Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Template synthesis of an intermediate in silver salt metathesis using a calix[4]arene-based diphosphine ligand†
Jack
Emerson-King
 and
Adrian B.
Chaplin
and
Adrian B.
Chaplin
 *
*
Department of Chemistry, University of Warwick, Gibbet Hill Road, Coventry, CV4 7AL, UK. E-mail: a.b.chaplin@warwick.ac.uk
First published on 3rd April 2023
Abstract
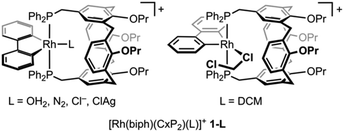
The synthesis and solid-state characterisation of the heterobimetallic rhodium(III)/silver(I) complex [Rh(2,2′-biphenyl)(CxP2)Cl]⊃Ag+ is described, where CxP2 is a trans-spanning calix[4]arene-based diphosphine and the silver cation is datively bound to the chloride ligand within the cavity of the macrocycle.
The activation of transition metal complexes by abstraction of halide ligands using silver(I) salts is a widely employed strategy in organometallic chemistry and catalysis.1 Mechanistic work by Mattson and Graham in 1981 substantiated a reaction sequence involving complexation of the silver(I) cation to the halide atom, before nucleophilic substitution and precipitation of the argentic salt from solution.2 Building on work by Reed and co-workers using weekly coordinating carborane anions,3 the first intermediate silver(I) halide adduct, [{CpMo(CO)3I}2(μ-Ag)2][CB11H12]2 was structurally corroborated in the solid-state by single crystal X-ray diffraction by Weller and co-workers in 2000.4 Notwithstanding facile onward reactivity, it is surprising to note that there have been only a handful of further well-defined examples over the intervening decades.5
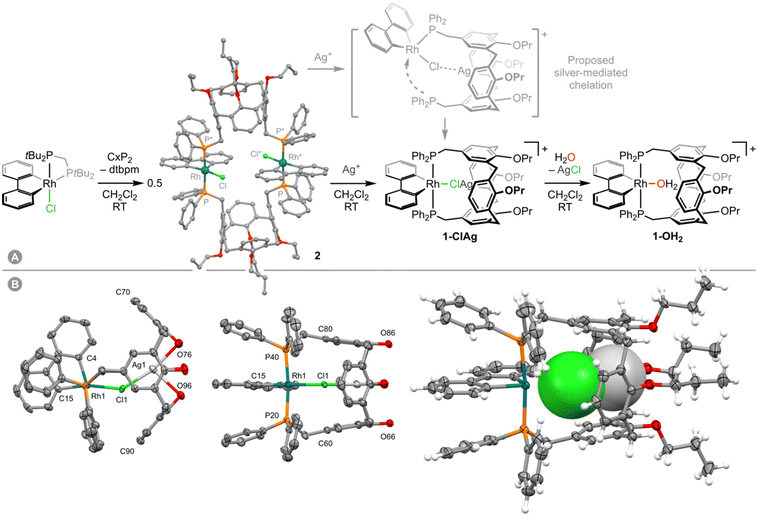
As part of our group's ongoing interest in cavitand-based ditopic ligands,6 we have recently become engaged in exploring the coordination chemistry of Kubas’ calix[4]arene diphosphine ligand CxP2.7 In a preceeding paper we described the preparation of mononuclear rhodium(III) aqua complex [Rh(biph)(CxP2)(OH2)][Al(ORF)4] (1-OH2; biph = 2,2′-biphenyl; RF = C(CF3)3) by substitution of trans-[Rh(biph)(PPh3)2(OH2)][Al(ORF)4] with CxP2 in THF.8 Seeking to access water-free, low-coordinate RhIII(biph) derivative 1, preparation and subsequent silver(I)-based halide abstraction of 1-Cl was targeted. During the course of this work, we discovered that the silver(I) cation templates assembly of heterobimetallic rhodium(III)/silver(I) complex [Rh(biph)(CxP2)Cl]⊃Ag+1-ClAg, which is a rare well-defined example of an intermediate in silver salt metathesis reactions.
Reasoning that chelation of CxP2 could still be induced upon chloride abstraction, 2 was carried forward and reacted with two equivalents of Ag[Al(ORF)4] in dichloromethane under argon at RT. Analysis of the resulting suspension by NMR spectroscopy indicated clean conversion into a new complex within 48 h rather than the expected dichloromethane adduct 1-DCM (δ31P 4.4, 1JRhP = 117 Hz).8 This new organometallic is characterised by a sharp 31P resonance at δ 13.9 (1JRhP = 120 Hz) significant downfield shifts of the aromatic 1H resonances of the calix[4]arene scaffold relative to 2 (p-ArH, 6.02→7.32; m-ArH, 5.63→7.11, m-ArP, 6.22→6.50), and assigned to mononuclear 1-ClAg, where the CxP2 ligand adopts the desired trans-spanning coordination mode and the silver cation is bound within the cavity of the calix[4]arene scaffold (Fig. 1A). This species is persistent at RT under argon or dinitrogen, but incredibly moisture sensitive. Repeated attempts to isolate analytically pure samples were frustrated by facile and irreversible reaction with adventitious water,10 resulting in the formation of aqua complex 1-OH2 with concomitant precipitation of AgCl. Indeed, on a preparative scale, deliberate addition of a slight excess of water to in situ generated 1-ClAg enabled isolation of the considerably more robust, air and moisture stable 1-OH2 as an orange solid in 77% yield from 2. Consistent with the assigned structure of 1-ClAg, only a slight perturbation to the 1H and 31P resonances occurs on formation of 1-OH2 (δ 13.2, 1JRhP = 120 Hz), alongside appearance of a distinctive 2H singlet at δ 0.84 for coordinated water.8 Most notably, one of the two unique OCH2 groups is shifted from 4.49→4.12 and we account for this change by coordination of the associated ether to silver in 1-ClAg.
Fortuitously, we have been able to structurally characterise 1-ClAg in the solid state through analysis of a co-crystalline sample formed with 1-OH2 (58%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42% relative occupancy; Fig. 1B). From the crystallographic disorder model, silver was identified within the cavity and found to exhibit a pseudo T-shaped metal coordination geometry with a Ag1–Cl1 distance of 2.490(2) Å and two dative bonding interactions with the flanking ether units of the calix[4]arene (Ag1–O76, 2.484(3) Å; Ag1–O96, 2.578(3) Å). The latter presumably provides a decisive enthalpic driving force for formation of 1-ClAg.
42% relative occupancy; Fig. 1B). From the crystallographic disorder model, silver was identified within the cavity and found to exhibit a pseudo T-shaped metal coordination geometry with a Ag1–Cl1 distance of 2.490(2) Å and two dative bonding interactions with the flanking ether units of the calix[4]arene (Ag1–O76, 2.484(3) Å; Ag1–O96, 2.578(3) Å). The latter presumably provides a decisive enthalpic driving force for formation of 1-ClAg.
Based on our observations, we propose conversion of 2 into 1-OH2 is initiated by capture of silver within the calix[4]arene scaffold. Chelation of CxP2 to rhodium is promoted by Cl→Ag+ bonding (Fig. 1A) and thereafter silver chloride is lost upon reaction with water, adventitious or deliberately added. This sequence further corroborates Mattson and Graham's mechanistic proposal for silver salt metathesis reactions, underscores the multifaceted ability of silver(I) cations to activate late transition metal complexes, and highlights the propensity of donor-functionalised cavitand ligands to orchestrate unusual metal-based reactivity.
Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
We gratefully acknowledge the EPSRC (DTA studentship to J. E.-K.) and Royal Society (UF100592, UF150675, A.B.C.) for financial support. Crystallographic data were collected using an instrument that received funding from the ERC under the European Union's Horizon 2020 research and innovation programme (grant agreement No. 637313).References
- J. F. Hartwig, Organotransition Metal Chemistry – From Bonding to Catalysis, University Science Books, 2010 Search PubMed.
- B. M. Mattson and W. A. G. Graham, Inorg. Chem., 1981, 20, 3186–3189 CrossRef CAS.
- (a) Z. Xie, T. Jelinek, R. Bau and C. A. Reed, J. Am. Chem. Soc., 1994, 116, 1907–1913 CrossRef CAS; (b) D. J. Liston, Y. J. Lee, W. R. Scheidt and C. A. Reed, J. Am. Chem. Soc., 1989, 111, 6643–6648 CrossRef CAS; (c) D. J. Liston, C. A. Reed, C. W. Eigenbrot and W. R. Scheidt, Inorg. Chem., 1987, 26, 2739–2740 CrossRef CAS.
- (a) N. J. Patmore, M. F. Mahon, J. W. Steed and A. S. Weller, J. Chem. Soc., Dalton Trans., 2001, 277–283 RSC; (b) N. J. Patmore, J. W. Steed and A. S. Weller, Chem. Commun., 2000, 1055–1056 RSC.
- (a) M. Carmona, L. Tejedor, R. Rodríguez, V. Passarelli, F. J. Lahoz, P. García-Orduña and D. Carmona, Chem. – Eur. J., 2017, 23, 14532–14546 CrossRef CAS PubMed; (b) G. Sipos, P. Gao, D. Foster, B. W. Skelton, A. N. Sobolev and R. Dorta, Organometallics, 2017, 36, 801–817 CrossRef CAS; (c) D. S. Bohle and Z. Chua, Organometallics, 2015, 34, 1074–1084 CrossRef CAS; (d) S. G. Weber, F. Rominger and B. F. Straub, Eur. J. Inorg. Chem., 2012, 2863–2867 CrossRef CAS; (e) A. Obenhuber and K. Ruhland, Organometallics, 2011, 30, 171–186 CrossRef CAS; (f) P. Paredes, J. Díez and M. P. Gamasa, Organometallics, 2008, 27, 2597–2607 CrossRef CAS; (g) V. G. Albano, M. D. Serio, M. Monari, I. Orabona, A. Panunzi and F. Ruffo, Inorg. Chem., 2002, 41, 2672–2677 CrossRef CAS PubMed.
- (a) R. Patchett, R. C. Knighton, J. D. Mattock, A. Vargas and A. B. Chaplin, Inorg. Chem., 2017, 56, 14345–14350 CrossRef CAS PubMed; (b) R. Patchett and A. B. Chaplin, Dalton Trans., 2016, 45, 8945–8955 RSC.
- X. Fang, B. L. Scott, J. G. Watkin, C. A. G. Carter and G. J. Kubas, Inorg. Chim. Acta, 2001, 317, 276–281 CrossRef CAS.
- J. Emerson-King, S. Pan, M. R. Gyton, R. Tonner-Zech and A. B. Chaplin, Chem. Commun., 2023, 59, 2150–2152 RSC.
- C. N. Iverson and W. D. Jones, Organometallics, 2001, 20, 5745–5750 CrossRef CAS.
- In line with the solution phase stability of the rhodium(III) dinitrogen analogue [Rh(biph)(CxP2)(N2)][Al(ORF)4] (1-N2, ref. 8).
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2244002 (1-ClAg) and 2244003 (2). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3dt00567d |
| This journal is © The Royal Society of Chemistry 2023 |