Open Access Article
Open Access ArticleAnalysis of microsamples by miniaturized magnetic-based pipette tip microextraction: determination of free cortisol in serum and urine from very low birth weight preterm newborns
José
Grau
a,
María
Moreno-Guzmán
b,
Luis
Arruza
c,
Miguel Ángel
López
de,
Alberto
Escarpa
 *de and
Alberto
Chisvert
*de and
Alberto
Chisvert
 *a
*a
aGICAPC Research Group, Department of Analytical Chemistry, University of Valencia, 46100 Burjassot, Valencia, Spain. E-mail: alberto.chisvert@uv.es
bDepartment of Chemistry in Pharmaceutical Sciences, Analytical Chemistry, Faculty of Pharmacy, Complutense University of Madrid, Plaza Ramón y Cajal s/n, 28040 Madrid, Spain
cDivision of Neonatology, Child and Teenager Institute, Clínico San Carlos Hospital IdISCC, Madrid, Spain
dDepartment of Analytical Chemistry, Physical Chemistry and Chemical Engineering, University of Alcala, Ctra. Madrid-Barcelona, Km. 33.600, Alcalá de Henares, 28802 Madrid, Spain. E-mail: alberto.escarpa@uah.es
eChemical Research Institute “Andres M. Del Río”, University of Alcala, Ctra. Madrid-Barcelona, Km. 33.600, Alcala de Henares, 28802 Madrid, Spain
First published on 26th January 2023
Abstract
Miniaturized magnetic-based pipette tip microextraction is presented as a sample preparation approach for microsamples. It involves quick dispersion of a diminutive amount of a magnetic sorbent material in a low-volume sample (10 μL) to entrap the target analytes. Next, the dispersion is aspirated using a (semi)automatic pipette through a pipette tip with a small cubic neodymium magnet inside, which retrieves the magnetic sorbent containing the analytes. After discarding the rest of the sample, the sorbent is properly rinsed by aspirating/dispensing deionized water, and then, the analytes are eluted by aspirating/dispensing an appropriate solvent. This approach was employed for the determination of free cortisol in serum and urine from very low birth weight preterm newborns, a vulnerable patient group who present low availability for sampling biological fluids. A magnetic immunosorbent made of a cortisol antibody was employed for the selective extraction, followed by liquid chromatography-tandem mass spectrometry. Good analytical features were obtained, such as limits of detection and quantification of 0.08 and 0.27 ng mL−1, respectively, linearity up to 50 ng mL−1 (R2 > 0.999), RSD values under 15% and relative recoveries between 91 and 111%. The cross-reactivity with other glucocorticoids (i.e., cortisone and prednisolone) was evaluated to show the selectivity of the extraction. Finally, the method applicability was demonstrated towards the determination of free cortisol in the serum and urine samples from low birth weight preterm newborns.
1. Introduction
The analysis of biological samples is usually challenging: firstly, because of the complexity of the matrices that need a rigorous sample treatment and, secondly, because of the sample volume limitation to guarantee the health of the patient under study.1,2 This becomes especially challenging during the diagnosis of pathologies of critically ill patients and/or newborns, even more in those with a very low birth weight.3,4 Due to the fragility of these groups, the sample amount available is reduced drastically to a few microliters. In this regard, the development of analytical methodologies to handle low-volume samples is in demand.Microextraction techniques do not completely solve this problem. The efforts devoted in this field have been traditionally focused, with certain exceptions commented below, on the miniaturization of the amounts of sorbents and/or on the volumes of organic solvents employed, leaving aside the reduction of the sample volume. Nevertheless, the reduction of the extracting phase enables, indirectly, the reduction of the sample volume from tens/hundreds of mL to a few mL, far away from the level that is required in the scenarios discussed above (i.e., μL). Furthermore, it should be said that reducing the sample volumes also presents benefits according to the Green Analytical Chemistry5 and Green Sample Preparation principles6 (i.e., minimizing extractant amounts, reducing analysis times and creating portable devices for on-site sample preparation).
At this point, it is worth emphasizing that a few methodologies have been adapted to work with small sample amounts. Among them, the ones that should be mentioned are miniaturized solid phase microextraction (SPME),7,8 microextraction by packed sorbents (MEPS)9 and pipette tip-based strategies such as pipette tip solid phase extraction (PT-SPE),10,11 dispersive pipette tip extraction (DPX)12,13 and, more recently, magnetic-based pipette tip microextraction.14 These pipette-tip based approaches have the advantage of being user-friendly, just needing aspirating/dispensing cycles with a (semi)automatic pipette to perform the extraction of the analytes and easily discard the sample for further steps (i.e., washing and desorption).
