Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of OH scavengers on the chemical composition of α-pinene secondary organic aerosol†
David M.
Bell
 *a,
Veronika
Pospisilova
ab,
Felipe
Lopez-Hilfiker
ab,
Amelie
Bertrand
a,
Mao
Xiao
a,
Xueqin
Zhou
a,
Wei
Huang‡
*a,
Veronika
Pospisilova
ab,
Felipe
Lopez-Hilfiker
ab,
Amelie
Bertrand
a,
Mao
Xiao
a,
Xueqin
Zhou
a,
Wei
Huang‡
 c,
Dongyu S.
Wang
a,
Chuan Ping
Lee
c,
Dongyu S.
Wang
a,
Chuan Ping
Lee
 a,
Josef
Dommen
a,
Josef
Dommen
 a,
Urs
Baltensperger
a,
Andre S. H.
Prevot
a,
Imad
El Haddad
a,
Urs
Baltensperger
a,
Andre S. H.
Prevot
a,
Imad
El Haddad
 a and
Jay G.
Slowik
*a
a and
Jay G.
Slowik
*a
aLaboratory of Atmospheric Chemistry, Paul Scherrer Institute, 5232 Villigen, Switzerland. E-mail: david.bell@psi.ch; jay.slowik@psi.ch
bTofwerk, 3600 Thun, Switzerland
cInstitute of Meteorology and Climate Research, Karlsruhe Institute of Technology, 76344 Eggenstein-Leopoldshafen, Germany
First published on 4th November 2022
Abstract
OH scavengers are extensively used in studies of secondary organic aerosol (SOA) because they create an idealized environment where only a single oxidation pathway is occurring. Here, we present a detailed molecular characterization of SOA produced from α-pinene + O3 with a variety of OH scavengers using the extractive electrospray time-of-flight mass spectrometer in our atmospheric simulation chamber, which is complemented by characterizing the gas phase composition in flow reactor experiments. Under our experimental conditions, radical chemistry largely controls the composition of SOA. Besides playing their desired role in suppressing the reaction of α-pinene with OH, OH scavengers alter the reaction pathways of radicals produced from α-pinene + O3. This involves changing the HO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RO2 ratio, the identity of the RO2 radicals present, and the RO2 major sinks. As a result, the use of the OH scavengers has significant effects on the composition of SOA, including inclusions of scavenger molecules in SOA, the promotion of fragmentation reactions, and depletion of dimers formed via α-pinene RO2–RO2 reactions. To date fragmentation reactions and inclusion of OH scavenger products into secondary organic aerosol have not been reported in atmospheric simulation chamber studies. Therefore, care should be considered if and when to use an OH scavenger during experiments.
RO2 ratio, the identity of the RO2 radicals present, and the RO2 major sinks. As a result, the use of the OH scavengers has significant effects on the composition of SOA, including inclusions of scavenger molecules in SOA, the promotion of fragmentation reactions, and depletion of dimers formed via α-pinene RO2–RO2 reactions. To date fragmentation reactions and inclusion of OH scavenger products into secondary organic aerosol have not been reported in atmospheric simulation chamber studies. Therefore, care should be considered if and when to use an OH scavenger during experiments.
Environmental significanceSecondary organic aerosol (SOA) is a major component of atmospheric particles, and is often simulated using laboratory studies in smog chambers. OH scavengers are a common additive to smog chambers, when reactions by other oxidants are investigated. OH scavengers are small organic molecules that possess too high volatility to contribute by themselves to formation of SOA. In this work, we demonstrate the impact of OH scavengers on the radical balance in smog chambers and its inclusion into SOA, which substantially alters SOA composition. This can have strong impacts on SOA yield parametrizations, volatility distribution determination and potentially the assessments of SOA toxicity and climate impacts. Therefore, the use of OH scavengers does not necessarily faithfully reproduce the processes occurring in the atmosphere. |
1 Introduction
Organic aerosol (OA) makes up between 20–90% of the global aerosol burden.1 Much of the OA in the atmosphere results from oxidation processes forming secondary species (secondary organic aerosol, SOA) that have low volatility, so after the transformation the formed molecules are more likely found in the particle phase than the gas phase.2 Quantitative investigation of these processes in the atmosphere is often impractical due to the large number of precursor volatile organic compounds (VOCs) and possible reaction pathways. Therefore, comparison of ambient measurements to laboratory studies provides the opportunity to study single VOCs oxidized under controlled conditions, thus disentangling complexities present in the atmosphere.3 Monoterpenes are prevalent VOC precursors and yield a substantial fraction of SOA globally, making them a frequent target for laboratory studies.4Perhaps the most studied ideal system in the laboratory is the ozonolysis of α-pinene. Ozonolysis of alkenes also produces OH radicals with yields up to 115%,5 providing a competing oxidation pathway which obscures the desired investigation of pure ozonolysis.6 For example, during α-pinene ozonolysis experiments, up to half of the α-pinene is estimated to react with OH.7 Therefore, the use of OH radical scavengers is standard practice to limit the oxidation to a single pathway and determine the corresponding yields of SOA formation,7–12 physical properties,13,14 and composition.9,11,15–17 The yields of SOA formation have been extensively studied with scavengers and have been found to vary substantially based on the scavenger identity, where small molecules (e.g. methanol, formaldehyde, propanol) have lower yields of SOA formation than cyclohexane.11 Overall, the use of a scavenger implicitly assumes that the scavenger exerts a negligible effect on SOA composition and properties.
By preventing the reaction of α-pinene with OH via an OH scavenger, a consequence is to limit the formation of OH oxidation products. While the reaction of α-pinene with O3 mainly produces C10H15O4,6 peroxy-radicals (RO2), in reactions with OH radicals the main radicals formed are C10H17O3,5.18,19 Therefore, studies have reported the reduction of highly oxygenated molecules (HOMs) produced through the C10H17Ox radical pathway, in the presence of an OH scavenger.18,20 However, as an undesired effect, the use of an OH scavenger also alters the fate of the RO2 radicals produced from ozonolysis and consequently the chemical composition of the resulting SOA. In the absence of nitrogen oxide (NO), RO2 radicals react with HO2 to form an alkoxy radical (RO) or hydroperoxides, or with other RO2 radicals to form an RO radical or different closed shell molecules, including ROOR′ dimers (see ESI†).19,21,22 The pathway to ROOR′ dimers is particularly important because these products have low volatility and are expected to be a major fraction of SOA.19,23–25 Changes in RO2 chemistry due to the use of scavengers alters the formation rates of dimers and the ability of RO2 radicals to undergo autoxidation, and these effects on SOA composition require systematic evaluation. On the one hand, the presence of some OH scavengers (e.g. H2, CO, alcohols, etc.) alters the HO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RO2 ratio,10,11 which may have an effect on dimer yields. On the other hand, the identity of the RO2 radicals formed will change because of the presence of a scavenger (e.g. cyclohexane, etc.), by replacing the RO2 radicals formed from the α-pinene + OH pathway with the scavenger + OH oxidation pathway. The products of the reaction between two RO2 radicals are strongly dependent on the radical structure, where the reaction branching ratios are in favor of dimer formation for larger radicals.18,19,21,26,27 Therefore, while RO2 radicals formed from small scavengers are expected to decrease dimer formation, radicals from larger scavengers are expected to recombine with the radicals from the VOC of interest forming mixed dimers. The latter have been observed in flow tube reactors18,26 and in the gas phase in smog chamber studies,27 but particle phase observations are currently lacking. Accordingly, OH scavengers can affect many aspects of the oxidation process, and their ubiquitous use in the atmospheric community necessitates the study of their effects on SOA formation and its composition.
