Violet phosphorus quantum dots†
Rongzheng
Zhao
,
Shuhao
Liu
,
Xuewen
Zhao
,
Mengyue
Gu
,
Yuhao
Zhang
,
Mengting
Jin
,
Yanhao
Wang
,
Yonghong
Cheng
and
Jinying
Zhang
 *
*
State Key Laboratory of Electrical Insulation and Power Equipment, Center of Nanomaterials for Renewable Energy (CNRE), School of Electrical Engineering, Xi'an Jiaotong University, Xi'an 710049, China. E-mail: jinying.zhang@mail.xjtu.edu.cn
First published on 22nd November 2021
Abstract
Violet phosphorus is another promising layered semiconducting elemental structure for electronic and optoelectronic applications. Violet phosphorus quantum dots (VPQDs) have unique optical and electronic properties due to quantum mechanics. However, the VPQDs are still unexplored since the structure of violet phosphorus and preparation of violet phosphorene have just been confirmed recently. The preparation and property investigation of VPQDs are urgent. A simple solvothermal method has been developed to effectively synthesize VPQDs. The size and fluorescence intensity of VPQDs are adjustable according to solvothermal parameters. An average lateral size of 3.16 ± 0.78 nm and an average height of 1.81 ± 0.57 nm (monolayer) have been obtained by the as-produced VPQDs, giving intense green fluorescence with excellent optical stability under conditions such as UV light irradiation, ionic strength, and pH environments. The VPQDs have also been demonstrated to be an effective fluorescent probe to selectively detect Cu2+ ions in the organic phase with a linear range of 10–300 μg mL−1 and a detection limit as low as 19.6 nM. The VPQDs are also promising nanostructures for photovoltaic materials, photocatalysts, photodetectors, or labeling live biological materials.
Introduction
Violet phosphorus (VP) has recently been demonstrated to be a more stable layered semiconducting phosphorus allotrope than black phosphorus.1 Violet phosphorus has a layer-dependent tunable band gap from 1.68 eV of bulk violet phosphorus to 2.02 eV of single layer violet phosphorene.2 Zero-dimensional (0D) quantum dots, another form of nanomaterials in addition to two-dimensional (2D) structures, have excellent electronic and optical properties due to their quantum confinement and edge effects. To date, graphene, molybdenum disulfide and black phosphorous quantum dots have been successfully prepared and used in photovoltaic devices,3 solar cells and biomedicine.4,5 Larger bandgaps with strong emission at visible wavelengths have been obtained by black phosphorus quantum dots (QDs).6 The preparation and properties of violet phosphorous quantum dots (VPQDs) are still unexplored since the lattice structure of violet phosphorus has just recently been confirmed by single crystal X-ray diffraction experiments.1 The VPQDs are promising candidates for photovoltaic materials, photocatalysts, photodetectors, or labeling live biological materials.Fluorescent probes for metal ion detection are one of the simplest applications of VPQDs. A series of QDs, such as carbon QDs,7 CdSe QDs,8 CdS QDs, and black phosphorus QDs, have been developed to detect metal ions.9,10 Copper is the third most abundant transition metal in the world. Cu2+ ions play various important roles in many physiological processes11–13 as an important substance in the human body.14,15 Additionally, it plays an important role in agriculture and industry since copper pollution will still be a very difficult problem in the future. Therefore, the development of a stable, reliable, and sensitive QD fluorescent probe to detect Cu2+ is urgent.
Herein, we for the first time report the synthesis of VPQDs based on a facile solvothermal method. An average lateral size of 3.16 ± 0.78 nm and an average height of 1.81 ± 0.57 nm (monolayer) have been obtained for the VPQDs by optimizing the synthesis parameters. A strong fluorescence band centered at green wavelength with excellent stability has been obtained by the VPQDs. The VPQDs have also been found to be effective fluorescent probes to detect Cu2+ in organic solutions with high sensitivity and selectivity.
Experimental
Preparation of VPQDs
Violet phosphorus polycrystals were produced by a chemical vapor transport method.1 A small amount of ground violet phosphorus (15.0 mg) was dispersed in 150 mL N-methyl-2-pyrrolidinone (NMP, AR, Shanghai Yien Chemical Technology) by ultrasonication in an ice bath at 500 W for 2 h. The violet phosphorus suspension (30 mL) was then transferred to a 50 mL Teflon-lined reactor under different conditions for solvothermal reaction. The suspension after the reaction was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min to obtain well dispersed VPQDs.
000 rpm for 20 min to obtain well dispersed VPQDs.
Detection of Cu2+ ions
The VPQDs in NMP (0.1 mg mL−1) prepared by solvothermal reaction at 150 °C for 24 h were adopted for metal ion detection. And 20 μL CuCl2 solution (AR, Aladdin) with different concentrations was introduced into 2 mL VPQDs in NMP, respectively. The mixtures were shaken and equilibrated for 10 min and then measured to obtain the corresponding fluorescence spectra. Different ions (300 μg mL−1) including Al3+, Ca2+, Fe3+, K+, Mg2+, Mn2+, Na+, Co2+, Cd2+, and Zn2+ in addition to Cu2+, by using their corresponding chlorides (AR, Aladdin), were adopted to measure the selective ion detection of the fluorescent probes.Results and discussion
The VPQDs were prepared by a solvothermal method16–21 in NMP. The van der Waals forces between different layers were easily overcome by boiling NMP, which intercalated into the interlayer gaps of violet phosphorus at high temperature. The lone electron pairs from the exposed phosphorus atoms with low steric hindrance were also easily shared with the electron acceptors from the solvent. The lone electron pairs are easily shared with oxygen from NMP, resulting in increasing defects of violet phosphorus. The functional groups are like “scissors” to further cut the violet phosphorene into VPQDs during solvothermal reaction. The VPQD suspension was homogeneously dispersed in NMP after centrifugation and collected for characterization and fluorescence measurements. Different solvothermal temperatures and times were studied to yield VPQDs with different sizes and fluorescence.Characterization of VPQDs
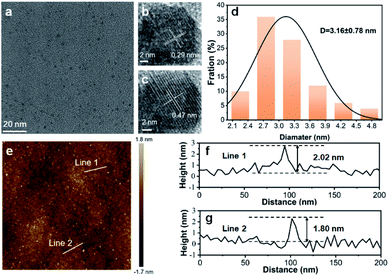
The as-prepared VPQDs (solvothermal temperature 150 °C, solvothermal time 24 h) were characterized by transmission electron microscopy (TEM) and atomic force microscopy (AFM). A uniform morphology was observed for the as-produced VPQDs by TEM (Fig. 1a). The average lateral size of the VPQDs was found to be 3.16 ± 0.78 nm (Fig. 1d) in statistics. The interplanar crystal spacings of 0.29 nm and 0.47 nm were easily observed from the high-resolution TEM (HRTEM) images of VPQDs (Fig. S1b and c†), which correspond to the {31![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) } and {014} planes of violet phosphorus crystals, respectively. The height of the as-produced VPQDs was measured by AFM (Fig. 1e). The representative heights of the VPQDs along lines 1 and 2 were measured to be around 1.80–2.02 nm (Fig. 1f and g), which are similar to the height of monolayer violet phosphorene, where the thickness of monolayer violet phosphorus is 1.1 nm with a gap between the substrate and violet phosphorene of about 0.7–0.9 nm. Analogous to the lateral size, the average height of the as-prepared VPQDs was deduced to be 1.81 ± 0.57 nm in statistics, indicating that the VPQDs are monolayered structures.
} and {014} planes of violet phosphorus crystals, respectively. The height of the as-produced VPQDs was measured by AFM (Fig. 1e). The representative heights of the VPQDs along lines 1 and 2 were measured to be around 1.80–2.02 nm (Fig. 1f and g), which are similar to the height of monolayer violet phosphorene, where the thickness of monolayer violet phosphorus is 1.1 nm with a gap between the substrate and violet phosphorene of about 0.7–0.9 nm. Analogous to the lateral size, the average height of the as-prepared VPQDs was deduced to be 1.81 ± 0.57 nm in statistics, indicating that the VPQDs are monolayered structures.
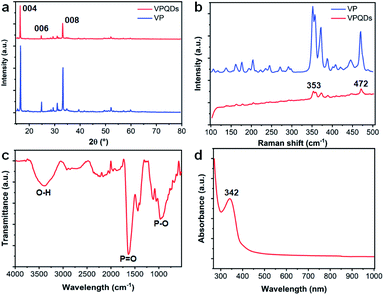
It was further confirmed by X-ray diffraction (XRD) that the VPQDs maintain the crystal structure of violet phosphorus. The VPQDs have strong 2θ diffraction peaks at 16.2°, 24.5° and 32.9° (Fig. 2a, red) corresponding to the {004}, {006} and {008} reflections of violet phosphorus, which are well consistent with those of bulk violet phosphorus (Fig. 2a, blue). Slightly blue shifts were observed for the XRD features of VPQDs, which are due to the size effects.
 | ||
| Fig. 2 (a) XRD patterns of VPQDs and bulk violet phosphorus. (b) Raman spectra of VPQDs and bulk violet phosphorus. (c) FT-IR spectrum of VPQDs. (d) UV-Vis absorption spectrum of VPQDs in NMP. | ||
Raman spectroscopy was also used to characterize the VPQDs (Fig. 2b, red). Four characteristic peaks at 353.2 (S2[P9]), 359.6 (S1[P8]), 372.6 (S2[P8]), and 471.9 cm−1 (Tg) with much weaker intensities than those from the bulk ones were observed for the VPQDs under ambient conditions. Additionally, the intensity ratio of the stretching vibrational modes of [P9] (353.2 cm−1, S2[P9]) to the tangential stretching mode of [P9] along the tubular axis (471.9 cm−1, Tg) was observed to change from 1.61 (bulk VP) to 1.86 (VPQDs). The stretching vibrational modes of [P9] are affected by the surrounding molecules more significantly than the tangential stretching mode of [P9] along the tubular axis after quantum size effects.
Strong Fourier transform infrared (FT-IR) peaks at 2358, 1661, 1140, and 1047 cm−1 were also observed for the VPQDs (Fig. 2c). The strong peak at 1047 cm−1 is attributed to the stretching vibration of the P–O–C bond.22 The strong FT-IR peaks at 1661 and 1140 cm−1 are attributed to the bending and stretching vibrations of the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, respectively.23 Additionally, the stretching vibration of the P–OH bond was found to have a broad absorption band at 2358 cm−1, indicating that the electron-donating functional groups were formed on the periphery and surface of VPQDs. Two weak peaks at 2922 cm−1 and 2854 cm−1 corresponding to the C–H stretching vibration of the alkyl group were also observed, indicating that a small amount of NMP was introduced into VPQDs. The peak at 3405 cm−1 is attributed to the O–H stretching vibration.24,25 The FT-IR spectrum of VPQDs indicates that the solvent molecules were introduced to connect with the phosphorus atoms (with exposed lone electron pairs) of violet phosphorus.
O bond, respectively.23 Additionally, the stretching vibration of the P–OH bond was found to have a broad absorption band at 2358 cm−1, indicating that the electron-donating functional groups were formed on the periphery and surface of VPQDs. Two weak peaks at 2922 cm−1 and 2854 cm−1 corresponding to the C–H stretching vibration of the alkyl group were also observed, indicating that a small amount of NMP was introduced into VPQDs. The peak at 3405 cm−1 is attributed to the O–H stretching vibration.24,25 The FT-IR spectrum of VPQDs indicates that the solvent molecules were introduced to connect with the phosphorus atoms (with exposed lone electron pairs) of violet phosphorus.
The VPQDs were also detected to have a strong absorption peak located at 342 nm and another strong absorption peak lower than 300 nm (Fig. 2d). The absorption of VPQDs is located in the UV region, further confirming the sp3 hybridization of phosphorus in VPQDs.
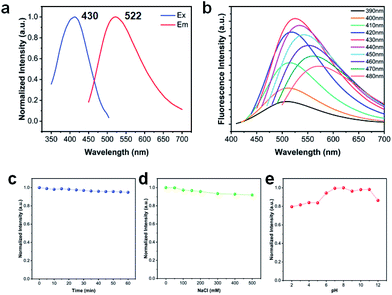
Strong fluorescence was obtained from VPQDs in NMP solution. The emission spectra were confirmed to be from VPQDs instead of NMP solvent by comparing the fluorescence spectra of VPQDs in NMP (Fig. S1a,† solid line) and NMP solvent (Fig. S1a,† dashed line) at different excitation wavelengths (Fig. S1†), where the fluorescence of NMP is much weaker than the emission of VPQDs in the visible range. The emission of NMP solvent was also observed to decrease with increasing excitation wavelength (Fig. S1†). The fluorescence of NMP totally disappears with an excitation wavelength longer than 430 nm. Therefore, 430 nm was adopted as the excitation wavelength since only emission from VPQDs was observed from the fluorescence spectra of VPQDs in NMP. A strong fluorescence band centered around 522 nm was obtained for the VPQDs with an excitation wavelength of 430 nm (Fig. 3a). The emission band center of VPQDs was also found to be slightly shifted at different excitation wavelengths (Fig. 3b), which is due to the different resonance of VPQDs with different bandgaps. The results are well consistent with the common phenomenon of various QDs due to broad size distribution, such as carbon dots, black phosphorus QDs, and MoS2 QDs.26–28
The strong green light emission of VPQDs is different from those of reported QDs with emission in the blue to purple range, such as carbon QDs (430 nm), black phosphorus QDs (450 nm) and MoS2 QDs (414 nm).6,29,30 The green fluorescence of VPQDs is preferred by bioimaging applications since the luminescence of biological tissues is mainly in the blue range.31 Nevertheless, the penetration of blue to violet light into biotissues is poor,32 which limits the optical imaging of deep tissue in vivo to hinder its biological applications. In addition, the absolute quantum efficiency of fluorescence is measured by using a FLS980 fluorescence spectrometer with a fluorescence quantum yield of 7.02%.
The time-resolved emission spectra of VPQDs were also studied to further understand the fluorescence properties of VPQDs (Fig. S2†). The time-resolved emission spectrum was fitted using a three-exponential function, I(t) = A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2
exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) + A3
exp(−t/τ2) + A3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ3), where A1, A2 and A3 are the fractional contributions of the fluorescence lifetimes τ1, τ2, and τ3, respectively. The excited state lifetimes of VPQDs with an excitation wavelength of 430 nm were calculated to be 1.60 ns (7.70%), 6.38 ns (53.40%), and 16.78 ns (38.90%), respectively. The short lifetime (1.60 ns) is related to the eigenstate of VPQDs, while the long lifetimes (6.38 ns and 16.78 ns) correspond to the surface state of VPQDs.33 The average lifetime of VPQDs was calculated to be 10.06 ns. Strong emission in the green light range with an average lifetime of 10.06 ns has been obtained from VPQDs. The averaged lifetime of VPQDs is longer than that of most reported QDs, facilitating bioimaging applications to remove background interference, where the lifetime of carbon QDs (6.76 ns) and MoS2 QDs (4.66 ns) is comparable to the decay time of autofluorescence of many biological samples.29,30
exp(−t/τ3), where A1, A2 and A3 are the fractional contributions of the fluorescence lifetimes τ1, τ2, and τ3, respectively. The excited state lifetimes of VPQDs with an excitation wavelength of 430 nm were calculated to be 1.60 ns (7.70%), 6.38 ns (53.40%), and 16.78 ns (38.90%), respectively. The short lifetime (1.60 ns) is related to the eigenstate of VPQDs, while the long lifetimes (6.38 ns and 16.78 ns) correspond to the surface state of VPQDs.33 The average lifetime of VPQDs was calculated to be 10.06 ns. Strong emission in the green light range with an average lifetime of 10.06 ns has been obtained from VPQDs. The averaged lifetime of VPQDs is longer than that of most reported QDs, facilitating bioimaging applications to remove background interference, where the lifetime of carbon QDs (6.76 ns) and MoS2 QDs (4.66 ns) is comparable to the decay time of autofluorescence of many biological samples.29,30
The fluorescence stability of VPQDs according to UV light irradiation, ionic strength, and pH environments was also investigated for potential biological applications. The fluorescence intensity of VPQDs after continuous irradiation using a UV lamp (365 nm) for 60 min was measured to be 94% of that before irradiation (Fig. 3c), indicating that the as-produced VPQDs have good UV bleaching stability. The fluorescence intensity of VPQDs with the addition of 500 mM NaCl was measured to be still about 90% of that of the intrinsic VPQDs (Fig. 3d), indicating good ion resistance stability of VPQDs. The fluorescence intensity of VPQDs was found to be almost constant under neutral or weak basic conditions (Fig. 3e). More than 80% of the fluorescence intensity of VPQDs was found to be maintained even under strong acid and base conditions, indicating good pH resistance stability. The fluorescence of the as-produced VPQDs has been demonstrated to have good stability under different conditions, suggesting that VPQDs are promising structures for biosensing and biomedicine.
The influence of solvothermal conditions on VPQDs
The effect of solvothermal temperature and time on the morphology of the as-prepared VPQDs was also investigated. The average lateral diameters of VPQDs prepared by solvothermal reaction at 150 °C, 180 °C and 210 °C for 12 h were measured to be 13.39, 6.91 and 8.07 nm, respectively, in statistics (Fig. S3†). The average particle size of VPQDs was found to decrease with increasing temperature from 150 °C to 180 °C and then increase with further increasing temperature (Fig. 4a, blue). The average heights of the as-produced VPQDs at 150, 180, and 210 °C for 12 h were measured to be 2.31, 1.89, and 2.07 nm, respectively (Fig. S5†). The average height of the VPQDs was also found to reach minimum at the solvothermal temperature of 180 °C (Fig. 4a, green), indicating that increasing the solvothermal temperature will also cause the aggregation of VPQDs.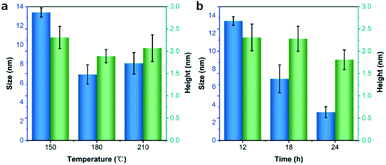 | ||
| Fig. 4 The size and height of VPQDs prepared at (a) different solvothermal temperatures and (b) different solvothermal time. | ||
The effect of solvothermal reaction time on the particle size of VPQDs at 150 °C was also studied. The statistical particle sizes of VPQDs were found to be 13.39 nm, 7.40 nm, and 3.16 nm for 12 h, 18 h, and 24 h, respectively (Fig. S4†). The average lateral size of VPQDs was found to decrease from 13.39 nm to 3.16 nm with increasing solvothermal reaction time from 12 h to 24 h (Fig. 4b, blue). The average heights of the as-prepared VPQDs at 150 °C for 12 h, 18 h, and 24 h were measured to be 2.31, 2.28, and 1.81 nm, respectively (Fig. S6†). The height of the VPQDs was also found to gradually decrease with increasing reaction time from 12 h to 24 h (Fig. 4b, green). The morphology of the VPQDs has been demonstrated to be easily tunable by adjusting the solvothermal reaction temperature and time, facilitating subsequent research on the thermodynamic, electrical, magnetic, optical and catalytic properties of VPQDs of different sizes. The solvothermal reaction at the temperature at 150 °C for long reaction time has been preferred to obtain VPQDs with small size.
The solvothermal reaction conditions have been demonstrated to tune the lateral size and thickness of the as-produced VPQDs, resulting in tunable fluorescence properties of VPQDs (Fig. S7†). The optimized fluorescence (∼520 nm) intensity of the as-prepared VPQDs upon excitation at 430 nm (Fig. 5a) was found to increase with increasing solvothermal temperature from 150 °C to 180 °C and then slightly decrease with further increasing solvothermal temperature, well consistent with the size and thickness tuning results. Additionally, the suitable reaction temperature might also promote the rearrangement of surface atoms, leading to the reduction of surface defects.34 High reaction temperature leads to faster reaction rates, resulting in the formation of a large number of non-radiative defects.35 The fluorescence spectra of the as-prepared VPQDs with different solvothermal times are shown in Fig. S8.† The optimized fluorescence intensity was found to reach maximum with a solvothermal reaction time of 18 h at 150 °C (Fig. 5b). However, the VPQDs from reaction (150 °C, 24 h) were found to have strong fluorescence with broad excitation wavelengths (Fig. S8c†), indicating a concentrated size distribution in a wider range. So the appropriate complete reaction time to produce VPQDs at 150 °C in NMP is about 24 h, well consistent with the QD preparation reaction requirements.36 The fluorescence of VPQDs has been demonstrated to be tunable by adjusting their solvothermal reaction conditions.
