Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reactivity of a series of triaryl borates, B(OArx)3, in hydroboration catalysis†
Tomasz
Sokolnicki‡
 abc,
Mashael M.
Alharbi‡
abc,
Mashael M.
Alharbi‡
 ad,
Yara
van Ingen
a,
Shahnaz
Rahim
ad,
Yara
van Ingen
a,
Shahnaz
Rahim
 ae,
Milan
Pramanik
ae,
Milan
Pramanik
 a,
Alberto
Roldan
a,
Alberto
Roldan
 a,
Jędrzej
Walkowiak
a,
Jędrzej
Walkowiak
 *c and
Rebecca L.
Melen
*c and
Rebecca L.
Melen
 *a
*a
aCardiff Catalysis Institute, Cardiff University, Translational Research Hub, Maindy Road, Cathays, Cardiff, CF24 4HQ Wales, UK. E-mail: MelenR@cardiff.ac.uk
bAdam Mickiewicz University, Faculty of Chemistry, Uniwersytetu Poznanskiego 8, 61-614, Poznan, Poland. E-mail: jedrzej.walkowiak@amu.edu.pl
cAdam Mickiewicz University, Center for Advanced Technology, Uniwersytetu Poznanskiego 10, 61-614, Poznan, Poland
dDepartment of Chemistry, King Faisal University, College of Science, P.O. Box 400, Al-Ahsa 31982, Saudi Arabia
eDepartment of Chemistry, Abdul Wali Khan University, Mardan, Pakistan
First published on 20th October 2023
Abstract
In this paper, we compare the reactivity of a series of triaryl borates B(OArx)3 as catalysts for the hydroboration of alkenes and alkynes. It was observed that commercially available B(OPh)3 performed the poorest, whereas catalysts with o-F atoms appeared to perform much better.
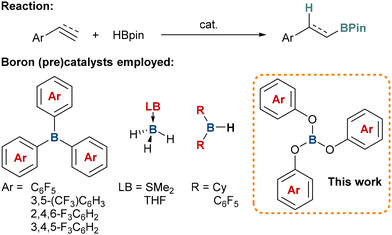
Organoboron compounds are versatile building blocks in organic synthesis as the high chemical reactivity of the boryl moiety allows for their multiple derivatisations, especially in Suzuki–Miyaura coupling reactions, giving access to numerous natural products and complex organic molecules.1 Therefore, novel approaches for the preparation of these reagents are highly sought after. Hydroboration is one of the simplest methods for the synthesis of a wide array of organoboranes. Typically, these reactions are promoted by precious transition metal complexes based on rhodium, ruthenium, palladium, platinum and others; however, an increasing focus on the application of cheaper and more Earth-abundant alternatives such as first-row transition metals2 and main group elements3 has recently been observed. In particular, boron Lewis acids have sparked growing attention (Scheme 1). For example, Hoshi described that dicyclohexylborane and 9-borabicyclo(3.3.1)nonane (9-BBN) can catalyse regioselective cis-hydroboration of alkynes with HBcat (catecholborane) at ambient temperature.4 Thomas reported that simple, commercially available borane adducts, H3B·THF and H3B·SMe2, can be used as effective catalysts for the hydroboration of alkynes and alkenes with HBpin (pinacolborane),5 and Okuda demonstrated that alkali metal hydridotriphenylborate complexes [(Me6TREN)M][HBPh3] (Me6TREN = tris{2-(dimethylamino)ethyl}amine) can serve as efficient catalysts for the hydroboration of a broad range of substrates with carbonyl groups.6 One area that has gained particular attention is where fluorinated aryl borates such as tris(pentafluorophenyl)borane [B(C6F5)3]7 or Piers’ borane [HB(C6F5)2] are used as pre-catalysts.8 We and Oestreich have later developed the use of other borane catalysts including tris[3,5-bis(trifluoromethyl)phenyl]borane,9 tris(2,4,6-trifluorophenyl)borane,10 and tris(3,4,5-trifluorophenyl)borane11 as effective catalysts for a range of hydroboration reactions. In these cases, the catalytic activity was generally found to be higher than that of the archetypical B(C6F5)3 catalyst.