RO2 ratio,10,11 which may have an effect on dimer yields. On the other hand, the identity of the RO2 radicals formed will change because of the presence of a scavenger (e.g. cyclohexane, etc.), by replacing the RO2 radicals formed from the α-pinene + OH pathway with the scavenger + OH oxidation pathway. The products of the reaction between two RO2 radicals are strongly dependent on the radical structure, where the reaction branching ratios are in favor of dimer formation for larger radicals.18,19,21,26,27 Therefore, while RO2 radicals formed from small scavengers are expected to decrease dimer formation, radicals from larger scavengers are expected to recombine with the radicals from the VOC of interest forming mixed dimers. The latter have been observed in flow tube reactors18,26 and in the gas phase in smog chamber studies,27 but particle phase observations are currently lacking. Accordingly, OH scavengers can affect many aspects of the oxidation process, and their ubiquitous use in the atmospheric community necessitates the study of their effects on SOA formation and its composition.
Here, we explore the chemical changes in α-pinene SOA forming in the presence and absence of different OH scavengers (butanol, cyclopentane, and cyclohexane). We utilize the extractive electrospray ionization time-of-flight mass spectrometer (EESI-TOF),28 as a soft ionization technique, to probe the changes on a molecular level. To understand the gas phase reactions leading to the observed molecules in the particle phase, we complement the SOA studies by also using a flow reactor.
2 Experimental
Studies were performed in Teflon atmospheric simulation chambers (27 m3 or 8 m3) at the Paul Scherrer Institute.29,30 The chambers are housed in temperature-controlled enclosures maintained at 20 ± 1 °C. The relative humidity (RH) for each experiment was 50%. Instrumentation included a proton-transfer mass spectrometer (PTR-MS, PTR-TOF-8000, Ionicon), an EESI-TOF including an atmospheric pressure time-of-flight mass spectrometer (Tofwerk), a scanning mobility particle sizer (SMPS, TSI model 3938) and an ozone gas monitor (Thermo 49C). Experiments were performed by injecting ozone into the chamber (200–500 ppb), followed by injection of an OH scavenger (if used), and then α-pinene (see Table S1† for details). Gas phase concentrations were monitored by a PTR-MS and for selected experiments a NO3-chemical ionization mass spectrometer (NO3-CIMS). The OH scavengers utilized were n-butanol (∼100 ppm), cyclopentane (200 ppm), and cyclohexane (200 ppm). These concentrations of OH scavenger resulted in OH reacting with the scavenger 99.9% of the time for all scavengers. The EESI-TOF provides highly time-resolved measurements (1 Hz) of the SOA molecular ions. The aerosol flow is continuously sampled and intersects with a spray of charged droplets doped with ∼100 ppm of NaI generated by a conventional fused silica electrospray capillary. The water-soluble portion of the aerosol is extracted into the droplets, which then yields intact SOA molecules in the form of Na+-adducts. Prior to interaction with the electrospray, a multi-walled charcoal denuder strips the gas phase constituents and leaves the aerosol, alone, to interact with the electrospray. The aerosol sample was regularly switched to a filter blank (4 min sample and 1 min filter) throughout the experiment to obtain regular background measurements. Detailed descriptions of the instrument can be found in lab studies,28,31 as well as in field studies.32,33 The particle phase mass concentrations were calculated using the size distributions obtained by the SMPS using a density of 1.2 g cm−3.34 The maximum mass concentrations are reported in Table 1 and are between 23–28 μg m−3 when no scavenger is present. The mass loadings are 12–16 μg m−3 when OH scavengers are used, similar to the reductions observed in Iinuma et al.9| Experiment # | Scavenger | Experimental setup | α-Pinene (ppb) | Scavenger (ppm) | O3 (ppb) | Mass loading (μg m−3) |
|---|---|---|---|---|---|---|
| 1 | No | Smog chamber | 25 | — | 160 | 23 |
| 2 | No | Smog chamber | 25 | — | 225 | 28 |
| 3 | No | Flow tube | 10 | — | 5000 | — |
| 4 | Butanol | Smog chamber | 25 | 200 | 250 | 16 |
| 5 | Butanol | Flow tube | 10 | 200 | 5000 | — |
| 6 | Cyclohexane | Smog chamber | 25 | 200 | 230 | 15 |
| 7 | Cyclohexane | Flow tube | 10 | 200 | 5000 | — |
| 8 | Cyclopentane | Smog chamber | 25 | 200 | 200 | 12 |
Flow-tube experiments were also performed in a ∼5 L glass vessel with a total flow rate of 20 L min−1, resulting in a residence time of ∼12 seconds at an RH of ∼5%. A constant source of α-pinene and ozone was injected into the flow tube, periodically an OH scavenger was additionally injected into the flow tube while maintaining a constant flow rate (see Table 1 for conditions). A condensation particle counter (CPC, TSI 3776, lower cut off 2.5 nm) continually monitored the particle number concentration and showed no particle formation in any experiment. During the flow-tube experiments the multi-channel denuder was removed from the EESI-TOF and the direct gas-phase products were detected. Backgrounds were assessed by comparing the signal observed by the EESI-TOF when only zero air was being passed through the flow tube both before and after each experiment, to achieve background levels. As mentioned above the total flow rate of the flow tube was 20 L min−1 and the flow rate from the flow tube to the EESI-TOF (∼0.5 m) was 10 L min−1, while the EESI-TOF sampled at 1 L min−1via a 3 cm long stainless steel tube. Below, the flow tube data is used to explore relative changes in the composition, and to verify products that are formed in the gas phase resulting from interactions between OH scavengers and α-pinene oxidation products. Therefore, absolute concentrations and reaction rates were not obtained. The experiments here were modelled with a 0-D box model (F0AM)35 using the chemical mechanism in MCM 3.3.1.36,37