Nevertheless, all these pipette tip-based approaches normally work with volumes between 0.2 and 5 mL –, amounts that, despite being quite low, are not recommendable in the case of critically ill patients, especially when newborns are involved. In this regard, the main objective of the present work was to develop a miniaturized version of the magnetic-based pipette tip microextraction for the analysis of microsamples (10 μL). For this purpose, a simple device, consisting of a cubic neodymium magnet encrusted in a conventional pipette tip, is designed. It is easy to build, affordable and portable for the purpose of on-site sample preparation. Moreover, the quickness of the method allows us to perform several analyses in a small amount of time, by increasing the sample throughput and allowing the implementation of point-of-care analysis for achieving faster diagnosis.15 In order to show the real applicability of this device, the determination of free cortisol in serum and urine from low weight birth preterm newborns has been selected as a proof-of-concept.
It should be said that serum cortisol is a good biomarker of adrenal malfunction in critically ill patients. Preterm neonates are at high risk of developing absolute or relative adrenal insufficiency in the first weeks of life. This blunted response to stress situations, such as sepsis, is the main underlying factor in the pathophysiology of severe arterial hypotension unresponsive to vasopressor therapy.16 In these sick babies, treatment with corticosteroids can contribute to normalising blood pressure and facilitating weaning from vasopressors. Diagnosis of this emergency situation is challenging and ideally requires the demonstration of low baseline cortisol levels and an insufficient response to adrenocorticotropin hormone (ACTH) stimulation (<12% increase from the baseline).17 However, the need for relatively large blood sample volumes and long processing times frequently makes the diagnosis of this condition impractical prior to the initiation of treatment. Therefore, steroid therapy is usually started before or even without laboratory results. This strategy may be dangerous due to the potential side effects of postnatal steroids in the preterm infant, so they should only be used if clearly indicated. A similar situation can be found in adult patients with septic shock. In this scenario, treatment with steroids is indicated when fluid resuscitation and vasopressors do not restore hemodynamic stability.18 While only unbound cortisol is active, the levels of serum cortisol have been traditionally obtained through the determination of total cortisol,19 being the most bound to cortisol-binding globulin (CBG).20 In recent years, determination of free cortisol has been proposed as a better alternative for the diagnosis of adrenal insufficiency in critically ill patients. For this purpose, different alternatives have been employed to separate the bound cortisol from the sample, among which ultrafiltration is one of the easiest and most employed methodologies.21
According to previous literature, one of the few studies available that measured free cortisol in sick preterm infants found a reference value of 1.37 ng mL−1 for this population.22 On the other hand, the analysis of urinary free cortisol (UFC) may be a good biomarker for the diagnosis of Cushing's syndrome.23
These analyses have been commonly performed by means of immunoassays.24 However, some immunosorbents could present cross-reactivity with similar compounds, thus obtaining non-specific signals as has been discussed by Raff et al.25 In the case of cortisol determination, cross-reactivity may occur due to the structural similarity to other glucocorticoids (e.g. cortisone).
All these aspects motivated us to develop a fast and reliable analytical method with the aim of achieving a quick diagnosis in fragile patients with scarce urine, and specially serum. Thus, a miniaturized version of magnetic-based pipette tip microextraction with a magnetic immunosorbent made of a cortisol antibody, followed by liquid chromatography-tandem mass spectrometry (LC-MS/MS), is proposed.
2. Experimental
2.1. Reagents and samples
All reagents and solvents were purchased from major suppliers. Cortisol (1 mg mL−1 in methanol) was obtained from Sigma-Aldrich (Steinheim, Germany). For the magnetic immunosorbent, a suspension of 30 mg mL−1 Dynabeads® (magnetic beads, MBs) covered with protein G was obtained from Thermo Fisher Scientific (Waltham, MA, USA). The cortisol antibody (4.35 mg mL−1) was purchased from East Coast Bio (Maryland Heights, MO, USA).Ultrapure deionized water was obtained from a Connect water purification system provided by Adrona (Riga, Latvia).
For buffer preparation, sodium chloride (NaCl) was obtained from Fisher Chemicals (Loughborough, UK), sodium dihydrogen phosphate monohydrate (NaH2PO4·H2O) and di-sodium hydrogen phosphate dodecahydrate (Na2HPO4·12H2O) were acquired from Scharlau (Barcelona, Spain), potassium chloride (KCl) was purchased from Panreac (Barcelona, Spain), and Tween 20 was obtained from Sigma-Aldrich (Steinheim, Germany). Finally, sodium hydroxide (NaOH) from Scharlau (Barcelona, Spain) was employed to adjust the pH.
LC-MS grade methanol (MeOH) from Honeywell (Seelze, Germany), LC-MS grade water from Panreac (Barcelona, Spain) and ammonium fluoride (for mass spectrometry) from Acros Organics (Geel, Belgium) were employed to prepare the mobile phase. Nitrogen used as a nebulizer and curtain gas in the MS/MS ion source was obtained using a NiGen LCMS 40-1 nitrogen generator from Claind S.r.l. (Lenno, Italy). On the other hand, extra pure nitrogen (>99.999%), used as a collision gas in the MS/MS collision cell, was provided by Praxair (Madrid, Spain).
2.2. Apparatus and materials
Strata X™-RP cartridges for the preparation of blank samples were obtained from Phenomenex (Torrance, CA, USA). Ultrafiltration tubes (0.5 mL, 30 kDA cut-off) for sample pre-treatment were obtained from Sigma-Aldrich (Steinheim, Germany).A Basic 20 pH meter from Crison (Alella, Spain) was used for the adjustment of pH.
An IMMULITE 2000 immunoassay system from Siemens Healthcare (Munich, Germany) was employed for the analysis of total cortisol.
A TS-100 thermo-shaker purchased from Biosan (Riga, Latvia) was employed for the immunosorbent preparation.
For the extraction devices, cubic neodymium magnets (1 × 1 mm) from Supermagnete (Gottmadingen, Germany) and 1–200 μL pipette tips from VWR (Radnor, PA, USA) were acquired.
An Agilent 1100 Series chromatography system from Agilent Technologies (Santa Clara, CA, USA) comprising a degasser, a programmable pump, an autosampler, and a thermostatic column oven with a Zorbax SB-C18 (50 mm length, 2.1 mm I.D., 1.8 μm) column, coupled to an Agilent 6410B Triple Quad MS/MS, was employed throughout the study.
2.3. Sample collection and pre-treatment
Serum and urine samples from a healthy adult volunteer were employed in the development and validation of the method. They were treated with Strata X™-RP to remove the endogenous cortisol. First, the cartridges were conditioned with 5 mL of MeOH and equilibrated with 10 mL of deionized water. Then, around 20 mL of sample were loaded, and the percolated sample was frozen at −20 °C until the moment of the analysis.Serum and urine samples from healthy preterm neonates were provided by the Clínico San Carlos Hospital (Madrid, Spain), and parental informed consent was obtained before the collection of samples. The premature newborns were included in this study because their samples were needed as part of the standard care in the intensive care unit and not for the only purpose of the present study. This study was approved by the Ethics Committee of both the University of Valencia and the Clínico San Carlos Hospital. Total cortisol was measured in the hospital laboratory to obtain reference values. The serum volume required was 10 μL, but at least 100 μL more were needed for the sample cup. Although results could be obtained in 30 minutes, the hospital samples were analyzed once daily; therefore the results can be delayed for several hours.
For the analysis of free cortisol, blood samples were first centrifuged (15 min, 6000 rpm) in order to isolate the serum. Serum was then ultrafiltered (30 kDA cut-off, 10 min, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) to separate the non-free cortisol, which could be recognized, and thus extracted, by the antibody in the extraction step. After that, 50 μL of sample were treated with 25 μL of ACN to separate the low-molecular weight proteins present in serum. Finally, the samples were centrifuged (5 min, 18
000 rpm) to separate the non-free cortisol, which could be recognized, and thus extracted, by the antibody in the extraction step. After that, 50 μL of sample were treated with 25 μL of ACN to separate the low-molecular weight proteins present in serum. Finally, the samples were centrifuged (5 min, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) and the supernatant was frozen at −80 °C until its analysis. On the other hand, the urine samples were centrifuged (5 min, 6000) rpm and kept at 4 °C until their analysis.
000 rpm) and the supernatant was frozen at −80 °C until its analysis. On the other hand, the urine samples were centrifuged (5 min, 6000) rpm and kept at 4 °C until their analysis.
2.4. Preparation of the standard and samples
250 mL of phosphate buffered saline (PBS) solution (0.1 M (pH 7.2)) was prepared. Bind and washing (B&W) solution (pH 8.2) for the preparation of the immunosorbent was prepared with Tween 20 (0.01% in PBS (pH 7.2)).A standard solution of 100 μg mL−1 of cortisol was prepared in methanol and kept at −20 °C. 10 μL of this solution was employed to prepare a 1 μg mL−1 intermediate solution in PBS (pH 7.2). Then, working standard solutions were prepared from 1–50 μg L−1 in PBS (pH 7.2).
2.5. Preparation of the immunosorbents
To prepare the immunosorbent, firstly, 1.5 μL of MBs (30 mg mL−1) were added to a 0.5 mL microcentrifuge tube and washed with 50 μL of B&W solution, and then the MBs were isolated by employing an external magnet and the supernatant was discarded. Afterwards, a solution of 10 μg mL−1 of cortisol antibody prepared in B&W solution was added and shaken at 1400 rpm at 25 °C for 5 minutes. After this, the immunosorbent was washed twice with 50 μL of PBS (pH 7.2).The prepared immunosorbent proved to be stable at 4 °C in PBS (pH 7.2) at least one week after being prepared. In this sense, several batches of the immunosorbent can be prepared during the first day of the week and they can be employed during the whole week.
2.6. Scheme of the microextraction device
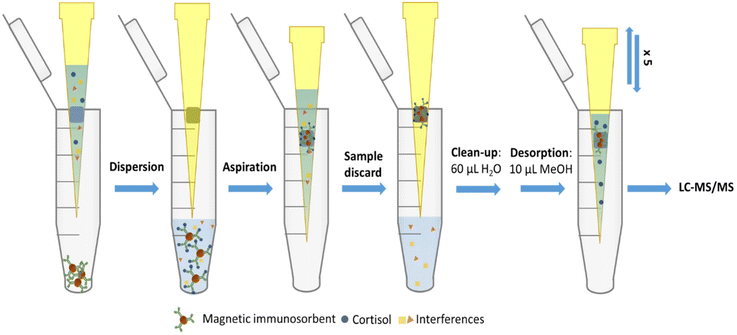
The microextraction was performed using a modified pipette tip. It consists of a conventional pipette tip (2–200 μL) with an internal cubic neodymium magnet (1 × 1 mm) encrusted. Compared with a previously designed device,14 there was no need of an external magnet to avoid the dragging of the internal magnet during the sample aspiration process. The cubic geometry within the inverted conical shape of the tip allows creating channels between the magnet and the tip wall, thus preventing clogging. A scheme of this device is presented in Fig. 1.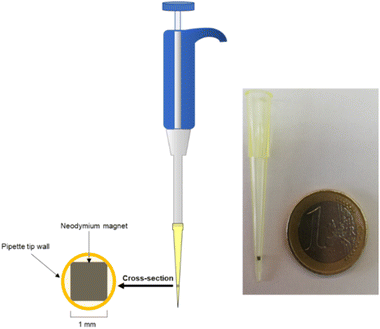 | ||
| Fig. 1 Scheme of the microextraction device, showing a cross-section with the encrusted magnet and the channels. | ||
Once the microextraction has been finished, the neodymium magnet can be retrieved by pushing it with a needle or a similar object. Then, the magnet was cleaned by just employing a piece of paper and soaking it in a small amount of acetone.
2.7. Microextraction procedure
For the microextraction process, 10 μL of the standard or sample were first diluted with 50 μL of PBS. Then, 60 μL of the diluted standard or sample solution were aspirated using the microextraction device described in section 2.6. After that, the solution was dispensed over the immunosorbent (preparation of which is described in section 2.5). The resultant dispersion was kept unaltered between 30 and 60 s; thus, within this time, it was aspirated using the microextraction device in such a way that the magnetic immunosorbent containing the entrapped analyte was retained on the magnet. The sample solution was then discarded and the pipette tip containing the retained immunosorbent with cortisol was washed with 60 μL of deionized water using two aspirating/dispensing cycles. Finally, 5 aspirating/dispensing cycles were performed with 10 μL of MeOH to desorb the analytes. Fig. 2 shows a scheme of the proposed method.2.8. LC-MS/MS analysis
Before its introduction into the LC-MS/MS instrument, 10 μL of the methanolic extract were diluted with 2.5 μL of deionized water to ensure a good subsequent analytical performance. Then, 5 μL were injected into the chromatographic system. The mobile phase was composed of solvent A (water, 0.5 mM ammonium fluoride) and solvent B (MeOH), and was prepared by isocratic elution employing a ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 (A
70 (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B) (v/v). The flow rate was set as 0.15 mL min−1 and the column temperature was kept at 40 °C. The total run time was 2.5 min.
B) (v/v). The flow rate was set as 0.15 mL min−1 and the column temperature was kept at 40 °C. The total run time was 2.5 min.
To measure cortisol, a triple quadrupole MS detector operating in positive electrospray ionization mode (ESI+, capillary voltage at 5 kV) was used. The gas temperature was set at 350 °C, the nebulizer gas flow rate was set at 11 L min−1, the nebulizer gas pressure was set at 50 psi, the collision energy was set at 21 V, the fragmentor was set at 155 V, and the dwell time used was 400 s. Multiple reaction monitoring (MRM) was used, where the m/z precursor → product ion transitions for quantification and for identification were 363 → 121 and 363 → 105, respectively.
3. Results and discussion
3.1. Optimization of the preparation of the immunosorbent
The immobilization time and the amount of antibody and MBs were studied to optimize the immunosorbent preparation. Each experiment was performed in triplicate by extracting 60 μL of a solution of 10 ng mL−1 of cortisol prepared in PBS (pH 7.2). The results are expressed as relative area (%). The performance of each experiment was analyzed during the same working session.As shown in Fig. 3a, increasing the immobilization time did not increase the analytical signal. Thus, it can be concluded that 5 min was sufficient to produce the immobilization of the antibody on the surface of the MBs and thus a time period of 5 min was selected.
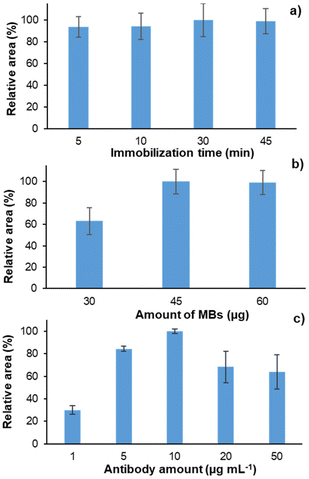 | ||
| Fig. 3 Optimization of the immunosorbent preparation: (a) the immobilization time; (b) MB amount; and (c) antibody concentration. The error bars show the standard deviation (N = 3). | ||
The amount of MBs employed was also studied. In this case, as shown in Fig. 3b, the signal increases when 45 μg of MBs (i.e., 1.5 μL of MB suspension) are employed. However, there were no significant differences when employing 45 or 60 μg. Higher amounts of MBs were not studied since the small magnet could not retain all of them, and the solid particles could be unintentionally introduced into the LC-MS/MS instrument, thus causing undesirable effects. Consequently, a smaller amount (i.e., 45 μg) was selected for further experiments.
Finally, as shown in Fig. 3c, increasing the amount of the cortisol antibody until 10 μg mL−1 increases the extraction capacity of the immunosorbent. Nevertheless, higher amounts of the cortisol antibody lead to a diminution of the signal. Since all the protein G interacts with the antibody, the molecules of the antibody begin to interact between them, reducing the active sites of the antibody, and thus, decreasing the signal. According to these results, 10 μg mL−1 was selected, since it provided the highest signal.
3.2. Optimization of the extraction/desorption variables
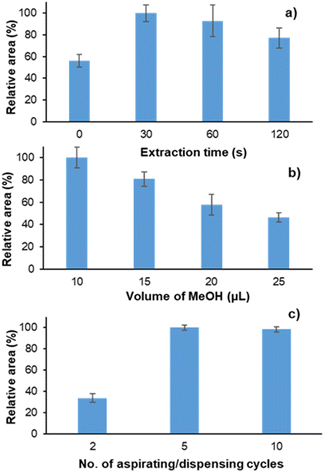
Once the preparation of the immunosorbent was concluded, different variables of the extraction/desorption method were studied. In this sense, the extraction time, the aspirating/dispensing cycles, and the desorption volume were tested. Each experiment was performed in triplicate by extracting 60 μL of a solution of 10 ng mL−1 of cortisol prepared in PBS (pH 7.2).The suspension of the immunosorbent in the sample was assayed at different times to see how the extraction/suspension time affected the extraction of cortisol. As shown in Fig. 4a, a time period between 30 and 60 s provided the maximum signal. After this time, part of the sorbent begins to be deposited at the bottom of the microcentrifuge tube, making its retrieval difficult for further steps. According to these results, the minimum time (i.e., 30 s) was selected in order to reduce the total analysis time.
The desorption volume was also studied. The results in Fig. 4b show that, as expected, using a greater amount of desorption solvent (i.e., MeOH) caused a decrease in the signal. 10 μL was then selected, since lower volumes were not recommended to ensure the correct intake of the injection volume using the LC-MS/MS instrument (i.e., 5 μL).
Finally, the number of aspirating/dispensing cycles was investigated. As shown in Fig. 4c, 2 cycles were not enough to achieve quantitative desorption. In this sense, 5 cycles were selected.
3.3. Analytical performance
The selectivity, limits of detection (LOD) and quantification (LOQ), linearity, repeatability and accuracy were studied to validate the analytical method.One of the advantages of working with LC-MS/MS is the possibility to study the cross-reactivity of the antibody, since some cortisol antibodies may interact with other glucocorticoids such as cortisone. To this end, a mixed solution of 10 ng mL−1 cortisol, cortisone and prednisolone was prepared in PBS (pH 7.2), and then this solution was extracted with the proposed method. An LC-MS/MS method from a previous work26 was employed for the simultaneous measurement of cortisol, cortisone and prednisolone. As can be seen in Fig. 5, no peaks were observed for cortisone and prednisolone, thus proving the good selectivity of the immunosorbent against cortisone and prednisolone.
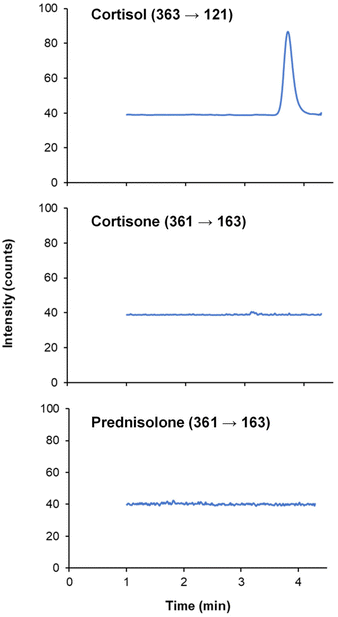 | ||
| Fig. 5 Chromatogram of a PBS solution spiked with 10 ng mL−1 of cortisol, cortisone and prednisolone after the magnetic-based pipette tip microextraction method. | ||
The LOD and LOQ were obtained after the extraction of 1 ng mL−1 of a PBS (pH 7.2) standard and after calculating which concentration provided 3 (LOD) and 10 (LOQ) times the signal-to-noise ratio. The values were 0.08 ng mL−1 and 0.27 ng mL−1, respectively.
The linearity was studied by extracting the standard solutions prepared in PBS (pH 7.2) at different concentrations. Calibration curves were constructed by plotting the cortisol area (Acortisol) versus the cortisol concentration in ng mL−1 (Ccortisol). The results indicated that the linearity reached up to 50 ng mL−1. To this end, the working range was set between the LOQ (i.e., 0.27 ng mL−1) and 50 ng mL−1 to cover the expected content in both healthy children (ca. 1 ng mL−1) and critically ill children (where the contents can be increased several times).27 The resultant calibration curve was Acortisol = 254.4Ccortisol + 3.4 with a determination coefficient (R2) of 0.9997.
For the study of repeatability, the RSD of five replicates extracted and analyzed during the same day (intraday) and five replicates in five consecutive days (interday) at three levels of concentration (i.e., 1, 10 and 20 ng mL−1) were measured. The values of RSD were under 10% for intraday and under 15% for interday.
With the aim of assessing matrix effects, blank serum and urine samples from a healthy adult (see section 2.3) were spiked at three levels of concentration (i.e., 1, 10 and 20 ng mL−1) and analyzed. The resultant concentrations were calculated by using a calibration curve obtained with standard solutions prepared in PBS (pH 7.2). Huge matrix effects were observed for both matrices. Different dilution ratios (i.e., 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) with PBS (pH 7.2) were then tested, thus showing that the matrix effects were negligible with a 1
5) with PBS (pH 7.2) were then tested, thus showing that the matrix effects were negligible with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dilution ratio. The results are presented in Table 1, showing relative recoveries between 91 and 111% for both samples.
5 dilution ratio. The results are presented in Table 1, showing relative recoveries between 91 and 111% for both samples.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 with PBS (pH 7.2)
5 with PBS (pH 7.2)
| Sample | Amount spiked (ng mL−1) | Relative recovery (%) |
|---|---|---|
| Serum | 1 | 100 ± 12 |
| 10 | 111 ± 2 | |
| 20 | 91 ± 7 | |
| Urine | 1 | 97 ± 11 |
| 10 | 110 ± 3 | |
| 20 | 91 ± 5 |
3.4. Analysis of real samples
Finally, five serum and five urine samples from very low birth weight preterm infants were analyzed to obtain the levels of free cortisol. The results from serum cortisol were compared with the amount of total cortisol obtained in the Clínico San Carlos Hospital. The results are shown in Table 2. Fig. 6 shows the chromatogram obtained for a serum sample and for a urine sample from one of the preterm newborns who participated in this study. The levels of free cortisol were found to be around one hundred times lower. It should be said that the determination of free cortisol levels by the proposed method makes use of different strategies to avoid potential interferences. On one hand, the ultrafiltration method separates the bound cortisol, thus ensuring that the signal obtained is only due to the free cortisol, and on the other hand, the use of an immunosorbent in the extraction step followed by LC-MS/MS avoids the non-specific interferences. Additionally, the levels of urinary free cortisol were also measured in the same children, obtaining similar values between them. Our results are in agreement with previous studies in sick preterm infants22 which found population reference values of 1.37 ng mL−1. Urinary free cortisol levels were also in the normal range for newborn children.28 Unfortunately, serum free cortisol was not measured in these newborns at the Clínico San Carlos Hospital, and therefore, comparison was not possible. Nevertheless, the total cortisol levels reported by the hospital were in accordance with the reference values for this population.16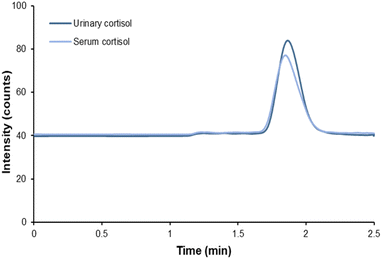 | ||
| Fig. 6 Chromatogram of the urine and serum samples from a preterm newborn after the magnetic-based pipette tip microextraction method. | ||
| Patient | Gestational age (weeks + days) | Birth weight (g) | Serum total cortisola (ng mL−1) | Serum free cortisol (ng mL−1) | Urinary free cortisol (ng mL−1) |
|---|---|---|---|---|---|
| a The immunoassay method was carried out routinely at Clínico San Carlos Hospital. | |||||
| 1 | 27 + 4 | 900 | 106 | 1.5 ± 0.2 | 2.9 ± 0.1 |
| 2 | 26 + 5 | 700 | 126 | 1.1 ± 0.1 | 0.49 ± 0.02 |
| 3 | 27 + 3 | 950 | 60 | 0.36 ± 0.04 | 0.63 ± 0.04 |
| 4 | 27 + 3 | 1100 | 104 | 1.39 ± 0.09 | 2.4 ± 0.2 |
| 5 | 27 + 5 | 930 | 138 | 1.0 ± 0.1 | 0.35 ± 0.05 |
3.5. Comparison with previous works
The presented method was compared with previous works that determined free cortisol in different biological matrices employing LC-MS/MS. This comparison is presented in Table 3.| Sample | Sample volume (μL) | Sample preparationa | LOQ (ng mL−1) | Relative recovery (%) | Ref. |
|---|---|---|---|---|---|
| a D: dilution; ED: equilibrium dialysis; ID: isotope dilution; LLE: liquid–liquid extraction; MPTME: magnetic-based pipette tip microextraction; SPE: solid phase extraction; and UF: ultrafiltration. | |||||
| Serum | 400 | ED + ID | 0.20 | 95–103 | 29 |
| Urine | 500 | UF + ID | 0.41 | 83.3–83.8 | 30 |
| Urine | 3000 | LLE | 1.13 | 78–94 | 31 |
| Serum, urine, saliva | 500 | UF + SPE | 0.17 | 95–108 | 32 |
| Serum, urine | 10 | UF + D + MPTME | 0.27 | 91–111 | This method |
The presented method shows a similar LOD compared with the other methods despite employing 40 to 50 times less sample amount. Regarding sample preparation, some of the presented methods29,30 used just isotopic dilution, thus reducing the sample treatment, but it is important to take note of the high amounts of samples used. For that reason, more sample treatment was necessary to overcome the matrix limitations and achieve higher sensitivity with smaller samples, since the volumes employed in the other works were unsuitable for working with low-weight newborns.
