ag20(C9H7)24Cl2 nanoclusters with similar kernels comprised of a hollow Au12 icosahedron surrounded by fullerene-like Ag20. The two Au24Ag20 monomers are further fused along different pathways into the Au12@Ag19-Au-Au12@Ag19 kernels and finally form Au43Ag38(C12H13)36Cl12 and Au43Ag38(C9H7)36Cl9 dimeric nanoclusters, respectively. The Au24Ag20 monomers and Au43Ag38 dimers offer a novel platform for atomic manufacturing on alloy nanoclusters to construct harmonious structures and unveil currently elusive properties such as electronic structures and catalytic properties.
Results and discussion
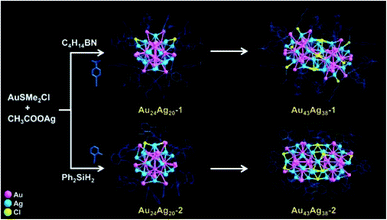
As shown in Fig. 1, based on a “cluster to cluster” strategy, four nanoclusters can be synthesized. AuSMe2Cl and CH3COOAg precursors were reduced by C4H14BN in the presence of 4-tert-butylphenylacetylene and sodium methoxide. Au24Ag20(C12H13)24Cl2 (abbreviated as Au24Ag20-1) was formed in a 12 hour reaction and then transformed into Au43Ag38(C12H13)36Cl12 (abbreviated as Au43Ag38-1) in another 12 hour reaction. With 2-methylphenylacetylene as the ligand and diphenylsilane as the reducing agent, Au24Ag20(C9H7)24Cl2 (denoted as Au24Ag20-2) was first obtained and further converted to Au43Ag38(C9H7)36Cl9 (denoted as Au43Ag38-2).Electrospray ionization mass (ESI-MS) and matrix-assisted laser desorption/ionization time of flight mass (MALDI-TOF-MS) spectrometry were carried out to confirm the cluster formula. The major peaks of monomers at m/z = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 995 and 9719 were assigned to [Au24Ag20(C12H13)24Cl2 + 2Cs+]2+ and [Au24Ag20(C9H7)24Cl2 − 2e]2+ (Fig. S1†), respectively. The mass peaks of dimers at m/z = 18
995 and 9719 were assigned to [Au24Ag20(C12H13)24Cl2 + 2Cs+]2+ and [Au24Ag20(C9H7)24Cl2 − 2e]2+ (Fig. S1†), respectively. The mass peaks of dimers at m/z = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 and 16
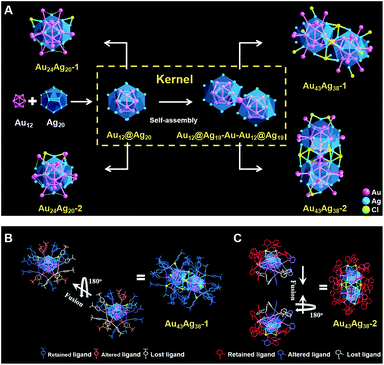
500 and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 916 were related to Au43Ag38(C12H13)35Cl12 and Au43Ag38(C9H7)35Cl9 (Fig. S2;† note that one alkyne ligand for Au43Ag38 was removed during the measurements), respectively. The total structures of the four clusters were determined by X-ray crystallography (Tables S1–S4†). Among them, the two monomers have identical kernels of Au12@Ag20, which can be viewed as a hollow Au12 icosahedral structure surrounded by a fullerene-like Ag20 shell (Fig. 2A). The kernels of dimers are generated by the fusion of two Au12@Ag20 units of monomers in a mode of Au12@Ag19–Au–Au12@Ag19 (Fig. 2). This is completely different from previous cases, in which the building blocks of nanoclusters are mainly based on icosahedral units.
916 were related to Au43Ag38(C12H13)35Cl12 and Au43Ag38(C9H7)35Cl9 (Fig. S2;† note that one alkyne ligand for Au43Ag38 was removed during the measurements), respectively. The total structures of the four clusters were determined by X-ray crystallography (Tables S1–S4†). Among them, the two monomers have identical kernels of Au12@Ag20, which can be viewed as a hollow Au12 icosahedral structure surrounded by a fullerene-like Ag20 shell (Fig. 2A). The kernels of dimers are generated by the fusion of two Au12@Ag20 units of monomers in a mode of Au12@Ag19–Au–Au12@Ag19 (Fig. 2). This is completely different from previous cases, in which the building blocks of nanoclusters are mainly based on icosahedral units.
