Open Access Article
Open Access ArticleSynthesis of carbinoxamine via α-C(sp3)–H 2-pyridylation of O, S or N-containing compounds enabled by non-D–A-type super organoreductants and sulfoxide- or sulfide HAT reagents†
Xian-Chao
Cui
a,
Hu
Zhang
a,
Yi-Ping
Wang
a,
Jian-Ping
Qu
 *b and
Yan-Biao
Kang
*b and
Yan-Biao
Kang
 *a
*a
aDepartment of Chemistry, University of Science and Technology of China, Hefei 230026, China. E-mail: ybkang@ustc.edu.cn
bInstitute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, China. E-mail: ias_jpqu@njtech.edu.cn
First published on 1st September 2022
Abstract
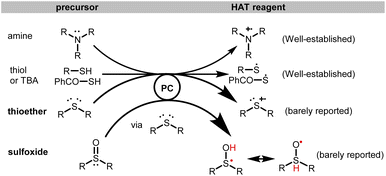
The radical cations of tertiary amines (R3N) have been well-established as the precursors of HAT reagents in photochemical transformations. Similarly, thiols and thioacids bearing SH groups have also been widely applied as HAT reagents. Despite the fact that sulfoxides (R2SO) and sulfides (RSR) also bear lone pairs of electrons, these compounds have been barely reported as HAT reagents in photocatalysis. On the other hand, the α-C–H 4-pyridylation of O or N-containing compounds has been documented, whereas 2-pyridylation remains challenging. However, the antihistamine and anticholinergic agent carbinoxamine is an ether bearing 2-pyridyl, which has not been obtained by the existing α-photoarylation of ether. In this work, we report the discovery of a non-donor–acceptor (D–A) type organic photoreductant CBZ6 and sulfoxide/sulfide synergistically catalyzed general α-C(sp3)–H arylation of ethers, thioethers and amines. By using as low as 1 mol% of CBZ6 as a recyclable organic photoreductant and sulfoxides or sulfides as a new type of HAT reagent, the 2- or 4-pyridylation of O, N, or S-containing compounds has been accomplished. This is the first base-free version of α-C–H 2-/4-pyridylation of O, N, or S-containing compounds. It is the first example of sulfoxides or sulfides working as HAT reagents. It is also the first general method for photocatalytic HAT 2-pyridylation of various ethers, amines or thioethers.
Introduction
Numerous diarylmethylalkyl ethers, sulfoxides and amines are pharmaceutical or bioactive molecules (Fig. 1). These compounds can be synthesized by the arylation of corresponding ether or amine precursors. For example, carbinoxamine is an antihistamine and anticholinergic agent, which might be prepared by the α-C–H 2-pyridylation of the corresponding ether (Fig. 1, bottom). The photocatalytic hydrogen atom transfer (HAT) is a straightforward route for α-C(sp3)–H functionalization of ethers, thioethers and amines.1–5 Compared to the alternative two-step SET-deprotonation pathway under photochemical conditions in the presence of bases,4–11 the HAT pathway3,12 does not need bases for deprotonation. Due to the mechanistic discrepancy, the regioselectivities of HAT and SET are usually quite different. For example, the C–H arylation of amines via the SET-deprotonation process normally favors cyclic amines on the non-benzylic positions.4–10 In contrast, the HAT process can accomplish the benzylic C–H arylation of amines.11,12 Despite the fact that a photochemical HAT process exhibits unique regioselectivity as well as catalytic efficiency, fewer examples for the HAT C(sp3)–H arylation have been documented.12 Nevertheless, the photo-arylation of ethers only goes through the HAT process rather than the SET-deprotonation process, which has been less reported than that of amines. In addition, with respect to a general problem in the α-C–H arylations of ethers or amines, 4-pyridylation has been frequently reported, whereas 2-pyridylation has been barely reported.13,14 However, the synthesis of carbinoxamine through the radical coupling between ethers and 2-pyridyl needs such 2-pyridylation (Fig. 1, bottom).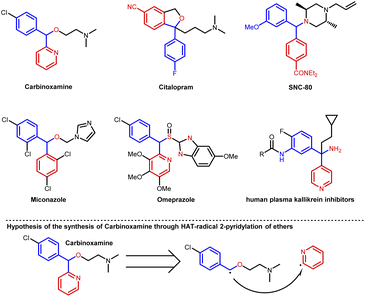 | ||
| Fig. 1 Pharmaceutical or bioactive molecules bearing diarylmethylalkyl ethers, sulfoxides or amines. | ||
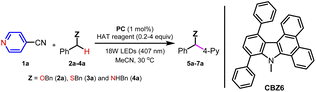
On the other hand, the radical cations of tertiary amines (R3N) have been widely applied as HAT reagents in the photochemical redox transformations. Similarly, thiols and thioacids bearing SH groups have also been applied as HAT reagents.15 Despite the fact that sulfoxides (R2SO) and thioethers (RSR) also bear lone pairs of electrons, these S-containing compounds have been barely reported as HAT reagents in photocatalysis (Fig. 2). In this work, we report our discovery of sulfoxides or sulfides as HAT reagents and an organic photoreductant CBZ6 synergistically catalyzed α-C(sp3)–H arylation of ethers, thioethers and amines. Both 2- and 4-pyridylation of O, N, or S-containing compounds has been accomplished.
