Open Access Article
Open Access ArticleElectrochemical synthesis of N,N′-disubstituted indazolin-3-ones via an intramolecular anodic dehydrogenative N–N coupling reaction†
Jessica C.
Bieniek
,
Michele
Grünewald
,
Johannes
Winter
,
Dieter
Schollmeyer
and
Siegfried R.
Waldvogel
 *
*
Department of Chemistry, Johannes Gutenberg University, Duesbergweg 10–14, Mainz 55128, Germany. E-mail: waldvogel@uni-mainz.de; Web: https://www.aksw.uni-mainz.de/
First published on 13th June 2022
Abstract
The use of electricity as a traceless oxidant enables a sustainable and novel approach to N,N′-disubstituted indazolin-3-ones by an intramolecular anodic dehydrogenative N–N coupling reaction. This method is characterized by mild reaction conditions, an easy experimental setup, excellent scalability, and a high atom economy. It was used to synthesize various indazolin-3-one derivatives in yields up to 78%, applying inexpensive and sustainable electrode materials and a low supporting electrolyte concentration. Mechanistic studies, based on cyclic voltammetry experiments, revealed a biradical pathway. Furthermore, the access to single 2-aryl substituted indazolin-3-ones by cleavage of the protecting group could be demonstrated.
Introduction
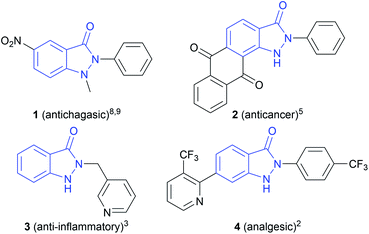
N-Heterocyclic structures play an important role in medicinal chemistry, as they are often found as core motifs in pharmaceuticals.1 Especially, indazolin-3-ones are attracting particular attention, showing a broad range of biological activities, such as analgesic,2 anti-inflammatory,3 antidiabetic,4 anticancer,5 antihyperlipidemic,6 antipsychotic,7 antichagasic,8,9 and antihypertensive10 properties. The high activity of the N,N′-disubstituted indazolin-3-one derivative 1 (Scheme 1) against the parasite Trypanosoma cruzi, which causes Chagas disease, is particularly noteworthy. Chagas disease is widespread in Latin America and only two approved drugs with strong side effects exist so far.8,9 Therefore, a direct and easy synthetic access to indazolin-3-one derivatives is of great interest.Conventionally, indazolin-3-ones can be synthesized by N–C bond formation, starting from hydrazino aryl compounds,11–17via N–N bond formation, starting from o-azidobenzamides,18 or by oxidative or reductive intramolecular N–N bond formation in o-aminobenzamides19–22 or o-nitrobenzamides,23–25 respectively. However, these methods involve harsh reaction conditions, such as high temperatures, strong bases or acids and the use of highly toxic and carcinogenic hydrazine or azide precursors containing leaving groups.11–18 Further, dangerous oxidizing and reducing agents in stoichiometric amounts or transition metal catalysts are necessary.19–26 This leads to a high amount of reagent waste, a poor atom economy and safety hazards.
Recently, various photochemical methods for the synthesis of indazolin-3-ones were published (Scheme 2).27–30 Despite the mild reaction conditions, one of the two reactants has to be used in excess, leading to increased reagent waste. Moreover, only very few 2-aryl substituted indazolin-3-one derivatives have been reported, and in most cases only poor yields were obtained.27–30
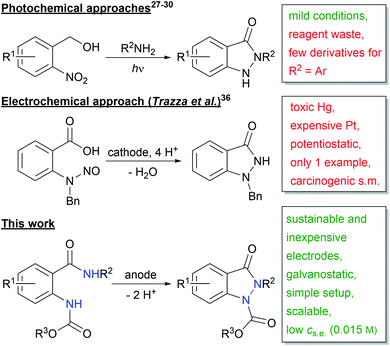 | ||
| Scheme 2 Photochemical and electrochemical approaches to indazolin-3-ones (Ar = aryl; s.m. = starting material; s.e. = supporting electrolyte). | ||
Another powerful and broadly applicable synthetic tool for the synthesis of N-heterocyclic structures is electro-organic chemistry.31 The direct use of inexpensive electric current as the redox reactant enables the replacement of polluting and dangerous oxidizing and reducing agents, diminishing reagent waste,32 increasing work safety, and lowering costs.33 For these reasons, electrochemistry can be considered a green technology.34,35
So far, the only electrochemical approach to indazolin-3-ones is the one published by Trazza et al. in 1995 (Scheme 2).36 However, they used highly toxic mercury and cost-intensive platinum electrodes, a carcinogenic nitrosamine precursor, a laborious potentiostatic reaction setup and additionally, their method was only applicable to one substrate.
Therefore, we herein report the first broadly applicable and sustainable electrochemical method for the synthesis of N,N′-disubstituted indazolin-3-ones, starting from readily available 2-(alkoxycarbonylamido)benzamide precursors, with mild reaction conditions, an easy experimental setup, sustainable and inexpensive electrode materials, low supporting electrolyte concentration and in high atom economy.
Results and discussion
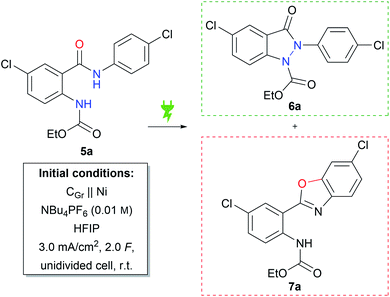
From earlier studies by the Waldvogel group it is known that the electrochemical generation of amidyl intermediates is a powerful tool for N–N and N–C coupling reactions to form various N-heterocycles.37–41 In order to avoid the use of toxic hydrazines, retrosynthetic considerations led to the idea of constructing the indazolin-3-one scaffold by intramolecular N–N bond formation, starting from amide bearing precursors. Compound 5a was chosen as the test substrate, which could be readily prepared in two reaction steps from 5-chloroanthranilic acid, first protecting the amino group with ethyl chloroformate, followed by an amide coupling reaction with 4-chloroaniline.42 Next, the previously published reaction conditions for the synthesis of phthalazin-1,4-diones39 were applied to substrate 5a, using nickel as the cathode material instead of platinum (Table 1, initial conditions). Gratifyingly, the desired indazolin-3-one 6a could be isolated in 58% yield, while benzoxazole 7a was formed as a side product in 11% yield. The molecular structure of 6a was confirmed by X-ray analysis (CCDC: 2148238).| Entry | Deviation from initial conditions | 6a (%) | 7a (%) | Conversionb,c (%) |
|---|---|---|---|---|
| a Substrate 5a (0.2 mmol), undivided 5 mL Teflon™ screening cell. CGr = isostatic graphite; CGC = glassy carbon; HFIP = 1,1,1,3,3,3-hexafluoro-2-propanol. b Yields determined by 1H NMR spectroscopy using 1,3,5-trimethoxybenzene as the internal standard. c Conversion based on the remaining starting material. d CGr cathode. | ||||
| 1 | None | 59 | 16 | 93 |
| 2 | CGC‖Ni | 59 | 16 | 92 |
| 3 | CGr‖Pt | 59 | 16 | 91 |
| 4 | C Gr ‖C Gr | 54 | 14 | 85 |
| 5 | HFIP/MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)d 1)d |
10 | 0 | 30 |
| 6 | NBu4BF4 (0.01 M)d | 52 | 12 | 83 |
| 7 | NMe4PF6 (0.01 M)d | 53 | 14 | 85 |
| 8 | NEt4PF6 (0.01 M)d | 54 | 14 | 85 |
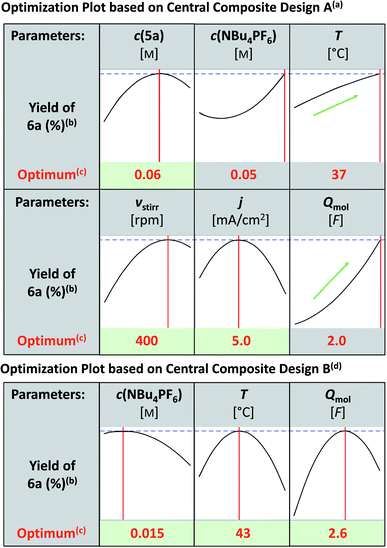
Taking this as the starting point, the reaction conditions were optimized. For detailed information, see the ESI.† In all optimization experiments the yields of 6a, 7a and the remaining starting material 5a were determined by 1H NMR spectroscopy using 1,3,5-trimethoxybenzene as the internal standard. First, different setups (undivided, quasi-divided, and divided) were evaluated. The undivided setup led to the best indazolin-3-one yield, so all further optimization experiments were conducted in undivided 5 mL Teflon™ screening cells.43 Next, various electrode materials were tested:
Regarding the anode material, isostatic graphite (CGr) and glassy carbon (CGC) resulted in the same yield of 59% (Table 1, entries 1 and 2). Due to cost reasons, isostatic graphite was further used as the anode material. With regard to the cathode materials, isostatic graphite, platinum and nickel turned out to be superior to other electrode materials and gave comparable yields of 54%, 59% and 59%, respectively (Table 1, entries 1, 3, and 4). However, isostatic graphite showed higher amounts of remaining starting material and was therefore most promising for subsequent optimization with respect to theoretically accessible yield. Additionally, the health hazards of nickel, as well as the limited availability and expected rising costs of both nickel and platinum led to the use of isostatic graphite as the cathode material. Interestingly, no other tested solvent besides 1,1,1,3,3,3-hexafluoro-2-propanole (HFIP) yielded the desired indazolin-3-one 6a in significant amounts, highlighting the outstanding solvation property of HFIP to stabilize radicals and intermediates.44 Variation of the supporting electrolyte showed that only NBu4BF4, NMe4PF6, NEt4PF6 and NBu4PF6 resulted in comparable indazolin-3-one yields (Table 1, entries 1, 6–8). Due to cost reasons NBu4PF6 was further used. Additives such as water, acetic acid and triethylamine decreased the yield of 6a. Optimization of continuous parameters, such as the concentrations of the starting material and supporting electrolyte, reaction temperature, stirring velocity, current density and applied charge, was performed by applying the statistics-based method Design of Experiments (DoE).35 This method enables the systematic execution of optimization experiments, providing evidence about main effects and interactions of the tested parameters, and has proven to be extremely effective for the optimization of reaction conditions.45 First, all the above mentioned six parameters were investigated by employing a central composite design based on a 26–2 fractional factorial design (Fig. 1). The modelled prediction of the optimum conditions revealed a clear yield maximum of 6a at a substrate concentration of 0.06 M, a stirring velocity of 400 rpm and a current density of 5.0 mA cm−2, while the optima of the temperature and applied charge were expected outside the investigated area. The curve profile of the supporting electrolyte concentration was physically questionable, which is why a second central composite design based on a full 23 factorial design was performed to investigate the influence of supporting electrolyte concentration, temperature and applied charge. This revealed the optimum reaction conditions at a supporting electrolyte concentration of 0.015 M, a reaction temperature of 43 °C and an applied charge of 2.6 F, yielding indazolin-3-one 6a in 65% isolated yield at full conversion with 17% byproduct (7a) formation. Thus, the product yield was increased by 7% and the reaction conditions were optimized in terms of sustainability by replacing nickel with isostatic graphite as the cathode material.
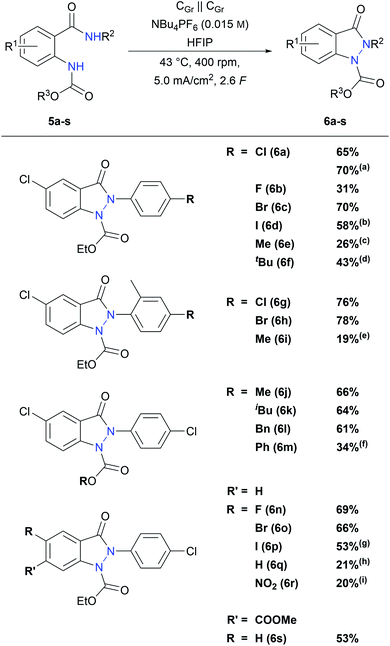
With these optimized conditions in hand, the reaction scope was examined. First, different anilide substituents were tested. As shown in Scheme 3, all p-halogen bearing anilides 5a–d led to the desired indazolin-3-ones 6a–d in moderate to very good yields. Especially, those with medium electron density on the aniline moiety (6a and 6c) show the best results with isolated yields up to 70%, while substrate 6b with the electron withdrawing p-fluoro substituent afforded a lower yield of 31%. Interestingly, indazolin-3-one 6d bearing an electrochemically poorly stable iodine substituent could be isolated in 58% yield, which enables further functionalization on the phenyl ring. Substrates 5g and 5h with an additional methyl group in the o-position resulted in enhanced indazolin-3-one yields of 76% and 78%, respectively. Furthermore, p-alkyl substituents on the anilide moiety were tolerated as well, and 6e, 6f and 6i could be isolated in yields up to 43%. This is in line with previous publications, where p-alkyl anilide precursors led to lower yields due to the possibility of side reactions at the benzylic position.40,41 Precursors with an unsubstituted anilide, an m-chloro substituted anilide, a tert-butyl amide or an unsubstituted amide moiety resulted in complex product mixtures with no or only traces of the indazolin-3-one product, indicating that p-substitution at the aniline ring is necessary for a successful indazolin-3-one formation. Electron withdrawing substituents on the anilide moiety, such as ester or nitro groups, led to complex product mixtures as well, whereas the electron rich p-methoxy substituted precursor was converted selectively to the corresponding benzoxazole byproduct in 65% 1H NMR yield.
Next, various substitutions on the carbamate moiety were investigated. Substrates with alkyl and benzyl substituents yielded indazolin-3-ones 6j, 6k and 6l in 66%, 64% and 61%, respectively, showing that sterically demanding carbamate substituents are perfectly tolerated as well. Moreover, indazolin-3-one 6m with a phenyl group on the carbamate moiety could be isolated in 34% yield. Precursors with a Boc or a tosyl protecting group could not be converted into the desired indazolin-3-ones. Substrates with an unprotected amine led to polymeric products, which is consistent with previous studies.46
Finally, derivatives with different anthranilic cores were studied. All halogen core substituted indazolin-3-ones 6n–p were obtained in good yields between 53% and 69%, while the yield of 6n with an electron withdrawing 5-fluoro substituent was even superior to that of the model derivative 6a. Indazolin-3-one 6q with an unsubstituted anthranilic core could be isolated in 21% yield as well. Interestingly, the redox sensitive nitro substituent was tolerated in the reaction and derivative 6r could be obtained in 20% yield, by applying quasi-divided reaction conditions. Furthermore, substrate 5s with an ester substituent in the m-position to the protected amino group could be converted to indazolin-3-one 6s in a good yield of 53%, displaying the broad substitution variability on the anthranilic core regarding functional groups and substitution patterns.
The excellent scalability of the reaction could be demonstrated by performing the electrolysis of test substrate 5a on a 10-fold scale (3.0 mmol of substrate, 60 mL beaker-type glass cell), yielding indazolin-3-one 6a in 70%, which is even superior to that of the small-scale reaction.
After having investigated the scope of the reaction, cyclic voltammetry (CV) studies were performed to get insights into the reaction mechanism. Detailed information about the interpretation of the cyclic voltammograms is provided in the ESI.† The proposed mechanism is shown in Scheme 4. First, direct oxidation of starting material 5 at the anode, followed by subsequent deprotonation, leads to intermediate I, bearing an amidyl radical, which might be stabilized by the π system of the anilide moiety and solvent HFIP. Next, the carbamate group of I is oxidized, succeeded by another deprotonation, leading to the biradical species II. Finally, the N–N bond is formed by recombination of the radicals, yielding indazolin-3-one 6. Concurrent to this mechanism, a twofold oxidation at the amide group is possible, resulting in the cationic species III. However, the generation of the amidyl cation III strongly depends on the electron density at the amide group, which is mainly influenced by the aryl ring attached to the amide nitrogen atom, leading to benzoxazole formation.38 As the counter reaction, hydrogen is generated at the cathode.
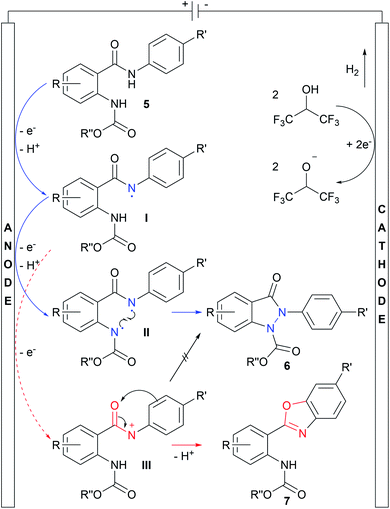 | ||
| Scheme 4 Proposed mechanism for the electrochemical synthesis of indazolin-3-ones based on CV studies and scope. Blue pathway: indazolin-3-one formation via biradical N–N bond linkage. Red pathway: benzoxazole formation as a side reaction through the previously reported cationic mechanism.38 Deprotonation steps occur via solvent molecules, releasing the protons at the cathode, resulting in hydrogen formation as the counter reaction. | ||
An experiment to cleave the alkoxycarbonyl protecting group of indazolin-3-one 6a was carried out, making single-substituted indazolin-3-ones with a 2-aryl substituent easily accessible (Scheme 5). Due to the complex conventional synthesis methods for introducing aryl substituents at the 2-position of indazolin-3-ones, 2-aryl bearing indazolin-3-ones have not yet been sufficiently studied regarding their biological activities. However, they could represent interesting leitmotifs for possible biologically active substances, highlighting the importance of the herein reported electrochemical synthesis, making 2-aryl substituted indazolin-3-ones easily accessible.
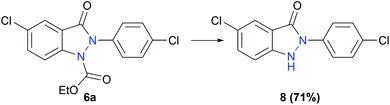 | ||
| Scheme 5 Deprotection of 6a yielding single substituted indazolin-3-one 8. Reaction conditions: NaOH, EtOH, 80 °C, 3 h. | ||
Conclusions
In summary, a direct, easy, and sustainable electrochemical synthesis of N,N′-disubstituted 2-aryl indazolin-3-ones with mild reaction conditions and broad applicability has been reported. The final reaction protocol was optimized by DoE and is characterized by its easy experimental setup, sustainable, readily available and inexpensive isostatic graphite electrodes, HFIP as a recyclable solvent and a low supporting electrolyte concentration. The broad applicability of this method could be demonstrated by synthesizing 19 indazolin-3-one derivatives in yields up to 78% and by the excellent scalability of the reaction. CV studies revealed that the reaction presumably proceeds via a biradical mechanism. Finally, deblocking of an electrochemically synthesized indazolin-3-one derivative demonstrated that single substituted 2-aryl indazolin-3-ones are easily accessible as well.Data availability
The ESI† is available and contains all experimental and analytical data.Author contributions
J. B., M. G. and J. W. designed and carried out the batch electrolysis experiments and analysed the data. S. W. supervised the project. J. B. and S. W. wrote the manuscript. D. S. performed the X-ray analysis and structural elucidation of the synthesized model derivative. All authors discussed the results and agreed to the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the Fonds der Chemischen Industrie (Kekulé Fellowship to J. C. Bieniek) is gratefully acknowledged. This work was supported by the Max Planck Graduate Center with the Johannes Gutenberg-Universität Mainz (MPGC Fellowship to J. C. Bieniek). We acknowledge the Deutsche Forschungsgemeinschaft (Wa1276/17-2) for financial support.References
- E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257 CrossRef CAS PubMed.
- S. R. Fletcher, E. McIver, S. Lewis, F. Burkamp, C. Leech, G. Mason, S. Boyce, D. Morrison, G. Richards, K. Sutton and A. B. Jones, Bioorg. Med. Chem. Lett., 2006, 16, 2872 CrossRef CAS PubMed.
- E. Tse, L. Butner, Y. Huang and I. H. Hall, Arch. Pharm. Pharm. Med. Chem., 1996, 329, 35 CrossRef CAS PubMed; K. A. M. Abouzid and H. S. El-Abhar, Arch. Pharmacal Res., 2003, 26, 1 CrossRef; P. Bruneau, C. Delvare, M. P. Edwards and R. M. McMillan, J. Med. Chem., 1991, 34, 1028 CrossRef PubMed; A. Roth, S. Ott, K. M. Farber, T. A. Palazzo, W. E. Conrad, M. J. Haddadin, D. J. Tantillo, C. E. Cross, J. P. Eiserich and M. J. Kurth, Bioorg. Med. Chem., 2014, 22, 6422 CrossRef PubMed; W. Yu, Z. Guo, P. Orth, V. Madison, L. Chen, C. Dai, R. J. Feltz, V. M. Girijavallabhan, S. H. Kim, J. A. Kozlowski, B. J. Lavey, D. Li, D. Lundell, X. Niu, J. J. Piwinski, J. Popovici-Muller, R. Rizvi, K. E. Rosner, B. B. Shankar, N.-Y. Shih, M. A. Siddiqui, J. Sun, L. Tong, S. Umland, M. K. C. Wong, D. Yang and G. Zhou, Bioorg. Med. Chem. Lett., 2010, 20, 1877 CrossRef.
- Y. Qian, D. Bolin, K. Conde-Knape, P. Gillespie, S. Hayden, K.-S. Huang, A. R. Olivier, T. Sato, Q. Xiang, W. Yun and X. Zhang, Bioorg. Med. Chem. Lett., 2013, 23, 2936 CrossRef CAS PubMed.
- N. Kawanishi, T. Sugimoto, J. Shibata, K. Nakamura, K. Masutani, M. Ikuta and H. Hirai, Bioorg. Med. Chem. Lett., 2006, 16, 5122 CrossRef CAS PubMed; H. Wang, H. Han and D. D. von Hoff, Cancer Res., 2006, 66, 9722 CrossRef PubMed.
- S. D. Wyrick, P. J. Voorstad, G. Cocolas and I. H. Hall, J. Med. Chem., 1984, 27, 768 CrossRef CAS PubMed.
- M. H. Norman, G. C. Rigdon, F. Navas III and B. R. Cooper, J. Med. Chem., 1994, 37, 2552 CrossRef CAS.
- A. Montero-Torres, M. C. Vega, Y. Marrero-Ponce, M. Rolón, A. Gómez-Barrio, J. A. Escario, V. J. Arán, A. R. Martínez-Fernández and A. Meneses-Marcel, Bioorg. Med. Chem., 2005, 13, 6264 CrossRef CAS PubMed.
- M. C. Vega, M. Rolón, A. Montero-Torres, C. Fonseca-Berzal, J. A. Escario, A. Gómez-Barrio, J. Gálvez, Y. Marrero-Ponce and V. J. Arán, Eur. J. Med. Chem., 2012, 58, 214 CrossRef CAS PubMed.
- A. Cappelli, C. Nannicini, A. Gallelli, G. Giuliani, S. Valenti, G. P. La Mohr, M. Anzini, L. Mennuni, F. Ferrari, G. Caselli, A. Giordani, W. Peris, F. Makovec, G. Giorgi and S. Vomero, J. Med. Chem., 2008, 51, 2137 CrossRef CAS PubMed.
- D. H. R. Barton, G. Lukacs and D. Wagle, J. Chem. Soc., Chem. Commun., 1982, 450 RSC.
- C. Stiff, D. R. Graber, A. Thorarensen, B. D. Wakefield, K. R. Marotti, E. P. Melchior, M. T. Sweeney, F. Han, D. C. Rohrer, G. E. Zurenko and D. L. Romero, Bioorg. Med. Chem. Lett., 2008, 18, 6293 CrossRef CAS PubMed.
- E. B. Elkaeed, J. An and A. M. Beauchemin, J. Org. Chem., 2017, 82, 9890 CrossRef CAS PubMed.
- N. Gu, S. Sun and J. Cheng, Tetrahedron Lett., 2018, 59, 1069 CrossRef CAS.
- S. Tanimori, Y. Kobayashi, Y. Iesaki, Y. Ozaki and M. Kirihata, Org. Biomol. Chem., 2012, 10, 1381 RSC.
- M. Tsujii, M. Sonoda and S. Tanimori, J. Org. Chem., 2016, 81, 6766 CrossRef CAS PubMed.
- R. C. Wheeler, E. Baxter, I. B. Campbell and S. J. F. Macdonald, Org. Process Res. Dev., 2011, 15, 565 CrossRef CAS.
- A. Y. Shaw, Y.-R. Chen and C.-H. Tsai, Synth. Commun., 2009, 39, 2647 CrossRef CAS.
- A. Correa, I. Tellitu, E. Dominguez and R. SanMartin, J. Org. Chem., 2006, 71, 3501 CrossRef CAS.
- A. Correa, I. Tellitu, E. Domínguez and R. SanMartin, Tetrahedron, 2006, 62, 11100 CrossRef CAS.
- S. W. Park, H. Choi, J.-H. Lee, Y.-J. Lee, J.-M. Ku, S. Y. Lee and T.-G. Nam, Arch. Pharmacal Res., 2016, 39, 302 CrossRef CAS.
- K. Govindan, T. Duraisamy, A. Jayaram, G. C. Senadi and W.-Y. Lin, Synthesis, 2022, 54, 1115 CrossRef CAS.
- Y. Bao, Z. Deng, J. Feng, W. Zhu, J. Li, J. Wan and G. Liu, Org. Lett., 2020, 22, 6277 CrossRef CAS.
- C. W. Bird, J. C. W. Chng, N. H. Rama and A. Saeed, Synth. Commun., 1991, 21, 545 CrossRef CAS.
- P. Bruneau, C. Delvare, M. P. Edwards and R. M. McMillan, J. Med. Chem., 1991, 34, 1028 CrossRef CAS PubMed.
- G. Dai, L. Yang and W. Zhou, Org. Chem. Front., 2017, 4, 229 RSC; S. Liu, L. Xu and Y. Wei, J. Org. Chem., 2019, 84, 1596 CrossRef CAS PubMed.
- N. Kraemer, C. J. Li, J. S. Zhu, J. M. Larach, K. Y. Tsui, D. J. Tantillo, M. J. Haddadin and M. J. Kurth, Org. Lett., 2019, 21, 6058 CrossRef CAS.
- J. S. Zhu, N. Kraemer, C. J. Li, M. J. Haddadin and M. J. Kurth, J. Org. Chem., 2018, 83, 15493 CrossRef CAS.
- T. Yang, H. Lu, R. Qiu, L. Hong, S.-F. Yin and N. Kambe, Chem.–Asian J., 2019, 14, 1436 CrossRef CAS PubMed.
- H.-J. Nie, A.-D. Guo, H.-X. Lin and X.-H. Chen, RSC Adv., 2019, 9, 13249 RSC.
- Y. Jiang, K. Xu and C. Zeng, Chem. Rev., 2018, 118, 4485 CrossRef CAS; R. Francke, Beilstein J. Org. Chem, 2014, 10, 2858 CrossRef PubMed.
- S. Möhle, M. Zirbes, E. Rodrigo, T. Gieshoff, A. Wiebe and S. R. Waldvogel, Angew. Chem., Int. Ed., 2018, 57, 6018 CrossRef CAS PubMed; A. Wiebe, T. Gieshoff, S. Möhle, E. Rodrigo, M. Zirbes and S. R. Waldvogel, Angew. Chem., Int. Ed., 2018, 57, 5594 CrossRef PubMed.
- J. Seidler, J. Strugatchi, T. Gärtner and S. R. Waldvogel, MRS Energy Sustain., 2020, 7, 42 CrossRef.
- B. A. Frontana-Uribe, R. D. Little, J. G. Ibanez, A. Palma and R. Vasquez-Medrano, Green Chem., 2010, 12, 2099 RSC; D. Pollok and S. R. Waldvogel, Chem. Sci., 2020, 11, 12386 RSC; J. L. Röckl, D. Pollok, R. Franke and S. R. Waldvogel, Acc. Chem. Res., 2020, 53, 45 CrossRef CAS PubMed.
- M. Dörr, M. M. Hielscher, J. Proppe and S. R. Waldvogel, ChemElectroChem, 2021, 8, 2621 CrossRef.
- G. Marrosu, R. Petrucci and A. Trazza, Electrochim. Acta, 1995, 40, 923 CrossRef CAS.
- T. Gieshoff, A. Kehl, D. Schollmeyer, K. D. Moeller and S. R. Waldvogel, Chem. Commun., 2017, 53, 2974 RSC; T. Gieshoff, D. Schollmeyer and S. R. Waldvogel, Angew. Chem., Int. Ed., 2016, 55, 9437 CrossRef CAS; A. Kehl, V. M. Breising, D. Schollmeyer and S. R. Waldvogel, Chem.–Eur. J., 2018, 24, 17230 CrossRef; Y. Yu, J.-S. Zhong, K. Xu, Y. Yuan and K.-Y. Ye, Adv. Synth. Catal., 2020, 362, 2102 CrossRef; P. Zhang, B. Li, L. Niu, L. Wang, G. Zhang, X. Jia, G. Zhang, S. Liu, L. Ma, W. Gao, D. Qin and J. Chen, Adv. Synth. Catal., 2020, 362, 2342 CrossRef.
- T. Gieshoff, A. Kehl, D. Schollmeyer, K. D. Moeller and S. R. Waldvogel, J. Am. Chem. Soc., 2017, 139, 12317 CrossRef CAS.
- A. Kehl, T. Gieshoff, D. Schollmeyer and S. R. Waldvogel, Chem.–Eur. J., 2018, 24, 590 CrossRef CAS PubMed.
- A. Kehl, N. Schupp, V. M. Breising, D. Schollmeyer and S. R. Waldvogel, Chem.–Eur. J., 2020, 26, 15847 CrossRef CAS PubMed.
- V. M. Breising, J. M. Kayser, A. Kehl, D. Schollmeyer, J. C. Liermann and S. R. Waldvogel, Chem. Commun., 2020, 56, 4348 RSC.
- M. T. Rudd, J. W. Butcher, K. T. Nguyen, C. J. McIntyre, J. J. Romano, K. F. Gilbert, K. J. Bush, N. J. Liverton, M. K. Holloway, S. Harper, M. Ferrara, M. DiFilippo, V. Summa, J. Swestock, J. Fritzen, S. S. Carroll, C. Burlein, J. M. DiMuzio, A. Gates, D. J. Graham, Q. Huang, S. McClain, C. McHale, M. W. Stahlhut, S. Black, R. Chase, A. Soriano, C. M. Fandozzi, A. Taylor, N. Trainor, D. B. Olsen, P. J. Coleman, S. W. Ludmerer and J. A. McCauley, ChemMedChem, 2015, 10, 727 CrossRef CAS; A. Numadate, Y. Mita, Y. Matsumoto, S. Fujii and Y. Hashimoto, Chem. Pharm. Bull., 2014, 62, 979 CrossRef.
- C. Gütz, B. Klöckner and S. R. Waldvogel, Org. Process Res. Dev., 2016, 20, 26 CrossRef.
- J. L. Röckl, D. Pollok, R. Franke and S. R. Waldvogel, Acc. Chem. Res., 2020, 53, 45 CrossRef CAS PubMed; B. Elsler, A. Wiebe, D. Schollmeyer, K. M. Dyballa, R. Franke and S. R. Waldvogel, Chem.–Eur. J., 2015, 21, 12321 CrossRef; O. Hollóczki, A. Berkessel, J. Mars, M. Mezger, A. Wiebe, S. R. Waldvogel and B. Kirchner, ACS Catal., 2017, 7, 1846 CrossRef; O. Hollóczki, R. Macchieraldo, B. Gleede, S. R. Waldvogel and B. Kirchner, J. Phys. Chem. Lett., 2019, 10, 1192 CrossRef PubMed; A. Kirste, S. Hayashi, G. Schnakenburg, I. M. Malkowsky, F. Stecker, A. Fischer, T. Fuchigami and S. R. Waldvogel, Chem.–Eur. J., 2011, 17, 14164 CrossRef PubMed; S. Lips, A. Wiebe, B. Elsler, D. Schollmeyer, K. M. Dyballa, R. Franke and S. R. Waldvogel, Angew. Chem., Int. Ed., 2016, 55, 10872 CrossRef PubMed.
- M. Hielscher, E. K. Oehl, B. Gleede, J. Buchholz and S. R. Waldvogel, ChemElectroChem, 2021, 8, 3904 CrossRef CAS; M. Dörr, J. L. Röckl, J. Rein, D. Schollmeyer and S. R. Waldvogel, Chem.–Eur. J., 2020, 26, 10195 CrossRef PubMed; M. M. Hielscher, B. Gleede and S. R. Waldvogel, Electrochim. Acta, 2021, 368, 137420 CrossRef.
- L. Schulz, M. Enders, B. Elsler, D. Schollmeyer, K. M. Dyballa, R. Franke and S. R. Waldvogel, Angew. Chem., Int. Ed., 2017, 56, 4877 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2148238. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2sc01827f |
| This journal is © The Royal Society of Chemistry 2022 |