Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An electrochemical modification strategy to fabricate NiFeCuPt polymetallic carbon matrices on nickel foam as stable electrocatalysts for water splitting†
Ziqi
Zhang
,
Yiduo
Li
,
Zhe
Zhang
,
He
Zheng
,
Yuxin
Liu
,
Yuxing
Yan
,
Chunguang
Li
,
Haiyan
Lu
*,
Zhan
Shi
 * and
Shouhua
Feng
* and
Shouhua
Feng
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P. R. China. E-mail: zshi@mail.jlu.edu.cn; luhy@jlu.edu.cn
First published on 5th July 2022
Abstract
Electrochemical modification is a mild and economical way to prepare electrocatalytic materials with abundant active sites and high atom efficiency. In this work, a stable NiFeCuPt carbon matrix deposited on nickel foam (NFFeCuPt) was fabricated with an extremely low Pt load (∼28 μg cm−2) using one-step electrochemical co-deposition modification, and it serves as a bifunctional catalyst for overall water splitting and achieves 100 mA cm−2 current density at a low cell voltage of 1.54 V in acidic solution and 1.63 V in alkaline solution, respectively. In addition, a novel electrolyte was developed to stabilize the catalyst under acidic conditions, which provides inspiration for the development of highly efficient, highly stable, and cost-effective ways to synthesize electrocatalysts.
Introduction
Hydrogen is one of the most desirable alternatives to fossil fuels due to its high energy density, carbon neutrality and environmental friendliness.1 Compared with the steam reforming process which accelerates fossil fuel depletion and CO2 emissions,2 electrochemical water splitting (EWS) is a desirable way to produce highly purified H2 since it can be powered by renewable energies without CO2 emissions.3To drive electrochemical water splitting with low power consumption, efficient, durable and affordable electrocatalysts with low overpotentials towards the hydrogen evolution reaction (HER)4 and oxygen evolution reaction (OER)5 are in urgent need.6 In particular, the OER remains a bottleneck for EWS owing to its sluggish kinetics.7 Although attempts have been made to use earth abundant transition metals8 and their corresponding compounds9 as electrocatalysts to reduce cost,10 their poor stability, excessive load and high overpotential partially meet the requirements of practical applications.11 On the other hand, noble metals such as platinum (Pt),12 iridium (Ir),13 ruthenium (Ru)14 and their corresponding oxides15 are used as benchmark electrocatalysts towards EWS, given their high efficiency and stability. Nevertheless, high cost and low abundance greatly hinder the further application of noble metal electrocatalysts.16 Therefore, it is desired to develop low-cost EWS catalysts exhibiting both dramatic catalytic performance and stability, and cost-effectiveness for large-scale industrial production.
Recently, it has been reported that electrochemical modification can be used to prepare highly dispersed noble metal modified carbon-based electrocatalysts.12 This method eliminates the need to overcome excessive surface free energy of highly dispersed noble metal atoms as the traditional pyrolysis method does.17 Moreover, a small quantity of noble metal ion containing solution is required.18,19 Since Pt dissolves in sulfuric acid at 1.1 V vs. RHE,20 the modification can be readily realized at ambient temperature and pressure.21
In this work, we fabricate a NiFeCuPt embedded carbon matrix uniformly dispersed on NF, denoted as NFFeCuPt hereafter, using a one-step electrochemical co-deposition method. The prepared electrode exhibits a low Pt load (ca. 28 μg cm−2, 1/5 of that in PtC) and low overpotentials at 65/98 mV, 273/328 mV, and 315/405 mV to drive a current density of 100 mA cm−2 for the HER, the OER and EWS in acidic (0.5 M H2SO4)/alkaline (1 M KOH) solutions, respectively, ranking it as one of the most efficient EWS catalysts reported to date.22 Furthermore, a strategy has also been developed to modify the prepared NF-based electrode to catalyze the HER under acidic conditions (0.5 M H2SO4) with long-term stability (>750 h). Semi in situ X-ray photoelectron spectra (XPS) were recorded to understand the formation process of a NiFeCuPt carbon matrix on NF. This controllable, simple and low-cost electrochemical modification method is expected to be a general method for preparing highly dispersed noble metal materials as EWS catalysts having high efficiency and desired stability.
Results and discussion
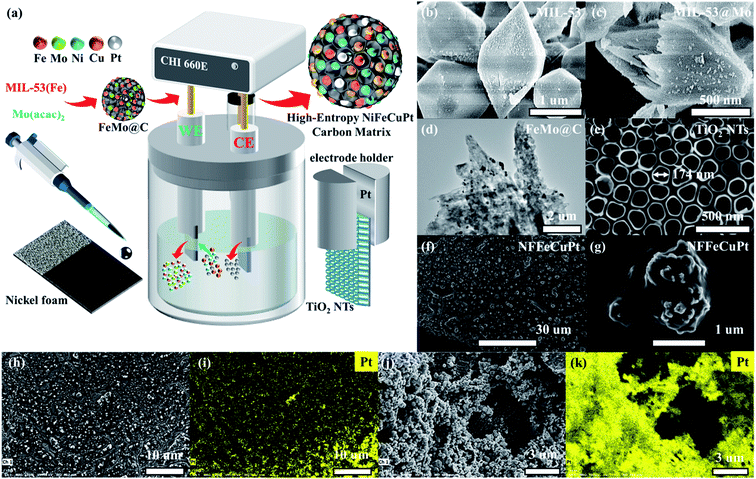
As schematically illustrated in Fig. 1a, NFFeCuPt was synthesized by using a three-electrode system. First, a mild hydrothermal process was carried out to synthesize MIL-53 (Fe) and yield uniform fusiform particles (Fig. 1b and S1a, b, ESI†). Then MIL-53 (Fe) and C10H14MoO6 were mixed in a methanol solution to obtain Mo doped MIL-53 (MIL-53@Mo) (Fig. 1c and S1c, ESI†). Finally, MIL-53@Mo was carbonized in a N2 atmosphere to obtain FeMo bimetallic carbide (FeMo@C) (Fig. 1d and S1d, S2, ESI†). It is evident that after carbonization, the metal particles are evenly dispersed throughout the entire FeMo@C. The corresponding XRD patterns are plotted and shown in Fig. S3, ESI.