Quantitative assessment of intracellular/extracellular dimethyl sulfoxide concentrations during freezing with low-temperature confocal Raman micro-spectroscopy†
Taijie
Zhan
 a,
Wenya
Niu
a,
Mengdong
Cui
a,
Hengxin
Han
a,
Ding
Wang
b and
Yi
Xu
*a
a,
Wenya
Niu
a,
Mengdong
Cui
a,
Hengxin
Han
a,
Ding
Wang
b and
Yi
Xu
*a
aInstitute of Bio-thermal Science and Technology, Shanghai Co-innovation Center for Energy Therapy of Tumors, Shanghai Technical Service Platform for Cryopreservation of Biological Resources, University of Shanghai for Science and Technology, Shanghai, 200093, China. E-mail: xuyi@usst.edu.cn
bSchool of Materials Science and Engineering, University of Shanghai for Science and Technology, Shanghai, 200093, China
First published on 19th October 2022
Abstract
Although cryopreservation plays an indispensable role in the clinical application of cell therapy, the research on the osmotic behavior of cells during freezing is still at the level of theoretical models, and quantitative experimental data are still lacking. Therefore, the Raman spectra of dimethyl sulfoxide (DMSO) solutions with different standard concentrations (5%–80% v/v) were recorded experimentally to establish a quantitative evaluation method with the intensity ratio of different labeled peaks to the hydrogen bonding peak (as the internal standard) of water molecules in relation to different DMSO concentrations. By using this method, the characteristics of quantitative changes in intra- and extracellular concentrations under three different freezing methods were explored, including direct freezing, ice seeding freezing and vitrification. It was found that the intracellular concentration (@ −50 °C) after the ice seeding (@ −7 °C) freezing (1 °C min−1) method could reach 41.6%–49.2%, significantly higher than that of the direct freezing method (1 °C min−1 to −50 °C) of 32.4%–39.1%. Moreover, it is worth noting that the quantitative values of concentrations (@ −50 °C) of the ice seeding freezing are more consistent with the primary saturation curve of the DMSO solution. Thus, for the first time, it was revealed from the experimental data that the fundamental reason for the improvement of cell survival after ice seeding operation was pre-dehydration, higher concentration and smaller osmotic pressure difference between the inside and outside of the cell. These results also confirmed the validity of the famous two-factor hypothesis and more work will be carried out in depth.
1. Introduction
Cryopreservation technology of the cells is crucial to meet the different spatial and temporal requirements of the cell therapy clinical application by storage of biological samples at cryogenic temperatures (below −60 °C) to completely arrest their bioactivities.1 At the same time, it is necessary to consider the cryo-injuries such as ice formation-induced mechanical stress and concentration-induced physiochemical deviation from physiological states during freezing/thawing.2–4 To mitigate the damage to cells, cryoprotectants (CPAs) have been introduced and widely used, such as the most classic dimethyl sulfoxide (DMSO). Slow freezing with 10% DMSO has become the gold standard for the preservation of cell samples.5–7 The underlying theory of slow freezing cryopreservation is summarized as the “two-factor hypothesis” offered by Mazur in the 1970s as the inverted ‘U’ shaped relationship between cell survival and the cooling rate.8,9 It is postulated that prolonged exposure to elevated extracellular concentration (solution injury) at the slow cooling and induction of intracellular ice formation (IIF) by rapidly cooling could significantly compromise the cell viability.4,8,9 However, the details of the hypothesis about the characterization of the injury process have not been fully understood, especially the synergistic effect of the osmotic behaviors of intracellular dehydration induced by crystallization of the extracellular solution during the freezing process. Therefore, a quantitative understanding of the process of extracellular ice crystal growth-induced changes in intracellular concentrations is crucial for optimizing cryopreservation protocols to maximize cell survival.In general, the quantitative method of the low-temperature freezing process of cells is differential scanning calorimetry (DSC), which can determine the unfrozen water content in the cells by the integral enthalpy of the exothermic signal peaks during different freezing cycles.10 However, it can only deduce the degree of dehydration and unfrozen water content of cells in the whole sample due to the inability to accurately analyze the permeation process of individual cells. The permeation process of single cells at low temperatures still remains in theoretical model analysis,11–17 and there is still no accurate experimental data analysis for the quantification of the intra- and extracellular solutions to the best of our knowledge.
Recently, Raman spectroscopy has been emerging as a powerful imaging tool to investigate the freezing responses of various types of cells cryopreserved in different cryoprotectants by different research groups.18–27 The visualization of the distribution of extracellular and intracellular ice, cryoprotectants, and biological signals in single cells during freezing was achieved by using the high spatial resolution of Raman spectroscopy.24,26 Furthermore, the Raman images were further analyzed to obtain the morphology and size of ice crystals to analyze the possible mechanism of intracellular ice formation.23,24 During the analysis of intracellular and extracellular solutions, the relative concentration changes in the surrounding environment of the cells were discussed, but the precise quantitative analysis of the intracellular and extracellular concentrations was not achieved, which also limited the researchers’ in-depth understanding of the osmotic equilibrium behavior during freezing. Although there have been preliminary attempts to use low-temperature Raman microscopy for quantification in early research,22 the specific quantitative method and detailed analysis process have not been given.
Therefore, in this work, the quantitative relationship between the intensity ratio of different labeled peaks and the concentration was established by obtaining the Raman spectral curves of DMSO solutions with different standard concentrations. After optimizing the intracellular spectrum acquisition method at low temperatures (laser irradiation in advance), this relationship was applied to the quantitative study of intracellular and extracellular cryoprotectant concentrations during three different freezing processes, including direct freezing, ice seeding freezing and vitrification, to analyze the characteristics of intracellular and extracellular permeation behavior. Finally, the essential reasons for ice seeding freezing with a better preservation effect were revealed by comparing the quantitative concentrations with the primary saturation curve, which verified the correctness of the two-factor hypothesis to a certain extent.
2. Experimental methods
2.1. Cell culture and preparation
A549 lung carcinoma cells (ATCC) were cultured in Dulbecco's modified Eagle's medium (DMEM, TBD Sciences technology company (Tianjin, China) supplemented with 10% (v/v) fetal bovine serum (FBS, Gibco)) and 1% (v/v) penicillin–streptomycin (Gibco). The cells were incubated under a humidified 5% CO2 atmosphere at 37 °C. Before the experiments, the cells were detached from culture flasks with trypsin/ethylene diamine tetraacetic acid (EDTA) (Gibco) digestion solution, centrifuged and resuspended twice in Dulbecco's phosphate buffered saline solution at 1500 rpm for 4 min to wash away the culture medium for further use. Cell suspensions were mixed with 20% DMSO solutions at a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to the targeted concentration for ∼3 min until CPA penetration was complete.
