Open Access Article
Open Access ArticleAdvanced (photo)electrocatalytic approaches to substitute the use of fossil fuels in chemical production†‡
Gabriele
Centi
 a,
Siglinda
Perathoner
a,
Siglinda
Perathoner
 *a,
Chiara
Genovese
*a and
Rosa
Arrigo
b
*a,
Chiara
Genovese
*a and
Rosa
Arrigo
b
aUniversity of Messina, Dept ChiBioFarAm, V.le F. Stagno D’Alcontres 32, 98166 Messina, Italy. E-mail: chiara.genovese@unime.it; perathon@unime.it
bUniversity of Salford, 336 Peel building, M5 4WT Manchester, UK
First published on 6th February 2023
Abstract
Electrification of the chemical industry for carbon-neutral production requires innovative (photo)electrocatalysis. This study highlights the contribution and discusses recent research projects in this area, which are relevant case examples to explore new directions but characterised by a little background research effort. It is organised into two main sections, where selected examples of innovative directions for electrocatalysis and photoelectrocatalysis are presented. The areas discussed include (i) new approaches to green energy or H2 vectors, (ii) the production of fertilisers directly from the air, (iii) the decoupling of the anodic and cathodic reactions in electrocatalytic or photoelectrocatalytic devices, (iv) the possibilities given by tandem/paired reactions in electrocatalytic devices, including the possibility to form the same product on both cathodic and anodic sides to “double” the efficiency, and (v) exploiting electrocatalytic cells to produce green H2 from biomass. The examples offer hits to expand current areas in electrocatalysis to accelerate the transformation to fossil-free chemical production.
1. Introduction
Defossilization of chemical production to meet targets, such as net-zero emissions by the year 2050, is a challenge influencing research directions.1–5 Catalysis is affected by this radical transformation for its crucial role in chemical processes with the electrification of the chemical processes being a central area of investigation today.6–9 Future chemical production will critically depend on developing new catalytic processes using directly renewable energy and alternative carbon raw materials to achieve an ultra-low carbon footprint.10,11Electrocatalysis has better perspectives for industrial implementation among the emerging areas of catalysis.12–15 In comparison to alternative catalytic technologies using directly renewable energy sources, such as photo- and plasma-catalysis, the main benefits are (i) knowledge for scaling-up and industrialisation of the processes, (ii) better process intensification by developing stacked electrocatalytic cells, and (iii) larger productivities by volume.14 The latter can be up to two orders of magnitude greater in electrocatalytic processes than in photo-catalytic approaches. Nevertheless, using solar energy directly makes the photoelectrocatalytic approach an attractive option. Plasma catalysis for applications, such as converting CO2 to CO, N2 to NOx, and CO2 reformation, is also a valuable option. Except for established areas, such as water and air treatment, photocatalysis still needs to overcome the productivity gap for industrial uses. We thus focus attention here on (photo)electrocatalytic technologies.
(Photo)electrocatalysis takes benefits from the developments in the area of electrochemistry. However, a difference with respect to electrolysers and fuel cells is the issue of selectivity.16–20
1.1 New catalysis for fossil-free chemical production
Electrochemistry has been known for over a century. Still, the interest in (photo)electrocatalysis has increased recently, with exponential growth over the last two decades reaching over 600 publications and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 citations per year.
000 citations per year.
Notwithstanding this impressive number of publications and reviews, the reactions investigated are mostly restricted to the conversion of small molecules (CO2, N2, and H2O) and a few biomass intermediates. Regarding reactors and experimental conditions, literature studies are often quite far from those necessary for possible industrial exploitation. Addressing the challenges of creating new fossil-free chemistry requires exploring novel solutions and approaches by identifying the limiting factors for exploitability and studying how to overcome them.10,11
Relevant progress has been made in understanding and improving the performance of the (photo)electrocatalysts.12–15 Still, we suggest that a primary limiting factor in the transition to fossil-free chemical production is the scarce effort to innovate through novel concepts and ideas for (photo)electrocatalytic devices and operations that explore new solutions, opportunities, challenges and value chains.
1.2 Aims and limits
A distinctive aspect of (photo)electrocatalysis is the presence of separate zones for oxidation and reduction reactions, offering creative clues to design innovative solutions and process intensification. Still, most literature studies focus on a single electrode. They do not consider the whole device enough and how to best use both cell sides. In addition, (photo)electrocatalytic devices are often studied as independent elements from their integration in the value chain. An electrolyte is necessary to close the ionic circuit, but electrocatalytic cell design can overcome the need for a liquid electrolyte. This different design also influences the selection of the electrode.These are some areas in which intensified research in (photo)electrocatalysis is necessary. In addition, we will show here that a lack of background studies exists in several relevant areas of application of (photo)electrocatalysis inspired by the analysis of the value chain, despite a large number of studies in the area.
The main feature of (photo)electrocatalysis is process intensification, e.g., producing in a single step the final product. Today, attention is given to power-to-X processes,21–26 where only the first step is electrified (the production of H2). The transition to a carbon-neutral emission would instead require accelerating the development of process intensification solutions based on the full electrification, such as the direct electrocatalytic synthesis of the final target products.10,11 An extension of the concept of process intensification is integrating the photo-active element (photoelectrocatalytic – PEC – devices).
These are the aspects discussed in this highlights contribution. As case examples, we use EU running or proposed research projects to provide a glimpse of the emerging directions. The examples discussed refer to novel directions based on limited background results. They are thus challenging, but partly speculative. Full feasibility cannot be demonstrated, nor extensive scientific detail and/or literature-supporting data be provided.
The aim is to foster exploring new opportunities driven by the value-chain analysis, rather than only developing disruptive catalysis.27 It is not a “traditional” review summarising the state-of-the-art innovations, but a perspective to stimulate readers’ creativity. It is organised into two main topics: innovate in (i) electrocatalysis and (ii) photo-electrocatalysis. The selected examples show emerging possibilities, but with limited background research. The background and motivations for exploring these directions will thus be presented, in addition to the possible novel (photo)electrocatalytic technology with a discussion of the (few) literature results as the basis to provide indications of the research opportunities.
2. Innovation in electrocatalytic devices
2.1 New approaches to green H2 vectors
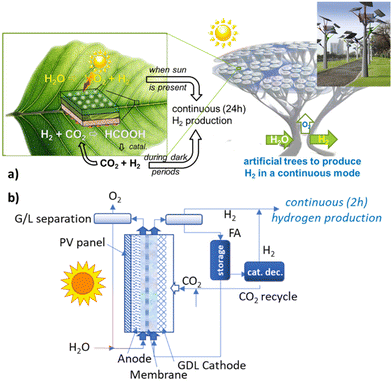
The first selected example involves the direct electrocatalytic synthesis of green H2 vectors to foster the creation of an H2 economy. The transition to carbon-neutral chemical production requires the electrification of the processes.28 Still, it suffers from the limits of (i) discontinuity and variability of electrical energy production from renewable resources, and (ii) insufficient local production by accounting for the multiple uses from mobility and building to applications in the process industry.It is thus necessary to develop technologies to store and transport long-distance renewable energy sources in a chemical form (chemical energy storage).29 These molecules, which mediate the production/availability of renewable energy and the requirements for industrial utilisation, are indicated as green energy vectors. In many process industries, mainly those that are energy-intensive, hydrogen is the candidate as an energy vector.2,4 Still, it suffers from storage and transport to long-distance issues. Direct onsite hydrogen production is considerably influenced by the limited time and availability (at low cost) of the renewable energy necessary to produce H2 with the continuity and amount required by energy-intensive processes.30 Still, onsite storage solutions for H2 are not very effective. Thus, green hydrogen vectors are a necessary element of an H2 economy panorama.10,28,31,32 An active research area is thus related to developing liquid hydrogen vectors, which facilitate transport and storage.32–35
The production of hydrogen vectors currently involves multistep technologies.28,31,32 H2 is typically produced by electrolysis, and then used to produce the hydrogen vectors in consecutive catalytic step(s). Among the limitations of this approach:
1. The presence of multiple steps, operating at different conditions and with different dynamics, makes the integration complex by impacting costs and energy efficiency.
2. The production of molecular H2 determines the presence of reversible reactions in consecutive steps, limiting the process efficiency (for example, when H2 is then used to produce NH3 catalytically).
The development of 2nd generation technologies, where the direct production of the hydrogen vectors is realised, overcomes the above drawbacks, potentially reducing costs and intensifying the process.10
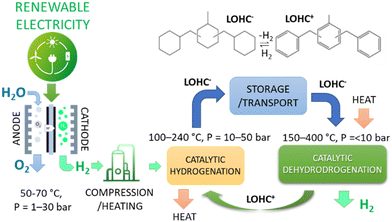
Motivations. Various hydrogen vectors have been proposed in the literature. Among them, liquid organic hydrogen carriers (LOHCs) have received considerable attention. LOHCs are organic molecules, typically polycyclic aromatics, which are catalytically hydrogenated or dehydrogenated.36–40 They are stable under multiple conversion cycles, have a good storage capacity (typically around 6–7% by weight), and are relatively safe to store and transport. This redox conversion allows the transport of H2 through this cycle.
LOHCs are liquid at ambient conditions, and have properties similar to diesel and gasoline. Therefore, their use allows a stepwise adaptation of the existing crude oil-based infrastructure. Avoiding extensive and costly changes in the energy infrastructure is mandatory to enable a smooth transition.
LOHCs are synthesised as a downstream process after the generation of green H2. Their production is an additional cost to be compensated only by the decrease in H2 transport and storage costs. Using LOHCs is generally advantageous for long-term storage/long-distance transport applications.39Fig. 1 schematically summarises the concept of LOHC, with an example of them.
The capital and operative expense costs are lowered by hydrogenating LOHC directly in an electrocatalytic device. This unit couples the two separate units of water electrolysis and catalytic hydrogenation in the conventional process (process intensification). A further advantage (explained later) is that the hydrogenation occurs via H+/e−, which jointly represent the equivalent of molecular H2.
Water electrolysers require high voltages (typically 1.8–2.6 V) for the reaction to proceed with sufficient current densities.41 The part that exceeds 1.23 V is the process overpotential. It represents the energy loss and nonideality in the electrochemical process. The main part of this overpotential is related to OER. Still, the cell aspects (resistance, bubble formation, etc.) and the reaction of H2 evolution (HER) significantly contribute.41 These energy losses can be reduced using (i) an alternative anodic reaction to OER, and (ii) H+/e− directly to hydrogenate LOHC+, as in the cited EPOCH project.
In addition, the catalytic hydrogenation of LOHC+ requires heat and pressurising the reactants (Fig. 1), requiring further energy. Furthermore, most hydrogenation reactions (relevant to forming H2 vectors) are reversible. They determine, for example, the need for high-pressure operations and large recycling, as exemplified in ammonia synthesis. These limitations are not present in the case of electrocatalytic hydrogenation via H+/e−, avoiding related limitations.
The direct LOHC+ hydrogenation led to process intensification and potential increased energy efficiency with respect to the conventional multistep process. The quantification of these aspects and impact on costs (capital and operative costs, e.g., CAPEX and OPEX, respectively) is still premature, being that the direct process is still to be developed. Nevertheless, it may be stated that a direct process offers advantages beyond process intensification. The cost of the integrated device is expected to be comparable to those of the H2 electrolysers (polymer electrolyte membrane type).
Challenges. Several challenges exist beyond identifying an appropriate selective electrocatalyst that combines high productivity (current density) and faradaic efficiency. The latter requires avoiding both side reactions of H+/e− recombination to H2 and other side hydrogenation reactions aside from the target to form LOHC+.
Cathodic reaction. There are still only limited studies on the electrocatalytic hydrogenation of LOHC+.42 One of the few studies that refers specifically to LOHC is a recent paper by Shiraz et al.43 They studied the proton-coupled electron transfer (PCET) reaction of 9-fluorenone/fluorenol as a model for the hydrogenation of LOHC. However, the latter involves the hydrogenation of the aromatic ring rather than the C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O to CH–OH reaction. In general, different reversible LOHCs have been proposed:42 (i) homocyclic compounds (to which belong the dibenzyltoluene illustrated in Fig. 1), (ii) nitrogen-containing compounds as N-heterocyclic compounds, amides or imides, urea/carbamates and nitriles, and (iii) oxygen-containing compounds as ketones, silane/alcohol or ester/alcohol pairs. However, the first class, particularly the molecules analogous to that presented in Fig. 1, show that the best LOHCs possess stability, cost, H2 storage capability, toxicity, and eco-toxicity, physical properties.