Earlier this year, we reported the synthesis of a range of fluorinated triaryl borates [B(OArF)3] with varying Lewis acidity, prepared by reacting various fluorophenols with BCl3.12 This concept stems from Britovsek's findings that the introduction of an O-atom spacer between the boron atom in B(C6F5)3 and the C6F5-aryl ring increases the Lewis acidity of the borate product B(OC6F5)3 relative to B(C6F5)3.13 In this project, we were interested in investigating the relative reactivity of these new borates in hydroboration catalysis.
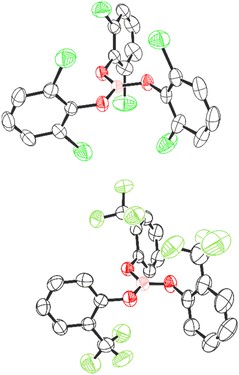
To begin, we synthesised a series of triaryl borates from the reaction of the parent phenol with BCl3 in CH2Cl2, and obtained B(OPh)31a from a commercial supplier. The borates prepared included B(OArF)3 (ArF = 2-FC6H4, 3-FC6H4, 4-FC6H4, 2,3-F2C6H3, 2,6-F2C6H3, 3,5-F2C6H3, 2,3,4-F3C6H2, 3,4,5-F3C6H2, 2,3,5,6-F4C6H, and C6F5) (compounds 1b–k, respectively). We also prepared two new borates B(OArx)3 (ArX = 2,6-Cl2C6H31l and 2-(CF3)C6H41m) for comparison. The rationale for this is that, in previous studies, the steric and electronic effects of the functional groups at the ortho-position were found to influence the efficacy of the pre-catalyst.9,11 These two new compounds could be recrystallised and characterised by single-crystal X-ray diffraction (Fig. 1).
The Lewis acidity of the two new boranes was then determined by both experimental and computational methods. Using the Gutmann–Beckett (GB) Lewis acidity test,14 the 31P NMR chemical shifts (δ ppm) of the Et3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(OArx)3 adducts were 78.9 and 78.7 ppm for 1l and 1m, respectively. The change in the 31P NMR chemical shift between the free phosphine oxide (δ = 52.5 ppm) and the adduct (Δδ) was determined to be 26.4 (1l) and 26.2 ppm (1m). Computational studies15 at the M06-2X+D3(0)/def2-QZVPP level of theory gave fluoride ion affinities (FIAs) of 336 (1l) and 400 (1m) kJ mol−1, and hydride ion affinities (HIA) of 317 (1l) and 381 (1m) kJ mol−1. Finally, the Lewis acidity was also determined using the global electrophilicity index (GEI),16 which gave values of 1.31 (1l) and 1.39 (1m) eV. In comparison to previously reported borates, 1l has the lowest Lewis acidity of the series when considering Gutmann–Beckett, HIA and FIA values, for example in comparison to the weakly Lewis acidic borate, B(OPh)3 (GB: Δδ = 23.0 ppm, FIA: 350 kJ mol−1, and HIA: 323 kJ mol−1). On the other hand, 1m shows higher Lewis acidity than previously reported ortho-substituted borates from Gutmann–Beckett, FIA and HIA [i.e., B(O(2-FC6H4))3 has GB: Δδ = 23.8 ppm, FIA: 351 kJ mol−1, HIA: 339 kJ mol−1], and comparable Lewis acidity to the most Lewis acidic borates, B(O(3,4,5-F3C6H2))3 and B(OC6F5)3.12 The GEI Lewis acidity metric is intrinsic, and considers the HOMO–LUMO gap rather than the coordination to an external probe and, from this, both 1l and 1m fall within the range of the reported values for F-substituted borates (GEI: 0.88–1.45 eV).12 This suggests that the larger chlorine substituents at both the ortho-positions in 1l hinder adduct formation over intrinsic factors significantly more than the previously explored fluorine substituents. However, higher Lewis acidity is observed for 1m with an ortho-CF3 group, which has comparable size to chlorine, from both intrinsic and extrinsic metrics, suggesting that the electronic effects of the electron-withdrawing CF3 group outweigh steric effects.
O → B(OArx)3 adducts were 78.9 and 78.7 ppm for 1l and 1m, respectively. The change in the 31P NMR chemical shift between the free phosphine oxide (δ = 52.5 ppm) and the adduct (Δδ) was determined to be 26.4 (1l) and 26.2 ppm (1m). Computational studies15 at the M06-2X+D3(0)/def2-QZVPP level of theory gave fluoride ion affinities (FIAs) of 336 (1l) and 400 (1m) kJ mol−1, and hydride ion affinities (HIA) of 317 (1l) and 381 (1m) kJ mol−1. Finally, the Lewis acidity was also determined using the global electrophilicity index (GEI),16 which gave values of 1.31 (1l) and 1.39 (1m) eV. In comparison to previously reported borates, 1l has the lowest Lewis acidity of the series when considering Gutmann–Beckett, HIA and FIA values, for example in comparison to the weakly Lewis acidic borate, B(OPh)3 (GB: Δδ = 23.0 ppm, FIA: 350 kJ mol−1, and HIA: 323 kJ mol−1). On the other hand, 1m shows higher Lewis acidity than previously reported ortho-substituted borates from Gutmann–Beckett, FIA and HIA [i.e., B(O(2-FC6H4))3 has GB: Δδ = 23.8 ppm, FIA: 351 kJ mol−1, HIA: 339 kJ mol−1], and comparable Lewis acidity to the most Lewis acidic borates, B(O(3,4,5-F3C6H2))3 and B(OC6F5)3.12 The GEI Lewis acidity metric is intrinsic, and considers the HOMO–LUMO gap rather than the coordination to an external probe and, from this, both 1l and 1m fall within the range of the reported values for F-substituted borates (GEI: 0.88–1.45 eV).12 This suggests that the larger chlorine substituents at both the ortho-positions in 1l hinder adduct formation over intrinsic factors significantly more than the previously explored fluorine substituents. However, higher Lewis acidity is observed for 1m with an ortho-CF3 group, which has comparable size to chlorine, from both intrinsic and extrinsic metrics, suggesting that the electronic effects of the electron-withdrawing CF3 group outweigh steric effects.
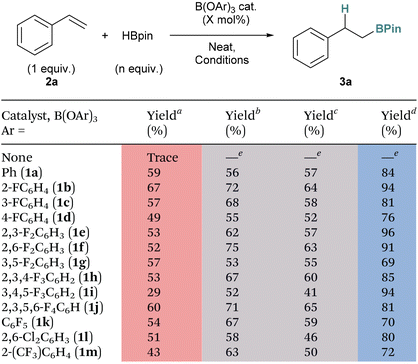
With a range of borate catalysts in hand, we were interested in understanding their comparative reactivity in the hydroboration reaction of unsaturated substrates. We began our investigations using styrene (2a) as the model substrate and HBpin as the hydroboration reagent under neat conditions, and measured the isolated yield (3a) of the borylated product under four different sets of reaction conditions: (a) HBpin (1.2 eq.), catalyst loading (10 mol%), temperature (50 °C), time (24 h); (b) HBpin (2 eq.), catalyst loading (2 mol%), temperature (80 °C), time (48 h); (c) HBpin (2 eq.), catalyst loading (5 mol%), temperature (80 °C), time (48 h); and (d) HBpin (2 eq.), catalyst loading (10 mol%), temperature (80 °C), time (48 h) (Table 1). Using these conditions, we found that the first set of conditions (a) gave the poorest overall results with the catalysts giving yields from 29% (1i) to 67% (1b). Upon changing the HBpin equivalents to 2, increasing the reaction temperature to 80 °C, and the time to 48 h (conditions (c)), the yields predictably increased for all catalysts (range: 69% (1g) to 96% (1e)) whilst keeping the catalyst loading the same. However, when we reduced the catalyst loading to 2 mol% or 5 mol% while keeping the other conditions the same (conditions (b) and (c), respectively), the yields expectedly decreased to 52% (1i)–75% (1f), but were not as low as those under the initial set of conditions (a) with the exception of catalysts 1a and 1g. While very good yields are obtained for conditions (d), we chose to use conditions (b) as the standard conditions for examining the catalysts in the hydroboration of other substrates as these allowed for better differentiation between the different activities of the catalysts.
| a Conditions: HBpin (1.2 eq.), catalyst loading (10 mol%), 50 °C, 24 h. b Conditions: HBpin (2 eq.), catalyst loading (2 mol%), 80 °C, 48 h. c Conditions: HBpin (2 eq.), catalyst (5 mol%), 80 °C, 48 h. d Conditions: HBpin (2 eq.), catalyst loading (10 mol%), 80 °C, 48 h. e Polymerisation of styrene was observed. Yields are isolated. |
|---|
|
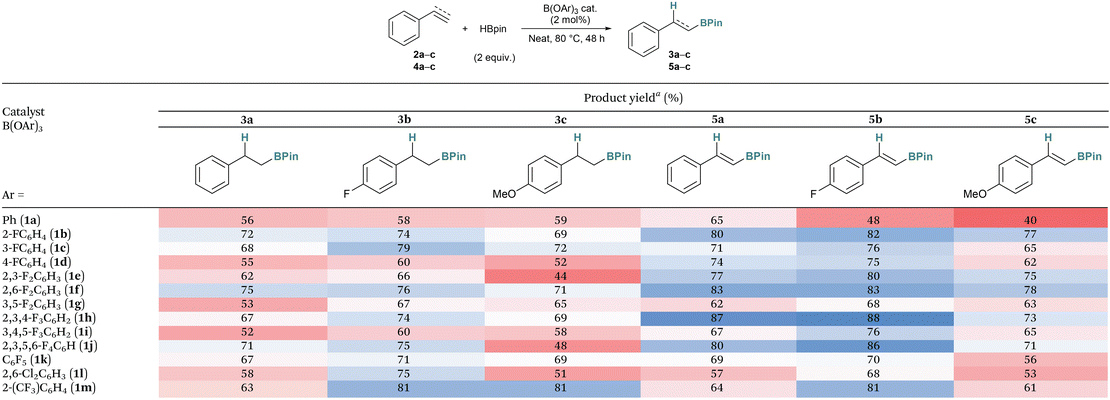
With these conditions in hand, we investigated different catalysts in the hydroboration of other substrates to compare their catalytic activity (Table 2). Initially, two further alkenes were trialed including the electron-deficient 4-fluorostyrene (2b) and electron-rich 4-methoxystyrene (2c). For all catalysts, the yields increased when using the electron-deficient substrate 2b (range: 58% (1a) to 81% (1m) versus 52% (1i) to 75% (1f)). Conversely, the yields were generally lower with the more electron-rich substrate 2c than those with 2b (range: 44% (1e) to 81% (1m)). These results are interesting as other Lewis acidic boranes such as tris[3,5-bis(trifluoromethyl)phenyl]borane were previously found to give trace products with substrate 2c.9
| a Yields are isolated. Colour scale from dark red to dark blue indicating low to high yields. |
|---|
|
Following this, we investigated the catalysts for the hydroboration of alkynes including electron-neutral phenyl acetylene (4a), electron-deficient 1-ethynyl-4-fluorobenzene (4b), and electron-rich 4-ethynylanisole (4c). For all substrates, the catalysts mostly performed better with the alkyne substrates (4) than the alkene substrates (2). When looking at the catalysts, the least Lewis acidic borate B(OPh)3 (1a) performed the poorest. The other borates, however, showed little trend between their Lewis acidity and their yield for the reaction. It was noticed, that the catalysts that had ortho-F atoms including B(O(2-FC6H4))3 (1b), B(O(2,6-F2C6H3))3 (1f), B(O(2,3,4-F3C6H2))3 (1h), and B(O(2,3,5,6-F4C6H))3 (1j) tended to perform better, showing higher yields for the majority of the reactions. Conversely, borates with no ortho-F atoms showed poorer activity including B(O(4-FC6H4))3 (1d), B(O(3,5-F2C6H3))3 (1g), and B(O(3,4,5-F3C6H2))3 (1i). Interestingly, by including ortho-Cl atoms, as in B(O(2,6-Cl2C6H3))3 (1l), the yield decreased relative to the fluorinated derivative 1f. The ortho-CF3 derivative B(O(2-(CF3)C6H4))3 (1m), on the other hand, gave very good yields for several substrates (2b, 2c, and 4b).
Oestreich proposed the formation of intermediary hydroboranes [HnBArF3−n]2 and [(ArF)(H)B(μ-H)2BArF2] as the active catalytic species when using tris[3,5-bis(trifluoromethyl)phenyl]borane.9 We similarly investigated the stoichiometric reactions between 1b and HBpin and found full conversion of both reagents to form 2 new species as revealed in the 1H NMR spectrum (see the ESI†). We hypothesise that the formation of a catalytically active hydroborate species is stabilised by ortho-fluorine substituents on the borates, and this accounts for their higher activity. Importantly, a control experiment with TMEDA suggested no involvement of B2H6, which may catalyse the reaction through “hidden boron catalysis” (see the ESI†).17
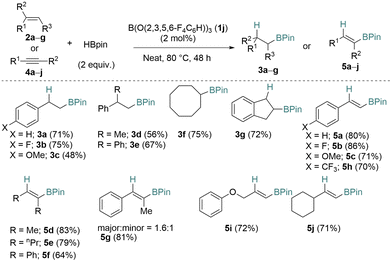
Using one of the best catalysts, B(O(2,3,5,6-F4C6H))3 (1j), we investigated a small scope of aliphatic, aromatic, and internal and terminal alkene or alkyne substrates (Scheme 2). Styrene derivatives (2a,b) worked well but a lower yield was observed with a p-OMe substituent (2c) generating 3c in, 48% yield.
The 1,1-disubstituted alkenes α-methyl styrene (2d) and 1,1-diphenylethylene (2e) also reacted well giving the hydroborated products 3d and 3e in 56% and 67% yields, respectively. The cyclic alkenes cyclooctene (2f) and indene (2g) also reacted very well giving products 3f (75%) and 3g (72%) in high yields. The terminal phenyl acetylenes gave excellent product yields of 70–86% for 5a–c and 5h. The internal aliphatic alkynes but-2-yne (4d), oct-4-yne (4e), and diphenylacetylene (4f) also reacted well giving the hydroborated products in 83% (5d), 79% (5e), and 64% (5f) yields, respectively. On the other hand, 1-phenyl-1-propyne (4g) gave 5g as a mixture of two regioisomers in a combined yield of 81% (major![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) minor = 1.6
minor = 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Non-aromatic acetylenes also reacted, (prop-2-yn-1-yloxy)benzene (4i) and ethynylcyclohexane (4j) yielded the desired hydroborated products 5i and 5j with 72% and 71% yields, respectively.
1). Non-aromatic acetylenes also reacted, (prop-2-yn-1-yloxy)benzene (4i) and ethynylcyclohexane (4j) yielded the desired hydroborated products 5i and 5j with 72% and 71% yields, respectively.
In conclusion, we have reported the reactivity of a series of triaryl borates B(OArx)3 as catalysts for the hydroboration of alkenes and alkynes. The catalysts tested included commercially available B(OPh)3, previously reported fluorinated triaryl borates, and two new borates with varying ortho-substituents B(OArx)3 (ArX = 2,6-Cl2C6H3 and 2-(CF3)C6H4). Although all catalysts were active in the reaction, it was observed that there was no obvious trend between their Lewis acidity and the reaction yield. However, commercially available B(OPh)3 performed the poorest, whereas catalysts with o-F atoms appeared to perform much better. One of the more active catalysts, B(O(2,3,5,6-F4C6H))3, was then trialed with a range of aliphatic, aromatic, and internal and terminal alkenes or alkynes.
Author contributions
TS, MAA, SR and MP conducted experimental work. YvI performed DFT calculations and X-ray crystallographic studies. TS, RLM and YvI wrote the paper. TS, MAA and YvI drafted the ESI.† All authors proofread and commented on the paper and the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
MAA acknowledges the Saudi Ministry of Education and King Faisal University (grant no. 3809). YvI and ARM acknowledge Supercomputing Wales for access to the Hawk HPC facility. SR acknowledges IRSIP (PIN code: IRSIP 52 PSc 28). TS and JW acknowledge the National Centre for Research and Development and the National Science Centre in Poland (POWR.03.02.00-00-I026/16 and UMO-2019/34/E/ST4/00068, respectively).References
- J. Carreras, A. Caballero and P. J. Pérez, Chem. – Asian J., 2019, 14, 329 CrossRef CAS PubMed; B. S. Kadu, Catal. Sci. Technol., 2021, 11, 1186 RSC.
- J. V. Obligacion and P. J. Chirik, J. Am. Chem. Soc., 2013, 135, 19107 CrossRef CAS PubMed; M. Espinal-Viguri, C. R. Woof and R. L. Webster, Chem. – Eur. J., 2016, 22, 11605 CrossRef PubMed; J. V. Obligacion and P. J. Chirik, Nat. Rev. Chem., 2018, 2, 15 CrossRef PubMed; W. Su, R.-X. Qiao, Y.-Y. Jiang, X.-L. Zhen, X. Tian, J.-R. Han, S.-M. Fan, Q. Cheng and S. Liu, ACS Catal., 2020, 10, 11963 CrossRef.
- M. A. Dureen, A. Lough, T. M. Gilbert and D. W. Stephan, Chem. Commun., 2008, 4303 RSC; P. Eisenberger, A. M. Bailey and C. M. Crudden, J. Am. Chem. Soc., 2012, 134, 17384 CrossRef CAS PubMed; A. Prokofjevs, A. Boussonnière, L. Li, H. Bonin, E. Lacôte, D. P. Curran and E. Vedejs, J. Am. Chem. Soc., 2012, 134, 12281 CrossRef PubMed; J. S. McGough, S. M. Butler, I. A. Cade and M. J. Ingleson, Chem. Sci., 2016, 7, 3384 RSC; D. M. C. Ould and R. L. Melen, Chem. – Eur. J., 2018, 24, 15201 CrossRef PubMed; M. Magre, M. Szewczyk and M. Rueping, Chem. Rev., 2022, 122, 8261 CrossRef PubMed.
- K. Shirakawa, A. Arase and M. Hoshi, Synthesis, 2004, 1814 CAS.
- N. W. J. Ang, C. S. Buettner, S. Docherty, A. Bismuto, J. R. Carney, J. H. Docherty, M. J. Cowley and S. P. Thomas, Synthesis, 2018, 50, 803 CrossRef CAS.
- D. Mukherjee, H. Osseili, T. P. Spaniol and J. Okuda, J. Am. Chem. Soc., 2016, 138, 10790 CrossRef CAS PubMed; D. Mukherjee, S. Shirase, T. P. Spaniol, K. Mashima and J. Okuda, Chem. Commun., 2016, 52, 13155 RSC; H. Osseili, D. Mukherjee, T. P. Spaniol and J. Okuda, Chem. – Eur. J., 2017, 23, 14292 CrossRef PubMed.
- A. Bismuto, M. J. Cowley and S. P. Thomas, Adv. Synth. Catal., 2021, 363, 2382 CrossRef CAS.
- M. Hoshi, K. Shirakawa and M. Okimoto, Tetrahedron Lett., 2007, 48, 8475 CrossRef CAS; M. Fleige, J. Möbus, T. vom Stein, F. Glorius and D. W. Stephan, Chem. Commun., 2016, 52, 10830 RSC.
- Q. Yin, S. Kemper, H. F. T. Klare and M. Oestreich, Chem. – Eur. J., 2016, 22, 13840 CrossRef CAS PubMed; Q. Yin, Y. Soltani, R. L. Melen and M. Oestreich, Organometallics, 2017, 36, 2381 CrossRef.
- J. R. Lawson, L. C. Wilkins and R. L. Melen, Chem. – Eur. J., 2017, 23, 10997 CrossRef CAS PubMed.
- J. L. Carden, L. J. Gierlichs, D. F. Wass, D. L. Browne and R. L. Melen, Chem. Commun., 2019, 55, 318 RSC.
- M. M. Alharbi, Y. van Ingen, A. Roldan, T. Kaehler and R. L. Melen, Dalton Trans., 2023, 52, 1820 RSC.
- G. J. Britovsek, J. Ugolotti and A. J. White, Organometallics, 2005, 24, 1685 CrossRef CAS.
- U. Mayer, V. Gutmann and W. Gerger, Chem. Mon., 1975, 106, 1235 CrossRef CAS; M. A. Beckett, G. C. Strickland, J. R. Holland and K. Sukumar Varma, Polymer, 1996, 37, 4629 CrossRef.
- L. Greb, Chem. – Eur. J., 2018, 24, 17881 CrossRef CAS PubMed; P. Erdmann, J. Leitner, J. Schwarz and L. Greb, ChemPhysChem, 2020, 21, 987 CrossRef PubMed.
- A. R. Jupp, T. C. Johnstone and D. W. Stephan, Dalton Trans., 2018, 47, 7029 RSC.
- A. D. Bage, T. A. Hunt and S. P. Thomas, Org. Lett., 2020, 22(11), 4107 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2281020 and 2281021. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3dt03333c |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |