Coupling of MAPbI3 microcrystals with conductive polyaniline for efficient visible-light-driven H2 evolution†
Wenbo
Li
abc,
Fang
Wang
abc,
Zhengguo
Zhang
abc,
Xiaohua
Ma
abc and
Shixiong
Min
 *abc
*abc
aSchool of Chemistry and Chemical Engineering, North Minzu University, Yinchuan, 750021, P. R. China. E-mail: sxmin@nun.edu.cn
bKey Laboratory of Chemical Engineering and Technology, State Ethnic Affairs Commission, North Minzu University, Yinchuan, 750021, P. R. China
cNingxia Key Laboratory of Solar Chemical Conversion Technology, North Minzu University, Yinchuan 750021, P. R. China
First published on 22nd November 2021
Abstract
Although the methylammonium lead iodide (MAPbI3) hybrid perovskite has shown great potential in photocatalytic H2 evolution, its performance is significantly suppressed due to the insufficient charge separation. Herein, a strongly coupled 3D/1D composite photocatalyst of MAPbI3 microcrystals with conductive polyaniline nanowires (PANI NWs) is developed for efficient photocatalytic H2 evolution in MAPbI3-saturated HI aqueous solution under visible light irradiation. The most active MAPbI3 (100 mg)–PANI NW (7 mg) composite exhibits a H2 evolution rate of 38.8 μmol h−1, which is 29 times higher than that of pristine MAPbI3. Spectroscopic and photoelectrochemical measurements reveal that the coupled PANI NWs can greatly improve the charge separation, thereby considerably enhancing the photocatalytic H2 evolution of the composite photocatalyst.
Photocatalytic H2 evolution represents a highly promising route to effectively convert solar energy to clean H2 energy.1–8 Recently, besides the conventional inorganic semiconductors (CdS and TiO2), a number of all-inorganic and hybrid perovskites have been identified as visible light photocatalysts for photocatalytic H2 evolution via splitting HX (X = I or Br) in aqueous solution.9–11 Among them, methylammonium lead iodide (MAPbI3), as a typical organic–inorganic hybrid perovskite, has been most widely studied for photocatalytic H2 evolution due to its suitable band gap and excellent light absorption capability.12 However, the rapid recombination of photogenerated electrons and holes in pristine MAPbI3 significantly suppresses its photocatalytic performance. To overcome this limitation, two main strategies, decorating the surface with cocatalysts (MoS2,13 CoP,14 Ni3C,15 MoC,16etc.) and engineering the band gap and charge transfer kinetics by halide substitution (MAPbBr3−xIx),17 have been adopted to further improve the photocatalytic H2 evolution performance of MAPbI3. In particular, it has been demonstrated that compositing MAPbI3 with conductive materials such as reduced graphene oxide (rGO),18 black phosphorus (BP),19 and Ti3C2Tx MXene20 is also an effective route to develop high-performance MAPbI3-based composite photocatalysts with enhanced charge separation efficiency and greatly improved photocatalytic performance for H2 evolution compared with the pristine MAPbI3.
Recently, owing to their high electrical conductivity and excellent chemical stability, conductive polymers such as polyaniline (PANI),21 polypyrrole (PPy),22 polythiophene (PTh),23 poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS),24 and their derivates have been extensively used as efficient electron or hole transfer media to boost the charge separation and thus enhance the photoelectrochemical conversion efficiency of perovskite solar cells. Of note, PANI is an abundant, low cost, and easily processable material that has also been employed as an efficient electron/hole acceptor and transporter to enhance the performance of a semiconductor photocatalyst by improving the charge separation.25 In addition, the PANI chains after doping bear a large number of positively charged –NH2+– groups that can effectively couple with hybrid perovskites through a strong ionic bond to form a strongly coupled composite.21 Moreover, the N atoms of amine groups on PANI chains bear abundant lone pair electrons, which are able to effectively capture H+ to facilitate the H2 evolution reaction on perovskite photocatalysts.26 Given the advantages of PANI, it could be envisioned that compositing conductive PANI with MAPbI3 would result in an efficient composite photocatalyst with enhanced charge separation and photocatalytic H2 evolution performance; however, there are still no reports on using PANI as a charge separation promoter to enhance the photocatalytic H2 evolution performance of the MAPbI3 microcrystals.
