Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Highly selective acid-catalyzed olefin isomerization of limonene to terpinolene by kinetic suppression of overreactions in a confined space of porous metal–macrocycle frameworks
Wei
He
,
Shohei
Tashiro
* and
Mitsuhiko
Shionoya
*
Department of Chemistry, Graduate School of Science, The University of Tokyo, Tokyo 113-0033, Japan. E-mail: shionoya@chem.s.u-tokyo.ac.jp
First published on 11th August 2022
Abstract
Correction for ‘Highly selective acid-catalyzed olefin isomerization of limonene to terpinolene by kinetic suppression of overreactions in a confined space of porous metal–macrocycle frameworks’ by Wei He et al., Chem. Sci., 2022, 13, 8752–8758, https://doi.org/10.1039/d2sc01561g.
The authors regret that there were errors in Fig. 2, Fig. 5 and Fig. 6 in the original article and Fig. S18 of the ESI. The stereochemistry of the chemical structural formulas for (−)-α-pinene (6) and (−)-β-pinene (7) was incorrectly reversed. The correct versions of the figures are shown below, and in the updated version of the ESI.
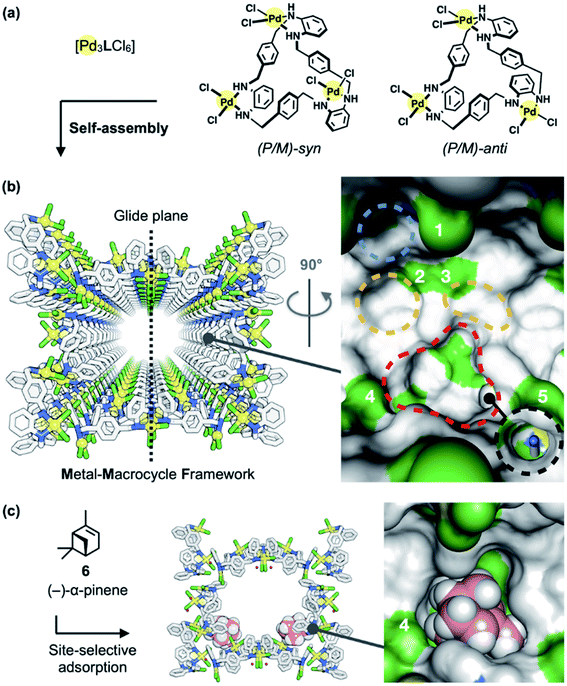 | ||
| Fig. 2 Metal–macrocycle framework (MMF). (a) Self-assembly of asymmetrically twisted PdII-macrocycles into (b) a porous crystal MMF (sticks model) with five enantiomeric pairs of binding pockets (surface model). (c) Previously reported site-selective adsorption of (−)-α-pinene (6) (space-filling model) on the channel surface of the MMF.1 Blue, yellow, red, or black dashed circles indicate the ceiling-, side-, bottom-, or tubular-pockets of the MMF, respectively. MMF: Pd, yellow; Cl, green; N, blue; C, grey. 6: C, pink; H, white. Hydrogen atoms attached to the MMF were omitted for clarity. Green or blue surface represents exposed Cl or N–H groups of the MMF, respectively. | ||
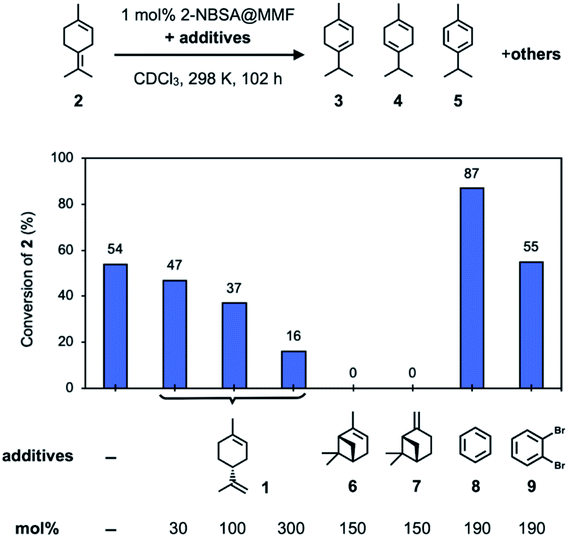 | ||
| Fig. 5 Investigation of the inhibitory effects of additives on the isomerization reaction of 2 using 2-NBSA@MMF at 25 °C for 102 h. | ||
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- S. Tashiro, T. Umeki, R. Kubota and M. Shionoya, Faraday Discuss., 2021, 225, 197–209 RSC.
| This journal is © The Royal Society of Chemistry 2022 |