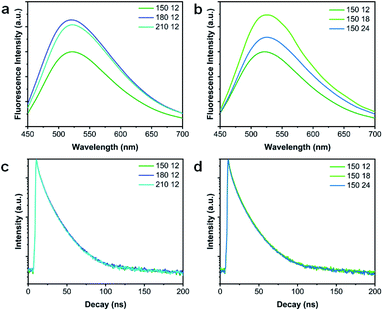 | ||
| Fig. 5 Fluorescence spectra of VPQDs at different preparation (a) temperatures and (b) times. Fluorescence decay curves of VPQDs at different preparation (c) temperatures and (d) time. | ||
The time-resolved emission spectra of the as-prepared VPQDs under different solvothermal conditions were also fitted using a three-exponential function (Fig. 5c and d). The fluorescence lifetime components and proportions of VPQDs are shown in Tables S1 and S2.† The lifetimes of VPQDs prepared under different solvothermal reaction conditions are not obviously different from each other with a value around 10 ns. The VPQDs prepared at 180 °C for 12 h were observed to have a slightly longer lifetime (10.19 ns). The VPQDs prepared at 150 °C for 18 h was demonstrated to have an even longer lifetime (10.44 ns).
Fluorescent probes for metal ion detection
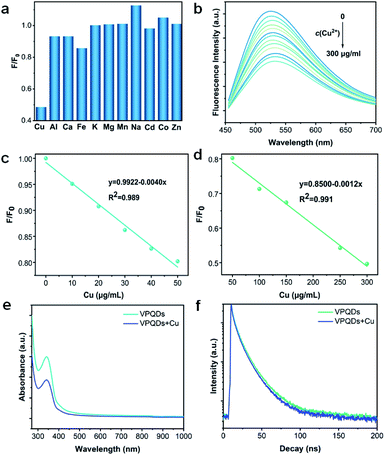
The VPQDs were then investigated as fluorescent probes for the detection of a series of metal ions (Cu2+, Al3+, Ca2+, Fe3+, K+, Mg2+, Mn2+, Na+, Co2+, Cd2+, and Zn2+) under the same conditions. Selective detection of Cu2+ was achieved using VPQDs (Fig. 6a). The fluorescence intensity of VPQDs was observed to be significantly quenched by the addition of Cu2+ compared to other metal ions. Different concentrations of Cu2+ were also added in VPQDs solution to investigate its detection sensitivity (Fig. 6b).The fluorescence intensity of VPQDs was observed to decrease monotonically at 520 nm with increasing concentration of Cu2+ in two interval ranges of 10–50 μg mL−1 (Fig. 6c) and 50–300 μg mL−1 (Fig. 6d) with linear correlation coefficients (R2) of 0.989 and 0.991, respectively. The limit of detection (LOD) was then calculated to be 0.0196 μM according to the triple standard deviation rule (S/N = 3),37 which is even more sensitive than that of metal sulfide,8,38,39 carbon,40 or black phosphorus QDs (Table S3†).10 The VPQDs have been demonstrated to be promising fluorescent probes for the detection of Cu2+.
The fluorescence quenching effects of Cu2+ to VPQDs is attributed to the interaction between VPQDs and Cu2+, where dynamic quenching and static quenching are two common modes. UV-Vis spectrophotometric analysis was adopted to distinguish between two quenching modes. The absorption intensity of the VPQDs was significantly reduced after the addition of Cu2+ (Fig. 6e), suggesting that the quenching mechanism between VPQDs and Cu2+ belongs to the static quenching mode.41 Time-resolve fluorescence spectroscopy was also adopted to further confirm the quenching mechanism. The lifetime was determined to be 9.92 ns after the addition of Cu2+, close to 10.06 ns for pristine VPQDs (Fig. 6f). The lifetime ratio of τ/τ0 tends to be 1, which further confirmed that static quenching mode plays the main role in the interaction between Cu2+ and VPQDs.41
Conclusions
We have successfully prepared VPQDs by a solvothermal method for the first time. The morphology, microstructure and fluorescence properties of the as-prepared VPQDs were systematically investigated. An average lateral size of 3.16 ± 0.78 nm and an average thickness of 1.81 ± 0.57 nm have been obtained for the VPQDs after solvothermal reaction at 150 °C for 24 h. The VPQDs have been found to be excitation-dependent and have a strong green fluorescence with excellent stability, facilitating biomaterial applications. The morphology and fluorescence properties of VPQDs have been successfully tuned by adjusting the solvothermal reaction temperature and time. The as-prepared VPQDs have also been demonstrated to be effective fluorescent probes for the selective detection of Cu2+ in the organic phases. The fluorescence quenching of Cu2+ to VPQDs has also been found to be attributed to the static quenching mechanism. A detection limit of 19.6 nM with high sensitivity has been deduced.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Chao Li and Hang Guo for their help in using Talos F200X and FLS980 in the Instrument Analysis Center of Xi'an Jiaotong University. Meanwhile, this research was supported by the National Natural Science Foundation of China (22175136 and 21771143), and the Fundamental Research Funds for the Central Universities.Notes and references
- L. Zhang, H. Huang, B. Zhang, M. Gu, D. Zhao, X. Zhao, L. Li, J. Zhou, K. Wu, Y. Cheng and J. Zhang, Angew. Chem., Int. Ed., 2020, 59, 1074–1080 CrossRef CAS.
- B. Zhang, Z. Wang, H. Huang, L. Zhang, M. Gu, Y. Cheng, K. Wu, J. Zhou and J. Zhang, J. Mater. Chem. A, 2020, 8, 8586–8592 RSC.
- Y. Li, Y. Hu, Y. Zhao, G. Shi, L. Deng, Y. Hou and L. Qu, Adv. Mater., 2011, 23, 776–780 CrossRef CAS.
- H. Yuan, Y. Zhao, Y. Wang, J. Duan, B. He and Q. Tang, J. Power Sources, 2019, 410–411, 53–58 CrossRef CAS.
- Q. Liu, B. Guo, Z. Rao, B. Zhang and J. R. Gong, Nano Lett., 2013, 13, 2436–2441 CrossRef CAS PubMed.
- L. Long, X. Niu, K. Yan, G. Zhou, J. Wang, X. Wu and P. K. Chu, Small, 2018, 14, 1803132 CrossRef.
- N. Murugan, M. Prakash, M. Jayakumar, A. Sundaramurthy and A. K. Sundramoorthy, Appl. Surf. Sci., 2019, 476, 468–480 CrossRef CAS.
- T.-W. Sung and Y.-L. Lo, Sens. Actuators, B, 2012, 165, 119–125 CrossRef CAS.
- C. Boonmee, T. Noipa, T. Tuntulani and W. Ngeontae, Spectrochim. Acta, Part A, 2016, 169, 161–168 CrossRef CAS.
- Z. Xu, L. Hu, J. Yuan, Y. Zhang, Y. Guo, Z. Jin, F. Long, Y. Long, H. Liang, S. Ruan and Y.-J. Zeng, Adv. Mater. Interfaces, 2020, 7, 1902075 CrossRef CAS.
- Y. Zhao, X.-B. Zhang, Z.-X. Han, L. Qiao, C.-Y. Li, L.-X. Jian, G.-L. Shen and R.-Q. Yu, Anal. Chem., 2009, 81, 7022–7030 CrossRef CAS.
- R. F. H. Viguier and A. N. Hulme, J. Am. Chem. Soc., 2006, 128, 11370–11371 CrossRef CAS PubMed.
- G. E. Cartwright and M. M. Wintrobe, Am. J. Clin. Nutr., 1964, 14, 224–232 CrossRef CAS.
- J. S. Valentine and P. J. Hart, Proc. Natl. Acad. Sci. U.S.A., 2003, 100, 3617 CrossRef CAS PubMed.
- B.-E. Kim, T. Nevitt and D. J. Thiele, Nat. Chem. Biol., 2008, 4, 176–185 CrossRef CAS.
- X. Zhang, H. Xie, Z. Liu, C. Tan, Z. Luo, H. Li, J. Lin, L. Sun, W. Chen, Z. Xu, L. Xie, W. Huang and H. Zhang, Angew. Chem., Int. Ed., 2015, 54, 3653–3657 CrossRef CAS.
- Y. Zhang, J. Di, X. Qian, M. Ji, Z. Tian, L. Ye, J. Zhao, S. Yin, H. Li and J. Xia, Appl. Catal. B Environ., 2021, 299, 120680 CrossRef CAS.
- F. Yuan, Z. Wang, X. Li, Y. Li, Z. a. Tan, L. Fan and S. Yang, Adv. Mater., 2017, 29, 1604436 CrossRef.
- L. Najafi, S. Bellani, B. Martín-García, R. Oropesa-Nuñez, A. E. Del Rio Castillo, M. Prato, I. Moreels and F. Bonaccorso, Chem. Mater., 2017, 29, 5782–5786 CrossRef CAS.
- B. Dong, C. Li, G. Chen, Y. Zhang, Y. Zhang, M. Deng and Q. Wang, Chem. Mater., 2013, 25, 2503–2509 CrossRef CAS.
- B. Liu, J. Xie, H. Ma, X. Zhang, Y. Pan, J. Lv, H. Ge, N. Ren, H. Su, X. Xie, L. Huang and W. Huang, Small, 2017, 13, 1601001 CrossRef PubMed.
- S. Ge, L. Zhang, P. Wang and Y. Fang, Sci. Rep., 2016, 6, 27307 CrossRef CAS PubMed.
- M. Lee, Y. H. Park, E. B. Kang, A. Chae, Y. Choi, S. Jo, Y. J. Kim, S.-J. Park, B. Min, T. K. An, J. Lee, S.-I. In, S. Y. Kim, S. Y. Park and I. In, ACS Omega, 2017, 2, 7096–7105 CrossRef CAS PubMed.
- L. Jayarathna, A. Bandara, W. J. Ng and R. Weerasooriya, J. Environ. Health Sci. Eng., 2015, 13, 54 CrossRef.
- M. Mehrali, E. Moghaddam, S. F. S. Shirazi, S. Baradaran, M. Mehrali, S. T. Latibari, H. S. C. Metselaar, N. A. Kadri, K. Zandi and N. A. A. Osman, ACS Appl. Mater. Interfaces, 2014, 6, 3947–3962 CrossRef CAS.
- A. Sharma, T. Gadly, A. Gupta, A. Ballal, S. K. Ghosh and M. Kumbhakar, J. Phys. Chem. Lett., 2016, 7, 3695–3702 CrossRef CAS PubMed.
- X. Ren, F. Zhang and X. Zhang, Chem. Asian J., 2018, 13, 1842–1846 CrossRef CAS.
- H. Dong, S. Tang, Y. Hao, H. Yu, W. Dai, G. Zhao, Y. Cao, H. Lu, X. Zhang and H. Ju, ACS Appl. Mater. Interfaces, 2016, 8, 3107–3114 CrossRef CAS PubMed.
- Q. Liang, W. Ma, Y. Shi, Z. Li and X. Yang, Carbon, 2013, 60, 421–428 CrossRef CAS.
- W. Dai, H. Dong, B. Fugetsu, Y. Cao, H. Lu, X. Ma and X. Zhang, Small, 2015, 11, 4158–4164 CrossRef CAS.
- L. Deng, S. V. Markova, E. S. Vysotski, Z.-J. Liu, J. Lee, J. Rose and B.-C. Wang, J. Biol. Chem., 2004, 279, 33647–33652 CrossRef CAS.
- S. H. C. Askes, G. U. Reddy, R. Wyrwa, S. Bonnet and A. Schiller, J. Am. Chem. Soc., 2017, 139, 15292–15295 CrossRef CAS PubMed.
- Z. Li, H. Yu, T. Bian, Y. Zhao, C. Zhou, L. Shang, Y. Liu, L.-Z. Wu, C.-H. Tung and T. Zhang, J. Mater. Chem. C, 2015, 3, 1922–1928 RSC.
- H. Ma, L. Pan, J. Wang, L. Zhang and Z. Zhang, Chin. Chem. Lett., 2019, 30, 79–82 CrossRef CAS.
- W. M. Girma, M. Z. Fahmi, A. Permadi, M. A. Abate and J.-Y. Chang, J. Mater. Chem. B, 2017, 5, 6193–6216 RSC.
- X. Hu, T. Chen, Y. Xu, M. Wang, W. Jiang and W. Jiang, J. Lumin., 2018, 200, 189–195 CrossRef CAS.
- A. Shrivastava and V. Gupta, Chron. Young Sci., 2011, 2, 21 CrossRef.
- T. Madrakian, S. Maleki and A. Afkhami, Sens. Actuators, B, 2017, 243, 14–21 CrossRef CAS.
- D.-W. H. Xuan Zhao, Y.-S. Wang, Y. Hu, C. Fu and X. Li, Chin. Phys. B, 2017, 26, 66102–066102 CrossRef.
- J. Xu, C. Wang, H. Li and W. Zhao, RSC Adv., 2020, 10, 2536–2544 RSC.
- M. Rahman and H. J. Harmon, Spectrochim. Acta, Part A, 2006, 65, 901–990 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ta09132h |
| This journal is © The Royal Society of Chemistry 2022 |