The tunability of oxygen evolution reaction performance in La0.67Sr0.33CoO3 van der Waals membrane
Yuan
Zhang
a,
Chen
Wang
a,
Hang
Xu
ab,
Ji
Qi
ab and
Ming
Feng
 *a
*a
aKey Laboratory of Functional Materials Physics and Chemistry of the Ministry of Education, Jilin Normal University, Changchun 130103, China. E-mail: mingfeng@jlnu.edu.cn
bFunctional Materials and Acousto-Optic Instruments Institute, School of Instrumentation Science and Engineering, Harbin Institute of Technology, Harbin 150080, China
First published on 25th November 2022
Abstract
Cobaltate has multiple valence states of 3d orbitals, and its oxygen evolution reaction (OER) performance can be adjusted by adjusting the oxygen concentration, internal electronic structure and connection between the lattice to afford superior OER activity. Therefore, cobaltate is considered a promising OER catalyst in alkaline media. Herein, the bending state of LSCO thin film was changed by directly applying mechanical bending, and the curvature diameter of the catalytic electrode was directly controlled. The results show that the OER activity of the LSCO thin film electrode considerably improved under an in situ strain of only ∼0.24%. It also showed that the volcanic trend in OER performance involves two parameters: the tolerance factor of Co and activation energy of oxygen.
1. Introduction
Cobaltite has been regarded as a promising catalyst candidate for the oxygen evolution reaction (OER) in alkaline media owing to its stable, active, and low-cost features. Moreover, cobaltite provides a platform for revealing the fundamental mechanism of catalysis at the atomic level, such as the link between the electronic structure and corresponding OER performance in single-crystal cobaltite.1 The long-range lattice ordering and controllable stoichiometry enable us to achieve the functionality of hybridization of 3d and 2p orbitals as a function of oxygen intermediate absorption/desorption ability. In the conventional strategies of OER tunability based on single-crystal cobaltite, such as LaCoO3, SrCoO3−δ and La1−xSrxCoO3, strain is an effective method to change the crystal field, thereby modulating electronic structures via the strong correlation of charge and lattice. Petrie et al. grew thin films on substrates with different lattice constants and improved the OER performance of SrCoO3−δ by adjusting the oxygen pressure.2 Kubicek et al. studied the effect of lattice strain on the epitaxial growth of La1−xSrxCoO thin films deposited using pulsed laser deposition (PLD). Their findings point to the application of lattice strain in existing hybrid conductive oxides to control oxygen surface exchange and diffusion dynamics for energy conversion.3 In the work of Liu et al. the photoconductivity of LCO thin films was regulated by epitaxial strain. Epitaxial strain LCO thin films with the same orientation were prepared on single-crystal substrates with different (001) orientations. The strain caused by lattice mismatch causes tetragonal distortion of the cobalt oxide octahedron in LCO films. The effect of epitaxial strain on the photoconductance of LCO films has been studied.4 It is observed that the strain effect always benefits from the interfacial lattice mismatch in single-crystal cobaltate heterostructures. Many studies have discovered that strain is an effective approach for targeting OER tunability. However, because interfacial strain requires different substrates with distinct lattice constants, comparison of such strain effect faces the severe challenge that interfacial strain may cause cobaltate stoichiometry offset, octahedral rotation, etc.5–8 Thus an in situ strain change on the OER performance of the single-crystal cobaltate is highly desired to maximally reduce the uncertainty from individual samples.Herein, we have conducted comprehensively studied the strain effects of single-crystal La0.67Sr0.33CoO3 (LSCO) van der Waals membrane on mica. The strain condition can be in situ modulated via curvature change owing to the flexibility of the mica substrate. Then, we found that both compressive and tensile strains give smaller current densities at 2 V compared to the zero strain conditions. With the increase in strain, the LSCO film exhibited worse OER activity under tensile and compressive strains.9,10 Under zero strain conditions, the corresponding current density was about 4.8 mA cm−2 at 2 V and was adjusted in situ with a 0.03% strain span. It was found that the corresponding current density was about 2.65 mA cm−2 and 2.32 mA cm−2 at the −0.12% and 0.12% strains, respectively. The negative value represents compressive, and the positive value represents tensile. However, on the filed cooling (FC) curve of the LSCO magnetic performance test, it was found that the tensile strain strengthened the exchange coupling effect between adjacent Co atoms. This suggests that two mechanisms are responsible for this volcano trend. Combining the measurement of the magnetic properties of strained LSCO, we found that the tolerant factor of Co and the activation energy of oxygen as a function of strain are separate mechanisms for the exchange coupling enhancement between neighboring Co atoms and the regulation of the O 2p orbital position.11–13
2. Experimental procedure
The LSCO thin films were epitaxially grown on mica substrates using PLD. The reason we choose mica as the substrate is that when the thickness of mica is less than 20 um, it produces flexibility, and its layered structure and the interaction between layers are very weak.14 The massive mica along the dissociation surface can be stripped into more than ten microns, even a few microns of mica sheet, which makes the mica substrate one of the good substrates for the growth of van der Waals epitaxial flexible films, which is necessary to apply in situ strain. Based on the above characteristics, our study adopts the method of ultrasonic stripping and tape stripping to obtain a single layer mica sheet with a thickness of about 10 um.The excimer laser wavelength was 248 nm, the energy of the laser was 310 mJ, and the repetition rate was set to 4 Hz. In the process of thin film sputtering, the substrate temperature was maintained at 550 °C, and the oxygen pressure was maintained at 26.7 Pa. The thickness of the mica obtained films was 40 nm. After sputtering, the film was naturally cooled to room temperature under a pressure of 26.7 Pa.15 The thickness of the exfoliated single-layer mica sheet was tested by a scanning electron microscope (SEM). The surface morphology of the mica before and after the LSCO film was measured by applying an atomic force microscope (AFM), and the structure of the film was analyzed using X-ray diffraction (XRD). To study the impact of the exchange interaction of the sample under different strain conditions (compression and tension), a superconducting quantum interference device (SQUID) was used to measure the FC curve of the film under an applied magnetic field of ±5000 Oe. The electrochemical characterization of LSCO/mica under different strain conditions was performed using an electrochemical workstation (model: solartron analytical 1400 cell test system).
3. Results and discussion
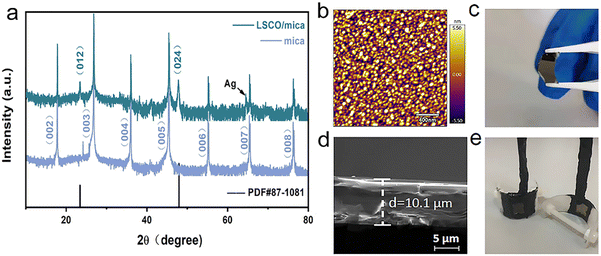
The XRD pattern of LSCO on the flexible mica substrate (θ–2θ scanning) is depicted in Fig. 1(a). In addition to the mica substrate peak, it can be observed that the (001) and (002) diffraction peaks of LSCO at 23.4° and 47.8° can be identified, respectively, indicating that the single-phase (001) LSCO film grows preferentially along the out-of-plane c axis.16–18 The LSCO diffraction peaks of other crystalline phases and any impurity peaks are not found in the XRD pattern. In Fig. 1(b), AFM images show the surface morphology of the LSCO film, and a dense surface structure can be clearly observed. For the LSCO film on the mica surface, the root-mean-square (RMS) of the roughness of the surface is ∼2.31 nm, which is within the predicted range.19 To calculate the curvature, the thickness of the mica substrate needs to be obtained. The cross-sectional images of LSCO/mica were taken under SEM. The thickness of the LSCO (40 nm) relative to the thickness of the mica substrate can be ignored. The thickness of the tested mica substrate is ∼10.1 μm, as shown in Fig. 1(c). Fig. 1(d and e) show the whole thin film, which can be bent easily by tweezers. It is confirmed that the quality of the prepared thin film is good. Under appropriate mechanical bending, there is no crack in the thin film sample. The prepared LSCO film was used as the physical model of the working electrode. We can adjust the curvature by adjusting the distance between the nuts to achieve the application of in situ strain.LSV was used to evaluate the OER catalytic activity of LSCO/mica films under tensile and compressive strain conditions. The standard three-electrode configuration test method was used.20 The OER measurement results of all LSCO films under different strain conditions are shown in Fig. 2. Flexible thin films can withstand different stresses under convex and concave mechanical bending conditions. In this case, concave bending causes compressive strain, while convex bending causes tensile strain. The strain exerted on the LSCO/mica film by bending is well defined and can be simply expressed by the formula 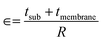 , where ∈, tsub, tmembrane, and R are the strain, substrate thickness, membrane thickness, and the radius of curvature, respectively. The film thickness (40 nm) here is negligible compared to the mica substrate (10 μm). To facilitate the distinction, we represent the positive value as tensile strain, the negative value represents compressive strain, and zero represents a sample without any deformation.