We report herein a 3D/1D heterostructured composite photocatalyst by coupling 3D MAPbI3 microcrystals with 1D PANI NWs via an in situ doping-induced assembly method for efficient photocatalytic H2 evolution from MAPbI3-saturated HI solution under visible light irradiation. The strongly anchored conductive PANI NWs can greatly improve the charge separation of MAPbI3. Consequently, the resulting MAPbI3–PANI NW composite photocatalyst with an optimal mass ratio of MAPbI3 to PANI exhibits a 29 times higher photocatalytic H2 evolution rate than pristine MAPbI3 microcrystals and relatively good stability.
The MAPbI3 microcrystals were first synthesized according to our previously reported procedures (see experimental details in the ESI†) and characterized by X-ray diffraction (XRD), revealing that MAPbI3 microcrystals are well-crystallized in the tetragonal phase without any other impurities (Fig. S1, ESI†). The HCl-doped PANI NWs were prepared through the facile interfacial polymerization method at room temperature (see experimental details in the ESI†). The dedoped PANI NWs were then obtained by reacting HCl-doped PANI NWs with NH3·H2O (1 M) followed by washing with water (Fig. S2, ESI†). Afterward, a series of MAPbI3–PANI NW composite photocatalysts were prepared by directly mixing 100 mg of MAPbI3 microcrystals with different amounts of dedoped PANI NWs in a MAPbI3-saturated aqueous HI/H3PO2 (v/v = 4/1) solution and directly used for the photocatalytic H2 evolution reaction without separation. Given that the MAPbI3–PANI NW composite photocatalyst prepared with 100 mg of MAPbI3 and 7 mg of PANI NWs exhibits the highest H2 evolution activity (see the results and discussion below), the following discussion is mainly on this sample unless noted.
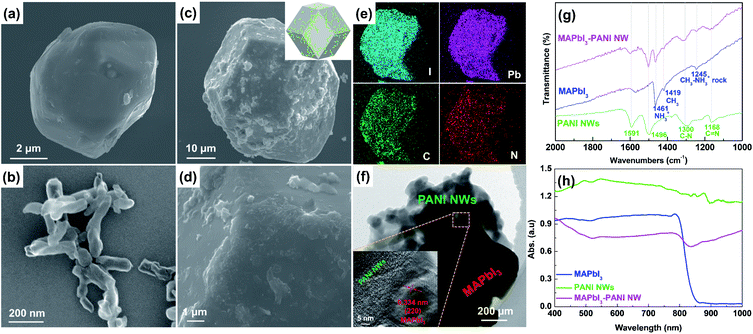
The XRD patterns (Fig. S1, ESI†) reveal that the resulting MAPbI3–PANI NW composite photocatalyst has the same tetragonal structure and good crystallinity as pristine MAPbI3 microcrystals, suggesting that the surface anchoring of PANI NWs can hardly change the bulk phase structure of the MAPbI3. In addition, no characteristic diffraction peaks related to PANI NWs can be found in the XRD pattern of the composite probably due to the low content and low crystallinity of PANI NWs. Fig. 1 shows the scanning electron microscopy (SEM) images of the pristine MAPbI3, PANI NWs, and MAPbI3–PANI NW composite photocatalyst. The pristine MAPbI3 possesses a cube-like morphology with a size of ∼10 μm and a relatively smooth surface (Fig. 1a), and the PANI NWs are short nanowires with diameters of 30–50 nm and lengths of 100–150 nm (Fig. 1b and S3, ESI†). For the MAPbI3–PANI NW composite photocatalyst, the surface of the MAPbI3 microcrystal becomes rougher (Fig. 1c) and a large number of PANI NWs are found to be firmly embedded on its surface (Fig. 1d). The corresponding energy dispersive X-ray (EDX) elemental maps (Fig. 1e) show a uniform distribution of I, Pd, and C on MAPbI3 particles and the distribution of N mainly occurs at the corner of MAPbI3 microcrystals, confirming the successful coupling of MAPbI3 and PANI NWs. As a result, the specific surface area of MAPbI3–PANI NW is enhanced to 3.5 m2 g−1, much higher than that of pristine MAPbI3 (0.1 m2 g−1) due to the presence of high-surface-area PANI NWs (57.4 m2 g−1) (Fig. S4, ESI†). The transmission electron microscopy (TEM) image in Fig. 1f indicates that the PANI NWs are in close contact with MAPbI3, and some PANI NWs are embedded inside the MAPbI3 microcrystals (Fig. S5, ESI†). The high-resolution