A dynamic anode boosting sulfamerazine mineralization via electrochemical oxidation†
Fengxia
Deng
 a,
Jinyu
Xie
a,
Orlando
Garcia-Rodriguez
d,
Baojian
Jing
a,
Yingshi
Zhu
a,
Zhonglin
Chen
a,
Jyh-Ping
Hsu
a,
Jinyu
Xie
a,
Orlando
Garcia-Rodriguez
d,
Baojian
Jing
a,
Yingshi
Zhu
a,
Zhonglin
Chen
a,
Jyh-Ping
Hsu
 c,
Jizhou
Jiang
c,
Jizhou
Jiang
 *b,
Shunwen
Bai
*a and
Shan
Qiu
*b,
Shunwen
Bai
*a and
Shan
Qiu
 *a
*a
aState Key Laboratory of Urban Water Resources Centre, School of Environment, Harbin Institute of Technology, Harbin, 150090, P. R. China. E-mail: baishunwen@hit.edu.cn; qiushan@hit.edu.cn
bSchool of Environmental Ecology and Biological Engineering, School of Chemistry and Environmental Engineering, Key Laboratory of Green Chemical Engineering Process of Ministry of Education, Wuhan Institute of Technology, Wuhan, Hubei 430205, P. R. China. E-mail: 027wi@163.com
cDepartment of Chemical Engineering, National Taiwan University, Taipei 10617, Taiwan
dCentre for Water Research, Department of Civil and Environmental Engineering, National University of Singapore, 1 Engineering Dr 2, Singapore 117576, Singapore
First published on 3rd November 2021
Abstract
Despite numerous efforts to treat wastewater with sulfonamides, their mineralization has rarely been achieved, resulting in the generation of more toxic by-products. In this study, greater than 75% mineralization of sulfamerazine (SMR) was achieved following 4 h of electrochemical oxidation. Remarkably, the Microtox® toxicity test confirmed the elimination of by-products with higher toxicity. The electrochemical treatment process was carried out using a dynamic oxygen-vacancy-mediated TiO2 anode (TiO2-OV@Ti-F), which comprised oxygen-deficient Ti4O7 coated on titanium-foam (Ti-F) via thermal spraying, allowing simultaneous high reactivity and mass transfer. SMR degradation followed a pseudo-first-order kinetics model, where the rate constant (kapp = 1.64 × 10−2 min−1) for the rotary TiO2-OV@Ti-F configuration was 1.98-fold greater than that of the static one (kapp = 8.30 × 10−3 min−1). This highlights the superiority of the rotary TiO2-OV@Ti-F anode for SMR decay. The high oxidation capabilities arose from: (i) the synergetic effect between the rotating system and the Ti4O7 coating; (ii) the enhanced mass transfer coefficient (3.49 × 10−5 m s−1) in the rotating configuration, as well as the increase in SMR degradation via direct oxidation, due to a low hole injection energy, as supported by density functional theory calculations; and (iii) boosted ˙OH formation achieved via removing the gas bubbles attached to the anode, along with lower adsorption energies for H2O and ˙OH. The results revealed that rotary TiO2-OV@Ti-F is a promising alternative for antibiotic wastewater treatment owing to its high organic mineralization and low level of energy consumption (0.29 kW per h per gTOC).
1. Introduction
The presence of antibiotics in wastewater and water at even trace concentrations is a critical environmental problem. Since antibiotics aim to elicit biological responses, they could cause adverse effects not only in aquatic systems, but also in any organism involved in the food chain.1 Furthermore, they are ubiquitous in the environment due to the large amounts of antibiotics consumed, along with their incomplete adsorption,2 which can result in the undesirable development of antibiotic-resistant bacteria. Sulfonamides are synthetic antibiotics that comprise more than 5000 compounds, which differ in their heterocyclic group; they are commonly used as growth promoters in animal husbandry and for other veterinary purposes.3 There are a considerable number of published studies regarding sulfonamide biodegradation; however, most of them agree that conventional biological treatment technologies, especially the activated sludge process, are insufficient to achieve complete removal due to the persistent and antimicrobial nature of these compounds.4–7 Furthermore, full mineralization is rarely verified even with advanced oxidation processes (AOPs), even though it has been found that the partial mineralization of sulfonamides leads to the generation of intermediates with higher toxicity.8 For example, the degradation of sulfamerazine (SMR), a typical sulfonamide antibiotic used extensively in animal husbandry to prevent and treat bacterial infections, has previously been reported to be readily achievable using AOPs; however, these investigations primarily indicated the formation of toxic intermediate products and poor mineralization to carbon dioxide.8–12Over the past few decades, electrochemical oxidation (EO) has emerged as a promising alternative to decontaminate refractory organics such as the aforementioned antibiotics due to its high reactivity and ease of operation. Comninellis13 proposed that the degradation of pollutants in EO can follow either a direct or indirect oxidation mechanism (eqn (1)). In the former, the target compounds are first transferred to active sites on the anode, followed by oxidation via direct electron transfer (DET).14,15 Additionally, organic pollutants can also be oxidized by heterogeneous/homogeneous reactive species formed at the anode, especially by non-active anodes with high oxygen overpotential.16,17
| Organic pollutant + direct oxidation + reactive species → CO2 + H2O | (1) |
| M + H2O → M(˙OH) + H+ + e− (M = the anode surface) | (2) |
| 3H2O → O3 + 6H+ + 6e− | (3) |
| 2H2O → H2O2 + 2H+ + 2e− | (4) |
| 2SO42− → S2O82− + 2e− | (5) |
| nTiO2 + oxygen vacancies (OVs) → TinO2n−1 | (6) |
The efficiency of EO largely depends on the type of anode; among them, boron-doped diamond (BDD) anodes have been widely reported due to their superior activity.18,19 The outstanding performance of BDD can be attributed to the weakly adsorbed hydroxyl radicals (M(˙OH)) from water oxidation (eqn (2)) along with DET.18,20,21 In addition, other reactive oxygen species, such as O3 (eqn (3)), H2O2 (eqn (4)), peroxy species (S2O82−, eqn (5)) and 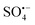 ,22–24 can also mediate the contaminant degradation.18,20,21 Unfortunately, the high cost of BDD (∼$7000 per m2) coupled with the scarcity of suitable substrates, has limited its commercial implementation mainly to disinfection systems.25
,22–24 can also mediate the contaminant degradation.18,20,21 Unfortunately, the high cost of BDD (∼$7000 per m2) coupled with the scarcity of suitable substrates, has limited its commercial implementation mainly to disinfection systems.25
In recent years, titanium oxides to which oxygen vacancies have been introduced (TinO2n−1, 4 < n < 10, such as Ti4O7 and Ti5O9, eqn (6)), known commercially as Magnéli-phase oxides, have been proposed as cost-efficient alternative electrodes for wastewater treatment with mineralization performances similar to BDD.26–29 Magnéli-phase oxides feature a distinct oxygen-deficient crystal structure for each n layer, resulting in improved electrochemical conductivity and corrosion resistance.30–32 Despite the fact that this enhanced electrochemical activity has a significant impact on the oxidation rate, the low mass transport of organics from and toward the electrode surface remains a bottleneck, because pollutant oxidation by short-lived ˙OH (<10 μs), is mostly confined to a distance of only 10 μm from the anode surface.33 Furthermore, although it is undesirable, the formation of oxygen bubbles on the anode surface is rather common, obstructing active sites and increasing the ohmic resistance.34,35 To overcome these drawbacks, the use of dynamic cylindrical electrodes has proven to be efficient, especially in heavy metal removal systems based on electrodeposition36 and electrocoagulation,37 but their application in electrochemical oxidation with Magnéli-phase oxides has only been reported in a few studies, which were mainly focused on the enhancement of mass transfer.38,39
In view of the above, it is of paramount importance to design a configuration that has not only good anodic reactivity, but also efficient mass transfer, and that allows satisfactory mineralization, as it is crucial to eliminate intermediates, which might pose environmental and ecological threats. Therefore, in this work, two novel approaches were combined: the study of the synergy between anodic reactivity and mass transfer using a Magnéli-phase oxide electrode with a rotational configuration, and the improvement of the mineralization efficiency of sulfamerazine to obtain biodegradable and less-toxic byproducts. To achieve this, we carried out: (i) construction of a rotary titanium oxide anode by introducing oxygen defects in TiO2 powder and using titanium-foam as substrate to develop a rotary TiO2-OV@Ti-F anode via thermal spraying; (ii) density functional theory (DFT) calculations to reveal the mass transfer and reactivity mechanisms associated with the rotary TiO2-OV@Ti-F anode during the electrochemical abatement of SMR; (iii) assessment of the toxicity, energy consumption, stability, and life cycle of the proposed anode; and (iv) evaluation of the environmental impact of its fabrication in order to provide critical quantitative foundations for its use in industrial applications.40