3.1 Experimental results
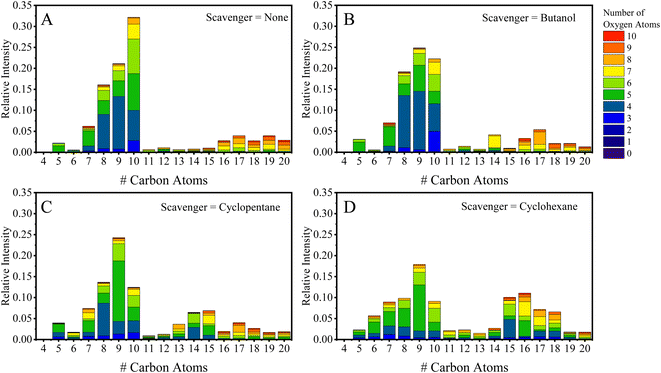
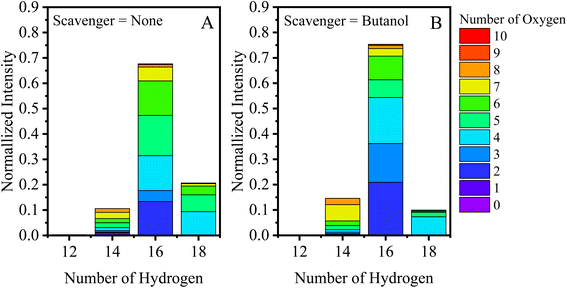
Previous work performed in our chambers utilizing α-pinene SOA, in Pospisilova et al., showed the composition of α-pinene to be highly time-dependent, and products from OH chemistry (C10H18Ox molecules) were found to be especially reactive with lifetimes below 30 minutes.31 Due to the complex evolution in composition, we will initially discuss EESI-TOF composition measurements performed at two experimental times: (1) within the first 30 minutes of the experiment; and (2) at the maximum of SOA mass. Fig. 1A–D show the carbon distribution at maximum mass with the bars colored by the number of oxygen atoms present for experiments without an OH scavenger, and with butanol, cyclopentane, and cyclohexane as OH scavengers. Fig. 1A shows the typical composition of α-pinene SOA formed without a scavenger at the time of maximum mass, binned in terms of the number of C and O atoms (x-axis and colors, respectively). Overall, C5–C10 molecules dominate in the monomer region and C14–C20 molecules in the dimer region, which together represent more than 90% of the total EESI-TOF signal observed in all scavenger-free experiments. The largest fraction of molecules formed contains 10 carbon atoms, and the hydrogen distribution for the C10 species consists mainly of H = 14, 16, and 18. Previous work shows it is possible to separate the contribution of different oxidation schemes (OH vs. O3) (e.g., C10H14Ox and C10H16Ox are formed from O3 chemistry, while C10H16Ox and C10H18Ox come from OH chemistry).18,19 Therefore, the C10H18Ox molecules can be used as an initial assessment for whether or not the OH chemistry pathway is depleted.Fig. 2A and B break down the C10 species observed by the EESI-TOF in terms of number of hydrogen atoms (H = 12, 14, 16, and 18) with number of oxygen atoms between 2–10 in experiments without an OH scavenger and butanol as an OH scavenger, respectively, 30 min after the addition of α-pinene. The C10H18Ox fraction without the scavenger is ∼25% of the total C10 contribution, consisting of O4–O7 molecules (Fig. 2A), while the butanol scavenger C10H18Ox fraction is only ∼15% (Fig. 2B). Overall, the fraction of C10H18Ox is significantly reduced for the latter case, and instead of spanning #O = 4–7, C10H18O4 is almost exclusively formed. C10H18O4 was found previously to decay away quickly in the particle phase, likely due to its high reactivity.31 While the majority of C10H18Ox molecules are formed through OH chemistry, C10H18O4 can also be formed via the reaction of the Criegee intermediate with H2O.15,19 Additionally, the change associated with the scavenger demonstrates that the C10H18Ox molecules (x = 5–7) are not a result of water clusters with C10H16Ox molecules, but rather formed via OH chemistry. Results when using cyclohexane as a scavenger are included in the ESI (Fig. S1A†), and agree with the results shown in Fig. 2, while formation of dimers via cyclopentane oxidation products complicates the analysis for that system (Fig. S1B†).
Fig. 1B–D show the carbon distribution at maximum mass when butanol (1B), cyclopentane (1C), and cyclohexane (1D) were used as OH scavengers. Comparing Fig. 1A (no scavenger) to 1B (butanol scavenger), there is depletion of the C10 molecules relative to the C9 molecules, which will be discussed further below. In the dimer region, the fraction of the C19–C20 molecules decreases from 6.8% (without scavenger) to 3.0% (with butanol). The C20H30–34Ox fraction measured by the NO3-CIMS is depleted (Fig. S2C and D†), which is consistent with previous flow tube studies18 and the particle phase composition (Fig. S3†). The C16–C18 region observes small changes on a relative scale (see Table S1†). Though, considering the mass concentration is lower for the scavenger experiments, the butanol and cyclopentane experiments exhibit lower absolute concentrations of C16–18 dimers, while the cyclohexane experiments have no difference relative to the no-scavenger experiment (excluding the mixed dimer products). The depletion of dimers comes from a change in the RO2 identities. For instance C14 molecules are not observed in the reaction without scavengers, while C14 molecules form via reactions between butanol radicals and α-pinene radicals. The main RO2 radical from the butanol scavenger is C4H9O3.10,18,26,37,38 C4H9O3 then reacts with the RO2 radicals from α-pinene ozonolysis, C10H15O4,6, to form the dominant C14 mixed dimers observed (C14H24O5,7) with an odd number of oxygen atoms. Another possibility to form C14 dimers could come from the reaction between the stabilized Criegee intermediate and the scavenger directly (i.e. C10H16O3 + C4H10O),17,39 the products of which would form C14H26O4. Based on the concentrations of the butanol and water in the chamber, approximately half of the reactivity of the Criegee should take place with butanol (assuming a reaction rate similar to propanol).40 However, C14H26O4 makes up less than 0.01% of the total EESI-TOF signal in the chamber, suggesting this pathway is not significant under our experimental conditions, or the species is too volatile to be in the particle phase. Overall, the formation of C14 molecules is a clear indicator that there exist unwanted effects of using scavengers on the chemistry occurring in the chamber.
Fig. 1C and D show that the ‘mixed dimers’ formed from the cycloalkane experiments are C16H26O5,7 and C15H24O5,7 for the cyclohexane and cyclopentane experiments, respectively, and preferentially form with odd-numbered oxygen atoms. If the formation pathway is the same as for butanol, then the molecules with an odd number of oxygen atoms must come from the mixture of an RO2 with even number of oxygen atoms + RO2 with odd number of oxygen atoms. The dominant α-pinene RO2 radicals are C10H15O4,6 and they must combine with either C6H11O3 or C5H9O3, respectively, to form the formula shown above. These formulae differ from the initial scavenger RO2 formed from the reaction with an OH radical, which are C6H11O2 (cyclohexane) and C5H9O2 (cyclopentane).41 Reaction schemes in the ESI (Schemes S1 and S2†) show how the initial RO2 can react with another RO2 to form an alkoxy radical, which can rapidly undergo a ring-opening reaction to form a second generation RO2 radical. These second-generation RO2 radicals (C6H11O3 – cyclohexane and C5H11O3 – cyclopentane) possess a formula matching the expected combination of scavenger and α-pinene oxidation products. An additional aspect in these experiments comes from the formation of dimers that have a carbon number equal to: Cscav + C10 − 1, which forms C15H24O4,6 in the cyclohexane experiment. In addition, there appears to be a systematic decrease in the C10 species with the increasing carbon content of the OH scavenger. For example, the C10 fraction decreases from 32% (no scavenger) to 23% (butanol), 12% (cyclopentane), and 9% (cyclohexane), which cannot be explained by changes in mass concentrations. Considering the scavenging of OH is effectively the same in all experiments (with scavengers) and the mass concentrations are similar, the observed differences should be attributed to radical reactions between the oxidation products of the scavengers and α-pinene. One possibility is that the reactions with RO2 radicals from cycloalkanes promote reactions via the alkoxy pathway which undergo subsequent fragmentation reactions. The cycloalkane experiments also exhibit the formation and inclusion of a small fraction of scavenger dimers (C12 – cyclohexane: see Fig. 1D, and C10 – cyclopentane: see Fig. S1B†), and small amounts of scavenger oxidation products (Fig. 1C and D), demonstrating three pathways for scavenger inclusions into SOA.
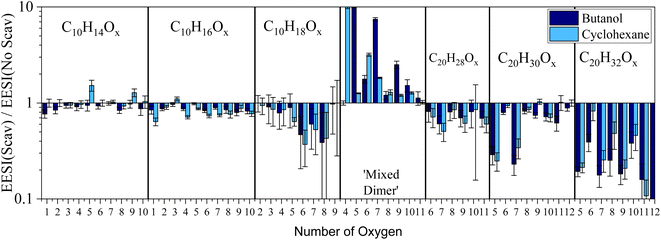
We further designed a flow-tube experiment to investigate the formation of the ‘mixed dimers’ in the gas phase from the systems discussed so far. The bar plot of Fig. 3 shows the gas-phase EESI-TOF signal (with scavenger)/EESI-TOF signal (without scavenger) for experiments with butanol and cyclohexane, with a CPC verifying particle number concentration <1 cm−3. The ESI (Fig. S4†) includes a time series to demonstrate how the injection of a scavenger influences the gaseous oxidation products in real-time. As we can see from Fig. 3, depletion of C10 molecules formed via OH chemistry are observed, with C10H16Ox depleted by 10–20%, and C10H18Ox depleted by up to 60% which is less than the depletion (80–90%) observed in the chamber experiment (Fig. 2). This could result from incomplete mixing in the flow tube, and does not result from changes in gas-particle partitioning due to the lower mass loadings with scavengers (presented in the ESI†). Unfortunately, the small signal-to-noise ratio for the C10H18Ox molecules results in relatively large error bars. Depletion of C20 dimers occurs with the addition of a scavenger, with C20H32Ox being depleted by 90% and exhibiting an even–odd oxygen atom behavior. Dimers with odd number of oxygen atoms are depleted because these molecules are formed from RO2–RO2 reactions of the OH (C10H17O3,5) and O3 reaction pathways (C10H15O4,6). C20H30O5,7 molecules are also depleted by up to 80%, while the rest of the C20H30Ox are only slightly diminished (by 30–50%), consistent with.18
For the experiments with a butanol scavenger, the main ‘mixed dimers’ formed have an odd number of oxygen atoms (C14H24O5,7,9,11), in good agreement with results from the smog chamber experiments, discussed above. In contrast, there is a difference between flow tube and the smog chamber results for the cyclohexane experiments where the principal number of oxygen atoms for the C16H26Ox ‘mixed dimers’ in Fig. 3 are even C16H26O4,6,8, which differs from the results from the smog chamber (odd) in Fig. 1D (C16H26O5 and O7). When modelling the oxidation processes with the 0-D box model (F0AM) from the flow tube and the smog chamber, the ratio of the C6H11O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C6H11O3 varies substantially between the two experiments (∼20 for smog chamber and 100–300 in the flow tube). The difference in the ratio comes from the time scale of the two experiments, and is not impacted by the concentration difference in the experiments. The initial RO2 radicals are still being formed in the flow tube, while longer times in the smog chamber allows the RO2 radicals to undergo further reactions (with other RO2 or HO2 radicals) forming second- and third-generation radicals. Therefore, differences in the ‘mixed dimer’ formed in the flow tube (O4 and O6) vs. the smog chamber (O5 and O7) reflect the distribution of the scavenger RO2 radicals present. Because the main ‘mixed dimer’ formed in the chamber comes from the second-generation scavenger RO2 radical (C6H11O3) despite the initial scavenger RO2 (C6H11O2) having a larger concentration demonstrates mixed dimer formation is faster between α-pinene-RO2 radicals and C6H11O3 when compared to C6H11O2. This is in agreement with the fact that dimer formation rates increase with the increase of the RO2 oxygen content.42,43
C6H11O3 varies substantially between the two experiments (∼20 for smog chamber and 100–300 in the flow tube). The difference in the ratio comes from the time scale of the two experiments, and is not impacted by the concentration difference in the experiments. The initial RO2 radicals are still being formed in the flow tube, while longer times in the smog chamber allows the RO2 radicals to undergo further reactions (with other RO2 or HO2 radicals) forming second- and third-generation radicals. Therefore, differences in the ‘mixed dimer’ formed in the flow tube (O4 and O6) vs. the smog chamber (O5 and O7) reflect the distribution of the scavenger RO2 radicals present. Because the main ‘mixed dimer’ formed in the chamber comes from the second-generation scavenger RO2 radical (C6H11O3) despite the initial scavenger RO2 (C6H11O2) having a larger concentration demonstrates mixed dimer formation is faster between α-pinene-RO2 radicals and C6H11O3 when compared to C6H11O2. This is in agreement with the fact that dimer formation rates increase with the increase of the RO2 oxygen content.42,43
In addition, the extent of C10 depletion differs depending on the identity of the scavenger in both the smog chamber (Fig. 1) and flow tube (Fig. 3). When considering the RO2 reaction pathways for each scavenger (shown in the ESI†), the fates of the RO2 radicals down the alkoxy pathway lead to different results. The butanol derived alkoxy radical terminates with the formation of HO2 and acetaldehyde. Scheme S1† shows the cyclohexane RO2 radicals going through an alkoxy radical until terminating with an HO2 radical. A consequence of the alkoxy pathway can be an enhancement of unimolecular fragmentation products,19 resulting in a shift in the carbon distribution away from C10 molecules to smaller carbon containing species (C7–9). Consequently, there is a shift in the carbon distribution toward smaller carbon containing molecules for all scavengers used in the chamber (Fig. 1) with the most substantial depletion occurring for the cycloalkanes in particular, which supports this explanation.
3.2 Modelling and discussion
Some of the changes observed from the use of scavengers comes from changes in the radical balance that occurs. Fig. S4† highlights the reactivity of α-pinene-RO2 with HO2, and RO2 radicals from either the scavenger or α-pinene, assuming general rates of RO2 + RO2 and RO2 + HO2 currently used in MCM 3.3.1. These results highlight the importance of HO2 in the butanol experiment because of the pathway to form butanal + HO2, which promotes RO2 radical termination to ROOH monomers over dimer formation. The dimer fraction in Table S1† (and Fig. 1) is roughly similar for all experiments, though the difference in mass loading between experiments will result in a change in the absolute concentration of the dimers. If the EESI results are presented in terms of the total mass flux of the EESI (attograms per second obtained by # s−1 × MW × 1 × 1018/Avogadro's number) the total dimer signal (C14–20) for the no scavenger experiment (4 ag s−1) is greater than the absolute dimer signal (2.7 ag s−1), consistent with the greater importance of RO2 + HO2. For the cycloalkane experiments, the absolute concentration of the dimer range (C14–20) is not dramatically different to the no-scavenger experiment (cyclopentane – 3.5 ag s−1, cyclohexane – 4.2 ag s−1), consistent with the importance of RO2 + RO2 dimer formation on SOA formation. Though, a systematic study on the rates of the RO2 + RO2 reactions and their branching ratios using a flow reactor would be needed to validate any quantitative modelling of these systems.Our results raise questions about previous studies that have used OH scavengers to examine SOA physical properties or chemical composition. It also raises the question: why have these products not been previously observed in SOA? Previous measurements of SOA formed in the presence of a scavenger have generally used techniques with harsh ionization processes with substantial fragmentation,17,34 or investigations of these molecules have not been a priority when employing offline techniques. Filter sampling techniques can also introduce artefacts and time that affords reactive species to degrade prior to analysis, as has been shown for reactive oxygen species from filter analysis.44 Therefore, filter extracts that have measured the chemical composition of SOA may not be an effective method for measuring potentially reactive species formed via RO2–RO2 reactions, including species that hydrolyze in the presence of water or other solvents.
Further, these results show the use of scavengers that form RO2 radicals is problematic because it can result in the incorporation of unwanted species into SOA (e.g. mixed dimers and scavenger oxidation products), as well as creating a radical environment that dramatically changes the monomer composition of SOA. Without accounting for sensitivity differences of different molecules measured by the EESI-TOF and using purely the relative intensities, shown in Fig. 1, the scavenger incorporated into SOA reaches nearly 20% for cyclohexane, while for the other scavengers the total fraction of artefacts decreases with decreasing carbon number of the scavenger (shown in Table S1†), down to ∼7% for butanol. This accounting includes the increase in C6 molecules for the cyclohexane experiment (see Fig. 1Avs. 1D) originating from inclusions of cyclohexane oxidation products. Similar increases are also observed in C12 molecules and the ‘mixed dimers’ (Fig. 1D). The change in the radical pathways and the incorporation of unwanted scavenger oxidation products in α-pinene SOA demonstrates the necessity to consider which OH scavenger to use and if to use an OH scavenger at all. Additionally, the atmosphere is rife with potential scavengers of OH radicals, and ultimately scavengers used within chambers should effectively mimic atmospheric conditions. Currently, many chamber experiments possess large concentrations of RO2 radicals, while in the atmosphere HO2 is a significant sink of RO2 radicals. Therefore, the goal should be to use scavengers that do not incorporate the scavenger and that produce an atmospherically relevant radical balance (e.g. HO2vs. RO2).
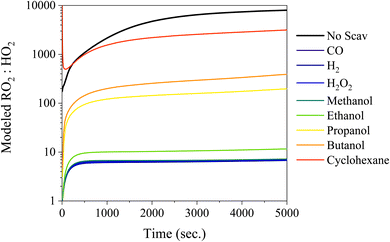
Scavengers such as H2, CO, and H2O2 are good candidates because they will only produce HO2 radicals, but the drawbacks of these scavengers include the large concentrations required (H2 – 2%), potential safety hazards (H2 and CO), and the uptake into the particle phase at elevated RH (H2O2). To probe the impact of the HO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RO2 ratio from different scavengers, a simple chamber box model using MCM37,38 simulated the RO2
RO2 ratio from different scavengers, a simple chamber box model using MCM37,38 simulated the RO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HO2 ratio in the chamber in Fig. 4 (the absolute concentrations are shown in Fig. S5†). For comparison, Fig. 4 also includes other scavengers such as, CO, H2, H2O2, and a series of alcohols, to probe the differences between RO2 and HO2 concentrations. RO2 concentrations are always at least one order of magnitude greater than the HO2 concentrations for all conditions explored here. Despite the large concentrations of RO2 radicals, the reaction rate between HO2–RO2 is ∼2 orders of magnitude faster than RO2–RO2 reactions, consequently HO2–RO2 will be the dominant reaction pathway when the RO2
HO2 ratio in the chamber in Fig. 4 (the absolute concentrations are shown in Fig. S5†). For comparison, Fig. 4 also includes other scavengers such as, CO, H2, H2O2, and a series of alcohols, to probe the differences between RO2 and HO2 concentrations. RO2 concentrations are always at least one order of magnitude greater than the HO2 concentrations for all conditions explored here. Despite the large concentrations of RO2 radicals, the reaction rate between HO2–RO2 is ∼2 orders of magnitude faster than RO2–RO2 reactions, consequently HO2–RO2 will be the dominant reaction pathway when the RO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HO2 is below 100 (e.g. CO, H2O2, H2, methanol, and ethanol). Reactions between HO2 and RO2 will also promote the formation of peroxide functional groups and inhibit formation of dimers via the RO2–RO2 pathway. Additionally, higher concentrations of HO2 more realistically capture the HO2
HO2 is below 100 (e.g. CO, H2O2, H2, methanol, and ethanol). Reactions between HO2 and RO2 will also promote the formation of peroxide functional groups and inhibit formation of dimers via the RO2–RO2 pathway. Additionally, higher concentrations of HO2 more realistically capture the HO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RO2 ratio present in the atmosphere as opposed to the RO2 dominant chemistry regime typically found in chambers.
RO2 ratio present in the atmosphere as opposed to the RO2 dominant chemistry regime typically found in chambers.
In summary, the roles of the scavengers in these experiments are multi-faceted because they influence the HO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RO2 ratio, the identity of the RO2 radicals present, and the fate of the RO2 radicals. The differences in the types of radicals produced in the gas phase (OH vs. HO2vs. scavenger-RO2vs. α-pinene-RO2) ultimately determines a substantial fraction of the composition of the SOA formed. Given many fundamental studies about SOA are performed in chambers or flow tubes with the presence of a scavenger, it is important to understand the role they will play in the chemistry taking place. This study shows significant changes in composition of α-pinene SOA as a function of OH scavenger and necessitates their further study and consideration.
RO2 ratio, the identity of the RO2 radicals present, and the fate of the RO2 radicals. The differences in the types of radicals produced in the gas phase (OH vs. HO2vs. scavenger-RO2vs. α-pinene-RO2) ultimately determines a substantial fraction of the composition of the SOA formed. Given many fundamental studies about SOA are performed in chambers or flow tubes with the presence of a scavenger, it is important to understand the role they will play in the chemistry taking place. This study shows significant changes in composition of α-pinene SOA as a function of OH scavenger and necessitates their further study and consideration.
Data availability
Data can be found at the Eurochamp Database of Atmospheric Simulation Chamber Studies (https://data.eurochamp.org/).Author contributions
Chamber investigations were performed by DB, VP, FL, AB, MX, XZ, and WH. Flow tube studies were investigated by DB, DSW, CPL. JS, AP, IEH and UB obtained funding for this work. DB prepared the manuscript with contributions from all co-authors.Conflicts of interest
The authors declare no conflict of interests with the performed work.Acknowledgements
This work was supported by the Swiss National Science Foundation (starting grant BSSGI0_155846, grant 200020_172602, grant 200021_169787) as well as the European Union's Horizon 2020 Research and Innovation Program through the EUROCHAMP-2020 Infrastructure Activity under grant agreement no. 730997. We would also like to thank Rene Richter for his assistance in installing and assembling the experimental setup.References
- J. L. Jimenez, M. R. Canagaratna, N. M. Donahue, A. S. Prevot, Q. Zhang, J. H. Kroll, P. F. DeCarlo, J. D. Allan, H. Coe, N. L. Ng, A. C. Aiken, K. S. Docherty, I. M. Ulbrich, A. P. Grieshop, A. L. Robinson, J. Duplissy, J. D. Smith, K. R. Wilson, V. A. Lanz, C. Hueglin, Y. L. Sun, J. Tian, A. Laaksonen, T. Raatikainen, J. Rautiainen, P. Vaattovaara, M. Ehn, M. Kulmala, J. M. Tomlinson, D. R. Collins, M. J. Cubison, E. J. Dunlea, J. A. Huffman, T. B. Onasch, M. R. Alfarra, P. I. Williams, K. Bower, Y. Kondo, J. Schneider, F. Drewnick, S. Borrmann, S. Weimer, K. Demerjian, D. Salcedo, L. Cottrell, R. Griffin, A. Takami, T. Miyoshi, S. Hatakeyama, A. Shimono, J. Y. Sun, Y. M. Zhang, K. Dzepina, J. R. Kimmel, D. Sueper, J. T. Jayne, S. C. Herndon, A. M. Trimborn, L. R. Williams, E. C. Wood, A. M. Middlebrook, C. E. Kolb, U. Baltensperger and D. R. Worsnop, Evolution of organic aerosols in the atmosphere, Science, 2009, 326, 1525–1529 CrossRef CAS PubMed.
- M. Hallquist, J. C. Wenger, U. Baltensperger, Y. Rudich, D. Simpson, M. Claeys, J. Dommen, N. M. Donahue, C. George, A. H. Goldstein, J. F. Hamilton, H. Herrmann, T. Hoffmann, Y. Iinuma, M. Jang, M. E. Jenkin, J. L. Jimenez, A. Kiendler-Scharr, W. Maenhaut, G. McFiggans, T. F. Mentel, A. Monod, A. S. H. Prévôt, J. H. Seinfeld, J. D. Surratt, R. Szmigielski and J. Wildt, The formation, properties and impact of secondary organic aerosol: current and emerging issues, Atmos. Chem. Phys., 2009, 9, 5155–5236 CrossRef CAS.
- M. Riva, L. Heikkinen, D. M. Bell, O. Peräkylä, Q. Zha, S. Schallhart, M. P. Rissanen, D. Imre, T. Petäjä, J. A. Thornton, A. Zelenyuk and M. Ehn, Chemical transformations in monoterpene-derived organic aerosol enhanced by inorganic composition, npj Clim. Atmos. Sci., 2019, 2, 2 CrossRef.
- H. Zhang, L. D. Yee, B. H. Lee, M. P. Curtis, D. R. Worton, G. Isaacman-VanWertz, J. H. Offenberg, M. Lewandowski, T. E. Kleindienst, M. R. Beaver, A. L. Holder, W. A. Lonneman, K. S. Docherty, M. Jaoui, H. O. T. Pye, W. Hu, D. A. Day, P. Campuzano-Jost, J. L. Jimenez, H. Guo, R. J. Weber, J. de Gouw, A. R. Koss, E. S. Edgerton, W. Brune, C. Mohr, F. D. Lopez-Hilfiker, A. Lutz, N. M. Kreisberg, S. R. Spielman, S. V. Hering, K. R. Wilson, J. A. Thornton and A. H. Goldstein, Monoterpenes are the largest source of summertime organic aerosol in the southeastern United States, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 2038 CrossRef CAS.
- A. R. Rickard, D. Johnson, C. D. McGill and G. Marston, OH Yields in the Gas-Phase Reactions of Ozone with Alkenes, J. Phys. Chem. A, 1999, 103, 7656–7664 CrossRef CAS.
- R. Atkinson, S. M. Aschmann, J. Arey and B. Shorees, Formation of OH radicals in the gas phase reactions of O3 with a series of terpenes, J. Geophys. Res.: Atmos., 1992, 97, 6065–6073 CrossRef CAS.
- K. M. Henry and N. M. Donahue, Effect of the OH Radical Scavenger Hydrogen Peroxide on Secondary Organic Aerosol Formation from α-Pinene Ozonolysis, Aerosol Sci. Technol., 2011, 45, 696–700 CrossRef.
- N. M. Donahue, K. E. Huff Hartz, B. Chuong, A. A. Presto, C. O. Stanier, T. Rosenhørn, A. L. Robinson and S. N. Pandis, Critical factors determining the variation in SOA yields from terpene ozonolysis: A combined experimental and computational study, Faraday Discuss., 2005, 130, 295–309 RSC.
- Y. Iinuma, O. Böge, Y. Miao, B. Sierau, T. Gnauk and H. Herrmann, Laboratory studies on secondary organic aerosol formation from terpenes, Faraday Discuss., 2005, 130, 279–294 RSC.
- M. D. Keywood, J. H. Kroll, V. Varutbangkul, R. Bahreini, R. C. Flagan and J. H. Seinfeld, Secondary Organic Aerosol Formation from Cyclohexene Ozonolysis: Effect of OH Scavenger and the Role of Radical Chemistry, Environ. Sci. Technol., 2004, 38, 3343–3350 CrossRef CAS PubMed.
- K. S. Docherty and P. J. Ziemann, Effects of Stabilized Criegee Intermediate and OH Radical Scavengers on Aerosol Formation from Reactions of β-Pinene with O 3, Aerosol Sci. Technol., 2003, 37, 877–891 CrossRef CAS.
- K. M. Henry, T. Lohaus and N. M. Donahue, Organic Aerosol Yields from α-Pinene Oxidation: Bridging the Gap between First-Generation Yields and Aging Chemistry, Environ. Sci. Technol., 2012, 46, 12347–12354 CrossRef CAS PubMed.
- E. Abramson, D. Imre, J. Beranek, J. Wilson and A. Zelenyuk, Experimental determination of chemical diffusion within secondary organic aerosol particles, Phys. Chem. Chem. Phys., 2013, 15, 2983–2991 RSC.
- R. K. Pathak, K. Salo, E. U. Emanuelsson, C. Cai, A. Lutz, Å. M. Hallquist and M. Hallquist, Influence of Ozone and Radical Chemistry on Limonene Organic Aerosol Production and Thermal Characteristics, Environ. Sci. Technol., 2012, 46, 11660–11669 CrossRef CAS PubMed.
- M. S. Claflin, J. E. Krechmer, W. Hu, J. L. Jimenez and P. J. Ziemann, Functional Group Composition of Secondary Organic Aerosol Formed from Ozonolysis of α-Pinene Under High VOC and Autoxidation Conditions, ACS Earth Space Chem., 2018, 2, 1196–1210 CrossRef CAS.
- M. L. Walser, Y. Desyaterik, J. Laskin, A. Laskin and S. A. Nizkorodov, High-resolution mass spectrometric analysis of secondary organic aerosol produced by ozonation of limonene, Phys. Chem. Chem. Phys., 2008, 10, 1009–1022 RSC.
- K. S. Docherty, W. Wu, Y. B. Lim and P. J. Ziemann, Contributions of Organic Peroxides to Secondary Aerosol Formed from Reactions of Monoterpenes with O3, Environ. Sci. Technol., 2005, 39, 4049–4059 CrossRef CAS PubMed.
- Y. Zhao, J. A. Thornton and H. O. T. Pye, Quantitative constraints on autoxidation and dimer formation from direct probing of monoterpene-derived peroxy radical chemistry, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 12142 CrossRef CAS PubMed.
- U. Molteni, M. Simon, M. Heinritzi, C. R. Hoyle, A.-K. Bernhammer, F. Bianchi, M. Breitenlechner, S. Brilke, A. Dias, J. Duplissy, C. Frege, H. Gordon, C. Heyn, T. Jokinen, A. Kürten, K. Lehtipalo, V. Makhmutov, T. Petäjä, S. M. Pieber, A. P. Praplan, S. Schobesberger, G. Steiner, Y. Stozhkov, A. Tomé, J. Tröstl, A. C. Wagner, R. Wagner, C. Williamson, C. Yan, U. Baltensperger, J. Curtius, N. M. Donahue, A. Hansel, J. Kirkby, M. Kulmala, D. R. Worsnop and J. Dommen, Formation of highly oxygenated organic molecules from α-pinene ozonolysis: chemical characteristics, mechanism, and kinetic model development, ACS Earth Space Chem., 2019, 3, 873–883 CrossRef CAS.
- C. M. Kenseth, Y. Huang, R. Zhao, N. F. Dalleska, J. C. Hethcox, B. M. Stoltz and J. H. Seinfeld, Synergistic O3 + OH oxidation pathway to extremely low-volatility dimers revealed in β-pinene secondary organic aerosol, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 8301 CrossRef CAS PubMed.
- R. R. Valiev, G. Hasan, V.-T. Salo, J. Kubečka and T. Kurten, Intersystem Crossings Drive Atmospheric Gas-Phase Dimer Formation, J. Phys. Chem. A, 2019, 123, 6596–6604 CrossRef CAS PubMed.
- J. J. Orlando and G. S. Tyndall, Laboratory studies of organic peroxy radical chemistry: an overview with emphasis on recent issues of atmospheric significance, Chem. Soc. Rev., 2012, 41, 6294–6317 RSC.
- F. Bianchi, J. Tröstl, H. Junninen, C. Frege, S. Henne, C. R. Hoyle, U. Molteni, E. Herrmann, A. Adamov, N. Bukowiecki, X. Chen, J. Duplissy, M. Gysel, M. Hutterli, J. Kangasluoma, J. Kontkanen, A. Kürten, H. E. Manninen, S. Münch, O. Peräkylä, T. Petäjä, L. Rondo, C. Williamson, E. Weingartner, J. Curtius, D. R. Worsnop, M. Kulmala, J. Dommen and U. Baltensperger, New particle formation in the free troposphere: a question of chemistry and timing, Science, 2016, 352, 1109–1112 CrossRef CAS PubMed.
- J. Tröstl, W. K. Chuang, H. Gordon, M. Heinritzi, C. Yan, U. Molteni, L. Ahlm, C. Frege, F. Bianchi, R. Wagner, M. Simon, K. Lehtipalo, C. Williamson, J. S. Craven, J. Duplissy, A. Adamov, J. Almeida, A.-K. Bernhammer, M. Breitenlechner, S. Brilke, A. Dias, S. Ehrhart, R. C. Flagan, A. Franchin, C. Fuchs, R. Guida, M. Gysel, A. Hansel, C. R. Hoyle, T. Jokinen, H. Junninen, J. Kangasluoma, H. Keskinen, J. Kim, M. Krapf, A. Kürten, A. Laaksonen, M. Lawler, M. Leiminger, S. Mathot, O. Möhler, T. Nieminen, A. Onnela, T. Petäjä, F. M. Piel, P. Miettinen, M. P. Rissanen, L. Rondo, N. Sarnela, S. Schobesberger, K. Sengupta, M. Sipilä, J. N. Smith, G. Steiner, A. Tomè, A. Virtanen, A. C. Wagner, E. Weingartner, D. Wimmer, P. M. Winkler, P. Ye, K. S. Carslaw, J. Curtius, J. Dommen, J. Kirkby, M. Kulmala, I. Riipinen, D. R. Worsnop, N. M. Donahue and U. Baltensperger, The role of low-volatility organic compounds in initial particle growth in the atmosphere, Nature, 2016, 533, 527–531 CrossRef PubMed.
- L. L. J. Quéléver, K. Kristensen, L. Normann Jensen, B. Rosati, R. Teiwes, K. R. Daellenbach, O. Peräkylä, P. Roldin, R. Bossi, H. B. Pedersen, M. Glasius, M. Bilde and M. Ehn, Effect of temperature on the formation of highly oxygenated organic molecules (HOMs) from alpha-pinene ozonolysis, Atmos. Chem. Phys., 2019, 19, 7609–7625 CrossRef.
- T. Berndt, W. Scholz, B. Mentler, L. Fischer, H. Herrmann, M. Kulmala and A. Hansel, Accretion Product Formation from Self- and Cross-Reactions of RO2 Radicals in the Atmosphere, Angew. Chem., Int. Ed., 2018, 57, 3820–3824 CrossRef CAS PubMed.
- G. McFiggans, T. F. Mentel, J. Wildt, I. Pullinen, S. Kang, E. Kleist, S. Schmitt, M. Springer, R. Tillmann, C. Wu, D. Zhao, M. Hallquist, C. Faxon, M. Le Breton, Å. M. Hallquist, D. Simpson, R. Bergström, M. E. Jenkin, M. Ehn, J. A. Thornton, M. R. Alfarra, T. J. Bannan, C. J. Percival, M. Priestley, D. Topping and A. Kiendler-Scharr, Secondary organic aerosol reduced by mixture of atmospheric vapours, Nature, 2019, 565, 587–593 CrossRef CAS PubMed.
- F. D. Lopez-Hilfiker, V. Pospisilova, W. Huang, M. Kalberer, C. Mohr, G. Stefenelli, J. A. Thornton, U. Baltensperger, A. S. H. Prevot and J. G. Slowik, An extractive electrospray ionization time-of-flight mass spectrometer (EESI-TOF) for online measurement of atmospheric aerosol particles, Atmos. Meas. Tech., 2019, 12, 4867–4886 CrossRef CAS.
- S. M. Platt, I. El Haddad, A. A. Zardini, M. Clairotte, C. Astorga, R. Wolf, J. G. Slowik, B. Temime-Roussel, N. Marchand, I. Ježek, L. Drinovec, G. Močnik, O. Möhler, R. Richter, P. Barmet, F. Bianchi, U. Baltensperger and A. S. H. Prévôt, Secondary organic aerosol formation from gasoline vehicle emissions in a new mobile environmental reaction chamber, Atmos. Chem. Phys., 2013, 13, 9141–9158 CrossRef.
- A. Metzger, J. Dommen, K. Gaeggeler, J. Duplissy, A. S. H. Prevot, J. Kleffmann, Y. Elshorbany, A. Wisthaler and U. Baltensperger, Evaluation of 1,3,5 trimethylbenzene degradation in the detailed tropospheric chemistry mechanism, MCMv3.1, using environmental chamber data, Atmos. Chem. Phys., 2008, 6453–6468 CrossRef CAS.
- V. Pospisilova, F. D. Lopez-Hilfiker, D. M. Bell, I. El Haddad, C. Mohr, W. Huang, L. Heikkinen, M. Xiao, J. Dommen, A. S. H. Prevot, U. Baltensperger and J. G. Slowik, On the fate of oxygenated organic molecules in atmospheric aerosol particles, Sci. Adv., 2020, 6, eaax8922 CrossRef CAS PubMed.
- L. Qi, M. Chen, G. Stefenelli, V. Pospisilova, Y. Tong, A. Bertrand, C. Hueglin, X. Ge, U. Baltensperger, A. S. H. Prévôt and J. G. Slowik, Organic aerosol source apportionment in Zurich using an extractive electrospray ionization time-of-flight mass spectrometer (EESI-TOF-MS) – Part 2: biomass burning influences in winter, Atmos. Chem. Phys., 2019, 19, 8037–8062 CrossRef CAS.
- G. Stefenelli, V. Pospisilova, F. D. Lopez-Hilfiker, K. R. Daellenbach, C. Hüglin, Y. Tong, U. Baltensperger, A. S. H. Prévôt and J. G. Slowik, Organic aerosol source apportionment in Zurich using an extractive electrospray ionization time-of-flight mass spectrometer (EESI-TOF-MS) – Part 1: Biogenic influences and day–night chemistry in summer, Atmos. Chem. Phys., 2019, 19, 14825–14848 CrossRef CAS.
- T. D. Vaden, D. Imre, J. Beranek, M. Shrivastava and A. Zelenyuk, Evaporation kinetics and phase of laboratory and ambient secondary organic aerosol, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 2190–2195 CrossRef CAS PubMed.
- G. M. Wolfe, M. R. Marvin, S. J. Roberts, K. R. Travis and J. Liao, The Framework for 0-D Atmospheric Modeling (F0AM) v3.1, Geosci. Model Dev., 2016, 9, 3309–3319 CrossRef.
- M. E. Jenkin, S. M. Saunders, V. Wagner and M. J. Pilling, Protocol for the development of the Master Chemical Mechanism, MCM v3 (Part B): tropospheric degradation of aromatic volatile organic compounds, Atmos. Chem. Phys., 2003, 3, 181–193 CrossRef CAS.
- S. M. Saunders, M. E. Jenkin, R. G. Derwent and M. J. Pilling, Protocol for the development of the Master Chemical Mechanism, MCM v3 (Part A): tropospheric degradation of non-aromatic volatile organic compounds, Atmos. Chem. Phys., 2003, 3, 161–180 CrossRef CAS.
- M. E. Jenkin, S. M. Saunders and M. J. Pilling, The tropospheric degradation of volatile organic compounds: a protocol for mechanism development, Atmos. Environ., 1997, 31, 81–104 CrossRef CAS.
- M. R. McGillen, B. F. E. Curchod, R. Chhantyal-Pun, J. M. Beames, N. Watson, M. A. H. Khan, L. McMahon, D. E. Shallcross and A. J. Orr-Ewing, Criegee Intermediate–Alcohol Reactions, A Potential Source of Functionalized Hydroperoxides in the Atmosphere, ACS Earth Space Chem., 2017, 1, 664–672 CrossRef CAS.
- H. J. Tobias and P. J. Ziemann, Kinetics of the Gas-Phase Reactions of Alcohols, Aldehydes, Carboxylic Acids, and Water with the C13 Stabilized Criegee Intermediate Formed from Ozonolysis of 1-Tetradecene, J. Phys. Chem. A, 2001, 105, 6129–6135 CrossRef CAS.
- S. M. Aschmann, A. A. Chew, J. Arey and R. Atkinson, Products of the Gas-Phase Reaction of OH Radicals with Cyclohexane: Reactions of the Cyclohexoxy Radical, J. Phys. Chem. A, 1997, 101, 8042–8048 CrossRef CAS.
- M. Schervish and N. M. Donahue, Peroxy radical kinetics and new particle formation, Environ. Sci.: Atmos., 2021, 1, 79–92 CAS.
- M. Schervish and N. M. Donahue, Peroxy radical chemistry and the volatility basis set, Atmos. Chem. Phys., 2020, 20, 1183–1199 CrossRef CAS.
- J. Zhou, E. A. Bruns, P. Zotter, G. Stefenelli, A. S. H. Prévôt, U. Baltensperger, I. El-Haddad and J. Dommen, Development, characterization and first deployment of an improved online reactive oxygen species analyzer, Atmos. Meas. Tech., 2018, 11, 65–80 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ea00105e |
| ‡ Now at: Institute for Atmospheric and Earth System Research/Physics, Faculty of Science, University of Helsinki, 00014 Helsinki, Finland. |
| This journal is © The Royal Society of Chemistry 2023 |