Open Access Article
Open Access ArticleDevelopment of 1,3-acetonedicarboxylate-derived glucoside amphiphiles (ACAs) for membrane protein study†
Ho Jin
Lee
 a,
Muhammad
Ehsan‡
a,
Xiang
Zhang
b,
Satoshi
Katsube
a,
Muhammad
Ehsan‡
a,
Xiang
Zhang
b,
Satoshi
Katsube
 c,
Chastine F.
Munk
d,
Haoqing
Wang
e,
Waqar
Ahmed
a,
Ashwani
Kumar
c,
Chastine F.
Munk
d,
Haoqing
Wang
e,
Waqar
Ahmed
a,
Ashwani
Kumar
 a,
Bernadette
Byrne
a,
Bernadette
Byrne
 f,
Claus J.
Loland
f,
Claus J.
Loland
 d,
Lan
Guan
c,
Xiangyu
Liu
b and
Pil Seok
Chae
d,
Lan
Guan
c,
Xiangyu
Liu
b and
Pil Seok
Chae
 *a
*a
aDepartment of Bionano Engineering, Hanyang University, Ansan 155-88, Korea. E-mail: pchae@hanyang.ac.kr
bBeijing Advanced Innovation Center for Structural Biology, School of Medicine, School of Pharmaceutical Sciences, Tsinghua University, 100084 Beijing, China
cDepartment of Cell Physiology and Molecular Biophysics, Center for Membrane Protein Research, School of Medicine, Texas Tech University Health Sciences Center, Lubbock, TX 79430, USA
dDepartment of Neuroscience, University of Copenhagen, Copenhagen, DK-2200, Denmark
eDepartment of Molecular and Cellular Physiology, Stanford University, California 94305, USA
fDepartment of Life Sciences, Imperial College London, London, SW7 2AZ, UK
First published on 19th April 2022
Abstract
Detergents are extensively used for membrane protein manipulation. Membrane proteins solubilized in conventional detergents are prone to denaturation and aggregation, rendering downstream characterization of these bio-macromolecules difficult. Although many amphiphiles have been developed to overcome the limited efficacy of conventional detergents for protein stabilization, only a handful of novel detergents have so far proved useful for membrane protein structural studies. Here, we introduce 1,3-acetonedicarboxylate-derived amphiphiles (ACAs) containing three glucose units and two alkyl chains as head and tail groups, respectively. The ACAs incorporate two different patterns of alkyl chain attachment to the core detergent unit, generating two sets of amphiphiles: ACA-As (asymmetrically alkylated) and ACA-Ss (symmetrically alkylated). The difference in the attachment pattern of the detergent alkyl chains resulted in minor variation in detergent properties such as micelle size, critical micelle concentration, and detergent behaviors toward membrane protein extraction and stabilization. In contrast, the impact of the detergent alkyl chain length on protein stability was marked. The two C11 variants (ACA-AC11 and ACA-SC11) were most effective at stabilizing the tested membrane proteins. The current study not only introduces new glucosides as tools for membrane protein study, but also provides detergent structure–property relationships important for future design of novel amphiphiles.
Introduction
Membrane proteins play crucial roles in a variety of cellular functions such as cell–cell recognition, signal transduction, material transport, and cell movement. The disorder in these proteins results in various diseases such as cancers, Alzheimer's and Parkinson's.1 In addition, these membrane-embedded bio-macromolecules are targets of more than 50% of current pharmaceuticals.2,3 Thus, membrane protein structures are an essential part of the rational drug design and development process.4,5 Membrane protein structures are determined through one or a combination of high-resolution techniques; X-ray crystallography, single particle cryo-electron microscopy (cryo-EM) and nuclear magnetic resonance (NMR) spectroscopy. Despite the importance of understanding structure and function of membrane proteins, the number of these bio-macromolecules with experimentally determined structure is significantly lower than that of soluble proteins.6 This is due mainly to the inherent amphiphilic architecture of membrane proteins, making them much more likely to aggregate or denature during purification and structural studies.7,8 Membrane-mimetic environments are necessary to preserve membrane protein structures in a state suitable for downstream characterization.9,10 Micellar systems formed by amphiphilic molecules are an effective surrogate for the cell membrane that shields the large hydrophobic portions of membrane proteins from the aqueous environment.11Small amphiphilic molecules, called detergents or surfactants, are widely used to maintain solubility and stability of membrane proteins outside their native membrane environment.11–13 Detergents both disrupt the hydrophobic interactions maintaining the membrane structure and form membrane protein–detergent complexes (PDCs). Conventional detergents such as n-dodecyl-β-D-maltoside (DDM), n-octyl-β-D-glucoside (OG) and lauryldimethylamine-N-oxide (LDAO) are widely used for membrane protein extraction. Since the first structure determination of the photosynthetic reaction centre of Rhodopseudomonas viridis using LDAO in 1985,14 detergents have been widely used for membrane protein structure determination. However, the canonical structure of these conventional detergents, a single hydrophobic chain and hydrophilic head group, provides insufficient structural diversity to stabilize many membrane proteins with varied structures.10,15,16
Over the past two decades, various approaches have been reported for the constitution of new amphipathic systems as exemplified by bicelles, membrane scaffold protein (MSP)- or styrene–maleic acid copolymer (SMA)-supported nanodiscs (NDs),17,18 amphiphilic polymers (e.g., amphipols (APols)19) and peptide-based detergents (e.g., lipopeptide detergents (LPDs) and β-peptides (BPs)).20,21 Although these systems are highly effective at preserving the native conformations of membrane proteins, most are ineffective at membrane protein extraction. In addition, they are often incompatible with protein crystallization.17 Thus a great deal of effort has been devoted to developing novel detergents with enhanced efficacy for membrane protein solubilization and stabilization. A number of novel detergents including neopentyl glycol-based amphiphiles [e.g., glucose neopentyl glycols (GNGs),22–24 maltose neopentyl glycols (MNGs)25–27 and neopentyl glycol-derived triglucosides (NDTs)28], rigid hydrophobic group-bearing detergents [e.g., chobimalt,29 glyco-diosgenin (GDN)30], facial amphiphiles (FAs)31,32 and hemi-fluorinated surfactants (HFSs)33 have been developed. Recently, novel detergents with distinctive hydrophilic structures were reported as exemplified by the penta-saccharide-bearing amphiphiles (PSEs)34 and oligoglycerol detergents (OGDs).35 In addition, stereoisomeric detergents [e.g., butane-1,2,3,4-tetraol-based maltosides (BTMs),36 norbornane-based maltosides (NBMs),37 and cyclopentane-based maltoside (CPMs)38] and carbohydrate-cored amphiphiles [mannitol-based amphiphiles (MNAs),39 scyllo-inositol-based glycosides (SIGs)40 and trehalose-cored maltosides (TCMs)]41 have been developed to facilitate membrane protein structure study. Of these novel small amphiphiles, it is notable that MNG-3 (commercial name: lauryl maltoside neopentyl glycol (LMNG)) has contributed to the determination of more than 200 membrane protein structures over the last 10 years.42 A glucoside version of LMNG, OGNG has been used for structural analysis of 17 membrane proteins.42 In addition, new detergents play an important role in maintaining protein integrity during reconstitution of purified membrane proteins into liposomes for structural and functional analysis. The resulting proteoliposomes enable structural determination of membrane proteins in the membrane environment. In this context, it is noteworthy that the use of LMNG in combination with auto-insertion allowed efficient liposome reconstitution of mammalian F-ATP synthase without the loss of key subunits.43,44 The successful examples of LMNG and OGNG highlight the crucial role of novel amphiphiles in membrane protein structural and functional studies.
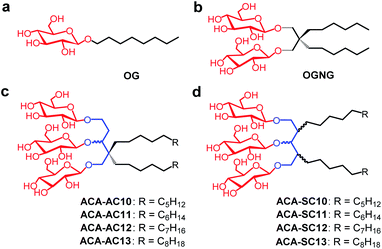
As new variants of OGNG, here we designed and prepared a class of detergents from a commercially available starting material, 1,3-acetonedicarboxylate. Three glucose units and two alkyl chains were attached to the 1,3-acetonedicarboxylate-derived core, resulting in detergents designated 1,3-acetonedicarboxylate-derived amphiphiles (ACAs) (Fig. 1). The alkyl chains were either attached to the central core symmetrically (ACA-Ss) or asymmetrically (ACA-As). These detergents were evaluated for membrane protein solubilization and stabilization, and a detergent comparison was made with a gold standard detergent (DDM) and/or two NG detergents (OGNG and LMNG). When multiple model membrane proteins were tested with these detergents, some ACAs, particularly C11 alkyl-chained detergents, conferred notably enhanced stability to the membrane proteins compared to the control agents.
Results and discussion
Detergent structures and physical characterizations
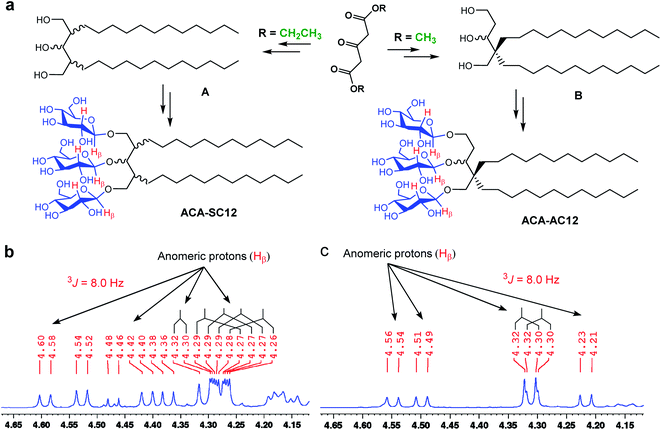
The new detergents contain two alkyl chains and three glucose units as tail and head groups, respectively. These detergents can be categorized into two sets according to the pattern of alkyl chain attachment to the central core (i.e., 1,3,5-pentane-triol). For one set, the alkyl chains are attached to two different carbons of the core (2C and 4C) and for the second set, both alkyl chains are attached to the same carbon of the core. We designated these detergents as asymmetrical ACAs (ACA-As) and symmetrical ACAs (ACA-Ss), respectively (Fig. 1c and d). The ACA-As include the OGNG scaffold within the molecular structure, but additionally bear an ethyl glucoside appendage in the hydrophilic region (Fig. 1). The ACA-Ss are structural isomers of the ACA-As. The new agents contain a hydrophobic linker (i.e., 1,3,5-pentane-triol) in the detergent core region, used to connect the head and tail groups. Thus, this class differs from many previous glucoside amphiphiles containing a hydrophilic linker in the same place, as exemplified by the NDTs and MNAs containing pentaerythritol and mannitol groups as linkers, respectively.28,38 The presence of the hydrophobic linker can be favorable for membrane protein structural studies as observed with OGNG and LMNG which contain a hydrophobic NG linker. The presence of an additional glucose unit in the hydrophilic region allowed us to increase the alkyl chain length of the ACAs up to C13 with no effect on water-solubility. This is in contrast to the C6 alkyl chain of OGNG and the limited water-solubility (∼1 wt%) of C7 alkyl-chained GNGs. The increase in the alkyl chain length compared to OGNG is important for optimising compatibility of detergent hydrophobic length with the hydrophobic dimensions of membrane proteins. In addition, the alkyl chain length of the ACAs varied from C10 to C13, necessary to find a detergent with an optimal hydrophilic–lipophilic balance (HLB), an important factor for stabilizing membrane proteins.45–47Diethyl (or methyl) 1,3-acetonedicarboxylate was used as the starting material for preparation of the ACAs (Fig. 2a). The ACA-Ss were synthesized in four synthetic steps: alkyl chain attachment to diethyl 1,3-acetonedicarboxylate, reduction of the carbonyl functional groups to alcohol, AgOTf-promoted β-selective glycosylation and global benzoyl group deprotection (Scheme S1†). In contrast, the ACA-As were prepared differently from the ACA-Ss. In order to attach two alkyl chains onto the same carbon of the central core, we utilized a previously reported protocol.48,49 This asymmetric introduction of the alkyl chains into the detergent scaffold requires two additional steps for preparation of the ACA-As compared to the symmetric molecules (Scheme S2†): conversion of the central ketone group to methyl enol ether and restoration of the ketone functional group from the methyl enol ether. All the synthesized detergents were characterized by NMR spectroscopy and mass spectrometry. In the NMR spectra of the ACAs, multiple doublet signals appear in the range of 4.20 to 4.70 ppm, a peak characteristic of β-anomeric protons (Fig. 2b, c and S1†). The β-stereochemistry of the anomeric carbon can be further identified through the vicinal coupling constant (3J). The coupling constant for the anomeric protons with the neighboring proton was observed to be 8.0 Hz, different from the typical value (4.0 Hz) for α-anomeric protons.50 Peak complexity observed for the anomeric protons in the NMR spectra of the ACA-As and ACA-Ss is due to the presence of stereo-chemically undefined C–O and/or C–C bonds, as indicated by the wavy lines in the chemical structures (Fig. 2).
High water-solubility of a detergent is essential for their use as a biochemical tool in membrane protein applications. Both sets of detergents (ACA-Ss and ACA-As) were highly soluble in water except ACA-SC13 and ACA-AC13, which gave ∼5.0 and <1.0 wt% water-solubility, respectively (Table 1). Due to this low water-solubility, ACA-AC13 was not further tested. Self-assembly properties of the individual detergents were investigated by measuring critical micelle concentrations (CMCs) and hydrodynamic radii (Rh) of their micelles formed in aqueous solution.51,52 All ACAs exhibit lower CMCs than DDM and OGNG (0.005–0.019 mM vs. 0.17 mM (DDM) and 1.0 mM (OGNG)), indicating strong hydrophobic interaction between the ACA detergent molecules. The increased hydrophobic interaction is mainly due to either the presence of two alkyl chains or of elongated alkyl chains. Detergent micelles of the ACAs varied from 2.4 nm to 13.9 nm depending on detergent symmetricity and alkyl chain length (Table 1). The micelle sizes of ACA-SC10/11, and ACA-AC10/11 were smaller than those of DDM and OGNG, while the micelles formed by the C12/C13 alkyl-chained ACAs were significantly larger compared to DDM, OGNG and the C10/C11 alkyl chain versions. Notably, the ACA-Ss gave lower CMCs and smaller micelle sizes than the ACA-As. For example, ACA-SC11 gave a lower CMC than ACA-AC11 (0.010 vs. 0.014 mM) and the micelle size of ACA-SC11 was smaller than that of ACA-AC11 (2.9 vs. 3.3 nm). These features are likely to be associated with the enhanced water-solubility of the ACA-Ss compared to their asymmetric counterparts; ACA-SC13 gave a higher water-solubility than ACA-AC13 (∼5 vs. <1.0 wt%). Detergent micelle size tends to increase with increasing alkyl chain length. A dramatic increase in detergent micelle size was observed, particularly when the alkyl chain length increases from C11 to C12 in both series (Table 1 and Fig. S2†). This correlation between detergent alkyl chain length and micelle size can be explained by a change in detergent molecular shape accompanied by increasing alkyl chain length. The incorporation of an additional carbon into the hydrophobic tail results in a detergent molecule with a more cylindrical shape, favorable for formation of large self-assemblies.53 Dynamic light scattering profiles indicate little polydispersity for self-assemblies formed by the ACA-Ss/As (Fig. S2†).
| Detergent | MWa | CMC (mM) | CMC (wt%) | R h (nm) | Solubilityc (wt%) |
|---|---|---|---|---|---|
| a Molecular weight of detergents. b Hydrodynamic radius of micelles determined at 1.0 wt% by dynamic light scattering. c Water-solubility at room temperature. d Data obtained from a literature.22 | |||||
| ACA-SC10 | 887.1 | ∼0.015 | ∼0.0013 | 2.4 ± 0.2 | >10 |
| ACA-SC11 | 915.2 | ∼0.010 | ∼0.0009 | 2.9 ± 0.3 | ∼10 |
| ACA-SC12 | 943.2 | ∼0.007 | ∼0.0007 | 8.3 ± 0.1 | ∼10 |
| ACA-SC13 | 971.3 | ∼0.005 | ∼0.0005 | 13.9 ± 1.1 | ∼5 |
| ACA-AC10 | 887.1 | ∼0.019 | ∼0.0017 | 2.7 ± 0.2 | >10 |
| ACA-AC11 | 915.2 | ∼0.014 | ∼0.0013 | 3.3 ± 0.2 | ∼10 |
| ACA-AC12 | 943.2 | ∼0.012 | ∼0.0011 | 8.9 ± 0.7 | ∼9 |
| OGNG | 568.7 | ∼1.0 | ∼0.058 | 4.4 ± 0.3 | >10 |
| DDM | 510.6 | 0.17 | 0.0087 | 3.4 ± 0.3 | >10 |
Detergent evaluation for membrane protein stability
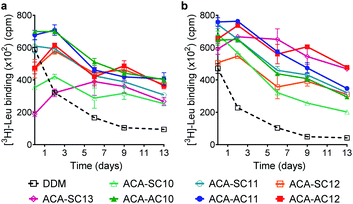
The ACAs were first evaluated with LeuT from the bacterium, Aquifex aeolicus.54 The transporter was first extracted from E. coli using 1.0 wt% DDM, followed by purification with 0.05 wt% of the same detergent. The DDM-purified LeuT was diluted into buffer solution containing DDM or the respective ACA-Ss/As to give a final detergent concentration of CMC + 0.04/0.2 wt% (Fig. 3). Protein stability is assessed by measuring the ability of the transporter to bind a radio-labeled substrate ([3H]-Leu) via scintillation proximity assay (SPA).55 The substrate binding ability was monitored at regular intervals over a 13 day incubation period at room temperature. At the detergent concentration of CMC + 0.04 wt%, initial activity (i.e., ligand binding) of LeuT solubilized in DDM was high compared to those of the ACAs, but this initial activity of the transporter in this detergent gradually decreased over time (Fig. 3a). In contrast, the ACA-solubilized transporters tended to give rather low initial [3H]-Leu binding compared to the DDM-solubilized protein, but binding activity recovered within two days (Fig. 3). Four ACA agents (ACA-AC10, ACA-AC11, ACA-AC12, and ACA-SC12) were clearly better than DDM at maintaining long-term LeuT stability under the conditions tested (Fig. S3 and Table S1†). At an increased detergent concentration of CMC + 0.2 wt%, the ACA efficacies for LeuT stabilization were in most cases enhanced compared to those obtained at CMC + 0.04 wt% (Fig. 3b). This enhancement was particularly notable for ACA-SC13. As a results, all ACAs were superior to DDM for LeuT stability under the conditions tested (Fig. S3 and Table S1†). Taken together these results reveal that all the ACA-solubilized transporters were more effective than DDM-solubilized protein at retaining Leu binding over the 13 day incubation period. The best performance was observed with ACA-AC11/AC12, followed by ACA-SC11 and ACA-SC13. There is no notable difference in detergent efficacy between the two series (ACA-As and ACA-Ss). Of note, OGNG was previously reported to be inferior to DDM for LeuT stability.22,56 Thus, these results indicate the potential utility of the ACAs in structural studies of membrane transporters.For further evaluation of the new detergents, we used the melibiose permease from Salmonella typhimurium (MelBSt).57–60 MelBSt expressed in E. coli membranes was extracted using 1.5 wt% of each detergent (DDM/ACAs) for 90 min at 0 °C. The protein extracts were further incubated at an elevated temperature (45, 55, or 65 °C) for another 90 min. The amounts of soluble MelBSt obtained in each individual condition were assessed by SDS-PAGE and Western blotting and presented as percentages (%) of the membrane-bound transporter (Fig. 4a). MelBSt extraction at 0 °C was carried out to estimate detergent efficiency for protein extraction. Further incubation of the MelBSt extracts at elevated temperatures provides further information on detergent efficacy for protein stabilization. At 0 °C, DDM was efficient at protein extraction, yielding nearly 100% soluble MelBSt. The long-alkyl chained ACAs such as ACA-AC12, ACA-SC12, and ACA-SC13 yielded much lower amounts of soluble MelBSt (less than 40%). In contrast, the C10 and C11 alkyl-chained ACAs were comparable to DDM (Fig. 4a and Table S2†). A similar trend was observed when the samples were further treated at 45 °C (Table S3†). The only difference was a slight increase in the amount of soluble MelBSt in the case of ACA-SC12/AC12. When the temperature was further increased to 55 °C, a dramatic difference in the amount of soluble MelBSt was found between the tested detergents (Table S4†). ACA-AC10 and ACA-SC13 yielded ∼10% soluble MelBSt, similar to DDM, whereas ACA-AC11 and ACA-SC11 gave ∼70% and ∼60% soluble MelBSt, respectively. Of note, it was reported that OGNG is inferior to DDM for MelB stability in previous studies.22,56 It is interesting to note that the ACAs behaved differently depending on the alkyl chain length. Specifically, the C10 alkyl chain members were as efficient as the C11 versions at protein extraction, but were less effective than the latter in terms of MelB stability. An opposite trend was observed for the C12 alkyl-chained ACAs. These detergents were inefficient at MelB extraction, but effective at maintaining MelB stability. The C11 alkyl-chained ACAs (ACA-AC11 and ACA-SC11) were not only efficient at MelBSt extraction, but were also effective at maintaining this protein in a soluble state at elevated temperature. Thus, these two detergents were selected to further evaluate their abilities to maintain MelBSt functionality. MelBSt functionality was assessed by monitoring fluorescence intensity changes associated with the ability of the transporter to bind the substrate (melibiose) and fluorescent ligand (dansyl-2-galactoside (D2G)).61 A high fluorescence intensity would be observed for functional transporter in the presence of D2G as the result of efficient Förster resonance energy transfer (FRET) from tryptophan (Trp) to D2G. Subsequent addition of melibiose results in a decrease in fluorescence intensity as this non-fluorescent substrate replaces D2G in the active site. Thus, monitoring fluorescence intensity over the course of D2G and melibiose additions allowed assessment of MelB functionality solubilized in the individual detergents. The DDM-extracted MelBSt retained functionality, as the increase and decrease in the fluorescence intensity were detected upon addition of first D2G and then melibiose (Fig. 4b). When a less stable MelB homologue from E. coli (MelBEc) was employed, however, DDM failed to yield a relevant fluorescence intensity in response to addition of D2G or melibiose. This result indicates that DDM-solubilized MelBEc had lost its sugar binding function, in marked contrast to the retention of protein function observed for ACA-AC11 and ACA-SC11 under the same conditions. Therefore, ACA-AC11 and ACA-SC11 are superior to DDM in maintaining MelB in a soluble and functional state.
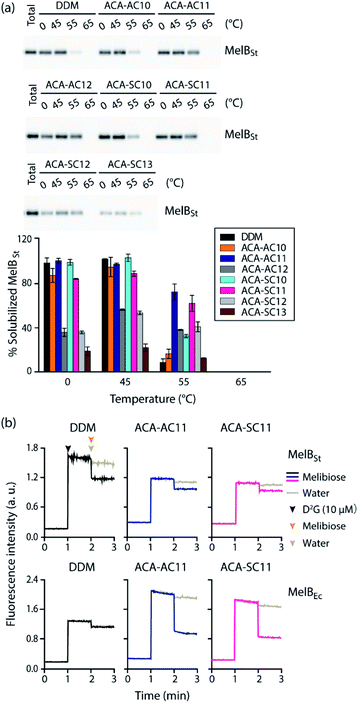 | ||
| Fig. 4 Thermostability of MelBSt solubilized in the new agents (ACA-As and ACA-Ss) (a) and MelBSt functionality in ACA-AC11 and ACA-SC11 (b). DDM was used as a control. 1.5 wt% of each detergent was used for protein extraction at 0 °C and the resulting MelBSt extracts were further incubated at 45, 55 or 65 °C for another 90 min. (a) After being subjected to ultracentrifugation, the individual samples were analyzed using SDS-PAGE and western blotting to estimate the amounts of soluble MelBSt. The amounts of soluble MelBSt are represented as relative percentages (%) of total MelBSt present in untreated membranes (expressed as ‘total’) in the histogram. Error bars, SEM; n = 2. The data were statistically analysed using one-way ANOVA (Tables S1–S3†). (b) MelB function was assessed by melibiose-mediated FRET reversal. Dansyl-2-galactoside (D2G) and excess melibiose were added to ACA-AC11/SC11-extracted MelB (MelBSt and MelBEc) at 1 min and 2 min time points, respectively. Fluorescence intensity was monitored over the course of the additions of the ligand and substrate. Control data was obtained by addition of water instead of melibiose. Melibiose addition is indicated in black (DDM), blue (ACA-AC11), and purple (ACA-SC11), and water addition is indicated in gray. | ||
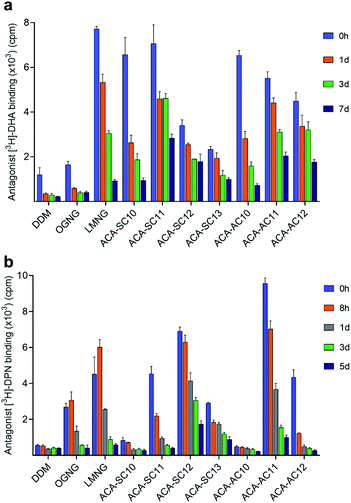
In order to evaluate the new detergents for G protein-coupled receptor (GPCR) stability, the human β2 adrenergic receptor (β2AR) was used for assessment.62DDM-purified receptor was exchanged into each detergent (i.e., DDM, OGNG, LMNG, ACA-Ss/As) via dilution. The final detergent concentration was 0.2 wt%. During a 7 day incubation at room temperature, we monitored the specific ligand binding ability of the receptor at regular intervals using the radio-labelled antagonist ([3H]-dihydroalprenolol (DHA)) (Fig. 5a).63 The receptor solubilized in DDM showed low ligand binding upon detergent exchange, also observed for OGNG-solubilized receptor. This low level of ligand binding is likely to indicate partial denaturation of the receptor in DDM micelles. When exchanged into the ACAs, the initial ability of the receptor to bind DHA increased significantly compared to DDM/OGNG. This enhanced DHA binding is likely due to refolding of the ‘partially-denatured’ receptor by the favourable action of the new detergents on the receptor, rescuing the receptor ability to bind the DHA ligand. The initial receptor activity (i.e., DHA binding) tended to decrease with increasing detergent alkyl chain length. Consequently, C10 and C11 alkyl-chained detergents showed initial high activity, while the C12 and C13 alkyl-chained ACAs gave intermediate activity upon detergent exchange. Interestingly, an opposite trend was obtained for detergent efficacy for long-term receptor stabilization. The C11/C12 alkyl-chained ACAs were better than the C10 alkyl-chained detergents in this regard (Fig. S4a, c and Table S5†). Combined together, ACA-SC11 and ACA-AC11 were the best of the ACAs and these ACAs were substantially better than or comparable to LMNG, a significantly optimized detergent for GPCR stability (Fig. 5a) (Fig. S4a, c and Table S5†). These detergents retained 40∼50% initial receptor activity after the 7 day incubation, while LMNG gave only 10% retention under the same conditions (Fig. S5a†). Thus, this result indicates that these detergents could be useful for GPCR structural studies.
Detergent evaluation was continued with another GPCR, the mouse μ-opioid receptor (MOR).64 The receptor was first purified in LMNG and exchanged into the respective ACA via dilution. The final detergent concentration was 0.1 wt%. MOR stability was measured by assessment of radioactive antagonist ([3H]-diprenorphine (DPN)) binding at regular intervals during a 5 day incubation at 4 °C (Fig. 5b).65 The receptor solubilized in DDM appeared to lose the ability to specifically bind ligand completely upon detergent exchange. Similar results were observed for the two C10 alkyl-chained ACAs, ACA-SC10 and ACA-AC10 (Fig. 5b). Interestingly, OGNG yielded the receptor with enhanced DPN binding compared to DDM (Fig. S4b, d and Table S6†). Use of LMNG led to a further increase in the initial receptor activity, but, similar to ACA-SC11 and ACA-AC12, the receptor solubilized in this maltoside exhibited rapid loss in DPN binding. The receptor in LMNG gave only 10% retention in the ligand binding of the receptor after the 5 day incubation (Fig. 5b). Remarkably, the receptors encapsulated in ACA-SC12/AC11 micelles not only showed higher initial activity than the LMNG-solubilized protein, but were more effective than the latter at preserving DPN binding capability long term (Fig. S4b, d and Table S6†). ACA-SC12-solubilized receptor retained ∼30% of initial ligand binding after the 5 day incubation (Fig. S5b†). Combined with the β2AR result, these findings for the MOR indicate that ACA-AC11, ACA-SC11, and ACA-SC12 hold significant potential for GPCR structural study.
In order to further explore whether the new detergents can be used for membrane protein extraction, we selected four ACAs (ACA-AC10, ACA-SC10, ACA-AC11, and ACA-SC11) for extraction of two GPCRs (β2AR and MOR). These detergents were selected as they generated large amounts of soluble MelBSt, close to levels obtained with DDM (Fig. 4). DDM and LMNG were used as controls as these detergents are widely used for GPCR extraction.66 When tested for β2AR extraction, DDM extracted the receptor quantitatively from the membranes (Fig. S6a†). Use of LMNG resulted in a large amount of soluble β2AR (∼80%), although slightly less than DDM. Of the tested ACAs, ACA-SC11 yielded the largest amount of soluble β2AR, followed by ACA-AC10 and ACA-SC10. The amount of β2AR solubilized by ACA-SC11 was more or less comparable to that achieved in DDM/LMNG. In contrast, the asymmetric version, ACA-AC11, yielded only low levels of soluble β2AR. A different trend was observed for MOR extraction. LMNG was slightly more effective than DDM at extracting this GPCR, likely related to the fact that MOR is more stable in LMNG. All tested ACAs except ACA-SC11 yielded similar levels of soluble receptor to DDM. Notably, ACA-AC11-solubilized MOR showed little protein aggregation, while DDM/LMNG-solubilized receptor appeared to undergo substantial aggregation (Fig. S6b†). Combined with the MelBSt results, these findings demonstrate that the new detergents can be used for membrane protein extraction as efficiently as DDM/LMNG.
Membrane proteins have diverse structures and thus their hydrophobic and hydrophilic dimensions vary. Consequently, these bio-macromolecules vary in terms of their tendency to denature and aggregate. The varied dimensions and diverse properties of membrane proteins imply a protein-specific nature of detergent efficacy for protein stabilization, as observed in the current study. For instance, ACA-AC12 was the most effective ACA at stabilizing LeuT, but this C12 alkyl-chained detergent was poor at stabilizing MOR. As another example, ACA-SC11 was inferior to ACA-SC12 at stabilizing MOR, but was the most effective of the ACA-Ss at stabilizing the other tested membrane proteins (LeuT, MelB and β2AR). Despite this apparent protein-specific nature of the detergents, ACA-AC11 was superior to DDM for stabilization of all the tested membrane proteins. The symmetric version of this detergent, ACA-SC11, also exhibited generally favourable behaviour toward stabilization of all tested membrane proteins except MOR. Notably, these C11 alkyl-chained ACAs (ACA-AC11 and ACA-SC11) showed enhanced GPCR stability even when compared to LMNG. This is a remarkable feature as glucoside detergents such as OG and OGNG are generally inferior to their maltoside counterparts (DDM and LMNG) with respect to protein stability. Furthermore, these C11 alkyl-chained ACAs (ACA-AC11 and ACA-SC11) gave high protein extraction efficiencies; ACA-AC11 and ACA-SC11 gave highly efficient MelBSt extraction, comparable to DDM, and were as efficient as DDM and LMNG at extracting β2AR and MOR, respectively. Thus, these detergents have potential in protein extraction, purification and structural studies.
Detergent efficacy for membrane protein stabilization tends to be significantly affected by a minor variation in detergent structure. In the current study, however, we could not find a meaningful difference in detergent efficacy for protein stabilization between the ACA-Ss and ACA-As. For instance, the ACA-As were generally better than their symmetric counterparts for LeuT stability, but the relative efficacy of these two sets for MOR stabilization varied depending on detergent alkyl chain length. For example, ACA-AC11 was better than ACA-SC11 at stabilizing this GPCR, whereas the opposite trend was observed for the C12 alkyl-chained ACAs. In the case of stability of β2AR and MelB, there was no substantial difference in detergent efficacy between the two sets of ACAs. This is somewhat unexpected considering their obvious structural differences. As the two alkyl chains were attached either to the same carbon or to different carbons of the central core, their alkyl chain density should be different. The alkyl chain density appears to be higher for the ACA-Ss, as supported by the lower CMCs and smaller micelle sizes compared to the ACA-As. In addition, due to the presence of the ethyl glucoside appendage, the hydrophilic group of the ACA-As is likely to be more flexible than that of the symmetric versions. The minor efficacy difference between the symmetric and asymmetric versions, despite the presence of their obvious structural differences, is likely attributed to the fact that several factors such as HLB, alkyl chain density/inter alkyl chain distance, and flexibility of detergent head group collectively affect detergent behaviour for protein stability. Thus, it is possible that one advantageous feature (e.g., flexibility of the hydrophilic group) is offset by detrimental feature (e.g., low alkyl chain density) for protein stability in the same set of detergents. A further study is necessary to explore this hypothesis.
It is noteworthy that detergent alkyl chain length was found to be highly important for protein stability in the current study. Of the ACA-As, the C11 version (ACA-AC11) was most effective at stabilizing the tested membrane proteins, followed by the C12 and C10 versions. A similar tendency was observed for the ACA-Ss. The only exception was MOR which was more stable in ACA-SC12 than the C11 version. As membrane proteins have a narrow range of hydrophobic widths (28–32 Å), the detergent alkyl chain length that is optimal for membrane protein stability should be limited.67,68 Furthermore, the alkyl chain length plays a central role in detergent hydrophobicity, a feature that strongly influences detergent–detergent and detergent–protein interactions in PDC environments. The short alkyl-chained ACAs (ACA-AC10 and ACA-SC10) are likely form relatively weak detergent–detergent and detergent–protein interactions, and are thus unlikely to be effective at preventing protein aggregation. In addition, the C10 alkyl chain of these detergents seems to be too short to effectively cover the hydrophobic surfaces of the membrane proteins. In contrast, the C12 alkyl-chained ACAs should support strong detergent–detergent and detergent–protein interactions, and therefore effective at preventing membrane protein aggregation. When it comes to compatibility with a protein's hydrophobic dimensions, however, the C12 alkyl-chain ACAs may be suboptimal due to the formation of micelles with large hydrophobic dimensions.69 The current study showed that the C11 alkyl-chained ACAs were best for protein stability in most cases, indicating that the C11 alkyl chain in the ACA architecture is hydrophobic enough to prevent protein aggregation and optimal for effective encapsulation of the hydrophobic surfaces of membrane proteins. It is notable that the C11 alkyl chain length has been found to be most effective for protein stability in the cases of other amphiphiles.28,34
Conclusions
Two sets of glucoside amphiphiles were designed and prepared with length and positional variations of the alkyl chains. These detergents (ACA-As and ACA-Ss) were evaluated with two transporters (LeuT and MelB) and two GPCRs (β2AR and MOR) in terms of protein solubilization and stabilization. The C11 versions (ACA-AC11 and ACA-SC11) are not only as efficient as DDM for protein extraction, but are also superior to the latter for membrane protein stabilization. Although they are glucoside detergents, ACA-AC11 and ACA-SC11/SC12 conferred markedly enhanced stability compared to the maltoside detergent (LMNG). Several advantageous features of these ACAs, such as convenient synthesis, high efficiency for membrane protein extraction and enhanced efficacy for membrane protein stabilization indicate their significant potential for the membrane protein manipulation and structural studies. The detailed analysis of the structure–efficacy relationship of the detergents provided here should facilitate new detergent design and development for membrane protein studies.Data availability
The data that support the findings of this study are available in the ESI† of this article.Author contributions
P. S. C. designed the new amphiphiles. H. J. L. and M. E. synthesized the amphiphiles. H. J. L., M. E., X. Z., S. K., C. F. M., H. W., W. A., and A. K. designed and performed the research and interpreted the data. P. S. C., X. L., L. G., C. J. L., and B. B. contributed to experimental design and data interpretation. P. S. C. and H. J. L. wrote the manuscript, with oversight from all the other authors.Conflicts of interest
The authors declare the following competing financial interest(s): P. S. C., H. J. L., and M. E. are inventors on a patent application describing the ACAs.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) (2021R1A2C2006067 and 2018R1A6A1A03024231 to P. S. C.). This work was also supported by the National Institutes of Health (grants R01GM122759 and R21NS105863 to L. G.).Notes and references
- J. P. Overington, B. Al-Lazikani and A. L. Hopkins, Nat. Rev. Drug Discovery, 2006, 5, 993–996 CrossRef CAS.
- J. Gong, Y. Chen, F. Pu, P. Sun, F. He, L. Zhang, Y. Li, Z. Ma and H. Wang, Curr. Drug Targets, 2019, 20, 551–564 CrossRef CAS PubMed.
- C. R. Sanders and J. K. Myers, Annu. Rev. Biophys. Biomol. Struct., 2004, 33, 25–51 CrossRef CAS PubMed.
- R. Santos, O. Ursu, A. Gaulton, A. P. Bento, R. S. Donadi, C. G. Bologa, A. Karlsson, B. Al-Lazikani, A. Hersey, T. I. Oprea and J. P. Overington, Nat. Rev. Drug Discovery, 2017, 16, 19–34 CrossRef CAS PubMed.
- S. H. White, Protein Sci., 2004, 13, 1948–1949 CrossRef CAS PubMed.
- https://www.wwpdb.org/ .
- M. J. Serrano-Vega, F. Magnani, Y. Shibata and C. G. Tate, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 877–882 CrossRef CAS PubMed.
- J. N. Israelachvili, D. J. Mitchell and B. W. Ninham, Biochim. Biophys. Acta, Biomembr., 1977, 470, 185–201 CrossRef CAS.
- Y. He, K. Wang and N. Yan, Protein Cell, 2014, 5, 658–672 CrossRef CAS PubMed.
- R. Phillips, T. Ursell, P. Wiggins and P. Sens, Nature, 2009, 459, 379–385 CrossRef CAS PubMed.
- G. G. Prive, Methods, 2007, 41, 388–397 CrossRef CAS PubMed.
- B. C. Choy, R. J. Cater, F. Mancia and E. E. Pryor, Jr, Biochim. Biophys. Acta, Biomembr., 2021, 1863, 183533 CrossRef CAS PubMed.
- R. M. Garavito and S. Ferguson-Miller, J. Biol. Chem., 2001, 276, 32403–32406 CrossRef CAS PubMed.
- J. Deisenhofer, O. Epp, K. Miki, R. Huber and H. Michel, Nature, 1985, 318, 618–624 CrossRef CAS PubMed.
- J. J. Lacapere, E. Pebay-Peyroula, J. M. Neumann and C. Etchebest, Trends Biochem. Sci., 2007, 32, 259–270 CrossRef CAS PubMed.
- J. U. Bowie, Curr. Opin. Struct. Biol., 2001, 11, 397–402 CrossRef CAS PubMed.
- A. Nath, W. M. Atkins and S. G. Sligar, Biochemistry, 2007, 46, 2059–2069 CrossRef CAS PubMed.
- J. Broecker, B. T. Eger and O. P. Ernst, Structure, 2017, 25, 384–392 CrossRef CAS PubMed.
- C. Tribet, R. Audebert and J. L. Popot, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 15047–15050 CrossRef CAS PubMed.
- C.-L. McGregor, L. Chen, N. C. Pomroy, P. Hwang, S. Go, A. Chakrabartty and G. G. Privé, Nat. Biotechnol., 2003, 21, 171–176 CrossRef CAS PubMed.
- H. Tao, S. C. Lee, A. Moeller, R. S. Roy, F. Y. Siu, J. Zimmermann, R. C. Stevens, C. S. Potter, B. Carragher and Q. Zhang, Nat. Methods, 2013, 10, 759–761 CrossRef CAS PubMed.
- H. E. Bae, C. Cecchetti, Y. Du, S. Katsube, J. S. Mortensen, W. Huang, S. Rehan, H. J. Lee, C. J. Loland, L. Guan, B. K. Kobilka, B. Byrne and P. S. Chae, Acta Biomater., 2020, 112, 250–261 CrossRef CAS PubMed.
- K. H. Cho, H. E. Bae, M. Das, S. H. Gellman and P. S. Chae, Chem.–Asian J., 2014, 9, 632–638 CrossRef CAS PubMed.
- P. S. Chae, R. R. Rana, K. Gotfryd, S. G. F. Rasmussen, A. C. Kruse, K. H. Cho, S. Capaldi, E. Carlsson, B. Kobilka, C. J. Loland, U. Gether, S. Banerjee, B. Byrne, J. K. Lee and S. H. Gellman, Chem. Commun., 2013, 49, 2287–2289 RSC.
- H. E. Bae, Y. Du, P. Hariharan, J. S. Mortensen, K. K. Kumar, B. Ha, M. Das, H. S. Lee, C. J. Loland, L. Guan, B. K. Kobilka and P. S. Chae, Chem. Sci., 2019, 10, 1107–1116 RSC.
- K. H. Cho, M. Husri, A. Amin, K. Gotfryd, H. J. Lee, J. Go, J. W. Kim, C. J. Loland, L. Guan, B. Byrne and P. S. Chae, Analyst, 2015, 140, 3157–3163 RSC.
- P. S. Chae, S. G. F. Rasmussen, R. R. Rana, K. Gotfryd, R. Chandra, M. A. Goren, A. C. Kruse, S. Nurva, C. J. Loland, Y. Pierre, D. Drew, J. L. Popot, D. Picot, B. G. Fox, L. Guan, U. Gether, B. Byrne, B. Kobilka and S. H. Gellman, Nat. Methods, 2010, 7, 1003–1008 CrossRef CAS PubMed.
- A. Sadaf, J. S. Mortensen, S. Capaldi, E. Tikhonova, P. Hariharan, O. de Castro Ribeiro, C. J. Loland, L. Guan, B. Byrne and P. S. Chae, Chem. Sci., 2016, 7, 1933–1939 RSC.
- S. C. Howell, R. Mittal, L. Huang, B. Travis, R. M. Breyer and C. R. Sanders, Biochemistry, 2010, 49, 9572–9583 CrossRef CAS PubMed.
- P. S. Chae, S. G. F. Rasmussen, R. R. Rana, K. Gotfryd, A. C. Kruse, A. Manglik, K. H. Cho, S. Nurva, U. Gether, L. Guan, C. J. Loland, B. Byrne, B. K. Kobilka and S. H. Gellman, Chem.–Eur. J., 2012, 18, 9485–9490 CrossRef CAS PubMed.
- S. C. Lee, B. C. Bennett, W. X. Hong, Y. Fu, K. A. Baker, J. Marcoux, C. V. Robinson, A. B. Ward, J. R. Halpert, R. C. Stevens, C. D. Stout, M. J. Yeager and Q. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, E1203–E1211 CrossRef CAS.
- P. S. Chae, K. Gotfryd, J. Pacyna, L. J. W. Miercke, S. G. F. Rasmussen, R. A. Robbins, R. R. Rana, C. J. Loland, B. Kobilka, R. Stroud, B. Byrne, U. Gether and S. H. Gellman, J. Am. Chem. Soc., 2010, 132, 16750–16752 CrossRef CAS PubMed.
- K. H. Park, C. Berrier, F. Lebaupain, B. Pucci, J. L. Popot, A. Ghazi and F. Zito, Biochem. J., 2007, 403, 183–187 CrossRef CAS PubMed.
- M. Ehsan, L. Ghani, Y. Du, P. Hariharan, J. S. Mortensen, O. Ribeiro, H. Hu, G. Skiniotis, C. J. Loland, L. Guan, B. K. Kobilka, B. Byrne and P. S. Chae, Analyst, 2017, 142, 3889–3898 RSC.
- L. H. Urner, I. Liko, H. Y. Yen, K. K. Hoi, J. R. Bolla, J. Gault, F. G. Almeida, M. P. Schweder, D. Shutin, S. Ehrmann, R. Haag, C. V. Robinson and K. Pagel, Nat. Commun., 2020, 11, 564 CrossRef CAS PubMed.
- M. Das, Y. Du, J. S. Mortensen, O. Ribeiro, P. Hariharan, L. Guan, C. J. Loland, B. K. Kobilka, B. Byrne and P. S. Chae, Chem. Sci., 2017, 8, 1169–1177 RSC.
- M. Das, Y. Du, O. Ribeiro, P. Hariharan, J. S. Mortensen, D. Patra, G. Skiniotis, C. J. Loland, L. Guan, B. K. Kobilka, B. Byrne and P. S. Chae, J. Am. Chem. Soc., 2017, 139, 3072–3081 CrossRef CAS PubMed.
- M. Das, F. Mahler, P. Hariharan, H. Wang, Y. Du, J. S. Mortensen, E. P. Patallo, L. Ghani, D. Glück, H. J. Lee, B. Byrne, C. J. Loland, L. Guan, B. K. Kobilka, S. Keller and P. S. Chae, J. Am. Chem. Soc., 2020, 142, 21382–21392 CrossRef CAS.
- H. Hussain, Y. Du, N. J. Scull, J. S. Mortensen, J. Tarrasch, H. E. Bae, C. J. Loland, B. Byrne, B. K. Kobilka and P. S. Chae, Chem.–Eur. J., 2016, 22, 7068 CrossRef CAS.
- A. Sadaf, M. Ramos, J. S. Mortensen, Y. Du, H. E. Bae, C. F. Munk, P. Hariharan, B. Byrne, B. K. Kobilka, C. J. Loland, L. Guan and P. S. Chae, ACS Chem. Biol., 2019, 14, 1717–1726 CrossRef CAS PubMed.
- M. Das, Y. Du, J. S. Mortensen, M. Ramos, L. Ghani, H. J. Lee, H. E. Bae, B. Byrne, L. Guan, C. J. Loland, B. K. Kobilka and P. S. Chae, Org. Biomol. Chem., 2019, 17, 3249–3257 RSC.
- H. J. Lee, H. S. Lee, T. Youn, B. Byrne and P. S. Chae, Chem, 2022, 8, 980–1013 CAS.
- A. Urbani, V. Giorgio, A. Carrer, C. Franchin, G. Arrigoni, C. Jiko, K. Abe, S. Maeda, K. Shinzawa-Itoh, J. F. M. Bogers, D. G. G. McMillan, C. Gerle, I. Szabo and P. Bernardi, Nat. Commun., 2019, 10, 4341 CrossRef PubMed.
- N. Mnatsakanyan, M. C. Llaguno, Y. Yang, Y. Yan, J. Weber, F. J. Sigworth and E. A. Jonas, Nat. Commun., 2019, 10, 5823 CrossRef CAS PubMed.
- J. Breibeck and A. Rompel, Biochim. Biophys. Acta, Gen. Subj., 2019, 1863, 437–455 CrossRef CAS PubMed.
- K. H. Cho, P. Hariharan, J. S. Mortensen, Y. Du, A. K. Nielsen, B. Byrne, B. K. Kobilka, C. J. Loland, L. Guan and P. S. Chae, ChemBioChem, 2016, 17, 2334–2339 CrossRef CAS PubMed.
- B. W. Berger, R. Y. Garcia, A. M. Lenhoff, E. W. Kaler and C. R. Robinson, Biophys. J., 2005, 89, 452–464 CrossRef CAS PubMed.
- A. Covarrubias-Zúñiga, L. S. Germán-Sanchez and J. G. Avila-Zárraga, Synth. Commun., 2003, 33, 3165–3172 CrossRef.
- A. Covarrubias-Zúñiga, J. G. Avila-Zárraga and D. Arias Salas, Synth. Commun., 2003, 33, 3173–3181 CrossRef.
- P. K. Agrawal, Phytochemistry, 1992, 31, 3307–3330 CrossRef CAS.
- R. C. Oliver, J. Lipfert, D. A. Fox, R. H. Lo, S. Doniach and L. Columbus, PLoS One, 2013, 8, e62488 CrossRef CAS.
- A. Chattopadhyay and E. London, Anal. Biochem., 1984, 139, 408–412 CrossRef CAS.
- J. Israelachvili, Colloids Surf., A, 1994, 91, 1–8 CrossRef CAS.
- G. Deckert, P. V. Warren, T. Gaasterland, W. G. Young, A. L. Lenox, D. E. Graham, R. Overbeek, M. A. Snead, M. Keller, M. Aujay, R. Huber, R. A. Feldman, J. M. Short, G. J. Olsen and R. V. Swanson, Nature, 1998, 392, 353–358 CrossRef CAS PubMed.
- M. Quick and J. A. Javitch, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 3603–3608 CrossRef CAS PubMed.
- M. Ehsan, S. Katsube, C. Cecchetti, Y. Du, J. S. Mortensen, H. Wang, A. Nygaard, L. Ghani, C. J. Loland, B. K. Kobilka, B. Byrne, L. Guan and P. S. Chae, ACS Chem. Biol., 2020, 15, 1697–1707 CrossRef CAS PubMed.
- A. S. Ethayathulla, M. S. Yousef, A. Amin, G. Leblanc, H. R. Kaback and L. Guan, Nat. Commun., 2014, 5, 3009 CrossRef PubMed.
- L. Guan, S. Nurva and S. P. Ankeshwarapu, J. Biol. Chem., 2011, 286, 6367–6374 CrossRef CAS PubMed.
- E. Cordat, I. Mus-Veteau and G. Leblanc, J. Biol. Chem., 1998, 273, 33198–33202 CrossRef CAS PubMed.
- L. Guan and P. Hariharan, Commun. Biol., 2021, 4, 931 CrossRef CAS.
- C. Maehrel, E. Cordat, I. Mus-Veteau and G. Leblanc, J. Biol. Chem., 1998, 273, 33192–33197 CrossRef CAS.
- D. M. Rosenbaum, V. Cherezov, M. A. Hanson, S. G. Rasmussen, F. S. Thian, T. S. Kobilka, H.-J. Choi, X.-J. Yao, W. I. Weis, R. C. Stevens and B. K. Kobilka, Science, 2007, 318, 1266–1273 CrossRef CAS.
- X. Yao, C. Parnot, X. Deupi, V. R. Ratnala, G. Swaminath, D. Farrens and B. Kobilka, Nat. Chem. Biol., 2006, 2, 417–422 CrossRef CAS.
- A. Manglik, A. C. Kruse, T. S. Kobilka, F. S. Thian, J. M. Mathiesen, R. K. Sunahara, L. Pardo, W. I. Weis, B. K. Kobilka and S. Granier, Nature, 2012, 485, 321–326 CrossRef CAS.
- A. W. Serohijos, S. Yin, F. Ding, J. Gauthier, D. G. Gibson, W. Maixner, N. V. Dokholyan and L. Diatchenko, Structure, 2011, 19, 1683–1690 CrossRef CAS.
- C. Le Bon, B. Michon, J. L. Popot and M. Zoonens, Q. Rev. Biophys., 2021, 54, e6 CrossRef CAS PubMed.
- M. Das, Y. Du, J. S. Mortensen, H. E. Bae, B. Byrne, C. J. Loland, B. K. Kobilka and P. S. Chae, Chem.–Eur. J., 2018, 24, 9860–9868 CrossRef CAS.
- Q. Zhang, X. Ma, A. Ward, W. X. Hong, V. P. Jaakola, R. C. Stevens, M. G. Finn and G. Chang, Angew. Chem., Int. Ed. Engl., 2007, 46, 7023–7025 CrossRef CAS PubMed.
- L. Columbus, J. Lipfert, K. Jambunathan, D. A. Fox, A. Y. Sim, S. Doniach and S. A. Lesley, J. Am. Chem. Soc., 2009, 131, 7320–7326 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2sc00539e |
| ‡ Current address: Department of Chemistry, Mirpur University of Science & Technology (MUST), Mirpur-10250 (AJK), Pakistan. |
| This journal is © The Royal Society of Chemistry 2022 |