Results and discussion
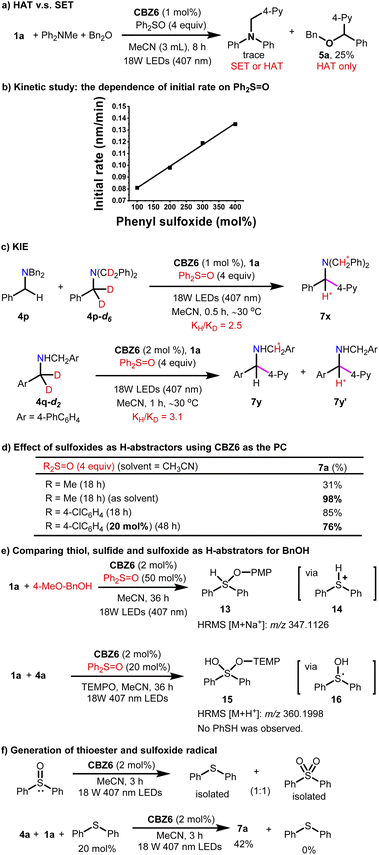
Photocatalysts (PCs) and HAT reagents were screened in α-C(sp3)–H 4-pyridylation of 1a with ether (2a), thioether (3a) and amine (4a) (Table 1). First, dibenzyl sulfoxide 8a was applied to investigate whether sulfoxide can act as a HAT reagent. It was found that 8a is efficient to afford α-pyridylation products 5a, 6a and 7a in 81%, 85%, and 82% yields, respectively (Table 1, entries 1–3). The same reaction conditions work for either ethers, thioethers or amines, suggesting the HAT pathway rather than the SET-deprotonation way because it is difficult for ethers to follow the SET pathway. Even the solvent DMSO was reactive without extra sulfoxides, and 98% 7a was obtained (entry 5). The reaction in the absence of sulfoxide 8a gives no conversion of starting materials (entry 4), indicating that the HAT reagent is necessary for this reaction. In comparison, several common PCs were screened, and no better results were achieved (entries 6–10).| Entry | PC | HAT reagent | Yield (%) |
|---|---|---|---|
| a 4 equiv. Ph2SO. b 20 mol% of HAT reagents. | |||
| 1a | CBZ6 | Ph2S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (8a) O (8a) |
5a, 81 |
| 2a | CBZ6 | Ph2S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (8a) O (8a) |
6a, 85 |
| 3a | CBZ6 | Ph2S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (8a) O (8a) |
7a, 82 |
| 4 | CBZ6 | None | 7a, 0 |
| 5 | CBZ6 | DMSO (no MeCN) | 7a, 98 |
| 6 | Eosin Y | DMSO (no MeCN) | 7a, trace |
| 7 | Rhodamine B | DMSO (no MeCN) | 7a, trace |
| 8 | Thioxanthen-9-one | DMSO (no MeCN) | 7a, 25 |
| 9 | Ir(ppy)3 | DMSO (no MeCN) | 7a, 40 |
| 10 | TBADT | DMSO (no MeCN) | 7a, 0 |
| 11 | None | DMSO (no MeCN) | 7a, 0 |
| 12b | CBZ6 | DABCO | 7a, 22 |
| 13b | CBZ6 | 1-Dodecanethiol (9a) | 7a, 55 |
| 14b | CBZ6 | iPr3SiSH (9b) | 7a, 23 |
| 15b | CBZ6 | 4-ClC6H5SH (9c) | 7a, 76 |
| 16b | CBZ6 | PhSPh (10) | 7a, 42 |
| 17b | CBZ6 | (4-ClC6H5)2S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (8b) O (8b) |
7a, 76 |
| 18 | PhC(O)SH (TBA) | K3PO4 in DMA (455 nm, ref. 12) | 7a, 63 |
| 19 | Ir(ppy)3 | 9a, 9c or 10 | 7a, 0 |
| 20 | Ir(ppy)3 | 4-tBuC6H4SH (9d) (425 nm, ref. 11) | 7a, trace |
Next, several potential HAT reagents were examined with a loading of 20 mol%. The tertiary amine DABCO is reactive but gives a low yield (entry 12). Thiols such as 1-dodecanethiol and iPr3SiSH are also reactive to give moderate yields even in the absence of bases (entries 13 and 14). Thiophenol 4-Cl–PhSH is a better HAT reagent than 1-dodecanethiol (entry 15 vs. 13). Surprisingly, diphenyl thioether 10 is also reactive as a HAT reagent (entry 16). Sulfoxide 8b is an even better HAT reagent than thioether 10, and 76% yield was obtained (entry 17). Thiobenzoic acid (TBA)-catalysis, which demonstrated good catalytic activity in the 4-pyridylation of amines,12 gave decreased yield (entry 18). In addition, the 4-pyridylation of dibenzyl amine 4a failed when using Ir(ppy)3 as a PC (entries 19 and 20). Finally, diphenyl sulfoxide 8a was chosen as the HAT agent under the standard reaction conditions. CBZ6 (1–5 mol%) was utilized as an organic photoreductant. Acetyl nitrile was the optimized solvent.
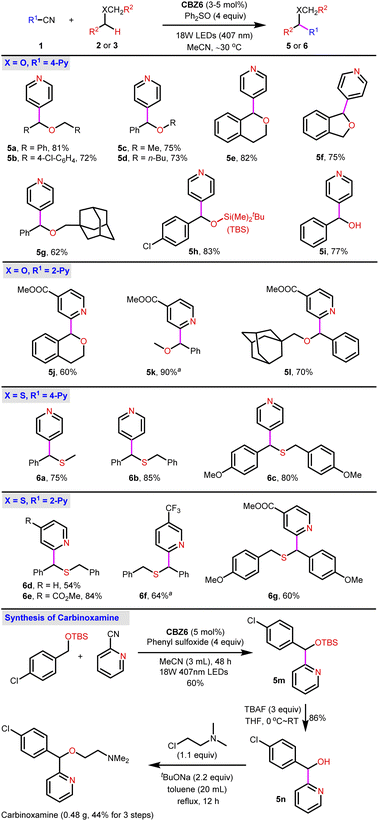
After the standard reaction conditions have been established, the scope of ethers, thioethers and amines was investigated. First, O-containing compounds including ethers and alcohols were subjected to the standard reaction conditions. What should be pointed out is that ethers are challenging substrates for photocatalytic α-C–H arylation via the SET pathway. The HAT pathway is a straightforward pathway for ethers. Nevertheless, only one example of α-C–H 4-pyridylation of dibenzyl ether with moderate yield (42% yield) was demonstrated in a previous study12 with noble metal PCs, whereas no example for 2-pyridylation has been documented.3,11,12 With respect to the alcohols, neither 2-pyridylation nor 4-pyridylation has been reported. Although the silyl-protected alcohols are suitable for 4-pyridylation, no example of 2-pyridylation has been documented. Therefore, the photocatalytic synthesis of carbinoxamine via the photocatalytic α-C–H 2-pyridylation has not been accomplished to date. To our delight, not only 4-pyridylation but also 2-pyridylation was achieved by the photocatalysis of CBZ6 with Ph2SO as the HAT reagent (Fig. 3). Both dibenzyl ethers (5a, 5b) and benzyl methyl/tert-butyl ethers (5c, 5d) afford good yields. Cyclic ethers (5e, 5f) gave comparable yields. The increase of steric hindrance leads to the decrease of yield to 62% (5g). The arylation of silyl ether (5h) and benzyl alcohol (5i) affords the desired products in 83% and 77% yields, respectively. The α-C–H 2-pyridylation has been barely reported for ethers. It was found that this CBZ6-sulfoxide system is a powerful tool for 2-pyridylation. The 2-pyridylation of ether was achieved in moderate to good yields (5j–l).
The α-C–H arylation of thioethers is normally achieved by Pummerer rearrangement.16 Fortunately, the 4-pyridylation of methyl benzyl ether or dibenzyl thioethers was achieved with 75–85% yields (6a–c) (Fig. 3). The α-C–H 2-pyridylation of thioethers was also successfully realized in the presence of the CBZ6-sulfoxide system providing 2-pyridylation products 6d–g in moderate yields.
The success of 2-pyridylation of ethers encouraged us to pursue the synthesis of carbinoxamine from a simple ether. A 3-step synthesis of carbinoxamine was accomplished with a total 44% yield via a key gram-scale 2-pyridylation of protected benzyl alcohol (Fig. 3, bottom).
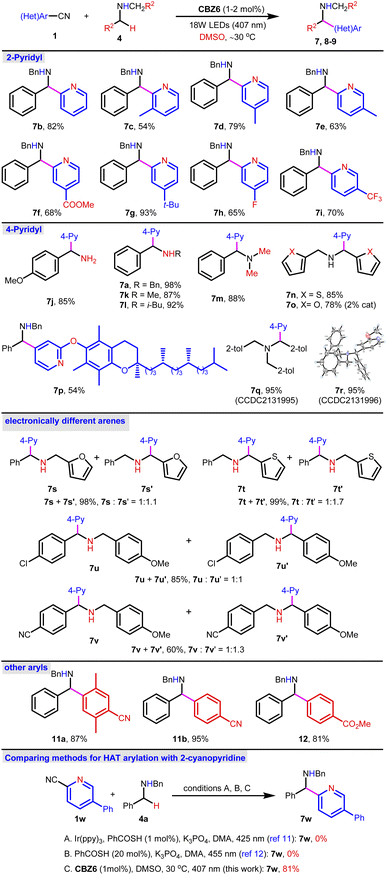
Compared to ethers or thioethers, amines are more reactive and might follow either the SET or HAT pathway for the α-arylation reactions under the irradiation of visible light. In previous reports, cyclic amines were widely examined in the photocatalytic 4-pyridylation via the SET-deprotonation pathway where bases were necessary for deprotonation. The arylation of acyclic amines has been less reported and was mainly concentrated on the non-benzylic positions. Thiobenzoic acid (TBA)-catalysis is the corner stone for the HAT arylation of amines.12 However, the 2-pyridylation of dibenzyl amine 4a fails without either Ir(ppy)3 or TBA PCs (Fig. 4, bottom, conditions A and B). Nevertheless, despite the fact that 4-pyridylation has been well established, the 2-pyridylation of either ethers or amines remains challenging. To test the catalytic ability of the CBZ6-sulfoxide system in 2-pyridylation, dibenzyl amine 4a was subjected to the standard reaction conditions using DMSO as a solvent and HAT reagent (Fig. 4, top). Product 7b was isolated in 82% yield. A range of nitriles were applied as 2-pyridylation reagents, and the desired products 7c–i were obtained in up to 93% yields. Besides the 2-pyridylation, the 4-pyridylation of amines was also smooth. The primary amine (7j), secondary amines (7a, k–l), and tertiary amine (7m) were all successful. The amines bearing heterocyclics (7n–o) as well as the arylation reagent bearing a 2-substituent (7p–r) were investigated, and the corresponding products were achieved in 53–95% yields. When amines bearing two electronically different arenes (7s–v) were used, a mixture of diastereomers was obtained in moderate to excellent yields. Other typical aryls were also examined (11a–b, 12) giving the desired products in good to excellent yields. All the above-mentioned examples indicate that the CBZ6-sulfoxide system is a general and efficient catalytic system for the α-C–H arylation of O, S, and N-containing compounds.
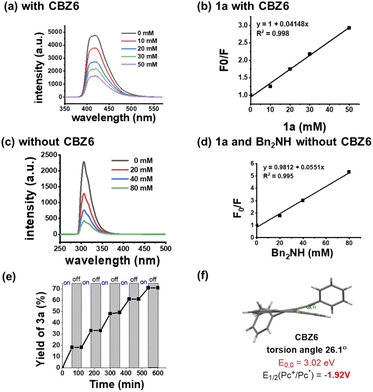
With respect to the reaction mechanism, the key point is the catalytic model of CBZ6 and the role of sulfoxides. Is it a SET or HAT process? The control experiments have been carried out to reveal the nature of this catalytic system. A mixture of Ph2NMe and Bn2O was treated under standard reaction conditions for 8 hours, and only 25% 5a was obtained (Fig. 5a). It is obvious that dibenzyl ether is more reactive than diphenyl methyl amine. Supposing ether can only follow the HAT pathway whereas amine can follow either the SET or HAT pathway, the above results support the HAT pathway.
The kinetic study shows a first-order dependence of the initial rate on the concentration of Ph2SO, indicating that sulfoxides directly participate in the rate determining step (Fig. 5b). The KIEs of 2.5 for 4p and 3.1 for 4q are consistent with the rate determining step of H-atom abstraction (Fig. 5c). The effect of sulfoxides was then investigated, and the results summarized in Fig. 5d indicate that sulfoxide works as a H-abstractor. The HRMS experiments detected intermediates 13 and 15, suggesting the existence of intermediates 14 and 16 (Fig. 5e). Thioester PhSPh proved to be generated from the decomposition of sulfoxide (Fig. 5f). When PhSPh was used as the HAT reagent, the arylation of 4a with 1a was achieved in 42%. Simultaneously, PhSPh disappeared and has been converted to Ph2SO. All the aforementioned evidence can prove that intermediate 16 is the catalytically active species for the HAT reagent in this arylation. Note that the reaction initialized from either thioesters or sulfoxides; the key intermediates as HAT reagents should be the same, probably radical 16.
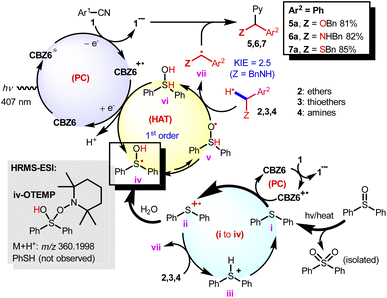
Fluorescence quenching experiments of CBZ6 illustrate the quenching effect with 4-cyanopyridine 1a (Fig. 6a). The Stern–Volmer plot shows that the excited state of CBZ6 has been quenched by 1a, and the quenching effect increased with the growth of the concentration of 1a (Fig. 6b). Although the quenching effect between 4-cyanopyridine and Bn2NH was detected at 307 nm, no quenching was observed in the range of around 407 nm (Fig. 6c and d), indicating that the EDA between 1a and 4a is not possible at 407 nm, therefore the SET model can be ruled out in this work. The on–off experiments prove the necessity of light.
Based on the experimental evidence demonstrated in Fig. 5 and 6, a proposed reaction mechanism is illustrated in Fig. 7. CBZ6 works as a redox neutral PC, and sulfoxides act as the HAT reagents. CBZ6 has been established as a powerful organic photoreductant (Fig. 6f).17,18 Thioether i is generated via the decomposition of sulfoxide under the irradiation of a LED at 407 nm. This thioether is oxidized to thioether radical cation ii by the CBZ6 radical cation which comes from the quenching effect with nitrile 1. Intermediate ii can abstract the α-H-atom of ethers or amines to form iii, which can be detected (Fig. 5e). It can also transform into radical iv, followed by the isomerization to O-centered radical v. Radical iv can be detected by a spin trapping reagent TEMPO with HRMS-ESI (M + H+ 360.1998). What should be noted is that PhSH has not been detected or isolated, suggesting that PhSH cannot be the reactive HAT reagent in this work. O-radical v abstracts the H atom of ethers or amines to yield α-C-radical vii, followed by the addition to a nitrile radical to afford target α-C–H arylation products 5–7. Intermediate vi is oxidized to regenerate iv by the CBZ6 radical cation. Because neither thiol nor thioether is observed in the reaction, the catalytically active species of the HAT reagent is assigned to radical iv or v. Radical iv is too bulky to abstract the H atom; v is believed to be the reactive form.