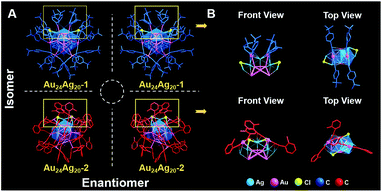
In terms of the monomers, the average Au–Au bond lengths of the Au12 icosahedral cores are very close (2.786 Å for Au24Ag20-1 and 2.785 Å for Au24Ag20-2). As shown in Fig. S3,† the average Au–Ag bond lengths between the Au12 core and Ag20 shell are 2.878 Å in Au24Ag20-1 and 2.868 Å in Au24Ag20-2, respectively, and both are shorter than those of bulk Au or bulk Ag, implying the strong metal bonding between the Au12 core and Ag20 shell. Of note, the Au–Au distances between the Au12 core and outer Au12 shell and between the Ag20 shell and outer Au12 shell are longer than those of bulk Au and Ag, indicating the weak interaction between the outermost Au atoms and Ag20 shell. Obviously, the surface arrangements of the two Au24Ag20 nanoclusters are different. Au24Ag20-1 has four binding types on the Ag5 faces (Fig. S4A–D†), while Au24Ag20-2 has three binding structures on the Ag5 faces (Fig. S4E–G†), although both Au24Ag20 nanoclusters exhibit such coordination modes on the staples: 12 as η3-μ1(Au), μ2(Ag), and μ2(Ag) and 12 as η2-μ1(Au) and μ2(Ag). The different binding motifs also lead to distinguishable steric arrangements of two Au and two Cl atoms at the top of the Au24Ag20 nanoclusters: symmetrically upward to form two parallel staples for Au24Ag20-1 (upper panel of Fig. 3); distinctively twisted to form two crossed staples for Au24Ag20-2 (lower panel of Fig. 3). The specific staples of the two nanoclusters might be acting as identification cards for identifying changes that have taken place in subsequent polymerizations.
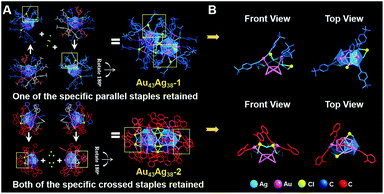
With respect to the dimers, their kernel structures are alike. The average Au–Au bond lengths of the Au12–Au–Au12 core are 2.790 Å in Au43Ag38-1 and 2.789 Å in Au43Ag38-2. The lengths of the Au–Au bonds directly connected to the central gold are the shortest in the whole core (Fig. S5†). The average Au–Ag lengths between the Au12–Au–Au12 core and Ag38 shell are 2.891 Å in Au43Ag38-1 and 2.911 Å in Au43Ag38-2, suggesting the weak interaction between the Au core and Ag shell, which differs from the monomeric cases. Additionally, the average Au–Au distances between the Au12–Au–Au12 core and outer Au18 shell are 2.856 Å in Au43Ag38-1 and 2.883 Å in Au43Ag38-2, suggesting strong a Au–Au force on the outer shell. The Au–Ag distances between the Ag38 shell and outer Au18 shell are longer than 3 Å for both Au43Ag38 nanoclusters and are consistent with the monomers. Moreover, clearly discernible surface motifs are presented in the Au43Ag38 clusters: Au43Ag38-1 has four binding types on the Ag5 faces with the coordination modes (Fig. S6†): 22 as η3-μ1(Au), μ2(Ag), and μ2(Ag) and 14 as η2-μ1(Au) and μ2(Ag); Au43Ag38-2 has five binding types on the Ag5 faces with the coordination modes (Fig. S7†): 24 as η3-μ1(Au), μ2(Ag), and μ2(Ag) and 12 as η2-μ1(Au), μ2(Ag). Both dimers contain 36 alkyne ligands, but Au43Ag38-1 has 12 Cl and Au43Ag38-2 has only 9 Cl. In addition, both dimers have six four-coordinated Cl atoms, but Au43Ag38-1 has another six one-coordinated Cl atoms and Au43Ag38-2 has another three two-coordinated Cl atoms. More interestingly, the specific staples as identification cards can also be found in the two dimeric nanoclusters (Fig. 4): the parallel staples on Au43Ag38-1 and the crossed staples on Au43Ag38-2.
