Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nanophase-photocatalysis: loading, storing, and release of H2O2 using graphitic carbon nitride†
Akalya
Karunakaran
ab,
Katie J.
Francis
a,
Chris R.
Bowen
b,
Richard J.
Ball
c,
Yuanzhu
Zhao
a,
Lina
Wang
a,
Neil B.
McKeown
 d,
Mariolino
Carta
d,
Mariolino
Carta
 e,
Philip J.
Fletcher
f,
Remi
Castaing
f,
Mark A.
Isaacs
e,
Philip J.
Fletcher
f,
Remi
Castaing
f,
Mark A.
Isaacs
 g,
Laurence J.
Hardwick
g,
Laurence J.
Hardwick
 hi,
Gema
Cabello
hij,
Igor V.
Sazanovich
hi,
Gema
Cabello
hij,
Igor V.
Sazanovich
 k and
Frank
Marken
k and
Frank
Marken
 *a
*a
aDepartment of Chemistry, University of Bath, Claverton Down, Bath BA2 7AY, UK. E-mail: f.marken@bath.ac.uk
bDepartment of Mechanical Engineering, University of Bath, Claverton Down, Bath BA2 7AY, UK
cDepartment of Architecture & Civil Engineering, University of Bath, Claverton Down, Bath BA2 7AY, UK
dEaStCHEM School of Chemistry, University of Edinburgh, Joseph Black Building, David Brewster Road, Edinburgh, Scotland EH9 3JF, UK
eDepartment of Chemistry, Swansea University, College of Science, Grove Building, Singleton Park, Swansea SA2 8PP, UK
fUniversity of Bath, Materials & Chemical Characterisation Facility, MC2, UK
gHarwellXPS, Research Complex at Harwell, STFC Rutherford Appleton Laboratory, Harwell Campus, Didcot, OX11 0FA, UK
hStephenson Institute for Renewable Energy, Department of Chemistry, University of Liverpool, Liverpool L69 7ZF, UK
iThe Faraday Institution, Harwell Campus, Didcot, OX11 0RA, UK
jSchlumberger Cambridge Research, High Cross, Madingley Road, Cambridge CB3 0EL, UK
kCentral Laser Facility, Research Complex at Harwell, STFC Rutherford Appleton Laboratory, Harwell Campus, Didcot OX11 OQX, UK
First published on 23rd May 2023
Abstract
A blue light mediated photochemical process using solid graphitic carbon nitride (g-C3N4) in ambient air/isopropanol vapour is suggested to be linked to “nanophase” water inclusions and is shown to produce approx. 50 μmol H2O2 per gram of g-C3N4, which can be stored in the solid g-C3N4 for later release for applications, for example, in disinfection or anti-bacterial surfaces.
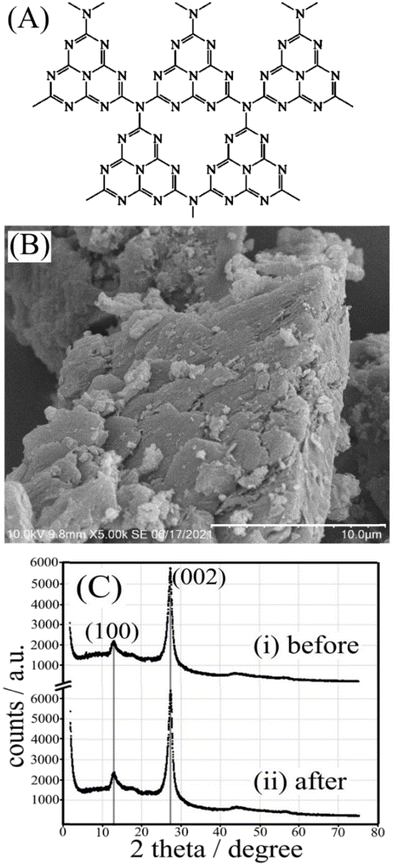
Graphitic carbon nitride (g-C3N4, see Fig. 1A, first synthesised by Berzelius and named by Liebig1) has a layered structure with many defects that can be produced at low cost and is employed in a number of important applications.2,3 Here, we explore the potential of g-C3N4 for the formation, storage, and release of hydrogen peroxide (H2O2). The photocatalytic formation of H2O2 has been widely reported4 and is useful to disinfect,5 bleach,6 or clean environments.7 In addition to effective production of H2O2 based on photo-catalysis8 from ambient oxygen and a hole quencher, there are reports of piezo-catalysis,9 which is indicative of a broader range of molecular activation processes for g-C3N4 at the microscopic scale. Singlet oxygen formation with g-C3N4 has been observed.10
Previously, when exploring the photochemical reactivity of g-C3N4 (as a solid powder or when immobilising g-C3N4 particles into an intrinsically microporous host material PIM-1)11 immersed in a liquid phase, we observed H2O2 production in the aqueous phase in the presence of hole quenchers such as glucose or Triton X-100. Here, isopropanol vapour is employed in order to provide a quencher during the corresponding solid-state photochemical process. In contrast to previous studies, here the photocatalytic process is performed with the g-C3N4 powder exposed to light in ambient air. H2O2 is released only later in the absence of light upon contact to water (eqn (1) and (2)).
| g-C3N4(H2O) + hv/quencher/O2 → g-C3N4(H2O2) | (1) |
| g-C3N4(H2O2) → g-C3N4 + H2O2(aq) | (2) |
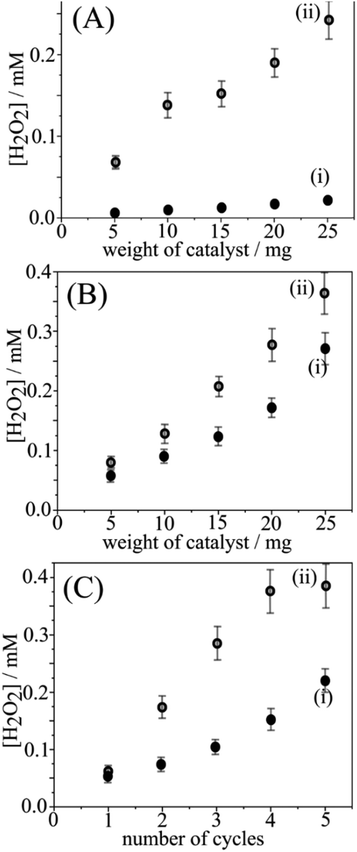
Data in Fig. 2A demonstrate the production of H2O2 (determined with para-nitrophenol and LC/MS detection; ESI†) when placing the photoactivated g-C3N4 powder into pure water. For the activated g-C3N4, typically 100–200 μM H2O2 in 2 cm3 water are released, whereas only traces of H2O2 are released without prior photoactivation. The release of H2O2 occurs immediately upon immersion and does not continue with time. Adding more g-C3N4 increases the H2O2 concentration in an approximately linear fashion. The number of moles of H2O2 released suggests approx. one H2O2 molecule for every 300 heptazine units in the g-C3N4 powder (or 0.06 wt% H2O2) assuming a bulk reaction. Fig. 2B shows data for the release of H2O2 (i) for g-C3N4 powder and (ii) for g-C3N4@PIM-1 composite (employing 17 wt% intrinsically microporous polymer PIM-1 to give a film on filter paper; see ESI†). As in previous studies,11,14 PIM-1 is employed as molecularly rigid material to make the photocatalyst easily accessible to the aqueous phase and allows easy recovery for re-use of the catalyst. Fig. 2B shows that both g-C3N4 powder and polymer-embedded g-C3N4 exhibit similar H2O2 release.
The data in Fig. 2C demonstrate the re-use of g-C3N4@PIM-1 with/without additional blue light treatment for up to five cycles. The intermittent blue light treatment clearly enhances the H2O2 release so that 0.5 mM H2O2 can be produced with only a few reaction cycles. The formation and release of H2O2 under blue light conditions can be discussed in terms of the following reaction steps:
| g-C3N4 + hv → g-C3N4* | (3) |
| g-C3N4* + isopropanol → g-C3N4− + H+(aq) + products | (4) |
| 2 g-C3N4− + O2 + 2 H+ → 2 g-C3N4(H2O2) | (5) |
| g-C3N4(H2O2) → g-C3N4 + H2O2(aq) | (6) |
Photoexcitation of g-C3N4 leads to an excited state (eqn (3)), which after charge separation produces a hole and an electron. The hole reacts with the isopropanol quencher to give protons and products (eqn (4)). The electrons, which have been reported to generate a strong electron paramagnetic resonance and blue coloration,15 can combine with ambient oxygen and protons to give trapped H2O2 (eqn (5)). The g-C3N4(H2O2) material can be stored for several weeks without significant loss of H2O2 content (Fig. 3B), and its eventual immersion into water releases H2O2 (eqn (6)). Thermogravimetric analysis of g-C3N4 before and after photoreaction (see ESI,† Fig. S9) reveals the presence of water (approx. 2.5 wt%), which is likely to be present as a “nanophase” in between layers and likely to play a crucial role (see eqn (4) during the solid state photochemical formation of H2O2. Heating the g-C3N4(H2O2) sample to 150 °C removes H2O and immediately destroys the H2O2. Intriguingly, a vacuum treatment (2h, approx. 10 mTorr) at room temperature also removes the H2O2.
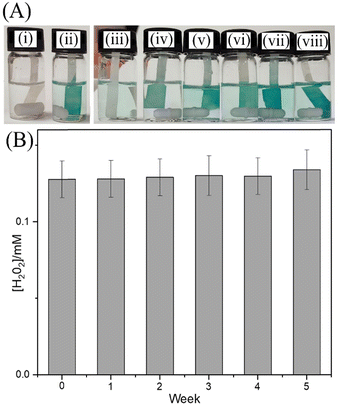
The release of H2O2 into aqueous media can be visualised with the 3,3′,5,5′-tetramethylbenzidine (TMB) colour reaction.16 The g-C3N4 treated with blue light and isopropanol was added 2 cm3 of deionized water. After 5 minutes, the solution was filtered with a 0.2 μm filter. The solution is added to the vial containing TMB and 0.1 M CH3COOH. Fig. 3A shows photographs of the test solutions (i) without and (ii) with 50 μM H2O2 intentionally added as reference. Samples (iii) to (viii) correspond to added weights (0, 5, 10, 15, 20, 25 mg) of g-C3N4 photochemically charged g-C3N4. A Nafion-impregnated filter paper was employed to bind the cationic oxidised form of TMB to amplify the blue colour signal. The colour reaction is consistent with the analysis of H2O2 concentration by mass spectrometry and suggests H2O2 release in the 50 to 500 μM range.
In conclusion, active sites for photo-generated H2O2 binding/storage in the solid g-C3N4 material appear to exist (approx. one bound H2O2 per 300 heptazine units), which are probably associated with nanophase water. Once bound, H2O2 in g-C3N4 remains stable over prolonged periods of time (Fig. 3B). However, a short vacuum treatment at room temperature or a heat treatment at 150 °C removes both the liquid nanophase H2O and any trapped H2O2.
Coatings of g-C3N4 could be employed to release H2O2 upon contact with water, for example as a disinfectant in hospital environments.20 Further studies of the nature of the H2O2 binding interaction, the effects of humidity and the nature of the hole quencher, and further g-C3N4 structural engineering provide future potential to achieve higher levels of H2O2 to be bound, stored, and released. Other types of photochemical gas conversions could be performed under illumination in solid state and with aqueous nanophase entrapment.
Akalya Karunakaran: data curation; formal Analysis; investigation; methodology; writing – review & editing. Katie J. Francis: data curation; investigation. Chris R. Bowen and Richard J. Ball: conceptualization; methodology; supervision; writing – review & editing. Yuanzhu Zhao and Lina Wang: data curation; investigation; writing – review & editing. Neil B. McKeown and Mariolino Carta: conceptualization; supervision; writing – review & editing. Philip J. Fletcher, Remi Castaing, and Mark Isaac: data curation; investigation; writing – review & editing. Laurence J. Hardwick: formal analysis; supervision; writing – review & editing. Gema Cabello and Igor V. Sazanovich: data curation; formal analysis; investigation; methodology; writing – review & editing. Frank Marken: conceptualization; project administration; resources; supervision; writing – original draft.
A. K. thanks the National Overseas Scholarship-India. L. J. H. and G. C. were supported by the UK Faraday Institution (EPSRC EP/S003053/1) through the Degradation Project (grant numbers FIRG001 and FIRG024). STFC is acknowledged for beam time on the ULTRA facility to carry out Kerr-gated experiments. We thank Alexander Cowan (University of Liverpool) for helpful discussion.
Conflicts of interest
There are no conflicts of interest to declare.Notes and references
- J. V. Liebig, Ann. Pharm., 1834, 10, 10 Search PubMed.
- Y. Wang, X. C. Wang and M. Antonietti, Angew. Chem., Int. Ed., 2012, 51(1), 68–89 CrossRef CAS PubMed.
- K. Z. Qi, S. Y. Liu and A. Zada, J. Taiwan Inst. Chem. Eng., 2020, 109, 111–123 CrossRef CAS.
- J. Q. Wen, J. Xie, X. B. Chen and X. Li, Appl. Surf. Sci., 2017, 391, 72–123 CrossRef CAS.
- L. L. Chen, W. P. Shi, T. D. Zhang, R. B. Zhou, X. Q. Jin, Y. Q. Zhou, W. J. Lin, W. H. Guo and D. C. Yin, 2D Mater., 2022, 9(4), 045034 CrossRef.
- Z. Haider, H. I. Cho, G. H. Moon and H. I. Kim, Catal. Today, 2019, 335, 55–64 CrossRef CAS.
- F. Ding, D. Yang, Z. W. Tong, Y. H. Nan, Y. J. Wang, X. Y. Zou and Z. Y. Jiang, Environ. Sci.: Nano, 2017, 4(7), 1455–1469 RSC.
- S. W. Cao, J. X. Low, J. G. Yu and M. Jaroniec, Adv. Mater., 2015, 27(13), 2150–2176 CrossRef CAS PubMed.
- C. Hu, F. Chen, Y. G. Wang, N. Tian, T. Y. Ma, Y. H. Zhang and H. W. Huang, Adv. Mater., 2021, 33(24), 2101751 CrossRef CAS PubMed.
- M. Tamtaji, A. Tyagi, C. Y. Young, P. R. Galligan, H. W. Liu, Z. J. Liu, R. Karimi, Y. T. Cai, A. P. Roxas, H. L. Wong and Z. T. Luo, ACS Appl. Nano Mater., 2021, 4(8), 7563–7586 CrossRef CAS.
- Y. Z. Zhao, L. N. Wang, R. Malpass-Evans, N. B. McKeown, M. Carta, J. P. Lowe, C. L. Lyall, R. Castaing, P. J. Fletcher, G. Kociok-Kohn, J. Wenk, Z. Y. Guo and F. Marken, ACS Appl. Mater. Interfaces, 2022, 14(17), 19938–19948 CrossRef CAS PubMed.
- F. Fina, S. K. Callear, G. M. Carins and J. T. S. Irvine, Chem. Mater., 2015, 27(7), 2612–2618 CrossRef CAS.
- Y. Shiraishi, S. Kanazawa, Y. Sugano, D. Tsukamoto, H. Sakamoto, S. Ichikawa and T. Hirai, ACS Catal., 2014, 4, 774–780 CrossRef CAS.
- Y. Z. Zhao, J. Dobson, C. Harabajiu, E. Madrid, T. Kanyanee, C. Lyall, S. Reeksting, M. Carta, N. B. McKeown, L. Torrente-Murciano, K. Black and F. Marken, Bioelectrochemistry, 2020, 134, 107499 CrossRef CAS PubMed.
- D. Hollmann, M. Karnahl, S. Tschierlei, K. Kailasam, M. Schneider, J. Radnik, K. Grabow, U. Bentrup, H. Junge, M. Beller, S. Lochbrunner, A. Thomas and A. Brückner, Chem. Mater., 2014, 26(4), 1727–1733 CrossRef CAS.
- L. N. Wang, M. Carta, R. Malpass-Evans, N. B. McKeown, P. J. Fletcher, P. Estrela, A. Roldan and F. Marken, J. Catal., 2011, 416, 253–266 CrossRef.
- Y. Z. Zhao, N. A. Al Abass, R. Malpass-Evans, M. Carta, N. B. McKeown, E. Madrid, P. J. Fletcher and F. Marken, Electrochem. Commun., 2019, 103, 1–6 CrossRef CAS.
- P. M. Budd, E. S. Elabas, B. S. Ghanem, S. Makhseed, N. B. McKeown, K. J. Msayib, C. E. Tattershall and D. Wang, Adv. Mater., 2004, 16, 456–457 CrossRef CAS.
- P. M. Budd, B. S. Ghanem, S. Makhseed, N. B. McKeown, K. J. Msayib and C. E. Tattershall, Chem. Commun., 2004, 230–231 RSC.
- Z. Y. Teng, N. L. Yang, H. Y. Lv, S. C. Wang, M. Z. Hu, C. Y. Wang, D. Wang and G. X. Wang, Chemistry, 2019, 5, 664–680 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc01442h |
| ‡ Experimental: g-C3N4 was prepared as reported previously.17 PIM-1 was prepared following a literature method.18,19 Films of g-C3N4@PIM-1 were obtained by drop casting. An amount of 5 mg of graphitic carbon nitride (measured with a Ohaus PX224 analytical balance) was added to the 1 cm3 solution of 1 mg PIM-1 in chloroform and sonicated for 15 minutes. The solution was drop-casted on filter paper with a size of 2 × 2 cm2. The sample was air dried to leave a layer of g-C3N4@PIM-1 on the filter paper (thickness approx. 0.25 mm). Further experimental details are provided in the ESI.† |
| This journal is © The Royal Society of Chemistry 2023 |