TEM (HRTEM) image (inset of Fig. 1f) further indicates that an intimate interface is formed between well-crystallized MAPbI3 and amorphous PANI NWs, further revealing the formation of a strongly coupled MAPbI3–PANI NW composite photocatalyst. The surface anchoring of PANI NWs on MAPbI3 microcrystals was further studied by Fourier transform infrared spectroscopy (FTIR) (Fig. 1g), which reveals that all the characteristic peaks of PANI NWs and MAPbI3 can be found in the FTIR spectrum of the MAPbI3–PANI NW composite photocatalyst, further confirming the presence of PANI NWs in the composite.27 The UV-vis diffuse reflectance spectra (Fig. 1h) indicate that the MAPbI3–PANI NW composite photocatalyst shows greatly enhanced light absorption towards the near-infrared region compared to pristine MAPbI3 due to the presence of PANI NWs. In addition, based on the corresponding Tauc plots (Fig. S6, ESI†), the band gap (Eg) of MAPbI3–PANI NW (1.26 eV) is much smaller than that of pristine MAPbI3 (1.49 eV) as a result of strong coupling interactions between MAPbI3 and PANI NWs. These results reveal that coupling PANI NWs with MAPbI3 would be effective in maximizing the light utilization efficiency and thus improving the photocatalytic H2 evolution activity.16,20
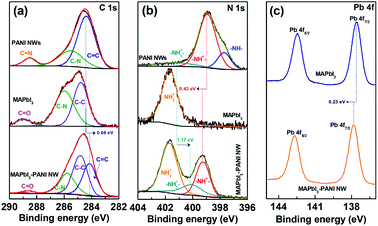
The successful combination of MAPbI3 and PANI NWs was further illustrated by X-ray photoelectron spectroscopy (XPS). As shown in Fig. S7, ESI,† all of the C, N, I and Pb elements can be found in the XPS survey spectrum of the MAPbI3–PANI NW composite photocatalyst, confirming the existence of PANI NWs. The high-resolution C 1s spectra in Fig. 2a indicate that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peak of PANI NWs in the MAPbI3–PANI NW composite photocatalyst presents a shift of 0.66 eV toward a lower binding energy as compared to that of pristine PANI NWs, and a more pronounced downshift (∼1.17 eV) is also found for the –NH2+– in the N 1s spectra (Fig. 2b). These results clearly suggest the formation of chemical interactions between PANI NWs and MAPbI3 microcrystals. Moreover, it should be noted that the –NH– peak of PANI NWs disappears in the composite, while the –NH+– peak is shifted to a higher binding energy by 0.43 eV.27 This can be ascribed to the in situ doping of PANI NWs when mixing with MAPbI3 in acidic HI/H3PO2 solution, which would effectively induce the assembly of MAPbI3 and PANI NWs to form a strongly coupled composite photocatalyst. In addition, as shown in Fig. 2c and S8, ESI,† the Pb 4f and I 3d peaks for the MAPbI3–PANI NW composite photocatalyst display shifts toward higher binding energies by 0.23 and 0.15 eV relative to pristine MAPbI3, respectively. This further suggests that the PANI NWs are strongly anchored on the surface of MAPbI3 microcrystals, leading to an efficient charge transfer between MAPbI3 and PANI NWs, which would greatly improve the charge separation and thus enhance the photocatalytic performance of the MAPbI3–PANI NW composite photocatalyst.16
C peak of PANI NWs in the MAPbI3–PANI NW composite photocatalyst presents a shift of 0.66 eV toward a lower binding energy as compared to that of pristine PANI NWs, and a more pronounced downshift (∼1.17 eV) is also found for the –NH2+– in the N 1s spectra (Fig. 2b). These results clearly suggest the formation of chemical interactions between PANI NWs and MAPbI3 microcrystals. Moreover, it should be noted that the –NH– peak of PANI NWs disappears in the composite, while the –NH+– peak is shifted to a higher binding energy by 0.43 eV.27 This can be ascribed to the in situ doping of PANI NWs when mixing with MAPbI3 in acidic HI/H3PO2 solution, which would effectively induce the assembly of MAPbI3 and PANI NWs to form a strongly coupled composite photocatalyst. In addition, as shown in Fig. 2c and S8, ESI,† the Pb 4f and I 3d peaks for the MAPbI3–PANI NW composite photocatalyst display shifts toward higher binding energies by 0.23 and 0.15 eV relative to pristine MAPbI3, respectively. This further suggests that the PANI NWs are strongly anchored on the surface of MAPbI3 microcrystals, leading to an efficient charge transfer between MAPbI3 and PANI NWs, which would greatly improve the charge separation and thus enhance the photocatalytic performance of the MAPbI3–PANI NW composite photocatalyst.16
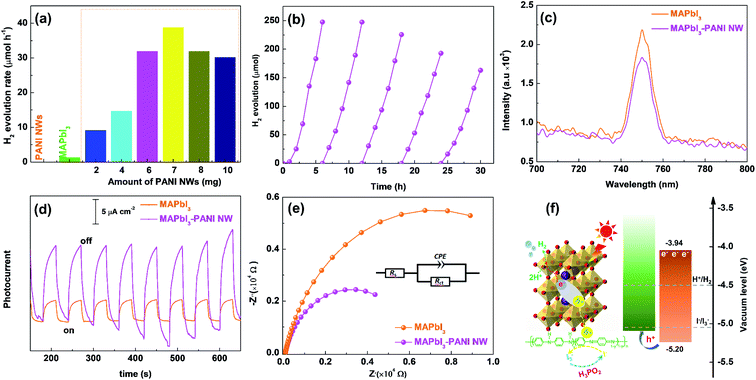
The photocatalytic H2 evolution performance of the as-prepared MAPbI3–PANI NW composite photocatalysts was evaluated in an MAPbI3-saturated aqueous HI/H3PO2 mixed solution under visible light irradiation (see experimental details in the ESI†). Fig. 3a shows the photocatalytic H2 evolution rates on MAPbI3–PANI NW composite photocatalysts prepared from 100 mg of MAPbI3 with different adding amounts of PANI NWs (2 to 10 mg), and those on pristine MAPbI3 and PANI NWs are included for comparison. Clearly, the pristine MAPbI3 shows a very low H2 evolution rate (1.32 μmol h−1). This is due to the fast charge recombination. On the other hand, the PANI NWs alone also show no activity toward H2 evolution under the same measurement conditions. Impressively, when PANI NWs were anchored on MAPbI3, the in situ formed MAPbI3–PANI NW composite photocatalysts show greatly enhanced photocatalytic H2 evolution activities compared to pristine MAPbI3. This clearly suggests that the PANI NWs are effective as a charge separation promoter to boost the charge separation and thus the photocatalytic performance of MAPbI3. The H2 evolution rate is greatly enhanced with increasing adding amount of PANI NWs and reaches the highest value of 38.8 μmol h−1 at 7 mg of PANI NWs, which is 29 times higher than that of pristine MAPbI3. The total amount of H2 evolved is 247.6 μmol and the turnover number (TON) is 3 in a 6 h reaction (Fig. S9, ESI†). The photocatalytic H2 evolution activity of the MAPbI3–PANI NW composite photocatalyst is also comparable to those of MAPbI3-based photocatalysts (Table S1, ESI†). Additionally, the apparent quantum efficiency of H2 evolution over this best photocatalyst is determined to be 0.3% at 420 nm (see details in the ESI†). Further increasing the adding amount of PANI NWs to 8 mg or more leads to a decreased performance since excessively anchored PANI NWs may interfere with the light absorption of MAPbI3. In addition, it should be mentioned that when PANI NWs were replaced by equal amounts of the HCl-doped PANI NWs to prepare the MAPbI3–PANI NW composite photocatalyst and other experimental conditions were maintained, the obtained H2 evolution rate dramatically reduced to 4.8 μmol h−1 (Fig. S10, ESI†). This can be explained by the fact that the HCl-doped PANI NWs are negatively charged (−10.9 mV) (Fig. S11, ESI†), and do not effectively couple with negatively charged MAPbI3 (−17.4 mV) in MAPbI3-saturated HI solution,15 thus leading to insufficient activity. In contrast, the dedoped PANI NWs are positively charged (+2.9 mV) and can readily couple with MAPbI3via electrostatic interactions to form a MAPbI3–PANI NW composite photocatalyst with strongly coupled interfaces, which are crucial to enhance the photocatalytic activity.
The photocatalytic H2 evolution stability of the MAPbI3–PANI NW composite photocatalyst was then tested by performing a continuous cycling reaction for a 30 h irradiation (with each cycle lasting 6 h). As shown in Fig. 3b, the H2 evolution activity is quite stable for the first three cycles, and then gradually decreases during the last two cycles. After the 30 h reaction, the colour of the reaction solution turned light yellow (Fig. S12, ESI†), indicating the partial decomposition of MAPbI3, as evidenced by XRD analysis (Fig. S13, ESI†). In addition, despite there being no obvious changes in the chemical composition and valence states of the composite (Fig. S14, ESI†), the SEM analysis (Fig. S15, ESI†) indicates that the MAPbI3–PANI NW composite photocatalyst breaks into small pieces and the intimate contact between MAPbI3 and PANI NWs is significantly damaged after the stability test. This leads to an unfavourable charge transfer from MAPbI3 to PANI NWs, thereby resulting in inferior stability.
To clarify the role of PANI NWs in improving the photocatalytic performance of the MAPbI3–PANI NW composite photocatalyst, the photoluminescence (PL) spectra of the samples were first measured. As presented in Fig. 3c, the pristine MAPbI3 microcrystals exhibit an intense PL emission at 750 nm upon excitation at 500 nm originating from the serious electron–hole recombination, which significantly quenches after anchoring PANI NWs. This suggests that a more efficient electron–hole separation is achieved in the MAPbI3–PANI NW composite photocatalyst.18 In addition, in comparison with the pristine MAPbI3, the photocurrent response of the MAPbI3–PANI NW composite photocatalyst is greatly enhanced (Fig. 3d). Mott–Schottky (MS) measurements were then performed to determine the conduction band (CB) and valence band (VB) levels of pristine MAPbI3 and MAPbI3–PANI NW, and the flat-band potentials (Efb) can be used to estimate the CB potentials (ECB). As shown in Fig. S16, ESI,† the ECB of pristine MAPbI3 and MAPbI3–PANI NW was determined to be −3.97 and −3.96 eV vs. the vacuum energy level, respectively. Based on the above results and the bandgap energy obtained by UV-vis-DRS, the VB potential (EVB) of pristine MAPbI3 and MAPbI3–PANI NW composite was calculated to be −5.46 and −5.20 eV vs. the vacuum energy level, respectively. Therefore, in combination with the reported EVB for PANI (−5.1 eV vs. vacuum energy level),21 it can be inferred that the photogenerated holes produced on MAPbI3 can migrate to PANI NWs to enable more efficient separation and transportation of the charges generated on MAPbI3.21 These favorable effects can be attributed to the presence of PANI NWs in the composite, which can be expected to provide conductive pathways for fast charge transfer, which is further confirmed by electrochemical impedance spectra (EIS). As shown in Fig. 3e, the MAPbI3–PANI NW composite photocatalyst exhibits a much smaller semicircle diameter and a much lower interfacial charge-transfer resistance (Rct: 6.7 kΩ) compared to pristine MAPbI3 (Rct: 14.0 kΩ), indicating that the coupled PANI NWs offer fast conductive pathways to boost charge transfer and would thus be expected to improve the charge separation.13,17
Based on the above experimental results, a photocatalytic H2 evolution mechanism on the MAPbI3–PANI NW composite photocatalyst is proposed, as illustrated in Fig. 3f. Upon visible light excitation, the photogenerated holes produced on MAPbI3 quickly transfer to the PANI NWs across the intimate interface between the two, and an efficient separation of electrons and holes is achieved. Since the electrochemical measurements show that the PANI NWs can hardly catalyze the proton reduction reaction but are an efficient catalyst for the I− oxidation reaction with a lower overpotential (Fig. S17 and S18, ESI†), the captured photogenerated holes by PANI NWs can be efficiently consumed for the I− oxidation to I3−. On the other hand, it has been verified that the ECB of MAPbI3 is located at ca. −3.96 eV versus the vacuum level, and thus the photogenerated electrons left on MAPbI3 will have sufficient driving force to reduce protons to H2.
In summary, we report a 3D/1D heterostructured composite photocatalyst by strongly coupling MAPbI3 microcrystals with PANI NWs for photocatalytic H2 evolution via splitting HI in MAPbI3-saturated aqueous solution under visible light irradiation. The most active MAPbI3–PANI NW exhibit a 29 times higher H2 evolution rate than that of pristine MAPbI3 and relatively good stability. The conductive PANI NWs provide fast charge transfer pathways that greatly improve the charge separation and enhance the photocatalytic H2 evolution activity. This work confirms the effectiveness of using conductive polymers as charge transfer promoters to enhance the photocatalytic performance of MAPbI3 and the new insights obtained can be extended to develop other all-inorganic and hybrid perovskite-based photocatalysts with enhanced performances.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 22162001), the Natural Science Foundation of Ningxia Province (Grant No. 2021AAC03194), the Graduate Student Innovation Project of North Minzu University (Grant No. YCX21138), the Foundation of Academic Top-notch Talent Support Program of North Minzu University (Grant No. 2019BGBZ08), the Leading Talents Program of Science and Technology Innovation in Ningxia Province (Grant No. 2020GKLRLX14), the Innovation and Entrepreneurship Projects for Returnees of Ningxia Province, and the Cooperative Scientific Research Project of Chunhui Plan of Ministry of Education of China (Grant No. 201900081).References
- D. D. Ren, W. N. Zhang, Y. N. Ding, R. C. Shen, Z. M. Jiang, X. Y. Lu and X. Li, Solar RRL, 2020, 4, 1900423 CrossRef CAS.
- R. C. Shen, K. L. He, A. P. Zhang, N. Li, Y. H. Ng, P. Zhang, J. Hu and X. Li, Appl. Catal. B Environ., 2021, 291, 120104 CrossRef CAS.
- H. T. Xu, R. Xiao, J. R. Huang, Y. Jiang, C. X. Zhao and X. F. Yang, Chin. J. Catal., 2021, 42, 107 CrossRef CAS.
- D. D. Ren, Z. Z. Liang, Y. H. Ng, P. Zhang, Q. J. Xiang and X. Li, Chem. Eng. J., 2020, 390, 124496 CrossRef CAS.
- C. Liu, Y. Feng, Z. T. Han, Y. Sun, X. Q. Wang, Q. F. Zhang and Z. G. Zou, Chin. J. Catal., 2021, 42, 164 CrossRef CAS.
- H. T. Xu, R. Xiao, J. R. Huang, Y. Jiang, C. X. Zhao and X. F. Yang, Chin. J. Catal., 2021, 42, 107 CrossRef CAS.
- F. F. Mei, Z. Li, K. Dai, J. F. Zhang and C. H. Liang, Chin. J. Catal., 2020, 41, 41 CrossRef CAS.
- J. R. Ran, J. Zhang, J. G. Yu, M. Jaroniecc and S. Z. Qiao, Chem. Soc. Rev., 2014, 43, 7787 RSC.
- X. M. Wang, H. Wang, H. F. Zhang, W. Yu, X. L. Wang, Y. Zhao, X. Zong and C. Li, ACS Energy Lett, 2018, 3, 1159 CrossRef CAS.
- W. B. Li, S. X. Min, F. Wang, Z. G. Zhang and D. W. Gao, Chem. Commun., 2021, 57, 1121 RSC.
- Z. H. Guan, Y. Q. Wu, P. Wang, Q. Q. Zhang, Z. Y. Wang, Z. K. Zheng, Y. Y. Liu, Y. Dai, M. H. Whangbo and B. B. Huang, Appl. Catal. B Environ., 2019, 245, 522 CrossRef CAS.
- S. Park, W. J. Chang, C. W. Lee, S. Park, H.-Y. Ahn and K. T. Nam, Nat. Energy, 2016, 2, 16185 CrossRef.
- F. Wang, X. Y. Liu, Z. G. Zhang and S. X. Min, Chem. Commun., 2020, 56, 3281 RSC.
- C. Cai, Y. Teng, J. H. Wu, J. Y. Li, H. Y. Chen, J. H. Chen and D. B. Kuang, Adv. Funct. Mater., 2020, 30, 2001478 CrossRef CAS.
- Z. J. Zhao, J. J. Wu, Y. Z. Zheng, N. Li, X. T. Li and X. Tao, ACS Catal., 2019, 9, 8144 CrossRef CAS.
- T. T. Zhang, J. F. Yu, J. Y. Huang, S. N. Lan, Y. B. Lou and J. X. Chen, Dalton Trans., 2021, 50, 10860 RSC.
- Y. Q. Wu, P. Wang, Z. H. Guan, J. X. Liu, Z. Y. Wang, Z. K. Zheng, S. Y. Jin, Y. Dai, M. H. Whangbo and B. B. Huang, ACS Catal., 2018, 8, 10349 CrossRef CAS.
- Y. Q. Wu, P. Wang, X. L. Zhu, Q. Q. Zhang, Z. Y. Wang, Y. Y. Liu, G. Z. Zou, Y. Dai, M. H. Whangbo and B. B. Huang, Adv. Mater., 2018, 30, 1704342 CrossRef PubMed.
- R. Li, X. T. Li, J. J. Wu, X. D. Lv, Y. Z. Zheng, Z. J. Zhao, X. Q. Ding, X. Tao and J. F. Chen, Appl. Catal., B, 2019, 259, 118075 CrossRef CAS.
- W. B. Li, F. Wang, Z. G. Zhang and S. X. Min, Chem. Commun., 2021, 57, 7774 RSC.
- J. W. Wei, F. R. Huang, S. N. Wang, L. Y. Zhou, Y. L. Xin, P. Jin, Z. Cai, Z. D. Yin, Q. Pang and J. Z. Zhang, Mater. Res. Bull., 2018, 106, 35 CrossRef CAS.
- H. Zarenezhada, T. Balkana, N. Solati, M. Halali, M. Askarie and S. Kaya, Sol. Energy, 2020, 207, 1300 CrossRef.
- H. C. Hsieh, C. Y. Hsiow, Y. A. Suc, Y. C. Liu, W. Chen, W. Y. Chiu, Y. C. Shih, K. F. Lin and L. Wang, J. Power Sources, 2019, 426, 55 CrossRef CAS.
- H. Wang, X. M. Wang, R. T. Chen, H. F. Zhang, X. L. Wang, J. H. Wang, J. Zhang, L. C. Mu, K. F. Wu, F. T. Fan, X. Zong and C. Li, ACS Energy Lett., 2019, 4, 40 CrossRef CAS.
- Z. G. Zhang, G. R. Xie, F. Wang and S. X. Min, Chem. Commun., 2021, 57, 663 RSC.
- J. X. Feng, S. Y. Tong, Y. X. Tong and G. R. Li, J. Am. Chem. Soc., 2018, 140, 5118 CrossRef CAS PubMed.
- T. Glaser, C. Müller, M. Sendner, C. Krekeler, O. E. Semonin, T. D. Hull, O. Yaffe, J. S. Owen, W. Kowalsky, A. Pucci and R. Lovrinčić, J. Phys. Chem. Lett., 2015, 6, 2913 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and addition data. See DOI: 10.1039/d1se01680f |
| This journal is © The Royal Society of Chemistry 2022 |