1 to the targeted concentration for ∼3 min until CPA penetration was complete.
2.2. Raman spectroscopy acquisition
A cryo-stage was fixed on an electric motor platform to move the frozen samples to the laser spot point for recording the local spectrum during the entire cooling cycle. The Raman spectrum was recorded using a Confocal Raman Micro-spectroscopy system (LabRAM HR Evolution, HORIBA Jobin Yvon Co. Ltd, Japan) with a 532 nm laser light source and a 50× objective lens with a long working distance and a numerical aperture (N.A.) of 0.5. It enables the spatial resolution of the spectral signal to be less than 1 μm on the XY plane, that is, the spectral signal acquired by confocal microscopy will not be disturbed by surrounding signals. Raman spectra were recorded in the range of 50–4500 cm−1 with an acquisition time of about 1 min.During measurement, about 1 μL of the cell suspension with the target concentration was transferred to the polished aluminum film carrier, covered with a 10 mm diameter sodium calcium glass coverslip and sealed with oil at the edges, as shown in Fig. 1. After that, the entire sample carrier is transferred to the upper surface of the cryo-stage (BCS-196, Linkam) with thermally conductive silicone oil. After the freezing stage cover is closed to seal the cryo-stage cavity, the entire freezing and rewarming process can be started.
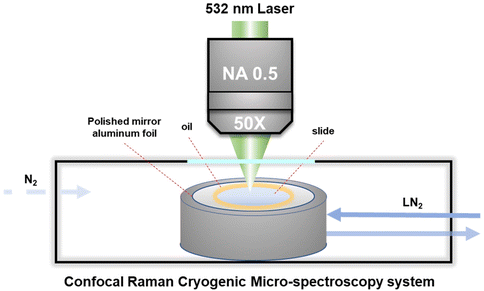 | ||
| Fig. 1 Schematic diagram of the Raman microscopy system monitoring the intracellular and extracellular concentrations during the freezing process. | ||
It should be noted here that for recording the cell spectra under low temperature conditions, it is necessary to introduce nitrogen gas into the freezing chamber to blow away the water vapor and fill the entire chamber with nitrogen gas, so as to avoid frost on the upper surface of the cover glass. In addition, in order to eliminate the strong background fluorescence signal of the cells under low temperature conditions, it is necessary to turn on the laser to irradiate the target cells about 1–2 minutes in advance for obtaining the Raman spectrum with obvious signal characteristics. Acquisition and post-processing were performed using Labspec software to establish the baselined smooth and determine the location and intensity of Raman peaks.
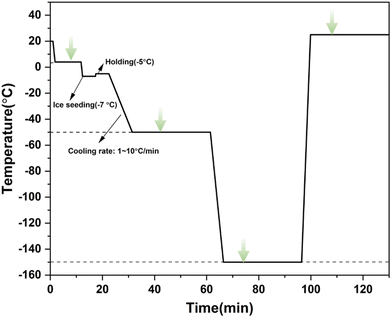
2.3. Freezing and warming procedure
In the experiment, the aforementioned experimental device was used to determine the changes in intracellular and extracellular concentrations during three different freezing processes, including direct slow freezing, ice seeding slow freezing and high-concentration vitrification.2.4. Principles of spectral quantification
The Raman peak intensity (I) is determined by the number of molecules N per unit volume, the scattered cross-section of the scattered light (dσ/dΩ), and the incident laser intensity I028 using the following relationship: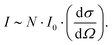 | (1) |
Accordingly, when the scattering cross-section dσ/dΩ does not change, and the intensity of the incident laser beam I0 remains stable, the intensity I of the obtained Raman peak is proportional to the sample concentration C. However, in the actual test process, the absolute intensity of peaks obtained by each measurement will vary significantly due to various environmental factors, resulting in errors in the multi-measurement process. Therefore, it is necessary to establish a quantitative method that can exclude interference.
The methods commonly used to calibrate spectral data include external standard method and internal standard method.29 In the external standard method, the external standard sample is tested before each sample test, and the peak intensity of all experimental spectra is corrected according to the change in the peak intensity of the standard sample.30 The external standard method is a common method for pre-calibration of spectroscopic instruments. The internal standard method includes two schemes. The first is to test the overall spectrum after adding the standard sample to the experimental sample (no interaction with experimental components, no spectral interference), and determine the relative proportion and concentration according to the change in the peak intensity of the standard sample. However, since the addition of other substances into the experimental system may affect the reaction process of the entire system, it is necessary to carefully consider the selection of standard samples. The other is to determine the final concentration by obtaining the ratio between the spectral peak intensity of a certain component (the solvent is generally selected as the standard for liquid samples) and other spectral peaks by comparing with the standard concentrated samples established earlier. Therefore, the second internal standard method was used to establish a quantitative method for different concentrations of DMSO solution in our work. The correlation relationships were established between the relative intensity ratio of the DMSO labeling peak and the hydrogen bond peak of water molecules and the volume concentration of the DMSO solution. The feasibility of the quantitative method has been verified at different concentrations and temperatures.
3. Results and discussion
3.1. Quantitative marker peak analysis
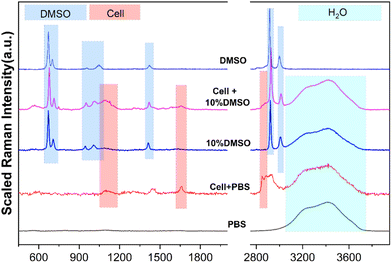
Before using Raman spectroscopy to conduct standard quantitative research on solutions, it is necessary to analyze the spectral characteristics of different substances in the solution system to determine the source of components corresponding to different characteristic peaks. As shown in Fig. 3, the spectral characteristic peaks from different sources in the mixed system were identified, respectively, including the pure substance DMSO, PBS buffer, cell in the PBS buffer, and cell in the CPA system. In the range of 200–4000 cm−1, the obvious characteristic peaks are classified into three different component sources: DMSO, cells and water (H2O), which are marked with blue, red and cyan shades, respectively. Detailed information on each characteristic peak is listed in Table 1 to provide a basis for subsequent quantification analysis.| Substances | Peaks/cm−1 | Assignment |
|---|---|---|
| DMSO | ∼675 | C–S–C asymmetric stretching |
| ∼712 | C–S–C symmetric stretching | |
| ∼947 | CH3 rocking | |
| ∼1014 | S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching O stretching |
|
| ∼1417 | CH3 asymmetric bending | |
| ∼2925 | CH3 symmetric stretching | |
| ∼3007 | CH3 asymmetric stretching | |
| Cells | ∼1650 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching amide I O stretching amide I |
| 1400–1490 | CH2 asymmetric deformation | |
| 2850–2890 | CH2 symmetric stretching | |
| H2O | 3100–3800 | Intramolecular hydrogen bond (O–H) |
3.2. Quantitative curve acquisition of standard concentration by the internal standard method
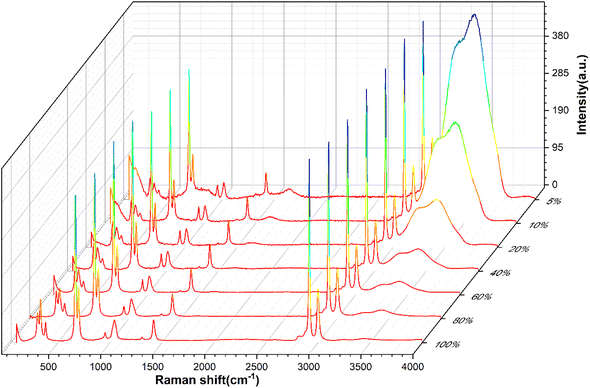
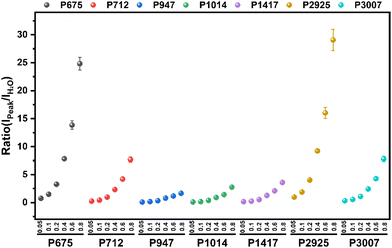
Full-range spectra of DMSO solutions with different standard volume concentrations (5%, 10%, 20%, 40%, 60%, 80%, 100%) were collected from 50 to 4000 cm−1. All raw spectra were baselined, smoothed, and adjusted to a uniform methyl peak intensity (∼2925 cm−1), as shown in Fig. 4. It can be found that with the increase of the concentration, the intramolecular hydrogen bond (or O–H bond 3100–3800 cm−1) peak of water molecules decreases rapidly, which is consistent with the actual situation that the relative content of water molecules in the solution gradually decreases, indicating that the relative concentration of the solution can be easily distinguished by the Raman spectrum curve.In order to quantitatively study the Raman spectrum of the solution, the intensities of the different labeled peaks of DMSO and the hydrogen bond (the highest point of the broad peak) on the same spectrum were obtained to calculate the intensity ratios of the different labeled peaks to the hydrogen bond peaks. The peak intensity ratios for different concentrations of DMSO solutions are shown in Fig. 5. In order to further analyze the relationship between the ratios of different peak intensities and concentrations, the fitting method was performed between the ratios of different labeled peaks and the solution concentration, and the fitting results are shown in Fig. S1.† The fitted relationship between the Raman peak ratio and concentration of different labeled peaks is summarized in Table 2. In fact, in the lower concentration range (<40%), the linear fitting can also obtain acceptable goodness of fit, but when the concentration is higher in a large range (>60%), the quadratic fitting is obviously better than the linear fitting. It can be found that the goodness of fit (R2) of the quadratic fitting equations of different marker peaks are all greater than 0.99, and the fitting error is small.
| Peaks/cm−1 | Relationship | R 2 |
|---|---|---|
| a Y = I_peaks/I_H2O; X = volume fraction of DMSO. | ||
| ∼675 | Y1 = 21.70X2 + 11.14X + 0.144 | 0.998 |
| ∼712 | Y2 = 7.874X2 + 2.598X + 0.094 | 0.996 |
| ∼947 | Y3 = 0.670X2 + 1.530X − 0.005 | 0.999 |
| ∼1014 | Y4 = 2.544X2 + 1.222X + 0.019 | 0.991 |
| ∼1417 | Y5 = 3.482X2 + 1.548X + 0.058 | 0.998 |
| ∼2925 | Y6 = 20.683X2 + 14.902X + 0.173 | 0.998 |
| ∼3007 | Y7 = 5.482X2 + 3.748X + 0.124 | 0.999 |
In order to verify the accuracy of the fitting equation, the Raman spectral curves of DMSO solutions with actual concentrations of 15%, 25%, 35% and 50% were tested. The error results between the analytical value and the true value of different solutions are obtained, as shown in Table S1.†
According to the error analysis results, it is found that there are four characteristic peaks at the lower concentration (15%) with larger deviations in the predicted concentration, which are ∼712, ∼947, ∼1014, and ∼1417 cm−1, respectively. There is a large error when these characteristic peaks are used to predict concentrations at low concentrations. The root mean square error of the whole method was 4.96% at 15% concentration, and the average recovery rate was 125.39%. However, the accuracy at higher concentrations was better than the low concentrations, the root mean square error was reduced to 0.84%, and the average recovery was 99.43% at 50% concentration. This is due to the low intensity of the labeled peak at low concentrations, and the interaction between the interference signal and the test labeled peak, resulting in low overall accuracy. However, when using characteristic peaks such as ∼673, ∼2925, and ∼3007 cm−1 for quantitative fitting, the fitting method still has a high accuracy even at low concentrations. In a high concentration solution, the content of the solute in solution increases, resulting in a marked peak intensity higher than the interference signal and higher fitting accuracy. Overall, the whole fitting estimation method can obtain a good quantitative predicted concentration.
On the other hand, in order to ensure the stability and consistency of the relative peak ratios of the Raman spectra at different temperatures, the Raman spectra of 45% concentration solution were collected at different temperatures as shown in Fig. S2.† The intensity ratio of each marker peak at different temperatures was calculated and the results are shown in Fig. S3(a).† It was found that the peak ratio remained at a uniform level without major changes with a decrease in temperature. After converting the peak ratio to concentration by using the fitting formula, it was found that the concentration basically maintains a small degree of deviation with a change in temperature as shown in Fig. S3(b),† especially for ∼673 and ∼2922 cm−1 as marker peaks, so the quantification standard fitting formula could be used to evaluate the concentration in the low temperature range. That is, the above fitting formula can be used for the subsequent quantitative evaluation of DMSO concentration.
