Polyoxometalate-based yolk@shell dual Z-scheme superstructure tandem heterojunction nanoreactors: encapsulation and confinement effects†
Chunxu
Wu
a,
Zipeng
Xing
 *a,
Bin
Fang
a,
Yongqian
Cui
a,
Zhenzi
Li
*b and
Wei
Zhou
*a,
Bin
Fang
a,
Yongqian
Cui
a,
Zhenzi
Li
*b and
Wei
Zhou
 *ab
*ab
aDepartment of Environmental Science, School of Chemistry and Materials Science, Key Laboratory of Functional Inorganic Material Chemistry, Ministry of Education of the People's Republic of China, Heilongjiang University, Harbin 150080, P. R. China. E-mail: xingzipeng@hlju.edu.cn; zwchem@hotmail.com; Fax: +86-451-8660-8240; Tel: +86-451-8660-8616
bShandong Provincial Key Laboratory of Molecular Engineering, School of Chemistry and Chemical Engineering, Qilu University of Technology, Shandong Academy of Sciences, Jinan 250353, P. R. China. E-mail: zhenzhenlee2014@163.com
First published on 17th November 2021
Abstract
Flower-ball-like ZnIn2S4@hollow dodecahedral polyoxometalate (K3PW12O40)@flower-shell-like ZnIn2S4/Ag2S yolk@shell dual Z-scheme superstructure tandem heterojunction nanoreactors are fabricated through a two-step hydrothermal method combined with a cation exchange strategy. Hollow dodecahedral K3PW12O40 acts as a bridge to form the special yolk@shell dual Z-scheme superstructure tandem heterojunction between two types of ZnIn2S4. Due to the encapsulation and confinement effects of the nanoreactors, the size of flower-ball-like ZnIn2S4 in hollow dodecahedral K3PW12O40 is limited to form a yolk@shell structure, which favors light multi-reflection. The surface plasmon resonance (SPR) effect of Ag2S nanoparticles extends the photoresponse to visible light and near-infrared regions. The resultant tandem heterojunction nanoreactors exhibit excellent photocatalytic degradation of tetracycline hydrochloride (∼99%) and hydrogen evolution (2107.3 μmol h−1 g−1) performance, which are several times higher than those of the pristine one. This is ascribed to the formation of a dual Z-scheme tandem heterojunction favoring spatial charge separation, the SPR of Ag2S and yolk@shell hollow structure benefiting light utilization and mass transfer, and the encapsulation and confinement effects of the special nanoreactors facilitating the complete catalytic reaction and stability. This work provides an efficient strategy for constructing highly efficient tandem heterojunction photocatalysts.
1. Introduction
In the past few decades, industrial production has made huge progress. However, with the rapid development of industry, there will be negative effects, namely environmental pollution and energy shortages.1 The pollution problem of tetracycline in environmental pollution is particularly prominent. The accumulation of tetracycline hydrochloride in the environment promotes the evolution of antibiotic resistance genes, which can lead to adverse ecological effects.2 The development of high-efficiency photocatalysts can use solar energy to degrade tetracycline hydrochloride and generate renewable energy. Among various photocatalytic materials, ZnIn2S4-based materials have unique characteristics due to their better physical and chemical properties in photocatalysis, and have shown wide applications in the degradation of tetracycline hydrochloride and hydrogen production. However, the photocatalytic performance of the traditional ZnIn2S4 material is limited by its low light absorption capacity which can be attributed to its relatively simple morphology. In order to overcome this problem, the layered structure of ZnIn2S4 can be compounded with other materials with a good structure or wide range of light absorption. Polyoxometalates are a series of molecular metal oxide clusters, and they possess a wide range of applications in the field of photocatalysis due to their unique properties in structure and geometry.3 K3PW12O40 is a Keggin-type polyoxometalate, which has attracted researchers' interest due to its suitable structure and geometry.3 Therefore, ZnIn2S4 and K3PW12O40 can be combined to fabricate highly efficient photocatalysts. However, the light absorption range of ZnIn2S4 and K3PW12O40 is relatively low, so it can be considered to combine them with a material with a wide light absorption range to construct a photocatalytic system. Ag2S is a material with a narrow band gap (∼0.87 eV) and excellent photoresponse and has high electron transport capabilities which can also generate plasmon resonance effects to enhance the photocatalytic performance.4,5 Li et al. prepared an Ag/Ag2S/Bi2MOO6 photocatalyst, which possessed excellent photocatalytic degradation performance of levofloxacin.6 Based on the above considerations, it is a feasible method to combine ZnIn2S4, K3PW12O40 and Ag2S to prepare photocatalysts which can effectively degrade tetracycline hydrochloride and produce hydrogen. In general, metal sulfides have shown great research value in aquatic products due to their unique photoelectric characteristics and suitable energy band structure.7–9 However, it is still a challenge to consider how to improve the performance of this photocatalyst in detail. Next, this paper will conceive how to specifically improve the performance of this photocatalyst from the two aspects of building a good structure and well-designed heterojunctions.In the field of photocatalysis, the rational design of the photocatalyst structure and morphology is critical to improve photocatalytic performance. For a long period of time, photocatalysts which have various structures have been prepared, such as the core@shell structure, hollow structure, layered structure and yolk@shell structure. The core@shell structure can effectively restrain the recombination of photoinduced electron–hole pairs and enhance the electron transport ability. Mishra et al. synthesized a SiO2/ZnIn2S4 photocatalyst with enhanced photocatalytic hydrogen production performance, which could be ascribed to the establishment of a core@shell structure which promoted the separation and transport of electron–hole pairs.10 Therefore, to establish a photocatalyst possessing a core–shell structure is a valuable research direction. However, the core in the core–shell structure is generally a solid structure, which cannot provide more active sites to promote surface catalytic activity. The hollow structure not only provides various light scattering and reflection paths to promote light absorption, but also provides a large specific surface area and abundant active sites to promote the adsorption of pollutants and surface catalytic activity compared with the solid structure. At the same time, the hollow shell possesses reduced volume-to-surface diffusion length to accelerate the separation of electrons and holes. Lou et al. prepared a hollow ZnIn2S4–In2O3 nanotube with excellent CO2 reduction performance.11 Therefore, the design of a unique hollow structure can provide a valuable idea for improving the photocatalytic performance. However, researchers have been working hard to construct photocatalysts with new structures based on hollow structures. In recent years, yolk@shell nanoreactors (YSNs) which are formed based on hollow structures have received continuous attention in the fields of metal–chalcogen batteries, sensors, and photocatalysis.12 It is worth noting that when YSNs are used as photocatalysts, they can provide specific functions such as encapsulation and confinement. YSNs can limit the size of the species (metals, metal sulfides, metal oxides, etc.) encapsulated in the hollow shell to prevent the degradation of photocatalytic performance caused by their polymerization and adjust the surface electron distribution of their active species to improve their catalytic activity.13–15 Cui et al. prepared metal nanoparticle@covalent organic framework nanoreactors which exhibited high catalytic activity and stability in reducing 4-nitrophenol sodium borohydride at room temperature, namely the improvement in catalytic performance and stability could be attributed to the contribution of the confinement effect of the nanoreactors.16 In addition, nanoreactors can provide a closed comprehensive catalytic environment, and the yolk and shell can independently catalyze different reactions. Xu et al. prepared a FeMn@hollow nanoreactor whose yolk and shell could independently catalyze different reactions for the direct synthesis of aromatic hydrocarbons from syngas with an aromatic hydrocarbon concentration as high as 1.9 g gFe−1 h−1.13 It is worth mentioning that if the species encapsulated in YSNs is a flower-like structure with a large specific surface area, it will provide more interfaces and incident light interactions to promote the light collection ability.17,18 Zhang et al. prepared a yolk@shell Co/C nanoreactor which exhibits more superior catalytic activity than solid Co/C nanoparticles.14 Li et al. synthesized a nanoreactor of nickel-containing carbon-shells which shows the best catalytic activity than catalysts possessing a solid structure.19 This can be attributed to the enhanced reaction rate caused by encapsulation and confinement effects. Generally speaking, YSNs have the advantages of effective mass transfer, high-efficiency electron transport, confinement effect and independent catalysis of the yolk and shell.20 In general, the structural design of photocatalysts for heterogeneous photocatalysis is significant.
The use of a well-designed type II heterojunction between semiconductors is a feasible way to promote photocatalytic performance.21,22 Shi et al. prepared a 2D/2D HCa2Nb3O10/g-C3N4 type II heterojunction, owing to its large 2D contact surface and matching band forming an effective type II heterojunction.23 Although the type II heterojunction improves the charge separation efficiency, the photoinduced electrons and holes are concentrated on the energy band with a lower redox potential, which weakens the redox capacity. Introducing the Z-scheme heterojunction has become an effective way to improve the photocatalytic performance due to its unique carrier migration path, superior carrier separation efficiency and high redox capacity.24–27 Zhang et al. proposed a strategy to covalently connect a covalent organic framework with a semiconductor, creating a stable organic–inorganic Z-scheme heterojunction for artificial photosynthesis.28 Chao et al. prepared a spontaneous Z-scheme nanosheet photocatalyst which could significantly improve the performance of photocatalytic hydrogen production.29 It can be seen that well-designed Z-scheme heterojunctions between semiconductors will significantly enhance the photocatalytic performance compared with the type II heterojunctions. What's more, unique dual Z-scheme tandem heterojunctions were proposed by researchers. Guo et al. realized the establishment of dual Z-scheme tandem heterojunctions by rationally designing the morphology and energy band structure, thereby preparing a WS2 quantum dots/MoS2@WO3-x core–shell hierarchical dual Z-scheme tandem heterojunction, which has a wide-spectral response and enhanced photocatalytic performance.30 Therefore, designing a distinct dual Z-scheme tandem heterojunction provides a valuable idea for improving the photocatalytic performance. Therefore, it might be necessary to consider the above factors and prepare a novel photocatalyst to explore its advantages in photocatalytic performance.
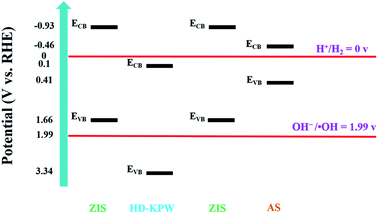
In this paper, novel flower-ball-like ZnIn2S4@hollow dodecahedral polyoxometalate (K3PW12O40)@flower-shell-like ZnIn2S4/Ag2S yolk@shell dual Z-scheme superstructure tandem heterojunction nanoreactors were prepared by a two-step hydrothermal method and a facile room temperature cation exchange strategy to effectively improve the photocatalytic degradation of tetracycline hydrochloride and hydrogen production performance. At the same time, a hierarchical yolk@shell structure was constructed and dual Z-scheme tandem heterojunctions were formed, thereby utilizing geometric and electronic effects together. The flower-ball-like ZnIn2S4 is encapsulated in hollow dodecahedral K3PW12O40, preventing its self-assembly polymerization namely confinement, thus enhancing its photocatalytic performance. The flower-shell-like ZnIn2S4 grown on the hollow dodecahedral K3PW12O40 substrate is beneficial to reduce the charge diffusion distance and further enhances the exposed catalytically active sites. The nanoreactor can provide a closed comprehensive catalytic environment. The hollow dodecahedral K3PW12O40 and ZnIn2S4 on its inner and outer surfaces form dual Z-scheme tandem heterojunctions. The valence band (VB) energy of the hollow dodecahedral K3PW12O40 and the conduction band (CB) energy of ZnIn2S4 on the inner and outer surfaces are 3.34 eV and −0.93 eV, respectively, thereby the oxidation and reducibility are strong. Thus, ˙OH are generated in the VB of hollow dodecahedral K3PW12O40 to effectively degrade tetracycline hydrochloride, and hydrogen is effectively produced in the CB of ZnIn2S4 on the inner and outer surfaces. The experimental results of photocatalytic degradation of tetracycline hydrochloride show that flower-ball-like ZnIn2S4@hollow dodecahedral K3PW12O40@flower-shell-like ZnIn2S4/Ag2S superstructure nanoreactors possess the highest degradation efficiency which is up to 99%. Next, hollow dodecahedral K3PW12O40, flower-ball-like ZnIn2S4@hollow dodecahedral K3PW12O40@flower-shell-like ZnIn2S4 nanoreactors and flower-ball-like ZnIn2S4@hollow dodecahedral K3PW12O40@flower-shell-like ZnIn2S4/Ag2S nanoreactors were researched emphatically by a series of characterization methods. The hydrogen production performance of flower-ball-like ZnIn2S4@hollow dodecahedral K3PW12O40@flower-shell-like ZnIn2S4/Ag2S nanoreactors is also much higher than that of any monomer material. This work provides a new idea for constructing a new type of photocatalyst with highly efficient performance.