† The FeMo@C ink was pipetted onto NF. After natural drying, the modified NF served as the working electrode (WE), and TiO2 nanotubes (TiO2 NTs) on the surface of a Ti sheet (Fig. 1e) as the counter electrode (CE), both of which were clamped with a platinum electrode holder and immersed in 0.5 M H2SO4, 117 mg L−1 of CuCl2·2H2O and 30 g L−1 H3BO3 as the electrolyte. The electrochemical modification was carried out by using cyclic voltammetry conducted from −0.6 to 0 V with a scan rate of 100 mV s−1 for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. During this process, all of the Mo and a fraction of Fe dissolved in acid and vacancies were created in FeMo@C, which could trap Ni, Cu and Pt atoms subsequently. And the Pt atoms were simultaneously dissolved from the Pt electrode holder into the electrolyte around the CE and then migrated to the WE area under an electric field force, and were finally co-deposited into the vacancies mentioned above with Ni and Cu atoms from electrolyte. It is assumed that in the process of electrochemical modification, the TiO2 NT CE creates a localized electric field that allows metal atoms, especially Pt atoms, to embed uniformly, precisely and robustly into the carbon matrix of FeMo@C (Fig. 1f–i) by forming chemical bonds, as verified by the subsequent semi-in situ XPS tests. If Pt foil was used as the CE rather than TiO2 NTs (NFFeCu–Pt), Pt particles will be randomly deposited on the surface of NF (Fig. 1j, k; and S4, ESI†) without the confined electric field, which will greatly reduce Pt atomic utilization. According to the EDS data (Fig. S5, ESI†), the content of Pt in NFFeCu–Pt is 47 wt% but it exhibits unsatisfactory catalytic performance and stability, which contributes in part to the fact that electrochemical modification is rarely used in the synthesis of catalysts. With the aid of the TiO2 NT CE, NFFeCuPt achieves a lower Pt load. EDS analysis (Fig. S6, ESI†) shows that the Pt content in NFFeCuPt is 4.3 wt%, which is close to the result (4.67 wt%) acquired from inductively coupled plasma (ICP) measurements. This result also shows that nearly all the Pt atoms in NFFeCuPt are distributed on the surface of the catalyst, making it more accessible to the reactants (H+ or OH−), rather than being wrapped inside the carbon matrix, resulting in invalid dead volume and a decline in the utilization rate of Pt atoms. In addition, the load of Pt in NFFeCuPt (ca. 28 μg cm−2) is about 1/5th of that in commercial 20 wt% Pt/C. More importantly, the Pt element is introduced only in one-step electrochemical modification without any pre- or post-treatments. As a source of Pt, the Pt electrode holder had no significant damage even after more than 100 NFFeCuPt sheets were manufactured (Fig. S7, ESI†). The proposed electrochemical modification method is able to maximize the utilization of Pt atoms from the perspective of atomic economy.23
000 cycles. During this process, all of the Mo and a fraction of Fe dissolved in acid and vacancies were created in FeMo@C, which could trap Ni, Cu and Pt atoms subsequently. And the Pt atoms were simultaneously dissolved from the Pt electrode holder into the electrolyte around the CE and then migrated to the WE area under an electric field force, and were finally co-deposited into the vacancies mentioned above with Ni and Cu atoms from electrolyte. It is assumed that in the process of electrochemical modification, the TiO2 NT CE creates a localized electric field that allows metal atoms, especially Pt atoms, to embed uniformly, precisely and robustly into the carbon matrix of FeMo@C (Fig. 1f–i) by forming chemical bonds, as verified by the subsequent semi-in situ XPS tests. If Pt foil was used as the CE rather than TiO2 NTs (NFFeCu–Pt), Pt particles will be randomly deposited on the surface of NF (Fig. 1j, k; and S4, ESI†) without the confined electric field, which will greatly reduce Pt atomic utilization. According to the EDS data (Fig. S5, ESI†), the content of Pt in NFFeCu–Pt is 47 wt% but it exhibits unsatisfactory catalytic performance and stability, which contributes in part to the fact that electrochemical modification is rarely used in the synthesis of catalysts. With the aid of the TiO2 NT CE, NFFeCuPt achieves a lower Pt load. EDS analysis (Fig. S6, ESI†) shows that the Pt content in NFFeCuPt is 4.3 wt%, which is close to the result (4.67 wt%) acquired from inductively coupled plasma (ICP) measurements. This result also shows that nearly all the Pt atoms in NFFeCuPt are distributed on the surface of the catalyst, making it more accessible to the reactants (H+ or OH−), rather than being wrapped inside the carbon matrix, resulting in invalid dead volume and a decline in the utilization rate of Pt atoms. In addition, the load of Pt in NFFeCuPt (ca. 28 μg cm−2) is about 1/5th of that in commercial 20 wt% Pt/C. More importantly, the Pt element is introduced only in one-step electrochemical modification without any pre- or post-treatments. As a source of Pt, the Pt electrode holder had no significant damage even after more than 100 NFFeCuPt sheets were manufactured (Fig. S7, ESI†). The proposed electrochemical modification method is able to maximize the utilization of Pt atoms from the perspective of atomic economy.23
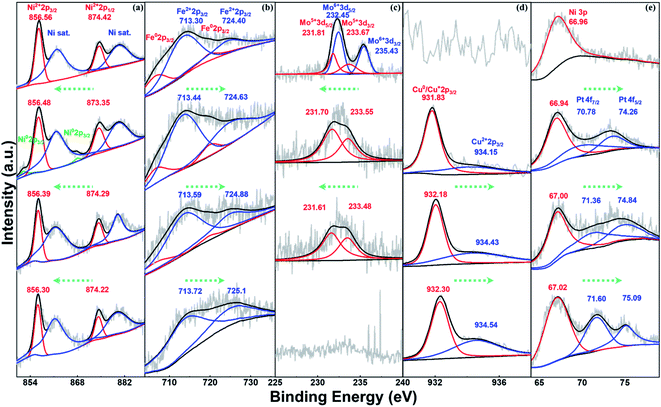
To investigate the formation process of NFFeCuPt during electrochemical modification, semi in situ XPS was applied to NFFeCuPt after different cycles of CV. As shown in Fig. 2a, two peaks of Ni 2p at 856.56 and 874.42 eV are ascribed to Ni2+ 2p3/2 and Ni2+ 2p1/2, respectively, along with two satellite peaks located respectively at 861.88 and 880.28 eV.24 The binding energy of Ni2+ is higher than that of the reported Ni–Nx sites25 which suggests that Ni exists mainly in the form of nickel oxide due to surface oxidization. With the increment in the cycles, nickel oxide is reduced at the cathode to form some elemental Ni which is then dissolved by sulfuric acid and the peaks disappear. Finally, nickel oxide on the surface gradually becomes elemental Ni and dissolves in sulfuric acid to form Ni2+ ions, which are adsorbed around the working electrode due to electrostatic action and then captured by the FeMo@C matrix under the reduction potential. The binding energy of Ni2+ decreases as the cycles of CV go up, indicating the transition from Ni–O to Ni–C bonds.26 As the cycles of CV go up, the unstable