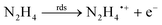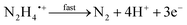Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electroanalytical overview: the electroanalytical sensing of hydrazine
Robert D.
Crapnell
 and
Craig E.
Banks
and
Craig E.
Banks
 *
*
Faculty of Science and Engineering, Manchester Metropolitan University, Chester Street, Manchester M1 5GD, UK. E-mail: c.banks@mmu.ac.uk; Tel: +44 (0)1612471196
First published on 29th September 2021
Abstract
In this overview, we explore the electroanalytical sensing of the important chemical reagent hydrazine, highlighting the plethora of electrochemical sensing strategies utilised from the first reports in 1951 to the present day. It is observed that a large proportion of the work developing electrochemical sensors for hydrazine focus on the use of metallic nanoparticles and some other surface modifications, although we note that the advantages of such strategies are often not reported. The use of nanoparticle-modified electrodes to this end is explored thoroughly, indicating that they allow the same electrochemical response as that of a macroelectrode made of the same material, with clear cost advantages. It is recommended that significant studies exploring the surface coverage/number of nanoparticles are performed to optimise electroanalytical devices and ensure that thin-layer effects are not producing false observations through electrocatalysis. Development of these sensor platforms has begun to transition away from classical macroelectrodes, toward more mass producible supporting electrodes such as screen-printed and inkjet-printed electrodes. We suggest significant advances in this area are still to be found. The vast majority of developed electroanalytical sensors for hydrazine are tested in aqueous based environments, such as tap, river and industrial effluent waters. There is significant scope for development of hydrazine sensors for gaseous environments and biologically relevant samples such as blood, serum and urine, aiming to produce sensors for accurate occupational exposure monitoring. Finally, we suggest that the levels of publications with independent validation of hydrazine concentrations with other well-established laboratory-based measurements is lacking. We believe that improving in these areas will lead to the development of significant commercial products for the electroanalytical detection of hydrazine.
Introduction: hydrazine
Hydrazine is an important chemical reagent, with the chemical formula N2H4, widely used in agricultural chemicals, air bags, pharmaceutical intermediates, photography chemicals, textile dyes, fuel for rockets, spacecraft and fuel cells and in boiler feed water systems where it acts a scavenger to remove traces of oxygen and reduces corrosion of metal pipes and fittings.1,2 The US National Institute for Occupational Safety and Health (NIOSH) reports that the recommended exposure limit (REL) in air is 0.03 ppm (2 h) and the permissible exposure limit 8 h total weight average (TWA) is 1 ppm, while the “Immediately Dangerous to Life or Health” (IDLH) is 50 ppm.3 The U.S. Environmental Protection Agency has classified hydrazine as an irritant and group B2, probable human carcinogen.4 The threshold limit value is reported to be no higher than 10 ppb.5,6 Due to hydrazine's industrial significance and toxicological effects, its sensing is widely explored. Methods for the detection of hydrazine sensing include: solid-phase spectrophotometry,2 gas chromatography-mass spectrometry,7 high-performance liquid chromatography-tandem mass spectrometry,8 and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry.9 Many sensors have been reported for the sensing of hydrazine, such as fluorescence,10,11 surface-enhanced Raman spectroscopy,12 chemiluminescence13 and electrochemistry to name just a few. In this overview we focus specifically on providing a thorough overview of the endeavours dedicated to the electroanalytical sensing of hydrazine.The electrochemical oxidation of hydrazine was studied as early as 1951 via polarography and oxide-coated platinum rotating disc electrodes.14,15 These works reported that the reaction in aqueous media yields nitrogen as a principal component and that the reaction proceeded more rapidly on an oxide-coated surface over that of a bare (clean) platinum surface. Bard explored the effect of electrode pre-treatment on the chronopotentiometric oxidation of hydrazine at a platinum electrode in aqueous solutions agreeing with Karp and Meites15 that a freshly prepared layer of platinum oxide plays an active role.16 Michlmayr and Sawyer explored the electrochemical oxidation of hydrazine, 1,1-dimethylhydrazine and 1,2-dimethylhydrazine in DMSO with chronopotentiometry, controlled-potential coulometry, and cyclic voltammetry at platinum electrodes, reporting that the overall reaction is a one-electron oxidation.17 Wang and Cao18 reported the first use of glassy carbon for the electrochemical oxidation of hydrazine, exploring the mechanism in both aqueous and non-aqueous media. Carbon based materials have the advantages of being economical, stable, having chemical inertness, give rise to relatively wide potential windows and low background current making them suitable for different types of electroanalysis, in-particular the sensing of hydrazine.
Compton et al. have recently provided a thorough overview of the electrochemical oxidation of hydrazine demonstrating that the phenomenological Butler–Volmer theory is not appropriate for interpreting the electrochemical process of hydrazine but rather reveals a strong potential dependence on the anodic transfer coefficient, consistent with the symmetric Marcus–Hush theory.19,20 Hydrazine in aqueous media has pKa1 and pKa2 values of 8.0 and −1.0 at 298 K respectively, which corresponds to the following chemical equilibria:19,20
| N2H5+ ⇆ N2H4 + H+ |
| N2H62+ ⇆ N2H5+ + H+ |
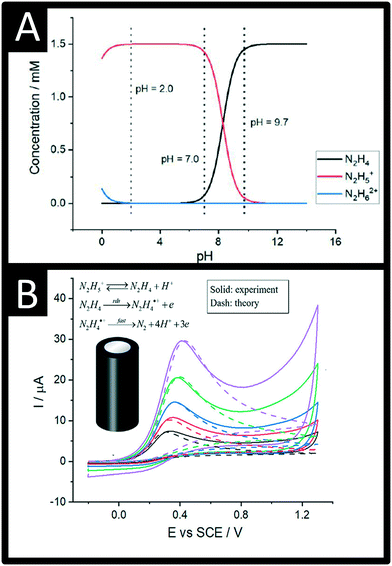 | ||
| Fig. 1 A: The speciation of hydrazine as a function of pH in pure water; B: Comparison between the experimental and DIGISIM-simulated voltammetry at a glassy carbon macroelectrode of 1.5 mM hydrazine supported by 0.1 M KNO3 at pH 9.7 (solid for experiment, dash for simulation). Simulation parameters: initial concentrations of N2H4, N2H5+, and H+ are 1.44 mM, 0.06 mM, and 2 × 10−10 M, respectively; formal potential of N2H4/N2H4˙+ is 0.14 V; reorganization energy is 0.35 eV; equilibrium constant of N2H4/N2H5+ is 5.0 × 10−9 M; diffusion coefficients of N2H4 and N2H5+ are 7.1 × 10−6 cm2 s−1 and 2.8 × 10−6 cm2 s−1, respectively; standard rate constant of the rds step is 4.5 × 10−5 cm s−1; second-order rate constant for the protonation of hydrazine is 1.1 × 105 M−1 s−1; figures reproduced with permission from ref. 19. Copyright 2021 American Chemical Society. | ||
The electrochemical oxidation of hydrazine at a glassy carbon electrode in aqueous media is shown in Fig. 1B along with fitting of the voltammetric profiles with DIGISIM, a commercial electrochemical simulation software. An excellent fit between theory and experiment is evident (Fig. 1B) and the electrochemical mechanism is described as:19,20
| N2H5+ ⇆ N2H4 + H+ |
A rigorous study by Compton et al.19,20 has elegantly shown that hydrazine is only electroactive in its unprotonated form, N2H4 whereas the protonated species N2H5+ is electro-inactive. Overall, the electrochemical oxidation of hydrazine occurs via a 4-electron process with the second step (see mechanism above) rate-determining step (rds). The standard rate constant of the rds step is 4.5 × 10−5 cm s−1; the second-order rate constant for the protonation of hydrazine is 1.1 × 105 M−1 s−1. The approximate estimation of the associated standard heterogeneous rate constant, k0, for the rate-determining step has been reported to be 4.5 (±1.0) × 10−5 cm s−1.19,20
Electroanalytical approaches
Table 1 provides an extensive overview of the current literature reported for the electroanalytical sensing of hydrazine, which shows the wide and diverse approaches utilised to measure this important analyte. From inspection of Table 1 we can observe that a plethora of electroanalytical approaches have been developed to provide electrochemical platforms for sensing hydrazine. Table 1 is in chronological order, showing one of the first studies utilised liquid chromatography with electrochemical detection in conjunction with cobalt phthalocyanine bulk modified carbon paste electrodes for the sensing of hydrazine over the range 10−3–10−8 M, with 6.4 pg (200 fmol) able to be routinely measured.21 Based on early electrochemical work, it is well known that the electrochemical oxidation of hydrazine is possible on palladium electrodes. Consequently, Liu et al.22 developed palladium nanoparticle modified carbon fiber microdisk array electrodes, which were employed in capillary electrophoresis for the simultaneous detection of hydrazine, methylhydrazine, and isoniazid. A wide linear range from 1 μM–5 mM was shown to be possible with a limit of detection (LOD) found to correspond to 1.2 pg. The authors successfully applied their sensor for the determination of hydrazine in spiked human urine, one of only a handful to determine hydrazine in this sample matrix.22 Wang et al. utilised capillary electrophoresis in conjunction with screen-printed carbon electrodes (SPEs) modified with palladium via electrodeposition, with low micromolar concentrations over the range 0.2–200 μM able to be readily detected.23 Capillary electrophoresis has not been widely used for hydrazine determination with electrochemistry and there is scope to explore this method further.| Electrode | Method of detection | Linear range | Limit of detection | Sample medium of real samples | Ref. |
|---|---|---|---|---|---|
| Key: Pd: palladium; CFMDE: carbon fibre microdisk electrode; ZnO: zinc oxide; NRs: nanorods; GCE: glassy carbon electrode NPs: nanoparticles; PEDOT–PSS: poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate); NR: not reported; MnO2: manganese oxide; MWCNTs: multi-walled carbon nanotubes; FePC: iron phthalocyanine; CoHCF: cobalt hexacyanoferrate; PoPD: poly(o-phenylenediamine); SPEs: screen-printed electrodes; CNTs: carbon nanotubes; ErGO: electrochemical reduced graphene oxide; BDD: boron-doped diamond; PDTYB: poly(4,5-dihydro-1,3-thiazol-2-ylsulfanyl-3-methyl-1,2-benzenediol); ITO: indium tin oxide; PANI: polyaniline; PAYR: poly(alizarin yellow R) Ag@C: Ag core–shell nanoparticles; PDDA: poly(diallyldimethylammonium chloride); CuHCF: copper(II) hexacyanoferrate nanocubes; CoPC: cobalt phthalocyanine; CPE: carbon paste electrode; LC-EC: liquid chromatography with electrochemical detection; EGN-IL: electrodeposited graphene ionic liquid composite film; PEDOT:PSS: poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate); CB: carbon black; CuS-OMC: copper sulfide-ordered mesoporous carbon; ZIF-8: zeolitic imidazolate framework-8 nanocrystals; PDI-GABA: perylene diimide derivative; PxDA: para benzylamine; CFCME: carbon fiber cylinder microelectrode; CoCuHCF: cobalt and copper hexacyanoferrate; ANSA: 1-amino-2-naphtol-4-sulfonic acid; PB: Prussian blue; CNP: nitrogen-doped carbon nanopolyhedra; PPy: polypyrrole; CNF: carbon nanofibre; VC@Ag: Vulcan carbon modified with silver nanoparticles; EDAC: ethylenediamine cellulose; PVP: poly(vinylpyrrolidone); AgNCs: silver nanocubes; 2-CDMA: 2-chloro-N′-[1-(2,5-dihydroxyphenyl) methylidene]aniline; polyCoTAPc: cobalt tetraamino phthalocyanine; EGNS: electroactivated graphite nanosheets; AG: activated graphite; GG-g-PAM: Guar gum grafted with poly(acrylamide); AuBPE: gold barrel plating electrode; PAMSA: poly(2-acryalamido-2-methyl-propane-sulfonic acid)-doped polyaniline layers; [hmim][PF6]: n-hexyl-3-methylimidazolium hexafluorophosphate; EFTA: ferrocene-derivative (ethyl2-(4-ferrocenyl[1,2,3]triazol-1-yl)acetate); NG: nitrogen-doped graphene; ZIF-67: cobalt-based zeolitic imidazolate framework; CTS; chitosan; poly(BCP): poly bromocresol purple; CCLP: calcium ions cross linked pectin film; FTO: fluorinated tin oxide glass; MBCPE: magnetic bar carbon paste electrode; DPB: 2-(3,4-dihydroxyphenyl) benzothiazole; FcD: 2,7-bis(ferrocenyl ethynyl)fluoren-9-one (2,7-BFE; 2PHC: 2-(4-oxo-3-phenyl-3,4-dihydroquinazolinyl)-N′-phenyl-hydrazinecarbothioamide; DHBPD: N,N′(2,3-dihydroxybenzylidene)-1,4-phenylene diamine; NPZ: nano P zeolite; ZSM-5: zeolite; CTAB: cetyltrimethyl ammonium bromide; NPC: N-doped porous carbon; OMC: ordered mesoporous carbon; BHD: bimetallic heterodimer; BCS: bimetallic core–shell; poly(CoOBImPc): cobalt(II) octabenzimidazolephthalocyanine; HRGO: honey reduced graphene oxide; TPP: platinum(II) tetraphenylporphyrin nanocomposite; NB: nile-blue; P-MWCNTs: polydopamine-modified multiwalled carbon nanotubes; MOF: zinc-metal organic frameworks; SSG: silicate sol–gel matrix; CH: choline film; CC: carbon cloth; APS: aminopropyl)triethoxysilane; AuTPP: gold tetra phenyl porphyrin; HM: redox mediator hematein; SPGEs: screen-printed graphene electrodes; BPPG: basal plane pyrolytic graphite electrode. | |||||
| CoPC/CPE | Cyclic voltammetry, LC-EC | 10−3–10−8 M | 6.4 pg (200 fmol) | NR | 21 |
| Pd NPs/CFMDE | Cyclic voltammetry, electrophoresis | 1 μM–5 mM | 1.2 pg | Spiked human urine | 22 |
| Chlorogenic acid/GCE | Amperometry | 0.05–3 mM | NR | NR | 52 |
| CoCuHCF/CFCME | Potentiometric | 1 μM–1 mM | 0.5 μM | NR | 53 |
| Pd/SPEs | Capillary electrophoresis | 0.2–200 μM | 1.5 μM | NR | 23 |
| FePc/Au | Square-wave voltammetry | 13–92 μM | 5 μM | NR | 54 |
| Pyrogallol red/GCE | Linear sweep | 5–600 μM | 1.7 μM | NR | 55 |
| CuCo – HCF/CPE | Amperometry | 0.1–12 mM | NR | NR | 56 |
| Pd-NPs/BDD | Linear sweep | 6.8–102 μM | 1.8 μM | NR | 31 |
| Pd-NPs – GC spheres/BPPG | Cyclic voltammetry | 100–600 μM | 2 μM | NR | 57 |
| ZnO – MWCNTs/GCE | Amperometry | 0.6 to 250 μM | 0.18 μM | Distilled water | 58 |
| Pd NPs – CNF/GCE | Differential pulse voltammetry | 10–4000 μM | 2.9 μM | NR | 59 |
| Curcumin – MWCNTs/GCE | Amperometry | 2–44 μM | 1.44 μM | NR | 60 |
| Au NPs/Ti | Cyclic voltammetry | 5–40 mM | NR | NR | 61 |
| Au-NPs – ZnO-MWCNTS/GCE | Amperometry | 0.5–1800 μM | 0.15 μM | Drinking water | 62 |
| TiO2 NPs – DHBPD/CPE | Differential pulse voltammetry | 0.01–4 μM | 9.15 nM | Drinking and river water | 63 |
| C@ZnO nanorod array | Cyclic voltammetry | 0.5–4 μM | 0.1 μM | NR | 62 |
| ZnO nanorod array | Cyclic voltammetry | 0.5–3 μM | 0.2 μM | NR | 62 |
| Pd/SPEs | Linear sweep voltammetry | 8–72 μM | 3.7 μM | NR | 38 |
| Pd NPs – PANI/GCE | Amperometry | 10–300 μM | 0.06 μM | NR | 64 |
| Pd micro-domains/SPEs | Linear sweep voltammetry | 12.5–175 μM | 8.9 μM | NR | 65 |
| Pd NPs/TiO2 | Cyclic voltammetry | 5–30 mM | 23 μM | NR | 66 |
| Ni(II) complex/GCE | Cyclic voltammetry | 1.0–100 mM | 0.4 μM | Drinking water | 67 |
| TiO2–Pt nanofibers/GCE | Amperometry | 2–1030 μM | 0.14 μM | Lake water | 68 |
| TiC – CNF arrays | Amperometry | 0.1–1635 μM | 0.026 μM | NR | 69 |
| Pd-NPs/MWCNTs/GCE | Differential pulse voltammetry | 2.5–700 μM | 1 μM | NR | 70 |
| CuO NPs/GCE | Amperometry | 0.1–600 μM | 0.03 μM | Drinking water and industrial wastewater | 71 |
| Pd/plaster-trodes | Cyclic voltammetry | 50–500 μM | 31 μM | NR | 72 |
| TiO2 NPs – quinizarine/CPE | Differential pulse voltammetry | 0.5–1900.0 μM | 77 nM | Drinking, river and wastewater | 73 |
| PANI-Ag-NPs/GCE | Amperometry | 20–90 μM | NR | NR | 74 |
| Pd-NPs-carbon/GCE | Cyclic voltammetry | NR | NR | NR | 75 |
| Au NPs – CH/GCE | Cyclic voltammetry | 0.5 μM–0.5 mM | 0.1 μM | Distilled water | 76 |
| Pd NPs – PAMSA/GCE | Amperometry | 40 μM–1 mM | 0.42 μM | NR | 77 |
| Mn2O3 nanofibres/GCE | Amperometry | Up to 644 μM | 0.3 μM | Lake water | 78 |
| ZnO nanorods/PET | Amperometry | 0.5–50 μM | 0.17 μM | NR | 79 |
| Pd NPs-CB/GCE | Cyclic voltammetry | 5–500 μM, 0.5–10 mM | 8.8 μM | NR | 80 |
| Nickel ferrite NPs – MWCNTs/GCE | Amperometry | 5–2500 μM | 1.5 μM | Drinking water | 81 |
| CoHCF – MWCNTs/graphite paste | Amperometry | 2.0–1200 μM | 0.91 μM | River water sample and pharmaceutical tablets | 82 |
| AuCu NPs-EGN-IL/GCE | Amperometry | 0.2–110 μM | 0.1 μM | Wastewater | 83 |
| Pd-NPs-decorated PoPD | Amperometry | 1 μM–1 mM | 96 nM | NR | 84 |
| Pt–SPEs | Amperometry | 50–500 μM | 0.12 μM | NR | 46 |
| TiO2 NPs/CPE | Cyclic voltammetry | 0.03–7 μM | 27.3 nM | Drinking water | 85 |
| MnO2–GO/GCE | Amperometry | 3 μM–1 mM | 0.16 μM | Lake water | 86 |
| PANI – ERGO/FTO | Linear sweep voltammetry | 0.01 μM–0.1 mM | 15 mM | NR | 87 |
| Vertically aligned ZnO nanorod/ITO | Cyclic voltammetry | 0.3 μM-0.3 mM | 515.7 μM | NR | 88 |
| Au nonporous/ITO | Amperometry | 5.00 nM–2.05 mM, | 4.37 nM | Drinking water | 89 |
| Ni(OH)2–MnO2-NPs/GCE | Linear sweep voltammetry | 5–18 mM | 0.12 μM | NR | 90 |
| PVP-AgNCs/GCE | Amperometry | 0.005–0.46 mM | 1.1 μM | NR | 91 |
| Pd NPs – EDAC/GCE | Differential pulse voltammetry | 5–150 μM | 1.5 μM | NR | 92 |
| Pd NPs – PEDOT/GCE | Amperometry | 0.5–30, 200–5000 μM | 0.8 μM | NR | 93 |
| SWCNT–SPEs | Cyclic voltammetry | 100–1000 μM | 54 μM | NR | 40 |
| Pd–SPEs | Cyclic voltammetry | 200–600 μM, 600–1000 μM | 4 μM | NR | 45 |
| Ag – CB NPs/GCE | Amperometry | 50–800 μM | 3.47 μM | NR | 94 |
| Au NPs/graphite pencil electrode | Amperometry | 0.05–250 μM | 42 mM | Drinking water | 95 |
| AuCu3/AuBPE | Amperometry | 0.1–1000 μM | 0.04 μM | Lake and ground water | 96 |
| Pt NPs/BDD | Differential pulse voltammetry | 10–500 μM | 3.3 μM | NR | 97 |
| WO3-NPs/Au | Amperometry | 100–1000 μM | 144.73 μM | NR | 98 |
| Pd-NPs – rGO/GCE | Amperometry | 0.04–200 μM | 7 nM | Wastewater | 32 |
| ERGO – CCLP-Au NPs/GCE | Amperometry | 10–600 nM, 0.6–197.4 μM | 1.6 nM | Drinking and rain water, urine | 99 |
| FcD – GO/CPE | Square wave voltammetry | 0.22 μM–0.3 mM | 98 nM | Drinking and river water | 100 |
| Pd NPs–GG-g-PAM | Cyclic voltammetry | 50 μM–0.6 mM, 0.6 mM–180 mM | 4.1 μM | NR | 101 |
| Ag NPs-PPy/GCE | Cyclic voltammetry | 0.5–1000 and 1000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μM 000 μM |
0.2 μM | Drinking and river water, industrial wastewater | 102 |
| Poly(BCP)–CNT/GCE | Amperometry | 0.5 μM–1 mM | 0.1 μM | NR | 103 |
| CoPC–SPEs | Cyclic voltammetry | 10–100 μM | 6.21 μM | NR | 47 |
| CoPC SPEs | Cyclic voltammetry | 10–100 μM | 9 μM | NR | 104 |
| Au NPs – AG/SPEs | Amperometry | 2 nM–936 μM | 0.57 nM | NR | 105 |
| Pd–Au NPs/GCE | Amperometry | 0.1–500 μM | 0.07 μM | Drinking water | 106 |
| Pd NPs – CoTAPc/GCE | Amperometry | 0.2–1 mM | 1.3 μM | NR | 107 |
| Au-NPs – PDTYB – MWCNTs/GCE | Cyclic voltammetry | 2–130 and 130–350 μM | 0.6 μM | NR | 108 |
| PdPt NPs – CNTS/GCE | Amperometry | 0.55–1200 μM | 0.28 μM | Drinking water | 109 |
| TiO2 NPs – Mn(III) salen/CPE | Square wave voltammetry | 0.03 μM–0.4 mM | 10 nM | Drinking and river water | 110 |
| Co3O4–MWCNTs/GCE | Amperometry | 20 μM–1.1 mM | 0.8 μM | Drinking water | 111 |
| Back-to-back CoPC SPEs | Cyclic voltammetry | 7.9–104 μM | 2.9 μM | NR | 48 |
| TiO2 nanowires | Amperometry (photoelectrochemistry) | 0–1000 μM | 1.9 μM | NR | 112 |
| Ag/ITO | Amperometry | 100–1700 μM | 0.5 μM | Drinking water | 113 |
| ANSA/CPE | Cyclic voltammetry | 50 μM–25 mM | 43 μM | NR | 114 |
| Ag-ZIF-8/CPE | Amperometry | 6–5000 μM | 1.6 μM | Drinking and river water | 115 |
| Au@Pd core–shell NPs-rGO/GCE | Amperometry | 2–40 μM | 0.08 μM | Drinking, pond and river water | 116 |
| PEDOT:PSS – Pd NPs/GCE | Amperometry | 0.4 to 100 μM | 0.12 μM | NR | 117 |
| Pd NPs – PxDA-rGO/GCE | Amperometry | 1 μM–7.4 mM | 0.17 μM | NR | 118 |
| Au-NPs – CNTs-ErGO/GCE | Amperometry | 0.3–319 μM | 65 nM | Drinking water | 119 |
| Fe3O4@SiO2−MWCNT – 2PHC-IL/CPE | Square wave voltammetry | 0.07 μM–0.5 mM | 40 nM | Drinking and river water | 120 |
| pDA/ITO | Cyclic voltammetry | 100 μM–10 mM | 1 μM | Drinking water | 121 |
| DPB – Fe3O4 NPs/MBCPE | Differential pulse voltammetry | 0.1–0.4 μM, 0.7–12.0 μM | 18 nM | Drinking and river water | 122 |
| GO – CTS – Pt NPs/GCE | Amperometry | 20 μM–10 mM | 3.6 μM | Wastewater | 123 |
| CoHCF-SSG/ITO | Cyclic voltammetry | 100–600 μM | NR | NR | 124 |
| Mesoporous Au–ZnO/GCE | Amperometry | 0.2–14.2 μM | 0.24 μM | NR | 125 |
| ZnO nanorods/glass | Amperometry | 0.8–101 μM | 0.08 μM | NR | 126 |
| NG-PVP – Au NPs/SPEs | Square wave voltammetry | 2–300 μM | 0.07 μM | High sugar fruit and vegetable samples | 41 |
| Au-NPs – poly(taurine)/GCE | Amperometry | 1000 μM | 0.05 μM | River water | 24 |
| Au–Pd NPs/GCE | Amperometry | 0.02–166.6 μM | 5 nM | NR | 127 |
| Cu nanoclusters/GCE | Amperometry | 1.04–425 μM | 1.04 μM | NR | 128 |
| CoS2-CNTs | Amperometry | 0.1 nM–1.0 mM | 0.1 nM | Industrial effluent and sea water | 129 |
| PEDOT-CuO/GCE | Amperometry | 0.5 μM–53 mM | 0.2 μM | Drinking and lake water | 130 |
| Pd NPs – EGNS/SPEs | Amperometry | 0.05–1415 μM | 4 nM | Wastewater | 39 |
| AuTPP-rGO/GCE | Amperometry | 20 nM to 198 nM | 3 nM | Ground, rain and river water | 34 |
| Cu NPs – MWCNTs/pencil graphite electrode | Amperometry | 0.1 to 800 μM | 70 nM | River, well and wastewater | 131 |
| Co3O4 nanosheets-rGO/CC | Amperometry | 5–470 μM | 0.14 μM | NR | 130 |
| Cu-NPs-PANI zeolites/GCE | Differential pulse voltammetry | 4 nM–800 μM | 1 nM | Drinking and river water | 132 |
| Pd NPs – MWCNTs/GCE | Cyclic voltammetry | 2–28 mM | NR | NR | 133 |
| MnO2 – VC@Ag NPs/GCE | Amperometry | 0.1–350 μM | 0.1 μM | Drinking water | 134 |
| Pd-NPs – rGO – MWCNTs/GCE | Amperometry | 1–1100 μM | 0.15 μM | Drinking water | 135 |
| ZrO2 NPs/Au electrode | Cyclic voltammetry | 20–90 μM | 1.05 μM | Well water, hand pump water and canal water | 136 |
| APS – Au NPs/GCE | Amperometry | 10 nM–12 μM | 10 mM | NR | 25 |
| TiO2 NPs/GCE | Cyclic voltammetry | 1 nM to 10 mM | 28.8 pM | Drinking and sea water, industrial effluent, PC bottle, PVC packaging bag | 137 |
| Pt-TPP – RGO/GCE | Amperometry | 13 nM to 232 μM | 5 nM | River, sea and rain water | 138 |
| RuO2 NPs – CTAB – GO/GCE | Cyclic voltammetry | 10 μM–1 mM | 2.3 μM | Drinking and river water | 139 |
| CuO – ZSM-5 NPs/CPE | Amperometry | 25 μM to 0.9 mM, 0.9–4.5 mM | 3.6 μM | Drinking and river water | 140 |
| PB-CNP-PPy/GCE | Amperometry | 0.75 μM–1.7 mM | 0.29 μM | Drinking water | 141 |
| Co–CeO2 nanoflake/steel electrode | Amperometry | 0.005–0.1, 0.13–0.37 μM | 0.006, 0.0012 μM | Drinking and river water | 142 |
| Ag-Zeolite NPs/CPE | Amperometry | 10 μM–0.4 mM, 0.4–4 mM | 1.5 μM | Drinking and river water | 143 |
| PDDA – CuHCF/SPEs | Amperometry | 0.03–536.6 μM | 0.01 μM | Drinking and lake water | 44 |
| Au@Pt-nanoflowers – GO/GCE | Amperometry | 0.8 μM–0.429 mM | 0.43 μM | Drinking water | 144 |
| Au NPs – ERGO/Au | Differential pulse voltammetry | 4–1000, 2000–8000 μM | 74 nM | Drinking water | 145 |
| CuO NPs – IL/CPE | Differential pulse voltammetry | 0.05–150 μM | 0.03 μM | Drinking, river and wastewater | 146 |
| CuO NPs – IL/CPE | Differential pulse voltammetry | 0.05–150 μM | 0.03 μM | Drinking, river and wastewater | 146 |
| PAYR – Ag@C/GCE | Amperometry | 0.3–3.6, 3.9–7.2 μM | 0.09 μM | Drinking and lake water | 147 |
| rGO-Co3O4@Au NPs/GCE | Amperometry | 10–620 μM | 0.44 μM | River, sea and lake water123 | 33 |
| Au NPs – MWCNTs – CTAB-GO/GCE | Differential pulse voltammetry | 1.0–1000 μM | 0.38 μM | Drinking and lake water | 148 |
| MnO2–N-doped RGO/GCE | Rotating disc | 20–100 μM | 0.09 μM | Drinking and lake water | 149 |
| CMDA/CPE | Square-wave voltammetry | 0.08–400 μM | 0.03 μM | Drinking, river and wastewater | 150 |
| Pd NPs – graphene/ITO | Amperometry | 0.1 μM to 2.5 mM | 0.02 μM | NR | 151 |
| Au–Cu NPs – NPZ/CPE | Amperometry | 0.01–150 mM | 0.04 μM | Drinking, rain and river water | 152 |
| CuO – CNTS/SPEs | Amperometry | 5 to 50 μM | 5 μM | NR | 43 |
| Polythiophene-mesoporous ZnO/GCE | Amperometry | 0.5–48 μM | 0.207 μM | NR | 153 |
| Cu3P@NH2-MIL-125(Ti)/GCE | Amperometry | 5 μM to 7.5 mM | 79 nM | Drinking and river water | 154 |
| Au@CeO2 NPs – RGO/GCE | Amperometry | 0 nM to 3 mM | 3 nM | Drinking, river and lake water | 155 |
| Au@Ni-BHD NPs-rGO/GCE | Amperometry | 0.2 μM to 1 mM, 1 mM to 9 mM | 0.06 μM | Drinking water | 156 |
| Au@Ni-BCS NPs-rGO/GCE | Amperometry | 3 μM to 1 mM, 1 mM to 20 mM | 0.9 μM | Drinking water | 156 |
| NiFeCo nanoalloy/GCE | Amperometry | 0.020–3080 μM | 6.4 nM | Drinking, lake, river, sewage water | 27 |
| CuO – OMC/GCE | Amperometry | 1 μM to 2.11 mM, 2.11 mM to 21.1 mM | 0.89 μM | NR | 155 |
| Pt–Pd NPS – ErGO/GCE | Amperometry | 0.007–5.5 mM | 1.7 μM | Drinking water | 157 |
| Curcumin-AgNPs – rGO-FeCo2O4 nanosheets/SPCE | Differential pulse voltammetry | 2.5–1200 μM | 23.7 nM | River and wastewater | 42 |
| FeCo oxide carbon spheres/GCE | Amperometry | 0.1–516.6 μM | 0.1 μM | River water | 158 |
| Sb2S3 – PDI-GABA/GCE | Amperometry | 10–250, 250–1300 μM | 0.05 nM | Drinking, river, sea water and industrial effluent | 159 |
| AuNPs@NPC-RGO/GCE | Amperometry | 0.05 to 1.00 μM | 9.6 nM | Drinking, river and lake water. Hydrazine sensing in an air environment | 35 |
| Ag NPs – ZIF-67/CPE | Amperometry | 4 to 326 μM, 326 to 4700 μM | 1.45 μM | Drinking and rainwater | 160 |
| β-Ni(OH)2/CPE | Amperometry | 1–1300 μM | 0.28 μM | Drinking water | 161 |
| P-MWCNTs – RGO/GCE | Amperometry | 1 μM–3 mM, 3–55 mM | 0.31 μM | Drinking and river water | 162 |
| ZnO-NRs/GCE | Cyclic voltammetry | NR | NR | NR | 163 |
| Au NPs-NiO nanosheets/GCE | Cyclic voltammetry | 0.0001–0.110 μM | 0.05 nM | NR | 164 |
| CdO NPs – MWCNTs/GCE | Amperometry | 0.01 nM–0.1 mM | 4 pM | Seawater, industrial effluent, PVC-food packaging bag | 165 |
| Co@SnO2 – HRGO/GCE | Cyclic voltammetry | NR | NR | NR | 166 |
| Poly(CoOBImPc) – rGO/GCE | Amperometry | 100–900 nM | 33 nM | Drinking and river water | 167 |
| Au NPs/graphite pencil electrode | Amperometry | 0.01 to 100 μM | 0.002 μM | Drinking and sea water | 168 |
| NiCo2S4 nanospheres/GCE | Amperometry | 1.7 μM to 7.8 mM | 0.6 μM | Drinking water | 169 |
| ZnO-NPs/PEDOT–PSS | Amperometry | 10–500 μM | 5 μM | Drinking and sea water | 50 |
| MnO2/GCE | Amperometry | 0.007–10 mM | 129 nM | Drinking water | 170 |
| CuS-OMC/GCE | Amperometry | 0.25–40 μM | 0.1 μM | Drinking and reverse osmosis water | 171 |
| AuNPs-rGO/Ni foam | Amperometry | 0.2–200 μM | 0.056 μM | Drinking water | 170 |
| Au–Pd NPs – rGO/GCE | Amperometry | 0.1–200 μM | 16 nM | River and lake water | 172 |
| Ru–Co NPs/GCE | Cyclic voltammetry | 0.0025–9.55 mM | 0.00025 mM | Drinking water | 173 |
| CeO2 NPs – NB/GC | Differential pulse voltammetry | 0.001–3.22 mM | 57 nM | Drinking water and industrial effluent | 174 |
| Rhodium acetamidate/CPE | Cyclic voltammetry | 0.1 μM–0.1 mM | 5 μM | NR | 175 |
| Zn-MOF – rGO/Au electrode | Amperometry | 0.001–100 μM | 8.75 nM | Drinking water | 176 |
| Au/Nd–Gd-titania nanotubes-Pd/Au electrode | Cyclic voltammetry | 0.01–10 μM | 0.15 μM | Irrigation water | 177 |
| Pt NPs/nanoporous gold electrode | Amperometry | 5–6105 μM | 1.03 μM | River and lake water | 178 |
| Ti3C2Tx – ZIF-8/GCE | Amperometry | 10–7700 μM | 5.1 μM | NR | 179 |
| SrTiO3 NPs – PANI/GCE | Amperometry | 16–58 μM | 0.95 μM | Drinking water | 180 |
| EFTA – [hmim][PF6] – CoS2-CNT/CPE | Differential pulse voltammetry | 0.03 to 500 μM | 0.015 μM | Drinking and river water | 181 |
| Ag@SO-gCN/FTO | Cyclic voltammetry | 8–110 μM | 0.143 μM | NR | 182 |
| HM-Al3+-(2D-MoS2)/SPGEs | Amperometry | 3.81–400 μM | 1.05 μM | Drinking and river water | 51 |
As can be seen from Table 1, a diverse range of metal and metal oxide in the form of nanoparticles, spheres, rods, cubes and more, are used for the electroanalytical sensing of hydrazine. The most common is the use of nanoparticles to modify an underlying supporting electrode, such as carbon electrodes, which allows electrical “wiring” of the nanoparticles. From an electroanalytical point of view, the electrochemical response of hydrazine at a bare glassy carbon electrode is reported to exhibit slow electrode kinetics with a voltammetric signal that occurs at high overpotentials with a relatively small electroanalytical signal. When the electrode is modified with the chosen nanoparticles, the electrochemical oxidation of hydrazine signal occurs at lower overpotentials compared to that seen at the bare electrode and with a larger electroanalytical signal.24,25 The reason for the improvements is based around arguments on the nanoparticles increased surface area, improved electrode kinetics, changed electronic structure and adsorption behaviour.26 Using such an approach provides advantages over that, of say, carbon based materials but economics need to be considered; generally, such a response is routinely reported to be “electrocatalytic”.
Annalakshmi et al.27 reported the sensing of hydrazine using trimetallic NiFeCo nanospheres supported upon a glassy carbon electrode, where the nanospheres were synthesized through a one-pot, facile hydrothermal methodology; see Fig. 2. The nanosphere modified electrode was reported to be an excellent electrocatalyst, where the bare glassy carbon electrode was reported to exhibit a high oxidation overpotential (+0.83 V), and no noticeable peak was observed compared to the nanosphere decorated glassy carbon electrode which exhibited a voltammetric signal at +0.56 V. The authors state that the modified electrode gives rise to an improved response, which might arise from its low impedance behaviour, nanospheres-like architecture and synergic effect among the metallic nanoparticles.27 The electroanalytical sensor was able to detect hydrazine over a large concentration range (0.020–3080 μM) with a very low LOD of 6.4 nM. The practical applicability of the sensor was successfully validated in spiked river, lake, tap, and sewage water with satisfactory recoveries.27 Generally, researchers overlook exploring a coverage study, that is, the effect of increasing the number of nanoparticles on the electrode surface against the electrochemical/electroanalytical response. One must not forget false electrocatalysis can result for thick porous layers of nanoparticles where the electrochemical response changes from that, at low monolayer coverage, of semi-infinite diffusion to that of thin layer diffusion for large coverages/porous layers.28
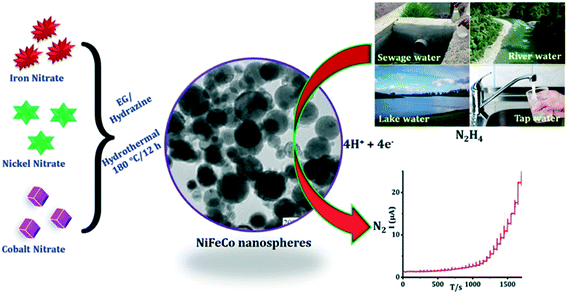 | ||
| Fig. 2 Schematic representation of trimetallic NiFeCo nanospheres synthesized through a one-pot, facile hydrothermal method. The practical applicability of the proposed novel HY sensor was successfully evaluated in real water samples with satisfactory recoveries. Figure reproduced with permission from ref. 27. Copyright 2019 Elsevier. | ||
If we consider that the case of nanoparticle modified electrodes have been extensively developed to sense hydrazine, let's again ask why would you adopt this strategy? The answer lies in not only the physical and chemical properties of nanoparticles differing greatly from that of the bulk material, but also the geometric configuration of the constructed electrochemical platform. If we consider an isolated hemispherical nanoparticle for a simple electrode reaction (e.g.: A + ne− ⇆ B), a diffusion-limited current (at suitably slow scan rates) is given by: Ilim = 2πnFDr[A]bulk, where n is the number of electrons transferred, r is the radius of the nanoparticle, [A]bulk is the bulk concentration of A, D is the diffusion coefficient of molecule A, and F is the Faraday constant. It is interesting to compare the limiting current for a spherical nanoparticle on an electrode surface which is described as: Ilim = 8.71nFDr[A]bulk. Note that that the difference between these equations is due to a ‘shielding effect’, produced as a result of the underling supporting electrode that reduces the limiting current.29 The above cases are for diffusion-limited cases, in essence when the voltammetric experiment is conducted at suitably slow scan-rates. When the scan rate of the voltammetric experiment is increased, the voltammetry deviates from that of the expected steady-state to that of a peak-shaped response.30 However, in reality it is very rare for electroanalysts to conduct electroanalytical measurements on a single nanoparticle. In most, if not all cases, electroanalysts will decorate a chosen electrode surface with nanoparticles; for example, see Table 1 for the various endeavours using a diverse range of nanoparticle compositions.
The voltammetry at nanoparticle-modified electrodes has been elegantly reported by Compton et al.,30 where the mass transport is different compared to that of a bulk electrode. In electroanalytical experiments, the modification of an electrode surface with nanoparticles results in a random array, that is, an assembly of nanoparticles randomly distributed over the supporting electrode surface. Fig. 3 shows simulations for a single reversible electron transfer process at a spherical nanoparticle array on an inert surface. Fig. 3 shows that four distinct categories arise, dependent upon the applied experimental voltage scan rates, which is represented in a dimensionless form: 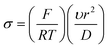 , where T is the temperature and R is the universal gas constant. As the voltammetric scan rate changes, so does the diffusion layer.28,30
, where T is the temperature and R is the universal gas constant. As the voltammetric scan rate changes, so does the diffusion layer.28,30
 | ||
| Fig. 3 A: Simulated concentration profiles at a diffusion domain containing a spherical particle. Category 1: σ = 1000. Category 2: σ = 10. Category 3: σ = 1. Category 4: σ = 0.01. For all categories R0 = 2. Concentration profiles were taken at the linear sweep's peak potential. B: Simulated linear sweep voltammetry of a reversible electron transfer at a spherical particle modified electrode. Scan rate σ = 0.01, θ varies from 10−4 to 0.1. Figures reproduced with permission from ref. 30. Copyright 2007 American Chemical Society. | ||
In the case of category 1, the diffusion layers are small, corresponding to fast voltammetric scan rates, and the nanoparticles are diffusionally independent. Mass transport in this regime is linear and the cyclic voltammetric profile is the classic peak shape and the peak current (Ip) is governed by the Randles–Ševčík equation, i.e.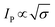 , while the chronoamperometric response is governed by the Cottrell equation, i.e.
, while the chronoamperometric response is governed by the Cottrell equation, i.e.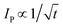 . In the case of category 2 (see Fig. 3), the voltammetric scan rate is slowed and the diffusion layers become larger where diffusional independence is still observed. In this category, diffusion is hemispherical (or convergent) and the resultant cyclic voltammetric responses are steady-state shaped (rather than peak shaped). In category 3, the scan rate is further slowed, and we see that the diffusional layers are no longer independent, but rather overlap. In this case, the cyclic voltammetric response is peak shaped but the absolute current is smaller than theoretically expected. Last, category 4, the diffusion layers heavily overlap, and mass transport is linear over the entire nanoparticle array. The observed voltammetric response is the same as that observed in category 1. Note that the reason for the nanoparticle size dependence of the peak potential is that this reflects a switch over between rate-determining electrode kinetics, which control the current before the peak potential, to rate determining mass transport (diffusion) after the peak potential.
. In the case of category 2 (see Fig. 3), the voltammetric scan rate is slowed and the diffusion layers become larger where diffusional independence is still observed. In this category, diffusion is hemispherical (or convergent) and the resultant cyclic voltammetric responses are steady-state shaped (rather than peak shaped). In category 3, the scan rate is further slowed, and we see that the diffusional layers are no longer independent, but rather overlap. In this case, the cyclic voltammetric response is peak shaped but the absolute current is smaller than theoretically expected. Last, category 4, the diffusion layers heavily overlap, and mass transport is linear over the entire nanoparticle array. The observed voltammetric response is the same as that observed in category 1. Note that the reason for the nanoparticle size dependence of the peak potential is that this reflects a switch over between rate-determining electrode kinetics, which control the current before the peak potential, to rate determining mass transport (diffusion) after the peak potential.
Next, if the voltammetric scan rate is fixed, what is the effect of changing the surface coverage? As shown in Fig. 3B, linear sweep voltammograms are shown for a fixed scan rate where the y axis is presented in terms of the dimensionless current per particle: j = i/NDrF[A], where N is the number of spherical particles present on the surface. The x-axis is presented in terms of a dimensionless potential, θ = F/RT(E − E0f). In Fig. 3B, the effect of the surface coverage, Θ upon the voltammetric response is shown from 0.1–10−4, where Θ = Nr2/A and A is the area of the supporting electrode. Fig. 3B elegantly shows that at low nanoparticle coverage (Θ = 10−4), the diffusion zones at each nanoparticle are diffusionally independent and a steady-state response is observed, falling within category 1 or 2. As the nanoparticle coverage is increased, the distance between each nanoparticle decreases and passes through category 3 finally arriving at category 4; the change in the voltammetric wave shape is easily visualised (Fig. 3). An important observation is the dimensionless current decreases since the region of the solution available to each nanoparticle is reduced.30 The above demonstrates that the voltammetric shape of a nanoparticle array will depend upon the scan rate and surface coverage of the nanoparticles.30
One interesting aspect most pertinent to electroanalysts is that when in category 4, where heavy diffusional overlap occurs at a nanoparticle random array, the current response (the electroanalytical response) is similar to that obtained if one had used a complete electrode (e.g. a film or a solid electrode) of the same material. This unique property is extremely useful in electroanalysis as in the category 4 region, a nanoparticle array yields a similar amount of electrolytic depletion to a macroelectrode of the same total area. Consequently, minimal amounts of expensive catalyst, such as platinum or gold, can be used to offer a maximal electrochemical response over that of a solid electrode comprised of platinum or gold with significant cost savings. This critical issue is generally overlooked/not reported when nanoparticle modified electrodes are utilised, particularly to the sensing of hydrazine.
To this end, Batchelor-McAuley and co-workers31 have explored the random distribution of palladium nanoparticles supported on a boron-doped diamond (BDD) electrode with that of a palladium plated BDD microelectrode array comprising 362 palladium 25 μm diameter microdisks. In comparison of the two electrodes, the palladium nanoparticle decorated BDD electrode exhibits low micro-molar detection and a highly linear response toward hydrazine (see Table 1). The authors suggest that it is likely that the palladium nanoparticles are also acting as an array of microelectrodes with AFM images of the modified electrode revealing nanoparticles that are close together which effectively makes them act as single, larger particles.31 One important aspect, is that both the array and the nanoparticle assemble provide lower detection limits and highly linear responses compared to that of palladium macroelectrodes. The beneficial cost implications of using palladium nano- or micro-particles in sensors compared to a palladium macroelectrode are evident and gives a substantial reason to pursue nanoparticle-decorated electrodes.
Rather than directly modify an electrode surface, as new 2D nanomaterials have appeared with reported beneficial properties, such as large surface areas and improved conductivity, these have been utilised to support various nanoparticle compositions.26 For example, palladium nanoparticles have been synthesized upon reduced graphene oxide (PdNPs – rGO) using an in situ polyol method; see Fig. 4.32 Palladium(II) ions and graphene oxide are reduced together with the reducing agent ethylene glycol and the size of the PdNPs can be readily controlled by varying the concentration of metal salt precursor from 3.7 nm up to 10.8 nm (diameter). Using rotating disk voltammetry, the PdNPs – rGO were modified upon a glassy carbon electrode and studied toward the detection of hydrazine where the smallest Pd nanoparticle size (3.7 nm) gave rise to the highest electroanalytical sensitivity and lowest limit of detection.32 This is attributed by the authors to be due to a higher electrochemical active surface area-to-volume ratio as well as to quantum confinement, for which the valence band centre of the PdNPs was shifted ca. +0.2 eV vs. Pd bulk leading to faster charge transfer.32 Using the 3.7 nm diameter PdNPs – rGO, a very low limit of detection of about 7 nM (at a rotation speed of 6000 rpm) was shown to be possible with a wide linear range of 0.04–200 μM. The sensor was shown to be highly selective to hydrazine without interference from uric acid, glucose, ammonia, caffeine, methylamine, ethylenediamine, hydroxylamine, n-butylamine, adenosine, cytosine, guanine, thymine, and L-arginine. The PdNPs – rGO based hydrazine sensor was shown to successfully determine hydrazine in spiked wastewater samples. Another approach has utilised reduced graphene oxide which is modified with cobalt oxide nanocubes@gold (rGO-Co3O4@Au) nanocomposites,33 fabricated using a one-pot hydrothermal synthesis (Fig. 5A). Using amperometry, the detection of hydrazine was shown to be viable over the range of 10–620 μM with a LOD of 0.44 μM. The effect of interferents were explored (NO3−, SO42−, Cl−, Ag+, Na+, K+, ethanol, 4-nitrophenol, ascorbic acid and glucose) which showed no detrimental effect upon the sensor. The sensing of hydrazine in spiked sea, lake and river water was shown to be viable. Following a similar approach, a gold tetra phenyl porphyrin (AuTPP) modified reduced graphene oxide nanocomposite film modified glassy carbon electrode (GCE) was fabricated and explored towards hydrazine sensing (see Fig. 5B). Initially graphene oxide (GO) was prepared from graphite by a modified Hummer's method, which was then mixed with the Au-TPP and drop cast upon a GCE surface and electrochemically reduced to prepare the final AuTPP – rGO/GCE sensor. Using amperometry, a linear 20 nM to 198 μM was shown to be possible with a very low LOD of 3 nM. The sensor was found to selectively detect hydrazine in the presence of 500 fold excess concentrations of a range of interfering ions and was shown to be viable for hydrazine sensing in spiked ground, rain and river water samples.34
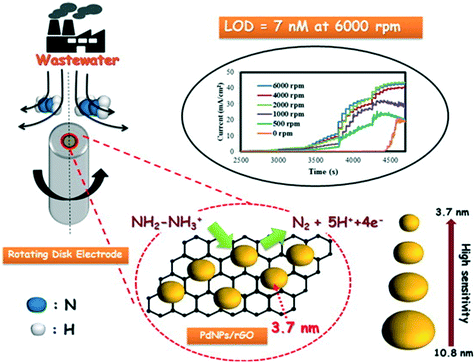 | ||
| Fig. 4 Palladium nanoparticles upon reduced graphene oxide (PdNPs/rGO) composites, synthesized using an in situ polyol method. Palladium(II) ions and graphene oxide are reduced together with a reducing agent, ethylene glycol. The size of the PdNPs can be controlled, which gives rise to improvements in the sensing of hydrazine via rotating disc electrochemistry. The approach is shown to be successful for determining hydrazine in wastewater samples. Figure reproduced with permission from ref. 32. Copyright 2014 American Chemical Society. | ||
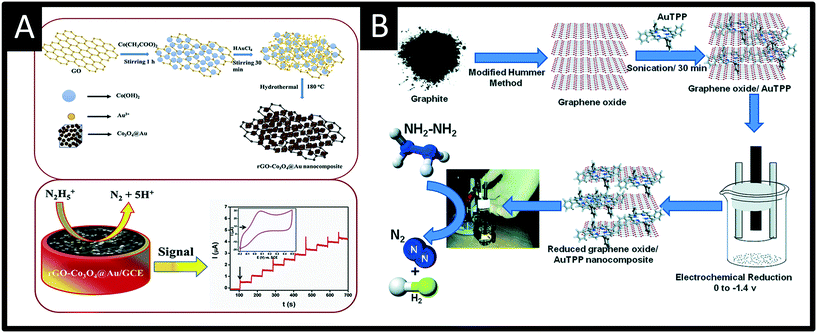 | ||
| Fig. 5 A: Summary of the synthesis of reduced graphene oxide-cobalt oxide nanocube@gold (rGO-Co3O4@Au) nanocomposite prepared using a one-pot hydrothermal synthesis. The nanocomposite is drop casted upon a glassy carbon electrode and successfully used to measure hydrazine via amperometry. B: Schematic representation for the preparation of reduced graphene oxide – gold tetra phenyl porphyrin nanocomposite glassy carbon modified electrode for hydrazine sensing. Figures reproduced from ref. 33 and 34. Copyright 2018 Elsevier and 2016 Royal Society of Chemistry. | ||
Zhang et al. utilised Au nanoparticles N-doped porous carbon anchored upon reduced graphene oxide nanosheets, supported upon a GCE which was fabricated via a confinement synthetic process in the frame structure of zeolitic imidazolate framework-67 (ZIF-67).35 The authors explored the role of the N-doped porous carbon and found that its incorporation provided a framework to immobilize Au nanoparticles. This prevents shifting and agglomeration, improving the wettability of rGO and therefore, avoiding irreversible restacking due to π–π interactions of rGO layers, which can reduce the performances of rGO-based support.35Fig. 6 shows a schematic illustration of the sensing mechanism (note the GCE is absent) which was explored toward sensing hydrazine in both aqueous and gaseous environments. In the aqueous solutions a linear detection from 0.05 to 1.00 μM was shown to be viable with an LOD of 9.6 nM, with the authors validating the sensor towards the sensing of hydrazine in spiked drinking, river and lake water. Notably, the authors explored the electrochemical platforms as the basis of a practical electrochemical gas sensor, which exhibited a LOD of 1.8 ppm with fast response/recovery. Those advantages of the electrochemical sensors based on AuNPs@NPC–rGO can be attributed to the key design element of sensing material with structural and compositional advantages leading to the synergy of AuNPs, NPC and rGO.35
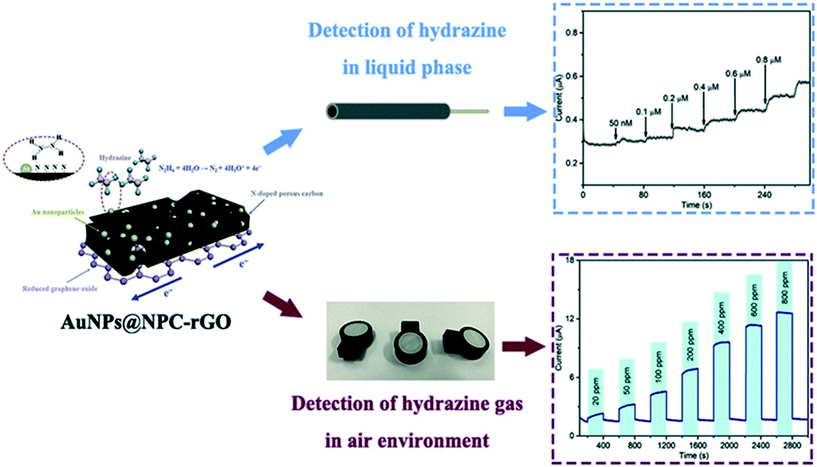 | ||
| Fig. 6 Schematic illustration of the sensing mechanism for Au nanoparticles-embedded N-doped porous carbon anchored on reduced graphene oxide (AuNPs@NPC-rGO) nanosheets, constructed by a confinement preparation process in the frame structure of zeolitic imidazolate framework-67 (ZIF-67). The sensor has been successfully demonstrated to sense hydrazine in both aqueous and gaseous environments. Figure reproduced with permission from ref. 35. Copyright 2019 Elsevier. | ||
In the majority of cases where modifiers are utilised, they generally use a solid electrode with the electrode of choice seemingly being a GCE. Another tacit is to utilise screen-printed graphite/carbon electrodes (SPEs), which offer numerous advantages such as low cost, flexibility of their design, high reproducibility and the ability to manufacture them with different designs and materials, while performing as a low cost electrode.36,37 For example, as we noted above, one of the first electroanalytical studies for the sensing of hydrazine utilised palladium nanoparticle modified SPEs, where the palladium was formed via electrodeposition.23 This has been extended to where palladium microdomain have been formed on SPEs, and via linear sweep voltammetry a linear detection range of 8–72 μM is possible with an LOD of 3.7 μM.38 Other variations include palladium nanoparticles on electroactivated graphite nanosheet modified SPEs,39 single-walled carbon nanotube (SWCNT) bulk modified SPEs,40 nitrogen-doped graphene-polyvinylpyrrolidone gold nanoparticle modified SPEs,41 curcumin-stabilized silver nanoparticle-coated reduced graphene oxide magnetic spinel (FeCo2O4) nanosheets supported upon SPEs,42 copper oxide nanoparticles/carbon nanotube modified SPEs43 and polydimethyldiallylamine stabilized copper(II) hexacyanoferrate nanocube modified SPEs.44 Metters et al. reported the first complete palladium screen-printed macroelectrodes45 which exhibited linear ranges from 200–600 μM and 600–1000 μM with a LOD of 4 μM possible in model aqueous solutions. This approach for hydrazine sensing has been extended to platinum SPEs, where a linear range and LOD was reported to be 50–500 μM and 0.12 μM respectively.46 In these instances, they are different from that of solid/bulk electrodes, as the metal on the surface of the SPEs is only microns thick (∼10 μm) and have obvious cost savings, but also allow mass-produced and highly reproducible electrodes to be fabricated, providing an avenue to bridge the gap between the laboratory and the field. The use of SPEs have been extended to cobalt(II) phthalocyanine – SPEs, (CoPc–SPEs), with the CoPc in the bulk of the SPEs demonstrating ultra-flexible sensors, which successfully measured hydrazine after extensive contortion giving rise to a LOD of 6.2 μM.47 Another approach utilising CoPc–SPEs48 is the concept of back-to-back electroanalytical sensors,47–49 where both sides of a plastic substrate are screen-printed upon. This utilises the usually redundant back of the screen-printed sensor, converting this “dead-space” into a further electrochemical sensor which results in improvements in the analytical performance.
Other recent advances following a similar theme are reported by Beduk and co-workers50 who have developed a fully inkjet-printed hydrazine sensor (Fig. 7A) which is comprised of a poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS) electrode functionalized with zinc oxide nanoparticles and encapsulated in a Nafion matrix. Using amperometry, the authors were able to show the inkjet-printed sensor was able to measure hydrazine over the range of 10 to 500 μM with a LOD of 5 μM and was successfully applied to measure hydrazine in spiked drinking and sea water samples. The ability to use inkjet printed electrochemical architectures provides another useful avenue to allow the mass production of cheap yet reproducible electroanalytical sensors. Other exciting work is by Villa-Manso et al.51 who have developed screen-printed graphene electrodes (Fig. 7B), which are modified with 2D-MoS2 and a AI3+ hematein (HM) complex which facilitates the electrocatalytic determination of hydrazine. The electrochemical mechanism is shown in Fig. 7B, which proceeds via an ECcat mechanism with a catalytic rate constant (kcat) found to be 8.1 (±0.1) × 104 M−1 s−1, confirming the AI3+ hematein complex has a high electrocatalytic activity. The sensor was shown to successfully determine hydrazine in spiked drinking and river water with good recoveries (96 and 90% respectively). Screen-printing and inkjet printing appear to be useful fabrication approaches for producing next generation disposable sensors that have scales of economy, allowing low-cost hydrazine sensors to be realised; future work should be directed to this endeavour.
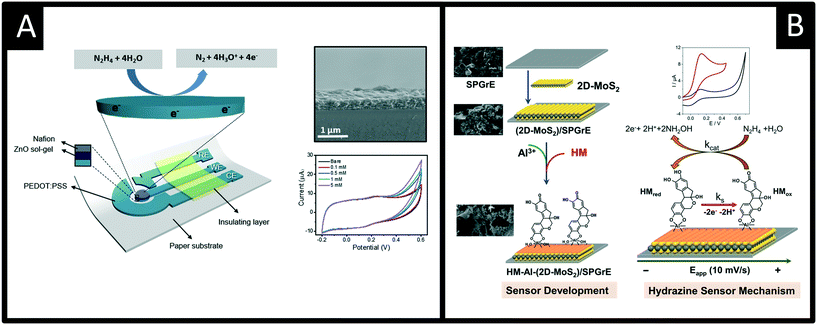 | ||
| Fig. 7 A: Schematic of the sensor comprising the working, reference and counter electrodes consisting of printed PEDOT:PSS, insulator, ZnO sol–gel and Nafion layers. Also shown is an SEM cross-section of the electrode surface and cyclic voltammetric responses using the ZnO-NPs/PEDOT–PSS in the presence of 0 (bare), 0,1, 0.5, 1, 5 mM of hydrazine in 0.1 M PBS (pH 7.4). B: A screen-printed graphene electrode modified with 2D-MoS2 and a AI3+ hematein (HM) complex, which provides an electrocatalytic determination of hydrazine. Figures reproduced with permission from ref. 50 and 51. Copyright 2020 Elsevier and 2021 Elsevier. | ||
Conclusions and outlook
We have overviewed the electroanalytical sensors that have been reported for the sensing of hydrazine. A large majority of electroanalytical sensors utilise metallic nanoparticles and other surface modifications, where the advantage of such is generally not explicitly stated. The use of nanoparticle modified electrodes allow the same electrochemical response as that of macroelectrode comprised of the same macro bulk electrode to be realised and have clear cost advantages. It is observed that coverage studies, exploring the amount/number of nanoparticles (coverage), are not routinely studied to optimise the electroanalytical sensor but also ensure that thin-layer effects are not giving rise to false observations of electrocatalysis. There is also scope to further explore the underlying supporting electrode, with screen-printed and inkjet-printed based sensors having clear advantages that can be further utilised. While there are a substantial number of academic papers reporting the determination of hydrazine sensing for aqueous based measurements, the majority, if not all, are still within the laboratory and there is not currently an electrochemical based hydrazine sensor commercially available; this is where screen-printed and inkjet-printed based sensor are the most suitable for hydrazine sensing, most likely with metallic modifications utilising the benefits of the nanoscale. We note that the majority, if not all electrochemical based hydrazine sensors are explored to aqueous samples, such as drinking, river and industrial effluent waters. Only a very limited number of papers explore gaseous environments and even fewer biological samples, such as its quantitative detection in blood/serum/urine for accurate occupational exposure monitoring; this is clearly another area for future development. Last, while electroanalytical hydrazine sensors are explored to a range of potential interferents and spiked water samples, independent validation with other well-established measurement techniques is lacking, which is likely hindering the uptake into a commercial product.Conflicts of interest
The authors confirm there are no conflicts to declare.References
- U. EPA, https://www.epa.gov/sites/default/files/2016-09/documents/hydrazine.pdf.
- P. Ortega-Barrales, A. Molina-Díaz, M. I. Pascual-Reguera and L. F. Capitán-Vallvey, Anal. Chim. Acta, 1997, 353, 115–122 CrossRef CAS.
- N. P. G. t. C. Hazards, DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Disease Control and Prevention National Institute for Occupational Safety and Health, September, 2007.
- U. S. E. P. Agency, Integrated Risk Information System (IRIS) on Hydrazine/Hydrazine Sulfate, National Center for Environmental Assessment, Office of Research and Development, Washington, DC, 1999 Search PubMed.
- S. Zelnick, D. Mattie and P. Stepaniak, Aviat., Space Environ. Med., 2003, 74, 1285 CAS.
- D. o. H. a. H. S. National Toxicology Program, https://ntp.niehs.nih.gov/ntp/roc/content/profiles/hydrazine.pdf.
- J.-A. Oh, J.-H. Park and H.-S. Shin, Anal. Chim. Acta, 2013, 769, 79–83 CrossRef CAS PubMed.
- J.-A. Oh and H.-S. Shin, J. Chromatogr. A, 2015, 1395, 73–78 CrossRef CAS PubMed.
- G.-C. Gil, Y.-G. Kim and B.-G. Kim, Anal. Biochem., 2008, 379, 45–59 CrossRef CAS PubMed.
- K. Wang, X. He, X. Yang and H. Shi, Acc. Chem. Res., 2013, 46, 1367–1376 CrossRef CAS PubMed.
- X.-Y. Zhang, Y.-S. Yang, W. Wang, Q.-C. Jiao and H.-L. Zhu, Coord. Chem. Rev., 2020, 417, 213367 CrossRef CAS.
- X. Gu and J. P. Camden, Anal. Chem., 2015, 87, 6460–6464 CrossRef CAS PubMed.
- G. E. Collins, S. Latturner and S. L. Rose-Pehrsson, Talanta, 1995, 42, 543–551 CrossRef CAS PubMed.
- S. Vivarelli, Ann. Chim., 1951, 41, 415 CAS.
- S. Karp and L. Meites, J. Am. Chem. Soc., 1962, 84, 906–912 CrossRef CAS.
- A. J. Bard, Anal. Chem., 1963, 35, 1602–1607 CrossRef CAS.
- M. Michlmayr and D. T. Sawyer, J. Electroanal. Chem. Interfacial Electrochem., 1969, 23, 375–385 CrossRef CAS.
- B. Wang and X. Cao, Electroanalysis, 1992, 4, 719–724 CrossRef CAS.
- R. Miao and R. G. Compton, J. Phys. Chem. Lett., 2021, 12, 1601–1605 CrossRef CAS PubMed.
- R. Miao, L. Chen and R. G. Compton, Sci. China: Chem., 2021, 64, 322–329 CrossRef CAS.
- K. M. Korfhage, K. Ravichandran and R. P. Baldwin, Anal. Chem., 1984, 56, 1514–1517 CrossRef CAS.
- J. Liu, W. Zhou, T. You, F. Li, E. Wang and S. Dong, Anal. Chem., 1996, 68, 3350–3353 CrossRef CAS PubMed.
- J. Wang, M. P. Chatrathi, B. Tian and R. Polsky, Electroanalysis, 2000, 12, 691–694 CrossRef CAS.
- Ç. C. Koçak, A. Altın, B. Aslışen and S. Koçak, Int. J. Electrochem. Sci., 2016, 11, 233–249 Search PubMed.
- G. Maduraiveeran and R. Ramaraj, J. Anal. Sci. Technol., 2017, 8, 14 CrossRef.
- R. D. Crapnell and C. E. Banks, Microchim. Acta, 2021, 188, 1–23 CrossRef PubMed.
- M. Annalakshmi, P. Balasubramanian, S.-M. Chen and T.-W. Chen, Sens. Actuators, B, 2019, 296, 126620 CrossRef CAS.
- R. G. Compton and C. E. Banks, Understanding Voltammetry, World Scientific, 3rd edn, 2018 Search PubMed.
- S. R. Belding, F. W. Campbell, E. J. F. Dickinson and R. G. Compton, Phys. Chem. Chem. Phys., 2010, 12, 11208–11221 RSC.
- I. Streeter, R. Baron and R. G. Compton, J. Phys. Chem. C, 2007, 111, 17008–17014 CrossRef CAS.
- C. Batchelor-McAuley, C. E. Banks, A. O. Simm, T. G. J. Jones and R. G. Compton, Analyst, 2006, 131, 106–110 RSC.
- A. Krittayavathananon, P. Srimuk, S. Luanwuthi and M. Sawangphruk, Anal. Chem., 2014, 86, 12272–12278 CrossRef CAS PubMed.
- M. M. Shahid, P. Rameshkumar, W. J. Basirunc, U. Wijayantha, W. S. Chiu, P. S. Khiew and N. M. Huang, Electrochim. Acta, 2018, 259, 606–616 CrossRef CAS.
- S. Sakthinathan, S. Kubendhiran, S.-M. Chen and P. Tamizhdurai, RSC Adv., 2016, 6, 56375–56383 RSC.
- Y. Zhang, Y. Zhang, D. Zhang, S. Li, C. Jiang and Y. Su, Sens. Actuators, B, 2019, 285, 607–616 CrossRef CAS.
- C. E. Banks, C. W. Foster and R. O. Kadara, Screen-Printing Electrochemical Architectures, Springer, 2016 Search PubMed.
- A. G.-M. Ferrari, S. J. Rowley-Neale and C. E. Banks, Talanta Open, 2021, 100032 CrossRef.
- N. A. Choudhry, M. Khairy, R. O. Kadara, N. Jenkinson and C. E. Banks, Electroanalysis, 2010, 22, 1831–1836 CrossRef CAS.
- C. Karuppiah, M. Velmurugan, S.-M. Chen, R. Devasenathipathy, R. Karthik and S.-F. Wang, Electroanalysis, 2016, 28, 808–816 CrossRef CAS.
- J. P. Metters, M. Gomez-Mingot, J. Iniesta, R. O. Kadara and C. E. Banks, Sens. Actuators, B, 2013, 177, 1043–1052 CrossRef CAS.
- C. Saengsookwaow, R. Rangkupan, O. Chailapakul and N. Rodthongkum, Sens. Actuators, B, 2016, 227, 524–532 CrossRef CAS.
- A. Mejri, A. Mars, H. Elfil and A. H. Hamzaoui, Microchim. Acta, 2019, 186, 561 CrossRef CAS PubMed.
- Neelam, G. Rani and M. Kumar, Sens. Transducers J., 2018, 223, 22–25 CAS.
- S. Ramaraj, R. Sakthivel, S.-M. Chen, S. Palanisamy, V. Velusamy, T.-W. Chen, S. Ramaraj and K. Pandian, Int. J. Electrochem. Sci., 2017, 12, 5567–5582 CrossRef CAS.
- J. P. Metters, F. Tan and C. E. Banks, J. Solid State Electrochem., 2013, 17, 1553–1562 CrossRef CAS.
- J. P. Metters, F. Tan, R. O. Kadara and C. E. Banks, Anal. Methods, 2012, 4, 1272–1277 RSC.