, where ∈, tsub, tmembrane, and R are the strain, substrate thickness, membrane thickness, and the radius of curvature, respectively. The film thickness (40 nm) here is negligible compared to the mica substrate (10 μm). To facilitate the distinction, we represent the positive value as tensile strain, the negative value represents compressive strain, and zero represents a sample without any deformation.
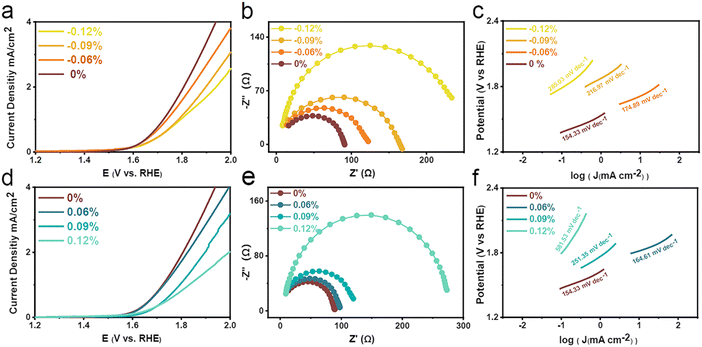
The OER performance under the strain effect of single-crystal LSCO van der Waals membrane on mica is shown in Fig. 2(a–e). Our data show that when compared with the zero-strain condition, both compressive and tensile strains exhibit a small current density at 2 V potential. Under the zero-strain condition, the OER activity was the highest. When the potential is 2 V, the current density is about 4.8 mA cm−2. As the distance between the nuts gradually decreases, the curvature of the physical model gradually increases, and a larger strain is generated. Based on the strain formula defined by us, the OER activity is the worst under the strains of −0.12% and 0.12%, and the current densities are 2.65 and 2.32 mA cm−2. Judging from the data, there is a linear relationship between strain and OER activity.21,22 With an increase in strain, the OER performance is worse. Fig. 2(b and e) show the charge transfer resistance at the electrode–electrolyte interface revealed by electrochemical impedance spectroscopy (EIS). The Nyquist diagram reveals that the semicircular arc diameter of the sample is the smallest under zero strain, indicating the best charge transfer ability. Based on the polarization curves of LSCO films under various strains, the Tafel curves (potential vs log(|current density|) are plotted, as shown in Fig. 2(c and f). The Tafel slope under zero strain is the smallest at 154.33 mv dec−1, indicating that it has excellent OER reaction kinetics compared with the compression and tensile conditions. This result agrees with the change trend of the LSV polarization curve and Nyquist plot, indicating whether it is tensile or compressive; with the increase in curvature, the LSCO van der Waals film is subjected to larger strain, and the OER performance tends to decrease.23–25 It is concluded that this strain is linearly related to the OER performance.
The stability of the flexible material under bending conditions was tested. The I–T curves are shown in Fig. 3(a). The chronoamperometry curves of the LSCO membrane without strain and with tensile, and compressive strains were tested within 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s under 2 V voltage, showing an approximate stable platform, which indicates that the change of strain does not destroy the stability of the LSCO membrane. Fig. 3(b) shows the LSV curves of the same sample after the first and the 200 times bending under various strains. It can be observed that after multiple bendings, the sample still retains the original catalytic activity under the corresponding strain. This indicates that it is not destructive to change OER activity by adjusting the strain.
000 s under 2 V voltage, showing an approximate stable platform, which indicates that the change of strain does not destroy the stability of the LSCO membrane. Fig. 3(b) shows the LSV curves of the same sample after the first and the 200 times bending under various strains. It can be observed that after multiple bendings, the sample still retains the original catalytic activity under the corresponding strain. This indicates that it is not destructive to change OER activity by adjusting the strain.
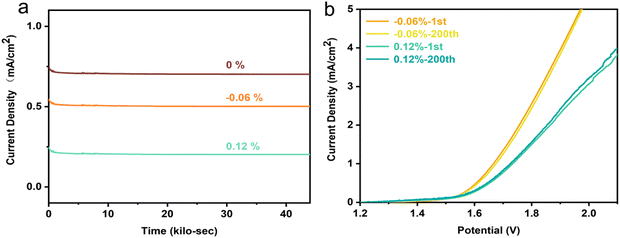 | ||
| Fig. 3 (a) Chronoamperometry curve of LSCO film at 2 V. (b) Comparison of LSV curves between the first bending specimen and the 200-times bending specimen. | ||
Fig. 4(a) shows the current density integration diagram of the sample under all strain conditions at 2 V potential. When the tensile strain is ∼0.06%, the OER activity of the sample is reduced by 40% compared with that of the sample without a bending state. The LSCO film OER performance decreases as the bending degree (strain) increases. Under the same compression strain, the OER activity of the LSCO film decreases with increasing strain, and under ∼0.12% compression strain, the OER activity decreases by ∼51.6% compared with that of the untreated film. In summary, compared with the LSCO film without any strain, both tensile and compressive strain can reduce the OER activity of LSCO in the electrochemical process. Here, we speculate that this rule is owing to the synergistic effect of exchange and oxygen activation energy, the orange line represents the linear relationship between the exchange mechanism and the strain, and the green line represents the linear relationship between the migration energy (activation energy) of oxygen and the strain.26–28
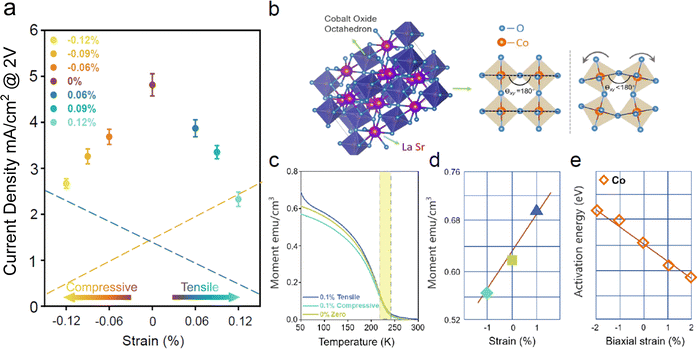 | ||
| Fig. 4 (a) Comparison of current density at 2 V potential. The error bar represents the standard deviation of the results of three independent measurements. The orange line represents the linear relationship between the exchange mechanism and strain and the green line represents the linear relationship between the activation energy of oxygen and strain. The synergy between the two is responsible for the overall OER activity. (b) Comparison of the Co–O bond angle deflection of CoO6 octahedron under stress with and without deflection. (c) FC curves of LSCO film under zero, tensile and compressive strain. (d) Summary of magnetization of LSCO thin films under zero strain, 1% tensile and 1% compressive strain at 50 K. (e) Mayeshiba et al. reported that to test the coupling relationship between strain and oxygen ion conductivity, the oxygen migration barrier was used as a simplified measurement of conductivity, and the increment (change) in strain migration per cent was introduced.28 | ||
For strain applications, one of the most important effects is ABO3 structure distortion. These distortions cause changes in the covalency of transition metal–oxygen bonds. The covalent strength changes the bond distance and electronic properties between atoms, including macroscopic functions, such as catalytic activity, electrochemical storage and ionic conductivity. Owing to our mechanical bending, the ideal CoO6 octahedron is distorted by force, as illustrated in Fig. 4(b). The crystal structure of LaSrCoO3 is shown in Fig. 4(b) (lower left corner of Fig. 4(b)). Based on the data in Fig. 4(b and c), the bond angle of Co–O was changed owing to the application of strain, resulting in a change in the Co tolerance factor. With an increase in tensile strain, the rotation of the octahedron position decreased. Therefore, compared with the bulk (zero strain), the strain did not change the bond length of Co–O. The smaller Co–O–Co bond angle increased the overlap of the electron cloud wave functions of Co and O, and the hybridization of the Co 3d–O 2p orbital increased, which inhibited the eg-level Yang–Taylor splitting and strengthened the double exchange effect, thereby increasing the magnetization. However, the electrochemical OER activity exhibited a small current density. We speculate that there is another mechanism responsible for this volcanic trend and that the activation energy of oxygen is also a function of strain, which is within the scope of our discussion.29–31
Mayeshiba et al. reported that to test the coupling relationship between strain and oxygen ionic conductivity, the oxygen migration barrier was used as a simplified measurement of conductivity, and an increment (change) in strain migration energy per percentage was introduced. The slope of each line is called the “DMEPS” value. It can be clearly observed that with the application of tensile and compressive strain, the migration energy of oxygen decreases monotonously.28 They concluded that with the increase in tensile strain (negative DMEP), the decrease in activation energy was important for the perovskite system, which was verified in our OER activity data. The green dotted line in Fig. 4(a) represents the linear relationship between strain and oxygen migration energy (activation energy).
In summary, the synergy of the two mechanisms makes the OER activity of LSCO/mica under tensile and compressive strains show a volcanic law after mechanical bending is applied, and the OER activity is the largest under zero strain. The synergy of the two mechanisms crosses. The octahedron deflects the Co–O bond angle in response to strain. When the Co–O–Co bond angle is 180°, the 3d–2p–3d orbitals overlap, and the electron cloud overlap area is smaller than other bond angles.32 Under the action of compressive strain, the small angle of θxy results in the opening of the band gap, which increases the orbital hybridization of Co and O. Fig. 4(d) shows the relationship between strain and magnetism at 50 K (The change trend of 300 K agrees with that of 50 K). As the compressive strain decreases, the magnetism increases and the OER activity increases as well. As the tensile strain increases, the magnetism increases, but the catalytic performance weakens. Therefore, the mechanism of the tolerance factor of Co plays a leading role under compressive strain. Fig. 4(e) shows the relationship between strain and activation energy. The activation energy of oxygen decreases as tensile strain increases. This is because when the stretching strain is positive, oxygen chemisorption is faster, and the potential energy of the whole process increases. In general, under normal circumstances, the reaction is not easy to proceed, and the catalytic performance decreases. Therefore, the activation energy of oxygen plays a leading role under tensile strain. Therefore, we conclude that the tolerance factor of Co and the activation energy of oxygen as a function of strain are independent mechanisms for the enhancement of the exchange coupling between adjacent Co atoms and the adjustment of O 2p orbitals.
4. Conclusion
This study reported that the OER activity of the van der Waals LSCO membrane was influenced by changes in strain. By adjusting the curvature, a total of about 51.6% of OER activity can be observed under an in situ strain modulation of about 0.24%, and two possible mechanisms under in situ strain are discussed. Combined with the electrochemical performance of strain LSCO and the measurement of magnetic properties, it was found that the tolerance factor of Co and the activation energy of oxygen are the functions of strain, which are independent mechanisms for the enhancement of the exchange coupling between adjacent Co atoms and the adjustment of the O 2p orbital position. The possible in situ strain operation provides a platform for solving the problem of multi-electron structure and the corresponding OER performance in single-crystal cobaltite and establishing a basic relationship between effective strain and OER activity. Octahedral distortion and O 2p orbital hybridization can be adjusted by introducing in situ strain into van der Waals LSCO films. The results are of great significance for tuning the OER properties of single-crystal cobaltite by introducing strain.5. Experimental section
Flexible LSCO membranes were prepared on mica using PLD. KrF excimer laser (λ = 248 nm) was used for PLD growth with a laser energy of 310 mJ pulse−1 and a repetition rate of 4 Hz. Then, the LSCO films were prepared at a growth oxygen pressure of 200 mTorr and a temperature of 550 °C. After growth, the prepared samples were cooled to room temperature at a constant growth oxygen pressure.The crystal structure of the films was studied using XRD. AFM is used to study surface morphology. The electrochemical measurements were carried out in 0.1 mol L−1 KOH solution using a three-electrode system at room temperature.33 A carbon rod and a Hg/HgO electrode were used as the counter electrode and reference electrodes, respectively. All potentials were converted to a reversible hydrogen electrode (RHE): E vs. RHE = E vs. HgO + 0.098 + 0.059 × pH. Unless otherwise stated, all potentials are relative to the RHE. The thin film sample was fixed on a flexible plastic mold, and the whole film was fixed on a copper plate to prepare a working electrode.34 The strain of the film can be adjusted continuously by changing the degree of bending of the plastic mold. To have no obvious contact resistance, a silver paste was applied between the LSCO film and copper sheet.
The working electrode is coated with an epoxy resin that is insulated, chemically resistant and flexible, thereby exposing only the surface of the LSCO film catalyst. The OER activity of the samples was measured under different strains.
LSV was performed at a scan rate of 5 mV s−1. EIS Nyquist plots were obtained by an AC signal of 1.7 V, 106–0.1 Hz and an amplitude of 5 mV.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21978110 and 52171210), Jilin Province Science and Technology Department Program (No. 20200201277JC, 20200201279JC, 20200201187JC and 20220201130GX).References
- W. T. Hong, M. Risch, K. A. Stoerzinger, A. Grimaud and J. Suntivich, Energy Environ. Sci., 2015, 8, 1404–1427 RSC.
- J. R. Petrie, H. Jeen, S. C. Barron, T. L. Meyer and H. N. Lee, J. Am. Chem. Soc., 2016, 138, 7252–7255 CrossRef CAS PubMed.
- M. Kubicek, Z. Cai, W. Ma, B. Yildiz, H. Hutter and J. Fleig, ACS Nano, 2013, 7, 3276–3286 CrossRef CAS PubMed.
- H. Liu, B. Guo, L. Wang, R. Xie, J. Yang, J. Li, X. Zhang, K. Zheng and J. Huo, Opt. Mater., 2021, 121, 111537 CrossRef CAS.
- A. Baena, L. Brey and M. J. Calderón, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83 Search PubMed.
- Y. Y. Zhao, H. W. Yang, Y. Liu, H. Kuang, M. Zhang, W. L. Zuo, J. Wang, F. X. Hu, J. R. Sun and B. G. Shen, IEEE Trans. Magn., 2015, 51, 1–4 Search PubMed.
- A. D. Rata, A. Herklotz, K. Nenkov, L. Schultz and K. Dorr, Phys. Rev. Lett., 2008, 100, 076401 CrossRef CAS PubMed.
- C. Yu, M. Tan, C. Tao, Y. Hou, C. Liu, H. Meng, Y. Su, L. Qiao and Y. Bai, J. Adv. Ceram., 2022, 11, 414–426 CrossRef CAS.
- A. Herklotz, D. Lee, E. J. Guo, T. L. Meyer, J. R. Petrie and H. N. Lee, J. Phys.: Condens. Matter, 2017, 29, 493001 CrossRef PubMed.
- H. J. Liu, C. K. Wang, D. Su, T. Amrillah, Y. H. Hsieh, K. H. Wu, Y. C. Chen, J. Y. Juang, L. M. Eng, S. U. Jen and Y. H. Chu, ACS Appl. Mater. Interfaces, 2017, 9, 7297–7304 CrossRef CAS PubMed.
- J. Suntivich, K. J. May, H. A. Gasteiger, J. B. Goodenough and Y. Shao-Horn, Science, 2011, 334, 1383–1385 CrossRef CAS PubMed.
- A. Grimaud, O. Diaz-Morales, B. Han, W. T. Hong, Y. L. Lee, L. Giordano, K. A. Stoerzinger, M. T. M. Koper and Y. Shao-Horn, Nat. Chem. Biol., 2017, 9, 457–465 CrossRef CAS PubMed.
- W. T. Hong, K. A. Stoerzinger, Y.-L. Lee, L. Giordano, A. Grimaud, A. M. Johnson, J. Hwang, E. J. Crumlin, W. Yang and Y. Shao-Horn, Energy Environ. Sci., 2017, 10, 2190–2200 RSC.
- L. Hu, X. Sun, F. Zhou, J. Qi, A. Wang, C. Wang, M. Liu and M. Feng, Ceram. Int., 2021, 47, 2672–2677 CrossRef CAS.
- H. Liu, J. Qi, M. Feng, H. Xu, R. Liu, N. Li, C. Wang, Y. Zhang and W. Lü, Adv. Sustainable Syst., 2021, 5, 2100073 CrossRef CAS.
- C. Yang, B. Liu, G. Liu, F. Diao, H. Yang and Y. Wang, Solid State Ionics, 2018, 319, 28–33 CrossRef CAS.
- Y. Dong, Z. Ma, Z. Luo, H. Zhou, D. D. Fong, W. Wu and C. Gao, Adv. Mater. Interfaces, 2019, 6, 1900644 CrossRef.
- Q. X. Zhu, W. Wang, X. Q. Zhao, X. M. Li, Y. Wang, H. S. Luo, H. L. W. Chan and R. K. Zheng, J. Appl. Phys., 2012, 111, 103702 CrossRef.
- L. Guo, G. Li and Z. Gan, J. Adv. Ceram., 2021, 10, 472–481 CrossRef CAS.
- P. Han, T. Tan, F. Wu, P. Cai, G. Cheng and W. Luo, Chin. Chem. Lett., 2020, 31, 2469–2472 CrossRef CAS.
- C. E. Beall, E. Fabbri and T. J. Schmidt, ACS Catal., 2021, 11, 3094–3114 CrossRef CAS.
- L. Sun, Z. Dai, L. Zhong, Y. Zhao, Y. Cheng, S. Chong, G. Chen, C. Yan, X. Zhang, H. Tan, L. Zhang, K. N. Dinh, S. Li, F. Ma and Q. Yan, Appl. Catal., B, 2021, 297, 120477 CrossRef CAS.
- D. A. Kuznetsov, B. Han, Y. Yu, R. R. Rao, J. Hwang, Y. Román-Leshkov and Y. Shao-Horn, Joule, 2018, 2, 225–244 CrossRef CAS.
- M. A. Ramos-Docampo, B. Rivas-Murias, B. Rodríguez-González and V. Salgueiriño, CrystEngComm, 2017, 19, 5542–5548 RSC.
- C. K. Xie, J. I. Budnick, W. A. Hines, B. O. Wells and J. C. Woicik, Appl. Phys. Lett., 2008, 93, 182507 CrossRef.
- H. Zhong, C. Campos-Roldán, Y. Zhao, S. Zhang, Y. Feng and N. Alonso-Vante, Focus Catal., 2018, 8, 559 Search PubMed.
- S. Wang, J. Hu, A. Wang, Y. Cui, B. Chen, X. Niu and F. Hao, J. Energy Chem., 2022, 66, 422–428 CrossRef CAS.
- T. Mayeshiba and D. Morgan, Chem. Phys., 2015, 17, 2715–2721 CAS.
- A. Cammarata and J. M. Rondinelli, J. Chem. Phys., 2014, 141, 114704 CrossRef PubMed.
- D. Fuchs, M. Merz, P. Nagel, R. Schneider, S. Schuppler and H. von Lohneysen, Rev. Lett., 2013, 111, 257203 CrossRef CAS PubMed.
- H. Xu, B. Wang, J. Qi, M. Liu, F. Teng, L. Hu, Y. Zhang, C. Qu and M. Feng, J. Adv. Ceram., 2022, 11, 515–521 CrossRef CAS.
- J. T. Mefford, X. Rong, A. M. Abakumov, W. G. Hardin, S. Dai, A. M. Kolpak, K. P. Johnston and K. J. Stevenson, Nat. Commun., 2016, 7 Search PubMed.
- X. Hu, Y. Li, X. Wei, L. Wang, H. She, J. Huang and Q. Wang, Adv. Powder Technol., 2022, 1, 100024 Search PubMed.
- J. Qi, H. Liu, H. Xu, L. Hu, C. Wang, Y. Zhang, M. Feng and W. Lu, ACS Appl. Mater. Interfaces, 2021, 13, 61267–61274 CrossRef CAS PubMed.
| This journal is © the Owner Societies 2023 |