2. Experiment section
The synthesis processes of hollow dodecahedral K3PW12O40, solid dodecahedral K3PW12O40, hollow spherical K3PW12O40 and solid spherical K3PW12O40 are shown in Fig. S1.† The synthesis process of yolk@shell flower-ball-like ZnIn2S4@hollow dodecahedral polyoxometalate@flower-shell-like ZnIn2S4/Ag2S nanoreactors is shown in Scheme 1. Details of the materials used throughout the experiment, characterization instruments, photocatalytic degradation activity test, photocatalytic hydrogen evolution and photoelectrochemical measurement section are presented in the ESI.†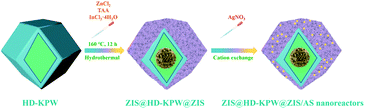 | ||
| Scheme 1 Schematic illustration of the synthesis of flower-ball-like ZnIn2S4@hollow dodecahedral K3PW12O40@flower-shell-like ZnIn2S4/Ag2S superstructure nanoreactors. | ||
2.1. Synthesis of hollow dodecahedral K3PW12O40
Hollow dodecahedral K3PW12O40 was synthesized by the hydrothermal method. KCl (0.3 g) and H3PW12O40·xH2O (0.5 g) were dissolved in a 100 mL beaker containing 50 mL deionized (DI) water. After stirring for 0.5 h, the obtained solution was transferred into a Teflon-lined stainless-steel autoclave and heated at 140 °C for 12 h. The suspension was washed several times with DI water and ethanol and dried at 60 °C for 6 h. The white powder was hollow dodecahedral K3PW12O40, which was named HD-KPW.2.2. Synthesis of flower-ball-like ZnIn2S4@hollow dodecahedral K3PW12O40@flower-shell-like ZnIn2S4 nanoreactors
Flower-ball-like ZnIn2S4@hollow dodecahedral K3PW12O40@flower-shell-like ZnIn2S4 nanoreactors were synthesized by the hydrothermal method. 0.08 g prepared HD-KPW were dispersed in a 100 mL beaker containing 50 mL DI water, then ZnCl2 (0.0136 g), TAA (0.015 g) and InCl3·4H2O (0.03 g) were added into the beaker. After stirring for 0.5 h, the solution was transferred into a Teflon-lined stainless-steel autoclave and heated at 160 °C for 12 h. The suspension was washed several times with DI water and ethanol and dried at 60 °C for 6 h. After adding HD-KPW and the precursor of ZnIn2S4, the hydrothermal temperature of forming ZnIn2S4 was higher than that of forming HD-KPW. This may lead to a general increase in the pore diameter of HD-KPW. Therefore, some of the Zn2+, In3+ and S2− ions enter the interior of hollow dodecahedral K3PW12O40 to self-assemble into flower-ball-like ZnIn2S4, and some of the Zn2+, In3+ and S2− ions self-assemble into flower-shell-like ZnIn2S4 on the outer surface of HD-KPW. The yellow powder was flower-ball-like ZnIn2S4@hollow dodecahedral K3PW12O40@flower-shell-like ZnIn2S4 nanoreactors, which were named ZIS@HD-KPW@ZIS. The separate self-assembled flower-ball-like ZnIn2S4 was synthesized under the same reaction conditions without the addition of HD-KPW as the substrate, and was named ZIS.2.3. Synthesis of flower-ball-like ZnIn2S4@hollow dodecahedral K3PW12O40@flower-shell-like ZnIn2S4/Ag2S superstructure nanoreactors
Flower-ball-like ZnIn2S4@hollow dodecahedral K3PW12O40@flower-shell-like ZnIn2S4/Ag2S superstructure nanoreactors were synthesized by a facile room temperature cation exchange method. Typically, ZIS@HD-KPW@ZIS (0.05 g) was dispersed into 15 mL of EtOH and ultrasonicated for 10 min, and then, a certain amount of AgNO3 EtOH solution (1 mM) was quickly added. A clear color change (from yellow to brown) was observed immediately after mixing these two solutions, suggesting the formation of Ag2S. After stirring for 60 min, the products were washed three times with deionized water and ethanol by centrifugation and then they were dried at 60 °C for 6 h. The brown powder was flower-ball-like ZnIn2S4@hollow dodecahedral K3PW12O40@flower-shell-like ZnIn2S4/Ag2S nanoreactors, which were named ZIS@HD-KPW@ZIS/AS.2.4. Synthesis of solid dodecahedral K3PW12O40
The difference from the synthesis process of hollow dodecahedral K3PW12O40 was that in the synthesis process of solid dodecahedral K3PW12O40, the amount of KCl and H3PW12O40·xH2O added as well as the temperature required for the hydrothermal method were different. The amount of KCl and H3PW12O40·xH2O was 0.15 and 0.3 g, respectively. The hydrothermal temperature was 120 °C. The rest of the synthesis steps were the same. The white powder was solid dodecahedral K3PW12O40, which was named SD-KPW.2.5. Synthesis of solid dodecahedral K3PW12O40@flower-shell-like ZnIn2S4
The difference from the synthesis process of flower-ball-like ZnIn2S4@hollow dodecahedral K3PW12O40@flower-shell-like ZnIn2S4 nanoreactors was that in the synthesis process of solid dodecahedral K3PW12O40@flower-shell-like ZnIn2S4, 0.08 g of prepared SD-KPW rather than HD-KPW was added. The rest of the synthesis steps were the same. The yellow powder was solid dodecahedral K3PW12O40@flower-shell-like ZnIn2S4, which was named SD-KPW@ZIS.2.6. Synthesis of solid dodecahedral K3PW12O40@flower-shell-like ZnIn2S4/Ag2S
The difference from the synthesis process of flower-ball-like ZnIn2S4@hollow dodecahedral K3PW12O40@flower-shell-like ZnIn2S4/Ag2S nanoreactors was that in the synthesis process of solid dodecahedral K3PW12O40@flower-shell-like ZnIn2S4/Ag2S, 0.05 g of prepared SD-KPW@ZIS rather than ZIS@HD-KPW@ZIS was added. The rest of the synthesis steps were the same. The brown powder was solid dodecahedral K3PW12O40@flower-shell-like ZnIn2S4/Ag2S, which was named SD-KPW@ZIS/AS.2.7. Synthesis of hollow spherical K3PW12O40
The difference from the synthesis process of hollow dodecahedral K3PW12O40 was that in the synthesis process of hollow spherical K3PW12O40, the amount of KCl and H3PW12O40·xH2O added as well as the temperature and time required for the hydrothermal method were different. The amount of KCl and H3PW12O40·xH2O was 0.15 and 0.3 g, respectively. The temperature and time required for the hydrothermal method were 100 °C and 6 h. The rest of the synthesis steps were the same. The white powder was hollow spherical K3PW12O40, which was named HS-KPW.2.8. Synthesis of flower-ball-like ZnIn2S4@hollow spherical K3PW12O40@flower-shell-like ZnIn2S4 nanoreactors
The difference from the synthesis process of flower-ball-like ZnIn2S4@hollow dodecahedral K3PW12O40@flower-shell-like ZnIn2S4 nanoreactors was that in the synthesis process of flower-ball-like ZnIn2S4@hollow spherical K3PW12O40@flower-shell-like ZnIn2S4 nanoreactors, 0.08 g of prepared HS-KPW rather than HD-KPW was added. The rest of the synthesis steps were the same. The yellow powder was flower-ball-like ZnIn2S4@hollow spherical K3PW12O40@flower-shell-like ZnIn2S4 nanoreactors, which were named ZIS@HS-KPW@ZIS.2.9. Synthesis of flower-ball-like ZnIn2S4@hollow spherical K3PW12O40@flower-shell-like ZnIn2S4/Ag2S nanoreactors
The difference from the synthesis process of flower-ball-like ZnIn2S4@hollow dodecahedral K3PW12O40@flower-shell-like ZnIn2S4/Ag2S nanoreactors was that in the synthesis process of flower-ball-like ZnIn2S4@hollow spherical K3PW12O40@flower-shell-like ZnIn2S4/Ag2S nanoreactors, 0.05 g of prepared ZIS@HS-KPW@ZIS rather than ZIS@HD-KPW@ZIS was added. The rest of the synthesis steps were the same. The brown powder was flower-ball-like ZnIn2S4@hollow spherical K3PW12O40@flower-shell-like ZnIn2S4/Ag2S nanoreactors, which were named ZIS@HS-KPW@ZIS/AS.2.10. Synthesis of solid spherical K3PW12O40
The difference from the synthesis process of hollow dodecahedral K3PW12O40 was that in the synthesis process of solid spherical K3PW12O40, the amount of KCl and H3PW12O40·xH2O added as well as the temperature and time required for the hydrothermal method were different. The amount of KCl and H3PW12O40·xH2O was 0.15 and 0.3 g, respectively. The temperature and time required for the hydrothermal method was 100 °C and 1 h. The rest of the synthesis steps were the same. The white powder was solid spherical K3PW12O40, which was named SS-KPW.2.11. Synthesis of solid spherical K3PW12O40@flower-shell-like ZnIn2S4
The difference from the synthesis process of flower-ball-like ZnIn2S4@hollow dodecahedral K3PW12O40@flower-shell-like ZnIn2S4 nanoreactors was that in the synthesis process of solid spherical K3PW12O40@flower-shell-like ZnIn2S4, 0.08 g of prepared SS-KPW rather than HD-KPW was added. The rest of the synthesis steps were the same. The yellow powder was solid spherical K3PW12O40@flower-shell-like ZnIn2S4, which was named SS-KPW@ZIS.2.12. Synthesis of solid spherical K3PW12O40@flower-shell-like ZnIn2S4/Ag2S
The difference from the synthesis process of flower-ball-like ZnIn2S4@hollow dodecahedral K3PW12O40@flower-shell-like ZnIn2S4/Ag2S nanoreactors was that in the synthesis process of solid spherical K3PW12O40@flower-shell-like ZnIn2S4/Ag2S, 0.05 g of prepared SS-KPW@ZIS rather than ZIS@HD-KPW@ZIS was added. The rest of the synthesis steps were the same. The brown powder was solid spherical K3PW12O40@flower-shell-like ZnIn2S4/Ag2S, which was named SS-KPW@ZIS/AS.2.13. Synthesis of Ag2S
Na2S·9H2O (0.0156 g) and AgNO3 (0.034 g) were dissolved in a 100 mL beaker containing 50 mL DI water. After stirring for 30 min, the precipitate was filtered and collected, and then dried at 60 °C overnight. The black powder was Ag2S, which was named AS.3. Results and discussion
3.1. Characterization and pollutant degradation analysis
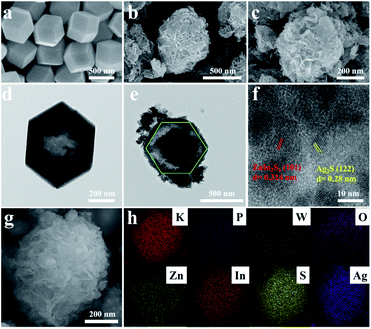
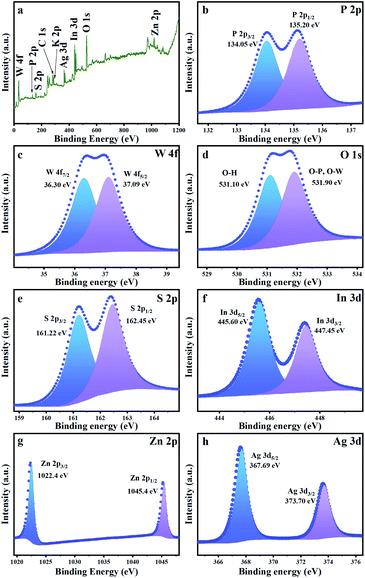
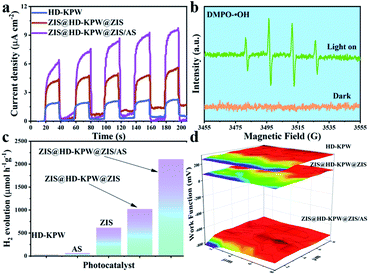
The transmission electron microscopy (TEM) images of a clear HD-KPW shell, flower-ball-like ZIS inside the HD-KPW shell and flower-shell-like ZIS on the outer surface of the HD-KPW shell are shown in Fig. 1e. In addition, due to the small size of AS quantum dots, they cannot be seen under this microscope magnification. In general, the yolk@shell structure of ZIS@HD-KPW@ZIS/AS which possesses an excellent structural rigidity is maintained perfectly and lots of pores are generated in the flower-ball-like cores, which is good for mass transfer and exposing more surface active sites.11 The high-resolution TEM image of two sets of characteristic interplanar spacing is shown in Fig. 1f. For ZIS and AS, the characteristic interplanar spacing of the (101) and (122) planes is calculated as approximately 0.324 and 0.28 nm, respectively.6,33 This shows the successful preparation of ZIS@HD-KPW@ZIS/AS, and the flower-ball-like ZnIn2S4 is encapsulated inside the HD-KPW shell and flower-shell-like ZIS successfully grows outside the HD-KPW shell. In the procedure of adding HD-KPW and the precursor of ZIS to form a composite, the hydrothermal temperature of forming ZIS was higher than that of generating HD-KPW, thereby possibly resulting in the pore size of HD-KPW to universally increase. Furthermore, some of the Zn2+, In3+ and S2− ions enter the interior of hollow dodecahedral K3PW12O40 to self-assemble into flower-ball-like ZnIn2S4, and some of the Zn2+, In3+ and S2− ions self-assemble into flower-shell-like ZnIn2S4 on the outer surface of HD-KPW. The SEM image in Fig. S2† shows that the individually grown ZnIn2S4 possess a good flower-like structure and a size of approximately 9 μm. In Fig. 1e, it is worth mentioning that the size of the flower-ball-like ZnIn2S4 which is encapsulated in HD-KPW is about 400 nm, which proves that the infinite growth of flower-ball-like ZnIn2S4 is confined by HD-KPW. In other words, flower-ball-like ZnIn2S4 is confined to the limited space of HD-KPW, thereby providing a limited catalytic environment, namely the confinement effect of ZIS@HD-KPW@ZIS/AS nanoreactors. The successful combination of HD-KPW, ZIS and AS and the formation of ZIS@HD-KPW@ZIS/AS are demonstrated by all the above mentioned results. In Fig. 1g and h, the coexistence of eight elements K, P, W, O, Zn, In, S and Ag in the entire nanocomposite is demonstrated using the SEM image of ZIS@HD-KPW@ZIS/AS and corresponding EDX maps. The formation of the nanocomposite is further proven using the EDX elemental spectrum of ZIS@HD-KPW@ZIS/AS shown in Fig. S3.†
The shape evolution of K3PW12O40 can be achieved by controlling the addition amount of KCl and H3PW12O40·xH2O and the temperature and time of the hydrothermal method. In the process of SD-KPW, 0.15 g of KCl and 0.3 g of H3PW12O40·xH2O were heated at 120 °C for 12 h under hydrothermal conditions. Fig. S4a–c† show the SEM images of SD-KPW, SD-KPW@ZIS, and SD-KPW@ZIS/AS photocatalysts, respectively. In the process of HS-KPW, 0.15 g of KCl and 0.3 g of H3PW12O40·xH2O were heated at 100 °C for 6 h under hydrothermal conditions. Fig. S5a and b† show the SEM images of HS-KPW and ZIS@HS-KPW@ZIS/AS photocatalysts, respectively. In the process of SS-KPW, 0.15 g of KCl and 0.3 g of H3PW12O40·xH2O were heated at 100 °C for 1 h under hydrothermal conditions. Fig. S6a and b† show the SEM images of SD-KPW and SD-KPW@ZIS/AS photocatalysts, respectively. The analysis results in Fig. S4, S5 and S6† indicate the successful combination of K3PW12O40, ZIS and AS and the formation of SD-KPW, SD-KPW@ZIS, SD-KPW@ZIS/AS, HS-KPW, ZIS@HS-KPW@ZIS, ZIS@HS-KPW@ZIS/AS, SD-KPW, SD-KPW@ZIS and SD-KPW@ZIS/AS. The possible driving forces for the evolution from spheres to dodecahedra and for the evolution from solid to hollow are extremely important to further understand this work. Through plenty of experiments, we found that the addition amount of KCl and H3PW12O40·xH2O and the temperature and time of the hydrothermal method taken had important roles in the growth of K3PW12O40. It is hard to explain the shape evolution mechanism of K3PW12O40 by a good deal of repeated experiments and research.34 However, previous work has provided some ideas for this (1) the formation of different shaped nanoparticles is related to their different growth rates, (2) the morphology of the particle changes from a sphere to a dodecahedron, and the particle size is maintained, and this is because the epitaxial interface is formed by the slight dissolution and re-precipitation of the nanocrystals.35,36 Hence, it is possible to change the experimental conditions (such as the amount of chemicals added, the temperature and time required by the hydrothermal method) to facilitate the growth rate of nanoparticles under different conditions and to form epitaxy through slight dissolution and re-precipitation of nanocrystals, thereby completing the transformation of K3PW12O40 particles from spheres into dodecahedra through the synergy of the above two reasons, and maintaining their particle sizes. As for the transformation mechanism of the shape of K3PW12O40 from a solid ball to a hollow ball, it can be considered the same as the possible transformation mechanism from a solid dodecahedron to a hollow dodecahedron.
In Fig. S7,† the phase analysis of HD-KPW, SD-KPW, HS-KPW and SS-KPW is compared and it can be seen that there is basically no change in the position of the diffraction peaks. Fig. S8† shows the comparative analysis of the phase analysis of ZIS@HD-KPW@ZIS, SD-KPW@ZIS, SD-KPW@ZIS, ZIS@HS-KPW@ZIS, and SS-KPW@ZIS and it can also be seen that there is basically no change in the position of the diffraction peaks. In Fig. S9,† the phase analysis of ZIS@HD-KPW@ZIS/AS, SD-KPW@ZIS/AS, ZIS@HS-KPW@ZIS/AS and SS-KPW@ZIS/AS is compared and it can also be seen that there is basically no change in the position of the diffraction peaks. Based on the analysis results in Fig. S7, S8 and S9,† the successful formation of HD-KPW, SD-KPW, HS-KPW, SS-KPW, ZIS@HD-KPW@ZIS, SD-KPW@ZIS, SD-KPW@ZIS, ZIS@HS-KPW@ZIS, SS-KPW@ZIS, ZIS@HD-KPW@ZIS/AS, SD-KPW@ZIS/AS, ZIS@HS-KPW@ZIS/AS and SS-KPW@ZIS/AS is proved. In addition, the XRD patterns of the twelve prepared samples are summarized in Fig. S10.†
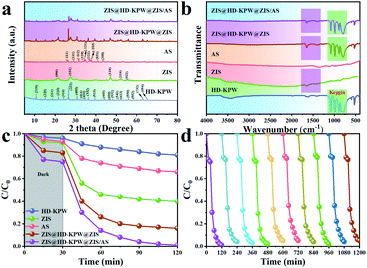
As shown in Fig. 2b, the chemical functional groups and structures of ZIS@HD-KPW@ZIS/AS were identified by Fourier transformed infrared (FT-IR) spectroscopy. For HD-KPW and ZIS@HD-KPW@ZIS/AS, peaks at 811, 890, 989 and 1080 cm−1 were the Keggin structure characteristic bands of POMs.39 Two peaks at 1610 and 1396 cm−1 corresponding to the surface absorbed water molecules and hydroxyl groups were observed over ZIS, ZIS@HD-KPW@ZIS and ZIS@HD-KPW@ZIS/AS.40 For AS, a peak at 975 cm−1 was the characteristic peak of Ag2S, but it was not obvious in ZIS@HD-KPW@ZIS/AS composites due to the low content of AS.41 This reveals that the original chemical functional groups and structures of the single ingredients are reserved after the formation of the heterojunction nanoreactors. Moreover, Fig. S11, S12 and S13† show the FT-IR patterns of HD-KPW, SD-KPW, HS-KPW and SS-KPW, that of ZIS@HD-KPW@ZIS, SD-KPW@ZIS, ZIS@HS-KPW@ZIS and SS-KPW@ZIS and that of ZIS@HD-KPW@ZIS/AS, SD-KPW@ZIS/AS, ZIS@HS-KPW@ZIS/AS and SS-KPW@ZIS/AS, respectively. And it can be seen that the characteristic peaks of the four morphologies of K3PW12O40 and the corresponding binary complexes and ternary complexes are almost the same.
Moreover, the photocatalytic degradation rates of ZIS@HD-KPW@ZIS and ZIS@HD-KPW@ZIS/AS composite samples and HD-KPW, ZIS and AS single samples are compared in Fig. 2c. Next, the photocatalytic degradation curves of the five samples with the apparent reaction rate constant k for the degradation of tetracycline hydrochloride are shown in Fig. S16.† Furthermore, the recycling test runs showed the obvious stability of the ZIS@HD-KPW@ZIS/AS nanoreactors, for which no significant decrease of the degradation rate was showed after five recycles (Fig. 2d). In Fig. S17,† the XRD diffraction peak of the ZIS@HD-KPW@ZIS/AS photocatalyst is basically unchanged after ten cycles, further indicating the high stability. In addition, compared with the selected photocatalysts in previous literature studies, ZIS@HD-KPW@ZIS/AS shows superior photocatalytic degradation performance of tetracycline hydrochloride (Table S1†).
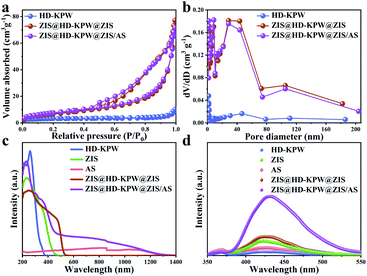
The optical properties of HD-KPW, ZIS, AS, ZIS@HD-KPW@ZIS and ZIS@HD-KPW@ZIS/AS photocatalysts (Fig. 4c) were detected using the UV-Vis-NIR diffuse reflectance spectra. HD-KPW and ZIS show strong absorption in the visible light region ending at ∼380 and ∼480 nm, respectively, while AS still exhibits extensive absorption in the near-infrared light range. After HD-KPW is combined with ZIS and AS, the light absorption edge of ZIS@HD-KPW@ZIS/AS shows a significant red shift compared with that of HD-KPW and ZIS. This is due to the introduction of AS having a near-infrared light response leading to a red shift. The actual photos of HD-KPW, ZIS@HD-KPW@ZIS and ZIS@HD-KPW@ZIS/AS are shown in Fig. S19 and S20.† When combined with yellow ZIS, the optical absorption intensity of HD-KPW decreases, and optical absorption intensity of ZIS@HD-KPW@ZIS increases when combined with black AS because the dark substance often possesses preferable light collection performance.52 The ZIS@HD-KPW@ZIS/AS photocatalyst shows apparent absorption peaks in the NIR range, which can be attributed to AS nanocrystals often showing an apparent peak in the NIR region. Furthermore, the ZIS@HD-KPW@ZIS/AS photocatalyst is responsive to light of various wavelengths from UV and visible light regions to the NIR region. This phenomenon may be due to the synergy effect of the optical cavity of the yolk@shell structure and the plasma nanocrystals.53 Moreover, the Tauc plots of (αhv)1/2vs. hv were plotted for the estimation of indirect transition band gaps (Fig. S21†). As shown in Fig. S21,† the band gap (Eg) energies of HD-KPW, ZIS and AS are 3.24, 2.59 and 0.87 eV, respectively.