2. Materials and methods
2.1 Reagents
Ammonium metavanadate (≥99.0%), 5,5-dimethyl-1-pyrroline N-oxide (DMPO, ≥98.0%), sulfamerazine (SMR, ≥99.5%), and tert-butanol (TBA, ≥99.5%) were purchased from Sigma-Aldrich, China. Disodium terephthalate (>9.0%) was obtained from Tokyo Chemical Industry. HPLC grade methanol (≥99.9%) and acetonitrile (≥99.9%) were purchased from Dikma Technologies. Pt sheet anodes (1 cm × 2 cm) and titanium-foam plates (Ti-F, 1 cm × 2 cm × 1 mm, aperture = 100 μm) were purchased from Suzhou Christie Foam Metal, China.2.2 Analytical methods
The concentration of H2O2 was measured using an ammonium metavanadate method.40,41 The O3 formed on the anode was detected using indigo indicator and ˙OH through the electron paramagnetic resonance spectra method, along with a terephthalic acid photoluminescence approach.42 Details of the SMR measurements and total organic carbon (TOC) evaluation can be found in Deng et al.43,44 Toxicity assessments were carried out at different time intervals using the Microtox method, which is based on determining the bioluminescence inhibition of the bacteria V. fischeri as described elsewhere.452.3 Synthesis of the TiO2-OV@Ti-F anode
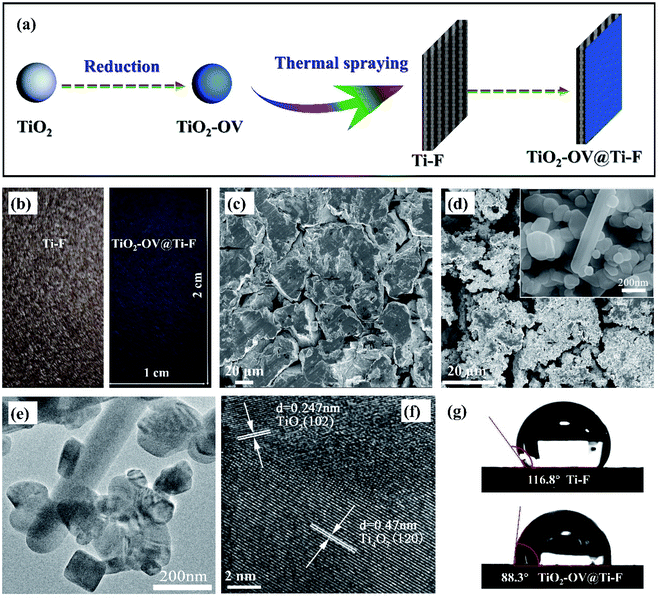
The Ti-F substrate was first degreased by rinsing it in acetone for 30 min in an ultrasonic bath, followed by oxide removal by immersing it in 0.5 M H2SO4. Finally, it was rinsed and washed with deionized water for further coating. TiO2 powder with oxygen vacancies (denoted as TiO2-OV) was prepared via the reduction of stoichiometric TiO2.46 Specifically, when exposed to a H2 atmosphere, the surficial oxygen atoms of TiO2 could be reduced into oxygen vacancies and H2O (eqn (7)).47 The obtained TiO2-OV was then coated on the pre-treated Ti-F substrate (TiO2-OV@Ti-F) via thermal spraying (Fig. 1a) under the following conditions: arc current = 530 A, voltage = 53 V, spraying speed = 30 mm s−1, powder feed rate = 28 g min−1 and spray distance = 90 mm. In addition, the analogous material with raw, unreduced TiO2 was also prepared for comparison (TiO2@Ti-F, Fig. S1†). Considering the wide bandgap (3.2 eV) of TiO2, electrons could hardly move freely in the insulator, and thus, we failed to conduct the related electrochemical experiments. However, the bandgap was narrowed via the introduction of oxygen vacancies.| H2 + surface O → H2O + oxygen vacancies (OVs) or H2 + 2Ti[4+]O2 → 2Ti[3+]OOH | (7) |
2.4 Characterization of the TiO2-OV@Ti-F anode
Scanning electron microscopy (SEM, ZEISS Sigma 500, Germany), X-ray diffraction (XRD, Bruker D8 ADVANCE, Germany), high resolution transmission electron microscopy (HRTEM), X-ray photoelectron spectroscopy (XPS, Thermo Fisher ESCALAB 250Xi, USA) and Fourier-transform infrared spectroscopy (FT-IR, PerkinElmer infrared spectrometer, USA) were used to characterize the physicochemical properties of the anodes as follows. Microscopic characterization of the electrodes and their surface elemental composition were analyzed using SEM-EDS. The chemical composition and crystal structure were identified via XRD with Cu Kα radiation with a 2 theta range from 10–80°. Chemical state analyses were determined using XPS with the binding energy of C 1s at 284.8 eV as a standard reference to correct the other elemental curves. FT-IR was used to assess the functional groups; the resolution was 4 cm−1 and the wavelength range was 400–4000 cm−1. The work function of the anodes was investigated using ultraviolet photoelectron spectroscopy (UPS, SPECS, Germany) using the He I (21.2 eV) resonance line; pure gold (4.9 eV) was utilized as a reference. The water contact angle, an indicator of wettability, was measured via a high temperature wetting angle meter (HuaYi, VAF-30) at room temperature.The electrochemical characterization was assessed using linear sweep voltammetry (LSV) and electrochemical impedance spectroscopy (EIS) with a three-electrode cell controlled by a workstation (CHI760E, Cheng Hua) in 50 mM Na2SO4 electrolyte. In a typical three-electrode system, Ag/AgCl (3 M KCl) served as the reference electrode, a Pt sheet (1 cm × 2 cm) as the counter electrode and, either Ti-F or TiO2-OV@Ti-F (1 cm × 2 cm) as the working electrode. LSV was conducted with a potential window range from the open-circuit potential (OCP) to 2.5 V with a scan rate of 5 mV s−1. EIS was carried out at the OCP with a frequency ranging from 100 kHz to 10 MHz and a potential amplitude of 10 mV.
To evaluate the contribution of direct electron transfer in SMR degradation, LSV was conducted for SMR concentrations ranging from 0 mg L−1 to 200 mg L−1 using the above-mentioned three-electrode system. The sweep potential was varied between 0.6 V and 1.5 V, with a scan rate of 5 mV s−1. The electrochemical active surface area coupled with the conductivity was measured by cyclic voltammetry (CV) at various scan rates ranging from 5 mV s−1 to 50 mV s−1 in a three-electrode cell with a 5 mM K3[Fe(CN)6] and 0.1 M KCl solution, for which the redox couple (eqn (8)) is well-characterized.44
| [Fe(CN)6]3− + e− → [Fe(CN)6]4− | (8) |
2.5 Electrochemical degradation and mineralization of SMR and its corresponding mass transfer coefficient (km)
A dynamic electrochemical system was designed for SMR treatment; the details are given in Fig. S2.† This comprised: (i) a cylindrical glass reactor with a working volume of 100 mL stirred by a magnetic bar at 200 rpm; (ii) a platinum (Pt) sheet (1 cm × 2 cm) cathode; (iii) a Ti-F (1 cm × 2 cm) or TiO2-OV@Ti-F (1 cm × 2 cm) anode and (iv) an electrical apparatus to control the rotation speed of the anode (60 W, 1200 rpm rotational speed), in which the direction was adjusted by a bevel gear. The mass transfer coefficient (km) in different configurations was calculated according to eqn (9) based on the method proposed in Fan's investigation.39 | (9) |
2.6 Theoretical calculations
The details for the life-cycle assessment (LCA) to study the environmental impacts of the TiO2-OV@Ti-F anode can be found in SI. 2, Tables S1–S5 and Fig. S3.† The LCA analysis aimed to study the environmental impacts of TiO2-OV@Ti-F anode with an emphasis on comparing it with two alternative anodes: 3DM-PbO2/Ti and BDD/Ti, both of which are based on a Ti substrate. Fig. S3† summarizes the procedure used to prepare these anodes. The 3DM-PbO2/Ti anode was chosen as a comparison. BDD/Ti was selected since it is regarded as the most promising anode, with high oxidation ability and a long life-span in electrochemical oxidation. The functional unit was considered as a 1 cm × 2 cm anodic plate and the system boundaries included all the chemicals, electricity and energy involved in the production of each anode.
3. Results and discussion
3.1 Physical characterization
The physical appearance of the electrodes used in this study is shown in Fig. 1b; as can be seen, the brown Ti-F base turned bluish after coating with TiO2-OV powder. This is in accordance with the crystalline structure of Ti4O7; further details will be given in the discussion of the XRD analysis.50 The morphological structure of the Ti-F base as observed using SEM is shown in Fig. 1c; it was composed of irregularly shaped sintered titanium particles with lengths of around 100 μm. After coating with TiO2-OV powder, the surface attained full coverage with small particles of ca. 100 nm in diameter showing a more porous structure than the raw base (Fig. 1d). In addition, the introduction of a large amount of oxygen vacancy defects to the TiO2 structure yielded a 10% increase in its oxygen content (Table S6†). The high-resolution TEM images (Fig. 1e and f) showed the formation of bar-shaped and cubic particles; the former had a width of ca. 100 nm and length exceeding 600 nm, and the latter a side length of ca. 100 nm. Both were composed of the (1 −2 0) and (1 0 4) crystallographic planes of Ti4O7. Inevitably, TiO2 was also present, which included its (1 0 −2) and (1 0 2) crystal faces.The properties of the electrode surface, particularly its hydrophilicity, play a key role in the adsorption/desorption of reactants, which is critical for anodic oxidation.51,52 Therefore, the contact angle of the pristine and treated anodes was measured to evaluate the variation in wettability after the introduction of OVs. As presented in Fig. 1g, the contact angle of deionized water on the pristine anode was 116.8°, and became 88.3° after the incorporation of OVs, implying that the OVs increased the hydrophilicity of the anode; this effect could be explained by the increased oxygen content on the electrode surface.53 This might be a good indicator of the adsorbed hydroxyl radicals (M(˙OH)) generated from water oxidation (eqn (2)).
Fig. 2a shows the results of the XRD analysis used to determine the crystalline structure of the Ti-F and TiO2-OV@Ti-F electrodes. The eight intense peaks at 35.09°, 38.42°, 40.17°, 52.95°, 62.84°, 70.66°, 76.17° and 77.29° were attributed to the diffraction of hexagonal titanium, corresponding to the (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (1 1 2) and (2 0 1) planes, respectively (JCPDS, card no. 044-1294).54 Additional peaks at 20.75°, 26.37°, 29.58°, 31.74°, 35.39°, 35.57°, 40.61°, 53.31°, 55.05°, 63.34° and 66.79° were observed following the application of the thermal spray coating. These diffraction peaks correspond to the (1 0 −2), (1 −2 0), (1 −2 −2), (1 0 −4), (2 0 −2), (1 0 4), (2 −1 3), (3 −2 2), (1 −4 −2), (3 0 4), and (1 4 −4) planes of Ti4O7, respectively (JCPDS, card no. 050-0787). Hence, the oxygen-defective TiO2-OV@Ti-F anode mainly contained Ti4O7, which is in good agreement with the HRTEM results and the appearance of a blueish colour after coating. Fig. 2b illustrates the FT-IR analysis of the surface vibration modes in the 400–4000 cm−1 region. The broad band centered at 3500 cm−1 was assigned to the stretching vibration of surface –OH or surface-adsorbed water. The band at 1092 cm−1 was attributed to Ti–O–Ti bending,55 while the peak at 798 cm−1 confirmed the vibration of the Ti–O chemical bond. The band at 468 cm−1 corresponded to the Ti–O–Ti bridge stretching and Ti–O stretching. Both the XRD and FT-IR results revealed that the oxygen-defective TiO2 contained mainly Ti4O7. The XPS spectra of the Ti-F and TiO2-OV@Ti-F electrode surfaces (Fig. 2c–f) depict the variation of Ti 2p and O 1s. The Ti0 2p3/2 and Ti0 2p1/2 XPS peaks of the raw titanium-foam anode were observed with binding energies (BEs) of 453.48 eV and 460.58 eV, respectively, along with the appearance of Ti4+ (457.59 eV and 463.33 eV) since titanium might be oxidized.56 As illustrated in Fig. 2e, the BE of Ti 2p shifted to a higher level, which could be attributed to the breakage of the Ti–O bond caused by oxygen vacancies after coating with oxygen-defective TiO2.46 The peaks with BEs of 464.11 eV and 458.01 eV could be fitted well to Ti4O7,57,58 as could those at 464.4 eV and 459.29 eV to TiO2. The results of the XPS measurements were in good agreement with those of XRD. The O1s XPS spectra for the Ti-F and TiO2-OV@Ti-F anodes are shown in Fig. 2d and f. The peak for the titanium metal base (Fig. 2d) at a BE of 529.31 eV could be attributed to O2− in the form of Ti–O–Ti from the newly formed oxidized film, while the peak at 531.70 eV confirmed the presence of surface hydroxyl groups, such as the terminal hydroxyl group (Ti–OH) and a bridging hydroxyl group (Ox–H), which were also previously confirmed by the FT-IR spectra.59,60 Both peaks experienced a red-shift to higher BE after coating (Fig. 2f), suggesting the appearance of oxygen vacancy defects next to Ti3+–O (BE 530.54 eV) and the loosely bound oxygen adsorbed onto the surface (BE 532.91 eV).
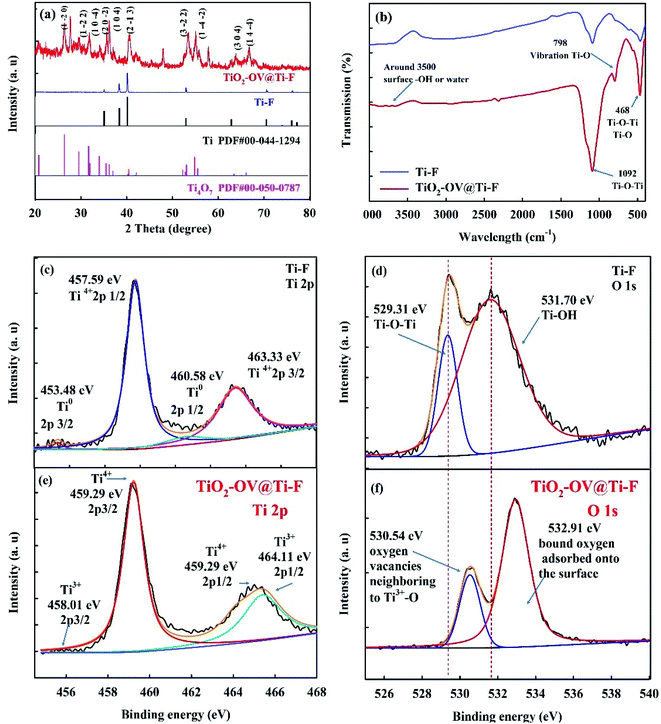 | ||
| Fig. 2 XRD diffraction patterns (a) and FT-IR spectra (b) of the Ti-F and TiO2-OV@Ti-F anodes. (c and e) Ti 2p and (d and f) O 1s XPS spectra of the Ti-F and TiO2-OV@Ti-F anodes. | ||
3.2 Electrochemical characterization
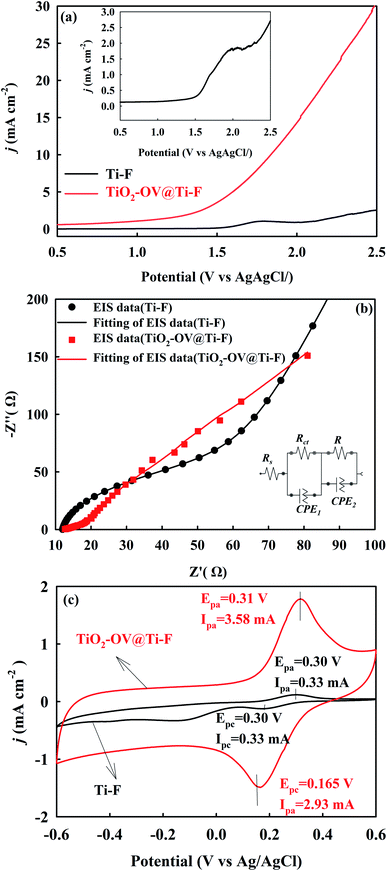
Previous DFT calculations revealed that oxygen-defective structures at the anode would inevitably affect its electronic nature (e.g., the charge density distribution).61 Hence, LSV and EIS were used to assess the variation in the electrochemical characteristics after the introduction of Ti4O7. A preliminary estimation based on the LSV curves in Fig. 3a suggested that the TiO2-OV@Ti-F anode could enhance the electrochemical activity, as a higher current response was observed compared to that for the Ti-F base anode. This might be attributed to the superior activity of Ti4O7. The oxidation peak of Ti-F located between 1.5 V and 2 V (inset to Fig. 3a) illustrated the oxidation of titanium on the Ti-F surface.50 However, this peak was absent in the case of the TiO2-OV@Ti-F anode, implying that it could resist anodic corrosion within the potential window ranging from 0.5 V from 2.5 V after modification with Ti4O7. EIS measurements were carried out utilizing the three-electrode setup described previously (Section 2.4) to better understand the electrochemical performance of each anode. The obtained Nyquist plots of each electrode in 50 mM Na2SO4 and the respective equivalent circuit that best fitted them are shown in Fig. 3b. The values of the charge transfer resistance (Rct) between the electrolyte and the electrode surface obtained from fitting equivalent circuits via the Nyquist plots were 43.4 Ω and 11.2 Ω for Ti-F and TiO2-OV@Ti-F, respectively, confirming that the electron transfer kinetics were enhanced on the defective anode, which could help to improve the mineralization efficiency of the model pollutant, as will be discussed further in the next section.To estimate the electrochemical active surface areas of both anodes, their CV curves in a solution containing 5 mM [Fe(CN)6]3− were recorded at various scan rates (Fig. S4†). The observed oxidation peak at 0.3 V and reduction peak at 0.165 V were attributed to the redox reaction of [Fe(CN)6]3−/[Fe(CN)6]4−. The CV curves of the Ti-F anode were qualitatively similar to those of the TiO2-OV@Ti-F anode, with the peak current rising gradually with increasing scan rate. However, the peak current for the TiO2-OV@Ti-F anode was ca. 10-fold that of the Ti-F anode (Fig. 3c), which implies an improvement in the electroactive surface area of the electrode due to the introduction of defective Ti4O7, providing more active sites for the oxidation of water and/or organic compounds; this could suggest a plausible enhancement in the mineralization efficiency of the process. The electrochemical active areas of the Ti-F and TiO2-OV@Ti-F anodes calculated using the Randles–Sevcik equation (eqn (10))44,62 were 1.08 and 13.66 cm2, respectively. This verified that the incorporation of oxygen vacancies could raise the electrochemical active surface area.
 | (10) |
3.3 Comparison of SMR degradation in the electrochemical oxidation system
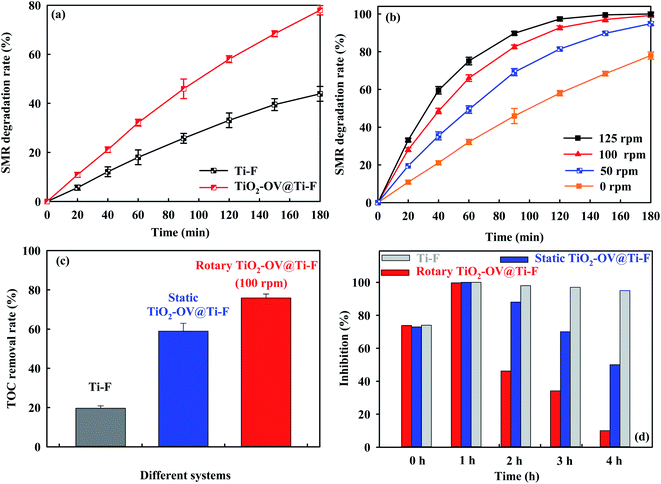
To evaluate their organic degradation performance in electrochemical oxidation, comparative SMR degradation/mineralization experiments were conducted using either a Ti-F or TiO2-OV@Ti-F anode and a Pt sheet cathode under the following conditions: 50 mg L−1 SMR, 50 mM Na2SO4, and a current density of 25 mA cm−2. After 3 h of electrolysis, the degradation efficiency of the TiO2-OV@Ti-F anode reached 78%, whereas the Ti-F anode only achieved 43.8%, as illustrated in Fig. 4a. In both cases, SMR degradation followed a pseudo-first-order kinetic rate. The rate constant for the TiO2-OV@Ti-F anode was kapp = 8.30 × 10−3 min−1, which was ca. 2.50-fold that of the Ti-F anode (kapp = 3.30 × 10−3 min−1). This highlighted the TiO2-OV@Ti-F anode's superior performance in SMR degradation via electrochemical oxidation.The performance of the proposed rotary system was also evaluated at various rotation speeds ranging from 0 to 125 rpm. As displayed in Fig. 4b, an appreciable improvement in the rate of SMR decay was observed, where the rate constants at 50 rpm, 100 rpm and 125 rpm were 1.64 × 10−2 min−1, 2.62 × 10−2 min−1 and 3.49 × 10−2 min−1, respectively, which were ca. 1.98-, 3.16- and 4.20-fold larger than those of the corresponding static system. Note that 100% SMR removal was achieved at 100 rpm and 125 rpm after 180 minutes of electrolysis, indicating that the rotational TiO2-OV@Ti-F anode system was effective at degrading the model pollutant.
Nevertheless, the formation of intermediate toxic products and poor mineralization to CO2 are the major concerns during sulfonamide degradation.44,62 Therefore, to highlight the merits of the proposed rotational TiO2-OV@Ti-F anode system, in addition to the SMR decay, the mineralization efficiency, along with the change in toxicity, during 4 h of electrolysis in three configurations was also evaluated for Ti-F and static and rotary TiO2-OV@Ti-F (100 rpm) systems. As shown in Fig. 4c, the TOC removal rate for the rotary TiO2-OV@Ti-F anode system reached ca. 76% at 4 h, which was ca. 1.29- and 3.86-fold greater than the rates for the static and raw systems, respectively. Toxicity results (Fig. 4d) showed an initial increase in the toxicity after 1 h of electrolysis, followed by a detoxification trend, which was more pronounced for the rotary TiO2-OV@Ti-F anode with a drop in inhibition percentage from 74% to 10% in the final stage of electrolysis. The drop in the inhibition rate for the dynamic anode was 3.2-fold greater than that for its static counterpart. However, the opposite trend was observed when Ti-F was used as the anode, with the inhibition rate rising from 74% to 95%. This meant that more hazardous intermediates were formed and persisted in the bulk since the raw Ti-F anode failed to mineralize them, which was in accordance with the modest TOC removal rate (no more than 20%) shown in Fig. 4c. Based on its much greater mineralization rate and enhanced detoxification performance, the rotating TiO2-OV@Ti-F anode was found to be a promising alternative for the treatment of wastewater containing SMR. The TiO2-OV@Ti-F anode was advantageous in terms of detoxification performance when compared to the Ti4O7 anode in the previous investigation.63 It even presented superiority compared to the high oxidation capacity of the BDD anode in antibiotic treatment.44 For example, an inhibition rate of approximately 60% was observed even after 6 h of electrolysis using BDD with a much lower sulfonamide antibiotic concentration (0.02 mM).18 The inhibition rate was only reduced to 25% when Fe2+ catalyst was added as a trigger coupled with BDD anodic oxidation.64 These comparisons highlight the superiority of the TiO2-OV@Ti-F anode in terms of detoxification performance. The high oxidation performance of the rotary TiO2-OV@Ti-F anode was attributed to two aspects: (i) the higher electroactive area of the fabricated TiO2-OV@Ti-F anode and (ii) the rotational mechanism, which allowed for a thinner diffusion layer on the anode/electrolyte interface, along with a less significant bubble curtain problem. In order to provide a better understanding of the superior performance of the rotary anode, its oxidation mechanism will be discussed in the following section.
3.4 SMR degradation mechanism using the rotary TiO2-OV@Ti-F anode
 | (11) |
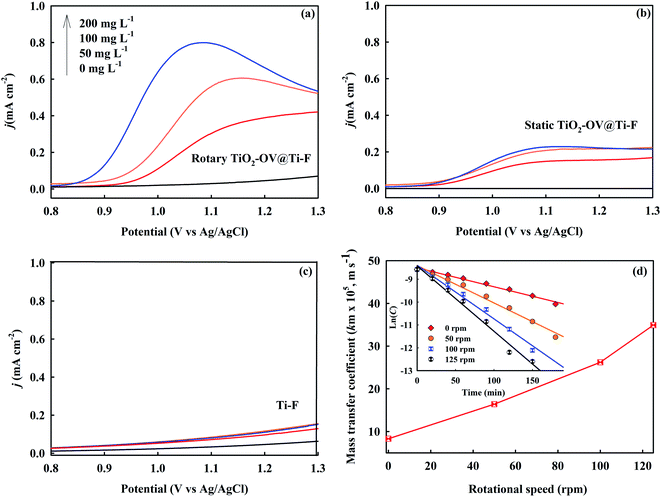
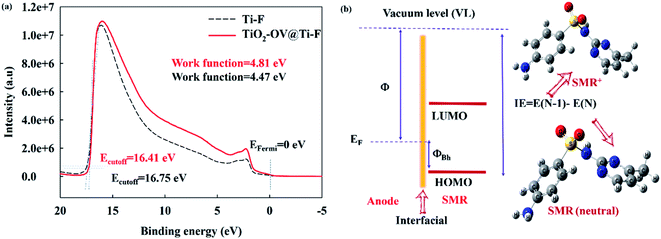
In general, the direct oxidation of SMR on the anode surface is composed of charge transfer and mass transport steps. In addition to the enhanced mass transport of SMR on the rotary TiO2-OV@Ti-F anode, the superior SMR decay could also be due to higher electron transfer on the TiO2-OV@Ti-F anode. To confirm this point, ultraviolet photoelectron spectroscopy coupled with density functional theory based on first principles was used to measure the work function (Φ, energy required to remove an electron from its Fermi level (EF) to the vacuum near the surface) of the anode and the interfacial hole injection energy (ΦBh), which is a crucial parameter to assess the efficiency of electron transfer on the electrode surface; the lower the ΦBh, the better the direct oxidation reaction on the anode. ΦBh is calculated by the Schottky–Mott rule (eqn (12)),69 while Φ could be estimated from the UPS spectra (Fig. 6a). It can be shown that through the oxygen-vacancy modification of the anode, Φ increased from 4.47 eV to 4.81 eV. The ionization energy (IE) of SMR was deduced from DFT calculations using B3LYP/6-31G(d) (Fig. 6b). The obtained results indicated that the ΦBh (2.57 eV) of the TiO2-OV@Ti-F anode is lower than that of the Ti-F anode (2.91 eV), supporting the better performance of the former in the direct electron transfer of SMR during EO.
| ΦBh = IE − Φ | (12) |
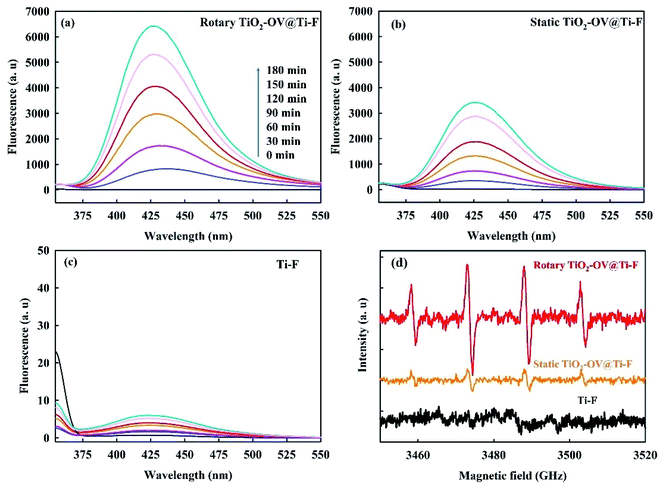
The terephthalic acid (TA) photoluminescence method coupled with the electron paramagnetic resonance (EPR) spectroscopy technique was used to detect the formation of ˙OH via water oxidation (eqn (2)) on the rotary (Fig. 7a) and static TiO2-OV@Ti-F (Fig. 7b) anodes, as well as the Ti-F (Fig. 7c) anode. With its high redox potential, TA resisted direct electron transfer oxidation during anodic oxidation and exclusively captured ˙OH to form fluorescent 2-hydroxyterephthalic acid (TAOH).23,70 The generation of ˙OH on both configurations of the TiO2-OV@Ti-F anode was demonstrated by the development of the fluorescent TAOH peak at an emission wavelength of 415 nm, as shown in Fig. 7a and b, which showed ˙OH peak intensities considerably higher than that of the raw Ti-F anode (Fig. 7c). This highlighted the effectiveness of the oxygen-defective TiO2 coating for ˙OH formation. Moreover, compared to that of the static anode, the intensity of the ˙OH peak was further remarkably enhanced when using the rotary TiO2-OV@Ti-F anode configuration. The signal of the adduct DMPO-˙OH in Fig. 7d (EPR) followed a similar trend as that in the terephthalic acid photoluminescence method. Note that the O3 and H2O2 generated at the anode viaeqn (3) and (4) were negligible in all the configurations (Fig. S5†). We concluded that in addition to the enhanced direct electron transfer oxidation, the high SMR decay on the rotary TiO2-OV@Ti-F anode was also attributed to greater ˙OH formation.
DFT calculations of Ti4O7 (previously identified as a typical component of the TiO2-OV@TiF anode) and TiO2 anatase (1 0 1) as a reference were used to determine the geometric configurations and energies of the precursor/product adsorption, dissociation, and detachment of ˙OH (Fig. 8) to provide theoretical evidence that Ti4O7 was able to enhance the production of ˙OH. The optimized structures of Ti4O7 and TiO2 anatase (1 0 1) with the lowest energies are shown in Fig. 8a and e, respectively. Moreover, the most stable Ti4O7 and TiO2 geometries during water oxidation for ˙OH formation (eqn (13)–(15)) are represented for the following key steps:71 (i) H2O adsorption (Fig. 8b and f), (ii) H2O dissociation (Fig. 8c and g), and (iii) ˙OH detachment (Fig. 8d and h). These optimized configurations were also used to calculate the energies involved during the H2O adsorption (Eads-water, eqn (16)), H2O dissociation (Edis-water, eqn (17)), and ˙OH desorption (Edetach-˙OH, eqn (18)).72 As can be inferred from Fig. 8, the energies of H2O adsorption on the TiO2-OV@Ti-F and TiO2 anodes are −0.21 Ha and −0.16 Ha, respectively, implying that the adsorption of H2O on these anodes is exothermic. The more negative adsorption energy suggests that the adsorption of H2O on Ti4O7 is more stable than that on TiO2, thereby favouring the subsequent generation of ˙OH. This could be attributed to the H-bond interactions introduced due to the presence of active sites such as surface titanol groups (–TiOH) when Ti4O7 was used as the anode.73 This is because the interaction between H2O and the anode surface is usually dominated by the van der Waals force, which is weaker than H-bond interactions.74 This provided a good explanation for the strong adsorption interaction of H2O on the Ti4O7 anode. After the H2O adsorption step, the degree of its subsequent dissociation into ˙OH and ˙H depended largely on the associated dissociation energy, which was 0.004 Ha on TiO2 anatase and −0.03 Ha on TiO2-OV@Ti-F. Since the dissociation of H2O in the latter is exothermic (thermodynamically favourable), TiO2-OV@Ti-F is the better choice for the formation of ˙OH.
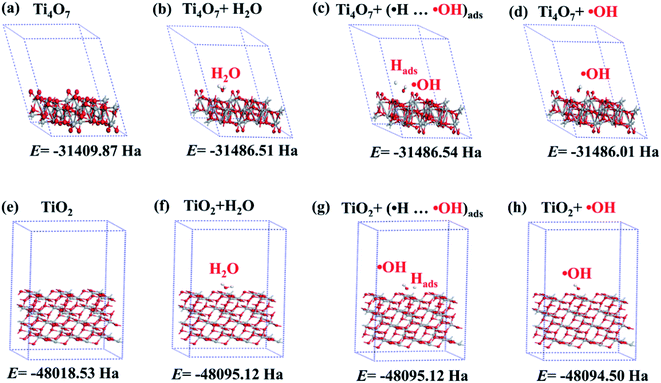 | ||
| Fig. 8 (a)–(h) Geometric configurations of Ti4O7, Ti4O7 + H2O, Ti4O7 + Hads⋯˙OH, Ti4O7 + ˙OH, TiO2, TiO2 + H2O, TiO2 + Hads⋯˙OH, and TiO2 + ˙OH with the corresponding calculated energies. | ||
Furthermore, only “non-active” anodes are able to generate weakly physisorbed ˙OH via H2O oxidation, and therefore, are suitable for the degradation of organics. In contrast, anodes with strongly chemisorbed ˙OH on their “active” surface sites are not suitable, because the strongly adsorbed ˙OH could recombine with ˙H, making it unavailable for further reactions. This implies that the desorption energy of ˙OH from the surface plays an important role in assessing the capability of organic mineralization. In our case, the energies for ˙OH desorption were −3.33 × 10−2 and −3.32 × 10−2 Ha for Ti4O7 and TiO2, respectively. The negative desorption energies indicated that the anodically generated ˙OH is prone to desorbing from the Ti4O7 anode and, therefore, is more available to react with organic compounds, as seen previously in Fig. 7.
| Anode surface + H2O → anode surface (H2O)ads | (13) |
| Anode surface (H2O)ads → anode surface (˙OH)ads + anode surface (˙H) | (14) |
| Anode surface (˙OH)ads + organics → surface + products | (15) |
| Eads-water = E(surface+water) − Esurface − Ewater | (16) |
| Edis-water = E(surface+H˙⋯˙OH) − E(surface+water) | (17) |
| Edetach-˙OH = E(surface+H˙⋯˙OH) − E(surface+H˙) − E˙OH | (18) |
The subscript ads denotes adsorbed species, Ewater and E˙OH are the energies of the free water (−76.41 Ha) and ˙OH (−78.83 Ha), respectively, Esurface is the surface energy prior to adsorption, and Eads-water and Eads-˙OH are the energies of the surface with adsorbed water and ˙OH, respectively.
In addition to the improvement of the electrode due to the TiO2-OV coating, the rotation was also important throughout the electrochemical process. As seen in Fig. S6,† side reactions such as oxygen evolution produced bubbles, which formed a bubble layer when the current density reached 25 mA cm−2. Due to their poor wettability, oxygen bubbles tend to adhere to and cover the TiO2-OV@Ti-F anode surface.75 In addition, their electrochemically inactive nature raised the resistivity and reduced the active surface area.76 As a result of the anode rotation, the produced shear force not only reduced the size of the attached bubbles, but also decreased the time for bubble nucleation and growth on the anode surface, resulting in a faster bubble detachment speed and a cell potential drop from 4.00 to 3.28 V at 25 mA cm−2. Therefore, compared to the static configuration, the rotating anode could minimize the surface bubble coverage and thus increase the concentration of ˙OH.
3.5 Energy consumption, stability, and environmental impacts of the rotary TiO2-OV@Ti-F anode
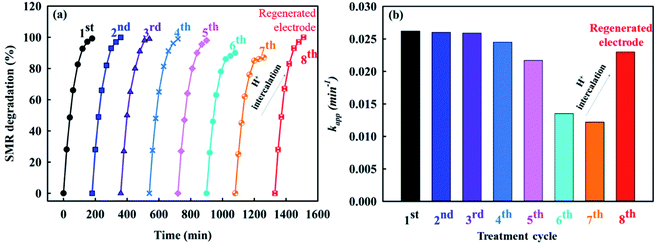
The energy consumption of an EO process is a key parameter to consider, especially when it comes to determining its potential for large-scale applications. Thus, we carried out a specific energy consumption (SEC) assessment, expressed as the amount of energy per unit mass of organic removed (kW h g−1) during the EO process.77 The SEC values for the static configuration using the Ti-F and TiO2-OV@Ti-F anodes were 0.87 kW h g−1 and 0.28 kW h g−1, respectively. The introduction of a rotating anode configuration could further reduce the SEC to 0.17 kW h g−1. The total energy consumption taking into account the electric power requirements of the rotary motor was 0.29 kW h g−1. This value is of the same order of magnitude as those of previous anodic oxidations in similar reactors. For example, 1.115 kW h g−1 TOC was seen for a non-porous Ti4O7 electrode,78 0.35 kW h g−1 COD for a PbO2–Ti4O7 electrode,79 and 0.25 kW h g−1 TOC for a Ti4O7 electrode.63 The low energy consumption of the rotary TiO2-OV@Ti-F anode was attributed to superior mass transfer efficiency, along with high reactivity including direct electron transfer and the generation of reactive species.In addition to the energy consumption, another crucial element to take into consideration is the stability of the electrode, which was evaluated through the SMR removal percentage after 160 minutes of treatment using the same rotary TiO2-OV@Ti-F anode over eight cycles. As shown in Fig. 9, the degradation of SMR remained consistent throughout five cycles of electrolysis, with no significant difference (nearly 100% SMR degradation). However, this was followed by a gradual decrease in the degree of degradation at the beginning of the sixth cycle. This anodic aging could be attributed to the active Ti3+ sites in the TiO2-OV@Ti-F anode being oxidized into Ti4+ sites followed with H+ discharge viaeqn (19). A cathodic reactivation method was then used to recover the TiO2-OV@Ti-F anode using H2SO4 electrolyte and an applied current density of 50 mA cm−2 for 30 minutes,80 during which H+ was incorporated into the Ti4+ sites to reverse reaction 19. As a consequence, the passivated surface was regenerated via H+ intercalation, followed by recovery of the degradation efficiency in the eighth electrolysis cycle. The Ti 2p results in Fig. S6c and d† confirm this point. The stability of the rotary TiO2-OV@Ti-F anode after five cycles could be acceptable compared to the 14.3% degradation loss in the Ti/PbO2 electrodes with Ti4O7 after five cycles.81 Despite the degradation loss, the TiO2-OV coating enhancement properties can be continuously regenerated to maintain its efficiency, allowing the continuous degradation of SMR. The morphology of the regenerated TiO2-OV@Ti-F electrode was further investigated using SEM, TEM and XPS, which showed a negligible change in morphology (Fig. S7†)
| Ti[3+]O2–H+ ↔ Ti[4+]O2 + H+ + e− | (19) |
The environmental impact of our rotational TiO2-OV@Ti-F anode (1 cm × 2 cm) was estimated using Monte Carlo simulations and compared to those of the 3DM-PbO2/Ti and BDD/Ti anodes. Tables S2, S4 and S5† summarize the results of the Monte Carlo simulations with a fluctuation range of ±10%, which were implemented for all the inventory data. The results revealed that all the examined anodes had a minor level of uncertainty. Among them, the TiO2-OV@Ti-F anode had the lowest total environmental impact with a 95% confidence interval of [0.009 points, 0.013 points], followed by the BDD/Ti anode and the 3DM-PbO2/Ti anode with 95% confidence intervals of [0.061 points, 0.083 points] and [0.036 points, 0.045 points], respectively.
