Open Access Article
Open Access ArticleControl of photoluminescence quantum yield and long-lived triplet emission lifetime in organic alloys†
Zhen
Xu‡
 ,
Duane
Hean‡
,
Duane
Hean‡
 ,
Jennifer
Yuan
,
Jennifer
Yuan
 and
Michael O.
Wolf
and
Michael O.
Wolf
 *
*
Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, BC V6T 1Z1, Canada. E-mail: mwolf@chem.ubc.ca
First published on 30th May 2022
Abstract
Two-component crystalline organic alloys with a wide range of compositional ratios (from 30% to 90% of one component) are employed to tune excited-state lifetimes and photoluminescence quantum yields (PLQYs). Alloy crystals exhibit homogeneous distribution of parent compounds by X-ray crystallography and differential scanning calorimetry. The alloys display a 1.5- to 5-fold enhancement in thermally activated delayed fluorescence (TADF) lifetime, compared to the parent compounds. PLQYs can also be tuned by changing alloy composition. The reverse intersystem crossing and long-lived lifetime of the parent compounds give rise to long-lived TADF in the alloys. Organic alloys enable tunability of both lifetime and efficiency, providing a new perspective on the development of organic long-lived emissive materials beyond the rules established for host–guest doped systems.
Introduction
Organic luminescent materials in which triplet excitons are effectively utilized are desired for numerous applications, including organic light-emitting diode (OLED) displays, anti-counterfeiting inks, photodynamic therapy, chemical sensors, and as bioimaging agents.1–7 These materials are alternatives to phosphorescent inorganic complexes and offer advantages including synthetic versatility, lower cost, and reduced toxicity. Controllable variation of the photophysical properties such as photoluminescence quantum yield (PLQY) and lifetime of organic luminescent materials is critical to obtain desired characteristics for specific applications. In this work, we use organic alloys to tune these properties.Organic alloys are homogeneous solid compositions formed by random occupancy of isostructural guest molecules in the crystal lattice of a host compound.8–10 Compositions can range from 0% to 100% of each of the two components, offering broad and continuous control over material properties.11 Organic alloys have been utilized to tune optoelectronic behavior and energy levels of organic semiconductors for applications such as organic photovoltaics (OPVs) and organic field-effect transistors (OFETs),12–15 in addition to adjusting the luminescence properties of organic co-assemblies.9,10,16 Compositional changes are usually limited due to challenges in preparing organic alloys by crystallization.9,12,14,17
In this work, we target two well-known photophysical phenomena, namely room temperature phosphorescence (RTP) and thermally activated delayed fluorescence (TADF) for our initial explorations of organic alloy systems. In RTP,1–4 photoluminescence may persist even after the photoexcitation source has been removed (so-called afterglow) due to its long lifetime (ms to s). TADF occurs when the singlet–triplet energy gap (ΔEST) is small (<0.2 eV), and excitons can repopulate the singlet state from triplet states via endothermic reverse intersystem crossing (RISC).18 TADF has been extensively used in OLEDs,5–7 however it remains challenging to develop TADF emitters with prolonged lifetime and afterglow,19–21 which would offer interesting opportunities to circumvent non-radiative decay of triplets and enable higher energy afterglow (typically RTP emitters phosphoresce above 500 nm).
Crystallization is usually required to stabilize triplet excitons for organic small molecule RTP emitters, thus prolonging triplet lifetime and enhancing efficiency.22–31 Another common approach to tuning triplet utilization via RTP and TADF in organic materials involves incorporating guest dopants in host matrices at low concentrations in order to modify the photophysical properties.32–43 Doped host–guest systems in the form of crystals, powders, or films can provide a rigid environment that promotes triplet emission. The concentrations of dopant guest compounds are relatively low (∼1 wt%), and increasing the amount of guest to above 10% can result in self-quenching and a decrease in phosphorescence efficiency and lifetime.34,38 It is also worth noting that when host and guest have strong charge-transfer interactions, organic long persistent luminescence (OLPL) can occur with hours of afterglow emission.44–46 OLPL differs from TADF and RTP in mechanism.46
Host compounds are typically non-emissive and serve as rigid matrices for triplet emission. Co-crystallization of a small percentage of a phosphorescent guest compound with a structurally similar non-emissive host resulted in 20-fold phosphorescence enhancement compared to the pure guest crystal alone.33 In some cases, interactions between host and guest compounds can lead to charge-separated states to give long-lived phosphorescence.40 Zhang and co-workers recently developed a doped host–guest system in which TADF was employed to realize both long-lived and efficient afterglow.47 The Tang group has developed host–guest systems where two emitters were used to enable a triplet energy trap or exciplex, allowing improved triplet efficiency and lifetime.39,41 Usually not much improvement is observed in lifetime by varying the dopant ratio.48 The limited compositional ratio range of host and guest compounds leads to limited tunability in lifetime and efficiency.28
Using organic alloys to tune TADF or RTP behavior by compositional changes is intriguing, and can potentially provide advantages beyond host–guest systems. Variation of the ratio of crystalline triplet emitters with similar chemical structures in the alloy offers dual tunability of both lifetime and efficiency, a feature that host–guest systems typically fail to offer. In addition, it also helps avoid the self-quenching that is commonly seen in doped host–guest systems at higher concentrations, which enables more compositional tunability than is possible in host–guest systems. The successful design of long-lived triplet luminescent organic alloys requires the two-component compounds to be structurally similar yet have contrasting photophysical properties. Ideally, one component has a higher PLQY and the other a longer emission lifetime, allowing the resulting alloys to potentially exhibit the merits of each compound. If the two compounds are isostructural, alloys can be formed over a large compositional range by recrystallization.
Due to recent progress on molecular design for RTP, we were able to select two reported compounds, (9H-carbazol-9-yl)(p-tolyl)methanone (CBZ-Me), and (4-bromophenyl)(9H-carbazol-9-yl)methanone (CBZ-Br) as the two alloy components (Fig. 1). CBZ-Me is an RTP emitter with 21 ms (@453 nm) and 340 ms (@530 nm) lifetime and a PLQY of 2.00%.26CBZ-Br, on the other hand, has two polymorphs, referred to as CBZ-Br and CBZ-Br-RTP (Fig. S2 and Table S1†).25,49 One polymorph (CBZ-Br) is isostructural with CBZ-Me and shows TADF, with a delayed lifetime of 3.4 μs and a PLQY of 18.0%. The other polymorph (CBZ-Br-RTP) only shows RTP. The difference between CBZ-Me and CBZ-Br in lifetime and PLQY is presumably the result of the heavy atom effect in CBZ-Br, which facilitates RISC, enhancing PLQY and decreasing the delayed lifetime.50 The methyl group, however, can prolong triplet lifetime by facilitating intermolecular interactions.24 In this work, we mix CBZ-Br and CBZ-Me in different ratios to make a series of organic alloys (MexBr10−x, where x = 3 to 10) obtained through recrystallization. This enables fine-tuning of PLQYs and triplet lifetimes and allows dialing in of photophysical properties for specific applications.
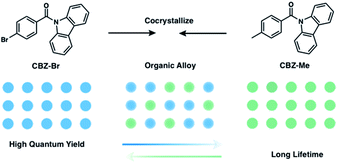 | ||
| Fig. 1 Chemical structures of parent compound CBZ-Me and CBZ-Br and the design principle of tunable organic alloys. | ||
Crystallography
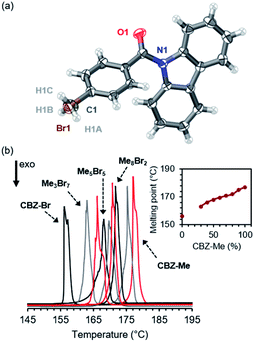
CBZ-Me and CBZ-Br are soluble in acetonitrile across the molar ratio range, allowing the growth of large (∼1 mm × ∼1 mm × ∼1 mm) high-quality alloy crystals (MexBr10−x, where x = 3 to 10) during slow evaporation experiments in this solvent (Fig. S3 and Table S2†). Acetonitrile promoted the crystallization of the CBZ-Br polymorph over the CBZ-Br-RTP polymorph allowing further access across the molar range by isostructural substitution. Attempts to produce organic alloy crystals with compositions containing CBZ-Br percentages higher than 70% were unsuccessful due to CBZ-Br crystallizing as a mixture of the CBZ-Br-RTP polymorph and alloy crystals with CBZ-Me compositions of approximately 30%.Similar to the crystals of the pure compounds CBZ-Me and CBZ-Br, the organic alloys are isostructural and crystallize in the P21/n space group with one molecule in the asymmetric unit. Crystallographically, the organic alloys are observed as substitutional disorder where the molecules occupy the exact fractional coordinates in the lattice (Fig. S4†), and the methyl and bromide groups are refined using a two-part disorder model (Fig. 2(a)). The site occupancy factors obtained from the crystal structures match the stoichiometric ratios determined by solution 1H NMR spectroscopy upon dissolution of the alloy crystals (Fig. S5 and Table S3†). With an increasing percentage of CBZ-Me in the alloy, the unit cell axes remain essentially unchanged with a slight decrease of 0.32° in the β angle tending toward the pure CBZ-Me structure (Fig. S6†). The unit cell volume was also found to decrease with increasing CBZ-Me percentage as the van der Waals volume of the methyl group occupies 1.53 Å3 less volume than the bromide group at the para position.51 Furthermore, short-range order (SRO) defects usually occur in disordered materials giving rise to diffuse scattering in addition to Bragg diffraction peaks.52,53 These organic alloys produce very low-intensity diffuse scattering, indicating that the SRO populations are very low and that the two components are homogenized within the lattice (Fig. S7†).
The homogeneity of the organic alloys was probed by differential scanning calorimetry (DSC) to assess the melting points of the parent compound and crystal alloys (Fig. 2(b)). CBZ-Br (TADF polymorph) melts at 156 °C, and with increasing CBZ-Me composition, the melting point of the crystal alloys increases with pure CBZ-Me melting at 177 °C. The linearity of the melting point range is consistent with the alloys being comprised of a single-phase system with homogenous distribution of the components in the crystals.11
Photophysics
The photophysical properties of the parent compounds CBZ-Me and CBZ-Br were examined in the single-crystal state. Liu and co-workers have raised the issue that carbazole isomers may induce long-lived phosphorescence.40 We did not observe any isomers in our recrystallized 9-H carbazole starting materials as evaluated by HPLC on the redissolved crystals, and the fluorescence spectra match those of the same compounds prepared by Liu and co-workers. The characteristic fine-structured localized phosphorescence of CBZ-Me is no longer observed, and instead, we obtain a broad featureless delayed spectrum, which resembles that of a structurally similar species, (4-chlorophenyl)(9H-carbazol-9-yl)methanone CPhCz (ESI Fig. 6(c), Nat. Mater., 2020, 14, 175–180†).40 This long-lived emission band has a TADF feature at 450 nm with a 38 ms lifetime (Fig. S8†). The delayed lifetime feature is present in more significant amounts at an elevated temperature while the intensity of the emission band below 450 nm gradually increases with increasing temperature. In the low-energy region >500 nm, the long-lived emission shows a much longer lifetime >300 ms, which can be assigned as RTP. The long-lived emission feature of CBZ-Me is present at relatively low amplitude compared to prompt fluorescence.CBZ-Br gives a similar microsecond delayed lifetime to that previously reported for this compound.43 A long-lived TADF feature was also observed with a lifetime of 76.3 ms, confirmed by the spectral overlap between the steady-state emission and the delayed photoluminescence at 1 ms (Fig. S9†). Similarly, in the lower-energy region, the lifetime of CBZ-Br is prolonged to 143 ms. Due to the long lifetimes, the delayed photoluminescence spectrum of this prolonged feature could not be accurately measured. CBZ-Br likely has a ‘hidden’ RTP state that can preserve triplet excitons for long-lived TADF emission and yield a longer lifetime above 500 nm.54
PLQY values of both parent compounds (44.0% for CBZ-Br and 11.8% for CBZ-Me) are greater than reported in the literature, possibly due to sample crystallinity or instrumental differences. We speculate that the lifetime and PLQY differences between CBZ-Me and CBZ-Br result from the heavy atom effect in CBZ-Br, which facilitates RISC and promotes TADF over RTP, enhancing RISC PLQY and resulting in a decrease in RTP lifetime. The methyl group facilitates improved molecular packing, which can prolong the RTP lifetime.24
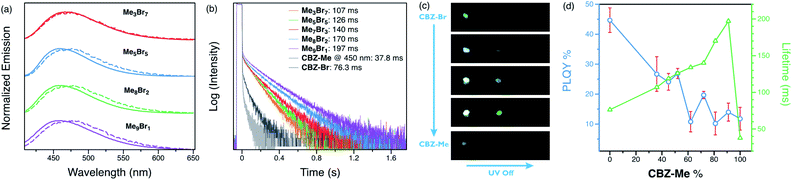
Steady-state and time-gated photoluminescence measurements were carried out on the alloy crystals. The steady-state emission maxima of the alloys are between those of the parent compounds (475 and 453 nm, respectively, for CBZ-Br and CBZ-Me), shifting slightly towards higher energy with increasing CBZ-Me composition (Fig. 3(a) and S10†). The time-gated spectra (1 ms delay) of the alloys are almost identical for all ratios, showing broad emission with maxima of 475 nm. Overall, they overlap well with the steady-state spectra, with slight differences observed in alloys with high CBZ-Me ratios. All the alloy crystals display prompt and delayed emission in their photoluminescence decays (Fig. 3(b)). The alloy crystal with the highest percentage of CBZ-Br, Me3Br7, shows the shortest delayed lifetime of 107 ms, which is still a 1.5-fold and 3-fold enhancement compared to CBZ-Br (76.3 ms), and CBZ-Me (@450 nm) (37.8 ms) delayed lifetimes, respectively. The amplitude of the delayed component is much larger than in the parent compounds. Me9Br1, on the other hand, shows an increase in lifetime (197 ms) by a factor of 2 compared to Me3Br7, while the delayed lifetimes of other alloys lie between Me3Br7 and Me9Br1.
When photoluminescence decays of the alloys were collected at 550 nm at room temperature, tails of longer decays were found with about 10% amplitude (Fig. S19†). The lifetimes of the alloys fall between those of CBZ-Br (143 ms) and CBZ-Me (404 ms) at 550 nm. Due to their low amplitudes and long lifetimes, the time-resolved spectra of these features could not be separated from the dominant delayed emission feature. However, we speculate they might originate from the RTP states of CBZ-Me and CBZ-Br. These results demonstrate that emission lifetime can be enhanced and systematically tuned by changing the compositional ratios of two parent compounds in alloy crystals.
The photoluminescence quantum yields of the alloys fall in the range between the two parent compounds. Generally, higher CBZ-Br content results in higher luminescence efficiency, which is the opposite of the observed trend in lifetimes. For example, Me3Br7 has a PLQY of 26.7%, while Me9Br1 shows only 14.0% efficiency. A digital single-lens camera was used to capture the afterglow of alloy crystals after removing UV excitation (Fig. 3(c)). Even though parent compound CBZ-Br has the highest overall luminescence efficiency and CBZ-Me displays the most extended RTP lifetime, the afterglow from the parent compounds is relatively weak and short, which can be attributed to the short delayed lifetime of CBZ-Br and the faint luminescence of CBZ-Me, in addition to the low amplitudes of long-lived species.
On the other hand, the alloy crystals emit much more intense long-lived emission, and increasing the percentage of CBZ-Me results in a slightly longer afterglow. Hence, it can be concluded that these organic alloys provide a wide range of tunability of both lifetime and efficiency with compositional ratio changes (Fig. 3(d)). It is possible to choose a ratio that results in either more emissive or longer-lived materials, depending on the desired properties for particular applications. Variable-temperature experiments were carried out to help elucidate the origin of the delayed emission. For Me5Br5, at temperatures below 200 K, structured emission peaks at 485 and 517 nm were observed, while emission >460 nm is much weaker in intensity. When the temperature is increased, the fine-structured band decreases dramatically and eventually becomes structureless. The vibronic character and temperature-dependence of the emission confirm that the phosphorescence arises from a locally excited state, not due to carbazole isomers at low temperature (Fig. 4(a) and S13–S18†).21,26,55 A broad emission band peaking at ∼460 nm starts growing with increasing temperature. The photoluminescence decay curves exhibit increasing amplitudes of the delayed features with shortened delayed lifetimes at elevated temperatures (Fig. 3(b)).
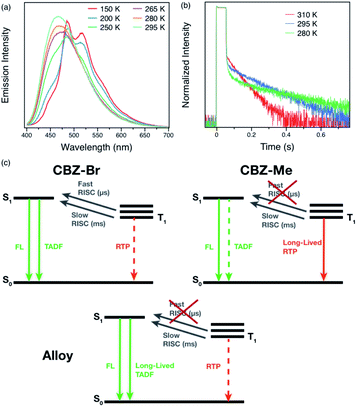 | ||
| Fig. 4 Variable-temperature (a) steady-state photoluminescence spectra and (b) phosphorescence decays of Me5Br5 under helium atmosphere. (c) Jablonski diagrams of parent compounds and Me5Br5. | ||
The excited-state pathways of the parent compounds are quite different from each other (Fig. 4(c)). CBZ-Br displays two RISC channels with distinct timescales (μs and ms), possibly originating from different triplet excited states.50 As a result, TADF is the major radiative pathway besides prompt fluorescence, resulting in a high PLQY. Even though the RTP state exists in CBZ-Br, it is overshadowed by strong TADF, as indicated by the well-overlapped steady-state and delayed spectra.54 On the other hand, CBZ-Me only exhibits slow RISC, and RTP is the dominant triplet radiative pathway, which leads to reduced emission efficiency. However, triplet excited states can function as traps and therefore give a longer lifetime.26 Since the two parent compounds exhibit identical packing modes and singlet–triplet energy gaps (∼0.2 eV), indicated by low-temperature measurements (Fig. S8 and S9†), it can be deduced that the electronic effects of the bromide and methyl groups are the main reason for the differences. The Br group is responsible for facilitating RISC50 while the methyl group results in the long-lived triplet excited state. When the two species are mixed to form alloys, the fast RISC channel is entirely suppressed by methyl involvement (Table S3 and Fig. S11(d)†). However, long-lived triplet states preserve the triplet exciton, followed by slow RISC facilitated by the Br group to emit TADF. Even though the PLQYs, as well as lifetimes above 550 nm, show almost linear changes by compositional variations, the fact that TADF lifetimes increase 1.5-fold (compared to CBZ-Br) to 5-fold (compared to CBZ-Me), proves the existence of interactions between two parent species. Mechanisms such as energy transfer or charge separation and recombination can be ruled out since the two-parent compounds share similar absorption spectra and oxidation potentials (Fig. S21†) due to their similar structure.
Conclusions
In conclusion, CBZ-Me and CBZ-Br have been crystallized together in various ratios to yield seven crystalline organic alloys (MexBr10−x, where x = 3 to 10). This is the first report employing organic alloys as long-lived emissive materials. The alloys exhibit long-lived TADF that gives strong afterglow emission, and TADF lifetimes show 1.5-fold (compared to CBZ-Br) to 5-fold (compared to CBZ-Me) enhancement compared to parent compounds. The compositional change offers broad tunability in both lifetime and PLQYs, which addresses the trade-off between efficiency and lifetime.Organic RTP and TADF materials have been of intense interest over the last few years, and several design principles for small molecules offering these characteristics have been established. With a rich library of emitters in hand, we believe that organic alloys provide an exciting opportunity to utilize existing molecular emitters to achieve better overall luminescence performance, promoting the development of RTP and TADF materials.
In addition, tremendous effort has been made towards altering long-lived luminescent properties by variations of host and guest compounds. In this work, we put forward an alternative strategy, organic alloys, to control photoluminescent properties by varying compositional ratios. Organic alloys minimize self-quenching using crystalline triplet emitters, thus providing a more comprehensive range of tunability over luminescence properties than the commonly used host–guest systems. Furthermore, this has the potential to be expanded into multi-component systems or to be used in combination with host–guest systems, which will offer numerous opportunities in both material design and practical applications.
Author contributions
Z. X. and D. H. contributed equally to this work. Z. X. conceived the idea and conducted steady-state and time-gated photoluminescence measurements. D. H. conducted the XRD and DSC measurements. J. Y. performed the photoluminescence quantum yield measurements. M. O. W. provided guidance throughout the project. All authors contributed to the writing of the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We acknowledge the Natural Sciences and Engineering Research Council of Canada (NSERC) for funding, Dr J. F. Britton, MAX Diffraction Facility, Department of Chemistry, McMaster University for diffuse X-ray diffraction experiments and interpretation of the data, and the Laboratory for Advanced Spectroscopy and Imaging Research (LASIR) for facility access. We would like to thank Dr Saeid Kamal for his assistance with spectroscopic studies.Notes and references
- Kenry, C. Chen and B. Liu, Nat. Commun., 2019, 10, 2111 CrossRef CAS PubMed.
- Q. Li, Y. Tang, W. Hu and Z. Li, Small, 2018, 14, 1801560 CrossRef PubMed.
- A. Forni, E. Lucenti, C. Botta and E. Cariati, J. Mater. Chem. C, 2018, 6, 4603–4626 RSC.
- W. Zhao, Z. He and B. Z. Tang, Nat. Rev. Mater., 2020, 5, 869–885 CrossRef CAS.
- Z. Yang, Z. Mao, Z. Xie, Y. Zhang, S. Liu, J. Zhao, J. Xu, Z. Chi and M. P. Aldred, Chem. Soc. Rev., 2017, 46, 915–1016 RSC.
- Y. Liu, C. Li, Z. Ren, S. Yan and M. R. Bryce, Nat. Rev. Mater., 2018, 3, 18020 CrossRef CAS.
- M. Godumala, S. Choi, M. J. Cho and D. H. Choi, J. Mater. Chem. C, 2019, 7, 2172–2198 RSC.
- E. M. Engler, B. A. Scott, S. Etemad, T. Penney and V. V. Patel, J. Phys. Chem., 1977, 99, 5909–5916 CAS.
- Y. Lei, Y. Sun, Y. Zhang, H. Zhang, H. Zhang, Z. Meng, W. Y. Wong, J. Yao and H. Fu, Nat. Commun., 2018, 9, 4358 CrossRef PubMed.
- H. Zhang, H. Wang, P.-C. Qian and W. Y. Wong, Nanoscale, 2020, 12, 16414–16419 RSC.
- M. Dabros, P. R. Emery and V. R. Thalladi, Angew. Chem., Int. Ed., 2007, 46, 4132–4135 CrossRef CAS PubMed.
- Y. Zhen, H. Tanaka, K. Harano, S. Okada, Y. Matsuo and E. Nakamura, J. Am. Chem. Soc., 2015, 137, 2247–2252 CrossRef CAS PubMed.
- X. Xu, T. Xiao, X. Gu, X. Yang, S. V. Kershaw, N. Zhao, J. Xu and Q. Miao, ACS Appl. Mater. Interfaces, 2015, 7, 28019–28026 CrossRef CAS PubMed.
- J.-H. Dou, Z.-A. Yu, J. Zhang, Y.-Q. Zheng, Z.-F. Yao, Z. Tu, X. Wang, S. Huang, C. Liu, J. Sun, Y. Yi, X. Cao, Y. Gao, J.-Y. Wang and J. Pei, J. Am. Chem. Soc., 2019, 141, 6561–6568 CrossRef CAS PubMed.
- X. Huang, X. Liu, K. Ding and S. R. Forrest, Mater. Horiz., 2020, 7, 244–251 RSC.
- Y.-Q. Sun, Y.-L. Lei, X.-H. Sun, S.-T. Lee and L.-S. Liao, Chem. Mater., 2015, 27, 1157–1163 CrossRef CAS.
- R. Natarajan, G. Magro, L. N. Bridgland, A. Sirikulkajorn, S. Narayanan, L. E. Ryan, M. F. Haddow, A. G. Orpen, J. P. H. Charmant, A. J. Hudson and A. P. Davis, Angew. Chem., Int. Ed., 2011, 50, 11386–11390 CrossRef CAS PubMed.
- F. B. Dias, T. J. Penfold and A. P. Monkman, Methods Appl. Fluoresc., 2017, 5, 012001 CrossRef PubMed.
- J. Chen, T. Yu, E. Ubba, Z. Xie, Z. Yang, Y. Zhang, S. Liu, J. Xu, M. P. Aldred and Z. Chi, Adv. Opt. Mater., 2019, 7, 1801593 CrossRef.
- J.-X. Wang, Y.-G. Fang, C.-X. Li, L.-Y. Niu, W.-H. Fang, G. Cui and Q. Z. Yang, Angew. Chem., Int. Ed., 2020, 59, 10032–10036 CrossRef CAS PubMed.
- Z. Mao, Z. Yang, C. Xu, Z. Xie, L. Jiang, F. L. Gu, J. Zhao, Y. Zhang, M. P. Aldred and Z. Chi, Chem. Sci., 2019, 10, 7352–7357 RSC.
- Y. Xiong, Z. Zhao, W. Zhao, H. Ma, Q. Peng, Z. He, X. Zhang, Y. Chen, X. He, J. W. Y. Lam and B. Z. Tang, Angew. Chem., Int. Ed., 2018, 57, 7997–8001 CrossRef CAS PubMed.
- N. Gan, X. Wang, H. Ma, A. Lv, H. Wang, Q. Wang, M. Gu, S. Cai, Y. Zhang, L. Fu, M. Zhang, C. Dong, W. Yao, H. Shi, Z. An and W. Huang, Angew. Chem., Int. Ed., 2019, 58, 14140–14145 CrossRef CAS PubMed.
- Z. Mao, Z. Yang, Z. Fan, E. Ubba, W. Li, Y. Li, J. Zhao, Z. Yang, M. P. Aldred and Z. Chi, Chem. Sci., 2019, 10, 179–184 RSC.
- S. Cai, H. Shi, J. Li, L. Gu, Y. Ni, Z. Cheng, S. Wang, W. W. Xiong, L. Li, Z. An and W. Huang, Adv. Mater., 2017, 29, 1701244 CrossRef PubMed.
- Y. Xie, Y. Ge, Q. Peng, C. Li, Q. Li and Z. Li, Adv. Mater., 2017, 29, 1606829 CrossRef PubMed.
- Z. Xu, C. Climent, C. M. Brown, D. Hean, C. J. Bardeen, D. Casanova and M. O. Wolf, Chem. Sci., 2021, 12, 188–195 RSC.
- Y. Shoji, Y. Ikabata, Q. Wang, D. Nemoto, A. Sakamoto, N. Tanaka, J. Seino, H. Nakai and T. Fukushima, J. Am. Chem. Soc., 2017, 139, 2728–2733 CrossRef CAS PubMed.
- W. Ye, H. Ma, H. Shi, H. Wang, A. Lv, L. Bian, M. Zhang, C. Ma, K. Ling, M. Gu, Y. Mao, X. Yao, C. Gao, K. Shen, W. Jia, J. Zhi, S. Cai, Z. Song, J. Li, Y. Zhang, S. Lu, K. Liu, C. Dong, Q. Wang, Y. Zhou, W. Yao, Y. Zhang, H. Zhang, Z. Zhang, X. Hang, Z. An, X. Liu and W. Huang, Nat. Mater., 2021, 20, 1539–1544 CrossRef CAS PubMed.
- Z. Yuan, J. Wang, L. Chen, L. Zou, X. Gong and X. Ma, CCS Chem., 2020, 2, 158–167 CrossRef CAS.
- L. Bian, H. Shi, X. Wang, K. Ling, H. Ma, M. Li, Z. Cheng, C. Ma, S. Cai, Q. Wu, N. Gan, X. Xu, Z. An and W. Huang, J. Am. Chem. Soc., 2018, 140, 10734–10739 CrossRef CAS PubMed.
- S. Guo, W. Dai, X. Chen, Y. Lei, J. Shi, B. Tong, Z. Cai and Y. Dong, ACS Mater. Lett., 2021, 3, 379–397 CrossRef CAS.
- O. Bolton, K. Lee, H.-J. Kim, K. Y. Lin and J. Kim, Nat. Chem., 2011, 3, 205–210 CrossRef CAS PubMed.
- O. Bolton, D. Lee, J. Jung and J. Kim, Chem. Mater., 2014, 26, 6644–6649 CrossRef CAS.
- D. Lee, O. Bolton, B. C. Kim, J. H. Youk, S. Takayama and J. Kim, J. Am. Chem. Soc., 2013, 135, 6325–6329 CrossRef CAS PubMed.
- M. S. Kwon, D. Lee, S. Seo, J. Jung and J. Kim, Angew. Chem., Int. Ed., 2014, 53, 11177–11181 CrossRef CAS PubMed.
- S. Hirata, K. Totani, J. Zhang, T. Yamashita, H. Kaji, S. R. Marder, T. Watanabe and C. Adachi, Adv. Funct. Mater., 2013, 23, 3386–3397 CrossRef CAS.
- N. Notsuka, R. Kabe, K. Goushi and C. Adachi, Adv. Funct. Mater., 2017, 27, 1703902 CrossRef.
- X. Zhang, L. Du, W. Zhao, Z. Zhao, Y. Xiong, X. He, P. F. Gao, P. Alam, C. Wang, Z. Li, J. Leng, J. Liu, C. Zhou, J. W. Y. Lam, D. L. Phillips, G. Zhang and B. Z. Tang, Nat. Commun., 2019, 10, 5161 CrossRef PubMed.
- C. Chen, Z. Chi, K. C. Chong, A. S. Batsanov, Z. Yang, Z. Mao, Z. Yang and B. Liu, Nat. Mater., 2020, 14, 175–180 Search PubMed.
- Y. Wang, H. Gao, J. Yang, M. Fang, D. Ding, B. Z. Tang and Z. Li, Adv. Mater., 2021, 33, 2007811 CrossRef CAS.
- L. Ma, Q. Xu, S. Sun, B. Ding, Z. Huang, X. Ma and H. Tian, Angew. Chem., Int. Ed., 2022, 61, e202115748 CAS.
- B. Ding, L. Ma, Z. Huang, X. Ma and H. Tian, Sci. Adv., 2021, 7, eabf9668 CrossRef CAS PubMed.
- R. Kabe and C. Adachi, Nature, 2017, 550, 384–387 CrossRef CAS PubMed.
- P. Alam, N. L. C. Leung, J. Liu, T. S. Cheung, X. Zhang, Z. He, R. T. K. Kwok, J. W. Y. Lam, H. H. Y. Sung, I. D. Williams, C. C. S. Chan, K. S. Wong, Q. Peng and B. Z. Tang, Adv. Mater., 2020, 32, 2001026 CrossRef CAS PubMed.
- Z. Lin, R. Kabe, K. Wang and C. Adachi, Nat. Commun., 2020, 11, 191 CrossRef CAS PubMed.
- X. Wang, Y. Sun, G. Wang, J. Li, X. Li and K. Zhang, Angew. Chem., Int. Ed., 2021, 60, 17138–17147 CrossRef CAS PubMed.
- B. Chen, W. Huang, X. Nie, F. Liao, H. Miao, X. Zhang and G. Zhang, Angew. Chem., Int. Ed., 2021, 60, 16970–16973 CrossRef CAS PubMed.
- Y. Liu, G. Zhan, P. Fang, Z. Liu, Z. Bian and C. Huang, J. Mater. Chem. C, 2017, 5, 12547–12552 RSC.
- Z. Xu, D. Hean, C. Climent, D. Casanova and M. O. Wolf, Mater. Adv., 2021, 2, 5777–5784 RSC.
- A. Bondi, J. Org. Chem., 1964, 68, 441–451 CAS.
- T. R. Welberry, R. L. Withers and J. C. Osborn, Acta Crystallogr., 1990, 46, 267–275 Search PubMed.
- T. R. Welberry and B. D. Butler, Chem. Rev., 1995, 95, 2369–2403 CrossRef CAS.
- X. Cai, Z. Qiao, M. Li, X. Wu, Y. He, X. Jiang, Y. Cao and S.-J. Su, Angew. Chem., Int. Ed., 2019, 58, 13522–13531 CrossRef CAS PubMed.
- H. Zhu, I. Badía-Domínguez, B. Shi, Q. Li, P. Wei, H. Xing, M. C. R. Delgado and F. Huang, J. Am. Chem. Soc., 2021, 143, 2164–2169 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic and computational details. CCDC 2116284–2116290. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2sc01922a |
| ‡ These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2022 |