The fluorescence intensities of the HD-KPW, ZIS, AS, ZIS@HD-KPW@ZIS and ZIS@HD-KPW@ZIS/AS photocatalysts are shown in Fig. 4d. It is worth noting that the fluorescence signal of ZIS@HD-KPW@ZIS/AS nanoreactors is markedly higher than that of others, namely a greater amount of ˙OH is generated. This reveals that the formation of ZIS@HD-KPW@ZIS/AS nanoreactors is advantageous to promote charge and photocatalytic activity.
3.2. Photocatalytic performance
The transient photocurrent responses of HD-KPW, ZIS@HD-KPW@ZIS and ZIS@HD-KPW@ZIS/AS are shown in Fig. 5a. It is obvious that ZIS@HD-KPW@ZIS/AS possesses the highest photocurrent response among all samples, and thereby this nanoreactor photocatalyst has the maximal photoinduced electron transfer efficiency. Based on the synergistic mechanism of the electronic effect which is caused by dual Z-scheme tandem heterojunctions and geometric effect which is caused by the yolk@shell structure of ZIS@HD-KPW@ZIS/AS, the electron interface transfer was obviously promoted. The above results show that the introduction of ZIS and AS promotes charge separation and could further improve the photocatalytic performance of the HD-KPW photocatalyst.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 which could be exactly observed, illustrating the generation of ˙OH in the photocatalytic process.
1 which could be exactly observed, illustrating the generation of ˙OH in the photocatalytic process.
Fig. S27a–c† shows the Mott–Schottky curves of HD-KPW, ZIS and AS. The estimated CB of HD-KPW, ZIS and AS is approximately 0.1, −0.93 and −0.46 eV vs. RHE, respectively. In addition, the CB potentials and VB potentials which are calculated via the formula Eg = EVB − ECB, and band gap energies which are calculated via the Tauc plots of HD-KPW, ZIS and AS are shown in Table S4.† The VB potentials calculated via XPS are consistent with the above results (Fig. S28†). The schematic diagram of the energy band structures is shown in Fig. 6.
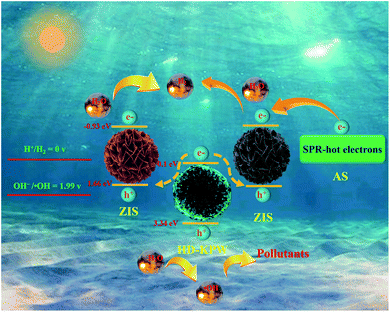
The possible photocatalytic charge transfer mechanism is shown in Fig. 7. It can be inferred that in the ZIS@HD-KPW@ZIS/AS nanoreactor photocatalyst, the photogenerated charge transfer path of the dual Z-scheme tandem mechanism would be followed. AS can give rise to the surface plasma resonance effect, which leads to the “hot electrons” from AS being immediately injected to the CB of flower-shell-like ZIS, thereby increasing the number of electrons. Under visible light irradiation, flower-ball-like ZIS, HD-KPW and flower-shell-like ZIS can produce photogenerated electron–hole pairs, which can be ascribed to account for their appropriate band gaps. Then the photoexcited electrons would migrate from the CB of HD-KPW to the VB of flower-ball-like and flower-shell-like ZIS, respectively. Furthermore, the electrons would recombine with the holes in the VB of flower-ball-like and flower-shell-like ZIS, respectively. Ultimately, photoinduced electrons accumulate in the CB of flower-ball-like and flower-shell-like ZIS, respectively. The photoinduced holes would aggregate in the VB of HD-KPW at the same time, namely the holes remaining in the VB of HD-KPW have enough energy to oxidize H2O, thereby forming ˙OH which can degrade tetracycline hydrochloride pollutants. In the meantime, the electrons remaining in the CB of flower-ball-like and flower-shell-like ZIS have enough energy to encounter H+ and synergistically release H2. HD-KPW acts as a bridge to form the special yolk@shell dual Z-scheme superstructure tandem heterojunction nanoreactors between two types of ZIS. The integrated tandem system greatly promotes the charge separation and effectively inhibits the electron–hole recombination. Moreover, the ZIS@HD-KPW@ZIS/AS tandem heterojunctions can effectively collect sunlight from UV-vis to infrared light. In general, the photogenerated charge transfer path of the well-designed ZIS@HD-KPW@ZIS/AS nanoreactor photocatalyst follows a dual Z-scheme tandem mechanism. This nanoreactor photocatalyst can not only facilitate the spatial charge separation and transfer of photoinduced charges, but also reserves the strong redox capacity in degrading tetracycline hydrochloride and releasing H2 efficiently. The photocatalytic process can be described using the eqn (1)–(4).
| Photocatalyst + hv → photocatalyst (e− + h+) | (1) |
| 2e− + 2H+ → H2 | (2) |
| h+ + OH− → ˙OH | (3) |
| h+/˙OH + tetracycline hydrochloride → H2O + CO2 | (4) |
In addition, this work also explores other possible tandem heterojunction mechanisms. The general idea is to prove the errors of these hypotheses by using mathematical contradiction, thereby supporting dual Z-scheme tandem mechanisms. As shown in Fig. S29a,† assuming that in ZIS@HD-KPW@ZIS/AS, the transfer path of photogenerated carriers follows the Z-scheme and type-II tandem mechanisms. In this assumption, the photogenerated charge transfer path between flower-ball-like ZIS and HD-KPW still follow the Z-scheme mechanism. The difference from the dual Z-scheme tandem mechanism is that the photoexcited electrons would migrate from the CB of flower-shell-like ZIS to that of HD-KPW and the holes would migrate from the VB of HD-KPW to that of flower-shell-like ZIS. Ultimately, photogenerated electrons aggregate in the CB of flower-ball-like ZIS. The photoinduced holes would accumulate in the VB of flower-shell-like ZIS at the same time. The holes remaining in the VB of flower-shell-like ZIS cannot oxidize H2O to form ˙OH because the VB potential (1.66 eV vs. RHE) of flower-shell-like ZIS is lower than the standard redox potential of OH−/˙OH (1.99 eV vs. RHE). However, the ˙OH has been detected by fluorescence and free radical quenching experiments, namely the transfer path of photogenerated carriers could not be allowed to follow the Z-scheme and type-II tandem mechanisms in the ZIS@HD-KPW@ZIS/AS nanoreactor photocatalyst.
As shown in Fig. S29b,† assuming that in ZIS@HD-KPW@ZIS/AS, the transfer path of photogenerated carriers follows the dual type-II tandem mechanism. Then the photoexcited electrons would migrate from the CB of flower-ball-like and flower-shell-like ZIS to that of HD-KPW, respectively. Then the holes would migrate from the VB of HD-KPW to that of flower-ball-like and flower-shell-like ZIS, respectively. Eventually, photogenerated electrons aggregate in the CB of HD-KPW. The photoinduced holes would accumulate in the VB of flower-ball-like and flower-shell-like ZIS at the same time, respectively. Similarly, the holes remaining in the VB of flower-shell-like ZIS cannot oxidize H2O to form ˙OH because the VB potential (1.66 eV vs. RHE) of flower-shell-like ZIS is lower than the standard redox potential of OH−/˙OH (1.99 eV vs. RHE). In addition, the electrons remaining in the CB of HD-KPW cannot release H2 because the CB potential which is 0.1 eV vs. RHE of HD-KPW is more positive than the standard redox potential of H+/H2 (0 eV vs. RHE). However, H2 has been detected by the photocatalytic H2 production experiment, namely the transfer path of photogenerated carriers could not be allowed to follow the dual type-II tandem mechanism in the ZIS@HD-KPW@ZIS/AS nanoreactor photocatalyst. In summary, the dual Z-scheme tandem heterojunction mechanism is the most reasonable one in the ZIS@HD-KPW@ZIS/AS nanoreactor photocatalyst.
4. Conclusions
In summary, the ZIS@HD-KPW@ZIS/AS superstructure nanoreactor photocatalyst showed excellent photocatalytic performance in degradation of tetracycline hydrochloride and hydrogen production. This superior photocatalytic performance might be attributed to the following reasons: (1) the hollow shell of the yolk@shell system provided various light scattering and reflection paths to promote light absorption; at the same time, the hollow shell possessed a reduced volume-to-surface diffusion length to accelerate the separation of electrons and holes. In addition, the confinement and encapsulation effects of the nanoreactors significantly promoted photocatalytic performance; (2) the establishment of dual Z-scheme tandem heterojunctions effectively improved the recombination of photogenerated carriers and prolonged the lifetime of charge carriers; (3) the introduction of AS expanded the light response range of the nanoreactors to the NIR region, making the light utilization efficiency high, and the SPR effect caused by AS could also enhance the photocatalytic performance; (4) compared with core@shell and hollow structures, the yolk@shell structure with a larger surface area contained adequate surface active sites to adsorb reactants, favoring the subsequent photocatalytic reaction; (5) based on the mesoporous structure of yolk@shell nanoreactors, it possesses efficient mass transfer.Author contributions
Chunxu Wu and Zipeng Xing: conceptualization, methodology. Chunxu Wu: data curation, writing – original draft preparation. Chunxu Wu, Bin Fang, and Yongqian Cui: visualization, investigation. Zipeng Xing, Zhenzi Li, and Wei Zhou: supervision. Chunxu Wu: software, validation. Chunxu Wu, Zipeng Xing, and Wei Zhou: writing – reviewing and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the support of this research by the National Natural Science Foundation of China (21871078 and 52172206), the Natural Science Foundation of Heilongjiang Province (JQ2019B001), the Natural Science Foundation of Shandong Province (ZR202103020770), the Heilongjiang Postdoctoral Startup Fund (LBH-Q14135), the Heilongjiang University Science Fund for Distinguished Young Scholars (JCL201802), and the Heilongjiang Provincial Institutions of Higher Learning Basic Research Funds Basic Research Projects (KJCX201909).Notes and references
- A. M. P. T. Pereira, L. J. G. Silva, C. S. M. Laranjeiro, L. M. Meisel, C. M. Lino and A. Pena, Sci. Total Environ., 2017, 609, 1182 CrossRef CAS PubMed.
- J. L. Guo, M. H. Huang, P. Gao, Y. Y. Zhang, H. S. Chen, S. Y. Zheng, T. W. Mu and X. B. Luo, Chem. Eng. J., 2020, 398, 125604 CrossRef CAS.
- L. Yang, J. Lei, J. M. Fan, R. M. Yuan, M. S. Zheng, J. J. Chen and Q. F. Dong, Adv. Mater., 2021, 33, 2005019 CrossRef.
- A. H. C. Khavar, G. Moussavi, A. R. Mahjoub, R. Luque, D. R. Padrón and M. Sattari, Sep. Purif. Technol., 2019, 229, 115803 CrossRef.
- W. L. Yu, J. Yin, Y. Li, B. Lai, T. Jiang, Y. Y. Li, H. W. Liu, J. L. Liu, C. Zhao, S. C. Singh, J. S. Chen, B. Lin, H. Idriss and C. L. Guo, ACS Appl. Energy Mater., 2019, 2, 2751 CrossRef CAS.
- S. J. Li, C. C. Wang, Y. P. Liu, B. Xue, W. Jiang, Y. Liu, L. Y. Mo and X. B. Chen, Chem. Eng. J., 2021, 415, 128991 CrossRef CAS.
- B. F. Li, W. J. Wang, J. W. Zhao, Z. Y. Wang, B. Su, Y. D. Hou, Z. X. Ding, W. J. Ong and S. B. Wang, J. Mater. Chem. A, 2021, 9, 10270–10276 RSC.
- Z. Y. Wang, B. Su, J. L. Xu, Y. D. Hou and Z. X. Ding, Int. J. Hydrogen Energy, 2020, 45, 4113–4121 CrossRef CAS.
- S. B. Wang, Y. Wang, S. L. Zhang, S. Q. Zang and X. W. Lou, Adv. Mater., 2019, 31, 1903404 CrossRef CAS.
- M. Mishra, Y. C. Huang, P. H. Wang, S. P. Liu, T. R. Lee and T. C. Lee, ACS Appl. Mater. Interfaces, 2021, 13, 4043 CrossRef CAS.
- S. B. Wang, B. Y. Guan and X. W. D. Lou, J. Am. Chem. Soc., 2018, 140, 5037 CrossRef CAS.
- C. J. Shi, S. Ye, X. W. Wang, F. N. Meng, J. X. Liu, T. Yang, W. Zhang, J. T. Wei, N. Ta, G. Q. Lu, M. Hu and J. Liu, Adv. Sci., 2021, 8, 2001987 CrossRef CAS.
- Y. F. Xu, G. Y. Ma, J. Y. Bai, Y. X. Du, C. Qin and M. Y. Ding, ACS Catal., 2021, 11, 4476 CrossRef CAS.
- M. Zhang, C. M. Xiao, X. Yan, S. S. Chen, C. H. Wang, R. Luo, J. W. Qi, X. Y. Sun, L. J. Wang and J. S. Li, Environ. Sci. Technol., 2020, 54, 10289 CrossRef CAS PubMed.
- R. P. Ye, X. Y. Wang, C. A. H. Price, X. Y. Liu, Q. H. Yang, M. Jaroniec and J. Liu, Small, 2021, 17, 1906250 CrossRef CAS PubMed.
- K. X. Cui, W. F. Zhong, L. Y. Li, Z. Y. Zhuang, L. Y. Li, J. H. Bi and Y. Yu, Small, 2019, 15, 1804419 Search PubMed.
- E. Boccalon, G. Gorrasi and M. Nocchetti, Adv. Colloid Interface Sci., 2020, 285, 102284 CrossRef CAS.
- S. B. Wang, B. Y. Guan, X. Wang and X. W. D. Lou, J. Am. Chem. Soc., 2018, 140, 15145 CrossRef CAS.
- B. Li, H. G. Nam, J. Zhao, J. Chang, N. Lingappan, F. Yao, T. H. Lee and Y. H. Lee, Adv. Mater., 2017, 29, 1605083 CrossRef.
- Y. Boyjoo, H. Shi, Q. Tian, S. M. Liu, J. Liang, Z. S. Wu, M. Jaroniec and J. Liu, Energy Environ. Sci., 2021, 14, 540 RSC.
- B. Y. Lin, S. S. Li, Y. N. Peng, Z. H. Chen and X. Wang, J. Hazard. Mater., 2021, 406, 124675 CrossRef CAS PubMed.
- X. F. Zhou, Y. X. Fang, X. Cai, S. S. Zhang, S. Y. Yang, H. Q. Wang, X. H. Zhong and Y. P. Fang, ACS Appl. Mater. Interfaces, 2020, 12, 20579 CrossRef CAS.
- J. W. Shi, L. H. Mao, C. Z. Cai, G. S. Li, C. Cheng, B. T. Zheng, Y. C. Hu, Z. S. Huang and X. W. Hu, Catal. Sci. Technol., 2020, 10, 5896 RSC.
- H. J. Yun, H. Lee, N. D. Kim, D. M. Lee, S. Yu and J. Yi, ACS Nano, 2011, 5, 4084 CrossRef CAS PubMed.
- L. F. Pan, S. H. Cao, R. Liu, H. Chen, F. Jiang and X. Wang, J. Colloid Interface Sci., 2019, 548, 284 CrossRef CAS.
- K. Maeda, ACS Catal., 2013, 3, 1486 CrossRef CAS.
- W. W. Xu, W. Tian, L. X. Meng, F. R. Cao and L. Li, Adv. Energy Mater., 2021, 11, 2003500 CrossRef CAS.
- M. Zhang, M. Lu, Z. L. Lang, J. Liu, M. Liu, J. N. Chang, L. Y. Li, L. J. Shang, M. Wang, S. L. Li and Y. Q. Lan, Angew. Chem., Int. Ed., 2020, 59, 6500 CrossRef CAS PubMed.
- Y. G. Chao, P. Zhou, N. Li, J. P. Lai, Y. Yang, Y. L. Zhang, Y. H. Tang, W. X. Yang, Y. P. Du, D. Su, Y. S. Tan and S. J. Guo, Adv. Mater., 2019, 31, 1807226 CrossRef PubMed.
- M. J. Guo, Z. P. Xing, T. Y. Zhao, Z. Z. Li, S. L. Yang and W. Zhou, Appl. Catal., B, 2019, 257, 117913 CrossRef CAS.
- J. Feng and Y. D. Yin, Adv. Mater., 2019, 31, 1802349 CrossRef.
- W. S. Wang, M. Dahl and Y. D. Yin, Chem. Mater., 2013, 25, 1179 CrossRef CAS.
- S. Y. Zhang, M. Du, Z. P. Xing, Z. Z. Li, K. Pan and W. Zhou, Appl. Catal., B, 2020, 262, 118202 CrossRef CAS.
- J. Z. He, H. Pang, W. Q. Wang, Y. Zhang, B. Yan, X. R. Li, S. J. Li and J. Chen, Dalton Trans., 2013, 42, 15637 RSC.
- M. H. Huang and C. Y. Chiu, J. Mater. Chem. A, 2013, 1, 8081 RSC.
- K. Okamoto, S. Uchida, T. Ito and N. Mizuno, J. Am. Chem. Soc., 2007, 129, 7378 CrossRef CAS PubMed.
- X. R. Li, H. G. Xue and H. Pang, Nanoscale, 2017, 9, 216 RSC.
- L. Ye and Z. H. Li, Appl. Catal., B, 2014, 160–161, 552 CrossRef CAS.
- Z. D. Yu, D. H. Wang, S. H. Xun, M. Q. He, R. Ma, W. Jiang, H. P. Li, W. S. Zhu and H. M. Li, Pet. Sci., 2018, 15, 870 CrossRef CAS.
- H. F. Li, H. T. Yu, S. Chen, H. M. Zhao, Y. B. Zhang and X. Quan, Dalton Trans., 2014, 43, 2888 RSC.
- C. L. Yu, L. F. Wei, W. Q. Zhou, D. D. Dionysiou, L. H. Zhu, Q. Shu and H. Liu, Chemosphere, 2016, 157, 250 CrossRef CAS PubMed.
- T. Zeng, X. L. Zhang, S. H. Wang, H. Y. Niu and Y. Q. Cai, Environ. Sci. Technol., 2015, 49, 2350 CrossRef CAS PubMed.
- A. Zeb, S. Sahar, U. Y. Qazi, A. H. Odda, N. Ullah, Y. N. Liu, I. A. Qazi and A. W. Xu, Dalton Trans., 2018, 47, 7344 RSC.
- S. D. Luo, S. Y. Dong, C. Lu, C. F. Yu, Y. W. Ou, L. Luo, J. Y. Sun and J. H. Sun, J. Colloid Interface Sci., 2018, 513, 389 CrossRef CAS PubMed.
- P. X. Jin, L. Wang, X. L. Ma, R. Lian, J. W. Huang, H. D. She, M. Y. Zhang and Q. Z. Wang, Appl. Catal., B, 2021, 284, 119762 CrossRef CAS.
- H. Liu, J. Zhang and D. Ao, Appl. Catal., B, 2018, 221, 433 CrossRef CAS.
- X. G. Wen, S. H. Wang, Y. T. Xie, X. Y. Li and S. H. Yang, J. Phys. Chem. B, 2005, 109, 10100 CrossRef CAS.
- C. Liu, J. Wang, J. S. Li, J. Z. Liu, C. H. Wang, X. Y. Sun, J. Y. Shen, W. Q. Han and L. J. Wang, J. Mater. Chem. A, 2017, 5, 1211 RSC.
- C. Liu, J. Wang, J. S. Li, R. Luo, X. Y. Sun, J. Y. Shen, W. Q. Han and L. J. Wang, Carbon, 2017, 114, 706 CrossRef CAS.
- Y. L. Qiu, Z. P. Xing, M. J. Guo, T. Y. Zhao, Y. Wang, P. Chen, Z. Z. Li, K. Pan and W. Zhou, J. Colloid Interface Sci., 2021, 582, 752 CrossRef CAS PubMed.
- Z. M. Cui, Z. Chen, C. Y. Cao, L. Jiang and W. G. Song, Chem. Commun., 2013, 49, 2332 RSC.
- M. J. Guo, T. Y. Zhao, Z. P. Xing, Y. L. Qiu, K. Pan, Z. Z. Li, S. L. Yang and W. Zhou, ACS Appl. Mater. Interfaces, 2020, 12, 40328 CrossRef CAS PubMed.
- J. Li, Y. Qin, C. Jin, Y. Li, D. L. Shi, L. S. Mende, L. H. Gan and J. H. Yang, Nanoscale, 2013, 5, 5009 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ta07800c |
| This journal is © The Royal Society of Chemistry 2022 |