Conclusions
In conclusion, we develop a general visible light organic photocatalytic α-C(sp3)–H arylation of ethers, thioethers and amines by using as low as 1 mol% of CBZ6 as a recyclable organic photoreductant and sulfoxides and thioethers as unique hydrogen abstractors. The first base-free photocatalytic α-C–H arylation of O, S and N-containing compounds is thus presented. Sulfoxides and thioethers have been first discovered as HAT reagents. 2-Pyridylation of ethers and amines is therefore enabled by the CBZ6-sulfoxide HAT system. Consequently, the antihistamine and anticholinergic agent carbinoxamine was synthesized by this method in total 44% yield on the gram scale. The insight into the mechanism as well as further applications of this method are being studied in our laboratory.Data availability
All experimental data and detailed experimental procedures are available in the ESI.†Author contributions
X.-C. C., H. Z. and Y.-P. W. performed experiments and characterization. X.-C. C., Y.-B. K. and J.-P. Q. conceived the research plan. Y.-B. K. and J.-P. Q. supervised the project, wrote the manuscript and secured funding.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Key R&D Program of China (No. 2021YFA1500100), the National Natural Science Foundation of China (22271268, 21922109, 21672196, and 21831007), the National Synchrotron Radiation Lab of the University of Science and Technology of China (UN2018LHJJ), and the Start-up Funding from Nanjing Tech University (39837134) for financial support.Notes and references
- L. Capaldo, D. Ravelli and M. Fagnoni, Chem. Rev., 2022, 122, 1875–1924 CrossRef CAS PubMed.
- N. Holmberg-Douglas and D. A. Nicewicz, Chem. Rev., 2022, 122, 1925–2016 CrossRef CAS.
- K. Qvortrup, D. A. Rankic and D. W. C. MacMillan, J. Am. Chem. Soc., 2014, 136, 626–629 CrossRef CAS PubMed.
- A. McNally, C. K. Prier and D. W. C. MacMillan, Science, 2011, 334, 1114–1117 CrossRef CAS PubMed.
- A. Noble and D. W. C. MacMillan, J. Am. Chem. Soc., 2014, 136, 11602–11605 CrossRef CAS PubMed.
- C.-C. Yan, L.-L. Li, Y.-X. Liu and Q.-M. Wang, Org. Lett., 2016, 18, 4686–4689 CrossRef CAS PubMed.
- J. A. Vega, J. M. Alonso, G. Méndez, M. Ciordia, F. Delgado and A. A. Trabanco, Org. Lett., 2017, 19, 938–941 CrossRef CAS PubMed.
- Z.-M. Sun, J.-C. Zhao, H.-W. Deng, L. Tian, B.-Q. Tang, K.-C. Liu and H.-Y. Zhu, Adv. Synth. Catal., 2020, 362, 1502–1508 CrossRef CAS.
- C. Xu, F.-Q. Shen, G.-F. Feng and J. Jin, Org. Lett., 2021, 23, 3913–3918 CrossRef CAS PubMed.
- H.-B. Zhao and D. Leonori, Angew. Chem., Int. Ed., 2021, 60, 7669–7674 CrossRef CAS.
- T. Ide, J. P. Barham, M. Fujita, Y. Kawato, H. Egami and Y. Hamashima, Chem. Sci., 2018, 9, 8453–8460 RSC.
- F. Kobayashi, M. Fujita, T. Ide, Y. Ito, K. Yamashita, H. Egami and Y. Hamashima, ACS Catal., 2021, 11, 82–87 CrossRef CAS.
- D. Lehnherr, Y. Lam, M. C. Nicastri, J. Liu, J. A. Newman, E. L. Regalado, D. A. DiRocco and T. Rovis, J. Am. Chem. Soc., 2020, 142, 468–478 CrossRef CAS PubMed.
- M. C. Nicastri, D. Lehnherr, Y. Lam, D. A. DiRocco and T. Rovis, J. Am. Chem. Soc., 2020, 142, 987–998 CrossRef CAS PubMed.
- For reviews on S-centered H-abstractors: F. Dénès, M. Pichowicz, G. Povie and P. Renaud, Chem. Rev., 2014, 114, 2587–2693 CrossRef PubMed.
- K. Colas, R. Martín-Montero and A. Mendoza, Angew. Chem., Int. Ed., 2017, 56, 16042–16046 CrossRef CAS.
- Z.-H. Luan, J.-P. Qu and Y.-B. Kang, J. Am. Chem. Soc., 2020, 142, 20942–20947 CrossRef CAS PubMed.
- H. Wang, J.-P. Qu and Y.-B. Kang, Org. Lett., 2021, 23, 2900–2903 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2131995 and 2131996. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2sc03504a |
| This journal is © The Royal Society of Chemistry 2022 |