It is worth pointing out that the unit cell of each Au24Ag20 monomer comprises two enantiomers, and hence the monomers are racemic (Fig. 3A). One Au24Ag20 isomer rotates 180° and then fuses with the other isomer by four or six Cl linkages to form Au43Ag38, in which two fusion units are chiral and the total Au43Ag38 clusters are thus mesomeric (Fig. 4A). Remarkably, Au43Ag38-1 is assembled from two chiral units along the diagonal direction, while Au43Ag38-2 is evolved from the vertical fusion of two chiral units. During the fusion process, each fusion unit of Au43Ag38-1 shows 5 alkyne rearranged, 6 alkyne detached and 4 Cl added (upper panel of Fig. 4A). However, when the fusion process happened to Au43Ag38-2, one fusion unit has 5 alkyne relocated, 6 alkyne left and 3 Cl fixed, whereas the other fusion unit shows 6 alkyne relocated, 6 alkyne and one Cl fell off, and another 3 Cl appended (lower panel of Fig. 4A).
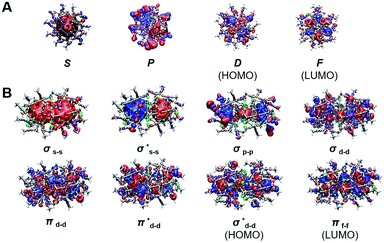
We next compared the electronic properties of monomeric and dimeric nanoclusters. The UV-vis absorption spectra of Au24Ag20 show four apparent peaks, while those of Au43Ag38 show five absorption peaks (Fig. S8†). It implies different electronic structures between monomers and dimers. We further performed density functional theory (DFT) calculations on the electronic configuration of these nanoclusters. From Fig. 5A, it is obvious that the Kohn–Sham (KS) orbital diagram of the Au24Ag20 monomer is similar to that of the orbitals of atoms, with the characteristics of s, p, d, and f atomic orbitals. Considering that Au24Ag20 has 18 valence electrons, it just forms an electron shell of 1s2|1p6|1d10, which is identical to the KS electron density diagram of typical superatomic Au25(SR)18− (Fig. S9A and B†).22 Therefore, Au24Ag20 can be classified into the category of superatomic clusters. Surprisingly, Au43Ag38 and Au38(SR)24 exhibit similar molecular orbital types (Fig. 5B, S9C and D†): bonding orbitals σ and π; anti-bonding orbitals σ* and π*.23 Obviously, these molecular orbitals can be viewed as linear combinations of different atomic orbitals of Au24Ag20 in different ways (head-to-head combination to obtain σ orbitals and side-to-side combination to form π orbitals). It is noted that due to the influence of the outer layer ligand, the molecular orbital diagram of the overall structure of Au43Ag38 and the orbital diagram of the inner core structure are different, e.g., the molecular orbital of the inner core has a δ orbital (Fig. S9D†). Nevertheless, this does not affect our qualitative judgement on the properties of the molecule. Overall, the configurations that can form superatoms are not only icosahedral but also diversified, and even alloy clusters also have superatomic or molecular-like characteristics.