free elemental Fe in FeMo@C slowly dissolve out since the peaks of Fe0 gradually vanish (Fig. 2b). Besides, the binding energy of Fe2+ keeps increasing, indicating that some originally unstable Fe2+ is reduced and dissolved. Eventually only stable Fe–C is retained in the carbon skeleton (Fig. S8, ESI†). This not only creates vacancies and defects for other metals to fill in, but also greatly increases the proportion of stable iron active centers. According to Fig. 2c, the binding energy of Mo 3d3/2 and Mo 3d5/2 is 235.43 eV and 232.45 eV for Mo6+ in MoO3 while the peaks at 233.67 and 231.81 eV can be ascribed to Mo5+.27,28 As the cycles of CV go up, MoO3 will first be destroyed and reduced to Mo5+. Then the binding energy of Mo5+ decreases gradually, indicating that Mo atoms slowly escape from the matrix lattice of FeMo@C. Eventually, Mo and its compounds dissolve completely in acidic electrolyte, which are also undetectable in ICP. In general, the steady dissolution of Mo and Fe creates many lattice defects and dredges the previously blocked pore structures for Ni, Cu and Pt atoms to anchor (Fig. S9 and S10, ESI†). As shown in Fig. 2d, the Cu 2p3/2 peaks appearing after 3000 cycles show two deconvoluted peaks at 931.83 and 934.15 eV, corresponding to Cu (0) or Cu(I) and Cu(II) species.29,30 This binding energy is smaller than the reported binding energy of the Cu-N bond,31 so it can be regarded as the Cu–C bond generated by Cu embedding into the FeMo@C matrix in the process of cathode deposition.32 Subsequently, the binding energy and the ratio of Cu2+ increase as the number of cycles goes up, which indicates that Cu atoms are continuously embedded in the skeleton throughout the cyclic voltammetry process. In addition, unlike Ni, Fe and Mo, the peak of Cu (0) finally exists, indicating that Cu ions form a very thin layer on the surface due to the reduction current. As shown in Fig. 2e, the Pt is not detected before CV, and only the Ni 3p peak can be observed which can be attributed to the NF base. After CVs for 3000 cycles, the peaks at 70.78 eV and 74.26 can be assigned to Pt 4f7/2 and Pt 4f5/2,33 which proves that the platinum sheet inside the electrode holder is indeed dissolved from the CE. And the Pt atoms migrate to the WE under the electric field force and are finally deposited on the surface of the WE.34 Compared with some existing literature studies,21,35 the binding energies shown in Fig. 2e demonstrate that Pt atoms are embedded in the carbon matrix in the form of Pt–C bonds rather than being dissociated in the pore structure of FeMo@C in the elemental form. This enhances the stability of Pt atoms, and the ligand effect can be used to regulate the adsorption energy of Pt towards H+ or OH−,36 which can greatly improve the performance of the catalyst. Meanwhile, the binding energy of Pt in NFFeCuPt is higher than that of Pt/C (Fig. S11a, ESI†). In addition, the peaks of Pt 4f in commercial Pt/C are sharper relative to the baseline, indicating a higher platinum content in commercial Pt/C than in NFFeCuPt. Last but not the least, no Ti 2p signal is detected by XPS (Fig. S11b, ESI†), indicating that Ti is not deposited on the WE. In fact, TiO2 NTs are quite stable at the operating voltage in this work. The surface of TiO2 NTs undergoes more than 100 electrochemical modification processes and the nanotube structure is still clear and visible (Fig. S12, ESI†).
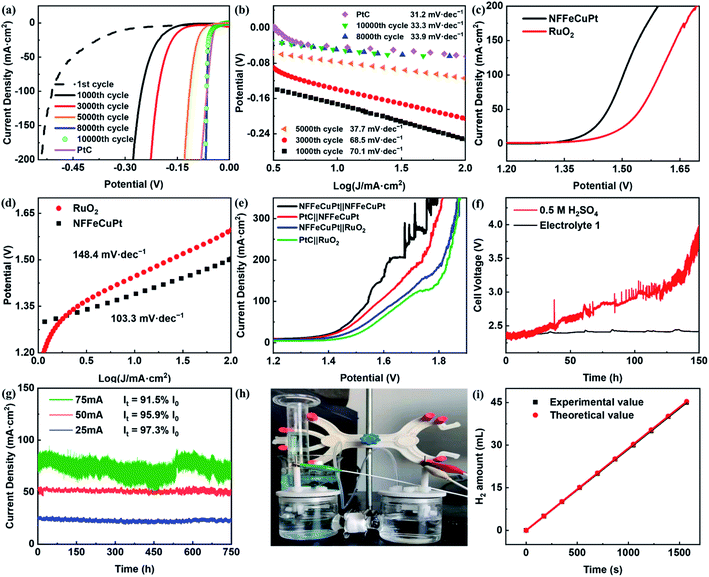
The HER catalytic performance was first tested in 0.5 M H2SO4. As shown in Fig. 3a, with the increase of the CV cycles, the HER overpotentials of NFFeCuPt gradually decrease. As a comparison, different CEs and WEs were applied for the electrochemical modification process (Fig. S13, ESI†), but the performances were not as satisfactory as that of NFFeCuPt, indicating that the confining electric field provided by the TiO2 NT CE, the dissolution–redeposition process of NF and the dissolution of Mo compounds are crucial to the catalytic performance. Although PtC shows a lower HER overpotential (33 mV) at 10 mA cm−2 compared to NFFeCuPt (40 mV), the latter exhibits a lower overpotential when the current density is greater than 100 mA cm−2. This is because Cu, as a weak adsorption element towards H, can effectively promote the desorption step of hydrogen, making Pt catalytic sites in NFFeCuPt less susceptible to poisoning at high current density than PtC.31 To gain insight into the kinetics and mechanism of the HER, the Tafel plots derived from the corresponding polarization curves are presented in Fig. 3b, in which the Tafel slope of NFFeCuPt (33.3 mV dec−1) is close to that of PtC (31.2 mV dec−1), indicating that the Tafel step is the rate determining step.37 The lower Tafel slope shows better HER kinetics.38 In an acidic electrolyte, H+ obtains an electron to form H atoms and adsorbed on the surface of NFFeCuPt (Volmer reaction, H3O+ + е− → Hads),39 followed by either an electrochemical desorption step (Heyrovsky step, Hads + H3O+ + е− → H2) or a chemical desorption step (Tafel step Hads + Hads → H2) to form H2.40 The theoretical Tafel slopes of the Volmer, Heyrovsky and Tafel reactions are −120, −40 and −30 mV dec−1, respectively.41 In comparison, the Tafel slope of NFFeCuPt is 33.3 mV dec−1 which demonstrates a Volmer–Tafel mechanism with the Tafel step as the rate determining step. A plausible mechanism of the HER under acidic conditions can be described as follows: H+ obtained an electron provided by the WE and adsorbed on the surface of NFFeCuPt (Volmer reaction, H3O+ + е− → Hads), and then two H atoms adsorbed by two adjacent active sites are chemically desorbed to form hydrogen (Tafel step Hads + Hads → H2) which is finally released from the surface of the catalyst. The electrochemical impedance spectra (EIS) were used to elucidate the charge transfer resistances of NFFeCuPt. NFFeCuPt has the lowest intrinsic resistance and charge transfer resistance among the other control groups (Fig. S14, ESI†), which facilitates the kinetics towards the HER.42
To examine the bifunctional EWS catalytic activity, the electrocatalytic OER performance of NFFeCuPt and RuO2 catalysts43 was also measured in 0.5 M H2SO4. As shown in Fig. 3c and d, NFFeCuPt (273 mV, 103.3 mV dec−1) exhibits a lower overpotential at 100 mA cm−2 and a smaller Tafel slope than RuO2 (367 mV, 148.4 mV dec−1), suggesting that NFFeCuPt is more favorable than RuO2 to catalyze the OER kinetically.44 Next, different electrode pairs were assembled and their EWS performance was tested. As shown in Fig. 3e, it can be found obviously that the NFFeCuPt‖NFFeCuPt pair possesses the best EWS performance of all, which reduces the overpotential by 128 mV at 100 mA cm−2 current density compared to the commercial PtC PtC‖RuO2 pair. However, NF was not a desirable supporter under acidic conditions especially as an anode. The NF would dissolve (Fig. S15, ESI†) when the oxidation potential was applied (this is also why the curve of current density fluctuates obviously when the voltage increases to a certain value as shown in Fig. 3e), and so, it is impossible for NF to serve as an anode for long-term electrolysis. As far as we know, Cu is an element that is often used for underpotential deposition on the surface of other noble metals to form dense monatomic layers for measuring electrochemical surface area.45 Inspired by this, we tried to protect the active sites of NFFeCuPt by electroplating a layer of Cu on the electrode. Therefore, a peculiar electrolyte has been developed that allows NFFeCuPt to stably catalyze the HER under acidic conditions. The electrolyte contained 0.5 M H2SO4, 117 mg L−1 of CuCl2·2H2O, 30 g L−1 of NaH2PO2, 30 g L−1 of C6H5Na3O7 and 30 g L−1 of H3BO3, and was named Electrolyte 1. It can be seen in Fig. 3f that NFFeCuPt exhibits worse stability in 0.5 M H2SO4 than in Electrolyte 1. This is probably because the Cu2+ in Electrolyte 1 can form a stable layer on the cathode surface due to the electric field force (Fig. S16, ESI†), which can protect the NF from being corroded by sulfuric acid.
To prove that Electrolyte 1 can help NFFeCuPt achieve enough stability for practical application, a potentiostatic test was carried out for NFFeCuPt in Electrolyte 1 with the initial current densities at 25 mA cm−2, 50 mA cm−2 and 75 mA cm−2. It can be seen clearly in Fig. 3g that the current density loss is inconspicuous even after working for 750 h, which is a breakthrough for the stability of NF under acidic conditions.46,47 In addition, with the increase of current density, the Cu film may become denser (Fig. S17, ESI†) due to a higher applied potential, preventing some active sites from accessing the reactants, which is also the reason for more current density loss. Since graphite rods will dissolve after serving as a CE for about 300 h (Fig. S18, ESI†), the WE (NFFeCuPt) and CE (RuTiIr) were connected to a copper wire to assemble a cell for a long-term stability test (Fig. S19, ESI†). The generated H2 gas was qualified by using a home-made drainage device (Fig. 3h)48 and a FE of 99.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ±
±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1% is obtained (Fig. 3I), indicating that the formed Cu film has little effect on FE. Next, galvanostatic and potentiostatic (Fig. S20, ESI†) measurements were carried out to investigate the stability of NFFeCuPt at high current density. Nevertheless, when the current density of the galvanostatic test exceeded 300 mA cm−2, there would be a noticeable decline after electrolysis for about 50 h (Fig. S21, ESI†). Interestingly, when the electrolyte solution was renewed, the current density can reach the initial value (Fig. S22, ESI†), which means that NFFeCuPt has not been damaged. This is probably due to the dissolution of the NF substrate at high current density (Fig. S23, ESI†). The SEM images of NFFeCuPt (after the potentiostatic test in Electrolyte 1) before and after the H2SO4 treatment (Fig. S24, ESI†) show the presence of a copper film. Notably, as the CE was no longer TiO2 NTs during the stability test, the carbon matrices on NF tended to agglomerate after the stability test (Fig. S24c and d, ESI†), which also proves the existence of the confining electric field effect of TiO2 NTs during the synthesis process of NFFeCuPt. Finally, the ECSA is estimated by using CVs to compare the intrinsic catalytic activity of NFFeCuPt (Fig. S25, ESI†). Although NFFeCu–Pt has a larger ECSA and a greater number of potential active sites, NFFeCuPt exhibits a much better catalytic performance (Fig. S13, ESI†), indicating that the active sites of the latter have higher intrinsic activity. Furthermore, due to the disordered aggregation of Pt (Fig. 1h and i) and excessive adsorption capacity of the H+ intermediate, Pt sites in NFFeCu–Pt are poisoned by H+ in the early stages of electrolysis (Fig. S26, ESI†) while NFFeCuPt has a stable catalytic activity due to the Cu sites promoting the desorption step.32 In conclusion, there are two major advantages of the electrochemical modification in this work. First, it can greatly improve the utilization rate of platinum atoms since it can construct catalytic sites with higher intrinsic activity compared to traditional methods. Secondly, it can simultaneously introduce Cu atoms, which are conducive to the H2 desorption step, into the carbon matrix by one-step co-deposition to avoid the Pt catalytic sites being poisoned by excessive adsorption of H+ under acidic conditions at high current density during the long-term electrolysis process.
1% is obtained (Fig. 3I), indicating that the formed Cu film has little effect on FE. Next, galvanostatic and potentiostatic (Fig. S20, ESI†) measurements were carried out to investigate the stability of NFFeCuPt at high current density. Nevertheless, when the current density of the galvanostatic test exceeded 300 mA cm−2, there would be a noticeable decline after electrolysis for about 50 h (Fig. S21, ESI†). Interestingly, when the electrolyte solution was renewed, the current density can reach the initial value (Fig. S22, ESI†), which means that NFFeCuPt has not been damaged. This is probably due to the dissolution of the NF substrate at high current density (Fig. S23, ESI†). The SEM images of NFFeCuPt (after the potentiostatic test in Electrolyte 1) before and after the H2SO4 treatment (Fig. S24, ESI†) show the presence of a copper film. Notably, as the CE was no longer TiO2 NTs during the stability test, the carbon matrices on NF tended to agglomerate after the stability test (Fig. S24c and d, ESI†), which also proves the existence of the confining electric field effect of TiO2 NTs during the synthesis process of NFFeCuPt. Finally, the ECSA is estimated by using CVs to compare the intrinsic catalytic activity of NFFeCuPt (Fig. S25, ESI†). Although NFFeCu–Pt has a larger ECSA and a greater number of potential active sites, NFFeCuPt exhibits a much better catalytic performance (Fig. S13, ESI†), indicating that the active sites of the latter have higher intrinsic activity. Furthermore, due to the disordered aggregation of Pt (Fig. 1h and i) and excessive adsorption capacity of the H+ intermediate, Pt sites in NFFeCu–Pt are poisoned by H+ in the early stages of electrolysis (Fig. S26, ESI†) while NFFeCuPt has a stable catalytic activity due to the Cu sites promoting the desorption step.32 In conclusion, there are two major advantages of the electrochemical modification in this work. First, it can greatly improve the utilization rate of platinum atoms since it can construct catalytic sites with higher intrinsic activity compared to traditional methods. Secondly, it can simultaneously introduce Cu atoms, which are conducive to the H2 desorption step, into the carbon matrix by one-step co-deposition to avoid the Pt catalytic sites being poisoned by excessive adsorption of H+ under acidic conditions at high current density during the long-term electrolysis process.
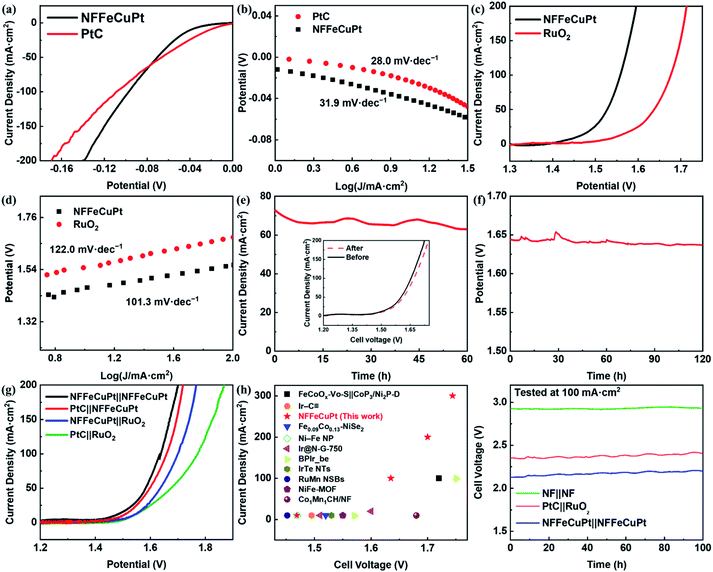
Since NF is not capable of catalyzing long-term OERs under acidic conditions, the EWS performance of NFFeCuPt under alkaline conditions (1 M KOH) is comprehensively evaluated to demonstrate its versatility. First, the overpotentials needed to achieve current densities of 10 mA cm−2 and 100 mA cm−2 are 39 mV and 97 mV for NFFeCuPt and 25 mV and 102 mV for PtC (Fig. 4a), which once again suggest that Cu atoms in NFFeCuPt can promote desorption steps to improve catalytic performance at higher current density. This can also be confirmed from the Tafel plots in Fig. 4b. Although in the range of small current density, PtC has a smaller Tafel slope (28.0 mV dec−1) compared with NFFeCuPt (31.9 mV dec−1), with the increase of current density, the Tafel slope of PtC tends to increase while that of NFFeCuPt does not. It is worth noting that the mechanism of the HER under alkaline conditions is slightly different from that under acidic conditions. Unlike the acidic condition which is rich in H+, under alkaline conditions, the dissociation of water (H2O → H+ + OH−) has a great impact on the HER since it is considered as the largest source of H+.49 Therefore, even the Tafel slope of NFFeCuPt is 31.9 mV dec−1 in 1 M KOH which is a Volmer–Tafel mechanism with the Tafel step as the rate determining step. The HER under alkaline conditions should proceed in this way: First, the dissociation of water (H2O → H+ + OH−) occurred in the catalytic sites to provide H+ for further use. Then the Volmer reaction (H2O + е− → Hads + OH−) and the Tafel step (Hads + Hads → H2) on the active sites.50
Next, the OER performance was also tested in 1 M KOH. NFFeCuPt (230 mV to 10 mA cm−2 and 330 mV to 100 mA cm−2) exhibited a lower overpotential (Fig. 4c) compared to RuO2 (310 mV to 10 mA cm−2 and 440 mV to 100 mA cm−2) and favorable kinetics with a lower Tafel slope (Fig. 4d). Additionally, according to some previous reports,51,52 the voltage and current required for in situ oxidation of nickel foam to form a layer of nickel oxide are far lower than the conditions for the OER to occur. Since the onset potential of the OER is at least 1.23 V vs. RHE, at this potential, in an alkaline, oxygen-rich environment, it can be reasonably assumed that oxygen atoms are inserted into the nickel lattice (Fig. S27, ESI†). Fortunately, the oxygen atoms inserted into the nickel lattice on the surface of NFFeCuPt can assist in the dissociation of water under alkaline conditions (H2O → H+ + OH−)53,54 which has a great impact on the EWS reaction49 since the oxygen atoms inserted into the nickel lattice form new and independent hydrolytic dissociation actives site.55 These dissociation sites can promote water dissociation (H2O → H+ + OH−, non-redox process) alone with the NFFeCuPt active sites facilitating the OER (4OH− + 4e− → O2 + H2O, redox process) respectively.56 In conclusion, the mechanism of the OER under alkaline conditions can be described as follows:57 the OH− loses an electron and adsorbs on the active sites of the WE (M + OH− → MOH + e−). Then the absorbed MOH intermediate loses an electron step by step and is converted into other oxygen-containing intermediates (MOH + OH− → MO + H2O + e−; MO + OH− → MOOH + e−) and finally the MOOH intermediate loses an electron and releases O2 (MOOH + OH− → M + O2 + H2O + e−). Moreover, the potentiostatic and chronopotentiometric test results in Fig. 4e and f show that NFFeCuPt has excellent stability under alkaline conditions towards the OER. For comparing the performance of different electrode pairs towards overall water splitting under alkaline conditions, a comparison was made between different catalysts supported by NF. It can be concluded as shown in Fig. 4g that in order to achieve a current density of 100 mA cm−2, cell voltages of 1.638 mV, 1.659 mV, 1.701 mV and 1.772 mV need to be applied to the following four electrode pairs including NFFeCuPt‖NFFeCuPt, NFFeCuPt‖RuO2, PtC‖NFFeCuPt and PtC‖RuO2, respectively. The overall water splitting performance of NFFeCuPt is superior to most of the other catalysts reported recently especially at high current density, including FeCoOx–Vo–S‖CoP3/Ni2P-D,48 Ir–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) ,22 Fe0.09Co0.13–NiSe2,15 Ni–Fe NP,58 Ir@N-G-750,59 BPIr_be,60 IrTe NTs,61 RuMn NSBs,62 NiFe-MOF,63 Co1Mn1CH/NF64 and so on (Fig. 4h). As for Pt in NFFeCuPt, the mass-specific activity is 848.9 A g−1 at an overpotential of 300 mV. Meanwhile, a two-electrode system was used to evaluate the stability of NFFeCuPt at a current density of 100 mA cm−2 using the galvanostatic method in 1 M KOH (Fig. 4i), indicating that the NFFeCuPt‖NFFeCuPt pair has not only comparable stability as commercial PtC‖RuO2, but also a lower overpotential towards EWS, which well meets the requirement of practical water splitting. In addition, the electrochemical performances of NFFeCuPt for the HER, the OER and EWS in a neutral environment (0.1 M PBS) were also recorded (Fig. S28, ESI†). Although the performances of NFFeCuPt exceed those of commercial noble-metal materials and are comparable to recently reported EWS materials,60 the high overpotentials (η10 = 369 mV for the HER; η10 = 684 mV for the OER; η10 = 1379 mV for EWS) still hinder the practical application of NFFeCuPt under neutral conditions. Last but not least, OER stability of NFFeCuPt was verified in a highly alkaline environment (Fig. S29, ESI†) at a higher current density (Fig. S30, ESI†). The extreme alkaline conditions can be applied in the OER during alkaline zinc–air battery charging.65
,22 Fe0.09Co0.13–NiSe2,15 Ni–Fe NP,58 Ir@N-G-750,59 BPIr_be,60 IrTe NTs,61 RuMn NSBs,62 NiFe-MOF,63 Co1Mn1CH/NF64 and so on (Fig. 4h). As for Pt in NFFeCuPt, the mass-specific activity is 848.9 A g−1 at an overpotential of 300 mV. Meanwhile, a two-electrode system was used to evaluate the stability of NFFeCuPt at a current density of 100 mA cm−2 using the galvanostatic method in 1 M KOH (Fig. 4i), indicating that the NFFeCuPt‖NFFeCuPt pair has not only comparable stability as commercial PtC‖RuO2, but also a lower overpotential towards EWS, which well meets the requirement of practical water splitting. In addition, the electrochemical performances of NFFeCuPt for the HER, the OER and EWS in a neutral environment (0.1 M PBS) were also recorded (Fig. S28, ESI†). Although the performances of NFFeCuPt exceed those of commercial noble-metal materials and are comparable to recently reported EWS materials,60 the high overpotentials (η10 = 369 mV for the HER; η10 = 684 mV for the OER; η10 = 1379 mV for EWS) still hinder the practical application of NFFeCuPt under neutral conditions. Last but not least, OER stability of NFFeCuPt was verified in a highly alkaline environment (Fig. S29, ESI†) at a higher current density (Fig. S30, ESI†). The extreme alkaline conditions can be applied in the OER during alkaline zinc–air battery charging.65
Conclusions
In conclusion, the synthesized NFFeCuPt catalyst exhibited superior electrocatalytic performance and stability for water splitting in acidic, neutral and alkaline environments. The proposed electrochemical modification demonstrated in this study can greatly improve the utilization rate of precious materials by accelerating the intrinsic activity of catalytic active sites and reducing the waste of noble metal atoms caused by redundant steps in traditional synthesis methods. Furthermore, the common approach used in this work can greatly improve the stability of a NF supporter towards the HER under acidic conditions. Such a cost-effective, simple and environmentally friendly electrochemical modification strategy provides alternative insights for fabricating low-load noble metal electrocatalysts with high activity and satisfactory stability, which can also be extended to the synthesis of various electrocatalytic materials at room temperature due to its desirable controllability.Data availability
All the calculation data and experimental data reported in this paper could be accessed from the authors.Author contributions
Ziqi Zhang: conceptualization, investigation, methodology, software, and drafting of the manuscript. Yiduo Li: Investigation and software. Zhe Zhang: software and formal analysis. He Zheng: analysis. Yuxin Liu: visualization and methodology. Yuxing Yan: visualization and methodology. Chunguang Li: conceptualization and resources. Haiyan Lu: data curation and funding acquisition. Zhan Shi: conceptualization, funding acquisition, project administration and supervision. Shouhua Feng: investigation and resources.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Foundation of Science and Technology Development of Jilin Province, China (20200801004GH), the National Natural Science Foundation of China (21621001, 21771084, and 21771077) and the 111 Project (B17020).Notes and references
- L. Yang, R. M. Liu and L. F. Jiao, Adv. Funct. Mater., 2020, 30, 1909618 CrossRef CAS.
- J. Wang, W. Cui, Q. Liu, Z. Xing, A. M. Asiri and X. Sun, Adv. Mater., 2016, 28, 215–230 CrossRef CAS PubMed.
- J. Zhang, W. Jia, S. Dang and Y. Cao, J. Colloid Interface Sci., 2020, 560, 161–168 CrossRef CAS PubMed.
- I. T. McCrum and M. T. M. Koper, Nat. Energy, 2020, 5, 891–899 CrossRef CAS.
- G. Hai, H. Gao, X. Huang, L. Tan, X. Xue, S. Feng and G. Wang, Chem. Sci., 2022, 13, 4397–4405 RSC.
- J. Hao, Z. Zhuang, K. Cao, G. Gao, C. Wang, F. Lai, S. Lu, P. Ma, W. Dong, T. Liu, M. Du and H. Zhu, Nat. Commun., 2022, 13, 2662 CrossRef CAS PubMed.
- J. Y. Xue, F. L. Li, Z. Y. Zhao, C. Li, C. Y. Ni, H. W. Gu, P. Braunstein, X. Q. Huang and J. P. Lang, Dalton Trans., 2019, 48, 12186–12192 RSC.
- J. Hao, Z. Zhuang, J. Hao, K. Cao, Y. Hu, W. Wu, S. Lu, C. Wang, N. Zhang, D. Wang, M. Du and H. Zhu, ACS Nano, 2022, 16, 3251–3263 CrossRef CAS PubMed.
- J. Hao, Z. Zhuang, J. Hao, C. Wang, S. Lu, F. Duan, F. Xu, M. Du and H. Zhu, Adv. Energy Mater., 2022, 2200579 CrossRef.
- Y. Wen, Z. Zhuang, H. Zhu, J. Hao, K. Chu, F. Lai, W. Zong, C. Wang, P. Ma, W. Dong, S. Lu, T. Liu and M. Du, Adv. Energy Mater., 2021, 11, 2102138 CrossRef CAS.
- N. Y. Cheng, L. Ren, G. Casillas, S. Zhou, J. C. Zhuang, L. Wang, X. Xu, S. X. Dou and Y. Du, Sustain. Energy Fuels, 2019, 3, 1757–1763 RSC.
- K. L. Zhou, Z. Wang, C. B. Han, X. Ke, C. Wang, Y. Jin, Q. Zhang, J. Liu, H. Wang and H. Yan, Nat. Commun., 2021, 12, 3783 CrossRef CAS PubMed.
- W. Sun, Y. Song, X.-Q. Gong, L.-m. Cao and J. Yang, Chem. Sci., 2015, 6, 4993–4999 RSC.
- M. Bellini, J. Bösken, M. Wörle, D. Thöny, J. J. Gamboa-Carballo, F. Krumeich, F. Bàrtoli, H. A. Miller, L. Poggini, W. Oberhauser, A. Lavacchi, H. Grützmacher and F. Vizza, Chem. Sci., 2022, 13, 3748–3760 RSC.
- Y. Sun, K. Xu, Z. Wei, H. Li, T. Zhang, X. Li, W. Cai, J. Ma, H. J. Fan and Y. Li, Adv. Mater., 2018, 30, e1802121 CrossRef PubMed.
- H. An, Y. Hu, N. Song, T. Mu, S. Bai, Y. Peng, L. Liu and Y. Tang, Chem. Sci., 2022, 13, 3035–3044 RSC.
- S. Cherevko, A. R. Zeradjanin, A. A. Topalov, N. Kulyk, I. Katsounaros and K. J. J. Mayrhofer, ChemCatChem, 2014, 6, 2219–2223 CrossRef CAS.
- H. Chen, X. Ai, W. Liu, Z. Xie, W. Feng, W. Chen and X. Zou, Angew. Chem., Int. Ed., 2019, 58, 11409–11413 CrossRef CAS PubMed.
- H. Zhang, P. An, W. Zhou, B. Y. Guan, P. Zhang, J. Dong and X. W. D. Lou, Sci. Adv., 2018, 4, eaao6657 CrossRef PubMed.
- L. Y. Xing, M. Hossain, M. Tian, D. Beauchemin, K. Adjemian and G. Jerkiewicz, Electrocatalysis, 2014, 5, 96–112 CrossRef CAS.
- M. Tavakkoli, N. Holmberg, R. Kronberg, H. Jiang, J. Sainio, E. I. Kauppinen, T. Kallio and K. Laasonen, ACS Catal., 2017, 7, 3121–3130 CrossRef CAS.
- Y. Peng, Q. M. Liu, B. Z. Lu, T. He, F. Nichols, X. Hu, T. Huang, G. Huang, L. Guzman, Y. Ping and S. W. Chen, ACS Catal., 2021, 11, 1179–1188 CrossRef CAS.
- Y. Lei, Y. Wang, Y. Liu, C. Song, Q. Li, D. Wang and Y. Li, Angew. Chem., Int. Ed., 2020, 59, 20794–20812 CrossRef CAS PubMed.
- B. Liu, Y. F. Zhao, H. Q. Peng, Z. Y. Zhang, C. K. Sit, M. F. Yuen, T. R. Zhang, C. S. Lee and W. J. Zhang, Adv. Mater., 2017, 29, 1606521 CrossRef PubMed.
- Y. M. Ma, Z. D. He, Z. F. Wu, B. Zhang, Y. Zhang, S. J. Ding and C. H. Xiao, J. Mater. Chem. A, 2017, 5, 24850–24858 RSC.
- X. Zhao, P. Pachfule, S. Li, J. R. J. Simke, J. Schmidt and A. Thomas, Angew. Chem., Int. Ed., 2018, 57, 8921–8926 CrossRef CAS PubMed.
- Z. C. Li, J. J. Ma, B. Zhang, C. X. Song and D. B. Wang, CrystEngComm, 2017, 19, 1479–1485 RSC.
- Y. Cao, H. X. Wang, R. M. Ding, L. C. Wang, Z. Liu and B. L. Lv, Appl. Catal. Gen., 2020, 589, 117308 CrossRef CAS.
- H. H. Wu, H. B. Li, X. F. Zhao, Q. F. Liu, J. Wang, J. P. Xiao, S. H. Xie, R. Si, F. Yang, S. Miao, X. G. Guo, G. X. Wang and X. H. Bao, Energy Environ. Sci., 2016, 9, 3736–3745 RSC.
- B. Volosskiy, H. Fei, Z. Zhao, S. Lee, M. Li, Z. Lin, B. Papandrea, C. Wang, Y. Huang and X. Duan, ACS Appl. Mater. Interfaces, 2016, 8, 26769–26774 CrossRef CAS PubMed.
- M. Kuang, Q. H. Wang, P. Han and G. F. Zheng, Adv. Energy Mater., 2017, 7, 1700193 CrossRef.
- Y. Li, X. Tan, R. K. Hocking, X. Bo, H. Ren, B. Johannessen, S. C. Smith and C. Zhao, Nat. Commun., 2020, 11, 2720 CrossRef CAS PubMed.
- K. L. Zhou, C. H. Wang, Z. L. Wang, C. B. Han, Q. Q. Zhang, X. X. Ke, J. B. Liu and H. Wang, Energy Environ. Sci., 2020, 13, 3082–3092 RSC.
- L. Zhang, L. Han, H. Liu, X. Liu and J. Luo, Angew. Chem., Int. Ed., 2017, 56, 13694–13698 CrossRef CAS PubMed.
- J. Q. Zhang, Y. F. Zhao, X. Guo, C. Chen, C. L. Dong, R. S. Liu, C. P. Han, Y. D. Li, Y. Gogotsi and G. X. Wang, Nat. Catal., 2018, 1, 985–992 CrossRef CAS.