- C. W. Foster, J. P. Metters, D. K. Kampouris and C. E. Banks, Electroanalysis, 2014, 26, 262–274 CrossRef CAS.
- A. P. Ruas de Souza, M. Bertotti, C. W. Foster and C. E. Banks, Electroanalysis, 2015, 27, 2295–2301 CrossRef CAS.
- A. P. Ruas de Souza, C. W. Foster, A. V. Kolliopoulos, M. Bertotti and C. E. Banks, Analyst, 2015, 140, 4130–4136 RSC.
- T. Beduk, E. Bihar, S. G. Surya, A. N. Castillo, S. Inal and K. N. Salama, Sens. Actuators, B, 2020, 306, 127539 CrossRef CAS.
- A. M. Villa-Manso, M. Revenga-Parra, M. Vera-Hidalgo, M. Vázquez Sulleiro, E. M. Pérez, E. Lorenzo and F. Pariente, Sens. Actuators, B, 2021, 345, 130385 CrossRef CAS.
- S. M. Golabi and H. R. Zare, J. Electroanal. Chem., 1999, 465, 168–176 CrossRef CAS.
- J.-W. Mo, B. Ogorevc, X. Zhang and B. Pihlar, Electroanalysis, 2000, 12, 48–54 CrossRef CAS.
- K. I. Ozoemena and T. Nyokong, Talanta, 2005, 67, 162–168 CrossRef CAS PubMed.
- A. A. Ensafi and E. Mirmomtaz, J. Electroanal. Chem., 2005, 583, 176–183 CrossRef CAS.
- A. Abbaspour and M. A. Kamyabi, J. Electroanal. Chem., 2005, 576, 73–83 CrossRef CAS.
- R. Baron, B. Šljukić, C. Salter, A. Crossley and R. G. Compton, Electroanalysis, 2007, 19, 1062–1068 CrossRef CAS.
- B. Fang, C. Zhang, W. Zhang and G. Wang, Electrochim. Acta, 2009, 55, 178–182 CrossRef CAS.
- H. Zhang, J. Huang, H. Hou and T. You, Electroanalysis, 2009, 21, 1869–1874 CrossRef.
- L. Zheng and J.-F. Song, Sens. Actuators, B, 2009, 135, 650–655 CrossRef CAS.
- Q. Yi and W. Yu, J. Electroanal. Chem., 2009, 633, 159–164 CrossRef CAS.
- C. Zhang, G. Wang, Y. Ji, M. Liu, Y. Feng, Z. Zhang and B. Fang, Sens. Actuators, B, 2010, 150, 247–253 CrossRef CAS.
- M. Mazloum-Ardakani, H. Rajabi, B. B. F. Mirjalili, H. Beitollahi and A. Akbari, J. Solid State Electrochem., 2010, 14, 2285–2292 CrossRef CAS.
- S. Ivanov, U. Lange, V. Tsakova and V. M. Mirsky, Sens. Actuators, B, 2010, 150, 271–278 CrossRef CAS.
- N. A. Choudhry, R. O. Kadara, N. Jenkinson and C. E. Banks, Electrochem. Commun., 2010, 12, 406–409 CrossRef CAS.
- Q. Yi, F. Niu and W. Yu, Thin Solid Films, 2011, 519, 3155–3161 CrossRef CAS.
- U. P. Azad and V. Ganesan, Electrochim. Acta, 2011, 56, 5766–5770 CrossRef CAS.
- Y. Ding, Y. Wang, L. Zhang, H. Zhang, C. M. Li and Y. Lei, Nanoscale, 2011, 3, 1149–1157 RSC.
- W. Zhang, K. Huo, Y. Jiang, L. Hu, R. Chen and P. K. Chu, Microchim. Acta, 2011, 175, 137 CrossRef CAS.
- J. Zhao, M. Zhu, M. Zheng, Y. Tang, Y. Chen and T. Lu, Electrochim. Acta, 2011, 56, 4930–4936 CrossRef CAS.
- Z. Yin, L. Liu and Z. Yang, J. Solid State Electrochem., 2011, 15, 821–827 CrossRef CAS.
- N. A. Choudhry and C. E. Banks, Analyst, 2011, 136, 1153–1156 RSC.
- M. Mazloum-Ardakani, Z. Taleat, H. Beitollahi and H. Naeimi, Nanoscale, 2011, 3, 1683–1689 RSC.
- P. Paulraj, N. Janaki, S. Sandhya and K. Pandian, Colloids Surf., A, 2011, 377, 28–34 CrossRef CAS.
- Y. Liang, Y. Zhou, J. Ma, J. Zhao, Y. Chen, Y. Tang and T. Lu, Appl. Catal., B, 2011, 103, 388–396 CrossRef CAS.
- J. Li, H. Xie and L. Chen, Sens. Actuators, B, 2011, 153, 239–245 CrossRef CAS.
- V. Lyutov and V. Tsakova, J. Electroanal. Chem., 2011, 661, 186–191 CrossRef CAS.
- Y. Ding, C. Hou, B. Li and Y. Lei, Electroanalysis, 2011, 23, 1245–1251 CrossRef CAS.
- K. N. Han, C. A. Li, M.-P. N. Bui, X.-H. Pham and G. H. Seong, Chem. Commun., 2011, 47, 938–940 RSC.
- J. Panchompoo, L. Aldous, C. Downing, A. Crossley and R. G. Compton, Electroanalysis, 2011, 23, 1568–1578 CrossRef CAS.
- B. Fang, Y. Feng, M. Liu, G. Wang, X. Zhang and M. Wang, Microchim. Acta, 2011, 175, 145 CrossRef CAS.
- X. Li, Z. Chen, Y. Zhong, F. Yang, J. Pan and Y. Liang, Anal. Chim. Acta, 2012, 710, 118–124 CrossRef CAS PubMed.
- L. Shang, F. Zhao and B. Zeng, Electroanalysis, 2012, 24, 2380–2386 CrossRef CAS.
- F. Liao, Z. Wang, T. Guo, T. Zhang and Z. Wu, J. Electroanal. Chem., 2012, 673, 38–42 CrossRef CAS.
- M. Mazloum-Ardakani, H. Rajabi and H. Beitollahi, Chin. Chem. Lett., 2012, 23, 213–216 CrossRef CAS.
- J. Lei, X. Lu, W. Wang, X. Bian, Y. Xue, C. Wang and L. Li, RSC Adv., 2012, 2, 2541–2544 RSC.
- S. Ameen, M. S. Akhtar and H. S. Shin, Sens. Actuators, B, 2012, 173, 177–183 CrossRef CAS.
- S. Ameen, M. Shaheer Akhtar and H. S. Shin, Talanta, 2012, 100, 377–383 CrossRef CAS PubMed.
- Y.-Y. Tang, C.-L. Kao and P.-Y. Chen, Anal. Chim. Acta, 2012, 711, 32–39 CrossRef CAS PubMed.
- M. U. Anu Prathap, V. Anuraj, B. Satpati and R. Srivastava, J. Hazard. Mater., 2013, 262, 766–774 CrossRef CAS PubMed.
- Y. Wang, X. Yang, J. Bai, X. Jiang and G. Fan, Biosens. Bioelectron., 2013, 43, 180–185 CrossRef CAS PubMed.
- H. Ahmar, S. Keshipour, H. Hosseini, A. R. Fakhari, A. Shaabani and A. Bagheri, J. Electroanal. Chem., 2013, 690, 96–103 CrossRef CAS.
- V. V. Kondratiev, T. A. Babkova and E. G. Tolstopjatova, J. Solid State Electrochem., 2013, 17, 1621–1630 CrossRef CAS.
- C. Tan, X. Xu, F. Wang, Z. Li, J. Liu and J. Ji, Sci. China: Chem., 2013, 56, 911–916 CrossRef CAS.
- M. Abdul Aziz and A.-N. Kawde, Talanta, 2013, 115, 214–221 CrossRef CAS PubMed.
- Y.-C. Chou, C.-Y. Tai, J.-F. Lee, T.-S. Chan and J.-M. Zen, Electrochim. Acta, 2013, 104, 104–109 CrossRef CAS.
- R. B. Channon, J. C. Newland, A. W. T. Bristow, A. D. Ray and J. V. Macpherson, Electroanalysis, 2013, 25, 2613–2619 CrossRef CAS.
- S. Shukla, S. Chaudhary, A. Umar, G. R. Chaudhary and S. K. Mehta, Sens. Actuators, B, 2014, 196, 231–237 CrossRef CAS.
- R. Devasenathipathy, V. Mani, S.-M. Chen, D. Arulraj and V. S. Vasantha, Electrochim. Acta, 2014, 135, 260–269 CrossRef CAS.
- S. E. Baghbamidi, H. Beitollahi and S. Tajik, Anal. Bioanal. Electrochem., 2014, 6, 91–105 Search PubMed.
- P. K. Rastogi, V. Ganesan and S. Krishnamoorthi, Electrochim. Acta, 2014, 125, 593–600 CrossRef CAS.
- K. Ghanbari, Synth. Met., 2014, 195, 234–240 CrossRef CAS.
- S. Koçak and B. Aslışen, Sens. Actuators, B, 2014, 196, 610–618 CrossRef.
- C. W. Foster, J. Pillay, J. P. Metters and C. E. Banks, Sens. Actuators, B, 2014, 14, 21905–21922 Search PubMed.
- C. Karuppiah, S. Palanisamy, S.-M. Chen, S. K. Ramaraj and P. Periakaruppan, Electrochim. Acta, 2014, 139, 157–164 CrossRef CAS.
- M. Shamsipur, Z. Karimi, M. A. Tabrizi and A. Shamsipur, Electroanalysis, 2014, 26, 1994–2001 CrossRef CAS.
- A. Maringa and T. Nyokong, Electroanalysis, 2014, 26, 1068–1077 CrossRef CAS.
- A. R. Fakhari, H. Ahmar, H. Hosseini and S. Kazemi Movahed, Sens. Actuators, B, 2015, 213, 82–91 CrossRef CAS.
- S. P. Kim, S. G. Lee, M. Y. Choi and H. C. Choi, J. Nanomater., 2015, 2015, 120485 Search PubMed.
- H. Mahmoudi Moghaddam, H. Beitollahi, S. Tajik, I. Sheikhshoaie and P. Biparva, Environ. Monit. Assess., 2015, 187, 407 CrossRef PubMed.
- J. Zhang, H. Liu, M. Dou, F. Wang, J. Liu, Z. Li and J. Ji, Electroanalysis, 2015, 27, 1188–1194 CrossRef CAS.
- M. Ongaro, M. Signoretto, V. Trevisan, A. M. Stortini and P. Ugo, Chemosensors, 2015, 3, 146–156 CrossRef CAS.
- R. Sivasubramanian and M. V. Sangaranarayanan, Sens. Actuators, B, 2015, 213, 92–101 CrossRef CAS.
- R. Ojani, V. Rahemi and J.-B. Raoof, J. Chin. Chem. Soc., 2015, 62, 90–96 CrossRef CAS.
- A. Samadi-Maybodi, S. Ghasemi and H. Ghaffari-Rad, Sens. Actuators, B, 2015, 220, 627–633 CrossRef CAS.
- S. Dutta, C. Ray, S. Mallick, S. Sarkar, A. Roy and T. Pal, RSC Adv., 2015, 5, 51690–51700 RSC.
- E. G. Tolstopjatova, V. V. Kondratiev and S. N. Eliseeva, J. Solid State Electrochem., 2015, 19, 2951–2959 CrossRef CAS.
- A. Ejaz, M. S. Ahmed and S. Jeon, Sens. Actuators, B, 2015, 221, 1256–1263 CrossRef CAS.
- Z. Zhao, Y. Sun, P. Li, W. Zhang, K. Lian, J. Hu and Y. Chen, Talanta, 2016, 158, 283–291 CrossRef CAS PubMed.
- H. Beitollahi, S. Tajik and S. Jahani, Electroanalysis, 2016, 28, 1093–1099 CrossRef CAS.
- J. Y. Lee, T. L. Nguyen, J. H. Park and B.-K. Kim, Sensors, 2016, 16, 647–657 CrossRef PubMed.
- A. Benvidi, S. Jahanbani, B.-F. Mirjalili and R. Zare, Chin. J. Catal., 2016, 37, 549–560 CrossRef CAS.
- D. Rao, Q. Sheng and J. Zheng, Sens. Actuators, B, 2016, 236, 192–200 CrossRef CAS.
- I. Kang, W.-S. Shin, S. Manivannan, Y. Seo and K. Kim, J. Electrochem. Sci. Technol., 2016, 7, 277–285 CrossRef CAS.
- A. A. Ismail, F. A. Harraz, M. Faisal, A. M. El-Toni, A. Al-Hajry and M. S. Al-Assiri, Mater. Des., 2016, 109, 530–538 CrossRef CAS.
- J. Hu, Z. Zhao, Y. Sun, Y. Wang, P. Li, W. Zhang and K. Lian, Appl. Surf. Sci., 2016, 364, 434–441 CrossRef CAS.
- Y. Liu, Z. Qiu, Q. Wan, Z. Wang, K. Wu and N. Yang, Electroanalysis, 2016, 28, 126–132 CrossRef CAS.
- X. Gao, C. Du, C. Zhang and W. Chen, ChemElectroChem, 2016, 3, 1266–1272 CrossRef CAS.
- M. M. Rahman, J. Ahmed, A. M. Asiri, I. A. Siddiquey and M. A. Hasnat, RSC Adv., 2016, 6, 90470–90479 RSC.
- F. Xu, Y. Liu, S. Xie and L. Wang, Anal. Methods, 2016, 8, 316–325 RSC.
- H. Heydari, M. B. Gholivand and A. Abdolmaleki, Mater. Sci. Eng., C, 2016, 66, 16–24 CrossRef CAS PubMed.
- B. Kaur, R. Srivastava and B. Satpati, Catal. Sci. Technol., 2016, 6, 1134–1145 RSC.
- F. Giroud, A. J. Gross, D. F. Junior, M. Holzinger, C. E. M. de Campos, J. J. S. Acuña, J. B. Domingos and S. Cosnier, J. Electrochem. Soc., 2016, 164, H3052–H3057 CrossRef.
- K. J. Babu, A. Zahoor, K. S. Nahm, M. A. Aziz, P. Vengadesh and G. G. Kumar, New J. Chem., 2016, 40, 7711–7720 RSC.
- J. Hu, Z. Zhao, J. Zhang, G. Li, P. Li, W. Zhang and K. Lian, Appl. Surf. Sci., 2017, 396, 523–529 CrossRef CAS.
- P. Bansal, G. Bhanjana, N. Prabhakar, J. S. Dhau and G. R. Chaudhary, J. Mol. Liq., 2017, 248, 651–657 CrossRef CAS.
- M. M. Rahman, V. G. Alfonso, F. Fabregat-Santiago, J. Bisquert, A. M. Asiri, A. A. Alshehri and H. A. Albar, Microchim. Acta, 2017, 184, 2123–2129 CrossRef CAS.
- S. Sakthinathan, S. Kubendhiran, S.-M. Chen, M. Govindasamy, F. M. A. Al-Hemaid, M. Ajmal Ali, P. Tamizhdurai and S. Sivasanker, Appl. Organomet. Chem., 2017, 31, e3703 CrossRef.
- Y. J. Yang, X. Hu and Z. Xu, Fullerenes, Nanotubes, Carbon Nanostruct., 2017, 25, 435–441 CrossRef CAS.
- S. Rostami, S. Naser Azizi and S. Ghasemi, New J. Chem., 2017, 41, 13712–13723 RSC.
- J. Zhao, P. Yue, S. Tricard, T. Pang, Y. Yang and J. Fang, Sens. Actuators, B, 2017, 251, 706–712 CrossRef CAS.
- S. Premlatha, P. Sivasakthi and G. N. K. Ramesh Bapu, J. Electroanal. Chem., 2017, 788, 107–117 CrossRef CAS.
- N. Salek Gilani, S. Naser AzizI and S. Ghasemi, Bull. Mater. Sci., 2017, 40, 177–185 CrossRef.
- Z. Yang, X. Zheng and J. Zheng, Chem. Eng. J., 2017, 327, 431–440 CrossRef CAS.
- H. M. A. Amin, M. F. El-Kady, N. F. Atta and A. Galal, Electroanalysis, 2018, 30, 1757–1766 CrossRef.
- N. Teymoori, J. B. Raoof, M. A. Khalilzadeh and R. Ojani, J. Iran. Chem. Soc., 2018, 15, 2271–2279 CrossRef CAS.
- A. Maleki, R. Rezaee, H. Daraei, B. Shahmoradi and N. Amini, J. Alloys Compd., 2018, 763, 997–1004 CrossRef CAS.
- Y. J. Yang and W. Li, Fullerenes, Nanotubes, Carbon Nanostruct., 2018, 26, 837–845 CrossRef CAS.
- P. Wuamprakhon, A. Krittayavathananon, N. Ma, N. Phattharasupakun, T. Maihom, J. Limtrakul and M. Sawangphruk, J. Electroanal. Chem., 2018, 808, 124–132 CrossRef CAS.
- F. Tahernejad-Javazmi, M. Shabani-Nooshabadi, H. Karimi-Maleh and H. Naeimi, Res. Chem. Intermed., 2018, 44, 5389–5401 CrossRef CAS.
- Y. He, X. Yang, Y. Huo, Q. Han and J. Zheng, Open Access Journal of Chemistry, 2018, 2, 15–22 Search PubMed.
- F. Amiripour, S. N. Azizi and S. Ghasemi, Biosens. Bioelectron., 2018, 107, 111–117 CrossRef CAS PubMed.
- M. Faisal, F. A. Harraz, A. E. Al-Salami, S. A. Al-Sayari, A. Al-Hajry and M. S. Al-Assiri, Mater. Chem. Phys., 2018, 214, 126–134 CrossRef CAS.
- M. Wang, L. Yang, B. Hu, Y. Liu, Y. Song, L. He, Z. Zhang and S. Fang, Appl. Surf. Sci., 2018, 445, 123–132 CrossRef CAS.
- H. Huang, T. Li, Y. Sun, L. Yu, C. Wang, R. Shen, W. Ye, D. Wang and Y. Li, Microchim. Acta, 2019, 186, 46–56 CrossRef PubMed.
- X. Zhang and J. Zheng, Appl. Surf. Sci., 2019, 493, 1159–1166 CrossRef CAS.
- S. Ghasemi, S. R. Hosseini, F. Hasanpoor and S. Nabipour, Microchim. Acta, 2019, 186, 601 CrossRef PubMed.
- D. M. Nguyen, L. G. Bach and Q. B. Bui, J. Pharm. Biomed. Anal., 2019, 172, 243–252 CrossRef CAS PubMed.
- Y. Gao, S. Zhang, W. Hou, H. Guo, Q. Li, D. Dong, S. Wu, S. Zhao and H. Zhang, Appl. Surf. Sci., 2019, 491, 267–275 CrossRef CAS.
- F. Asadi, S. N. Azizi and S. Ghasemi, J. Mater. Sci.: Mater. Electron., 2019, 30, 5410–5420 CrossRef CAS.
- A. Avanes, M. Hasanzadeh-Karamjavan and G. Shokri-Jarcheloo, Microchim. Acta, 2019, 186, 441 CrossRef PubMed.
- X. Zhang and J. Zheng, Microchim. Acta, 2020, 187, 89 CrossRef CAS PubMed.
- R. Wahab, N. Ahmad, M. Alam and J. Ahmad, Vacuum, 2019, 165, 290–296 CrossRef CAS.
- R. Ahmad, T. Bedük, S. M. Majhi and K. N. Salama, Sens. Actuators, B, 2019, 286, 139–147 CrossRef CAS.
- M. M. Rahman, M. M. Alam and K. A. Alamry, J. Ind. Eng. Chem., 2019, 77, 309–316 CrossRef CAS.
- S. Ramanathan, E. Elanthamilan, A. Obadiah, A. Durairaj, P. SanthoshKumar, J. Princy Merlin, S. Ramasundaram and S. Vasanthkumar, J. Electron. Mater., 2019, 48, 542–550 CrossRef CAS.
- M. Nemakal, S. Aralekallu, I. Mohammed, S. Swamy and L. K. Sannegowda, J. Electroanal. Chem., 2019, 839, 238–246 CrossRef CAS.
- İ. Teoman, S. Karakaya and Y. Dilgin, Anal. Lett., 2019, 52, 2041–2056 CrossRef.
- C. Duan, Y. Dong, Q. Sheng and J. Zheng, Talanta, 2019, 198, 23–29 CrossRef CAS PubMed.
- P. Wuamprakhon, A. Krittayavathananon, S. Kosasang, N. Ma, T. Maihom, J. Limtrakul, N. Chanlec, P. Kidkhunthod and M. Sawangphruk, Inorg. Chem., 2020, 59, 15595–15605 CrossRef CAS PubMed.
- G. Srinidhi, S. Sudalaimani, K. Giribabu, S. J. S. Basha and C. Suresh, Microchim. Acta, 2020, 187, 359 CrossRef CAS PubMed.
- H. Zhou, L. Chen, S. Li, S. Huang, Y. Sun, Y. Chen, Z. Wang, W. Liu and X. Li, J. Colloid Interface Sci., 2020, 566, 473–484 CrossRef CAS PubMed.
- A. L. Lavanya, K. G. Bala Kumari, K. R. S. Prasad and P. K. Brahman, Int. J. Environ. Anal. Chem., 2020, 1–16, DOI:10.1080/03067319.2020.1726333.
- N. S. K. Gowthaman, H. Ngee Lim, V. Balakumar and S. Shankar, Ultrason. Sonochem., 2020, 61, 104828–110438 CrossRef CAS PubMed.
- E. S. Gil and L. T. Kubota, J. Braz. Chem. Soc., 2000, 11, 304–310 CrossRef CAS.
- S. Rani, S. Kapoor, B. Sharma, S. Kumar, R. Malhotra and N. Dilbaghi, J. Alloys Compd., 2020, 816, 152509–152519 CrossRef CAS.
- K. M. Emran, S. M. Ali and H. E. Alanazi, J. Electroanal. Chem., 2020, 856, 113661–113669 CrossRef CAS.
- Y. Pei, M. Hu, Y. Xia, W. Huang, Z. Li and S. Chen, Sens. Actuators, B, 2020, 304, 127416–127424 CrossRef CAS.
- Y. Yao, X. Han, X. Yang, J. Zhao and C. Chai, Chin. J. Chem., 2021, 39, 330–336 CrossRef CAS.
- M. Faisal, M. A. Rashed, M. M. Abdullah, F. A. Harraz, M. Jalalah and M. S. Al-Assiri, J. Electroanal. Chem., 2020, 879, 114805–114818 CrossRef CAS.
- S. Tajik, H. Beitollahi, R. Hosseinzadeh, A. Aghaei Afshar, R. S. Varma, H. W. Jang and M. Shokouhimehr, ACS Omega, 2021, 6, 4641–4648 CrossRef CAS PubMed.
- A. Mohammad, M. E. Khan, M. R. Karim, M. H. Cho and T. Yoon, Ceram. Int., 2021, 47, 23578–23589 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |