Open Access Article
Open Access ArticleIminyl radical-triggered relay annulation for the construction of bridged aza-tetracycles bearing four contiguous stereogenic centers†
Kun
Jiang
a,
Shi-Jun
Li
 b,
Qing-Peng
Liu
a,
Ning
Yu
a,
Yu-Lin
Li
a,
Yu-Qiang
Zhou
a,
Kui-Cheng
He
a,
Jing
Lin
a,
Ting-Yu
Zheng
a,
Jian
Lang
a,
Yu
Lan
b,
Qing-Peng
Liu
a,
Ning
Yu
a,
Yu-Lin
Li
a,
Yu-Qiang
Zhou
a,
Kui-Cheng
He
a,
Jing
Lin
a,
Ting-Yu
Zheng
a,
Jian
Lang
a,
Yu
Lan
 *b and
Ye
Wei
*b and
Ye
Wei
 *a
*a
aSchool of Chemistry and Chemical Engineering, Southwest University, Chongqing, 400715, China. E-mail: weiye712@swu.edu.cn
bCollege of Chemistry, Institute of Green Catalysis, Zhengzhou University, Zhengzhou, Henan 450001, China. E-mail: lanyu@cqu.edu.cn
First published on 30th May 2022
Abstract
Bridged tetracyclic nitrogen scaffolds are found in numerous biologically active molecules and medicinally relevant structures. Traditional methods usually require tedious reaction steps, and/or the use of structurally specific starting materials. We report an unprecedented, iminyl radical-triggered relay annulation from oxime-derived peresters and azadienes, which shows good substrate scope and functional group compatibility, and can deliver various bridged aza-tetracyclic compounds with complex molecular topology and four contiguous stereogenic centers (dr > 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in a single operation. This transformation represents the first example of trifunctionalization of iminyl radicals through simultaneous formation of one C–N and two C–C bonds. DFT calculation studies were conducted to obtain an in-depth insight into the reaction pathways, which revealed that the reactions involved an interesting 1,6-hydrogen atom transfer process.
1) in a single operation. This transformation represents the first example of trifunctionalization of iminyl radicals through simultaneous formation of one C–N and two C–C bonds. DFT calculation studies were conducted to obtain an in-depth insight into the reaction pathways, which revealed that the reactions involved an interesting 1,6-hydrogen atom transfer process.
Introduction
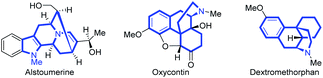
Bridged aza-tetracyclic skeletons represent a structurally complex and important class of polycyclic frameworks, and such skeletons are found in numerous alkaloids and biologically active natural products,1 such as alstoumerine,2a as well as commercial pharmaceuticals such as oxycontin2b and dextromethorphan (Scheme 1).2c Besides, the interesting structures have also led their use as useful intermediates for the construction of a series of valuable molecules.3 Owing to the intriguing structures, diverse biological activities and synthetic potentials associated with bridged aza-tetracycles, tremendous attention has been paid to their efficient syntheses. Traditional methods usually require tedious reaction steps, and/or the use of structurally specific starting materials that are often difficult to access.4,5 As such, the construction of bridged aza-tetracyclic scaffolds using readily available starting materials in a one-step manner would be a highly attractive way.Radical relay annulation is emerging as a useful synthetic tool enabling access to polycyclic frameworks in one step, such as bicyclic, tricyclic, and sometimes tetracyclic structures.6 Nevertheless, the synthetic potential of such a strategy towards the formation of bridged aza-tetracyclic skeletons has been significantly underdeveloped. Thus, new protocols remain in high demand for generating more structurally new bridged aza-tetracycles with an aim to increase their molecular diversity and complexity. This work describes an unprecedented radical relay annulation from oxime-derived peresters and azadienes, which delivers various bridged aza-tetracyclic compounds with complex molecular topology and four contiguous stereogenic centers (dr > 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Moreover, DFT calculation studies revealed that the reactions involved an interesting 1,6-hydrogen atom transfer process. Significantly, this transformation, to the best of our knowledge, represents the first example of trifunctionalization of iminyl radicals through simultaneous formation of one C–N and two C–C bonds.
1). Moreover, DFT calculation studies revealed that the reactions involved an interesting 1,6-hydrogen atom transfer process. Significantly, this transformation, to the best of our knowledge, represents the first example of trifunctionalization of iminyl radicals through simultaneous formation of one C–N and two C–C bonds.
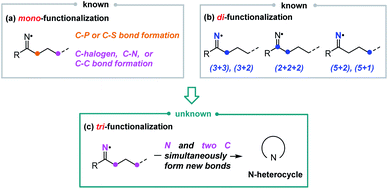
Iminyl radicals, one of the most important classes of nitrogen-centered radicals, have attracted much attention from synthetic chemists. A series of transformations based on iminyl radicals have been successfully realized,7 such as C-halogenation,8 -phosphorylation,9 -sulfonylation,10 -azidation,11 -alkenylation,12 and -alkynylation (Scheme 2a).13 Moreover, iminyl radical-based intermolecular annulations, including (3 + n), (2 + 2 + 2), and (5 + n) annulations, have also been disclosed, by which various N-heterocycles were generated (Scheme 2b).14 In these reactions, iminyl radicals either provided one atom (mono-functionalization) or two atoms (di-functionalization) to generate new chemical bonds. In terms of the functionalization of iminyl radicals, to the best of our knowledge, only mono- and di-functionalization fashions were revealed, which might limit their synthetic applications to some extent. In order to increase the atom utilization and enrich the structural diversity and complexity of organic molecules, we envisioned to challenge the tri-functionalization of iminyl radicals, in which multiple new chemical bonds (e.g., three new chemical bonds) would be generated in one step (Scheme 2c).
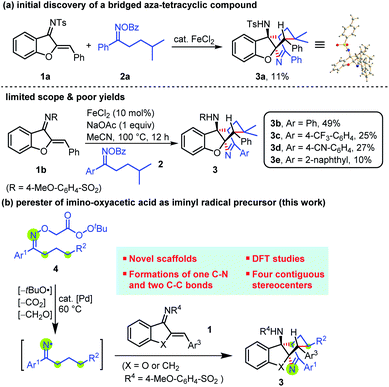
Our group14l,m has great interest in the iminyl radical-triggered relay annulation involving 1,5-hydrogen atom transfer15,16 for the synthesis of N-heterocycles. During the survey of iminyl radical-induced (5 + n) annulation, we obtained a bridged aza-tetracyclic compound (3a) from the reaction of aurone-derived azadienes (1a) with isopentyl(phenyl) ketone-derived oxime (2a) (Scheme 3a). The structure of 3a was unambiguously confirmed by single-crystal X-ray diffraction.17 In this reaction, one C–N and two C–C bonds were newly formed accompanying the generation of two new bridged rings. This reaction not only involves the trifunctionalization of iminyl radicals, but also generates structurally novel bridged nitrogen skeletons. After numerous experiments,18 we found that the reaction of azadiene (1b) and 2a afforded a bridged aza-tetracyclic compound 3b in 49% yield in the presence of FeCl2 (10 mol%) and NaOAc (1 equiv.) at 100 °C in MeCN within 12 h. Unfortunately, the yield cannot be further improved. More regrettably, such reaction conditions were only amenable to limited substrate scope, and the yields were generally below 30% (e.g., 3c, 3d, and 3e). A key problem of this reaction might be the high reaction temperature used which led to rapid decomposition of the oximes.
Our recent study on the oxime transformation suggested that the use of high reaction temperature might be partially responsible for the N–O bond cleavage.14m In order to make this step occur at lower temperature, peresters 4 derived from the corresponding imino-oxyacetic acids were synthesized, which was inspired by Forrester's seminal work on iminyl radical transformations.19 He demonstrated that the perester can undergo thermolysis to generate the iminyl radical. Moreover, it has been known that various transition metals can facilitate the homolysis of the weak O–O bond in peroxides.20 Based on these significant studies, we envisaged that 4 could be converted into the same iminyl radicals at lower temperature in the presence of a suitable transition metal, which would involve the sequential release of a tert-butoxy radical, formaldehyde, and carbon dioxide. As such, the desired bridged aza-tetracyclic compounds might also be generated (Scheme 3b). To verify our hypothesis, a plethora of experiments were conducted, and some important results obtained during the process are displayed in Table 1.
| Entry | Catalyst | Additive | Yieldb (%) |
|---|---|---|---|
a Reaction conditions: 1b (0.3 mmol), 4a (0.2 mmol), catalyst (0 or 10 mol%), additive (0 or 1 equiv.), MeCN (2 mL), 60 °C, 12 h, in a sealed tube, under Ar.
b Isolated yields; dr > 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
c Reaction was run at 40 °C.
d NaOAc (0.5 equiv.) was used. 1.
c Reaction was run at 40 °C.
d NaOAc (0.5 equiv.) was used.
|
|||
| 1 | None | NaOAc | 15 |
| 2 | FeCl2 | NaOAc | 41 |
| 3 | Fe(acac)3 | NaOAc | 32 |
| 4 | CuBr | NaOAc | 11 |
| 5 | CuBr2 | NaOAc | Trace |
| 6 | PdCl2 | NaOAc | 36 |
| 7 | Pd(OAc)2 | NaOAc | 35 |
| 8 | Pd2(dba)3 | NaOAc | 40 |
| 9 | Pd(PPh3)2Cl2 | NaOAc | 60 |
| 10 | Pd(PCy3)2Cl2 | NaOAc | 43 |
| 11 | Pd(PPh3)2Cl2 | None | 10 |
| 12 | Pd(PPh3)2Cl2 | PivONa | 35 |
| 13 | Pd(PPh3)2Cl2 | PhCO2Na | 13 |
| 14 | Pd(PPh3)2Cl2 | NaOAc | Tracec |
| 15 | Pd(PPh3)2Cl2 | NaOAc | 67d |
Results and discussion
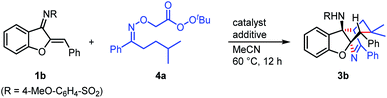
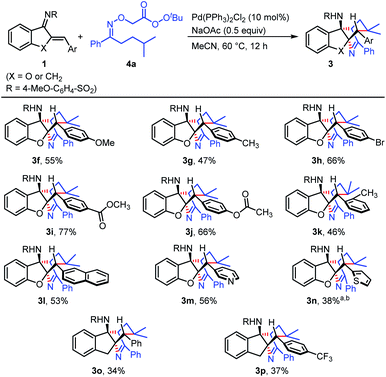
Initially, aurone-derived azadiene (1b) and an oxime-derived perester (4a) were chosen as model substrates to optimize the reaction conditions toward the formation of the target product (3b).18 We set the reaction temperature at 60 °C for the screening of transition metals. A control experiment showed that the product was obtained in 15% yield in the absence of a transition metal (entry 1). Since iron and copper salts are usually used to promote the O–O bond homolysis of peroxides, a series of iron and copper salts were studied (entries 2–5). The results showed that FeCl2 and Fe(acac)3 can promote the transformation (entries 2 and 3), while CuBr and CuBr2 inhibited the reaction (entries 4 and 5). Gratifyingly, it was found that palladium salts were beneficial for the reaction (entries 6–10), and Pd(PPh3)2Cl2 (ref. 21) exhibited the best reactivity, using which a 60% yield was obtained (entry 9). It is noteworthy that NaOAc was important for this transformation, because a very low yield was observed without such an additive (entry 11). By replacing NaOAc with PivONa or PhCO2Na, the reaction took place albeit 35 and 13% yields were obtained, respectively (entries 12 and 13). In addition, decreasing the reaction temperature from 60 to 40 °C was detrimental to the transformation (entry 14). Finally, reducing the amount of NaOAc from 1 to 0.5 equiv. afforded a 67% yield of 3b (entry 15).With the optimal reaction conditions in hand, we subsequently evaluated the substrate scope with respect to the azadienes using 4a as another substrate (Scheme 4). Azadienes bearing methoxy (3f) and methyl (3g) groups at the para position of the aryl ring showed moderate reactivity, delivering the target products in 55 and 47% yields, respectively. In addition, azadienes with electron-withdrawing groups on the aromatic ring, such as bromo (3h) and ester (3i and 3j) groups, reacted with 4a to give rise to the products in moderate to good yields. The reaction gave 46% yield in the case of an azadiene with an ortho-methyl substitution on the aryl ring (3k). Note that, this protocol was applicable to some heterocycle-containing azadienes, including pyridine (3m) and thiophene (3n), which displayed moderate reactivity in the transformations. Furthermore, 1-indanone-derived azadienes were also found to be potential substrates to participate in the synthesis of the bridged tetracyclic N-heterocycles, which produced the target products in synthetically useful yields (3o and 3p). Note that, in all cases, the target products were formed with excellent diastereoselectivity (>19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
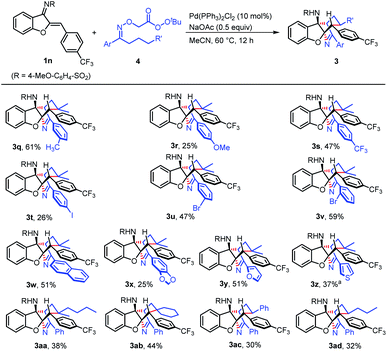
This approach can be applied to a variety of oxime-derived peresters containing diverse functionalities (Scheme 5). The peresters bearing methyl (3q), methoxy (3r), trifluoromethyl (3s), iodo (3t), bromo (3u and 3v), and naphthyl (3w) groups took part in the reactions to generate the corresponding bridged aza-tetracyclic compounds in synthetically useful yields. Significantly, heterocyclic moieties, including benzodioxole (3x), furan (3y), and thiophene (3z) also participated in the transformations. Besides the di-methyl group located at the γ position of the perester, methyl/n-butyl (3aa) and cyclohexyl (3ab) groups were also suitable. Not only tertiary carbon radicals, but also secondary carbon radicals can be formed, which can participate in the reactions to deliver the target products (3ac and 3ad). In contrast, butyrophenone-derived perester failed to react, probably due to the low stability of the corresponding primary carbon radical.
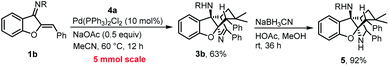
Our method is suitable for gram-scale reactions, which was demonstrated by a 5 mmol scale reaction between 1b and 4a. The obtained bridged aza-tetracyclic product 3b can easily undergo reduction to generate a tetracyclic secondary amine 5 in excellent yield (Scheme 6).
To obtain insight into the reaction mechanism, we performed some experiments (Scheme 7). A reaction between 1b and 4a cannot generate the desired product 3b in the presence of 3 equiv. of TEMPO as a radical scavenger, and significant amounts of radical-trapping product 6 were formed (Scheme 7a). These results suggested that a γ-carbon radical might be formed in the reaction. In addition, intermolecular competitive experiments were carried out. Under otherwise identical conditions, the azadiene bearing a trifluoromethyl group (1n) showed better reactivity, probably because it would be inclined to react with a nucleophilic carbon radical (Scheme 7b). Besides, the N–O bond might be more easily cleavable for 4d containing a trifluoromethyl group, and thus, the reaction of 4d with 1n was faster than the reaction between 4c and 1n (Scheme 7c).
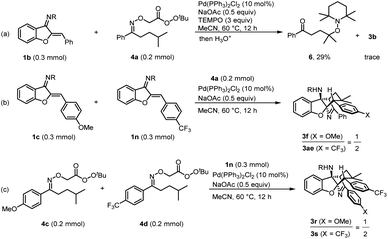 | ||
| Scheme 7 Mechanistic studies: (a) a radical-trapping experiment. (b) and (c) Intermolecular competitive reactions. R = 4-MeO–C6H4–SO2. | ||
To further understand the mechanism of this interesting reaction from the oxime-derived peresters and azadienes, density functional theory (DFT) calculation has been performed at the M06-2X/Def2-SVP level of theory (Scheme 8).22 Iminyl radical M1 could be obtained from the homolytic cleavage of the peroxy bond of 4a in the presence of a palladium salt,23 which is exergonic by 14.5 kcal mol−1 with the release of carbon dioxide, formaldehyde, and a tert-butoxy radical. Then, 1,5-hydrogen atom transfer occurs via transition state TS1 to deliver a tertiary alkyl radical M2 with an energy barrier of 13.5. kcal mol−1. The generated radical M2 could be caught by azadiene 1bvia transition state TS2 with a free energy barrier of only 1.0 kcal mol−1. The formation of M3 is exergonic by 25.2 kcal mol−1. This species is a stable intermediate that might be caused by the spin distribution. As shown in Scheme 8b, two possible resonance structures for the intermediate M3 could be drawn, which reveals either a carbon radical character (M3) or amino radical character (M3′). The further spin density calculation proved this point. The spin densities of carbon and nitrogen atoms are 0.504 and 0.380, respectively. Based on this idea, two possible pathways starting from either of these radical atoms have been proposed and considered by DFT calculations. From carbon radical resonance M3, an intramolecular radical addition into the imine moiety takes place via transition state TS3 with a free energy barrier of 18.7 kcal mol−1. The generated benzylic radical M4 undergoes 1,6-hydorgen transfer via transition state TS4 with a free energy barrier of 21.7 kcal mol−1 to afford carbon radical M5. Followed by another intramolecular radical addition to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond via transition state TS5, the precursor of product Pre-P could be obtained, which can undergo oxidation to yield the final product 3b. The free energy barrier for the radical addition step is detected to be 19.4 kcal mol−1. From the alternative amino radical resonance M3′, 1,7-hydrogen atom transfer would occur via transition state TS6 to generate M6 with a quite higher free energy barrier of 23.4 kcal mol−1. After that, the relay intramolecular radical additions via transition states TS7 and TS8 would also provide the common precursor of product Pre-P. The computed free energy barriers for these two steps are 25.0 kcal mol−1 and 13.7 kcal mol−1, respectively. The calculated results clearly show that the second pathway would be unfavorable.
C double bond via transition state TS5, the precursor of product Pre-P could be obtained, which can undergo oxidation to yield the final product 3b. The free energy barrier for the radical addition step is detected to be 19.4 kcal mol−1. From the alternative amino radical resonance M3′, 1,7-hydrogen atom transfer would occur via transition state TS6 to generate M6 with a quite higher free energy barrier of 23.4 kcal mol−1. After that, the relay intramolecular radical additions via transition states TS7 and TS8 would also provide the common precursor of product Pre-P. The computed free energy barriers for these two steps are 25.0 kcal mol−1 and 13.7 kcal mol−1, respectively. The calculated results clearly show that the second pathway would be unfavorable.
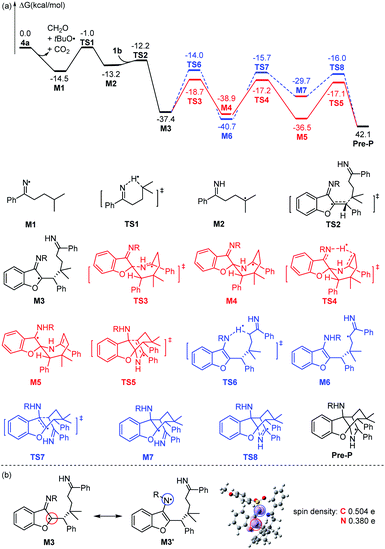 | ||
| Scheme 8 (a) The free energy barrier for the transformation. (b) The resonance structure and the spin density population for the M3. R = 4-MeO–C6H4–SO2. | ||
Conclusions
In conclusion, we have established a new radical relay annulation protocol with oxime-derived peresters and azadienes as readily available substrates. This method shows good substrate scope and functional group tolerance, and generates a series of structurally interesting bridged aza-tetracyclic compounds with complex molecular topology and four contiguous stereogenic centers (dr > 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). These molecules might be recognized as privileged scaffolds in drug discovery. Our work represents the first example of the trifunctionalization of iminyl radicals by means of simultaneous formation of one C–N and two C–C bonds. The exploitation of more iminyl radical-triggered relay annulation for the efficient construction of N-heterocyclic skeletons is ongoing in our research group.
1). These molecules might be recognized as privileged scaffolds in drug discovery. Our work represents the first example of the trifunctionalization of iminyl radicals by means of simultaneous formation of one C–N and two C–C bonds. The exploitation of more iminyl radical-triggered relay annulation for the efficient construction of N-heterocyclic skeletons is ongoing in our research group.
Data availability
All experimental data, and detailed experimental procedures are available in the ESI.†Author contributions
K. J. and Y. W. conceived the project and prepared the manuscript. S.-J. L. and Y. L. performed the DFT calculation. Q.-P. L., N. Y., Y.-L. L., Y.-Q. Z., K.-C. H., J. L., T.-Y. Z., and J. L. performed experiments and analyzed the data.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Science Foundation of China (No. 21772231), Natural Science Foundation of Chongqing (No. cstc2020jcyj-msxm2042), Southwest University (No. SWU118129, SWU120046), and Fundamental Research Funds for the Central Universities (No. XDJK2019AA003) is greatly appreciated.Notes and references
- (a) U. Anthonic, C. Christophersen and P. H. Nielsen, Alkaloids: Chemical and Biological Perspectives, ed. S. W. Pelletier, Elsevier, New York, 1999, vol. 14, pp. 163–236 Search PubMed; (b) G. Wang, W. Tang and R. R. Bidigare, Terpenoids as therapeutic drugs and pharmaceuticals agents, in Natural Products: Drug Discovery and Therapeutic Medicine, ed. L. Zhang and A. L. Demain, Humana, Totowa, 2005, pp. 197–227 Search PubMed.
- (a) N. Keawpradub, P. J. Houghton, E. Eno-Amooquaye and P. J. Burke, Planta Med., 1997, 63, 97–101 CrossRef CAS; (b) J. V. Pergolizzi, R. Taylor, J. A. LeQuang and R. B. Raffa, J. Pain Res., 2018, 11, 301–311 CrossRef CAS; (c) E.-J. Shin, S.-Y. Nah, W.-K. Kim, K. H. Ko, W.-K. Jhoo, Y.-W. Lim, J. Y. Cha, C.-F. Chen and H.-C. Kim, Br. J. Pharmacol., 2005, 144, 908–918 CrossRef CAS PubMed.
- (a) A. B. Smith, B. A. Wexler, C.-Y. Tu and J. P. Konopelski, J. Am. Chem. Soc., 1985, 107, 1308–1320 CrossRef CAS; (b) S. Tanimori, T. Sunami, K. Fukubayashi and M. Kirihata, Tetrahedron, 2005, 61, 2481–2492 CrossRef CAS.
- For recent reviews, see: (a) C. Avendaño and J. C. Menéndez, Bicyclic 6-6 Systems with One Bridgehead (ring junction) Nitrogen Atom: No Extra Heteroatom, in Comprehensive Heterocyclic Chemistry III, ed. A. R. Katritzky, C. A. Ramsden, E. F. V. Scriven and R. J. K. Taylor, Elsevier, 2008, vol. 12, pp. 1–75 Search PubMed; (b) K. Speck and T. Magauer, Beilstein J. Org. Chem., 2013, 9, 2048–2078 CrossRef PubMed.
- For recent examples, see: (a) H.-Y. Lee, S. Lee, B. G. Kim and J. S. Bahn, Tetrahedron Lett., 2004, 45, 7225–7229 CrossRef CAS; (b) Y. S. Tran and O. Kwon, Org. Lett., 2005, 7, 4289–4291 CrossRef CAS PubMed; (c) P. B. Finn, B. P. Derstine and S. M. Sieburth, Angew. Chem., Int. Ed., 2016, 55, 2536–2539 CrossRef CAS PubMed; (d) M. A. Haque and C. K. Jana, Chem.–Eur. J., 2017, 23, 13300–13304 CrossRef CAS PubMed.
- For recent reviews, see: (a) U. Wille, Chem. Rev., 2013, 113, 813–853 CrossRef CAS PubMed; (b) A. Studer and D. P. Curran, Angew. Chem., Int. Ed., 2016, 55, 58–102 CrossRef CAS; (c) J. Xie, H. Jin and A. S. K. Hashmi, Chem. Soc. Rev., 2017, 46, 5193–5203 RSC; (d) M. P. Plesniak, H.-M. Huang and D. J. Procter, Nat. Rev. Chem., 2017, 1, 0077 CrossRef; (e) Y. Okada and K. Chiba, Chem. Rev., 2018, 118, 4592–4630 CrossRef CAS; (f) H.-M. Huang, M. H. Garduño-Castro, C. Morrill and D. J. Procter, Chem. Soc. Rev., 2019, 48, 4626–4638 RSC; (g) C.-H. Xu, Y. Li, J.-H. Li, J.-N. Xiang and W. Deng, Sci. China: Chem., 2019, 62, 1463–1475 CrossRef CAS.
- For recent reviews, see: (a) M. Kitamura and K. Narasaka, Bull. Chem. Soc. Jpn., 2008, 81, 539–547 CrossRef CAS; (b) J. C. Walton, Acc. Chem. Res., 2014, 47, 1406–1416 CrossRef CAS PubMed; (c) H. Huang, X. Ji, W. Wu and H. Jiang, Chem. Soc. Rev., 2015, 44, 1155–1171 RSC; (d) X. Zhu and S. Chiba, Chem. Soc. Rev., 2016, 45, 4504–4523 RSC; (e) D. S. Bolotin, N. A. Bokach, M. Y. Demakova and V. Y. Kukushkin, Chem. Rev., 2017, 117, 13039–13122 CrossRef CAS PubMed; (f) N. J. Race, I. R. Hazelden, A. Faulkner and J. F. Bower, Chem. Sci., 2017, 8, 5248–5260 RSC; (g) L. M. Stateman, K. M. Nakafuku and D. A. Nagib, Synthesis, 2018, 50, 1569–1586 CrossRef CAS PubMed; (h) J. Davies, S. P. Morcillo, J. J. Douglas and D. Leonori, Chem.–Eur. J., 2018, 24, 12154–12163 CrossRef CAS PubMed; (i) S. P. Morcillo, Angew. Chem., Int. Ed., 2019, 58, 14044–14054 CrossRef CAS PubMed.
- E. M. Dauncey, S. P. Morcillo, J. J. Douglas, N. S. Sheikh and D. Leonori, Angew. Chem., Int. Ed., 2018, 57, 744–748 CrossRef CAS PubMed.
- J. Ke, Y. Tang, H. Yi, Y. Li, Y. Cheng, C. Liu and A. Lei, Angew. Chem., Int. Ed., 2015, 54, 6604–6607 CrossRef CAS PubMed.
- X. Tang, L. Huang, Y. Xu, J. Yang, W. Wu and H. Jiang, Angew. Chem., Int. Ed., 2014, 53, 4205–4208 CrossRef CAS PubMed.
- R. O. Torres-Ochoa, A. Leclair, Q. Wang and J. Zhu, Chem.–Eur. J., 2019, 25, 9477–9484 CrossRef CAS PubMed.
- (a) X. Shen, J.-J. Zhao and S. Yu, Org. Lett., 2018, 20, 5523–5527 CrossRef CAS PubMed; (b) L. Chen, L.-N. Guo, Z.-Y. Ma, Y.-R. Gu, J. Zhang and X.-H. Duan, J. Org. Chem., 2019, 84, 6475–6482 CrossRef CAS.
- Z. Li, R. O. Torres-Ochoa, Q. Wang and J. Zhu, Nat. Commun., 2020, 11, 403–409 CrossRef CAS PubMed.
- (a) Y. Wei and N. Yoshikai, J. Am. Chem. Soc., 2013, 135, 3756–3759 CrossRef CAS PubMed; (b) X. Tang, L. Huang, C. Qi, W. Wu and H. Jiang, Chem. Commun., 2013, 49, 9597–9599 RSC; (c) Q. Wu, Y. Zhang and S. Cui, Org. Lett., 2014, 16, 1350–1353 CrossRef CAS PubMed; (d) M.-N. Zhao, Z.-H. Ren, L. Yu, Y.-Y. Wang and Z.-H. Guan, Org. Lett., 2016, 18, 1194–1197 CrossRef CAS PubMed; (e) H. Huang, J. Cai, X. Ji, F. Xiao, Y. Chen and G.-J. Deng, Angew. Chem., Int. Ed., 2016, 55, 307–311 CrossRef CAS PubMed; (f) W. W. Tan, Y. J. Ong and N. Yoshikai, Angew. Chem., Int. Ed., 2017, 56, 8240–8244 CrossRef CAS; (g) C. Zhu, R. Zhu, H. Zeng, F. Chen, C. Liu, W. Wu and H. Jiang, Angew. Chem., Int. Ed., 2017, 56, 13324–13328 CrossRef CAS; (h) B. Zhao, H.-W. Liang, J. Yang, Z. Yang and Y. Wei, ACS Catal., 2017, 7, 5612–5617 CrossRef CAS; (i) H.-B. Yang and N. Selander, Chem.–Eur. J., 2017, 23, 1779–1783 CrossRef CAS PubMed; (j) D. Bai, X. Wang, G. Zheng and X. Li, Angew. Chem., Int. Ed., 2018, 57, 6633–6637 CrossRef CAS PubMed; (k) J. Yang, B. Zhao, Y. Xi, S. Sun, Z. Yang, Y. Ye, K. Jiang and Y. Wei, Org. Lett., 2018, 20, 1216–1219 CrossRef CAS PubMed; (l) W. Liang, K. Jiang, F. Du, J. Yang, L. Shuai, Q. Ouyang, Y.-C. Chen and Y. Wei, Angew. Chem., Int. Ed., 2020, 59, 19222–19228 CrossRef CAS PubMed; (m) F. Du, S.-J. Li, K. Jiang, R. Zeng, X.-C. Pan, Y. Lan, Y.-C. Chen and Y. Wei, Angew. Chem., Int. Ed., 2020, 59, 23755–23762 CrossRef CAS.
- For recent reviews regarding the radical-triggered 1,5-HAT reactions, see: (a) J. Robertson, J. Pillai and R. K. Lush, Chem. Soc. Rev., 2001, 30, 94–103 RSC; (b) Ž. Čeković, Tetrahedron, 2003, 59, 8073–8090 CrossRef; (c) S. Chiba and H. Chen, Org. Biomol. Chem., 2014, 12, 4051–4060 RSC; (d) M. Yan, J. C. Lo, J. T. Edwards and P. S. Baran, J. Am. Chem. Soc., 2016, 138, 12692–12714 CrossRef CAS PubMed; (e) L. Capaldo and D. Ravelli, Eur. J. Org. Chem., 2017, 2056–2071 CrossRef CAS PubMed; (f) X.-Q. Hu, J.-R. Chen and W.-J. Xiao, Angew. Chem., Int. Ed., 2017, 56, 1960–1962 CrossRef CAS PubMed; (g) L. M. Stateman, K. M. Nakafuku and D. A. Nagib, Synthesis, 2018, 50, 1569–1586 CrossRef CAS PubMed; (h) X. Wu and C. Zhu, Chem. Commun., 2019, 55, 9747–9756 RSC; (i) G. Kumar, S. Pradhan and I. Chatterjee, Chem.–Asian J., 2020, 15, 651–672 CrossRef CAS PubMed; (j) D.-Q. Dong, J.-C. Song, S.-H. Yang, Q.-X. Qin, Z.-L. Wang, E.-X. Zhang, Y.-Y. Sun, Q.-Q. Han and S. Yue, Chin. Chem. Lett., 2022, 33, 1199–1206 CrossRef CAS.
- For recent examples regarding the radical-triggered 1,5-HAT reactions, see: (a) Y.-F. Wang, H. Chen, X. Zhu and S. Chiba, J. Am. Chem. Soc., 2012, 134, 11980–11983 CrossRef CAS PubMed; (b) P. Yu, J.-S. Lin, L. Li, S.-C. Zheng, Y.-P. Xiong, L.-J. Zhao, B. Tan and X.-Y. Liu, Angew. Chem., Int. Ed., 2014, 53, 11890–11894 CrossRef CAS PubMed; (c) G. J. Choi, Q. Zhu, D. C. Miller, C. J. Gu and R. R. Knowles, Nature, 2016, 539, 268–271 CrossRef CAS PubMed; (d) J. C. K. Chu and T. Rovis, Nature, 2016, 539, 272–275 CrossRef PubMed; (e) J. Zhang, Y. Li, F. Zhang, C. Hu and Y. Chen, Angew. Chem., Int. Ed., 2016, 55, 1872–1875 CrossRef CAS PubMed; (f) C. Wang, K. Harms and E. Meggers, Angew. Chem., Int. Ed., 2016, 55, 13495–13498 CrossRef CAS PubMed; (g) E. A. Wappes, K. M. Nakafuku and D. A. Nagib, J. Am. Chem. Soc., 2017, 139, 10204–10207 CrossRef CAS PubMed; (h) W. Yuan, Z. Zhou, L. Gong and E. Meggers, Chem. Commun., 2017, 53, 8964–8967 RSC; (i) A. Hu, J.-J. Guo, H. Pan, H. Tang, Z. Gao and Z. Zuo, J. Am. Chem. Soc., 2018, 140, 1612–1616 CrossRef CAS PubMed; (j) Y. Xia, L. Wang and A. Studer, Angew. Chem., Int. Ed., 2018, 57, 12940–12944 CrossRef CAS; (k) H. Guan, S. Sun, Y. Mao, L. Chen, R. Lu, J. Huang and L. Liu, Angew. Chem., Int. Ed., 2018, 57, 11413–11417 CrossRef CAS PubMed; (l) S. P. Morcillo, E. M. Dauncey, J. H. Kim, J. J. Douglas, N. S. Sheikh and D. Leonori, Angew. Chem., Int. Ed., 2018, 57, 12945–12949 CrossRef CAS; (m) X.-X. Wu, H. Zhang, N.-N. Tang, Z. Wu, D.-P. Wang, M.-S. Ji, Y. Xu, M. Wang and C. Zhu, Nat. Commun., 2018, 9, 3343 CrossRef PubMed; (n) Z. Li, Q. Wang and J. Zhu, Angew. Chem., Int. Ed., 2018, 57, 13288–13292 CrossRef CAS PubMed; (o) D. Kurandina, D. Yadagiri, M. Rivas, A. Kavun, P. Chuentragool, K. Hayama and V. Gevorgyan, J. Am. Chem. Soc., 2019, 141, 8104–8109 CrossRef CAS PubMed; (p) S. Wu, X. Wu, D. Wang and C. Zhu, Angew. Chem., Int. Ed., 2019, 58, 1499–1503 CrossRef CAS PubMed; (q) K. Wu, L. Wang, S. Colón-Rodríguez, G.-U. Flechsig and T. Wang, Angew. Chem., Int. Ed., 2019, 58, 1774–1778 CrossRef CAS PubMed.
- CCDC 2048559 (3a) contains supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre.
- Please see the ESI† for more details.
- (a) A. R. Forrester, M. Gill, C. J. Meyer, J. S. Sadd and R. H. Thomson, J. Chem. Soc., Perkin Trans. 1, 1979, 606–611 RSC; (b) A. R. Forrester, M. Gill, J. S. Sadd and R. H. Thomson, J. Chem. Soc., Perkin Trans. 1, 1979, 612–615 RSC.
- (a) M. S. Kharasch and G. Sosnovsky, J. Am. Chem. Soc., 1958, 80, 756 CrossRef CAS; (b) J. Eames and M. Watkinson, Angew. Chem., Int. Ed., 2001, 40, 3567–3571 CrossRef CAS; (c) M. B. Andrus and J. S. Lashley, Tetrahedron, 2002, 58, 845–866 CrossRef CAS; (d) S. Wertz, D. Leifert and A. Studer, Org. Lett., 2013, 15, 928–931 CrossRef CAS PubMed; (e) K. Foo, E. Sella, I. Thomé, M. D. Eastgate and P. S. Baran, J. Am. Chem. Soc., 2014, 136, 5279–5282 CrossRef CAS PubMed; (f) W. Jian, L. Ge, Y. Jiao, B. Qian and H. Bao, Angew. Chem., Int. Ed., 2017, 56, 3650–3654 CrossRef CAS PubMed; (g) B. Qian, S. Chen, T. Wang, X. Zhang and H. Bao, J. Am. Chem. Soc., 2017, 139, 13076–13082 CrossRef CAS PubMed; (h) Y. Li, L. Ge, M. T. Muhammad and H. Bao, Synthesis, 2017, 49, 5263–5284 CrossRef CAS.
- Pd(PPh3)Cl2 (99.9%) was purchased from Strem Chemicals.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 Search PubMed.
- Y. Lan, Computational Methods in Organometallic Catalysis, Wiley-VCH, Germany, 2021 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: All experimental data, and detailed experimental procedures. CCDC 2048559. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2sc01548j |
| This journal is © The Royal Society of Chemistry 2022 |