- K. Jiang, B. Liu, M. Luo, S. Ning, M. Peng, Y. Zhao, Y. R. Lu, T. S. Chan, F. M. F. de Groot and Y. Tan, Nat. Commun., 2019, 10, 1743 CrossRef PubMed.
- S. Liu, Y. Shen, Y. Zhang, B. Cui, S. Xi, J. Zhang, L. Xu, S. Zhu, Y. Chen, Y. Deng and W. Hu, Adv. Mater., 2022, 34, e2106973 CrossRef PubMed.
- J. Wang, F. Xu, H. Jin, Y. Chen and Y. Wang, Adv. Mater., 2017, 29, 1605838 CrossRef PubMed.
- H. Gao and M. K. Stenstrom, Water Environ. Res., 2019, 91, 101–110 CrossRef CAS PubMed.
- J. Huang, C. Du, J. Nie, H. Zhou, X. Zhang and J. Chen, Electrochim. Acta, 2019, 326, 134982 CrossRef CAS.
- M. Li, X. Liu, Y. Xiong, X. Bo, Y. Zhang, C. Han and L. Guo, J. Mater. Chem. A, 2015, 3, 4255–4265 RSC.
- J. Pan, S. W. Yu, Z. W. Jing, Q. T. Zhou, Y. F. Dong, X. D. Lou and F. Xia, Small Struct., 2021, 2, 2100076 CrossRef CAS.
- E. A. Paoli, F. Masini, R. Frydendal, D. Deiana, C. Schlaup, M. Malizia, T. W. Hansen, S. Horch, I. E. L. Stephens and I. Chorkendorff, Chem. Sci., 2015, 6, 190–196 RSC.
- L. Li, P. Wang, Q. Shao and X. Huang, Adv. Mater., 2021, 33, e2004243 CrossRef PubMed.
- C. Wei, S. Sun, D. Mandler, X. Wang, S. Z. Qiao and Z. J. Xu, Chem. Soc. Rev., 2019, 48, 2518–2534 RSC.
- K. Wang, H. Du, S. He, L. Liu, K. Yang, J. Sun, Y. Liu, Z. Du, L. Xie, W. Ai and W. Huang, Adv. Mater., 2021, 33, e2005587 CrossRef PubMed.
- X. Chen, J. Wan, J. Wang, Q. Zhang, L. Gu, L. Zheng, N. Wang and R. Yu, Adv. Mater., 2021, 33, e2104764 CrossRef PubMed.
- L. Zhuang, Y. Jia, H. Liu, Z. Li, M. Li, L. Zhang, X. Wang, D. Yang, Z. Zhu and X. Yao, Angew. Chem., Int. Ed., 2020, 59, 14664–14670 CrossRef CAS PubMed.
- Y. Jiao, W. Z. Hong, P. Y. Li, L. X. Wang and G. Chen, Appl. Catal. B Environ., 2019, 244, 732–739 CrossRef CAS.
- M. Gong, W. Zhou, M.-C. Tsai, J. Zhou, M. Guan, M.-C. Lin, B. Zhang, Y. Hu, D.-Y. Wang, J. Yang, S. J. Pennycook, B.-J. Hwang and H. Dai, Nat. Commun., 2014, 5, 4695 CrossRef CAS PubMed.
- L. Wang, Micro & Nano Lett., 2017, 12, 166–169 CrossRef CAS.
- V. S. Kumbhar, M. H. Cho, J. Lee, W. K. Kim, M. Lee, Y. R. Lee and J.-J. Shim, New J. Chem., 2017, 41, 10584–10591 RSC.
- L. Zhao, Y. Zhang, Z. Zhao, Q. H. Zhang, L. B. Huang, L. Gu, G. Lu, J. S. Hu and L. J. Wan, Natl. Sci. Rev., 2020, 7, 27–36 CrossRef CAS PubMed.
- J. Dong, Z. Qian, P. Xu, M.-F. Yue, R.-Y. Zhou, Y. Wang, Z.-A. Nan, S. Huang, Q. Dong, J.-F. Li, F. R. Fan and Z.-Q. Tian, Chem. Sci., 2022, 13, 5639–5649 RSC.
- J. Wang, S. Mao, Z. Liu, Z. Wei, H. Wang, Y. Chen and Y. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 7139–7147 CrossRef CAS PubMed.
- Y. F. Xu, M. R. Gao, Y. R. Zheng, J. Jiang and S. H. Yu, Angew. Chem., Int. Ed., 2013, 52, 8546–8550 CrossRef CAS PubMed.
- J. Wang, K. Li, H.-x. Zhong, D. Xu, Z.-l. Wang, Z. Jiang, Z.-j. Wu and X.-b. Zhang, Angew. Chem., Int. Ed., 2015, 54, 10530–10534 CrossRef CAS PubMed.
- B. H. R. Suryanto, Y. Wang, R. K. Hocking, W. Adamson and C. Zhao, Nat. Commun., 2019, 10, 5599 CrossRef CAS PubMed.
- X. J. Wu, B. M. Feng, W. Li, Y. L. Niu, Y. A. Yu, S. Y. Lu, C. Y. Zhong, P. Y. Liu, Z. Q. Tian, L. Chen, W. H. Hu and C. M. Li, Nano Energy, 2019, 62, 117–126 CrossRef CAS.
- J. Mei, T. He, J. Bai, D. Qi, A. Du, T. Liao, G. A. Ayoko, Y. Yamauchi, L. Sun and Z. Sun, Adv. Mater., 2021, e2104638 CrossRef PubMed.
- Z. Q. Wang, P. Wang, H. G. Zhang, W. J. Tian, Y. Xu, X. N. A. Li, L. Wang and H. J. Wang, J. Mater. Chem. A, 2021, 9, 18576–18581 RSC.
- L. Li, L. Bu, B. Huang, P. Wang, C. Shen, S. Bai, T. S. Chan, Q. Shao, Z. Hu and X. Huang, Adv. Mater., 2021, 33, e2105308 CrossRef PubMed.
- J. Duan, S. Chen and C. Zhao, Nat. Commun., 2017, 8, 15341 CrossRef CAS PubMed.
- T. Tang, W. J. Jiang, S. Niu, N. Liu, H. Luo, Y. Y. Chen, S. F. Jin, F. Gao, L. J. Wan and J. S. Hu, J. Am. Chem. Soc., 2017, 139, 8320–8328 CrossRef CAS PubMed.
- W. Lao-atiman, K. Bumroongsil, A. Arpornwichanop, P. Bumroongsakulsawat, S. Olaru and S. Kheawhom, Front. Energy Res., 2019, 7, 15 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2sc02845j |
| This journal is © The Royal Society of Chemistry 2022 |