3.3. Quantitative study of intracellular and extracellular concentrations during freezing and warming processes
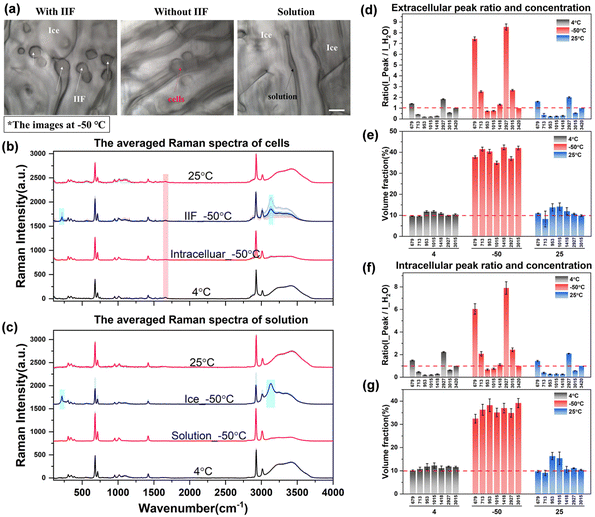
After acquiring the spectra of solutions with different concentrations and obtaining the standard fitting formula of the peak intensity with concentration, it was used to study the characteristics of intracellular and extracellular concentration changes during the process of cell cryopreservation, including direct slow freezing, ice seeding slow freezing and high-concentration vitrification.Fig. 6(a) shows the images of cells at −50 °C in different regions after direct slow freezing. It can be found that the cells and ice crystals are basically embedded together without significant boundaries, and the ice crystals may grow into the cells to generate intracellular ice. The Raman spectra showed that the solution in the gap was not in the crystalline state, but in an unfrozen phase state after concentration changes. The corresponding Raman spectral curves for each part are shown in Fig. 6(b) and (c). It can be found that in the unfrozen state (4 °C and 25 °C), the Raman spectrum inside and outside the cell shows the major characteristic peaks of water molecules and DMSO. In the frozen state, there will be a large number of ice crystals outside the cell, and a significant ice crystal peak can be found by directly collecting the ice crystal region spectrum (Ice_−50 °C). However, it should be noted that when the Raman spectral signal was collected from the ice crystal region, a large number of DMSO characteristic peaks also appeared in the spectrum of the ice crystal phase, which was inconsistent with the spectral characteristics of pure water crystals (Raman characteristic peaks of DMSO do not exist in the 200–2000 cm−1 range). This is inconsistent with the traditional understanding that ice crystals will exclude solutes during crystallization, and only water molecules will assemble into ice crystals. Our experimental results directly verify that water molecules may be mixed with a certain number of DMSO molecules during crystallization, and then the Raman spectral characteristics of DMSO molecules will be displayed when the ice crystal region spectrum is collected. From this point of view, the existing theoretical prediction models of the intracellular concentration in the unfrozen solution during the cell freezing process are ideal, ignoring the loss of this part of the solute, and need to be revised in future work to obtain more accurate prediction results.
In addition to the Raman characteristics of extracellular ice crystals, the Raman spectrum of extracellular solution (solution_−50 °C) showed that the hydrogen bond peaks of water molecules were significantly reduced and the proportion of DMSO-labeled peaks was significantly increased. According to the previous results of different concentrations, the concentration of the extracellular solution increased significantly, which will inevitably lead to an increment in the intracellular concentration. Analysis of the cell spectra showed that most of the intracellular spectra had significant Raman peaks of ice crystals, which indicated intracellular ice formation (IIF_−50 °C) in most cells. However, there are still a small number of cells without intracellular ice formation, as shown by the Raman spectrum of intracellular cells (intracellular_−50 °C), which indicates that the intracellular concentration has increased to a large extent.
In order to further discuss the variation law of concentration, the quantitative fitting formula established above was used to convert the Raman characteristic peak ratios under different conditions into quantitative concentrations. The relevant results are shown in Fig. 6(d)–(g).
It can be seen from the peak ratio of intracellular and extracellular solutions that the relative peak ratios of ∼673 and ∼2927 cm−1 labeled peaks were the largest, which is still very obvious even at low concentrations, so it is suitable as a spectral quantitative indicator. From the data of quantified concentration, before the freezing process (4 °C), the intracellular and extracellular concentrations were very close to the initial concentration of 10%. After the direct freezing procedure, the extracellular concentration directly rose to the range of 35.0% to 42.3%, and the intracellular concentration was slightly lower than the extracellular concentration, ranging from 32.4% to 39.2%. It can be seen from the relative changes of intracellular and extracellular concentrations that when the extracellular solution crystallizes first, the concentration of the extracellular solution gradually increases. A small number of cells in the ice crystal gap gradually dehydrate, the cell volume gradually shrinks, and the intracellular concentration increases. However, due to the formation of intracellular ice in most cells, the accurate acquisition of the hydrogen bond peak intensity of free water molecules cannot be achieved. Therefore, the internal concentration of cells with intracellular ice formation cannot be determined by the aforementioned quantitative method. And the intracellular ice formation will cause serious damage to the cells, and it should be avoided as much as possible.
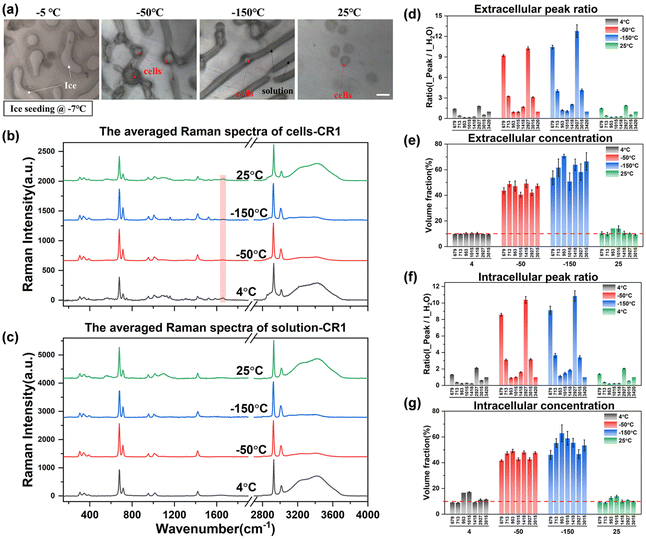
In the complete cryopreservation process, the cell samples also need to be transferred to liquid nitrogen for long-term storage. Therefore, after recording the spectrum at −50 °C, we tried to further cool down the samples to −150 °C to record the spectrum. However, due to the further cooling process, the ice crystals will grow to cover the entire region, making the boundary between the solution and the ice crystals more difficult to distinguish. The acquisition of extracellular and intracellular spectra at −150 °C is difficult to achieve, so the Raman spectral data at −150 °C cannot be displayed during the direct slow freezing process. This problem can be avoided in the ice seeding slow freezing process, and a more stable and accurate spectral signal can be obtained.
Observing the intracellular spectrum at −50 °C after the ice seeding freezing method, there was no ice crystal peak in the intracellular spectrum, that is, no intracellular ice was formed, which is mutually confirmed with the picture taken at −50 °C in Fig. 7(a). Compared with the non-frozen state (4 °C or 25 °C), the ratio of the intensity of each peak to the hydrogen bond peak changed significantly, indicating that the concentration of the solution inside and outside the cell increased significantly. After further cooling to −150 °C, the concentration will rise further. And the Raman spectral peak ratios at different temperatures were converted into quantitative concentrations using quantitative fitting methods, and the results are shown in Fig. 7(d)–(g).
It can be seen that the extracellular concentration is stable at 10% before freezing or after rewarming, while the intracellular concentration is not consistent. The more accurate quantitative labeled peaks are still at ∼679, ∼713, ∼2925, and ∼3015 cm−1, while ∼953 and ∼1015 cm−1 and other labeled peaks are not stable during quantification. By comparing the original spectrum, it can be found that there is a slight background fluorescence phenomenon in the intracellular spectrum. With the decrease of temperature, the background fluorescence of intracellular proteins, lipids and other biological macromolecules will also be significantly enhanced at low temperatures, and it is the most significant at −150 °C. By turning on the laser to irradiate the sample in advance, the intracellular fluorescence is eliminated by the pre-excitation method, and then a better intracellular Raman spectral signal at low temperatures can be obtained during spectral scanning. However, there is no significant background fluorescence phenomenon in the extracellular solution, and the subsequent quantification process can be carried out after simple preprocessing of the original extracellular Raman spectrum.
After eliminating the fluorescence, the Raman peak signals at −50 °C and −150 °C were obtained to calculate the concentration at each temperature. The concentration of the extracellular solution at −50 °C will rise to the range of 40.5%–49.2%, and the concentration of the intracellular solution is in the range of 41.6%–49.2%, which are very close. The overall concentration is significantly higher than that of direct slow freezing, and the reasons for this will be analyzed in the subsequent discussion section. After equilibrating at −50 °C for 20 min and rapidly cooling down to −150 °C, the concentration of the extracellular solution will further increase to 50.7%–70.7%, and the intracellular concentration is 46.0%–62.9%. The quantified concentration of different labeled peaks has a large fluctuation range, so it is difficult to directly analyze the difference between intracellular and extracellular concentrations. Therefore, we compare the quantification concentrations of intracellular and extracellular solutions together, as shown in Fig. 8.
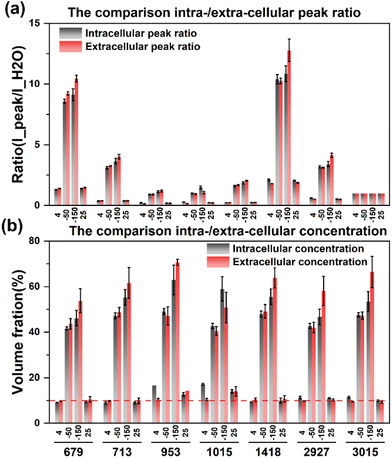 | ||
| Fig. 8 Comparison of intracellular and extracellular peak ratios (a) and concentrations (b) during the ice seeding slow freezing method. | ||
The results showed that the intracellular concentration was lower than the extracellular concentration, which was consistent with the fact that the concentration increased after the extracellular crystallization occurred first during the freezing process, and then induced the process of intracellular dehydration leading to the increase of the intracellular concentration. Moreover, the difference between intracellular and extracellular concentrations was different after the cooling processes. When the temperature was slowly cooled to −50 °C, the difference between the intracellular and intracellular concentrations was small, but after the rapid cooling to −150 °C in the second step, the difference between the extracellular and intracellular concentrations increased significantly, which further verified that the extracellular crystallization induced intracellular dehydration.
Similarly, the drastic changes in the rapid cooling process also indicate that the osmotic damage caused by the large concentration difference between intracellular and extracellular solutions needs to be considered in the second step. In addition, the higher concentration difference may be caused by the slower intracellular migration at low temperatures. Insufficient intracellular dehydration during rapid cooling may also cause ice crystal regrowth or devitrification of cells during rewarming, thereby damaging cell viability. Therefore, the control of the rapid cooling process in the second step also needs attention, and it is necessary to coordinate the relationship between the vitrification behavior of the remaining high-concentration solution in the cell and the slow infiltration process of the intracellular water at low temperatures.
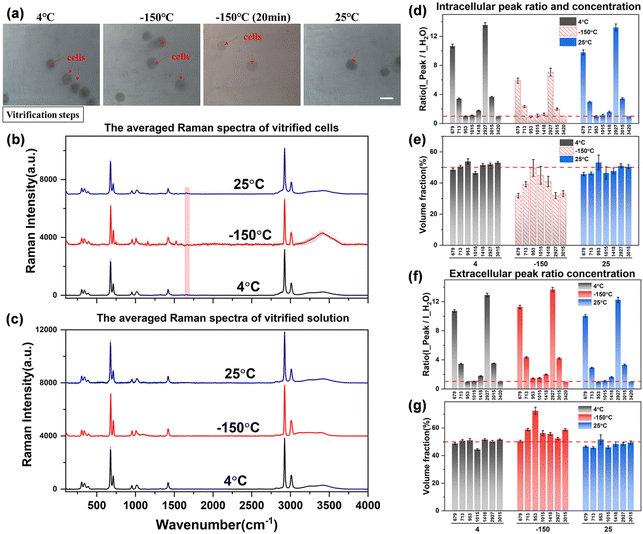
From the bright field images at different temperatures, the entire field of view does not change significantly during the vitrification process as shown in Fig. 9(a). The entire solution system maintains a transparent state, and the surrounding environment of the cells does not change, that is, the vitrification state at low temperatures cannot be distinguished from the brightfield images. However, the vitrification and the crystalline state can be distinguished significantly by Raman spectroscopy.35 From the observation of the characteristics of the hydrogen bond range of the extracellular spectrum at −150 °C, it is found that the higher right shoulder peak (∼3420 cm−1) of the hydrogen bond peak at room temperature obviously drifts to a lower wavenumber and becomes equal in intensity with the left shoulder peak (∼3220 cm−1). The spectral characteristics are very similar to the high-density amorphous (HDA) ice state,36,37 which confirms the vitrification state of the solution after high concentration rapid freezing.
However, the intracellular spectrum will generate a strong background fluorescence signal at −150 °C, and it is difficult to obtain the intracellular Raman spectrum signal with a flat baseline and obvious peaks in the entire spectral range. As shown in Fig. 9(b), the hydrogen bond range of the intracellular Raman spectrum at −150 °C has been completely deformed. We also tried to eliminate part of the background fluorescence by the aforementioned laser pre-irradiation method, but still could not get a higher-quality intracellular Raman spectrum curve. Therefore, it is speculated that the intracellular components are transformed into another phase state at this time, that is, the macromolecular substances in the cell are solidified into a vitrified state, and the solidified intracellular substance leads to a strong Raman fluorescence phenomenon. This phenomenon can also be observed from intracellular Raman spectroscopy when the temperature is cooled to −150 °C during direct or ice seeding slow freezing. This may be one of the Raman characteristics of the vitrification state of intracellular substances, but the law is not yet conclusive, and more perfect experiments are needed to verify it.
The obtained Raman spectral curve is further quantified, and the results are presented in Fig. 9(d)–(g). It should be noted that the quantitative data of the intracellular concentration is not the actual concentration as shown in the middle histogram at −150 °C in Fig. 9(e), which is due to the influence of intracellular fluorescence at low temperatures. Therefore, the highest Raman peak intensity in the hydrogen bond range of the intracellular Raman spectrum (3000–3800 cm−1) was used as the denominator to calculate the peak ratio and fit the subsequent quantitative concentration. The obtained nominal concentration is not the actual intracellular concentration, so it is marked with a dashed fill in the histogram. However, there is no significant fluorescence phenomenon in the vitrification state of the extracellular solution, and the Raman spectrum curve with a high signal-to-noise ratio can be obtained from the extracellular spectrum at low temperatures, so the quantitative concentration is relatively more accurate. As shown in Fig. 9(g), the quantitative concentration at −150 °C is close to that at 4 °C and 25 °C, and the overall concentration is maintained at the 50% concentration level. Although the hydrogen bonds of the extracellular solution are shifted to lower wavenumbers and the highest peak on the right is slightly reduced, the overall peak intensity remains basically unchanged. Among them, there is a large error in the quantitative calculation of the characteristic peak at ∼953 cm−1. This characteristic peak is not stable, which may be due to the molecular structure transformation process of the –CH3 methyl group inside the DMSO molecules during the vitrification process, which leads to a significant decrease in the Raman characteristic peak. However, the interaction principle at the molecular level of the methyl group (–CH3) still needs more work to further explore this interference factor, which is not the research content of this work for the time being.
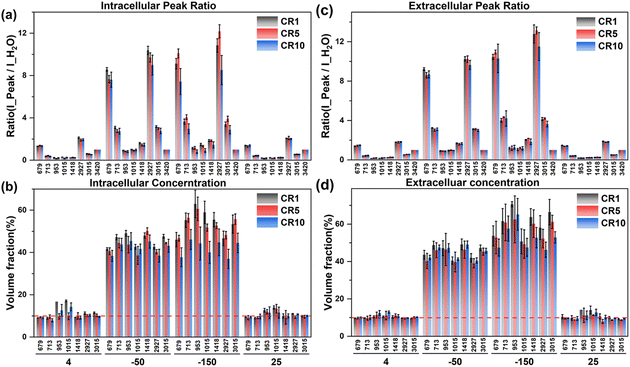 | ||
| Fig. 10 The intracellular and extracellular Raman spectral peak ratio (a and c) and concentration quantification (b and d) at different cooling rates during ice seeding slow freezing. | ||
According to the intracellular concentration changes, the final concentration at −50 °C tends to decrease as the cooling rate increases. At a faster cooling rate, the concentration of the intracellular cryoprotectant was lower and the intracellular water content was higher, and there was no significant change in the extracellular concentration at different cooling rates, which was generally higher than the intracellular concentration. Therefore, the rapid cooling process is not conducive to the dehydration of the cells during the freezing process. During the second step of rapid cooling to −150 °C, due to the lower concentration of the intracellular cryoprotectant, the higher water content is not conducive to the complete vitrification of the entire cell. The minimum intracellular concentration at −150 °C was only ∼40% at 10 °C min−1. The lower intracellular concentration and the high proportion of water content make it easier to produce ice crystal regrowth during cell rewarming, and generate more intracellular ice, resulting in significantly lower cell viability.
3.4. Analysis of concentration variation during slow freezing
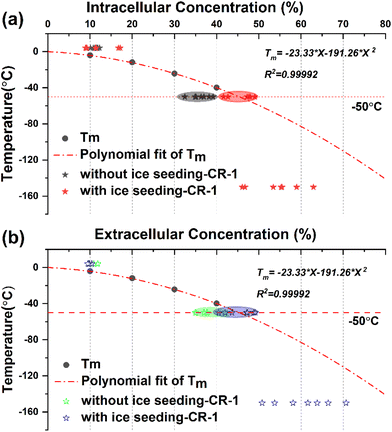
In order to explore the law of changes in the concentrations of intracellular and extracellular solutions during cell freezing, a comparative analysis was carried out by combining the primary saturation curve (melting temperature curve) of DMSO solution with the actual measured concentration of extracellular and intracellular cryoprotectants. The corresponding data are shown in Fig. 11.The melting temperature Tm of DMSO solution with different concentrations was obtained by the DSC heat flow curve, and the primary saturation temperature curve represented by the corresponding concentration change of the saturated solution under different temperature conditions was fitted using the equation Tm = −23.33 × X − 191.26 × X2.
By marking the concentration points before and after direct freezing (without ice seeding-CR1) and ice seeding slow freezing (with ice seeding-CR1) at a cooling rate of 1 °C min−1, it can be found that the intracellular and extracellular concentration points of the direct slow freezing method are all below the primary saturation curve. In other words, the water content inside and outside the cell is in a supercooled state at −50 °C in the direct slow freezing process, and it is easy to crystallize after being disturbed to damage the cells. However, in the ice seeding slow freezing process, the extracellular and intracellular concentrations in the frozen state are closer to or overlap the primary saturation curve. At this concentration, the cell–solution system is more stable and less susceptible to interference, with the least amount of ice crystals, which is beneficial for cell survival.
Based on the above analysis, by distinguishing the concentration changes in different freezing processes and comparing the primary saturation curve of the DMSO solution system, it was found that one of the essential reasons for the improvement of the survival rate of cells cryo-preserved by the ice seeding method is that the concentration change process is more consistent with the primary saturation curve and the smaller intracellular osmotic pressure difference. Accordingly, it is found that the basic principle of the ice seeding slow freezing method is similar to the liquidus tracing method for tissue cryopreservation,38–41 which is a cryopreservation procedure without the freezing state by gradient loading the cryoprotectant into the tissue sample at low temperatures. On the cellular scale, the experimental phenomenon and secret that the change process of intracellular and extracellular concentrations during the process of ice seeding slow freezing coincides with the primary saturation curve were discovered and demonstrated for the first time, which also indirectly verifies the correctness of the famous two-factor hypothesis.
3.5. Discussion on the characteristics of intracellular and extracellular changes during different freezing processes
Although there are still some unstable factors during the Raman quantification experiment (such as the interference of intracellular background fluorescence at low temperatures, the instability of the ∼953 cm−1 peak, etc.), the quantitative concentration range change process during the Raman experiment can still be applied to analyze the law of cell cryopreservation. By conducting Raman spectroscopy measurement experiments in different freezing processes, the variation rules of intracellular and intracellular concentrations in different freezing processes were obtained, and the possible cell damage factors were discussed respectively. Finally, a schematic diagram of the hypothesis related to cells in different freezing processes was proposed, as shown in Fig. 12.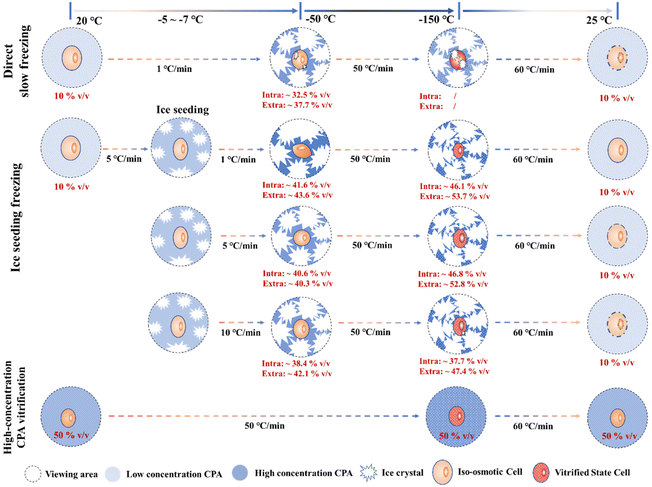 | ||
| Fig. 12 Schematic diagram of the intracellular quantified concentration (∼675 cm−1 peak) and freezing state during different freezing processes. | ||
In the process of direct slow freezing, the instant freezing of the cell suspension will lead to intracellular ice formation, which is not conducive to the migration of intracellular water, resulting in a lower intracellular concentration in the frozen state (−50 °C). This makes the cells not only damaged by ice crystals during the direct freezing process, but also increases the possibility of cell damage during the rewarming process, which is not conducive to cell cryopreservation.
In the process of ice seeding freezing, the concentration of the cryoprotectant is initially concentrated through the ice seeding operation, which can generate a good osmotic environment to facilitate the gradual dehydration of cells during the cooling process. The ice seeding operation can also effectively adjust the shape of ice crystals, so that the growth process of ice crystals will not damage the cell structure, which greatly improves the final survival of cells. However, the rapid cooling process (5 °C min−1 and 10 °C min−1) after the ice seeding operation is not conducive to the gradual dehydration of cells, resulting in a low concentration of the intracellular cryoprotectant. That is, the high intracellular water content may lead to the occurrence of ice crystal regrowth during the rewarming process, thereby damaging the cells.
For the rapid vitrification process of the high-concentration system, it is necessary to load high-concentration CPAs into the cell in advance, and the key factor is the toxicity of high-concentration CPAs. During the rapid vitrification process, the extracellular and intracellular solutions will not change significantly. When the temperature drops below the glass transition temperature, the glass transition will occur in the extracellular solution. At this time, according to our spectroscopic results at −150 °C, it is speculated that the glass transition also occurs in the intracellular region, although the morphology will not change. Therefore, although the cell morphology does not change significantly during the vitrification process, indicating that no cell structure damage has occurred, it is still necessary to pay attention to the devitrification phenomenon during the rewarming stage and the toxicity of high-concentration CPAs.
4. Conclusion
Due to the fact that only theoretical models and the lack of experimental data are used for cell osmotic behavior at low temperatures, the quantitative research of the intra- and extracellular permeation behavior of the cryoprotectant-mixed cell suspension solution system during the cooling process was carried out in this work. After optimizing the intracellular spectrum acquisition method, the characteristics of quantitative concentration changes of intra- and extracellular concentrations under three different freezing methods were explored. The quantified concentration values of the ice seeding freezing method were more consistent with the primary saturation curve of the DMSO solution. In addition, the reason for the obvious improvement of the cell survival effect after ice seeding freezing was revealed as the relatively smaller osmotic pressure difference and the higher termination concentration, which may ultimately be beneficial for intracellular vitrification during the second rapid cooling process. In addition, the concentrated intracellular solution during the freezing process, the water osmotic migration process, and the possible partial vitrification of the cells after rapid freezing all significantly support the validity of the two-factor hypothesis from the view of the experiment. Although there are some characteristic peak quantification data with low accuracy, more accurate quantitative marker peak selection and continuous monitoring of the whole freezing process need to be further developed.Author contributions
Taijie Zhan: conceptualization, methodology, investigation, formal analysis, and writing – original draft; Wenya Niu: investigation and formal analysis; Mengdong Cui: investigation; Hengxin Han: investigation; Ding Wang: resources; and Yi Xu: methodology, supervision, and funding acquisition.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (No. 52076140).References
- H. Huang, X. He and M. L. Yarmush, Nat. Biomed. Eng., 2021, 5, 793–804 CrossRef.
- D. Gao and J. K. Critser, ILAR J., 2000, 41, 187–196 CrossRef CAS PubMed.
- P. Mazur, S. P. Leibo and E. H. Y. Chu, Exp. Cell Res., 1972, 71, 345–355 CrossRef CAS.
- P. Mazur, in Life in the Frozen State, ed. B. J. Fuller, N. Lane and E. E. Benson, CRC Press, Boca Raton, 1st edn, 2004, pp. 3–65, DOI:10.1201/9780203647073.ch1.
- S.-A. Ock and G.-J. Rho, Cell Transplant., 2011, 20, 1231–1239 Search PubMed.
- D. M. Clarke, D. J. Yadock, I. B. Nicoud, A. J. Mathew and S. Heimfeld, Cytotherapy, 2009, 11, 472–479 CrossRef CAS.
- R. Li, R. Johnson, G. Yu, D. H. McKenna and A. Hubel, Cytotherapy, 2019, 21, 943–957 CrossRef CAS PubMed.
- P. Mazur, Science, 1970, 168, 939–949 CrossRef CAS.
- P. Mazur, Am. J. Physiol.: Cell Physiol., 1984, 247, C125–C142 CrossRef CAS PubMed.
- S. Mori, J. Choi, R. V. Devireddy and J. C. Bischof, Cryobiology, 2012, 65, 242–255 CrossRef CAS PubMed.
- M. Toner, E. G. Cravalho and M. Karel, J. Appl. Phys., 1990, 67, 1582–1593 CrossRef.
- J. O. M. Karlsson, E. G. Cravalho and M. Toner, J. Appl. Phys., 1994, 75, 4442–4455 CrossRef.
- J. Saenz, M. Toner and R. Risco, J. Phys. Chem. B, 2009, 113, 4853–4864 CrossRef CAS PubMed.
- L. Weng, W. Li and J. Zuo, Cryobiology, 2010, 61, 194–203 CrossRef CAS.
- G. Zhao, H. Takamatsu and X. He, J. Appl. Phys., 2014, 115, 144701 CrossRef.
- J. D. Benson, in Cryopreservation and Freeze-Drying Protocols, ed. W. F. Wolkers and H. Oldenhof, Springer New York, New York, NY, 2015, pp. 83–120, DOI:10.1007/978-1-4939-2193-5_3.
- J. D. Benson, in Cryopreservation and Freeze-Drying Protocols, ed. W. F. Wolkers and H. Oldenhof, Springer US, New York, NY, 2021, pp. 129–172, DOI:10.1007/978-1-0716-0783-1_4.
- J. Solocinski, Q. Osgood, M. Wang, A. Connolly, M. A. Menze and N. Chakraborty, Cryobiology, 2017, 75, 134–143 CrossRef CAS PubMed.
- A. Abazari, N. Chakraborty, S. Hand, A. Aksan and M. Toner, Biophys. J., 2014, 107, 2253–2262 CrossRef CAS PubMed.
- A. Kreiner-Møller, F. Stracke and H. Zimmermann, Cryobiology, 2014, 69, 41–47 CrossRef PubMed.
- A. Kreiner-Møller, F. Stracke and H. Zimmermann, CryoLetters, 2013, 34, 248–254 Search PubMed.
- J. Dong, J. Malsam, J. C. Bischof, A. Hubel and A. Aksan, Biophys. J., 2010, 99, 2453–2459 CrossRef CAS PubMed.
- K. Pollock, G. Yu, R. Moller-Trane, M. Koran, P. I. Dosa, D. H. McKenna and A. Hubel, Tissue Eng., Part C, 2016, 22, 999–1008 CrossRef CAS.
- G. Yu, Y. R. Yap, K. Pollock and A. Hubel, Biophys. J., 2017, 112, 2653–2663 CrossRef CAS PubMed.
- R. Li, G. Yu, S. M. Azarin and A. Hubel, Tissue Eng., Part C, 2018, 24, 289–299 CrossRef CAS.
- G. Yu, R. Li and A. Hubel, Langmuir, 2019, 35, 7388–7395 CrossRef CAS PubMed.
- R. Li, K. Hornberger, J. R. Dutton and A. Hubel, Front. Bioeng. Biotechnol., 2020, 8, 1–13 CrossRef.
- E. Smith and G. Dent, in Modern Raman Spectroscopy – A Practical Approach, ed. E. Smith and G. Dent, John Wiley & Sons Ltd, England, 2004, pp. 71–92, DOI:10.1002/0470011831.ch3.
- B. Schrader, in Infrared and Raman Spectroscopy, ed. B. Schrader, Federal Republic of Germany, New York, 1995, pp. 411–463, DOI:10.1002/9783527615438.ch05.
- R. N. Favors, Y. Jiang, Y. L. Loethen and D. Ben-Amotz, Rev. Sci. Instrum., 2005, 76, 033108 CrossRef.
- K. Wakabayashi, Y. Maeda, K. Ozutsumi and H. Ohtaki, J. Mol. Liq., 2004, 110, 43–50 CrossRef CAS.
- A. F. Palonpon, M. Sodeoka and K. Fujita, Curr. Opin. Chem. Biol., 2013, 17, 708–715 CrossRef CAS PubMed.
- B. Kann, H. L. Offerhaus, M. Windbergs and C. Otto, Adv. Drug Delivery Rev., 2015, 89, 71–90 CrossRef CAS PubMed.
- L. L. Zaghi, M. B. D. Berteli, J. D. S. de Freitas, O. B. Q. de Oliveira, A. D. Lopes, S. P. Ruiz, J. S. do Valle, G. A. Linde and N. B. Colauto, J. Microbiol. Methods, 2020, 176, 106030 CrossRef PubMed.
- T. Zhan, Y. Xu, D. Wang, M. Cui, X. Li and X. Wang, J. Mol. Liq., 2021, 325, 114658 CrossRef CAS.
- O. Mishima and Y. Suzuki, Nature, 2002, 419, 599–603 CrossRef CAS PubMed.
- H. Kanno, K. Tomikawa and O. Mishima, Chem. Phys. Lett., 1998, 293, 412–416 CrossRef CAS.
- J. Farrant, Nature, 1965, 205, 1284–1287 CrossRef CAS.
- B. C. Elford and C. A. Walter, Nature, New Biol., 1972, 236, 58–60 CrossRef CAS PubMed.
- B. C. Elford and C. A. Walter, Cryobiology, 1972, 9, 82–100 CrossRef CAS.
- D. E. Pegg, L. Wang and D. Vaughan, Cryobiology, 2020, 93, 12–17 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The quantitative relationship of different labeled peaks and their verification under different conditions. See DOI: https://doi.org/10.1039/d2an01288j |
| This journal is © The Royal Society of Chemistry 2023 |