The total environmental impact resulted from aggregating three environmental categories: ecosystem quality, human health, and resources (Fig. 10). Climate change, human toxicity, and fossil fuel depletion were the factors that contributed most to each environmental category for the anodic materials studied, accounting for, on average, 70.55, 72.01, and 96.75%, respectively. It is worth noting that the amount of titanium plate assumed in each of the three cases was the same, and therefore, so was its environmental impact contribution. However, it was observed that the chemicals used to carry out the anode coating played an important role: the contribution of the TiO2-OV coating was 83.38% of the total impact, followed by 3DM-PbO2 (22.92%), and BDD (13.14%). This is because consuming ethanol and methanol during the fabrication process increases the life cycle environmental consequences. For 3DM-PbO2/Ti, ethanol accounted for 60.56% of the total impact (39.5 g of ethanol cm−2), and for BDD/Ti, methanol accounted for 83.62% of the total impact (126.7 g of methanol cm−2). Nevertheless, the crucial factor that determined the total environmental impact was the methodology used to apply the coating; while 3DM-PbO2/Ti and BDD/Ti used a sol–gel method and hot filament chemical vapor deposition, respectively, our methodology consisted of thermal spraying, which allowed us to minimize the usage of chemicals and thus reduce the environmental impact.
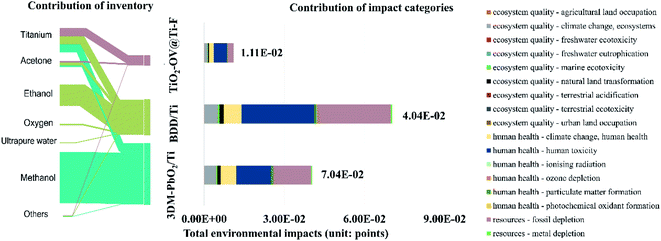 | ||
| Fig. 10 An analysis of the contributions of the inventory and the impact categories to the total environmental impacts of the anodic materials. | ||
4. Conclusions
These results demonstrated that the mineralization of SMR using our rotating TiO2-OV@Ti-F anode was enhanced, resulting in a substantial decrease in the effluent toxicity. The high oxidation ability was attributed to enhanced reactivity and an enhanced mass transfer coefficient. The mass transfer coefficient of the rotary TiO2-OV@Ti-F anode was 3.49 × 10−5 m s−1, which was ca. 320% higher than that of the conventional static one (8.3 × 10−6 m s−1). The estimated ΦBh value of the Ti-F anode was 2.91 eV, while that of the TiO2-OV@Ti-F anode was reduced to 2.57 eV, implying that electron transfer on the surface of the latter is easier. Furthermore, the rotary TiO2-OV@Ti-F anode showed a lower desorption energy for ˙OH and a larger active surface area, both of which favour the formation of ˙OH. The rotary configuration also allowed for the removal of gas bubbles from the surface of the anode, which improved the production of reactive species and at the same time reduced the EO energy consumption. The results obtained in this study provide not only a detailed description of the SMR degradation mechanisms via EO, but also the required information for the design and fabrication of efficient and environmentally friendly anodes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by the National Natural Science Foundation of China (No. 52000052, No. 52070056, and No. 62004143), the State Key Laboratory of Urban Water Resource and Environment (Harbin Institute of Technology, No. 2021TS26), the Central Government Guided Local Science, Technology Development Special Fund Project (No. 2020ZYYD033), the China Postdoctoral Science Foundation (No. 2020M681103), the Opening Fund of Key Laboratory for Green Chemical Process of Ministry of Education of Wuhan Institute of Technology (No. GCP202101), and the Fundamental Research Funds for the Central Universities (No. AUEA5750303621). We also appreciate support from Heilongjiang Province Touyan Team and the valuable suggestions of Prof. Andrew T. S. Wee from the National University of Singapore. This work is dedicated to celebrating the 50th anniversary of Wuhan Institute of Technology.References
- S. B. Kumar, S. R. Arnipalli and O. Ziouzenkova, Antibiotics in food chain: the consequences for antibiotic resistance, Antibiotics, 2020, 9, 688, DOI:10.3390/antibiotics9100688.
- M. Bilal, S. S. Ashraf, D. Barceló and H. M. N. Iqbal, Biocatalytic degradation/redefining "removal" fate of pharmaceutically active compounds and antibiotics in the aquatic environment, Sci. Total Environ., 2019, 691, 1190–1211, DOI:10.1016/j.scitotenv.2019.07.224.
- A. C. Reis, B. A. Kolvenbach, O. C. Nunes and P. F. X. Corvini, Biodegradation of antibiotics: The new resistance determinants - part I, New Biotechnol., 2020, 54, 34–51, DOI:10.1016/j.nbt.2019.08.002.
- N. H. Tran, H. Chen, M. Reinhard, F. Mao and K. Y. Gin, Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes, Water Res., 2016, 104, 461–472, DOI:10.1016/j.watres.2016.08.040.
- J. Chen and S. Xie, Overview of sulfonamide biodegradation and the relevant pathways and microorganisms, Sci. Total Environ., 2018, 640–641, 1465–1477, DOI:10.1016/j.scitotenv.2018.06.016.
- Z. Li, R. Dai, B. Yang, M. Chen, X. Wang and Z. Wang, An electrochemical membrane biofilm reactor for removing sulfonamides from wastewater and suppressing antibiotic resistance development: Performance and mechanisms, J. Hazard. Mater., 2021, 404, 112510–124198, DOI:10.1016/j.jhazmat.2020.124198.
- S. Qiu, W. Tang, S. Yang, J. Xie, D. Yu, O. Garcia-Rodriguez, J. Qu, S. Bai and F. Deng, A microbubble-assisted rotary tubular titanium cathode for boosting Fenton's reagents in the electro-Fenton process, J. Hazard. Mater., 2022, 424, 127403, DOI:10.1016/j.jhazmat.2021.127403.
- H. Yang, S. Zhuang, Q. Hu, L. Hu, L. Yang, C. Au and B. Yi, Competitive reactions of hydroxyl and sulfate radicals with sulfonamides in Fe2+/S2O82− system: Reaction kinetics, degradation mechanism and acute toxicity, Chem. Eng. J., 2018, 339, 32–41, DOI:10.1016/j.cej.2018.01.106.
- S. Wang and J. Wang, A novel strategy of successive non-radical and radical process for enhancing the utilization efficiency of persulfate, Chemosphere, 2020, 245, 125555–125576, DOI:10.1016/j.chemosphere.2019.125555.
- F. Zhu, J. Pan, Q. Zou, M. Wu, H. Wang and G. Xu, Electron beam irradiation of typical sulfonamide antibiotics in the aquatic environment: Kinetics, removal mechanisms, degradation products and toxicity assessment, Chemosphere, 2021, 274, 129713–129725, DOI:10.1016/j.chemosphere.2021.129713.
- Y. Ma, K. Zhang, C. Li, T. Zhang and N. Gao, Oxidation of sulfonamides in aqueous solution by UV-TiO2-Fe(VI), BioMed Res. Int., 2015, 2, 973942–974965, DOI:10.1155/2015/973942.
- H. Li, H. Jiang, C. Liu, C. Zhu and X. P. Zhu, Electrochemical oxidation of sulfonamides with Boron-doped Diamond and Pt anodes, Chemistryopen, 2019, 8, 1421–1428, DOI:10.1002/open.201900250.
- C. Comninellis, Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment, Electrochim. Acta, 1994, 39, 1857–1862, DOI:10.1016/0013-4686(94)85175-1.
- A. M. Zaky and B. P. Chaplin, Porous substoichiometric TiO2 anodes as reactive electrochemical membranes for water treatment, Environ. Sci. Technol., 2013, 47, 6554–6563, DOI:10.1021/es401287e.
- D. S. Carretero, C. Huang, J. Tzeng and C. Huang, The recovery of sulfuric acid from spent piranha solution over a dimensionally stable anode (DSA) Ti-RuO2 electrode, J. Hazard. Mater., 2021, 406, 124658–124678, DOI:10.1016/j.jhazmat.2020.124658.
- J. Radjenovic and D. L. Sedlak, Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water, Environ. Sci. Technol., 2015, 49, 11292–11302, DOI:10.1021/acs.est.5b02414.
- M. Pierpaoli, M. Szopińska, B. K. Wilk, M. Sobaszek, A. Łuczkiewicz, R. Bogdanowicz and S. Fudala-Książek, Electrochemical oxidation of PFOA and PFOS in landfill leachates at low and highly boron-doped diamond electrodes, J. Hazard. Mater., 2021, 403, 123606–123620, DOI:10.1016/j.jhazmat.2020.123606.
- Y. Shin, H. Yoo, Y. Ahn, M. S. Kim, K. Lee, S. Yu, C. Lee, K. Cho, H. Kim and J. Lee, Electrochemical oxidation of organics in sulfate solutions on boron-doped diamond electrode: Multiple pathways for sulfate radical generation, Appl. Catal., B, 2019, 254, 156–165, DOI:10.1016/j.apcatb.2019.04.060.
- S. Garcia-Segura, M. M. S. G. Eiband, J. V. de Melo and C. A. Martínez-Huitle, Electrocoagulation and advanced electrocoagulation processes: A general review about the fundamentals, emerging applications and its association with other technologies, J. Electroanal. Chem., 2017, 801, 267–299, DOI:10.1016/j.jelechem.2017.07.047.
- A. J. Dos Santos, S. Garcia-Segura, S. Dosta, I. G. Cano, C. A. Martínez-Huitle and E. Brillas, A ceramic electrode of ZrO2-Y2O3 for the generation of oxidant species in anodic oxidation. Assessment of the treatment of Acid Blue 29 dye in sulfate and chloride media, Sep. Purif. Technol., 2019, 228, 115747–115760, DOI:10.1016/j.seppur.2019.115747.
- E. Brillas, A. Thiam and S. Garcia-Segura, Incineration of acidic aqueous solutions of dopamine by electrochemical advanced oxidation processes with Pt and BDD anodes, J. Electroanal. Chem., 2016, 775, 189–197, DOI:10.1016/j.jelechem.2016.04.054.
- H. Monteil, Y. Péchaud, N. Oturan and M. A. Oturan, A review on efficiency and cost effectiveness of electro- and bio-electro-Fenton processes: Application to the treatment of pharmaceutical pollutants in water, Chem. Eng. J., 2019, 376, 119577–119599, DOI:10.1016/j.cej.2018.07.179.
- L. Gan, Y. Wu, H. Song, C. Lu, S. Zhang and A. Li, Self-doped TiO2 nanotube arrays for electrochemical mineralization of phenols, Chemosphere, 2019, 226, 329–339, DOI:10.1016/j.chemosphere.2019.03.135.
- X. Li, F. Ma, Y. Li, H. Zhang, J. Min, X. Zhang and H. Yao, Enhanced mechanisms of electrocatalytic-ozonation of ibuprofen using a TiO2 nanoflower-coated porous titanium gas diffuser anode: Role of TiO2 catalysts and electrochemical action in reactive oxygen species formation, Chem. Eng. J., 2020, 389, 124411–124425, DOI:10.1016/j.cej.2020.124411.
- O. Garcia-Rodriguez, E. Mousset, H. Olvera-Vargas and O. Lefebvre, Electrochemical treatment of highly concentrated wastewater: A review of experimental and modeling approaches from lab- to full-scale, Crit. Rev. Environ. Sci. Technol., 2020, 1–70, DOI:10.1080/10643389.2020.1820428.
- L. Wang, J. Lu, L. Li, Y. Wang and Q. Huang, Effects of chloride on electrochemical degradation of perfluorooctanesulfonate by Magnéli phase Ti4O7 and boron doped diamond anodes, Water Res., 2020, 170, 115254–115270, DOI:10.1016/j.watres.2019.115254.
- D. Huang, K. Wang, J. Niu, C. Chu, S. Weon, Q. Zhu, J. Lu, E. Stavitski and J. Kim, Amorphous Pd-loaded Ti4O7 electrode for direct anodic destruction of perfluorooctanoic acid, Environ. Sci. Technol., 2020, 54, 10954–10963, DOI:10.1021/acs.est.0c03800.
- M. Lin, D. M. Bulman, C. K. Remucal and B. P. Chaplin, Chlorinated byproduct formation during the electrochemical advanced oxidation process at magnéli phase Ti4O7 electrodes, Environ. Sci. Technol., 2020, 2, 12–34, DOI:10.1021/acs.est.0c03916.
- M. Lanzarini-Lopes, S. Garcia-Segura, K. Hristovski and P. Westerhoff, Electrical energy per order and current efficiency for electrochemical oxidation of p-chlorobenzoic acid with boron-doped diamond anode, Chemosphere, 2017, 188, 304–311, DOI:10.1016/j.chemosphere.2017.08.145.
- S. Almassi, Z. Li, W. Xu, C. Pu, T. Zeng and B. P. Chaplin, Simultaneous adsorption and electrochemical reduction of n-Nitrosodimethylamine using carbon-Ti4O7 composite reactive electrochemical membranes, Environ. Sci. Technol., 2019, 53, 928–937, DOI:10.1021/acs.est.8b05933.
- Y. Jing, S. Almassi, S. Mehraeen, R. J. LeSuer and B. P. Chaplin, The roles of oxygen vacancies, electrolyte composition, lattice structure, and doping density on the electrochemical reactivity of Magnéli phase TiO2 anodes, J. Mater. Chem. A, 2018, 6, 23828–23839, 10.1039/C8TA03719A.
- H. Malik, S. Sarkar, S. Mohanty and K. Carlson, Modelling and synthesis of Magnéli Phases in ordered titanium oxide nanotubes with preserved morphology, Sci. Rep., 2020, 10, 8050–8065, DOI:10.1038/s41598-020-64918-0.
- S. Zhang, M. Sun, T. Hedtke, A. Deshmukh, X. Zhou, S. Weon, M. Elimelech and J. Kim, Mechanism of heterogeneous Fenton reaction kinetics enhancement under nanoscale spatial confinement, Environ. Sci. Technol., 2020, 54, 10868–10875, DOI:10.1021/acs.est.0c02192.
- J. Teng, G. Liu, J. Liang and S. You, Electrochemical oxidation of sulfadiazine with titanium suboxide mesh anode, Electrochim. Acta, 2020, 331, 135441–135556, DOI:10.1016/j.electacta.2019.135441.
- Q. Wang, A. Zhang, P. Li, P. Héroux, H. Zhang, X. Yu and Y. Liu, Degradation of aqueous atrazine using persulfate activated by electrochemical plasma coupling with microbubbles: removal mechanisms and potential applications, J. Hazard. Mater., 2021, 403, 124087–124099, DOI:10.1016/j.jhazmat.2020.124087.
- J. A. Yáñez-Varela, A. Alonzo-Garcia, I. González-Neria, V. Mendoza-Escamilla, G. Rivadeneyra-Romero and S. A. Martínez-Delgadillo, Experimental and numerical evaluation of the performance of the electrochemical reactor operated with static and dynamic electrodes in the reduction of hexavalent chromium, Chem. Eng. J., 2020, 390, 124575, DOI:10.1016/j.cej.2020.124575.
- N. Nippatlapalli and L. Philip, Assessment of novel rotating bipolar multiple disc electrode electrocoagulation-flotation and pulsed plasma corona discharge for the treatment of textile dyes, Water Sci. Technol., 2020, 81, 564–570, DOI:10.2166/wst.2020.137.
- C. Yang, Y. He, K. Li, Y. Yao, R. Cao, Y. Wang and J. Jia, Degrading organic pollutants and generating electricity in a dual-chamber rotating-disk photocatalytic fuel cell (RPFC) with a TiO2 nanotube array anode, Res. Chem. Intermed., 2015, 41, 5365–5377, DOI:10.1007/s11164-014-1637-2.
- T. Fan, Y. Cai, G. Chu, Y. Luo, L. Zhang and J. Chen, A novel rotating multielectrodes reactor for electrochemical oxidation process intensification, Ind. Eng. Chem. Res., 2019, 58, 2396–2404, DOI:10.1021/acs.iecr.8b05736.
- N. Sherborne and N. Galic, Modeling sublethal effects of chemicals: application of a simplified dynamic energy budget model to standard ecotoxicity data, Environ. Sci. Technol., 2020, 54, 7420–7429, DOI:10.1021/acs.est.0c00140.
- A. Farhat, Removal of persistent organic contaminants by electrochemically activated sulfate, Environ. Sci. Technol., 2015, 49, 14326–14333, DOI:10.1021/acs.est.5b02705.
- F. Deng, H. Olvera-Vargas, O. Garcia-Rodriguez, S. Qiu, F. Ma, Z. Chen and O. Lefebvre, Unconventional electro-Fenton process operating at a wide pH range with Ni foam cathode and tripolyphosphate electrolyte, J. Hazard. Mater., 2020, 396, 122641–122655, DOI:10.1016/j.jhazmat.2020.122641.
- F. Deng, S. Li, M. Zhou, Y. Zhu, S. Qiu, K. Li, F. Ma and J. Jiang, A biochar modified nickel-foam cathode with iron-foam catalyst in electro-Fenton for sulfamerazine degradation, Appl. Catal., B, 2019, 256, 111789–117796, DOI:10.1016/j.apcatb.2019.117796.
- F. Deng, H. Olvera-Vargas, O. Garcia-Rodriguez, Y. Zhu, J. Jiang, S. Qiu and J. Yang, Waste-wood-derived biochar cathode and its application in electro-Fenton for sulfathiazole treatment at alkaline pH with pyrophosphate electrolyte, J. Hazard. Mater., 2019, 377, 249–258, DOI:10.1016/j.jhazmat.2019.05.077.
- F. Deng, H. Olvera-Vargas, O. Garcia-Rodriguez, S. Qiu, F. Ma, Z. Chen and O. Lefebvre, Unconventional electro-Fenton process operating at a wide pH range with Ni foam cathode and tripolyphosphate electrolyte, J. Hazard. Mater., 2020, 396, 122641–122655, DOI:10.1016/j.jhazmat.2020.122641.
- K. B. Ibrahim, W. Su, M. Tsai, S. A. Chala, A. W. Kahsay, M. Yeh, H. Chen, A. D. Duma, H. Dai and B. Hwang, Robust and conductive Magnéli Phase Ti4O7 decorated on 3D-nanoflower NiRu-LDH as high-performance oxygen reduction electrocatalyst, Nano Energy, 2018, 47, 309–315, DOI:10.1016/j.nanoen.2018.03.017.
- S. Selcuk, X. Zhao and A. Selloni, Structural evolution of titanium dioxide during reduction in high-pressure hydrogen, Nat. Mater., 2018, 17, 923–928, DOI:10.1038/s41563-018-0135-0.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman, and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- X. Zhang, J. Jiang, A. A. Suleiman, B. Jin, X. Hu, X. Zhou and T. Zhai, Hydrogen-assisted growth of ultrathin Te flakes with giant gate-dependent photoresponse, Adv. Funct. Mater., 2019, 29, 1906585–1906599, DOI:10.1002/adfm.201906585.
- J. R. Smith, F. C. Walsh and R. L. Clarke, Electrodes based on Magnéli phase titanium oxides: the properties and applications of Ebonex® materials, J. Appl. Electrochem., 1998, 28, 1021–1033, DOI:10.1023/a:1003469427858.
- Z. Chen, W. Lai, Y. Xu, G. Xie, W. Hou, P. Zhanchang, C. Kuang and Y. Li, Anodic oxidation of ciprofloxacin using different graphite felt anodes: Kinetics and degradation pathways, J. Hazard. Mater., 2021, 405, 124262–124286, DOI:10.1016/j.jhazmat.2020.124262.
- J. Qu, Y. Liu, L. Cheng, Z. Jiang, G. Zhang, F. Deng, L. Wang, W. Han and Y. Zhang, Green synthesis of hydrophilic activated carbon supported sulfide nZVI for enhanced Pb(II) scavenging from water: Characterization, kinetics, isotherms and mechanisms, J. Hazard. Mater., 2021, 403, 123607–123634, DOI:10.1016/j.jhazmat.2020.123607.
- O. Garcia-Rodriguez, Y. Y. Lee, H. Olvera-Vargas, F. Deng, Z. Wang and O. Lefebvre, Mineralization of electronic wastewater by electro-Fenton with an enhanced graphene-based gas diffusion cathode, Electrochim. Acta, 2018, 276, 12–20, DOI:10.1016/j.electacta.2018.04.076.
- H. Shen, M. Lin, L. Wang, Z. Huang, X. Wu, X. Jiang, Q. Li, C. Chen, J. Zhao, G. Jing and C. Yuan, Experimental and theoretical investigation of the enhancement of the photo-oxidation of Hg0 by CeO2-modified morphology-controlled anatase TiO2, J. Hazard. Mater., 2021, 406, 124535–124550, DOI:10.1016/j.jhazmat.2020.124535.
- J. Maragatha, C. Rani, S. Rajendran and S. Karuppuchamy, Microwave synthesis of nitrogen doped Ti4O7 for photocatalytic applications, Phys. E, 2017, 93, 78–82, DOI:10.1016/j.physe.2017.05.020.
- L. Hao, K. Miyazawa, H. Yoshida and Y. Lu, Visible-light-driven oxygen vacancies and Ti3+ co-doped TiO2 coatings prepared by mechanical coating and carbon reduction, Mater. Res. Bull., 2018, 97, 13–18, DOI:10.1016/j.materresbull.2017.08.023.
- D. Kundu, R. Black, E. J. Berg and L. F. Nazar, A highly active nanostructured metallic oxide cathode for aprotic Li-O2 batteries, Energy Environ. Sci., 2015, 8, 1292–1298, 10.1039/C4EE02587C.
- K. Gurung, M. C. Ncibi, M. Shestakova and M. Sillanpää, Removal of carbamazepine from MBR effluent by electrochemical oxidation (EO) using a Ti/Ta2O5-SnO2 electrode, Appl. Catal., B, 2018, 221, 329–338, DOI:10.1016/j.apcatb.2017.09.017.
- X. Chen, L. Liu, P. Y. Yu and S. S. Mao, Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals, Science, 2011, 331, 746–758, DOI:10.1126/science.1200448.
- E. McCafferty and J. P. Wightman, Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative XPS method, Surf. Interface Anal., 1998, 26, 549–564, DOI:10.1002/(SICI)1096-9918(199807)26:8.
- H. Wang, D. Yong, S. Chen, S. Jiang, X. Zhang, W. Shao, Q. Zhang, W. Yan, B. Pan and Y. Xie, Oxygen-vacancy-mediated exciton dissociation in BiOBr for boosting charge-carrier-involved molecular oxygen activation, J. Am. Chem. Soc., 2018, 140, 1760–1766, DOI:10.1021/jacs.7b10997.
- F. Shahzad, S. A. Zaidi and C. M. Koo, Highly sensitive electrochemical sensor based on environmentally friendly biomass-derived sulfur-doped graphene for cancer biomarker detection, Sens. Actuators, B, 2017, 241, 716–724, DOI:10.1016/j.snb.2016.10.144.
- N. Oturan, S. O. Ganiyu, S. Raffy and M. A. Oturan, Sub-stoichiometric titanium oxide as a new anode material for electro-Fenton process: Application to electrocatalytic destruction of antibiotic amoxicillin, Appl. Catal., B, 2017, 217, 214–223, DOI:10.1016/j.apcatb.2017.05.062.
- E. Bocos, N. Oturan, M. Á. Sanromán and M. A. Oturan, Elimination of radiocontrast agent Diatrizoic acid from water by electrochemical advanced oxidation: Kinetics study, mechanism and mineralization pathway, J. Electroanal. Chem., 2016, 772, 1–8, DOI:10.1016/j.jelechem.2016.04.011.
- H. Olvera-Vargas, J. Rouch, C. Coetsier, M. Cretin and C. Causserand, Dynamic cross-flow electro-Fenton process coupled to anodic oxidation for wastewater treatment: Application to the degradation of acetaminophen, Sep. Purif. Technol., 2018, 203, 143–151, DOI:10.1016/j.seppur.2018.03.063.
- A. Kapałka, G. Fóti and C. Comninellis, Kinetic modelling of the electrochemical mineralization of organic pollutants for wastewater treatment, J. Appl. Electrochem., 2008, 38, 7–16, DOI:10.1007/s10800-007-9365-6.
- J. Wang, T. Li, M. Zhou, X. Li and J. Yu, Characterization of hydrodynamics and mass transfer in two types of tubular electrochemical reactors, Electrochim. Acta, 2015, 173, 698–704, DOI:10.1016/j.electacta.2015.05.135.
- M. C. Santos, Y. A. Elabd, Y. Jing, B. P. Chaplin and L. Fang, Highly porous Ti4O7 reactive electrochemical water filtration membranes fabricated via electrospinning/electrospraying, AIChE J., 2016, 62, 508–524, DOI:10.1002/aic.15093.
- E. Goiri, P. Borghetti, A. El-Sayed, J. E. Ortega and D. G. de Oteyza, Multi-component organic layers on metal substrates, Adv. Mater., 2016, 28, 1340–1368, DOI:10.1002/adma.201503570.
- D. Bejan, E. Guinea and N. J. Bunce, On the nature of the hydroxyl radicals produced at boron-doped diamond and Ebonex® anodes, Electrochim. Acta, 2012, 69, 275–281, DOI:10.1016/j.electacta.2012.02.097.
- S. Siahrostami and A. Vojvodic, Influence of adsorbed water on the oxygen evolution reaction on oxides, J. Phys. Chem. C, 2015, 119, 1032–1037, DOI:10.1021/jp508932x.
- R. Jaimes, J. Vazquez-Arenas, I. González and M. Galván, Theoretical evidence of the relationship established between the HO radicals and H2O adsorptions and the electroactivity of typical catalysts used to oxidize organic compounds, Electrochim. Acta, 2017, 229, 345–351, DOI:10.1016/j.electacta.2017.01.120.
- H. Shi, Y. Wang, C. Li, R. Pierce, S. Gao and Q. Huang, Degradation of perfluorooctanesulfonate by reactive electrochemical membrane composed of magnéli Phase titanium suboxide, Environ. Sci. Technol., 2019, 53, 14528–14537, DOI:10.1021/acs.est.9b04148.
- Q. Wang, A. Zhang, P. Li, P. Héroux, H. Zhang, X. Yu and Y. Liu, Degradation of aqueous atrazine using persulfate activated by electrochemical plasma coupling with microbubbles: removal mechanisms and potential applications, J. Hazard. Mater., 2021, 403, 124087–124099, DOI:10.1016/j.jhazmat.2020.124087.
- H. Matsushima, T. Iida and Y. Fukunaka, Observation of bubble layer formed on hydrogen and oxygen gas-evolving electrode in a magnetic field, J. Solid State Electrochem., 2012, 16, 617–623, DOI:10.1007/s10008-011-1392-x.
- H. Matsushima, T. Iida and Y. Fukunaka, Gas bubble evolution on transparent electrode during water electrolysis in a magnetic field, Electrochim. Acta, 2013, 100, 261–264, DOI:10.1016/j.electacta.2012.05.082.
- F. Deng, H. Olvera-Vargas, O. Garcia-Rodriguez, S. Qiu, J. Yang and O. Lefebvre, The synergistic effect of nickel-iron-foam and tripolyphosphate for enhancing the electro-Fenton process at circum-neutral pH, Chemosphere, 2018, 201, 687–696, DOI:10.1016/j.chemosphere.2018.02.186.
- M. F. Silva Barni, L. I. Doumic, R. A. Procaccini, M. A. Ayude and H. E. Romeo, Layered platforms of Ti4O7 as flow-through anodes for intensifying the electro-oxidation of bentazon, J. Environ. Manage., 2020, 263, 110403–110423, DOI:10.1016/j.jenvman.2020.110403.
- X. Li, H. Xu and W. Yan, Fabrication and characterization of a PbO2-TiN composite electrode by Co-deposition method, J. Electrochem. Soc., 2016, 163–178 DOI:10.1149/2.0261610JES.
- Y. Jing, S. Almassi, S. Mehraeen, R. J. LeSuer and B. P. Chaplin, The roles of oxygen vacancies, electrolyte composition, lattice structure, and doping density on the electrochemical reactivity of Magnéli phase TiO2 anodes, J. Mater. Chem. A, 2018, 6, 23828–23839, 10.1039/C8TA03719A.
- D. Li, S. Wang, Y. Tian, H. Ma, C. Ma, Y. Fu and X. Dong, Preparation and photoelectrocatalytic performance of Ti/PbO2 electrodes modified with Ti4O7, Chemistryselect, 2018, 3, 5098–5105, DOI:10.1002/slct.201703181.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ta08095d |
| This journal is © The Royal Society of Chemistry 2022 |