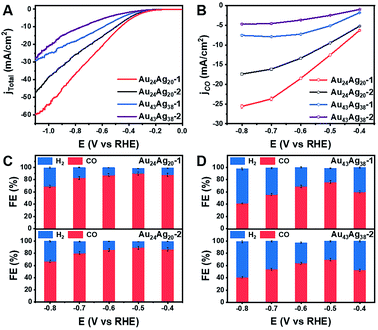
More importantly, the inherent ability of electron transport in monomeric and dimeric nanoclusters is readily differentiated in their electrochemical impedance spectra (Fig. S10†). The semicircular diameters of the Au24Ag20 clusters are smaller than those of the Au43Ag38 clusters, meaning that the electron transport in the monomers might be faster than that in the dimers. Given the inherent advantages of employing the cluster catalysts in electronic properties, we envisioned the CO2 reduction reaction (CO2RR) involving the electron transfer process to explore the catalytic properties of the monomers and dimers. We were pleased to observe different reactivities of the four nanocluster catalysts for the CO2RR and the high performances were achieved in the monomers. From the linear scanning voltammetry (LSV) curves of Au24Ag20 and Au43Ag38 in CO2-saturated solutions of 0.5 M KHCO3, the monomers showed higher current density than the dimers (Fig. 6A). Meanwhile, the CO partial current density was arranged in descending order as Au24Ag20-1 > Au24Ag20-2 > Au43Ag38-1 > Au43Ag38-2 (Fig. 6B). Notably, the monomers showed much higher faradaic efficiency (FE) toward CO than the dimers in the voltage range from −0.4 to −0.8 V (Fig. 6C and D). 90% CO FE was obtained at a voltage of −0.5 V over the Au24Ag20 catalysts. The results were partially related to their atomic-packing structures (individual-core vs. dual-core) and surface motif arrangements (parallel vs. crossed).24,25 Further studies are ongoing to elucidate the roles of superatomic and molecular-like electronic properties on cluster catalysis, which so far remain elusive.
Conclusions
In summary, we have successfully implemented the evolution of racemic Au24Ag20 monomers into mesomeric Au43Ag38 dimers and mapped out significant differences in steric configurations and electronic structures between the monomeric and dimeric nanoclusters. Our studies show that the Au24Ag20 monomers exhibit more efficient reactivity than the Au43Ag38 dimers for CO2 reduction processes. This work not only provides a strategy for hierarchical assembly of metal nanoclusters to tune their structure and functionality, but also provides a paradigm of the monomeric and dimeric alloy nanoclusters to find applications in challenging chemical reactions.Data availability
The X-ray crystallographic structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) with deposition numbers (CCDC: 2129383 for Au43Ag38(C12H13)36Cl12; CCDC: 2129384 for Au24Ag20(C9H7)24Cl2; CCDC: 2129385 for Au43Ag38(C9H7)36Cl9; CCDC: 2129386 for Au24Ag20(C12H13)24Cl2).Author contributions
Y. Z. conceived the project. J. X. synthesized the nanoclusters and grew the crystal. L. X. and Y. P. conducted the calculations. X. C. and S.T. did the catalytic tests. A. T. and X. L. analyzed the crystal data. All authors wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge financial support from National Natural Science Foundation of China (22125202; 91961121) and Programs for High-Level Entrepreneurial and Innovative Talents Introduction of Jiangsu Province.Notes and references
- T. Wang, J. Zhuang, J. Lynch, O. Chen, Z. Wang, X. Wang, D. LaMontagne, H. Wu, Z. Wang and Y. C. Cao, Science, 2012, 338, 358–363 CrossRef CAS PubMed.
- C. Zeng, Y. Chen, K. Kirschbaum, K. J. Lambright and R. Jin, Science, 2016, 354, 1580–1584 CrossRef CAS PubMed.
- Z. Lei, X. Pei, Z. Jiang and Q. Wang, Angew. Chem., Int. Ed., 2014, 53, 12771–12775 CrossRef CAS PubMed.
- I. Chakraborty and T. Pradeep, Chem. Rev., 2017, 117, 8208–8271 CrossRef CAS PubMed.
- Y. Song, F. Fu, J. Zhang, J. Chai, X. Kang, P. Li, S. Li, H. Zhou and M. Zhu, Angew. Chem., Int. Ed., 2015, 54, 8430–8434 CrossRef CAS PubMed.
- R. Huang, Y. Wei, X. Dong, X. Wu, C. Du, S. Zang and T. C. W. Mak, Nat. Chem., 2017, 9, 689–697 CrossRef CAS PubMed.
- L. Shi, L. Zhu, J. Guo, L. Zhang, Y. Shi, Y. Zhang, K. Hou, Y. Zheng, Y. Zhu, J. Lv, S. Liu and Z. Tang, Angew. Chem., Int. Ed., 2017, 56, 15397–15401 CrossRef CAS PubMed.