4. Conclusions
In this study, a miniaturized magnetic-based pipette-tip microextraction approach has been successfully developed as a new way for the analysis of small sample volumes (10 μL), thus providing new insights into the analysis of microsamples. This technique allows the reduction of not only the sample volume, but also the amount of organic solvents and sorbents, thus minimizing the quantity of waste in accordance with the ten principles of Green Sample Preparation. The proposed method has been successfully validated and applied to the determination of free cortisol in serum and urine samples from low birth weight preterm newborns, obtaining excellent results. The analysis of these kinds of samples is critical due to the restricted volume available from vulnerable patients. The combination of the employment of immunosorbents, LC-MS/MS and ultrafiltration has been proven to eliminate the non-specific interaction and the interferences from non-free cortisol. In this sense, this new technique is an excellent strategy for the analysis of reduced sample volumes due to its simplicity, greenness, low price and quickness, thus offering a feasible tool in the diagnosis of disorders by means of the analysis of microsamples.Author contributions
J. Grau: conceptualization, methodology, writing – original draft, investigation, data analysis and visualization; M. Moreno Guzmán: supervision, investigation, and writing – review and editing; L. Arruza: investigation, resources, and writing – review and editing; M. A. López: methodology, supervision, and writing – review and editing; A. Escarpa: conceptualization, funding acquisition, resources, supervision, and writing – review and editing; and A. Chisvert: conceptualization, funding acquisition, resources, supervision, and writing – review and editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
Grant PID2020-118924RB-I00 funded by the Spanish Ministry of Science and Innovation (MCIN/AEI/10.13039/501100011033) is greatly appreciated. This article is based on the work from the National Thematic Network on Sample Treatment (RED-2018-102522-T) of the Spanish Ministry of Science, Innovation and Universities, and the Sample Preparation Study Group and Network supported by the Division of Analytical Chemistry of the European Chemical Society. The authors are very grateful to Dr María José Torrejón from Clínico San Carlos Hospital for processing the clinical samples and obtaining the total cortisol levels.References
- M. Abdel-Rehim, S. Pedersen-Bjergaard, A. Abdel-Rehim, R. Lucena, M. M. Moein, S. Cárdenas and M. Miró, J. Chromatogr. A, 2020, 1616, 460790 CrossRef CAS PubMed.
- Z. Niu, W. Zhang, C. Yu, J. Zhang and Y. Wen, TrAC, Trends Anal. Chem., 2018, 102, 123–146 CrossRef CAS.
- A. Molinero-Fernandez, M. A. Lopez and A. Escarpa, Anal. Chem., 2020, 92, 5048–5054 CrossRef CAS PubMed.
- L. García-Carmona, M. C. González and A. Escarpa, TrAC, Trends Anal. Chem., 2019, 118, 29–42 CrossRef.
- A. Gałuszka, Z. Migaszewski and J. Namieśnik, TrAC, Trends Anal. Chem., 2013, 50, 78–84 CrossRef.
- Á. I López-Lorente, F. Pena-Pereira, S. Pedersen-Bjergaard, V. G. Zuin, S. A. Ozkan and E. Psillakis, TrAC, Trends Anal. Chem., 2022, 148, 116530 CrossRef.
- E. Boyaci, B. Bojko, N. Reyes-Garcés, J. J. Poole, G. A. Gómez-Ríos, A. Teixeira, B. Nicol and J. Pawliszyn, Sci. Rep., 2018, 8, 1167 CrossRef PubMed.
- T. Vasiljevic, V. Singh and J. Pawliszyn, Talanta, 2019, 199, 689–697 CrossRef CAS PubMed.
- M. Abdel-Rehim, Anal. Chim. Acta, 2011, 711, 119–128 CrossRef PubMed.
- S. Seidi, M. Tajik, M. Baharfar and M. Rezazadeh, TrAC, Trends Anal. Chem., 2019, 118, 810–827 CrossRef CAS.
- Naeemullah and M. Tuzen, Microchem. J., 2019, 147, 1147–1154 CrossRef CAS.
- D. C. M. Bordin, M. N. R. Alves, E. G. de Campos and B. S. De Martinis, J. Sep. Sci., 2016, 39, 1168–1172 CrossRef CAS PubMed.
- E. Carasek, L. Morés and R. D. Huelsmann, Anal. Chim. Acta, 2022, 1192, 339383 CrossRef CAS PubMed.
- J. Grau, J. L. Benedé, A. Chisvert and A. Salvador, Anal. Chim. Acta, 2022, 1221, 340117 CrossRef CAS PubMed.
- B. M. Cummins, F. S. Ligler and G. M. Walker, Biotechnol. Adv., 2016, 34, 161–176 CrossRef PubMed.
- N. Kumbhat and S. Noori, Clin. Perinatol., 2020, 47, 549–562 CrossRef PubMed.
- O. Hochwald, L. Holsti and H. Osiovich, J. Perinatol., 2012, 32, 412–417 CrossRef CAS PubMed.
- L. Evans, A. Rhodes, W. Alhazzani, M. Antonelli, C. M. Coopersmith, C. French, F. R. MacHado, L. McIntyre, M. Ostermann, H. C. Prescott, C. Schorr, S. Simpson, W. Joost Wiersinga, F. Alshamsi, D. C. Angus, Y. Arabi, L. Azevedo, R. Beale, G. Beilman, E. Belley-Cote, L. Burry, M. Cecconi, J. Centofanti, A. C. Yataco, J. De Waele, R. P. Dellinger, K. Doi, B. Du, E. Estenssoro, R. Ferrer, C. Gomersall, C. Hodgson, M. H. Møller, T. Iwashyna, S. Jacob, R. Kleinpell, M. Klompas, Y. Koh, A. Kumar, A. Kwizera, S. Lobo, H. Masur, S. McGloughlin, S. Mehta, Y. Mehta, M. Mer, M. Nunnally, S. Oczkowski, T. Osborn, E. Papathanassoglou, A. Perner, M. Puskarich, J. Roberts, W. Schweickert, M. Seckel, J. Sevransky, C. L. Sprung, T. Welte, J. Zimmerman and M. Levy, Crit. Care Med., 2021, 49, 1974–1982 CrossRef PubMed.
- N. Molenaar, A. B. Johan Groeneveld, H. M. Dijstelbloem, M. F. C. De Jong, A. R. J. Girbes, A. C. Heijboer and A. Beishuizen, Intensive Care Med., 2011, 37, 1986–1993 CrossRef CAS PubMed.
- I. Perogamvros, D. W. Ray and P. J. Trainer, Nat. Rev. Endocrinol., 2012, 8, 717–727 CrossRef CAS PubMed.
- C. J. Pretorius, J. P. Galligan, B. C. McWhinney, S. E. Briscoe and J. P. J. Ungerer, Clin. Chim. Acta, 2011, 412, 1043–1047 CrossRef CAS PubMed.
- H. E. Vezina, C. M. Ng, D. M. Vazquez, J. D. Barks and V. Bhatt-Mehta, Pediatr. Crit. Care Med., 2014, 15, 546–553 CrossRef PubMed.
- A. Luo, E. T. M. El Gierari, L. M. Nally, L. R. Sturmer, D. Dodd and R. Z. Shi, Steroids, 2019, 146, 65–69 CrossRef CAS PubMed.
- U. Turpeinen and E. Hämäläinen, Best Pract. Res., Clin. Endocrinol. Metab., 2013, 27, 795–801 CrossRef CAS PubMed.
- H. Raff, R. J. Auchus, J. W. Findling and L. K. Nieman, J. Clin. Endocrinol. Metab., 2015, 100, 395–397 CrossRef CAS PubMed.
- J. Grau, J. L. Benedé, A. Chisvert and A. Salvador, J. Chromatogr. A, 2021, 1652, 462361 CrossRef CAS PubMed.
- P. Poomthavorn, R. Lertbunrian, A. Preutthipan, A. Sriphrapradang, P. Khlairit and P. Mahachoklertwattana, Intensive Care Med., 2009, 35, 1281–1285 CrossRef CAS PubMed.
- R. Wang, M. F. Hartmann and S. A. Wudy, J. Steroid Biochem. Mol. Biol., 2021, 205, 105774 CrossRef CAS PubMed.
- F. Kirchhoff, J. Briegel and M. Vogeser, Clin. Biochem., 2011, 44, 894–899 CrossRef CAS PubMed.
- L. Bianchi, B. Campi, M. R. Sessa, G. De Marco, E. Ferrarini, R. Zucchi, C. Marcocci, P. Vitti, L. Manetti, A. Saba and P. Agretti, J. Endocrinol. Invest., 2019, 42, 1299–1305 CrossRef CAS PubMed.
- M. Dasenaki, M. Papatzani, E. Gounari, P. Magnisali, N. Papadopoulo-Marketou, C. Kanaka-Gantenbein, P. Moutsatsou and N. S. Thomaidis, Anal. Lett., 2019, 52, 2764–2781 CrossRef CAS.
- G. Montskó, Z. Tarjányi, E. Mezösi and G. L. Kovács, Anal. Bioanal. Chem., 2014, 406, 2333–2341 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2023 |