O to CH–OH reaction. In general, different reversible LOHCs have been proposed:42 (i) homocyclic compounds (to which belong the dibenzyltoluene illustrated in Fig. 1), (ii) nitrogen-containing compounds as N-heterocyclic compounds, amides or imides, urea/carbamates and nitriles, and (iii) oxygen-containing compounds as ketones, silane/alcohol or ester/alcohol pairs. However, the first class, particularly the molecules analogous to that presented in Fig. 1, show that the best LOHCs possess stability, cost, H2 storage capability, toxicity, and eco-toxicity, physical properties.
The catalytic hydrogenation of dibenzyltoluene to perhydrodibenzyl-toluene is realised typically at 30–50 bar and 150–200 °C on Ru- or Ni-based catalysts.44 RANEY® nickel is an effective catalyst.45 However, there are no studies on the electrocatalytic hydrogenation of dibenzyltoluene. However, the electrocatalytic hydrogenation of toluene using Ni-RANEY®-based electrodes has been reported.46 Noble-metal-based cathodes such as the Ru–Ir alloy show excellent properties.47 However, using Ni-RANEY® electrodes would be preferable over the noble-metal-based electrocatalysts for sustainability and cost motivations.
Even if the specific electrocatalytic hydrogenation of dibenzyltoluene has not yet been reported in the literature, it may be thus considered feasible.
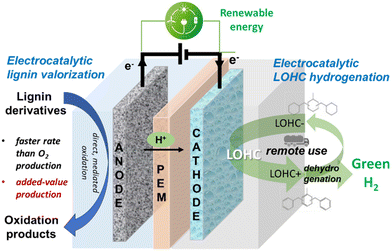
Anodic reaction. As mentioned above, the valorisation of the anodic reaction over the conventional OER is crucial for cost and energy intensity reduction. It is a primary advantage of the solution explored in EPOCH technology (Fig. 1). In water electrolysers, the O2 produced has a low, often negligible, value. In addition, OER requires a high overpotential and shows sluggish kinetics.48,49 Producing a higher-value chemical instead of O2 can make the overall process a competitive one. In addition, the selected reaction should improve the kinetics of the process and reduce the overpotential. Again, changing the anodic reaction combines multiple advantages.
The EPOCH-proposed technology offers additional benefits deriving from the synergic joining of multiple areas of renewable energy panorama. Selecting the target anodic reaction to have comparable production volumes to the cathodic reaction and compatible markets is a crucial factor of success. Many alternatives to OER have been proposed, from wastewater treatment to the oxidation of different organic compounds, such as glycerol, ethanol, and others.50,51
Coupling with the biorefinery, especially with the valorisation of lignin derivatives, is a valuable option. A high volume of waste lignin is potentially available, and high-added-value products can be produced.52–54 In addition, often in biorefineries, green electricity by biowaste combustion or gasification is available.55,56
Limitations. Notwithstanding the potential relevance of the electrocatalytic selective oxidation of lignin and its derivatives, there are limited studies reported in the literature in this direction. Yang et al.57 reviewed recent achievements in electrocatalytic lignin oxidation, and compared them with enzymatic oxidation and the mediated oxidation by molecules, such as TEMPO (2,2,6,6-tetramethylpiperidine-N-oxyl) and PINO (phthalimide-N-oxyl). The authors commented on a few studies using heterogeneous electrocatalysts, mainly based on noble metals such as Pt, Au, and Ir. Among the non-noble-based electrocatalysts, the best results were obtained with lead/lead-oxide-based anodes. Even if these anodes are active in oxidative lignin conversion, a broad range of products is usually obtained. A further general issue is the electrode fouling caused by the polymerisation of the reactants and/or products.
Garedew et al.58 also discussed lignin valorisation and the use of electrochemistry as a greener route for the process. In addition to noble metal and lead-based electrocatalysts, they also discussed nickel-, cobalt-, and nickel–cobalt-based electrodes. A specific section was also dedicated to the electrochemical degradation of lignin for hydrogen co-production. Among the challenges indicated are the complexity of the lignin polymer and its resistance to degradation, the limited choice in the electrolytes that can be used, and the difficulty in obtaining both selective products or complete mineralisation, as well as anode degradation.
Du et al.,59 Movil-Cabrera et al.,60 Bateni et al.,61 and Wijaya et al.62 also reviewed the electrochemical lignin conversion, but the analyses were not significantly different from those discussed above. However, the analysis of these results does not allow for identifying a proper target anodic reaction for coupling with the LOHC electrocatalytic hydrogenation, which meets the criteria indicated before.
The LOHC+ hydrogenation was also not extensively investigated in the literature.63–66 The need to overcome limitations by the reaction equilibrium was discussed in the literature, but electrocatalysis was not considered among the possible solutions.67 There are no reports in the literature on attempts to integrate LOHC hydrogenation and lignin or lignin derivatives electrocatalytic oxidation into a single device, nor examples of coupling the LOHC electrocatalytic hydrogenation with other biorefinery waste conversions. The simplified scheme of the integrated device is illustrated in Fig. 2.
The concept presented in Fig. 2 is an example of the possible new directions for electrocatalysis, in which there is a lack of studies. It couples different sectors of applications for a sustainable future (green hydrogen vectors and biorefinery). It exemplifies a new valuable research direction to expand the current studies in electrocatalysis, mainly focused on water electrolysis and CO2 or N2 electroreduction.
However, many studies have been reported on the electro-oxidation of biobased chemicals. As examples, the electrochemical oxidation of 5-hydroxymethylfurfural (HMF),68–70 furfural,71–74 and glycerol75–77 may be cited. However, they never arrived at developing a process for the mismatch between volumes required by H2 carriers and the chemicals produced by these biobased chemicals, as well as the limited synergies between the two value chains and sectors.
While these examples provide valuable inspiration for electrocatalysts to be used and optimal operation conditions, the main issue in developing an industrial-relevant case for the direct electrocatalytic production of LOHC is the identification of a proper target anodic reaction that brings the cited benefits of reduction in costs and energy intensity, but have a value chain that integrates well with that of the LOHC sector and brings effective synergies.
Ammonia is considered a preferential hydrogen vector for applications in marine transport,78,79 despite some limitations.80 Several companies (Alfa Laval, Hafnia, Haldor Topsoe, Vestas, and Siemens Gamesa) recently prepared a report with a comprehensive and up-to-date overview of the applicability, scalability, cost, and sustainability of NH3 as a marine fuel.81
The current technology to produce green ammonia is a three-step process: (1) produce H2 by electrolysis, (2) separate N2 from the air, and (3) thermo-catalytically convert H2 + N2 to ammonia. The latter stage is similar to the conventional Haber–Bosch (HB) unit for industrial ammonia production, but with the catalysts and operative conditions adapted to operate under milder conditions due to coupling with the electrolyser. The catalyst under these milder operative conditions shows a higher sensitivity to deactivation.
Several companies, including Stamicarbon, Kapsom, Harold Topsoe, Thyssenkrupp, and Siemens Energy, propose this green ammonia process. Several large projects in Saudi Arabia, Australia, Germany and Japan have started.82 However, costs are still high. Nazemi and El-Sayed83 indicated that the green ammonia synthesis would be cost-competitive with the conventional HB process, having an estimated production cost of around 143 ± 14 USD. To be competitive, green H2 production should have an energy input lower than 6 MW h per tonNH3 (now around 7.7 MW h) and a price of renewable electricity below 0.025 USD per kW h (now higher, but depends on many aspects).
Thus, an increase in the process energy efficiency would be required, together with a reduction in renewable electricity costs. This reduction is difficult to achieve in green ammonia synthesis via electrolysis, followed by HB catalytic synthesis. The degree of improving energy efficiency in the two separate steps is limited, and energy is required to compress H2 from the electrolyser to the pressure required for HB operations. The HB catalytic step is an exothermic reversible reaction between molecular H2 and N2. The related thermodynamic constraints determine high-pressure operations and large recycling. Furthermore, energy efficiency in the HB step largely depends on the possibility of an efficient heat recovery, which is less effective in small-medium scale plants as would be necessary for a distributed production of ammonia as a hydrogen vector.
In a direct electrocatalytic synthesis of NH3, the hydrogen-equivalent (H+/e−) rather than molecular H2 is involved in the hydrogenation of N2, and the reaction mechanism is different from heterogeneous catalysis.84 Operations proceed under mild conditions, and a single unit, whose cost could be comparable in principle to that of the electrolyser, allows for direct ammonia synthesis rather than having a downstream catalytic step operating at higher pressure and temperatures. For these reasons, various authors consider it necessary to move to the 2nd generation technologies of direct electrocatalytic synthesis of ammonia from N2.11,28,83,85Fig. 3 shows the concept of a direct electrocatalytic device to produce ammonia from the air at ambient conditions.
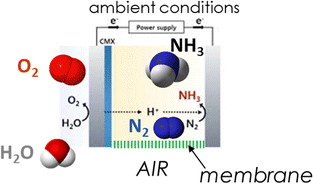 | ||
| Fig. 3 Concept of a direct electrocatalytic device to produce ammonia from the air at ambient conditions. | ||
Direct electrocatalytic synthesis of ammonia. The direct electrocatalytic synthesis of ammonia from N2 is an area of fast-rising research. Over 600 papers on this topic have been published in the last two years. The studies focus on the electrocatalysts and conditions to minimise the side formation of H2, which reduces the faradaic efficiency,86–91 even if questions still exist about the reliability of the results, e.g., if the N in ammonia is derived from N2 rather than N-impurities.92 Among the remarkable developments, Xue et al.93 showed about 68% faradaic efficiency in Au electrocatalyst nanoparticles by increasing their electron density through donor–acceptor couples.
On the other hand, the current densities at the maximum faradaic efficiency (FE) are about 0.1 mA cm−2, while current densities should be three orders of magnitude higher to consider exploitability. This result exemplifies, among others, that a large gap to reaching the conditions for industrialisation exists even within the relevant scientific progress. Industrial targets can be roughly indicated in FE above 80% at current densities of at least 80–100 mA cm−2.94 The cell design should allow for scalability, and avoid the use of electrolytes unsuitable for industrial operations to guarantee stable operations in continuous mode.
Most current studies and electrocatalyst design criteria focus on mechanistic aspects as the tool to obtain the targets. We showed94 that despite the great range of proposed reaction mechanisms, the electrocatalytic results fail within a limited range of faradaic efficiency versus current density values. This observation suggests that the existing methodological approaches do not allow the correct identification of the crucial aspects to boost performance.
In addition, many other aspects have to be improved in parallel: (i) realise high stability in extended operations, (ii) design of efficacious continuous-flow reactors with a configuration suitable for scale-up, (iii) an easy, low-cost ammonia recovery, (iv) the use of non-critical and not-costly raw materials, (v) avoiding the use of electrolytes, which may cause problems of corrosion and long-term stability, etc.
Despite many literature studies, only a few studies address these aspects. Tuning the current approach and extending the concepts and methodologies used, including reactor design and the direct coupling with air, is required to accelerate the implementation of a distributed production of H2 vectors.
Producing ammonia directly from the air. Fig. 3 illustrates a device to make NH3 directly from the air rather than from N2 produced by air separation. There are still no studies in this direction. However, conceptually, it can be a direction to create artificial-leaf-type devices (coupled with a photovoltaic panel) to produce ammonia. We can thus imagine having future artificial trees that directly produce H2 in remote areas in the form of an easily storable and transportable vector (ammonia). For such a device, it is mandatory to integrate the N2 separation from the air in the device.
It may be questioned whether this is feasible or even necessary. All current studies in direct electrocatalytic NH3 synthesis use rather pure N2, and the effect of oxygen impurities has not been analysed. Although membrane units for up to 99.9% or over are commercially available, they require multistep separation stages, making the process costly in energetic terms.95 In addition, in a model of artificial-leaf devices, it is necessary to integrate the N2 separation component in the unit. However, in a different approach, where the decentralised ammonia production unit still has a larger capacity than a single artificial-leaf type device, it is likely preferable to have a separate air separation unit. Thus, the need to integrate the N2 separation component from the air in the device depends on the target.
Conceptually, the air-separation unit is different from the membrane-like element indicated in Fig. 3. In commercial membrane units, the separation factor is related to the rate of transport of N2 and O2 through the membrane, with oxygen diffusing faster through the membrane (typically 5–10 times more quickly).95 Pressure is the driving force. A single-stage unit typically allows an N2 flow (retentate side) having around 90% concentration in N2. In the membrane-like element integrated into the electrocatalytic device (Fig. 3), it is necessary to diffuse N2 selectively and block O2 transport. Thus, a different transport mechanism is essential via, for example, metal-complexes binding N2 selectively rather than O2.96,97
This topic of functional elements for the selective transport of N2 in membranes is a novel development area. Some preliminary studies, however, indicate the feasibility. Yoon et al.96 showed that mesoporous metal–organic framework materials containing accessible Cr(III) sites could capture N2 over O2.
On the other hand, N2-selective group V metallic membranes are known.97 Thus, from a conceptual perspective, it is feasible to indicate the device scheme reported in Fig. 3, although the membrane-like elements are still unavailable.
Out of the membrane availability, some O2 will still be present together with N2. It is thus necessary to explore whether it could be feasible for the ammonia synthesis catalyst to have some oxygen in the N2 feed, and determine the oxygen-limiting concentration. Despite over 600 papers on this topic in the last two years, none has analysed the crucial question of the impact of the presence of O2 in the N2 flow. O2 has likely stronger binding than N2 and higher reducibility.
On the other hand, there is a rich dinitrogen coordination chemistry,98 although the possibility of selective binding and activation of N2 rather than O2 was never systematically explored.
Nitrite/nitrate can be electrocatalytically reduced to ammonia faster than N2.99,100 Therefore, the oxygen in nitrite/nitrate does not poison the electrocatalysts for ammonia reduction. It may also be possible to consider an oxygen-assisted mechanism, in which N2 first converts to the NOx species, and successively reduced onsite to ammonia. This is a less efficient redox mechanism because it requires more electrons/protons. On the other hand, the electrocatalyst reduction of NOx to NH3 is faster and more selective than the direct reduction of N2 to ammonia.101 Long et al.101 reported experimental and theoretical results showing that on copper foams, reaction rates of over 500 μmol h−1 cm−2 and FE over 90% are possible at the low potential of −0.9 V vs. RHE. In addition, long-term stability (over 100 h) was shown. Ko et al.102 also showed that >80% FE is obtained in the electrocatalytic reduction of NO on copper. N2 oxidation to nitrogen oxides is also a faster process than the N2 reduction,103,104 as also discussed later.
Thus, it is not unfeasible to develop an electrocatalyst for direct ammonia production in the presence of some oxygen in the feed. However, this is a topic largely unexplored, even if crucial from an application perspective.
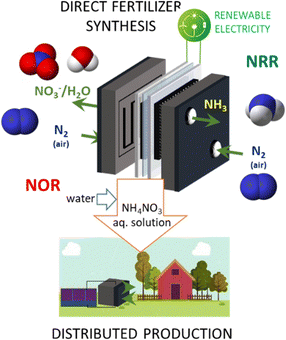
2.2 Fertilisers directly from the air
The concept presented in Fig. 3 could be extended to directly produce fertilisers. The separation of N2 from the air could be in a separate unit or integrated into the device.Nitrate ions are highly mobile/soluble in soil water, and can be assimilated directly by the root system of plants. The ammonium ion is the counter-ion and acts as a reservoir, then transformed to nitrate by the enzymes present in the soil. Its role is thus to double the nitrogen content of the fertiliser.
Commercially, ammonium nitrate is produced as an aqueous solution. Still, to transport, it is necessary to make a solid first by concentrating the ammonium nitrate solution in an evaporator or concentrator, and then spraying the concentrated melt into the top of a prilling tower. Besides safety aspects (ammonium nitrate is an explosive), this prilling (or alternative granulating) process is energy intensive (around 8500 MJ per ton N) and costly.
Dai et al.105 showed the possibility of preparation by direct electrochemical oxidation of nitrogen on ZnFexCo2−xO4 spinel oxides in an alkaline electrolyte. They reported a nitrate production rate of ∼130 μmol h−1 gMO−1 at an applied potential of 1.6 V versus the reversible hydrogen electrode (RHE). The faradaic efficiency, however, is only a few percentages, attributed to low N2 solubility. Spinel ZnFe0.4Co1.6O4 exhibits the highest nitrate yield.
Guo et al.106 used atomically dispersed Fe-based catalysts on N-doped carbon nanosheets. They reported a nitrate yield of 6.12 μmol mg−1 h−1 (2.45 μmol cm−2 h−1) and Faraday efficiency of 35.6%. The proposed reaction mechanism indicates Fe atoms as active centres for NOR, able to elongate the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond through hybridisation between the Fe 3d orbitals and N 2p orbitals. This hybridisation activates N2 molecules and triggers the subsequent NOR.
N bond through hybridisation between the Fe 3d orbitals and N 2p orbitals. This hybridisation activates N2 molecules and triggers the subsequent NOR.
Limited additional results on direct N2 to nitrate electro-oxidation have been reported, but are based on noble-metal electrocatalysts. A theoretical study107 indicates a noble metal, such as IrO2 (110), as an excellent catalyst because it limits the side oxygen evolution reaction. Avoiding this side reaction is undoubtedly a challenge. However, the results of Guo et al.106 evidence that alternative designing characteristics for the electrocatalyst exist aside from the oxide electrocatalysts considered by Anand et al.107
Therefore, even if very limited studies exist on the NOR reaction, they indicate a promising direction to explore, and the feasibility of combining NOR and NRR reactions in a single device (Fig. 4).
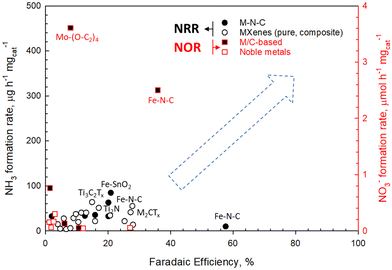
Fig. 5 reports selected examples of literature results in nitrogen reduction and oxidation reactions (NRR and NOR, respectively). The dashed arrow indicates the gap in the electrocatalytic results to meet conditions for industrial exploitability.
Two main classes of materials are emerging: (i) double transition metal MXene (a class of two-dimensional carbides, nitrides, and carbonitrides), and (ii) nitrogen-doped carbon materials (single-atom catalysts). However, Fig. 5 shows that significant improvements are still necessary. In addition, some results need further studies to verify reproducibility and stability.
Fig. 5 shows the feasibility of coupling NRR and NOR in a single device for producing fertilisers directly from N2 and, in the future, directly from the air. The last challenge has still not been attempted in the literature. Some EU projects, for example, the Horizon Europe FERTIGEN (CL4-2022-TWIN-TRANSITION) proposal from which Fig. 4 and 5 are derived, attempt to investigate this novel route. However, the disruptive character of this approach with respect to more conventional ones fails to be adequately understood. Nevertheless, we discuss the need to explore these novel directions by electrocatalysis to expand the range of possibilities.
N2 fixation with non-thermal plasma can be divided into (i) ammonia synthesis reaction by nitrogen reduction, and (ii) the direct production of NOx or NO3−/NO2− by oxidation.112 In both cases, the energy utilisation rate is still very low. However, the second path is better in terms of energy efficiency than the first.109 It is preferable to produce fertilisers directly in a distributed approach. However, coupling with a tailored catalyst would be necessary.
In direct plasma oxidation to nitrogen oxides, some main issues to solve to exploit this route could be identified: (i) coupling effectively with a catalyst to improve the performances, particularly the formation of NO2 with respect to NO, (ii) increasing the energy efficiency under relevant reaction conditions (atmospheric pressure), and (iii) scalability of the results considerably, depending on the plasma reactor. Intensification of research is necessary to solve these many challenges. The research in this direction is at a low technology readiness level.
2.3 Tandem/paired electrocatalytic reactions
– Use of renewable electricity to drive the process.
– Presence of an in situ generation of the redox reactants (hydrogen equivalents, active oxygen species).
– Process intensification; a single unit allows for realising complex multistep processes.
Electrocatalytic devices are thus truly crucial technologies for future chemical production. However, their potentialities are still underestimated, and most literature studies do not attempt to study them.
The main issue in pairing reactions at the cathodic and anodic sides of the electrocatalytic cell is to identify good examples from an industrial perspective, which overcome limitations from different markets and market dynamics of the products. An example is the coupling of cathodic reactions such as CO2RR with the anodic degradation of organics in wastewater.114–116
Notwithstanding this possibility being known and investigated for many years, it never reached commercial use, except in niche cases. This choice of relevant reactions and industrial sectors to be coupled must be addressed, in addition to the technical issues. It is similar to what was discussed before for the LOHC case.
The other relevant challenge is developing electrocatalysts and operative conditions that match the requirements of anodic and cathodic reactions and their optimal coupling (e.g., balance in terms of reaction rates, electron and H+/OH− flow, etc.). It is often assumed that anodic and cathodic reactions can be independently developed. The optimal electrocatalysts could be significantly different when both reactions are simultaneously studied, instead of using another reaction or sacrificial agents at the counter electrode. Literature results on this crucial question, however, are scarce.
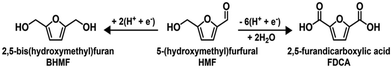
Synthesis of monomers for PEF. An example is the production of the two monomers used in the production poly(ethylene 2,5-furanoate) (PEF), a biobased polymer to substitute the fossil-based polyethylene terephthalate.117,118 The latter is synthesised from furan dicarboxylic acid (FDCA) and mono ethylene glycol. The electrocatalytic oxidation and reduction of sugars can lead to these chemicals.
The electrosynthesis of FDCA was reported in many studies.119–122 Differently, the electrocatalytic reductive cleavage of sugars to obtain ethylene glycol has been rarely investigated. Even less studied is the tandem process of producing FDCA and ethylene glycol at the two sides of the electrocatalytic cell. Studying these reactions and the related electrocatalytic reactor was the objective of the EU H2020 project TERRA (Tandem Electrocatalytic reactor for energy/resource efficiency and process intensification, project no. 677471).123
The alternative is the production of 2,5-bis(hydroxymethyl)furan (BHMF) instead of ethylene glycol:124,125
5-(Hydroxymethyl)furfural (HMF) can be obtained from glucose (a primary C6 sugar obtained by cellulose depolymerisation) by isomerisation to fructose. The latter is then dehydrated to HMF.
HMF can then be transformed by electro-oxidation to FDCA in a paired electrocatalytic reactor with BHMF production by electroreduction.125 At the anode side, the electro-oxidation is realised using (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) as an organic redox mediator to enhance the performance. A combined faradaic efficiency (FE) as high as 139% to BHMF and FDCA was obtained,125 or even higher up to nearly 190%.124 The FE higher than 100% is derived from the fact that the same reactant is converted at both the cathodic and anodic sides.
Although BHMF could be considered an equivalent ethylene glycol diol for polyester production, aliphatic diols such as ethylene glycol or more rigid diols such as isosorbide are necessary to obtain good polymer characteristics.126 BHMF needs to be converted to products, such as 1,6-hexanediol or 1,2,6-hexanetriol, or polymerised with chemicals other than FDCA to produce bio-based polymers.127 Therefore, the benefit of making the monomers simultaneously at the two electrocatalytic cell sides for the same polymer is not present in the case of BHMF coproduction with FDCA.
Electrocatalytic synthesis of adipic acid. Other industrial-relevant examples of paired/tandem electrocatalytic reactions exist. One of them is the production of bio-adipic acid, one of the most relevant dicarboxylic acids from an industrial point of view. Among the different possible routes,128,129 the direct electrocatalytic synthesis summarised in Fig. 6 is one of the challenging possibilities. It is explored in the frame of the EU H2020 PERFORM project (PowerPlatform: establishment of platform infrastructure for highly selective electrochemical conversions, project 820723). Limited literature data are available on the target electrocatalytic reactions,130–132 particularly the cathodic reaction. The PERFORM project aims to develop a pilot plan unit to demonstrate process feasibility.
 | ||
| Fig. 6 Scheme of the direct electrocatalytic synthesis of adipic acid from glucose in a paired/tandem electrocatalytic cell. | ||
Among the developments realised in this project are (i) NiFe oxide supported on Ni-foam as an anodic material for the electrocatalytic oxidation of glucose to glucaric acid, and (ii) supported PbO2 electrocatalysts for the oxidation of furfural to maleic acid, as documented in the project web site (https://www.performproject.eu). In contrast, other developments are still maintained as confidential. A general review on the electrosynthesis of biobased chemicals using carbohydrates as a feedstock was published by Vedovato et al.133 This review, besides discussing state of the art innovations, gives recommendations of the research needs, choice of electrocatalyst and electrolyte, and for upscaling the technology from an industrial perspective.
Tandem-paired electrocatalytic conversion of xylose into δ-valerolactone. There are different electrocatalytic cell design possibilities to pair two electrocatalytic reactions.134 The conventional is when the two reactions occur in different reactor compartments, separated by a membrane. The latter could be bipolar to allow different electrolyte conditions (for example, pH). The two reactions are only coupled through the protons or hydroxyl ions passing the membrane to close the cycle and the flow of electrons connecting the two electrodes.
When the membrane is not present, reactions between the products formed at the two electrodes are eventually mediated by other redox chemicals in the electrolyte. The intermediate products can react with each other, generating a coupling product, or the product generated at one electrode can then be further converted at the other electrode.
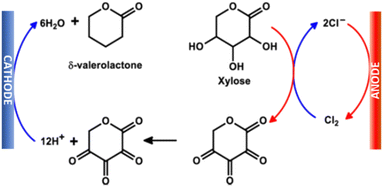
This possibility is exemplified in the tandem paired electrolysis of xylose into δ-valerolactone in an undivided cell (Fig. 7). James et al.135 reported the electro-deoxygenation of xylose to δ-valerolactone on porous Pb electrodes, but with HCl conversion to Cl2 as a counter-reaction on the RuOx–TiO2 anode. The latter, for safety concerns, cannot be used industrially.
Lucas et al.134 discussed several other examples of the electrochemical conversion of biobased molecules: polyols, carbohydrates, heterocyclic, and other chemicals.
Although excellent performances have been obtained in some cases at the laboratory scale, several challenges were still identified by Lucas et al.134 for the industrialisation of these electrocatalytic processes. The main issues are summarised below:
– Low faradaic efficiency and presence of competitive HER/OER reactions.
– Cost in downstream processing due to low product concentrations and complex product streams; needs to recycle the electrolyte.
– Slow conversion rates compared to non-electrochemical techniques.
– Low selectivity in targeting specific chemicals within real mixtures; the role of impurities.
– Electrode stability due to fouling; electrocatalyst stability.
– Reactor scale-up and moving from half-cell to continuous whole-cell processes.
– Membrane crossover of chemical species.
– Calibrating simultaneous tandem electrocatalysis on the anode and cathode in terms of rates, market sizes, technical variables.
– Establishment of compatibility with intermittent power.
In addition, in studies of single reactions, the counter-reaction is typically water oxidation or reduction. The overpotential for these reactions and electron/proton exchanges are likely different from when the pairing with the other target reaction is made. The electrolyte could also be different, and this also drastically influences the behaviour.
Thus, the assumption that an electrocatalytic cell's cathodic and anodic reactions can be independently developed is an oversimplification, even if typically made.
It should also be noted that most of the studies in electrocatalytic reactions operate at room temperature, and the effect of the temperature is often not investigated. There are material stability issues both on the electrocatalysts side and especially on the membrane, but operations above room temperature may often offer a key to enhancing performance.
Finally, it is usually assumed that the same temperature should be used at both the cathodic and anodic sides. The possibility of having different temperatures at the anodic and cathodic sides, up to a 50–100 °C gradient, is a technically feasible option. It has scarcely been investigated in the literature, but offers a novel key to optimising the two reactions at the anodic and cathodic sides. The cited EU TERRA project explored this possibility.
These examples illustrate the possibilities of pairing electrocatalytic reactions, but often industrial applicability and use within an advanced biorefinery value chain have not yet been convincingly proven.137 Besides the technical challenges above, a top-down approach would be necessary to identify the proper targets in integration within biorefinery schemes, such as the volume produced, feeds available and product quality demand, costs and availability of renewable energy sources, and competitive alternatives. On the contrary, research has been mainly driven from a bottom-up approach. Even with recent advances, mechanistic data are still limited to designing ad-doc electrocatalysts given a target reaction. A general toolbox of electrocatalysts to use is not available.
In addition, a new framework of electrocatalytically based reactions is required to develop a new chemistry based on renewable resources alternative to fossil fuel use.10–12 Rather than just coupling the conversion of biobased molecules with reactions such as HER, OER or CO2RR, new electrocatalytic methods should be developed to use the active intermediates generated in the electrocatalytic conversion of small molecules. Examples are (i) H* and OH* from H2O conversion, (ii) CO* and *CxHy from CO2 reduction, (iii) *NHy by N2 reduction or *NO by N2 oxidation; the asterisk indicates active surface intermediates produced during the electrocatalytic conversion.
Reactions to explore include (i) direct coupling of in situ generated intermediates, (ii) tandem reactions using in situ generated chemicals, and (iii) the eventual coupling between electro- and heterogeneous catalysis.
A full set of novel possibilities exists beyond the actual studies in electrocatalysis. Exploring these routes is the grand challenge to innovate electrocatalysis, not just scaling up and exploiting the current approaches.
2.4 Electrodriven production of green H2 from biomass
The previous discussion focused on pairing reactions for products derived from biomass transformation. Other alternatives to exploit the potentialities of electrocatalytic devices were scarcely investigated.Interesting is the dehydrogenation of organic chemicals to produce in an electrocatalytic cell both H2 and electricity together with the dehydrogenated (added-value) organic. Different from endothermic catalytic dehydrogenation, this electro-driven process produces protons and electrons, which migrate on the other side of the electrocatalytic cell, forming pure H2 on the cathode (Fig. 8). Ding et al.138 demonstrated that ethane could be selectively converted to ethylene with the coproduction of electricity and H2 using BaZr0.1Ce0.7Y0.1Yb0.1O3−δ as a solid-oxide electrolyte, Ni on the anodic side, and a perovskite as the cathode.
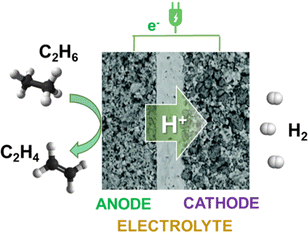 | ||
| Fig. 8 Schematic illustration of the ethane to ethylene + H2 coproduction on solid proton-conductive membranes in an electrochemical cell. | ||
The ethylene selectivity was close to 100%, but the hydrogen generation rate was low, around 0.45 mol cm−2 per day at 400 °C. The reason is the low ionic conductivity of the electrolyte. However, the concept could be extended to other reactions using better conductive protonic membranes, which need more reactive substrates than alkanes.
This concept was explored by Zhang et al.,139 who studied the electrocatalytic oxidation of glycerol on Pt/C in an anion-exchange membrane electrocatalytic cell, with the production of H2 on the cathodic side and the cogeneration of electricity (Fig. 9).
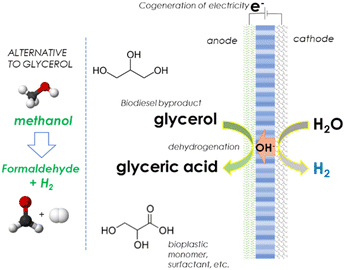 | ||
| Fig. 9 Concept of using an electrocatalytic cell for dehydrogenating chemicals combined with the coproduction of H2 and electricity in an anion-exchange membrane electrocatalytic cell. | ||
Zhang et al.139 used an anion-exchange membrane electrocatalytic cell and a Pt/C anode catalyst for glycerol dehydrogenation with cogenerating electricity and green H2. The anode overpotential determines the oxidation product distribution. Higher anode overpotentials favour bond breaking, thus lowering the C3 acids’ selectivity. The highest selectivity of C3 acids (glyceric acid + tartronic acid) reaches 91%. Higher current densities require operating above room temperature, up to 80 °C, which lowers the selectivity.
Fig. 9 indicates that the methanol-to-formaldehyde conversion is a valuable possible alternative. Formaldehyde is commercially produced from methanol on a Mton scale by catalytic oxidative dehydrogenation or dehydrogenation. The electrocatalytic process thus offers an alternative possibility for a low-carbon process. However, very few literature studies have reported this possibility,140,141 and they were two-three decades old.
3. Innovation in photo-electrocatalytic devices
Integrating a photo-active element in the electrocatalytic (EC) cell offers the possibility to develop devices that do not depend on the external availability of renewable energy, and thus on their large variability. They thus allow the development of artificial-leaf devices and artificial trees for a distributed production of chemicals and fuels.142–147While attention is typically focused only on solar-to-fuel efficiency, many other challenges are present, from the possibility of extending the limited range of uses explored today (essentially water splitting, CO2 reduction and more recently ammonia production from N2)146 to developing compact and efficient devices,148 extending the time of operations beyond the period of illumination and overcoming limitations by OER. Some of the possibilities will be discussed in the following sections.
3.1 Extending the time of operations and improving efficiency by decoupling anodic and cathodic reactions
On average, depending on the mix of sources used to produce the renewable electricity, from 4 to 8 h is the daytime when renewable electricity could be considered available. Thus, the device has to be amortised annually for a time ranging from 1000 to 2000 h, instead of the conventional 8000 h considered in chemical processes. On the other hand, storage of renewable electricity is still costly, and not efficient on a large scale and seasonally.
As an example, the following factors mainly determine the production cost of green H2 by electrolysis:
(i) The amortisation time (the cost of electrolytic H2 increases very steeply for insufficient electrolyser up-time, e.g., <2000 h per year);149
(ii) The cost of renewable electricity.150
The second aspect depends on the energy market rather than on technology solutions. Integrating a photo-active element in the device, such as a photovoltaic (PV) module, can overcome this dependence on strong fluctuations. This is the main motivation to develop photoelectrocatalytic (PEC) devices, and also called artificial leaf or photosynthesis. We use here the extended concept of artificial leaf devices as those that use solar light to produce chemicals or fuels without external bias or use sacrificial agents.151 The definition is thus independent of the device's configuration, whether wireless or not, or the use of a PV module to drive the process rather than photo-electrode.
A technological challenge is to explore solutions to overcome the first limitations, e.g., the operations only when solar light is present. The combination of a battery with the PV module to operate also during the dark period is not effective for an artificial tree (or analogous) solution due to the still inadequate energy density capacities of the batteries. A flow battery152 should be integrated into a PEC cell, realizing a solar flow battery design.153 However, this integration is highly challenging and alternative solutions should be identified.
(i) Introduces a redox function that allows a temporal decoupling of the anodic and cathodic processes.
(ii) The kinetics of the Ni(OH)2 to NiOOH reaction is faster than that of OER, and shows a lower overpotential because it is a one-electron reaction rather than a 4e− as OER; the result is better productivity and energy efficiency.
(iii) it avoids using a membrane to separate the anodic and cathodic zones, resulting in cost reduction.
Such a type of configuration also facilitates electrocatalytic operations under pressure. Several studies have explored this possibility,155–160 although limited mainly to water electrolysis as the application. An Israelian company (H2Pro) is also trying to commercialise the device.
However, these studies focus on taking advantage of the latter two aspects (the increased energy efficiency of the electrolysis, from less than 75% to over 90%), and not the potential benefit of the change in electrochemical cell architecture offered by this spatio-temporal decoupling. In addition, limited studies are present in the literature on the following aspects:
– To use the concept of spatiotemporal decoupling in PEC devices.155,161
– Applying it to reactions other than water electrolysis, such as the direct conversion of N2 to ammonia.162
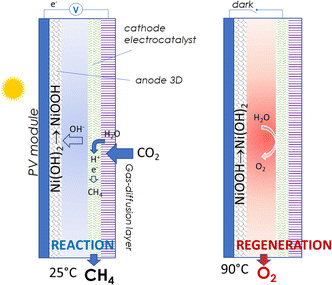
Using this solid-state redox approach for the anode could improve the energy efficiency, reaction rate and flexibility of operations also in PEC devices for chemical energy storage. An example is the PEC device to produce methane from CO2 (solar-to-fuel), presented in Fig. 10.
Several studies have been reported in the literature on the photoelectrochemical conversion of CO2 into fuels, including methane, as reviewed, for example, by Kumaravel et al.163 However, a specific configuration such as that presented in Fig. 10 has not been reported insofar. It reduces the potential necessary to drive the CO2 methanation, and thus increases energy and solar-to-fuel efficiency. Among the best electrocatalysts for CO2 to methane are modified copper nanoparticles, with FE above 70%.164
Ideally, the anode capacity should allow the PEC cell to operate at all daytime hours, with the regeneration occurring at night. The determining factor is the amount of Ni-hydroxide available for the reaction at the anode. 3D High-capacity anodes should be thus developed.165–167 Still, the operation times are of the order of a few hours.
It should be noted that the possibility of spatial decoupling of the cathodic and anodic reactions through the electrolyte circulation makes it possible to consider a design where the solid anodic state anodic component is in one place (for example, on the roof), and the cathodic part of the cell is in a different (dark) location.
Redox mediators can be present instead in the liquid phase (the electrolyte),168 overcoming the capacity limits of the solid-state redox anode. There is an increasing interest in this approach for spatiotemporal decoupling, although still focused on water electrolysis and not applied to the promotion of devices for solar-to-fuel energy storage.
Wang et al.169 used sodium nickel hexacyanoferrate as a redox reservoir to produce electrochemically strong oxidants. Frey et al.170 demonstrated the possibility of a Ce-mediated dual-use (energy storage and H2 production) cell. The high reduction potential of the CeIII/CeIV redox couple allows the decoupling of the water-splitting reaction into a charging cell that produces H2 and a discharging cell that produces electricity. Ho et al.171 reported a PEC cell where a Ni-electrode generates O2 from OH− at the anode, and the cathode V3+ is reduced to V2+. Then, the V-containing electrolyte is transported to a different reactor, where a MoCx-based catalyst produces H2 and forms V3+ again for subsequent reduction. Although the averaged diurnal solar-to-hydrogen (STH) energy conversion efficiency was limited (3.7%), this solution represents an interesting advancement towards realising PEC devices with continuous operations.
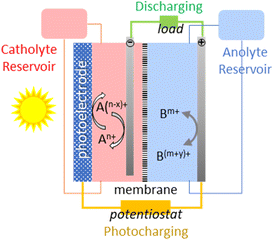
The concept of solar flow batteries should now be introduced to further understand these possibilities.
The principle of operations of SFB is summarised in Fig. 11. Solar energy is absorbed by a photoelectrode, and the photoexcited carriers convert the redox couples, i.e., store the solar energy in the electrolytes. When electricity is needed, the charged-up redox couples will be discharged on the electrodes, as in normal RFB, to generate the electricity. Catholite and anolyte reservoirs will allow for modulating the storage capacity to the needs.
Integration of this approach directly into that of a PEC device, where the aim is to produce chemicals/fuels rather than a reversible redox change in an inorganic ion, is not straight. It should be noted that organic redox batteries are also possible.176 However, this does not markedly change the issue of integrating SFB and PEC devices.
The examples presented in the previous section evidence some possible direction, but an optimal solution still has not been clearly identified. Research in this direction should be intensified because it offers significant relevant possibilities to exploit PEC devices.
Spatiotemporal decoupling thus offers a range of exciting solutions for innovation in electro- and photo-electro catalytic devices integrating redox mediators. These concepts will allow for the design of next-generation PEC devices for distributed production of fuels/chemicals. They offer many innovation opportunities that are still largely unexplored. However, the study of all these possibilities is just starting.
3.2 Doubling the efficiency by producing the same product on both electrocatalytic sides
Another challenge scarcely explored for PEC devices is the possibility of producing the same product at both the anode and cathode sides to formally “double” the efficiency. In addition, downstream separation costs often determine the overall process's feasibility. Thus, producing the same chemical in an electrocatalytic cell on both sides and avoiding the need for downstream operations may appear to be an impossible challenge. However, this is the objective of the EU H2020 DECADE project (distributed chemicals and fuels production from CO2 in photoelectrocatalytic devices, project 862030).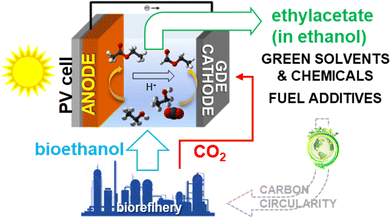 | ||
| Fig. 13 Simplified scheme of the electrocatalytic approach to producing ethyl acetate at both sides of the electrocatalytic cell. | ||
On the anode side, bioethanol from biorefinery is oxidised to acetate, which further reacts with ethanol to form ethyl acetate. On the cathode side, CO2 emitted in the biorefinery is fed to a gas diffusion cathodic electrode (GDE) to be converted to acetate, which reacts with ethanol to produce ethyl acetate. Ethyl formate forms as a side product. For the target uses, the additional presence of ethyl formate is not critical. In this way, carbon circularity is also enhanced.
A photovoltaic module is integrated into the device to use direct solar energy. Thus, this concept of a PEC device combines the two challenges of synthesising a single product at both the anode and cathode sides, which can be directly used without downstream separation. Both these aspects are critical for process economics.
The same concept can be used for the valorisation of methanol production rather than for biorefinery streams. The device can synthesise methyl formate from methanol by adapting the electrocatalysts. This way, CO2 from methanol plants is reused to produce a higher-added-value chemical.
There are limited studies on the electrocatalytic conversion of ethanol to ethyl acetate,178,179 while there several studies on CO2 reduction to acetate and/or formate.180–183 In addition to the low conductivity of ethanol, the main issue is that the same electrocatalysts change behaviour in an ethanol environment rather than an aqueous solution. This change is possibly related to the absence of the equilibria of CO2 with mono- and bi-carbonate present in water. However, this is another missing aspect of the literature. Thus, new electrocatalytic chemistry and materials have to be discovered. On the other hand, there are still insufficient scientific bases to design tailored electrocatalysts for operations under less conventional conditions and electrolytes.
At the anode, glycerol (a byproduct of biodiesel production) is electro-oxidised to glycolic acid (GC), while at the cathode side, GC is produced by oxalic acid reduction. The latter is made in a bio-reactor by biowaste fermentation, followed by electrochemical oxalate to oxalic acid extraction. GC is a monomer for green poly-glycolic acid production, a high-value polymer. GC is also widely used (i) in the textile industry as a dyeing and tanning agent, (ii) in food processing as a flavouring agent and a preservative, and (iii) in the pharmaceutical industry as a skin care agent. It is a high-added-value chemical produced from biowaste, according to this process scheme.
However, few studies have been reported in this direction. No one addressed the tandem electrolyser with glycerol to GC on the anode side and oxalic acid to GC on the cathodic side. However, separate studies on these two reactions have been reported on glycerol to GC electro-oxidation,184–187 and oxalic acid to GC electro-reduction.188–191
It may be argued that in organic chemistry, the stable product of glycerol oxidation is glyceraldehyde, which quickly isomerises to dihydroxyacetone. The next two-electron oxidation does not form the acid, but the pyruvaldehyde, as observed in most biochemical enzyme cycles. However, various authors demonstrate the feasibility of the electro-oxidation of glycerol to GC.184–187 Kim et al.184 used nanostructured Au catalysts at the anode of an electrochemical cell, reporting a glycerol conversion of ∼51% and GC selectivity ∼47% at 1.0 V (vs. RHE). The selectivity to GC was attributed to the enhanced facet-dependent OH adsorption, especially at the (100) and (110) sites. Lee et al.185 reported a selectivity to GC up to 72% with a yield of 66% using an anode based on carbon-black and Amberlyst-15. Brix et al.186 used a nickel boride electrocatalyst, forming GC and other products. At a potential of 0.4–45 V vs. Ag/AgCl/KCl (3 M), GC is the main product. Santiago et al.187 used LaNiO3 and LaCoO3 perovskite as anodes, forming nearly equimolar amounts of formate and glycolate. Thus, different performances have been shown depending on the electrode, but up to high selectivities to GC have been demonstrated in glycerol electro-oxidation.
The reasons for the differences to that observed in organic and enzymatic chemistry are still unclear, but likely related to the different reaction mechanisms. On the other hand, this aspect demonstrates how different and novel reaction mechanisms could be present in electrocatalysis.
Oxalic electrocatalytic reduction to GC has also been demonstrated. Abramo et al.188 used as electrocatalysts an ordered array of vertically aligned TiO2 nanotubes, on top of which some small partially-reduced titania nanoparticles are present. The synergy between these two elements is necessary.
GC with faradaic selectivities up to about 60% was observed. Masaaki et al.189 used TiO2 on Ti mesh or Ti felt as a cathode to obtain similar faradaic selectivities. Zhao et al.190 used roughened TiO2 film electrodes, forming mainly glyoxylic acid. This molecule is an intermediate in the hydrogenation of oxalic acid to GC. De Luca et al.191 used g-C3N4 decorated TiO2 nanotube thin films as cathodic electrodes for the selective reduction of oxalic acid. They found that g-C3N4 promotes the behaviour with FE to GC up to 76%.
Although the tandem electrocatalytic approach was not reported in the literature, the independent cathodic and anodic reactions were proven feasible.
Oxalic acid, rather than being produced by fermentation of biowaste, can also be made from CO2.192 This way offers the possibility of developing a new value chain for C2 products from CO2 by using electrocatalysis. This was the objective of the EU H2020 project OCEAN (Oxalic acid from CO2 using electrochemistry at demonstration scale, project no. 767798; https://www.aspire2050.eu/ocean). The electrocatalytic process was demonstrated up to a pilot unit scale. It is a prototype electrochemical reactor with a stack of electrochemical cells of about 0.4 m2 working at current densities of at least 1.5 kA m−2 to convert 250 g of CO2 into formate per hour. The formate is then thermally converted to oxalic acid, being that this solution is preferable over the direct electrocatalytic synthesis of oxalic acid. Sn-based electrodes convert CO2 to formate with high FE at high current densities.
4. Conclusions
We have provided some examples, without being exhaustive of all the possibilities, of novel valuable directions for innovation in (photo)electrocatalysis by starting from the analysis of the needs in some relevant value chains.These examples and approaches highlight the demand to expand the current area of studies, mainly focused on a restricted range of options. We focused on running or proposed EU projects in which we were involved because it is often difficult to have precise insights into them otherwise. However, for confidentiality, only published data were discussed here.
These projects indicate the innovative effort to realise the challenge of electrifying chemical production. However, as discussed in this manuscript, the fundamental studies to support these novel, industrially-valuable directions are often lacking, despite the impressive number of publications on electrocatalysis and related aspects.
Often, this determines an inadequate recognition of the potential of these novel directions because there is a lack of fundamental background studies. This is an egg and chick problem. The aim of this feature article was thus to remark on the need for a scientific effort to open the horizons in (photo)electrocatalysis to new directions. On the other hand, the example of GC from glycerol demonstrates how electrocatalysis could produce chemicals that are otherwise difficult to synthesise by organic or enzymatic chemistry.
Other relevant research areas were discussed earlier11 in outlining the feasibility of fossil-free chemical production. The new areas of development indicated were the following: (i) benzene direct electrocatalytic hydroxylation to phenol, (ii) novel electrocatalytic routes by coupling the products of the conversion of small molecules (CO2, N2, H2O) with the in situ functionalisation of biobased molecules, (iii) electro-carboxylation of olefins, diolefins or aromatics, (iv) use of the reactive intermediates formed in the electrocatalytic conversion of small molecules to make new paths, and (v) the electrocatalytic conversion of biomethane.
These developments and those discussed here require improving the fundamental bases for (photo)electrocatalysis. Among the different necessary aspects: (i) the design of advanced (photo)electrocatalytic reactors, (ii) the study of multiphasic (photo)electrocatalytic reactors, (iii) understanding of the differences, including in mechanistic aspects, between (photo)electrocatalysis and conventional heterogeneous catalysis, (iv) develop new approaches and explore novel (complex) pathways, and (v) analyse the complex interplay determining behaviour (rational design of three-phase boundary address the selectivity challenges of electrocatalysts).
Creating a new value chain for chemical production to substitute fossil fuels requires advances in the (photo)electrocatalysis approach and exploring new possibilities, some of which are discussed here as an example. It is necessary to accelerate the progress in this area by exploring new solutions, reactions and topics rather than focusing only on mechanistic aspects and developing new catalysts.
Notwithstanding the many studies in electrocatalysis, there is still too limited research on creative directions. The most research effort is restricted to a few areas, such as CO2 or N2 electrocatalytic reduction. Meeting the ambitious net-zero emissions target by 2050 requires accelerating research in the directions outlined in this highlight manuscript.
Abbreviation
| BHMF | Bis(hydroxymethyl)furan |
| CAPEX | Capital expenditures |
| CO2RR | CO2 reduction reaction |
| EU | European Union |
| FA | Formic acid |
| FDCA | Furan dicarboxylic acid |
| FE | Faradaic efficiency |
| GC | Glycolic acid |
| HB | Haber–Bosch (process) |
| HER | Hydrogen evolution reaction |
| HMF | 5-(Hydroxymethyl)furfural |
| LOHC | Liquid organic hydrogen carriers |
| NOR | Nitrogen oxidation reaction |
| NRR | Nitrogen reduction reaction |
| OER | Oxygen evolution reaction |
| PCET | Proton-coupled electron transfer |
| PEC | Photoelectrocatalytic |
| PEF | Poly(ethylene 2,5-furanoate) |
| OPEX | Operative expenditures |
| RFB | Redox flow battery |
| RHE | Reverse hydrogen electrode |
| SFB | Solar flow battery |
Author contributions
All authors equally contributed to the realisation of this manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The EU supported this work with the ERC Synergy SCOPE project (810182), EU DECADE (nr. 862030) and EPOCH (nr. 101070976) projects. G. C. also thanks the Alexander von Humboldt-Stiftung/Foundation (Humboldt Research Award).References
- P. Gabrielli, M. Gazzani and M. Mazzotti, Ind. Eng. Chem. Res., 2020, 59, 7033 CrossRef.
- K. M. Van Geem and B. M. Weckhuysen, MRS Bull., 2021, 46, 1187 CrossRef.
- S. Perathoner, K. M. Van Geem, G. B. Marin and G. Centi, Chem. Commun., 2021, 57, 10967 RSC.
- F. Ausfelder (Dechema), Defossilization in the chemical industry – challenges and opportunities, presented at International Energy Agency (IEA) workshop Deep decarbonisation in Industry – Final Report, Vienna (Austria) Oct. 2019.
- S. Brethauer and M. H.-P. Studer, Chimia, 2021, 75, 788 CrossRef CAS PubMed.
- G. Papanikolaou, G. Centi, S. Perathoner and P. Lanzafame, Chin. J. Catal., 2022, 43, 1194 CrossRef CAS.
- Z. J. Schiffer and K. Manthiram, Joule, 2017, 1, 10 CrossRef.
- G. Centi and S. Perathoner, Catal. Today, 2022, 387, 216 CrossRef CAS.
- A. I. Stankiewicz and H. Nigar, React. Chem. Eng., 2020, 5, 1005 RSC.
- G. Papanikolaou, G. Centi, S. Perathoner and P. Lanzafame, ACS Catal., 2022, 12, 2861 CrossRef CAS PubMed.
- G. Centi and S. Perathoner, Green Chem., 2022, 24, 7305 RSC.
- C. Tang, Y. Zheng, M. Jaroniec and S.-Z. Qiao, Angew. Chem., Int. Ed., 2021, 60, 19572 CrossRef CAS PubMed.
- A. Yazdani and G. G. Botte, Engineering, 2020, 29, 89 Search PubMed.
- S. Perathoner and G. Centi, Catal. Today, 2019, 330, 157 CrossRef CAS.
- Q. Tu, A. Parvatker, M. Garedew, C. Harris, M. Eckelman, J. B. Zimmerman, P. T. Anastas and C. Ho Lam, Environ. Sci. Technol., 2021, 55, 3240 CrossRef CAS PubMed.
- X. Guo, J. Gu, S. Lin, S. Zhang, Z. Chen and S. Huang, J. Appl. Chem. Sci., 2020, 142, 5709 CAS.
- T. Tang, Z. Wang and J. Guan, Adv. Funct. Mater., 2022, 32, 2111504 CrossRef CAS.
- H. Li, X. Qin, T. Jiang, X.-Y. Ma, K. Jiang and W.-B. Cai, ChemCatChem, 2019, 11, 6139 CrossRef CAS.
- G.-F. Chen, S. Y. Ren, L. L. Zhang, H. Cheng, Y. R. Luo, K. H. Zhu, L.-X. Ding and H. H. Wang, Small Methods, 2019, 3, 1800337 CrossRef.
- C. Choi, G. Ho Gu, J. Noh, H. S. Park and Y. Jung, Nat. Commun., 2021, 12, 4353 CrossRef CAS PubMed.
- C. Wulf, P. Zapp and A. Schreiber, Front. Energy Res., 2020, 8, 191 CrossRef.
- M. Sterner and M. Specht, Energies, 2020, 14, 6594 CrossRef.
- A. C. Ince, C. O. Colpan, A. Hagen and M. F. Serincan, Fuel, 2021, 304, 121354 CrossRef CAS.
- M. Hermesmann, K. Gruebel, L. Scherotzki and T. E. Mueller, Renewable Sustainable Energy Rev., 2021, 138, 110644 CrossRef CAS.
- G. Centi and S. Perathoner, Catal. Today, 2020, 342, 4 CrossRef CAS.
- Z. Chehade, C. Mansilla, P. Lucchese, S. Hilliard and J. Proost, Int. J. Hydrogen Energy, 2019, 44, 27637 CrossRef CAS.
- S. Abate, K. Barbera, G. Centi, P. Lanzafame and S. Perathoner, Catal.: Sci. Technol., 2016, 6, 2485 RSC.
- G. Centi and S. Perathoner, Catal. Today, 2022 DOI:10.1016/j.cattod.2022.10.017.
- R. Schlögl, Green Chem., 2021, 23, 1584 RSC.
- IRENA (International Renewable Energy Agency), Green Hydrogen Cost Reduction: Scaling up Electrolysers to Meet the 1.5 °C Climate Goal, IRENA, Abu Dhabi 2020.
- Hydrogen Council, Path to hydrogen competitiveness. A cost perspective, Hydrogen Council, Brussels (Belgium) 2020.
- N. Salmon and R. Bañares-Alcántara, Sustainable Energy Fuels, 2021, 5, 2814 RSC.
- IEA (International Energy Agency), Global Hydrogen Review 2022, IEA, Paris 2022.
- Z. Abdin, A. Zafaranloo, A. Rafiee, W. Mérida, W. Lipiński and K. R. Khalilpour, Renewable Sustainable Energy Rev., 2020, 120, 109620 CrossRef CAS.
- D. Guilbert and G. Vitale, Clean Technol., 2021, 3, 881 CrossRef.
- P. Preuster, C. Papp and P. Wasserscheid, Acc. Chem. Res., 2017, 50, 74 CrossRef CAS PubMed.
- M. Markiewicz, Y. Q. Zhang, A. Bösmann, N. Brückner, J. Thöming, P. Wasserscheid and S. Stolte, Energy Environ. Sci., 2015, 8, 1035 RSC.
- P. C. Rao and M. Yoon, Energies, 2020, 13, 6040 CrossRef CAS.
- M. Niermann, S. Drünert, M. Kaltschmitt and K. Bonhoff, Energy Environ. Sci., 2019, 12, 290 RSC.
- V. Yadav, G. Sivakumar, V. Gupt and E. Balaraman, ACS Catal., 2021, 11, 14712 CrossRef CAS.
- M. Wang, Z. Wang, X. Gong and Z. Guo, Renewable Sustainable Energy Rev., 2014, 29, 573 CrossRef CAS.
- J.-Y. Cho, H. Kim, J.-E. Oh and B. Y. Park, Catalysts, 2021, 11, 1497 CrossRef CAS.
- H. G. Shiraz, M. Vagin, T.-P. Ruoko, V. Gueskine, K. Karoń, M. Łapkowski, T. Abrahamsson, T. Ederth, M. Berggren and X. Crispin, J. Energy Chem., 2022, 73, 292 CrossRef CAS.
- K. Sisáková, N. Podrojková, R. Oriňaková and A. Oriňak, Energy Fuels, 2021, 35(9), 7608 CrossRef.
- A. Ali, G. U. Kumar and H. J. Lee, Mater. Today: Proc., 2021, 45, 1123 CAS.
- T. Chiba, M. Okimoto, H. Nagai and Y. Takata, Bull. Chem. Soc. Jpn., 1983, 56, 719 CrossRef CAS.
- Y. Inami, H. Ogihara, S. Nagamatsu, K. Asakura and I. Yamanaka, ACS Catal., 2019, 9, 2448 CrossRef CAS.
- K. S. Exner and H. Over, ACS Catal., 2019, 9, 6755 CrossRef CAS.
- B. You and Y. Sun, Acc. Chem. Res., 2018, 51, 1571 CrossRef CAS PubMed.
- J. Jack, W. Zhu, J. L. Avalos, J. Gong and Z. J. Ren, Green Chem., 2021, 23, 7917 RSC.
- M. T. Bender, X. Yuan and K.-S. Choi, Nat. Commun., 2020, 11, 4594 CrossRef CAS PubMed.
- P. Sivagurunathan, T. Raj, C. S. Mohanta, S. Semwal, A. Satlewal, R. P. Gupta, S. K. Puri, S. S. V. Ramakumar and R. Kumar, Chemosphere, 2021, 268, 129326 CrossRef CAS PubMed.
- S. Sethupathy, G. M. Morales, L. Gao, H. Wang, B. Yang, J. Jiang, J. Sun and D. Zhu, Bioresour. Technol., 2022, 347, 126696 CrossRef CAS PubMed.
- L. Cao, I. K. M. Yu, Y. Liu, X. Ruan, D. C. W. Tsang, A. J. Hunt, Y. S. Ok, H. Song and S. Zhang, Bioresour. Technol., 2018, 269, 465 CrossRef CAS PubMed.
- T. Rafione, M. Marinova, L. Montastruc and J. Paris, Appl. Therm. Eng., 2014, 73, 74 CrossRef.
- B. Annevelink, L. G. Chavez, R. van Ree and I. V. Gursel, Global biorefinery status report 2022, IEA Bioenergy: Biorefining in a circular economy, 2022.
- C. Yang, S. Maldonado and C. R. J. Stephenson, ACS Catal., 2021, 11, 10104 CrossRef CAS.
- M. Garedew, F. Lin, B. Song, T. M. DeWinter, J. E. Jackson, C. M. Saffron, C. H. Lam and P. T. Anastas, ChemSusChem, 2020, 13, 4214 CrossRef CAS PubMed.
- X. Du, H. Zhang, K. P. Sullivan, P. Gogoi and Y. Deng, ChemSusChem, 2020, 13, 4318 CrossRef CAS PubMed.
- O. Movil-Cabrera, A. Rodriguez-Silva, C. Arroyo-Torres and J. A. Staser, Biomass Bioenergy, 2016, 88, 89 CrossRef CAS.
- F. Bateni, R. Ghahremani and J. A. Staser, J. Appl. Electrochem., 2021, 51, 65 CrossRef CAS.
- Y. P. Wijaya, K. J. Smith, C. S. Kim and E. L. Gyenge, Green Chem., 2020, 22, 7233 RSC.
- J.-Y. Cho, H. Kim, J.-E. Oh and B. Y. Park, Catalysts, 2021, 11, 1497 CrossRef CAS.
- H. Jorschick, P. Preuster, A. Bösmann and P. Wasserscheid, Sustainable Energy Fuels, 2021, 5, 1311 RSC.
- X. Chen, C. H. Gierlich, S. Schötz, D. Blaumeiser, T. Bauer, J. Libuda and R. Palkovits, ACS Sustainable Chem. Eng., 2021, 9, 6561 CrossRef CAS.
- T. W. Kim, H. Jeong, J. H. Baik and Y.-W. Suh, Chem. Lett., 2022, 51, 239 CrossRef CAS.
- K. Müller, T. Skeledzic and P. Wasserscheid, Energy Fuels, 2021, 35, 10929 CrossRef.
- Y. Yang and T. Mu, Green Chem., 2021, 23, 4228 RSC.
- H. Liu and W. Li, Curr. Opin. Electrochem., 2021, 30, 100795 CrossRef CAS.
- Y. Zhou, Y. Shen and H. Li, Appl. Catal., B, 2022, 317, 121776 CrossRef CAS.
- K. Li and Y. Sun, Chem. – Eur. J., 2018, 24, 18258 CrossRef CAS PubMed.
- H. Wu, J. Song, H. Liu, Z. Xie, C. Xie, Y. Hu, X. Huang, M. Hua and B. Han, Chem. Sci., 2019, 10, 4692 RSC.
- A. M. Román, J. C. Hasse, J. W. Medlin and A. Holewinski, ACS Catal., 2019, 9, 10305 CrossRef.
- S. R. Kubota and K.-S. Choi, ACS Sustainable Chem. Eng., 2018, 6, 9596 CrossRef CAS.
- T. Li and D. A. Harrington, ChemSusChem, 2021, 14, 1472 CrossRef CAS PubMed.
- C. Coutanceau, S. Baranton and R. S. Bitty Kouamé, Front. Chem., 2019, 7, 100 CrossRef CAS PubMed.
- L. Fan, B. Liu, X. Liu, N. Senthilkumar, G. Wang and Z. Wen, Energy Technol., 2021, 9, 2000804 CrossRef CAS.
- J. Sousa Cardoso, V. Silva, R. C. Rocha, M. J. Hall, M. Costa and D. Eusébio, J. Cleaner Prod., 2021, 296, 126562 CrossRef.
- N. Salmon and R. Bañares-Alcántara, Sustainable Energy Fuels, 2021, 5, 2814 RSC.
- S. Chatterjee, R. K. Parsapur and K.-W. Huang, ACS Energy Lett., 2021, 6, 4390 CrossRef CAS.
- Alfa Laval, Hafnia, Haldor Topsoe, Vestas, Siemens Gamesa, Ammonfuel An industrial view of ammonia as a marine fuel, August 2020. Available on 08/08/2022 at https://www.topsoe.com/hubfs/DOWNLOADS/DOWNLOADS%20-%20White%20papers/Ammonfuel%20Report%20Version%2009.9%20August%203_update.pdf.
- A. H. Tullo, Is ammonia the fuel of the future?, Chem. Eng. News, 2021, 99, 8 Search PubMed.
- M. Nazemi and M. A. El-Sayed, Acc. Chem. Res., 2021, 54(23), 4294 CrossRef CAS PubMed.
- D. Mallamace, G. Papanikolaou, S. Perathoner, G. Centi and P. Lanzafame, Int. J. Mol. Sci., 2020, 22, 139 CrossRef PubMed.
- F. Jiao and B. J. A. M. Xu, Adv. Mater., 2019, 31, 1805173 CrossRef PubMed.
- Y. Ren, C. Yu, X. Tan, H. Huang, Q. Wei and J. Qiu, Energy Environ. Sci., 2021, 14, 1176 RSC.
- Y. Tanabe and Y. Nishibayashi, Chem. Soc. Rev., 2021, 50, 5201 RSC.
- N. Morlanes, S. P. Katikaneni, S. N. Paglieri, A. Harale, B. Solami, S. M. Sarathy and J. A. Gascon, Chem. Eng. J., 2021, 408, 127310 CrossRef CAS.
- X. Han, Q. Gao, Z. Yan, M. Ji, C. Long and H. Zhu, Nanoscale, 2021, 13, 1515 RSC.
- Y. Fu, Y. Liao, P. Li, H. Li, S. Jiang, H. Huang, W. Sun, T. Li, H. Yu, K. Li, H. Li, B. Jia and T. Ma, Coord. Chem. Rev., 2022, 460, 214468 CrossRef CAS.
- S. Chen, S. Perathoner, C. Ampelli and G. Centi, Stud. Surf. Sci. Catal., 2019, 178, 31–46 CrossRef CAS.
- J. Kibsgaard, J. K. Nørskov and I. Chorkendorff, ACS Energy Lett., 2019, 2986–2988 CrossRef CAS.
- Z.-H. Xue, S.-N. Zhang, Y.-X. Lin, H. Su, G.-Y. Zhai, J.-T. Han, Q.-Y. Yu, X.-H. Li, M. Antonietti and J.-S. Chen, J. Appl. Chem. Sci., 2019, 141, 14976 CAS.
- G. Centi and S. Perathoner, Small, 2021, 17, 2007055 CrossRef CAS PubMed.
- M. Bozorg, B. Addis, V. Piccialli, Á. Ramírez-Santos, C. Castel, I. Pinnau and E. Favre, Chem. Eng. Sci., 2019, 207, 1196 CrossRef CAS.
- J. W. Yoon, H. Chang, S. J. Lee, Y. K. Hwang, D. Y. Hong, S. K. Lee, J. S. Lee, S. Jang, T. U. Yoon, K. Kwac, Y. Jung, R. S. Pillai, F. Faucher, A. Vimont, M. Daturi, G. Férey, C. Serre, G. Maurin, Y. S. Bae and J. S. Chang, Nat. Mater., 2017, 16, 526 CrossRef CAS PubMed.
- S. Liguori, K. Lee and J. Wilcox, J. Membr. Sci., 2019, 585, 52 CrossRef CAS.
- B. A. MacKay and M. D. Fryzuk, Chem. Rev., 2004, 104, 385 CrossRef CAS PubMed.
- Z. Wang, D. Richards and N. Singh, Catal. Sci. Technol., 2021, 11, 705 RSC.
- W. Jung and Y. J. Hwang, Mater. Chem. Front., 2021, 5, 6803 RSC.
- J. Long, S. Chen, Y. Zhang, C. Guo, X. Fu, D. Deng and J. Xiao, Angew. Chem., Int. Ed., 2020, 59, 9711 CrossRef CAS PubMed.
- B. H. Ko, B. Hasa, H. Shin, Y. Zhao and F. Jiao, J. Am. Chem. Soc., 2022, 144, 1258 CrossRef CAS PubMed.
- H. Wan, A. Bagger and J. Rossmeisl, J. Phys. Chem. Lett., 2022, 13, 8928 CrossRef CAS PubMed.
- Y. Wang, T. Li, Y. Yu and B. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202115409 CAS.
- C. Dai, Y. Sun, G. Chen, A. C. Fisher and Z. J. Xu, Angew. Chem., Int. Ed., 2020, 59, 9418 CrossRef CAS PubMed.
- Y. Guo, S. Zhang, R. Zhang, D. Wang, D. Zhu, X. Wang, D. Xiao, N. Li, Y. Zhao, Z. Huang, W. Xu, S. Chen, L. Song, J. Fan, Q. Chen and C. Zhi, ACS Nano, 2022, 16, 655 CrossRef CAS PubMed.
- M. Anand, C. S. Abraham and J. K. Nørskov, Chem. Sci., 2021, 12, 6442 RSC.
- B. S. Patil, F. J. J. Peeters, G. J. van Rooij, J. A. Medrano, F. Gallucci, J. Lang, Q. Wang and V. Hessel, AIChE J., 2018, 64, 526 CrossRef CAS.
- L. R. Winter and J. G. Chen, Joule, 2021, 5, 300 CrossRef CAS.
- C. Sze, B. Wang, J. Xu, J. Rivas-Davila and M. A. Cappelli, RSC Adv., 2021, 11, 37886 RSC.
- R. K. Sharma, H. Patel, U. Mushtaq, V. Kyriakou, G. Zafeiropoulos, F. Peeters, S. Welzel, M. C. M. van de Sanden and M. N. Tsampas, ACS Energy Lett., 2021, 6(2), 313 CrossRef CAS.
- H. Chen, D. Yuan, A. Wu, X. Lin and X. Li, Environ. Prog. Sustain. Energy., 2021, 3, 201 Search PubMed.
- Y. Kwon, K. J. P. Schouten, J. C. van der Waal, E. de Jong and M. T. M. Koper, ACS Catal., 2016, 6, 6704 CrossRef CAS.
- C. Candia-Onfray, S. Rojas, M. V. Boldrin Zanoni and R. Salazar, Curr. Opin. Electrochem., 2021, 26, 100669 CrossRef CAS.
- Á. Vass, B. Endrődi and C. Janáky, Curr. Opin. Electrochem., 2021, 25, 100621 CrossRef.
- S. Sabatino, A. Galia, G. Saracco and O. Scialdone, ChemElectroChem, 2017, 4, 150 CrossRef CAS.
- E. de Jong, A. Visser, A. Sousa Dias, C. Harvey and G.-J. M. Gruter, Polymers, 2022, 14, 943 CrossRef CAS PubMed.
- X. Fei, J. Wang, J. Zhu, X. Wang and X. Liu, ACS Sustainable Chem. Eng., 2020, 8, 8471 CrossRef CAS.
- D. A. Giannakoudakis, J. C. Colmenares, D. Tsiplakides and K. S. Triantafyllidis, ACS Sustainable Chem. Eng., 2021, 9, 1970 CrossRef CAS.
- Y. Zhao, M. Cai, J. Xian, Y. Suna and G. Li, J. Mater. Chem. A, 2021, 9, 20164 RSC.
- A. D. K. Deshan, L. Atanda, L. Moghaddam, D. W. Rackemann, J. Beltramini and W. O. S. Doherty, Front. Chem., 2020, 8, 659 CrossRef CAS PubMed.
- M. Sajid, X. Zhao and D. Liu, Green Chem., 2018, 20, 5427 RSC.
- G. Centi, G. Iaquaniello and S. Perathoner, BMC Chem. Eng., 2019, 1, 1 CrossRef.
- X. H. Chadderdon, D. J. Chadderdon, T. Pfennig, B. H. Shanks and W. Li, Green Chem., 2019, 21, 6210 RSC.
- H. Liu, T.-H. Lee, Y. Chen, E. W. Cochran and W. Li, ChemElectroChem, 2021, 8, 2817 CrossRef CAS.
- X. Fei, J. Wang, X. Zhang, Z. Jia, Y. Jiang and X. Liu, Polymers, 2022, 14, 625 CrossRef CAS PubMed.
- J. Zhang, T. Wang, X. Tang, L. Peng, J. Weiand and L. Lin, BioRes., 2018, 13, 7137 Search PubMed.
- E. Skoog, J. H. Shin, V. Saez-Jimenez, V. Mapelli and L. Olsson, Biotechnol. Adv., 2018, 36, 2248 CrossRef CAS PubMed.
- M. Lang and H. Li, ChemSusChem, 2022, 15, e202101531 CrossRef CAS PubMed.
- G. Moggia, T. Kenis, N. Daems and T. Breugelmans, ChemElectroChem, 2020, 7, 86 CrossRef CAS.
- W.-J. Liu, Z. Xu, D. Zhao, X.-Q. Pan, H.-C. Li, X. Hu, Z.-Y. Fan, W.-K. Wang, G.-H. Zhao, S. Jin, G. W. Huber and H.-Q. Yu, Nat. Commun., 2020, 11, 265 CrossRef CAS PubMed.
- G. Moggia, J. Schalck, N. Daems and T. Breugelmans, Electrochim. Acta, 2021, 374, 137852 CrossRef CAS.
- V. Vedovato, K. Vanbroekhoven, D. Pant and J. Helsen, Molecules, 2020, 25, 3712 CrossRef CAS PubMed.
- F. W. S. Lucas, R. G. Grim, S. A. Tacey, C. A. Downes, J. Hasse, A. M. Roman, C. A. Farberow, J. A. Schaidle and A. Holewinski, ACS Energy Lett., 2021, 6, 1205 CrossRef CAS.
- O. O. James, W. Sauter and U. Schröder, ChemSusChem, 2017, 10, 2015 CrossRef CAS PubMed.
- J. Na, B. Seo, J. Kim, C. W. Lee, H. Lee, Y. J. Hwang, B. K. Min, D. K. Lee, H.-S. Oh and U. Lee, Nat. Commun., 2019, 10, 5193 CrossRef PubMed.
- S. Abate, P. Lanzafame, S. Perathoner and G. Centi, ChemSusChem, 2015, 8, 2854 CrossRef CAS PubMed.
- D. Ding, Y. Zhang, W. Wu, D. Chen, M. Liu and T. He, Energy Environ. Sci., 2018, 11, 1710 RSC.
- Z. Zhang, L. Xin and W. Li, Appl. Catal., B, 2012, 119–120, 40 CrossRef CAS.
- I. Yamanaka and K. Otsuka, Electrochim. Acta, 1989, 34, 211 CrossRef CAS.
- C. L. Childers, H. Huang and C. Korzeniewski, Langmuir, 1999, 15, 786 CrossRef CAS.
- A. Kumar, V. Hasija, A. Sudhaik, P. Raizada, Q. Van Le, P. Singh, T.-H. Pham, T. Y. Kim, S. Ghotekar and V.-H. Nguyen, Chem. Eng. J., 2022, 430, 133031 CrossRef CAS.
- H. Zhou, R. Yan, D. Zhang and T. Fan, Chem. – Eur. J., 2016, 22, 9870 CrossRef CAS PubMed.
- S. Bensaid, G. Centi, E. Garrone, S. Perathoner and G. Saracco, ChemSusChem, 2012, 5, 500 CrossRef CAS PubMed.
- P. D. Nguyen, T. M. Duong and P. D. Tran, J. Sci.: Adv. Mater. Devices, 2017, 2, 399 Search PubMed.
- V. Romano, G. D’Angelo, S. Perathoner and G. Centi, Energy Environ. Sci., 2021, 14, 5760 RSC.
- D. K. Dogutan and D. G. Nocera, Acc. Chem. Res., 2019, 52, 314 CrossRef PubMed.
- F. Tavella, D. Giusi and C. Ampelli, Curr. Opin. Green Sustainable Chem., 2022, 35, 100604 CrossRef CAS.
- J. Proost, Int. J. Hydrogen Energy, 2019, 44, 4406 CrossRef CAS.
- International Renewable Energy Agency (IRENA), Green Hydrogen Cost Reduction: Scaling up Electrolysers to Meet the 1.5 °C Climate Goal, IRENA, Abu Dhabi, 2020.
- V. Romano, G. D’Angelo, S. Perathoner and G. Centi, Energy Environ. Sci., 2021, 14, 5760 RSC.
- E. Sánchez-Díez, E. Ventosa, M. Guarnieri, A. Trovò, C. Flox, R. Marcilla, F. Soavi, P. Mazur, E. Aranzabe and R. Ferret, J. Power Sources, 2021, 481, 228804 CrossRef.
- P. Lu, P. Leung, H. Su, W. Yang and Q. Xu, Appl. Energy, 2021, 282, 116210 CrossRef CAS.
- G. Centi, ChemSusChem, 2022, 15, e202200007 CrossRef CAS PubMed.
- P. Ifkovits, J. M. Evans, M. C. Meier, K. M. Papadantonakis and N. S. Lewis, Energy Environ. Sci., 2021, 14, 4740 RSC.
- X. Liu, J. Chi, B. Dong and Y. Sun, ChemElectroChem, 2019, 8, 215 Search PubMed.
- P. J. McHugh, A. D. Stergiou and M. D. Symes, Adv. Energy Mater., 2020, 10, 2002453 CrossRef CAS.
- F. Zhang and Q. Wang, ACS Mater. Lett., 2021, 3, 641 CAS.
- F. Wang, W. Li, R. Wang, T. Guo, H. Sheng, H.-C. Fu, S. S. Stahl and S. Jin, Joule, 2021, 5, 149 CrossRef CAS.
- J. Huang, Y. Xie, L. Yan, B. Wang, T. Kong, X. Dong, Y. Wang and Y. Xia, Energy Environ. Sci., 2021, 14, 883 RSC.
- A. Landman, H. Dotan, G. E. Shter, M. Wullenkord, A. Houaijia, A. Maljusch, G. S. Grader and A. Rothschild, Nat. Mater., 2017, 16, 646 CrossRef CAS PubMed.
- X. Wang, J. Yang, M. Salla, S. Xi, Y. Yang, M. Li, F. Zhang, M.-K. Zhu, S. Huang, S. Huang, Y.-W. Zhang and Q. Wang, Angew. Chem., Int. Ed., 2021, 60, 18721 CrossRef CAS PubMed.
- V. Kumaravel, J. Bartlett and S. C. Pillai, ACS Energy Lett., 2020, 5, 486 CrossRef CAS.
- R. Zhao, P. Ding, P. Wei, L. Zhang, Q. Liu, Y. Luo, T. Li, S. Lu, X. Shi, S. Gao, A. M. Asiri, Z. Wang and X. Sun, Adv. Funct. Mater., 2021, 31, 2009449 CrossRef CAS.
- J. Li, L. Jiang, S. He, L. Wei, R. Zhou, J. Zhang, D. Yuan and S. P. Jiang, Energy Fuels, 2019, 33, 12052 CrossRef CAS.
- A. Landman, S. Hadash, G. E. Shter, A. Ben-Azaria, H. Dotan, A. Rothschild and G. S. Grader, Adv. Funct. Mater., 2021, 31, 2008118 CrossRef CAS.
- N. Hayek, A. Landman, Y. Halpern, I. Slobodkin, E. S. Davydova and A. Rothschild, J. Mater. Chem. A, 2022, 10, 726 RSC.
- Y. T. Yan, J. H. Lin, J. Cao, S. Guo, X. H. Zheng, J. C. Feng and J. L. Qi, J. Mater. Chem. A, 2019, 7, 24486 RSC.
- Fi Wang, W. Li, R. Wang, T. Guo, H. Sheng, H.-C. Fu, S. S. Stahl and S. Jin, Joule, 2021, 5, 149 CrossRef CAS.
- D. Frey, J. Kim, Y. Dvorkin and M. A. Modestino, Cell Rep. Phys. Sci., 2020, 1, 100226 CrossRef CAS.
- A. Ho, X. Zhou, L. Han, I. Sullivan, C. Karp, N. S. Lewis and C. Xiang, ACS Energy Lett., 2019, 4, 968 CrossRef CAS.
- W. J. Li, H. C. Fu, L. S. Li, M. Caban-Acevedo, J. H. He and S. Jin, Angew. Chem., Int. Ed., 2016, 55, 13104 CrossRef CAS PubMed.
- W. J. Li, H. C. Fu, Y. Z. Zhao, J. H. He and S. Jin, Chem, 2018, 4, 2644 CAS.
- W. Li, E. Kerr, M.-A. Goulet, H.-C. Fu, Y. Zhao, Y. Yang, A. Veyssal, J.-H. He, R. G. Gordon, J. M. Aziz and S. Jin, Adv. Energy Mater., 2019, 9, 1900918 CrossRef.
- X. Wei, W. Pan, W. Duan, A. Hollas, Z. Yang, B. Li, Z. Nie, J. Liu, D. Reed, W. Wang and V. Sprenkle, ACS Energy Lett., 2017, 2, 2187 CrossRef CAS.
- P. K. Leung, A. A. Shah, L. Sanz, C. Flox, J. R. Morante, Q. Xu, M. R. Mohamed, C. Ponce-de-León and F. C. Walsh, J. Power Sources, 2017, 360, 243 CrossRef CAS.
- C. Ampelli, D. Giusi, M. Miceli, T. Merdzhanova, V. Smirnov, U. Chime, O. Astakhov, A. Martin Fernandez, F. Veenstra, F. Garcés-Pineda, J. Gonzalez-Cobos, M. García-Tecedor, S. Gimenez, W. Jaegermann, G. Centi, J. Pérez-Ramírez, J. Galan-Mascaros and S. Perathoner, Energy Environ. Sci., 2022 Search PubMed , submitted.
- L. Dai, Q. Qin, X. Zhao, C. Xu, C. Hu, S. Mo, Y. O. Wang, S. Lin, Z. Tang and N. Zheng, ACS Cent. Sci., 2016, 2, 538 CrossRef CAS PubMed.
- S. H. Langer and J. C. Card, J. Mol. Catal., 1987, 42, 331 CrossRef CAS.
- Y. Liu, S. Chen, X. Quan and H. Yu, J. Appl. Chem. Sci., 2015, 137, 11631 CAS.
- B. Zha, C. Li and J. Li, J. Catal., 2020, 382, 69 CrossRef CAS.
- R. De, S. Gonglach, S. Paul, M. Haas, S. S. Sreejith, P. Gerschel, U.-P. Apfel, T. H. Vuong, J. Rabeah, S. Roy and W. Schöfberger, Angew. Chem., Int. Ed., 2020, 59, 10527 CrossRef CAS PubMed.
- C. Genovese, C. Ampelli, S. Perathoner and G. Centi, Green Chem., 2017, 19, 2406 RSC.
- D. Kim, L. S. Oh, Y. C. Tan, H. Song, H. J. Kim and J. Oh, ACS Catal., 2021, 11, 14926 CrossRef CAS.
- C. S. Lee, M. K. Aroua, W. A. W. Daud, P. Cogne, Y. Pérès and M. A. Ajeel, Front. Chem., 2019, 7, 110 CrossRef CAS PubMed.
- A. C. Brix, D. M. Morales, M. Braun, D. Jambrec, J. R. C. Junqueira, S. Cychy, S. Seisel, J. Masa, M. Muhler, C. Andronescu and W. Schuhmann, ChemElectroChem, 2021, 8, 2336 CrossRef CAS.
- P. V. B. Santiago, C. C. Lima, J. L. Bott-Neto, P. S. Fernández, C. A. Angelucci and J. Souza-Garcia, J. Electroanal. Chem., 2021, 896, 115198 CrossRef CAS.
- F. P. Abramo, F. De Luca, R. Passalacqua, G. Centi, G. Giorgianni, S. Perathoner and S. Abate, J. Energy Chem., 2022, 68, 669 CrossRef CAS.
- S. Masaaki, H. Shinichi, C. Xuedong, Y. Miho, S. Masaaki and Y. Miho, Sci. Rep., 2017, 7, 17032 CrossRef PubMed.
- F. Zhao, F. Yan, Y. Qian, Y. Xu and C. Ma, J. Electroanal. Chem., 2013, 698, 31 CrossRef CAS.
- F. De Luca, R. Passalacqua, F. P. Abramo, S. Perathoner, G. Centi and S. Abate, Chem. Eng. Trans., 2021, 84, 37 Search PubMed.
- E. Schuler, M. Demetriou, N. R. Shiju and G.-J. M. Gruter, ChemSusChem, 2021, 14, 3636 CrossRef CAS PubMed.
Footnotes |
| † Presented by G. Centi as plenary lecture at 19th Nordic Symposium on Catalysis, Espoo (Finland), 6–8th June 2022 and the Symposium on Materials for Emerging Energy Materials, 19 and 20th May 2022, IMDEA Energy Institute, Madrid – Spain. |
| ‡ This manuscript summarises concepts and discussions that emerged from the large EU initiative SUNERGY (fossil-free fuels and chemicals for a climate-neutral Europe; https://www.sunergyinitiative.eu). |
| This journal is © The Royal Society of Chemistry 2023 |