- Z. Wang, M. Wang, Y. Li, P. Luo, T. Jia, R. Huang, S. Zang and T. C. W. Mak, J. Am. Chem. Soc., 2018, 140, 1069–1076 CrossRef CAS PubMed.
- G. Deng, B. K. Teo and N. Zheng, J. Am. Chem. Soc., 2021, 143, 10214–10220 CrossRef CAS PubMed.
- H. Qian, W. T. Eckenhoff, Y. Zhu, T. Pintauer and R. Jin, J. Am. Chem. Soc., 2010, 132, 8280–8281 CrossRef CAS PubMed.
- Y. Pei, Y. Gao and X. Zeng, J. Am. Chem. Soc., 2008, 130, 7830–7832 CrossRef CAS PubMed.
- Y. Shichibu, Y. Negishi, T. Watanabe, N. K. Chaki, H. Kawaguchi and T. Tsukuda, J. Phys. Chem. C, 2007, 111, 7845–7847 CrossRef CAS.
- R. Jin, C. Liu, S. Zhao, A. Das, H. Xing, C. Gayathri, Y. Xing, N. L. Rosi, R. R. Gil and R. Jin, ACS Nano, 2015, 9, 8530–8536 CrossRef CAS PubMed.
- S. Yuan, C. Xu, W. Liu, J. Zhang, J. Li and Q. Wang, J. Am. Chem. Soc., 2021, 143, 12261–12267 CrossRef CAS PubMed.
- S. Jin, X. Zou, L. Xiong, W. Du, S. Wang, Y. Pei and M. Zhu, Angew. Chem., Int. Ed., 2018, 57, 16768–16772 CrossRef CAS PubMed.
- E. Ito, S. Takano, T. Nakamura and T. Tsukuda, Angew. Chem., Int. Ed., 2021, 60, 645–649 CrossRef CAS PubMed.
- X. Liu, G. Saranya, X. Huang, X. Cheng, R. Wang, M. Chen, C. Zhang, T. Li and Y. Zhu, Angew. Chem., Int. Ed., 2020, 59, 13941–13946 CrossRef CAS PubMed.
- Z. Lei, J. Li, Z. Nan, Z. Jiang and Q. Wang, Angew. Chem., Int. Ed., 2021, 60, 14415–14419 CrossRef CAS PubMed.
- P. Yuan, R. Zhang, E. Selenius, P. Ruan, Y. Yao, Y. Zhou, S. Malola, H. Häkkinen, B. K. Teo, Y. Cao and N. Zheng, Nat. Commun., 2020, 11, 2229 CrossRef CAS PubMed.
- Z. Wu, Y. Du, J. Liu, Q. Yao, T. Chen, Y. Cao, H. Zhang and J. Xie, Angew. Chem., Int. Ed., 2019, 58, 8139–8144 CrossRef CAS PubMed.
- M. D. Nardi, S. Antonello, D.-E. Jiang, F. Pan, K. Rissanen, M. Ruzzi, A. Venzo, A. Zoleo and F. Maran, ACS Nano, 2014, 8, 8505–8512 CrossRef PubMed.
- C. M. Aikens, Acc. Chem. Res., 2018, 51, 3065–3073 CrossRef CAS PubMed.
- Q. Tang, G. Hu, V. Fung and D.-E. Jiang, Acc. Chem. Res., 2018, 51, 2793–2802 CrossRef CAS PubMed.
- H. Seong, V. Efremov, G. Park, H. Kim, J. S. Yoo and D. Lee, Angew. Chem., Int. Ed., 2021, 60, 14563–14570 CrossRef CAS PubMed.
- M. R. Narouz, K. M. Osten, P. J. Unsworth, R. W. Y. Man, K. Salorinne, S. Takano, R. Tomihara, S. Kaappa, S. Malola, C. Dinh, J. D. Padmos, K. Ayoo, P. J. Garrett, M. Nambo, J. H. Horton, E. H. Sargent, H. Häkkinen, T. Tsukuda and C. M. Crudden, Nat. Chem., 2019, 11, 419–425 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2129383–2129386. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc07178e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |