Open Access Article
Open Access ArticleImplementation of rare isotopologues into machine learning of the chemical inventory of the solar-type protostellar source IRAS 16293-2422
Zachary T. P.
Fried
 *a,
Kin Long Kelvin
Lee
*a,
Kin Long Kelvin
Lee
 b,
Alex N.
Byrne
b,
Alex N.
Byrne
 c and
Brett A.
McGuire
c and
Brett A.
McGuire
 *de
*de
aDepartment of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. E-mail: zfried@mit.edu
bAccelerated Computing Systems and Graphics Group, Intel Corporation, 2111 NE 25th Ave., Hillsboro, OR 97124, USA
cDepartment of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA
dDepartment of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. E-mail: brettmc@mit.edu
eNational Radio Astronomy Observatory, Charlottesville, VA 22903, USA
First published on 9th May 2023
Abstract
Machine learning techniques have been previously used to model and predict column densities in the TMC-1 dark molecular cloud. In interstellar sources further along the path of star formation, such as those where a protostar itself has been formed, the chemistry is known to be drastically different from that of largely quiescent dark clouds. To that end, we have tested the ability of various machine learning models to fit the column densities of the molecules detected in source B of the Class 0 protostellar system IRAS 16293-2422. By including a simple encoding of isotopic composition in our molecular feature vectors, we also examine for the first time how well these models can replicate the isotopic ratios. Finally, we report the predicted column densities of the chemically relevant molecules that may be excellent targets for radioastronomical detection in IRAS 16293-2422B.
1 Introduction
The observation of interstellar molecules is a central component of astrochemical studies. Molecular species have shaped our understanding of star1 and planet formation,2 can trace stellar outflows,3 interstellar shocks,4 and protoplanetary disks,5 and can serve as probes of the physical conditions of interstellar sources such as the temperature.6 However, until recently, in order to model interstellar abundances and predict new molecules for detection, observations have relied on complex chemical models based on a vast network of interconnected reactions (e.g. Ruaud et al.,7 Wakelam et al.8). While these astrochemical models can be excellent tools to explore specific chemical processes that occur in space, their predictive ability can also be quite limited for several reasons (e.g. McGuire et al.9). Firstly, these models are by definition incomplete representations of the true chemical complexity of the interstellar medium because network expansions rely on human input. Additionally, the networks are oftentimes dependent on uncertain extrapolated rate constants.10In an attempt to predict molecular abundances without the need for complete networks, Lee et al.11 introduced a novel methodology involving machine learning. A major benefit of their approach contrasts traditional astrochemical modeling, as it requires no prior knowledge of the conditions of an interstellar source or any reaction pathways involving the previously detected molecules. Instead, abundances are expressed purely in terms of a chemical vector space. Simple regression algorithms were shown to significantly outperform traditional astrochemical models in reproducing the abundances of molecules already observed, and provided a straightforward way to extrapolate to yet undetected molecules.
An interstellar source for which this machine learning technique could be effectively applied is the Class 0 protostar IRAS 16293-2422B (hereafter referred to as IRAS 16293B). IRAS 16293B is one component of the protostellar system IRAS 16293, which is located in the L1689 region of the ρ Ophiuchus cloud complex. Interferometric observations initially revealed two protostellar sources in IRAS 16293 (source A and source B), separated by around 5.1′′.12–14 Further high-resolution studies then confirmed that source A is in fact composed of two compact sources (source A1 and A2), making IRAS 16293 a triple protostellar system.15 Extensive observations have been made of this source with the Atacama Large Millimeter/submillimeter Array (ALMA) as part of the Protostellar Interferometric Line Survey (PILS) program.16 The submillimeter spectrum toward IRAS 16293B is especially rich with more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 features detected.16 The line widths of the spectral peaks are also extremely narrow for a star forming region (∼1 km s−1 FWHM), which significantly reduces line confusion and makes this an excellent source for molecular detections.
000 features detected.16 The line widths of the spectral peaks are also extremely narrow for a star forming region (∼1 km s−1 FWHM), which significantly reduces line confusion and makes this an excellent source for molecular detections.
The predictive power of the machine learning method introduced by Lee et al.11 may be especially useful for IRAS 16293B since a large portion of the molecular lines in the interstellar line survey remain unassigned. In fact, as of 2018, Taquet et al.17 noted that approximately 70% of the 5σ transitions identified in the ALMA Band 6 dataset were unidentified. If successful, this method might be able to provide an unbiased list of astrochemical targets not yet detected but which might be abundant enough to be contributing to the unidentified molecular lines. If subsequently detected, these molecules and their abundances could then be used to further constrain both the machine learning model and traditional network-based astrochemical models of low mass protostars. These models provide invaluable insight into the chemical processes and conditions relevant to the formation of stars similar to our Sun.
One aspect of interstellar chemistry that was not treated in the work of Lee et al.11 was the incorporation of isotopically substituted species. While certainly such rare isotopologues are present and detectable in TMC-1,18 detections of these species are more common toward IRAS 16293B and therefore provide substantially more insight into the chemical and physical history of this source. Therefore, it is desirable to update the machine learning model to include isotopologues. Molecules in IRAS 16293B consistently display isotopic ratios that are enhanced compared to the mean solar value and other interstellar sources, especially deuterium (D) and 13C substituted species. In fact, the deuterated isotopologues of ethanol, ketene, acetaldehyde, formic acid, formamide, and isocyanic acid were all first detected toward this source.19,20 Various doubly and triply deuterated species have been detected as well (e.g. Ilyushin et al.,21 Persson et al.22). Additionally, the 12C/13C ratios of dimethyl ether, methyl formate, ethanol, and glycolaldehyde toward IRAS 16293B are all much lower than the 12C/13C ratio of the local ISM.16,19 By convention, the deuterium ratios are reported as D/H while the 13C ratios are reported in the inverse manner. Therefore, a high D/H ratio and low 12C/13C ratio both denote isotopic enhancement.
A large portion of the remaining unassigned spectral peaks are predicted to arise from isotopically substituted species. In fact, Jørgensen et al.16 note that only 25% of the transitions correspond to the most common organic molecules detected in hot cores, including formaldehyde, methanol, methyl cyanide, isocyanic acid, ethanol, acetaldehyde, methyl formate, dimethyl ether, and ketene. Following this, they predict that the majority of the remaining transitions are likely related to various isotopically substituted molecules as well as more complex organic species. Thus, it is also vital to accurately model the column densities of isotopically substituted molecules so that the high abundance isotopologues in this source can be predicted, measured as needed in the laboratory, and their signals in the PILS survey identified and assigned.
Additionally, machine learning predictions of isotopic ratios are also useful since these ratios can act as tracers of the evolutionary history of an interstellar source along with the conditions, timescales, and pathways of molecular formation. For example, deuterium fractionation relies on gas-phase isotope exchange reactions that are strongly dependent on the temperature. Consequently, the deuterium fraction is a tracer of the conditions of the interstellar environment during molecular formation, with a high D/H ratio (i.e. high deuterium fraction) indicating cold formation temperatures.23,24 Therefore, accurate prediction of isotopic ratios would allow us to gain insight into the details of molecular formation and source history without requiring a dedicated search for these isotopically-substituted species that are often present in fairly low abundance.
In this work we apply the machine learning technique introduced by Lee et al.11 to IRAS 16293B. The machine learning pipeline used for this project along with the isotopic encoding is described in Section 3. Section 4.1 then presents the ability of the supervised machine learning regressors to model the molecular column densities in this source. Using these trained regression models, we obtain an unbiased list of predicted high-abundance targets for astronomical observation. Analysis of these molecular targets is presented in Section 4.2. Next, in Section 4.3 we test the ability of the regressors to model the isotopic ratios in this source. Finally, a list of high predicted column density isotopologues is provided in Section 4.4.
2 Dataset
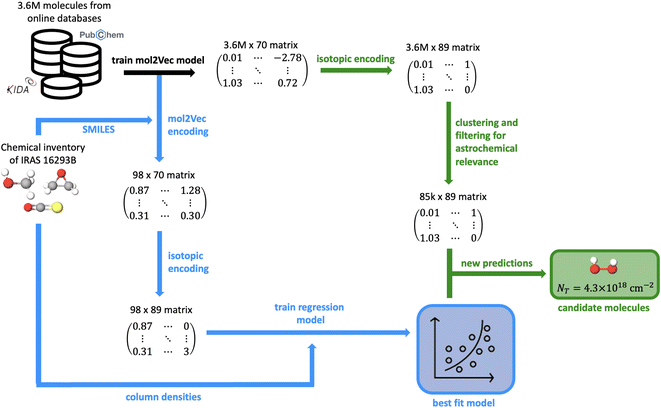
The molecules included in the dataset for this work were mostly detected through observations from the PILS survey.16 IRAS 16293A is an edge-on disk system while IRAS 16293B has a face-on orientation. This results in the line widths of the spectral peaks toward source A being much wider.16 Consequently, there is much more overlap of the spectral peaks, making the identification of individual signals more challenging and resulting in fewer definitive molecular detections toward source A. Our analysis therefore focused solely on IRAS 16293B. Most molecules were detected at a one-beam (0.5′′) offset position from the continuum peak of source B in the south-west direction. The coordinates of this one-beam offset position are αJ2000 = 16h32m22s.58, δJ2000 = −24°28′32.8′′. A few of the species, however, were detected at a half-beam offset. For these molecules, the column densities have been reduced by a factor of 2.136 to account for this different pointing position.25 In total, our dataset includes 98 molecules. Of these, 43 are main isotopologues and 55 are isotopically substituted species. All molecules in the dataset are listed in Table 5 in the Appendix. Our dataset contains 27 deuterium substituted species, 15 13C substituted species, two 15N substituted species, four 34S substituted species, two 33S substituted species, two 17O substituted species, and three 18O substituted species. Additionally, several doubly deuterated molecules along with one triply deuterated molecule is included.3 Model description
The machine learning pipeline has three main components: (1) molecular featurization, (2) modeling the column densities in IRAS 16293B using supervised regressors, and (3) prediction of high abundance astrochemical targets. An outline of this entire process is depicted in Fig. 8 in the Appendix. In this work, the process of molecular featurization and regression is quite similar to the methods introduced by Lee et al.11 In summary, a Mol2vec26 model is first trained on a dataset of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634
634![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 046 molecules collected from various online databases like Pubchem,27 ZINC,28 and the NASA PAH database.29–31 This trained embedding model then creates 70 dimensional feature vectors for all molecules in the dataset, in addition to the detected interstellar species.
046 molecules collected from various online databases like Pubchem,27 ZINC,28 and the NASA PAH database.29–31 This trained embedding model then creates 70 dimensional feature vectors for all molecules in the dataset, in addition to the detected interstellar species.
In order to include isotopologues in the training set and investigate isotopic ratios, it was necessary to encode isotopic composition in the feature vectors. In its current form, Mol2vec is not able to fully capture isotopic information. For example, it creates unique vectors for deuterium-substituted molecules but not for molecules that are substituted with 13C. This is because the molecular substructures are first encoded using Morgan fingerprints, which do not by-default capture differences in 13C-substituted isotopologues (e.g. the default RDKit-constructed32 Morgan fingerprint33 of H2CO and H213CO are identical). To differentiate between each of the isotopologues in our dataset, we ensure that the Mol2vec-generated vectors are identical for all isotopologues of the same species and then add 19 extra dimensions that encode isotopic information as well as the chemical environment of the isotopic substitution (Fig. 1). The isotopic encoding is designed as follows:
• Dimensions 1–9: number of D, 34S, 33S, 36S, 13C, 17O, 18O, 15N, and 37Cl atoms in the molecule.
• Dimensions 10–12: whether the 13C atoms are sp, sp2 or sp3 hybridized.
• Dimension 13: whether the substituted 13C atom is bonded to oxygen.
• Dimension 14–15: whether a deuterium atom is bonded to carbon or oxygen.
• Dimension 16: number of non-hydrogen atoms in the molecule.
• Dimensions 17–18: whether there is an oxygen or carbon atom two bonds away from the substituted deuterium.
• Dimension 19: number of deuterium atoms bonded to carbonyl carbons.
The RDKit module was used to obtain hybridization and bonding information.32 Because of the limited number of unique isotopologues in our dataset, the selection of these hand-picked features was largely dependent on which chemical substructures are present in enough molecules to constitute a reasonably sized training set. More specifically, each of the isotopic features denoted by dimensions 10–19 are present in at least three molecules in the dataset. Each of the selected features also has a notable impact on the average isotopic ratio.
Additionally, Mol2vec was unable to differentiate between several conformers of the detected molecules. Examples include ethylene glycol (for which both the aGg′ and gGg′ conformers were detected) as well as monodeuterated CH2DCH2OH and CH2DOCH3. For consistency, we inputted the column density of the most stable or abundant conformer in each case.
Using the resulting feature vectors as inputs and the log10 column densities as outputs, the data was split 80/20 into training and testing sets. In order to mitigate data leakage, all isotopologues of the same molecule were assigned to either the training or testing set. The datapoints were then bootstrapped with Gaussian noise in order to increase the effective dataset size to 800 and control overfitting.
These resulting training and testing sets were then fed into two separate supervised machine learning regressors: Gaussian process regression (GPR)34 and Bayesian ridge regression (BR). These models learn relationships between the vector components to map the molecular features to the column density data. Each of the models were implemented with the SCIKIT-LEARN Python module.35 We determined the optimal hyperparameters for each model by first splitting the data into training and testing sets and then running a 5-fold grid search on the training data.
GPR is a nonparametric model that defines a probability distribution over all functions that can map the molecular descriptors to the column densities. It is therefore able to handle nonlinear relationships in the data. A kernel provides the model with prior knowledge regarding the shape and smoothness of the functions. Similarly to Lee et al.,11 the kernel we used was a linear combination of the rational quadratic, dot product, and white noise kernels. Along with the kernel function, additional hyperparameters include a noise value added to the kernel matrix diagonal that denotes the inherent Gaussian noise of the training observations.
BR is a linear regressor that takes a probabilistic approach to optimize the ridge regression model coefficients. It does this by using a gamma distribution prior for the regularization coefficients. These parameters are then optimized through maximization of the log marginal likelihood. For this regressor, the hyperparameters define the shape and inverse scale of the Gamma distribution priors over the various model parameters.
Similarly to Lee et al.,11 a linear model is included in order to provide a baseline performance using an extremely simple model with a limited number of parameters. Bayesian ridge was specifically chosen due to its ability to report prediction uncertainties, which allows us to gauge the confidence level of the predicted values. A GPR model then displays the ability of a more complex model to improve upon this baseline regressor. GPR was also chosen due to its probabilistic and nonlinear nature. The reported uncertainties are fairly informative and interpretable since they can be linked directly to the designed covariance matrix.
Following the training of the regression models, the column densities of the molecules that are most chemically similar to those detected in IRAS 16293B were predicted using the trained models. K-means clustering with k = 10 was used to cluster the entire dataset of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634
634![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 046 feature vectors. Each of the molecules detected in IRAS 16293B was assigned to a single cluster. Thus, we only considered the molecules assigned to this cluster when searching for detectable new species.
046 feature vectors. Each of the molecules detected in IRAS 16293B was assigned to a single cluster. Thus, we only considered the molecules assigned to this cluster when searching for detectable new species.
When analyzing the ability of the regressors to model the molecules in the training set and subsequently predict the column densities of the species in the testing set, we were limited to the molecules that have been previously detected toward IRAS 16293B. We were therefore only able to gauge the performance of these models on molecules that are relevant to this fairly small and homogeneous dataset. Consequently, we proceeded to remove the molecules that had no or few chemically similar examples in the dataset since the models did not have sufficient training examples to learn the required relationships for these species and we had no ability to gauge the accuracy of the model predictions. For this additional filtering, we removed molecules that contained atoms other than hydrogen, carbon, sulfur, nitrogen, and oxygen because all of the previously detected molecules were predominantly composed of these atoms. Additionally, we also removed the remaining free radical species because nitrous oxide is the only free radical for which a column density has been derived toward IRAS 16293B. This minuscule training set of free radicals resulted in the models not being able to sufficiently handle these molecules. For example, the free radical counter-parts of various molecules had much higher predicted column densities than the observed values of the parent species. This is very unlikely due to the instability of free radicals and the general under abundance of radicals in protostellar sources. In fact, while 36.5% of interstellar molecules were first detected in star-forming regions, only 13.5% of radical species were first detected in these protostellar sources.36 This under abundance may be due to the larger gas-phase chemical inventory and warmer kinetic temperatures leading to a greater number of destruction partners for these highly reactive species. Following these filtering steps, the column densities of the remaining 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 863 molecules were then predicted using the previously trained regression models.
863 molecules were then predicted using the previously trained regression models.
4 Results and discussion
4.1 Regression analysis
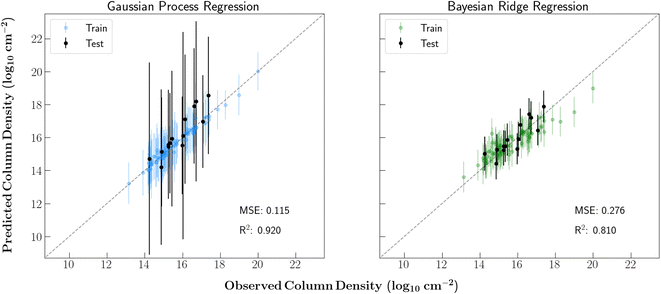
Fig. 2 shows the training and testing results of each regression model. The train/test splits were consistent in each case. Just as shown in Lee et al.11 's study of TMC-1, each of the regressors were able to accurately model the column densities in this source. The strong performance on the test set also provides confidence that the models can generalize well to relevant molecules that were not included in the training set.Additionally, although the BR model showed an ability to precisely model the column densities with lower uncertainties than the GPR regressor, it was mainly limited by its linear mapping. With our current isotope encoding, a linear model will be unable to fully capture the relevant isotopic fractionation. For example, the difference between a singly and doubly deuterated molecule is in-part denoted with a 2 instead of a 1 in a single vector dimension. That said, the difference in the column densities of singly and doubly deuterated species is typically not simply 2/1 and can differ significantly between molecules. Thus, for the remainder of the analysis, a GPR model was used since a nonlinear mapping was required.
The large error bars on the GPR predictions are in part due to the small size of the dataset. Additional molecular detections (especially of main isotopologues) toward this source will allow for further constrained predictions. Moreover, despite the overall strong performance of the GPR model, this regressor overpredicts ethylene glycol (OHCH2CH2OH) and dimethyl ether (CH3OCH3) by over one order of magnitude. This prediction inaccuracy is likely because these molecules have few nearby neighbors in the training set. In fact, these molecules are the 10th and 12th furthest species from any neighbor in the dataset, respectively. Ethylene glycol is especially unique in that it is the only molecule in the dataset containing two hydroxyl groups. Additionally, ethylene glycol's nearest neighbors are methoxymethanol and ethanol, each of which are more abundant.
Another notable prediction error is the slight overprediction of chloromethane and underprediction of methanol since it highlights the shortcomings of our molecular featurization. Mol2vec creates molecular feature vectors by combining vector representations of chemical substructures. Therefore, small molecules with some shared substructures have extremely similar feature vectors. In this case, Mol2vec generates similar feature vectors for all molecules that contain a methyl group bonded to a single heteroatom. Despite obvious chemical differences, the resulting vector representations of chloromethane and methanol are therefore very similar. Since methanol is one of the most abundant molecules in the dataset and chloromethane is one of the least abundant, the resulting prediction errors are fairly unsurprising.
In order to test the efficacy of the chosen kernel for the GPR model, we predicted the column density of cimetidine C10H16N6S. This molecule is far more complex than any other species in our dataset and would certainly have an extremely low abundance in the interstellar medium. Therefore, an effective kernel would also produce a low column density prediction for this species. Ultimately, the trained GPR model predicted this molecule to have a column density of 6.94 × 106 cm−2, which is nearly eight orders of magnitude lower than any main isotopologue in the dataset. This provides confidence that the model is not over-fitting to the dataset and simply learning to predict each column density to be in the range of the detected species.
4.2 Targets for astrochemical study
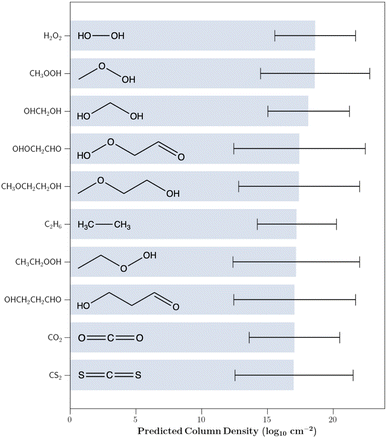
Using the trained GPR model, we then predicted the column densities of the aforementioned 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 863 astrochemically relevant molecules assigned to the same cluster as the IRAS 16293B detections. To enhance the confidence in our predicted values and further limit our investigation to chemically relevant molecules, our analysis was solely focused on species for which the 1σ prediction uncertainty was less than five orders of magnitude; there were 242 such species. Fig. 3 shows the 10 molecules with the highest predicted column densities. All predictions are provided in the associated GitHub repository.
863 astrochemically relevant molecules assigned to the same cluster as the IRAS 16293B detections. To enhance the confidence in our predicted values and further limit our investigation to chemically relevant molecules, our analysis was solely focused on species for which the 1σ prediction uncertainty was less than five orders of magnitude; there were 242 such species. Fig. 3 shows the 10 molecules with the highest predicted column densities. All predictions are provided in the associated GitHub repository.
The chemical composition of the predicted molecules is displayed in Table 1. Oxygenated hydrocarbons were typically predicted to be in high abundance while those containing nitrogen and sulfur were predicted to have lower column densities. Of the 20 highest predicted column density molecules, 15 contain at least one oxygen atom, while 2 contain a nitrogen atom, and 1 contains a sulfur atom. The preference for oxygen-substituted molecules is not surprising since the most abundant detected species in IRAS 16293B are carbon monoxide (CO), methanol (CH3OH), formaldehyde (H2CO), methyl formate (HCOOCH3), dimethyl ether (CH3OCH3), carbonyl sulfide (OCS), and ethanol (CH3CH2OH) – each of which contain an oxygen atom.
| 20 highest predicted abundance molecules | 20 lowest predicted abundance molecules | |
|---|---|---|
| Mean # of oxygen atoms | 1.65 | 0.75 |
| Mean # of nitrogen atoms | 0.10 | 1.05 |
| Mean # of sulfur atoms | 0.10 | 0.75 |
| Mean degree of unsaturation | 0.75 | 1.65 |
| Mean # of heavy atoms | 3.85 | 4.25 |
| Mean molecular weight (amu) | 59.66 | 74.35 |
Highly saturated molecules were also predicted to be very abundant in IRAS 16293B. This is to be expected in a protostellar source since hydrogenation is very efficient on grain surfaces. Therefore, many of the species that are sublimated from grains as the protostar heats the surroundings are highly saturated (e.g. Linnartz et al.,37 Fedoseev et al.,38 Woon,39 Garrod et al.40).
The predictions also display a preference for lighter molecules that contain less heavy atoms. This also matches the detected chemical inventory in IRAS 16293B, in which the seven highest abundance molecules each contain four or less heavy atoms.
The proceeding subsections highlight the astrochemical relevance of some of the molecules with the highest predicted column densities in Fig. 3. It is important to note that while a high column density is beneficial for interstellar molecular detectability, various additional factors must also be considered including the magnitude of the dipole moment, the spectral pattern, and intrinsic line strengths.
4.3 Isotope ratios
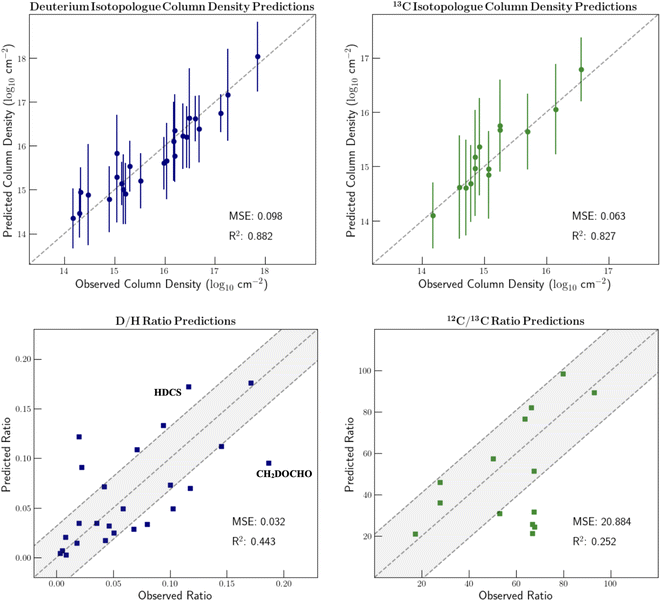
Since isotopic composition was encoded in our molecular feature vectors, we proceeded to test the regressors' ability to predict isotopic ratios. As noted previously, if the machine learning model can accurately predict isotope ratios, information about the evolutionary history of the source and the molecular formation can be deciphered. That said, with such a simple encoding of the isotopic information in our feature vectors as well as the relatively small collection of isotopically substituted molecules, modeling this nuanced chemistry will be a challenge. Our discussion will solely focus on 13C and D substituted isotopologues because of the extremely small sample size of all other minor isotopes.Fig. 4 displays the predicted column densities of the D and 13C substituted isotopologues along with the corresponding isotopic ratios. These predictions stem from 5-fold cross validation on the isotopically substituted data. In this process, the isotopologues are split into five subsets of training and validation data. In each iteration, 20% of the isotopically substituted molecules are left out of the training set. The model is then trained on all molecules in the dataset besides the 20% of rare isotopologues that were assigned to the validation set.
Because the deuterium and 13C ratios are reported in inverse fashions, the mean squared errors of the two ratio plots differ dramatically. The points within the shaded regions denote the molecules for which the prediction error is less than the mean absolute prediction error. For the D/H ratios, the mean absolute error is 0.032. For the 12C/13C ratios, the mean absolute error is 20.9.
The column density predictions for isotopically substituted molecules are typically extremely accurate. However, when considering isotopic ratios, the range of realistic values is quite limited; therefore, a small prediction error is very notable. For example, deuterated acetaldehyde in IRAS 16293B is observed to have a D/H ratio of 7.98%. A column density under-prediction by 0.3 orders of magnitude would result in a predicted ratio of approximately 4.00%. This ratio suggests very different temperatures and timescales of formation.
There are a few molecules for which the ratio prediction is especially inaccurate. These species are labelled on Fig. 4. The prediction errors of HDCS and CH2DOCHO are especially notable since they highlight the shortcomings of using hand-picked descriptors. When encoding the chemical environment of the deuterium substitution, vector dimensions are included that denote whether there is a carbon or oxygen atom two bonds away from the deuterium atom. However, there was no consideration of whether the atom in this position is sulfur since HDCS is the only molecule in the dataset in which this is the case. Therefore, this important chemical environment information is not provided to the model, thus leading to a large prediction error. Additionally, with simple hand-picked features, the model isn't always able to capture the nuances of isotopic fractionation. For example, the isotopic encoding of CH2DOCHO is very similar to that of CH2DOH. Because CH2DOH has a D/H ratio of only around 7%, the model inaccurately predicts CH2DOCHO to have approximately the same ratio.
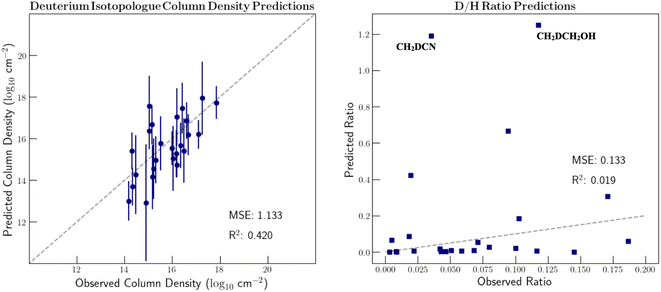
Preferably we could include a more nuanced encoding of isotopic composition that better captures the local chemical environment instead of simple hand-picked features. However, with only 27 deuterated molecules and 15 13C substituted species, the dataset of unique isotopologues is too small to learn the required relationships with a complex featurization. As mentioned previously, Mol2vec is sensitive to some, but not all, isotopic substitutions. It can, however, create unique vectors for deuterated species. Therefore, we tested the ability to learn deuterium ratios from the original Mol2vec-produced vectors that more fully consider chemical context. These results are shown in Fig. 5. The D/H ratio of formic acid is omitted from the graph since a ratio of around 6 is predicted which skews the ability to view the remaining points. As can be seen, these predictions are far less accurate than when hand-picked features were used. This is because the vector representations of many of the deuterated species are quite dissimilar to the main isotopologue in this case. In fact, the vector representation of CH2DCH2OH is closer to that of propanal than that of CH3CH2OH. In order to include more detailed isotopic information in the feature vectors and thus accurately model isotopic fractionation, it is clear that we require additional isotopologue detections.
Finally, in order to test the predictive ability of the model on isotopologues for which no column density has been derived, we proceeded to predict the 12C/13C ratio of CO and CN with the trained GPR model from Section 4.1. For these two molecules (along with H2CO), a linear 12C/13C trend has been defined as a function of galactocentric distance (DGC). The formulae of these galactocentric gradients are displayed in eqn (1)–(3)
| 12CO/13CO = (5.41 ± 1.07)kpc−1 × DGC + (19.03 ± 7.90) | (1) |
| 12CN/13CN = (6.01 ± 1.19)kpc−1 × DGC + (12.28 ± 9.33) | (2) |
| H212CO/H213CO = (7.60 ± 1.79)kpc−1 × DGC + (18.05 ± 10.88) | (3) |
Using a galactocentric distance of 8.043 kpc for IRAS 16293, the range of expected ratios along with the ratios predicted by the GPR model are shown in Table 2. For reference, the observed 12C/13C ratio of H2CO is listed as well.22 The Galactocentric distance of IRAS 16293 was computed using the ASTROPY Python module.62 The values used in this calculation were the distances from the Earth to both the Galactic Center and IRAS 16293 (8.178 kpc and 141 pc (ref. 63 and 64)) as well as their respective sky coordinates.
| Molecule | Galactocentric gradient 12C/13C ratio | Predicted 12C/13C ratio | Observed 12C/13C |
|---|---|---|---|
| CO | 46.04–79.05 | 50.85 | — |
| CN | 41.72–79.52 | 27.34 | — |
| H2CO | 53.90–104.46 | — | 52.92 |
The observed 12C/13C ratio of formaldehyde toward IRAS 16293B is very near the lower bound of the galactocentric trend error bars at 8.043 kpc. Interestingly, the GPR model predicts the CO and CN ratios to also be fairly near the lower bounds of the respective ratio gradients. This matches the observed trends of various other molecules in IRAS 16293B, which typically show high levels of 13C substitution. A high abundance of 13CO could stem from favorable ion-neutral isotope exchange reactions in the cold interstellar gas before CO freeze-out.65 Complex organic species that were then formed on grain surfaces from CO following freeze-out would inherit this small 12C/13C ratio. The 13C enhancement in organic molecules would be even more notable at later timescales since laboratory experiments have shown that 12CO desorbs slightly more efficiently than 13CO.66 This enables 12CO to sublimate from the grain at a lower temperature. Therefore, as the protostar begins to heat the surroundings to around the CO sublimation temperature, the more efficient 12CO sublimation would result in grain surfaces that are further enhanced with 13CO.
Overall, while the machine learning regressor is not precise enough to adequately model the exact isotopic ratios, the 13CO and 13CN predictions show that it still is able to learn the general overabundance of 13C in the organic species of IRAS 16293B.
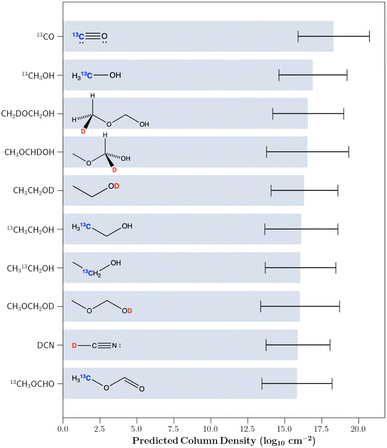
4.4 Isotopologue targets for astrochemical study
As noted in Section 1, because of the extreme isotopic fractionation in this source, multiple groups have predicted that a significant portion of the unidentified spectral peaks in the line survey arise from isotopically substituted species.16,17 Additionally, Fig. 4 shows that the GPR regressor is able to precisely model column densities of the isotopically substituted molecules. Therefore, using the trained GPR model we proceeded to predict the column densities of the D and 13C mono-substituted isotopologues of the previously identified molecules in IRAS 16293B for which no accurate column density has been derived. Due to the inaccurate predictions of the main isotopologues of ethylene glycol, chloromethane and dimethyl ether, the predictions of the rare isotopologues of these species were omitted. The highest 10 predicted column density isotopologues are displayed in Fig. 6. All predictions are provided in the associated GitHub repository.Of these highest 10 predicted column density species, both 13C-substituted ethanol isotopologues and OD substituted ethanol were marginally detected toward IRAS 16293B. While we did not include these molecules in our training set, Jørgensen et al.19 derived tentative column densities for these species. These tentatively derived column densities along with the values predicted by the GPR model are listed in Table 3. As can be seen, all predictions closely match the tentative column densities. Additionally, while transitions corresponding to 13CH3OH and DCN have been identified toward IRAS 16293, no derived column density is listed.67
| Molecule | Tentative column density (cm−2) | Predicted column density (cm−2) |
|---|---|---|
| CH3CH2OD | 1.1 × 1016 | 2.15 × 1016 |
| 13CH3CH2OH | 9.1 × 1015 | 1.33 × 1016 |
| CH313CH2OH | 9.1 × 1015 | 1.18 × 1016 |
Therefore, the remaining molecules of interest are the three deuterated isotopologues of methoxymethanol along with the 13C isotopologue of methyl formate. Beyond these largest 10 predicted column density isotopologues, another high predicted abundance isotopologue is the deuterated isotopologue of methoxyethane (CH3CH2OCH2D). As mentioned previously, while large column densities are beneficial for detection, various other factors impact the detectability of a molecule. For all of these molecules, we therefore simulated their spectra toward IRAS 16293B using the predicted column densities in order to assess their true detectability. The microwave and sub-mm spectra of 13CH3OCHO have been experimentally studied and assigned, thus making interstellar detection currently possible. In fact, this rare isotopologue was detected in the Orion molecular cloud.68 However, this particular isotopologue is not present in the CDMS molecular spectroscopy database.69 All of the other aforementioned deuterated isotopologues have not been studied experimentally.
In order to simulate the spectra of these previously unstudied isotopologues, the rotational constants must first be calculated. A low-cost method to obtain molecular rotational constants for isotopically substituted species can be achieved by combining experimental data and ab initio calculations. In this process, the experimental rotational and distortion constants of the main isotopologue are first collected. The A, B, and C rotational constants of the parent species are then calculated at a given level of theory and basis set. For our work, we ran the calculations with the PSI4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 Python package and used the M06-2X functional with the 6-311++G(d,p) basis set. Assuming that the geometry remains constant upon isotopic substitution, the same computational methods are then used to calculate rotational constants of the rare isotopologues. Finally, it is assumed that the scaling factor between the experimental and calculated rotational constants is the same for the main isotopologues and the isotopically substituted molecules. For example, the B rotational constant of the isotopically substituted species can be calculated using eqn (4).
70 Python package and used the M06-2X functional with the 6-311++G(d,p) basis set. Assuming that the geometry remains constant upon isotopic substitution, the same computational methods are then used to calculate rotational constants of the rare isotopologues. Finally, it is assumed that the scaling factor between the experimental and calculated rotational constants is the same for the main isotopologues and the isotopically substituted molecules. For example, the B rotational constant of the isotopically substituted species can be calculated using eqn (4).
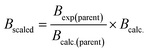 | (4) |
The experimental distortion constants and dipole moments of the main isotopologues were used as-is for the isotopically substituted molecules.
Following the rotational constant calculations, a rotational line catalog was generated using Pickett's SPCAT.71 Only the A, B, and C rotational constants and distortion constants (when available) were used. Internal rotational was not considered during the catalog simulations. The molsim72 Python package was then used to simulate the spectra of the isotopologues toward IRAS 16293B with the predicted column densities. Molsim assumes that the molecular emission can be described by a single excitation temperature, and accounts for the effects of optical depth. For the simulations, the excitation temperature and vlsr of the main isotopologue were used. A source size of 0.5′′, beam diameter of 0.5′′, and line width of 1.0 km s−1 were used for each simulation.
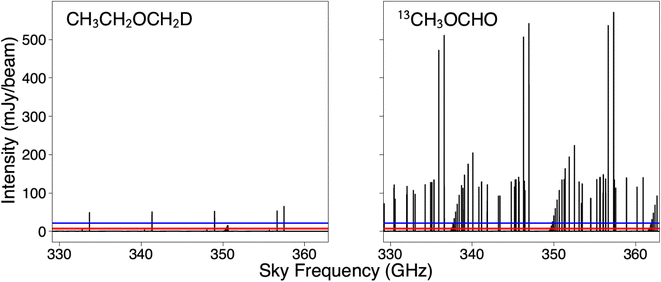
Since ALMA Band 7 (329.147–362.896 GHz) is fully covered with the PILS observations, this frequency range is the predominant focus of our spectral simulations. Given the noise level of the PILS observations,16 any transition with a peak intensity stronger than ∼21 mJy per beam should be detectable at a 3σ significance. Analysis of the spectral simulations can be seen in Table 4. The simulated spectra of the 13C-substituted methyl formate isotopologue and the deuterated methoxyethane isotopologue toward IRAS 16293B are presented in Fig. 7.
| Molecule | # of 3σ transitions | # of 1σ transitions | Intensity of strongest transition (mJy per beam) |
|---|---|---|---|
| 13CH3OCHO | 81 | 86 | 571.0 |
| CH3CH2OCH2D | 12 | 18 | 64.8 |
| CH2DOCH2OH | 0 | 0 | 0.39 |
| CH3OCHDOH | 0 | 0 | 0.18 |
| CH3OCH2OD | 0 | 0 | 3.92 × 10−8 |
Despite having higher predicted column densities, the rare isotopologues of methoxymethanol are predicted to have much weaker spectral peaks than the other isotopologues considered due to the limited dipole moment. That said, with several transitions predicted to be stronger than 3σ, CH3CH2OCH2D is an excellent candidate for experimental study for astrochemical purposes. Additionally, since the spectrum of 13CH3OCHO has already been collected and assigned, we recommend that this molecule be searched for in the PILS data.
5 Conclusions
In this work, we applied the machine learning pipeline introduced by Lee et al.11 to source B of the Class 0 protostellar system IRAS 16293-2422. In order to include isotopologues in the dataset, we also concatenated a simple encoding of the isotopic composition to the feature vectors. Gaussian process regression and Bayesian ridge regression were both able to accurately model the column densities of the detected molecules in this source. The trained Gaussian process regression model then provided a list of 242 well-constrained targets for astrochemical study. Small, oxygenated, and fairly saturated hydrocarbons were predicted to be in high abundance in this protostellar source. While the column density predictions of isotopologues were quite precise, the nuances of isotopic ratios were only modeled with moderate accuracy. Additional isotopologue detections will be required to allow for a more complex encoding of isotopic substitution that better captures local chemical environment. Finally, since it has been predicted that many of the unassigned transitions in the PILS survey arise from isotopically substituted molecules, we provided a list of 92 isotopologue column density predictions.This machine learning method has now been shown to effectively model the molecular column densities in two separate interstellar sources and the resulting trained regression models can be used to predict molecular species that are likely abundant in these various regions of interstellar space. However, these same techniques can be readily applied to terrestrial chemical mixture identifications as well. For example, if a researcher is able to reliably identify a fairly small number of chemical components present in an environmental sample along with their abundances, these supervised regressors could be trained and used to predict other components and contaminants of that mixture.
6 Appendix
| Formula | SMILES | Observed column density | Reference |
|---|---|---|---|
| CO | [C−]#[O+] | 20.0000 | Drozdovskaya et al.25 |
| CH3OH | CO | 19.0000 | Jørgensen et al.16,19 |
| H2CO | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
18.2800 | Persson et al.22 |
| CH3CH2OH | CCO | 17.3600 | Jørgensen et al.19 |
| CH3OCH3 | COC | 17.3800 | Jørgensen et al.19 |
| HCOOCH3 | COC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
17.4100 | Jørgensen et al.19 |
| CH2OHCHO | O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCO CCO |
16.5100 | Jørgensen et al.16 |
| CH3COOH | CC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)O O)O |
15.4500 | Jørgensen et al.16 |
| CH3CHO | CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
17.0800 | Jørgensen et al.19 |
| c-C2H4O | C1CO1 | 15.7300 | Lykke et al.73 |
| HCOOH | O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CO CO |
16.7500 | Jørgensen et al.19 |
| aGg′-(CH2OH)2 | OCCO | 16.7200 | Jørgensen et al.16 |
| CH3OCH2OH | COCO | 17.1500 | Manigand et al.52 |
| C2H5CHO | CCC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
15.3400 | Lykke et al.73 |
| (CH3)2CO | CC(C)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
16.2300 | Lykke et al.73 |
| NH2CHO | NC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
15.9800 | Coutens et al.20 |
| HCN | C#N | 16.7000 | Drozdovskaya et al.25 |
| CH3CN | CC#N | 16.6000 | Calcutt et al.74 |
| CH3NC | [C−]#[N+]C | 14.3000 | Calcutt et al.75 |
| HNCO | N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
16.5700 | Ligterink et al.76 |
| HC3N | C#CC#N | 14.2600 | Calcutt et al.74 |
| H2S | S | 17.2300 | Drozdovskaya et al.59 |
| OCS | O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S S |
17.4000 | Drozdovskaya et al.59 |
| CH3SH | CS | 15.6800 | Drozdovskaya et al.59 |
| CS | [C−]#[S+] | 15.5900 | Drozdovskaya et al.59 |
| H2CS | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S S |
15.1100 | Drozdovskaya et al.59 |
| SO | O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S S |
14.6400 | Drozdovskaya et al.59 |
| CH3Cl | CCl | 14.6600 | Fayolle et al.77 |
| C2H3CHO | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
14.5300 | Manigand et al.78 |
| C3H6 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC CC |
16.6200 | Manigand et al.78 |
| CH3CCH | C#CC | 16.0400 | Calcutt et al.79 |
| t-C2H5OCH3 | CCOC | 16.2600 | Manigand et al.52 |
| C3H4O2 | O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CO CO |
15.0000 | Coutens et al.80 |
| CH3NCO | CN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
15.6000 | Ligterink et al.76 |
| C2H5CN | CCC#N | 15.5600 | Calcutt et al.74 |
| C2H3CN | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC#N CC#N |
14.8700 | Calcutt et al.74 |
| CH2CO | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
16.6800 | Jørgensen et al.19 |
| HONO | O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NO NO |
14.9500 | Coutens et al.81 |
| NO | [N]![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
16.3000 | Ligterink et al.82 |
| CH3C(O)NH2 | CC(N)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
14.9500 | Ligterink et al.82 |
| SO2 | O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
15.1100 | Drozdovskaya et al.59 |
| t-HCOOH | O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CO CO |
16.7500 | Jørgensen et al.19 |
| CH2NH | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N N |
14.9031 | Ligterink et al.82 |
| H213CO | [13CH2]![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
16.5563 | Persson et al.22 |
| H2C17O | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) [17O] [17O] |
14.8573 | Persson et al.22 |
| H2C18O | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) [18O] [18O] |
15.3617 | Persson et al.22 |
| HDCO | [2H]C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
17.1139 | Persson et al.22 |
| D2CO | [2H]C([2H])![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
16.2041 | Persson et al.22 |
| D213CO | [2H][13C]([2H])![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
14.3424 | Persson et al.22 |
| HC15N | C#[15N] | 14.3979 | Drozdovskaya et al.25 |
| 13CH3CN | [13CH3]C#N | 14.7782 | Calcutt et al.74 |
| CH313CN | C[13C]#N | 14.6990 | Calcutt et al.74 |
| CH3C15N | CC#[15N] | 14.2041 | Calcutt et al.74 |
| CH2DCN | [2H]CC#N | 15.1461 | Calcutt et al.74 |
| CHD2CN | [2H]C([2H])C#N | 14.3010 | Calcutt et al.74 |
| 34SO2 | O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) [34S [34S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O] O] |
14.6021 | Drozdovskaya et al.59 |
| O13CS | O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) [13C [13C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S] S] |
15.6990 | Drozdovskaya et al.59 |
| OC34S | O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) [34S] [34S] |
16.0000 | Drozdovskaya et al.59 |
| OC33S | O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) [33S] [33S] |
15.4771 | Drozdovskaya et al.59 |
| 18OCS | [18O]![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S S |
14.6990 | Drozdovskaya et al.59 |
| C34S | [C−]#[34S+] | 14.3010 | Drozdovskaya et al.59 |
| C33S | [C−]#[33S+] | 13.9031 | Drozdovskaya et al.59 |
| C36S | [C−]#[36S+] | 13.1461 | Drozdovskaya et al.59 |
| HDCS | [2H]C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S S |
14.1761 | Drozdovskaya et al.59 |
| HDS | [2H]S | 16.2041 | Drozdovskaya et al.59 |
| HD34S | [2H][34SH] | 15.0000 | Drozdovskaya et al.59 |
| CD3OH | [2H]C([2H)([2H)O | 16.4914 | Ilyushin et al.21 |
| CH2DOH | [2H]CO | 17.8513 | Jørgensen et al.19 |
| CH3OD | [2H]OC | 17.2553 | Jørgensen et al.19 |
| a-CH3CHDOH | [2H]C(C)O | 16.3617 | Jørgensen et al.19 |
| CH3OCDO | [2H]C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)OC O)OC |
16.1761 | Jørgensen et al.19 |
| CH2DOCHO | [2H]COC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
16.6812 | Jørgensen et al.19 |
| CHDCO | [2H]C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
15.3010 | Jørgensen et al.19 |
| 13CH3OCH3 | CO[13CH3] | 16.1461 | Jørgensen et al.19 |
| CH3CDO | [2H]C(C)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
15.9823 | Jørgensen et al.19 |
| H13COOH | O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) [13CH]O [13CH]O |
14.9191 | Jørgensen et al.19 |
| CHD2OCHO | [2H]C([2H)OC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
16.0414 | Manigand et al.83 |
| CH337Cl | C[37Cl] | 14.3424 | Fayolle et al.77 |
| NH2CDO | [2H]C(N)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
14.3222 | Coutens et al.20 |
| NH213CHO | N[13CH]![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
14.1761 | Coutens et al.20 |
| DNCO | [2H]N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
14.4771 | Coutens et al.20 |
| HN13CO | N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) [13C] [13C]![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
14.6021 | Coutens et al.20 |
| CHDOHCHO | [2H]C(O)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
15.5211 | Jørgensen et al.16 |
| CH2ODCHO | [2H]OCC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
15.1761 | Jørgensen et al.16 |
| CH2OHCDO | [2H]C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)CO O)CO |
15.2148 | Jørgensen et al.16 |
| CH318OH | C[18OH] | 16.2718 | Jørgensen et al.16 |
| 13CH2CO | [13CH2]![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
14.8513 | Jørgensen et al.19 |
| CH213CO | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) [13C] [13C]![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
14.8513 | Jørgensen et al.19 |
| 13CH3CHO | [13CH3]C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
15.2553 | Jørgensen et al.19 |
| CH313CHO | C[13CH]![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
15.2553 | Jørgensen et al.19 |
| t-DCOOH | [2H]C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)O O)O |
15.0414 | Jørgensen et al.19 |
| t-HCOOD | [2H]OC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
15.0414 | Jørgensen et al.19 |
| a–a-CH2DCH2OH | [2H]CCO | 16.4313 | Jørgensen et al.19 |
| Asym-CH2DOCH3 | [2H]COC | 16.6128 | Jørgensen et al.19 |
Data availability
The code, analysis scripts, and datasets supporting this article are available on GitHub at https://github.com/zfried/IRAS_ML_Predictions.Author contributions
Zachary Fried: software, formal analysis, investigation, data curation, writing – original draft, visualization. Kelvin Lee: methodology, supervision, writing – review & editing. Alex Byrne: software, writing – review & editing. Brett McGuire: supervision, writing – review & editing, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank C. W. Coley for providing helpful insight regarding the Mol2vec molecular featurization and the inclusion of isotopic information in Morgan fingerprints. The National Radio Astronomy Observatory is a facility of the National Science Foundation operated under cooperative agreement by Associated Universities, Inc.Notes and references
- E. F. van Dishoeck and G. A. Blake, Annu. Rev. Astron. Astrophys., 1998, 36, 317–368 CrossRef CAS PubMed.
- K. I. Öberg, R. Murray-Clay and E. A. Bergin, Astrophys. J., 2011, 743, L16 CrossRef.
- R. Bachiller, M. Pérez Gutiérrez, M. S. N. Kumar and M. Tafalla, Astron. Astrophys., 2001, 372, 899–912 CrossRef CAS.
- P. Schilke, C. M. Walmsley, G. Pineau des Forets and D. R. Flower, Astron. Astrophys., 1997, 321, 293–304 CAS.
- A. Ginsburg, B. McGuire, R. Plambeck, J. Bally, C. Goddi and M. Wright, Astrophys. J., 2019, 872, 54 CrossRef CAS.
- P. T. P. Ho, A. H. Barrett, P. C. Myers, D. N. Matsakis, A. L. Cheung, M. F. Chui, C. H. Townes and K. S. Yngvesson, Astrophys. J., 1979, 234, 912–921 CrossRef CAS.
- M. Ruaud, V. Wakelam and F. Hersant, Mon. Not. R. Astron. Soc., 2016, 459, 3756–3767 Search PubMed.
- V. Wakelam, J.-C. Loison, E. Herbst, B. Pavone, A. Bergeat, K. Béroff, M. Chabot, A. Faure, D. Galli, W. D. Geppert, D. Gerlich, P. Gratier, N. Harada, K. M. Hickson, P. Honvault, S. J. Klippenstein, S. D. L. Picard, G. Nyman, M. Ruaud, S. Schlemmer, I. R. Sims, D. Talbi, J. Tennyson and R. Wester, Astrophys. J., Suppl. Ser., 2015, 217, 20 CrossRef.
- B. A. McGuire, P. B. Carroll, N. M. Dollhopf, N. R. Crockett, J. F. Corby, R. A. Loomis, A. M. Burkhardt, C. Shingledecker, G. A. Blake and A. J. Remijan, Astrophys. J., 2015, 812, 76 CrossRef.
- E. Hébrard, M. Dobrijevic, P. Pernot, N. Carrasco, A. Bergeat, K. M. Hickson, A. Canosa, S. D. Le Picard and I. R. Sims, J. Phys. Chem. A, 2009, 113, 11227–11237 CrossRef PubMed.
- K. L. K. Lee, J. Patterson, A. M. Burkhardt, V. Vankayalapati, M. C. McCarthy and B. A. McGuire, Astrophys. J., Lett., 2021, 917, L6 CrossRef CAS.
- A. Wootten, Astrophys. J., 1989, 337, 858 CrossRef CAS.
- L. G. Mundy, A. Wootten, B. A. Wilking, G. A. Blake and A. I. Sargent, Astrophys. J., 1992, 385, 306 CrossRef CAS.
- L. W. Looney, L. G. Mundy and W. J. Welch, Astrophys. J., 2000, 529, 477–498 CrossRef.
- M. J. Maureira, J. E. Pineda, D. M. Segura-Cox, P. Caselli, L. Testi, G. Lodato, L. Loinard and A. Hernández-Gómez, Astrophys. J., 2020, 897, 59 CrossRef CAS.
- J. K. Jørgensen, M. H. D. v. d. Wiel, A. Coutens, J. M. Lykke, H. S. P. Müller, E. F. v. Dishoeck, H. Calcutt, P. Bjerkeli, T. L. Bourke, M. N. Drozdovskaya, C. Favre, E. C. Fayolle, R. T. Garrod, S. K. Jacobsen, K. I. Öberg, M. V. Persson and S. F. Wampfler, Astron. Astrophys., 2016, 595, A117 CrossRef.
- V. Taquet, E. F. v. Dishoeck, M. Swayne, D. Harsono, J. K. Jørgensen, L. Maud, N. F. W. Ligterink, H. S. P. Müller, C. Codella, K. Altwegg, A. Bieler, A. Coutens, M. N. Drozdovskaya, K. Furuya, M. V. Persson, M. L. R. v. Hoff, C. Walsh and S. F. Wampfler, Astron. Astrophys., 2018, 618, A11 CrossRef.
- A. M. Burkhardt, E. Herbst, S. V. Kalenskii, M. C. McCarthy, A. J. Remijan and B. A. McGuire, Mon. Not. R. Astron. Soc., 2018, 474, 5068–5075 CrossRef CAS.
- J. K. Jørgensen, H. S. P. Müller, H. Calcutt, A. Coutens, M. N. Drozdovskaya, K. I. Öberg, M. V. Persson, V. Taquet, E. F. v. Dishoeck and S. F. Wampfler, Astron. Astrophys., 2018, 620, A170 CrossRef.
- A. Coutens, J. K. Jørgensen, M. H. D. v. d. Wiel, H. S. P. Müller, J. M. Lykke, P. Bjerkeli, T. L. Bourke, H. Calcutt, M. N. Drozdovskaya, C. Favre, E. C. Fayolle, R. T. Garrod, S. K. Jacobsen, N. F. W. Ligterink, K. I. Öberg, M. V. Persson, E. F. v. Dishoeck and S. F. Wampfler, Astron. Astrophys., 2016, 590, L6 Search PubMed.
- V. V. Ilyushin, H. S. P. Müller, J. K. Jørgensen, S. Bauerecker, C. Maul, Y. Bakhmat, E. A. Alekseev, O. Dorovskaya, S. Vlasenko, F. Lewen, S. Schlemmer, K. Berezkin and R. M. Lees, Astron. Astrophys., 2022, 658, A127 CrossRef CAS.
- M. V. Persson, J. K. Jørgensen, H. S. P. Müller, A. Coutens, E. F. v. Dishoeck, V. Taquet, H. Calcutt, M. H. D. v. d. Wiel, T. L. Bourke and S. F. Wampfler, Astron. Astrophys., 2018, 610, A54 CrossRef.
- W. D. Watson, Rev. Mod. Phys., 1976, 48, 513–552 CrossRef CAS.
- T. J. Millar, A. Bennett and E. Herbst, Astrophys. J., 1989, 340, 906 CrossRef.
- M. N. Drozdovskaya, E. F. van Dishoeck, M. Rubin, J. K. Jørgensen and K. Altwegg, Mon. Not. R. Astron. Soc., 2019, 490, 50–79 CrossRef CAS.
- S. Jaeger, S. Fulle and S. Turk, J. Chem. Inf. Model., 2018, 58, 27–35 CrossRef CAS PubMed.
- S. Kim, J. Chen, T. Cheng, A. Gindulyte, J. He, S. He, Q. Li, B. A. Shoemaker, P. A. Thiessen, B. Yu, L. Zaslavsky, J. Zhang and E. E. Bolton, Nucleic Acids Res., 2021, 49, D1388–D1395 CrossRef CAS PubMed.
- T. Sterling and J. J. Irwin, J. Chem. Inf. Model., 2015, 55, 2324–2337 CrossRef CAS PubMed.
- C. Boersma, C. W. Bauschlicher, A. Ricca, A. L. Mattioda, J. Cami, E. Peeters, F. S. de Armas, G. P. Saborido, D. M. Hudgins and L. J. Allamandola, Astrophys. J., Suppl. Ser., 2014, 211, 8 Search PubMed.
- C. W. Bauschlicher, A. Ricca, C. Boersma and L. J. Allamandola, Astrophys. J., Suppl. Ser., 2018, 234, 32 CrossRef.
- A. L. Mattioda, D. M. Hudgins, C. Boersma, C. W. Bauschlicher, A. Ricca, J. Cami, E. Peeters, F. S. de Armas, G. P. Saborido and L. J. Allamandola, Astrophys. J., Suppl. Ser., 2020, 251, 22 Search PubMed.
- RDKit: Open-Source Cheminformatics, http://www.rdkit.org Search PubMed.
- H. L. Morgan, J. Chem. Doc., 1965, 5, 107–113 CrossRef CAS.
- C. E. Rasmussen and C. K. I. Williams, Gaussian Processes for Machine Learning, 2005 Search PubMed.
- F. Pedregosa, G. Varoquaux, A. Gramfort, V. Michel, B. Thirion, O. Grisel, M. Blondel, P. Prettenhofer, R. Weiss, V. Dubourg, J. Vanderplas, A. Passos, D. Cournapeau, M. Brucher, M. Perrot and E. Duchesnay, J. Mach. Learn. Res., 2011, 12, 2825–2830 Search PubMed.
- B. A. McGuire, Astrophys. J., Suppl. Ser., 2022, 259, 30 CrossRef.
- H. Linnartz, S. Ioppolo and G. Fedoseev, Int. Rev. Phys. Chem., 2015, 34, 205–237 Search PubMed.
- G. Fedoseev, H. M. Cuppen, S. Ioppolo, T. Lamberts and H. Linnartz, Mon. Not. R. Astron. Soc., 2015, 448, 1288–1297 CrossRef CAS.
- D. E. Woon, Astrophys. J., 2002, 569, 541 CrossRef CAS.
- R. T. Garrod, S. L. Widicus Weaver and E. Herbst, Astrophys. J., 2008, 682, 283–302 CrossRef CAS.
- P. Bergman, B. Parise, R. Liseau, B. Larsson, H. Olofsson, K. M. Menten and R. Güsten, Astron. Astrophys., 2011, 531, L8 CrossRef.
- F. Du, B. Parise and P. Bergman, Astron. Astrophys., 2012, 538, A91 Search PubMed.
- B. Parise, P. Bergman and F. Du, Astron. Astrophys., 2012, 541, L11 CrossRef.
- M. A. Thelen, P. Felder and J. Robert Huber, Chem. Phys. Lett., 1993, 213, 275–281 CrossRef CAS.
- D. McNally, Sci. Prog., 1965, 53, 83–87 CAS.
- J. Cernicharo, N. Marcelino, E. Roueff, M. Gerin, A. Jiménez-Escobar and G. M. M. Caro, Astrophys. J., Lett., 2012, 759, L43 CrossRef.
- M. Tyblewski, T. Ha, R. Meyer, A. Bauder and C. E. Blom, J. Chem. Phys., 1992, 97, 6168–6180 CrossRef CAS.
- G. R. Möhlmann, J. Raman Spectrosc., 1987, 18, 199–203 CrossRef.
- H. Matsuura, M. Yamamoto and H. Murata, Spectrochim. Acta, Part A, 1980, 36, 321–327 CrossRef.
- R. S. Ryabova, G. I. Voloshenko, V. D. Maiorov and G. F. Osipova, Russ. J. Appl. Chem., 2002, 75, 22–24 CrossRef CAS.
- C. Zhu, N. F. Kleimeier, A. M. Turner, S. K. Singh, R. C. Fortenberry and R. I. Kaiser, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2111938119 CrossRef CAS PubMed.
- S. Manigand, J. K. Jørgensen, H. Calcutt, H. S. P. Müller, N. F. W. Ligterink, A. Coutens, M. N. Drozdovskaya, E. F. v. Dishoeck and S. F. Wampfler, Astron. Astrophys., 2020, 635, A48 CrossRef CAS.
- J. Cernicharo, N. Marcelino, E. Roueff, M. Gerin, A. Jiménez-Escobar and G. M. M. Caro, Astrophys. J., Lett., 2012, 759, L43 CrossRef.
- P. Buckley and M. Brochu, Can. J. Chem., 1972, 50, 1149–1156 CrossRef CAS.
- M. J. Mumma, M. A. DiSanti, N. D. Russo, M. Fomenkova, K. Magee-Sauer, C. D. Kaminski and D. X. Xie, Science, 1996, 272, 1310–1314 CrossRef CAS PubMed.
- L. B. D'Hendecourt and M. Jourdain de Muizon, Astron. Astrophys., 1989, 223, L5–L8 Search PubMed.
- E. F. van Dishoeck, F. P. Helmich, T. de Graauw, J. H. Black, A. C. A. Boogert, P. Ehrenfreund, P. A. Gerakines, J. H. Lacy, T. J. Millar, W. A. Schutte, A. G. G. M. Tielens, D. C. B. Whittet, D. R. Boxhoorn, D. J. M. Kester, K. Leech, P. R. Roelfsema, A. Salama and B. Vandenbussche, Astron. Astrophys., 1996, 315, L349–L352 CAS.
- M. D. Ward, I. A. Hogg and S. D. Price, Mon. Not. R. Astron. Soc., 2012, 425, 1264–1269 CrossRef CAS.
- M. N. Drozdovskaya, E. F. van Dishoeck, J. K. Jørgensen, U. Calmonte, M. H. D. van der Wiel, A. Coutens, H. Calcutt, H. S. P. Müller, P. Bjerkeli, M. V. Persson, S. F. Wampfler and K. Altwegg, Mon. Not. R. Astron. Soc., 2018, 476, 4949–4964 CrossRef CAS.
- Y. Zhou, D.-H. Quan, X. Zhang and S.-L. Qin, Res. Astron. Astrophys., 2020, 20, 125 CrossRef CAS.
- J. Wang, J. H. Marks, A. M. Turner, A. A. Nikolayev, V. Azyazov, A. M. Mebel and R. I. Kaiser, Phys. Chem. Chem. Phys., 2023, 25, 936–953 RSC.
- The Astropy Collaboration, Astrophys. J., 2022, 935, 167 CrossRef.
- R. Abuter, A. Amorim, M. Bauböck, J. P. Berger, H. Bonnet, W. Brandner, Y. Clénet, V. C. d. Foresto, P. T. d. Zeeuw, J. Dexter, G. Duvert, A. Eckart, F. Eisenhauer, N. M. F. Schreiber, P. Garcia, F. Gao, E. Gendron, R. Genzel, O. Gerhard, S. Gillessen, M. Habibi, X. Haubois, T. Henning, S. Hippler, M. Horrobin, A. Jiménez-Rosales, L. Jocou, P. Kervella, S. Lacour, V. Lapeyrère, J.-B. L. Bouquin, P. Léna, T. Ott, T. Paumard, K. Perraut, G. Perrin, O. Pfuhl, S. Rabien, G. R. Coira, G. Rousset, S. Scheithauer, A. Sternberg, O. Straub, C. Straubmeier, E. Sturm, L. J. Tacconi, F. Vincent, S. v. Fellenberg, I. Waisberg, F. Widmann, E. Wieprecht, E. Wiezorrek, J. Woillez and S. Yazici, Astron. Astrophys., 2019, 625, L10 CrossRef.
- S. A. Dzib, G. N. Ortiz-León, A. Hernández-Gómez, L. Loinard, A. J. Mioduszewski, M. Claussen, K. M. Menten, E. Caux and A. Sanna, Astron. Astrophys., 2018, 614, A20 CrossRef.
- W. D. Langer, T. E. Graedel, M. A. Frerking and P. B. Armentrout, Astrophys. J., 1984, 277, 581–604 CrossRef CAS.
- L. R. Smith, M. S. Gudipati, R. L. Smith and R. D. Lewis, Astron. Astrophys., 2021, 656, A82 CrossRef CAS.
- J. K. Jørgensen, T. L. Bourke, Q. N. Luong and S. Takakuwa, Astron. Astrophys., 2011, 534, A100 CrossRef.
- M. Carvajal, L. Margulès, B. Tercero, K. Demyk, I. Kleiner, J. C. Guillemin, V. Lattanzi, A. Walters, J. Demaison, G. Wlodarczak, T. R. Huet, H. Møllendal, V. V. Ilyushin and J. Cernicharo, Astron. Astrophys., 2009, 500, 1109–1118 CrossRef CAS.
- H. S. P. Müller, F. Schlöder, J. Stutzki and G. Winnewisser, J. Mol. Struct., 2005, 742, 215–227 CrossRef.
- D. G. A. Smith, L. A. Burns, A. C. Simmonett, R. M. Parrish, M. C. Schieber, R. Galvelis, P. Kraus, H. Kruse, R. Di Remigio, A. Alenaizan, A. M. James, S. Lehtola, J. P. Misiewicz, M. Scheurer, R. A. Shaw, J. B. Schriber, Y. Xie, Z. L. Glick, D. A. Sirianni, J. S. O'Brien, J. M. Waldrop, A. Kumar, E. G. Hohenstein, B. P. Pritchard, B. R. Brooks, H. F. Schaefer, A. Y. Sokolov, K. Patkowski, A. E. DePrince, U. Bozkaya, R. A. King, F. A. Evangelista, J. M. Turney, T. D. Crawford and C. D. Sherrill, J. Chem. Phys., 2020, 152, 184108 CrossRef CAS PubMed.
- H. M. Pickett, J. Mol. Spectrosc., 1991, 148, 371–377 CrossRef CAS.
- B. A. McGuire and K. Lee, molsim, 2020, https://zenodo.org/record/4122749 Search PubMed.
- J. M. Lykke, A. Coutens, J. K. Jørgensen, M. H. D. v. d. Wiel, R. T. Garrod, H. S. P. Müller, P. Bjerkeli, T. L. Bourke, H. Calcutt, M. N. Drozdovskaya, C. Favre, E. C. Fayolle, S. K. Jacobsen, K. I. Öberg, M. V. Persson, E. F. v. Dishoeck and S. F. Wampfler, Astron. Astrophys., 2017, 597, A53 Search PubMed.
- H. Calcutt, J. K. Jørgensen, H. S. P. Müller, L. E. Kristensen, A. Coutens, T. L. Bourke, R. T. Garrod, M. V. Persson, M. H. D. v. d. Wiel, E. F. v. Dishoeck and S. F. Wampfler, Astron. Astrophys., 2018, 616, A90 Search PubMed.
- H. Calcutt, M. R. Fiechter, E. R. Willis, H. S. P. Müller, R. T. Garrod, J. K. Jørgensen, S. F. Wampfler, T. L. Bourke, A. Coutens, M. N. Drozdovskaya, N. F. W. Ligterink and L. E. Kristensen, Astron. Astrophys., 2018, 617, A95 CrossRef.
- N. F. W. Ligterink, A. Coutens, V. Kofman, H. S. P. Müller, R. T. Garrod, H. Calcutt, S. F. Wampfler, J. K. Jørgensen, H. Linnartz and E. F. van Dishoeck, Mon. Not. R. Astron. Soc., 2017, 469, 2219–2229 CrossRef CAS.
- E. C. Fayolle, K. I. Öberg, J. K. Jørgensen, K. Altwegg, H. Calcutt, H. S. P. Müller, M. Rubin, M. H. D. van der Wiel, P. Bjerkeli, T. L. Bourke, A. Coutens, E. F. van Dishoeck, M. N. Drozdovskaya, R. T. Garrod, N. F. W. Ligterink, M. V. Persson and S. F. Wampfler, Nat. Astron., 2017, 1, 703–708 CrossRef.
- S. Manigand, A. Coutens, J.-C. Loison, V. Wakelam, H. Calcutt, H. S. P. Müller, J. K. Jørgensen, V. Taquet, S. F. Wampfler, T. L. Bourke, B. M. Kulterer, E. F. v. Dishoeck, M. N. Drozdovskaya and N. F. W. Ligterink, Astron. Astrophys., 2021, 645, A53 CrossRef CAS.
- H. Calcutt, E. R. Willis, J. K. Jørgensen, P. Bjerkeli, N. F. W. Ligterink, A. Coutens, H. S. P. Müller, R. T. Garrod, S. F. Wampfler and M. N. Drozdovskaya, Astron. Astrophys., 2019, 631, A137 CrossRef CAS.
- A. Coutens, J.-C. Loison, A. Boulanger, E. Caux, H. S. P. Müller, V. Wakelam, S. Manigand and J. K. Jørgensen, Astron. Astrophys., 2022, 660, L6 CrossRef CAS.
- A. Coutens, N. F. W. Ligterink, J.-C. Loison, V. Wakelam, H. Calcutt, M. N. Drozdovskaya, J. K. Jørgensen, H. S. P. Müller, E. F. v. Dishoeck and S. F. Wampfler, Astron. Astrophys., 2019, 623, L13 Search PubMed.
- N. F. W. Ligterink, H. Calcutt, A. Coutens, L. E. Kristensen, T. L. Bourke, M. N. Drozdovskaya, H. S. P. Müller, S. F. Wampfler, M. H. D. v. d. Wiel, E. F. v. Dishoeck and J. K. Jørgensen, Astron. Astrophys., 2018, 619, A28 Search PubMed.
- S. Manigand, H. Calcutt, J. K. Jørgensen, V. Taquet, H. S. P. Müller, A. Coutens, S. F. Wampfler, N. F. W. Ligterink, M. N. Drozdovskaya, L. E. Kristensen, M. H. D. v. d. Wiel and T. L. Bourke, Astron. Astrophys., 2019, 623, A69 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |