Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photoreductive β-aminoalkylation with amino acids affords functionalized γ-aminoketones for nucleoside mimics†
Sebastian O.
Klein
 ab,
Adina A.
Baniahmad
ab,
Adina A.
Baniahmad
 a and
Manfred
Jung
a and
Manfred
Jung
 *ab
*ab
aInstitute of Pharmaceutical Sciences, University of Freiburg, Albertstrasse 25, 79104 Freiburg, Germany. E-mail: manfred.jung@pharmazie.uni-freiburg.de
bCIBSS – Centre for Integrative Biological Signalling Studies, University of Freiburg, Germany
First published on 23rd January 2023
Abstract
We developed a facile photoreductive and stereoselective β-aminoalkylation of a crowded enone by blue LED light irradiation using a wide variety of α-amino acids in order to access 5′-amino substituted carbasugar nucleosides for SAM-based methyltransferase inhibitors. This photochemical method provides highly functionalized carbasugar mimics for nucleoside analogue synthesis.
S-Adenosyl methionine (SAM) is the second most abundant cosubstrate in the human body. It is the major methyl-donor reagent for essential methylation reactions.1 The methyl group is transferred by methyltransferases which exhibit their catalytic activity in a multitude of biological substrates e.g. DNA, RNA, neurotransmitters, and proteins.2 As such methyltransferases play a pivotal role in many biological processes they were recognized as interesting and promising drug targets. Over the past decades the FDA approved several methyltransferase-inhibitors3e.g. azacytidine (2005)4 and tazemetostat (2020).5 Moreover, the PRMT5-inhibitor onametostat and the DOT1L inhibitor pinometostat are clinical candidates.6 This shows the significant therapeutic potential of methyltransferase inhibitors.7 Moreover, 5′-substitued carbocyclic nucleosides received much attention in the development of new antitumor and antiviral therapeutic agents.8 For instance, the natural occurring carbocyclic nucleosides (−)-aristeromycin and (−)-neplanocin A led to the design of the antiviral agents carbavir and abacavir.9 In addition, carbocyclic nucleosides such as 8-azapurine carbocyclic hydrazones10 and fluorocyclopentenyl cytosines11 have anticancer activity.12,13
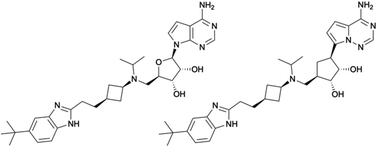
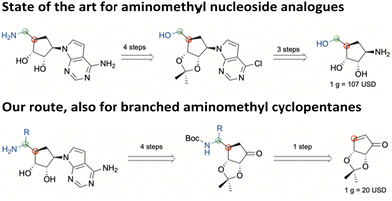
In this regard, SAM-derived nucleosides represent a promising scaffold. Hence, an efficient synthetic pathway is desirable as the current synthesis for functionalized 5′-amino substituted carbasugar nucleosides involves multistep synthesis.6,14,15 Within this work, we report an innovative and highly efficient 5-step synthesis approach for the design of 5′-substituted carbasugar nucleoside analogues. This approach is amenable to diverse alterations towards promising biological active compounds, e.g. the DOT1L inhibitor pinometostat6 or its recent derived analogue MU1656 (Fig. 1).6 Carbasugar nucleosides were chosen due to their higher lipophilicity and metabolic stability as compared to the natural ribose.8
Akita and co-worker as well as Ronghua and co-workers showed the successful photochemically addition of organoborates and α-amino acids to cyclopentenone with a yield of up to 54%, respectively.16,17 Using chiral Lewis acids as a catalyst, Yoon and colleagues were able to demonstrate the effective efficient enantioselective addition of photogenerated α-amino radicals to Michael acceptors18 while our approach was substrate induced diastereoselectivity (Scheme 1).
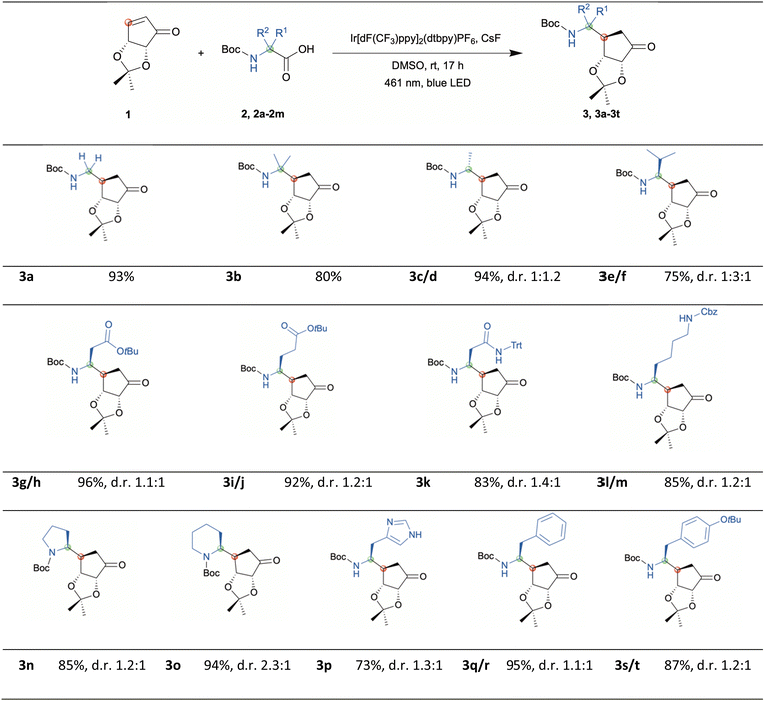
We herein describe the highly efficient photochemical redox reaction between various α-amino acids and an α-β-unsaturated carbasugar mimic driven by blue LED light leading to Boc-protected primary amines. These intermediates can serve for further applications such as the design of 5′-amino carbasugar nucleosides. First Boc-protected glycine was tested in the presence of 3 mol% of the photocatalyst Ir[dF(CF3)ppy]2(dtbpy)PF6, KH2PO4 in DMF at rt under blue LED light irradiation (via a 40W A160WE Tuna Blue lamp from Kessil).19 In this case we achieved the desired coupling product with high efficiency (example 3a, yield 93%), while switching to Boc-protected alanine resulted in a poor reaction efficiency where only traces could be detected presumably due to poor solubility of α-amino acids in DMF. Hence, KH2PO4 was exchanged to CsF and DMSO was chosen as solvent since high-dielectric solvents are required.20 With this new setup the desired coupling product with Boc-Ala-OH was obtained with high yield (examples 3c & 3d, yield 94%). Based on the newly applied protocol, we next explored the scope of the α-amino acid component. As shown in Scheme 2 the reaction was performed for all entries at room temperature and the desired products were obtained with high yields. In addition, two negative experiments using Boc-Phe-OH were performed to rule out the possibility of a solely thermally driven reaction. For this purpose, a reaction was carried out with photocatalyst and without the use of irradiation. Another negative experiment was conducted with irradiation but no photocatalyst present. HPLC analysis of both trials revealed no conversion (depicted in ESI†).
The photocatalytic decarboxylation of α-amino acids generates radicals mediated by a single-electron transfer mechanism.16 Those α-amino radicals undergo conjugate addition on the convex side of the carbasugar mimic. Only one diastereomer was found regarding the newly formed chiral center at the carbasugar mimic. However, by this radical formation the stereocenter at the former α-amino acid site generates the product as mixture of two diastereomers except for the achiral examples 3a & 3b of course. For the examples 3c–3j, 3l, 3m, and 3q–3t the diastereomers were separated by flash column chromatography and the diastereomeric ratio was determined by isolated yields. Under standard conditions examples 3k and 3n–3p were isolated as mixtures, thus the diastereomeric ratio was determined by NMR-experiments of the purified material. With the help of 2D-NOESY experiments (shown in ESI†) for examples 3e & 3f we were able to assign the diastereomers. With these mild conditions a broad range of functional groups was accepted including esters, amides, and carbamates. The benzyl carbamate (CBz-NR2) is tolerated as well and can be used in alternative to tert-butyloxycarbonyl (Boc) protected amino acids. In addition, the successful application could be demonstrated for various acyclic α-amino acids with aliphatic and aryl side chains as well as for the non-proteinogenic acyclic α-amino acid Boc-methylalanine. Moreover, excellent results were achieved for the cyclic amino acids proline and pipecolic acid. In the next step, the products were converted to nucleosides as potential starting point towards potent biologically active compounds.
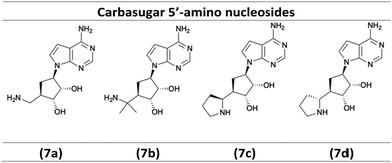
As illustrated in Scheme 3, the diastereoselective reduction of compounds 3a, 3b, and 3n with NaBH4 in MeOH yielded the desired alcohols 4a–4c (72–90%). Then, a Mitsunobu-reaction was employed to introduce the base for the target nucleoside analogues shown in Scheme 4.21 Aromatic substitution with ammonia in 1,4-dioxane at 100 °C afforded the products 5a–5c with a yield of 67–75%.22 Subsequently, acidic treatment with TFA afforded the desired 5′-amino carbasugar nucleoside analogues 7a–7d shown in Scheme 4.
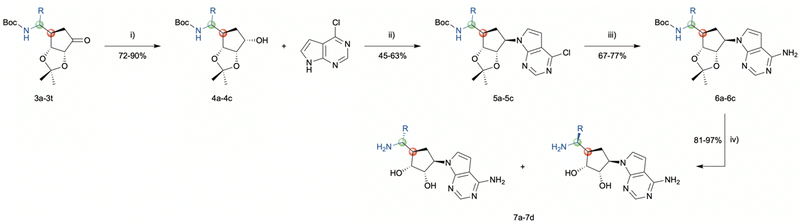 | ||
| Scheme 3 Reagents and conditions: (i) NaBH4, MeOH, 0 °C, 1 h; (ii) PPh3, DBAD, PhMe, 0–60 °C, 17 h; (iii) NH3, 1,4-dioxane, 100 °C, 17 h; (iv) TFA/H2O, rt, 3 h. | ||
In conclusion, we were able to demonstrate the first efficient and versatile photochemical decarboxylative coupling between readily available α-amino acids and a highly crowded enone. This mild protocol affords rapidly functionalized carbasugar mimics for the design of 5′-amino carbasugar nucleosides which can serve as a starting point towards biologically active compounds, e.g. for protein lysine and arginine methyltransferases.
All experimental and characterization data are available in the ESI.†
S. O. K.: conceptualization, investigation, methodology, writing – original draft; A. A. B.: methodology, investigation, writing – review & editing; M. J.: conceptualization, funding acquisition, supervision, writing – review & editing.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) through CRC 992 (Project ID 192904750) and under Germany's Excellence Strategy (CIBSS – EXC-2189 – Project ID 390939984). A special thanks goes to Philipp Eberhardt for his support.
Conflicts of interest
There are no conflicts to declare.References
- M. Fontecave, M. Atta and E. Mulliez, Trends Biochem. Sci., 2004, 29, 243–249 CrossRef CAS PubMed.
- P. A. Frey, Chem. Rev., 2003, 103, 2129–2148 CrossRef CAS PubMed.
- K. Nepali and J.-P. Liou, J. Biomed. Sci., 2021, 28, 27 CrossRef CAS PubMed.
- E. Kaminskas, A. T. Farrell, Y.-C. Wang, R. Sridhara and R. Pazdur, Oncologist, 2005, 10, 176–182 CrossRef CAS PubMed.
- A. Mullard, Nat. Rev. Drug Discovery, 2020, 19, 156 Search PubMed.
- P. Khirsariya, P. Pospíšil, L. Maier, M. Boudný, M. Babáš, O. Kroutil, M. Mráz, R. Vácha and K. Paruch, J. Med. Chem., 2022, 65, 5701–5723 CrossRef CAS PubMed.
- R. Ferreira de Freitas, D. Ivanochko and M. Schapira, Molecules, 2019, 24, 4492 CrossRef CAS PubMed.
- L. S. Jeong and J. A. Lee, Antiviral Chem. Chemother., 2004, 15, 235–250 CrossRef CAS PubMed.
- H. R. Moon, W. J. Choi, H. O. Kim and L. S. Jeong, Tetrahedron: Asymmetry, 2002, 13, 1189–1193 CrossRef CAS.
- Y. Wang, H. Yan, C. Ma and D. Lu, Bioorg. Med. Chem. Lett., 2015, 25, 4461–4463 CrossRef CAS PubMed.
- W. J. Choi, H.-J. Chung, G. Chandra, A. Varughese, L. X. Zhao, H. W. Lee, A. Nayak, M. S. Majik, H. O. Kim, J.-H. Kim, Y. B. Lee, C. H. Ahn, S. K. Lee and L. S. Jeong, J. Med. Chem., 2012, 55, 4521–4525 CrossRef CAS PubMed.
- V. P. Rajappan and S. W. Schneller, Bioorg. Med. Chem., 2003, 11, 5199–5201 CrossRef CAS PubMed.
- J. A. S. Iii, R. N. Comber, R. J. Gray, R. B. Gilroy and J. A. Montgomery, J. Med. Chem., 1993, 36, 2102–2106 CrossRef PubMed.
- R. Schüle, E. Metzger, S. Wang, M. Jung, N. Barthes, B. Breit and D. Sarraf, WO2020/058358A1, 2020.
- E. J. Olhava, R. Chesworth, K. W. Kuntz, V. M. Richon, R. M. Pollock and S. R. Daigle, WO2012/075381A1, 2012.
- K. Miyazawa, T. Koike and M. Akita, Adv. Synth. Catal., 2014, 356, 2749–2755 CrossRef CAS.
- Z. R. Shi Zhifang, Guangdong Chem. Ind., 2014, 4, 2–3 Search PubMed.
- L. Ruiz Espelt, I. S. McPherson, E. M. Wiensch and T. P. Yoon, J. Am. Chem. Soc., 2015, 137, 2452–2455 CrossRef CAS PubMed.
- J. C. Deforest, R. A. Samame, G. Suryn, A. Burtea and S. D. Rychnovsky, J. Org. Chem., 2018, 83, 8914–8925 CrossRef CAS PubMed.
- Z. Zuo and D. W. C. MacMillan, J. Am. Chem. Soc., 2014, 136, 5257–5260 CrossRef CAS PubMed.
- J. Luengo, R. A. Leal and H. Lin, WO2018/085818A1, 2018.
- P. Naus, O. Caletkova, P. Konec and M. Hocek, J. Med. Chem., 2014, 57, 1097–1110 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc06071j |
| This journal is © The Royal Society of Chemistry 2023 |