Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pre-zeolite framework super-MIEC anodes for high-rate lithium-ion batteries†
Shitong
Wang‡
a,
Lijiang
Zhao‡
b,
Yanhao
Dong
 *ac,
He
Zhu
d,
Yang
Yang
ef,
Haowei
Xu
a,
Baoming
Wang
g,
Yakun
Yuan
hi,
Yang
Ren
d,
Xiaojing
Huang
j,
Wei
Quan
kl,
Yutong
Li
c,
Yimeng
Huang
g,
Charles M.
Settens
m,
Qi
He
a,
Yongwen
Sun
e,
Hua
Wang
a,
Zunqiu
Xiao
c,
Wenjun
Liu
n,
Xianghui
Xiao
j,
Riqiang
Fu
*ac,
He
Zhu
d,
Yang
Yang
ef,
Haowei
Xu
a,
Baoming
Wang
g,
Yakun
Yuan
hi,
Yang
Ren
d,
Xiaojing
Huang
j,
Wei
Quan
kl,
Yutong
Li
c,
Yimeng
Huang
g,
Charles M.
Settens
m,
Qi
He
a,
Yongwen
Sun
e,
Hua
Wang
a,
Zunqiu
Xiao
c,
Wenjun
Liu
n,
Xianghui
Xiao
j,
Riqiang
Fu
 o,
Qiang
Li
p,
Yong S.
Chu
j,
Zhongtai
Zhang
c,
Qi
Liu
o,
Qiang
Li
p,
Yong S.
Chu
j,
Zhongtai
Zhang
c,
Qi
Liu
 d,
Andrew M.
Minor
fq,
Junying
Zhang
d,
Andrew M.
Minor
fq,
Junying
Zhang
 *b,
Zilong
Tang
*b,
Zilong
Tang
 *c and
Ju
Li
*c and
Ju
Li
 *ag
*ag
aDepartment of Nuclear Science and Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. E-mail: dongyh@mit.edu; liju@mit.edu
bSchool of Physics, Beihang University, Beijing 100191, China. E-mail: zjy@buaa.edu.cn
cState Key Lab of New Ceramics and Fine Processing, School of Materials Science and Engineering, Tsinghua University, Beijing 100084, China. E-mail: tzl@tsinghua.edu.cn
dDepartment of Physics, City University of Hong Kong, Kowloon, Hong Kong 999077, China
eDepartment of Engineering Science and Mechanics and Materials Research Institute, The Pennsylvania State University, University Park, PA 16802, USA
fNational Center for Electron Microscopy, Molecular Foundry, Lawrence Berkeley National Laboratory, Berkeley, CA 94702, USA
gDepartment of Materials Science and Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA
hSchool of Mechanical Engineering, Shanghai Jiao Tong University, Shanghai 200240, China
iZhangjiang Institute for Advanced Study, Shanghai Jiao Tong University, Shanghai 201203, China
jNational Synchrotron Light Source II, Brookhaven National Laboratory, Upton, NY 11973, USA
kChina Automotive Battery Research Institute Co., Ltd., Beijing 101407, China
lGeneral Research Institute for Nonferrous Metals, Beijing 100088, China
mMaterials Research Laboratory, Massachusetts Institute of Technology, Cambridge, MA 02139, USA
nAdvanced Photon Source, Argonne National Laboratory, Argonne, IL 60439, USA
oNational High Magnetic Field Laboratory, Florida State University, Tallahassee, FL 32310, USA
pBeijing Advanced Innovation Center for Materials Genome Engineering, Institute of Solid State Chemistry, University of Science and Technology Beijing, Beijing 100083, China
qDepartment of Materials Science and Engineering, University of California, Berkeley, CA 94702, USA
First published on 14th December 2022
Abstract
Zeolites, Prussian blue analogues (PBAs), and metal–organic frameworks (MOFs) rely on surface-like internal pore diffusions, which have generically low activation barriers to enable the rapid uptake of chemical species. Here we show that Wadsley–Roth oxides (WROs) with pore diameters of 2.5 Å < d < 2.8 Å, while excluding molecules, enable very rapid diffusion of Li+ in single-crystal particles >10 μm size. This supports full charge cycles at high rates of ∼30C, which would rival the filling up of gasoline vehicles, while reducing the contact and side reactions with the electrolyte and enhancing the cycle life up to 10 000 cycles. Pore diffusion in WRO mixed ionic and electronic conductors (MIECs) differs from that in lithium intercalation compounds in the off-centered Li storage and low-coordination saddle points for migration. The reduced topological constraints per atom and large free volume in the host also lead to abnormally low or even negative thermal expansion and soft phonons, similar to other open frameworks such as zeolites, PBAs, and MOFs. Based on these guidelines, we have synthesized new composition (Nb9W4Ti4O42.5) and crystal size-coarsened H-Nb2O5 (>20 μm single crystals) with unprecedented performance.
Broader contextThe key components in lithium-ion batteries are battery electrodes, which must be superfast mixed ionic and electronic conductors (super-MIECs) with an effective activation energy of less than 250 meV. Across different material classes, diffusion energy barriers less than 250 meV are rare for lattice diffusion, yet frequently encountered in surface diffusion that usually requires a host framework with a pore diameter d > 2 Å. Well-known frameworks such as zeolites with internal pore diameters ranging from ∼3 Å to 10 Å and other nanoporous materials can take up large quantities of H2O (molecular diameter 2.8 Å), H2, and CO2 molecules. However, these large-pore open frameworks will not store too many alkali metal atoms due to their excessively large pore sizes, and are thus not suitable for energy-dense fast-charging lithium-ion batteries. Here we define “pre-zeolite” frameworks to mean crystals with percolating open pores with a diameter smaller than the size of H2O, and therefore excluding water adsorption and molecular adsorption in general, while allowing surface-diffusion like superfast transport of Li+. This work offers unique physical insights and a robust approach to developing super-MIEC anodes for high-rate batteries. It also bridges two general material families—nanoporous framework materials and conductive oxides. |
Introduction
Mixed ionic and electronic conductors (MIECs) are widely used in solid oxide fuel/electrolysis cells, batteries, electrochromic devices, neuromorphic computing, etc.1–5 Lithium-ion battery (LIB)'s cathode and anode must be MIECs, and need to store large quantities of Li0 on-demand in the lattice interior, accompanied by the redox of certain host elements, often transition metals (TMs). Rapid Li+ and e− transport (thus effective Li0 “atomic” diffusivity DLi) should be maintained at all depths of discharge (DODs), which is the most crucial factor for fast charging and high discharge power batteries used in heavy transportation (e.g., boats, trains, and trucks), industrial equipment (e.g., cranes), household products, and electrical-grid regulation.5–15 Drawing an analogy to superionic solid electrolytes, we define super-MIECs in the LIB context to mean having an effective activation energy Q of DLi inside the MIEC of less than 250 meV or about 10 kBT at T = 300 K, which would give DLi ∼ νLihLi2e−10 ∼ 5 × 10−13 m2 s−1, for a typical hopping trial frequency νLi = 1 THz and a hopping distance hLi = 1 Å. This means in t = 100 seconds (the typical duration it takes to fill up a gasoline car), or in a full charge/discharge cycle at 36C, the diffusion distance L = (2DLit)1/2 = 10 μm, which is the desirable battery electrode particle size for slurry coating. Note that a super-MIEC with a large DOD range would allow fully dense, single-crystal particles of 10 μm size to be used without requiring electrolyte infiltration into polycrystalline secondary particles, greatly increasing the volumetric energy density and reducing the side reactions. This is superior to nano Li4Ti5O12 (the most widely studied and commercialized anode for fast charging16) with low DLi. Some reported oxide anodes can be viewed as super-MIECs12,17–19 (especially for Nb-based materials with >1 μm size, Table S1, ESI†), which can support a full charge/discharge cycle within several minutes.The dual demands of large “Li adsorption” per volume and maintaining DLi ≥ 5 × 10−13 m2 s−1 put stringent requirements on transition-metal oxide MIECs. Across material classes, diffusion barriers generically less than 250 meV are frequently encountered only in surface diffusion. Diffusion in tight-fitting atomic channels for the Li+ cation (Shannon ionic diameters of 1.18 Å for 4-fold coordinated, 1.52 Å for 6-fold coordinated and 1.84 Å for 8-fold coordinated Li+), such as inside LiFePO4, typically gives Q values ranging from 270 meV to 500 meV.20 By tight-fitting, we mean that the diffusing species at some point strongly interact with the host on two or more sides (e.g., in the LiCoO2 lattice; stoichiometric LiCoO2 in a fully lithiated state has sluggish Li+ diffusivity), unlike surface diffusion where the mobile species mainly interact with the host on one side. If the host framework has a large enough pore diameter > 2 Å, then this allows for the Li+ to adsorb on the side wall of the pore, rather than be constrained at the center of a channel. Adsorption/uptake of external species is well-known in the realm of framework materials and molecular sieves. For example, zeolites have internal pore diameters ranging from ∼3 Å to 10 Å, which can take up large quantities of H2O (molecular diameter 2.8 Å), H2, and CO2 molecules.21 The word “zeolite” originated from its hygroscopicity, which literally meant “stones that give off water steam when heated”.22 The open aluminosilicate framework of zeolite A often gives entropic elasticity and a negative coefficient of thermal expansion (CTE) when dehydrated, which however turns positive in its fully hydrated state, when the pore is filled up.23 Well-known framework crystals also include Prussian blue analogues (PBAs) and metal–organic frameworks (MOFs). These open frameworks often have a negative CTE, tolerance for a wide variety of molecules inside the pores, and surface-diffusion-like rapid mass transport for molecules that can fit inside the pores, making them ideal gas storage media.24–26 Some PBAs and MOFs are even electronically conductive, making them super-MIECs.27–29 But these large-pore open frameworks, even though generally showing diffusivity >5 × 10−13 m2 s−1, will not store too many alkali metal atoms per volume due to the excessively large pore sizes, and thus are not optimal for high-volumetric-energy density fast-charging electrodes.
As H2O is the second smallest simple molecule (slightly larger than NH3), a basic question is if it is possible to exclude molecular adsorption, while still allowing alkali ions to have surface-like adsorption and diffusion on the sidewalls of the “internal pore surfaces” of the framework structures. In this work, we define a pre-zeolite framework to mean crystals with percolating open pores with a diameter smaller than 2.8 Å, and therefore exclude water adsorption and generally all molecular adsorptions, while allowing surface-diffusion-like transport of Li+. We are mainly interested in multi-valent TM-containing pre-zeolite frameworks, and will show that these frameworks, if electronically conductive, are generally all super-MIECs. We will demonstrate the structural and chemical design criteria for such pre-zeolite frameworks, in particular Wadsley–Roth structures containing multi-valent Nb, W, and Ti and having an anion-to-cation ratio (ACR) around 2.5 (mostly between 2.33 and 2.8). While some of the compositions have been shown before, the universality and robustness of these open-pore design rules provide a cornucopia of surface-diffusion-like high-capacity super-MIECs, that would allow 10 μm sized single crystals with >30C charging/discharging rates, rivaling fossil-fuel vehicles in the charging rate.
Results and discussion
Wadsley–Roth pore with 2.5 Å < d < 2.8 Å is a sufficient condition for ultrafast Li+ diffusion
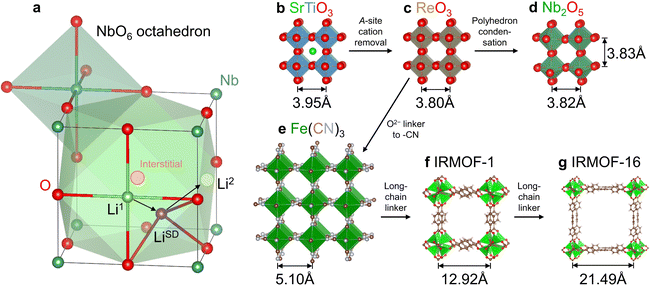
We selected a representative Wadsley–Roth oxide (WRO) H-Nb2O5, and conducted first-principles calculations to clarify the physical picture of surface-like diffusion. As shown in Fig. S1a, ESI,† there are 14 open pores within the a–c plane of an H-Nb2O5 unit cell containing 28 Nb and 70 O. To study the storage and migration mechanism of Li+ in the dilute lithiated state, we added one Li atom into a 1 × 3 × 1 supercell (containing 84 Nb and 210 O). Pore P6 (the same as P3 by symmetry) was picked randomly and Li+ was placed on the interstitial site in the center of a pore surrounded by 12 O (consisting of six square planes, including two within the a–c plane perpendicular to the b axis and four parallel to the b axis on the sidewalls of the cage; see the schematic for a cubic cage in Fig. 1 where lattice distortions were neglected for simplicity). In conventional transition-metal oxides, such high-symmetry interstitial sites are typically preferred sites for Li+ storage, for example, octahedral sites in layered LiCoO2 and lithiated Li4Ti5O12 (Li7Ti5O12) and tetrahedral sites in spinel LiMn2O4. However, this does not apply to H-Nb2O5, whose interstitial site (pore) is so large that a pore-centered Li+ would automatically relax to a sidewall and adopt a square planar geometry (such as sites Li1 and Li2 in Fig. 1(a)). We refer to such one-sided behavior as “surface adsorption” like. Such a storage mechanism has been reported in ReO3 experimentally using the diffraction technique30 and in Nb2TiO731 and Nb12WO3332 by atomistic simulations.We next used the climbing image nudged elastic band (NEB) method to calculate the migration path and energy barrier for Li+ hopping between two neighbouring square-planar sites from one on the sidewall to the one in the a–c plane. We found the former has an energy of 130 meV lower than the latter, with a forward migration barrier of 190 meV and a backward barrier of 60 meV (Fig. S1b, ESI†). Such low barriers are surface-diffusion like, which supports the super-MIEC behavior. Yet a more striking feature is the saddle-point configuration, where the coordination number of Li+ decreases from 4 in the square-planar ground-state (see the schematic Li+ migration pathway from sites Li1 to Li2 in Fig. 1(a)) to a remarkably low coordination of 3 (marked as LiSD), which is rare for lattice diffusion in crystals (The critical role of the ultra-low coordination number at the saddle point of Li+ hopping was not realized in previous studies.31,32). We recall that surface diffusion can take place via low-coordination-number adatoms which reside on adsorption sites on the surface plane, and it does not cost much elastic energy because these adatoms can veer into the vacuum half-space (with zero moduli) instead of the solid. The analogy is thus: the saddle-point Li+ veers into the pore with a large free volume like an adatom, and this minimizes elastic energy penalty, which typically applies to the crowded saddle-point configuration and slows down diffusion due to steric hindrance (for example, octahedral-to-tetrahedral-to-octahedral Li+ diffusion in LiCoO2). Therefore, one can justifiably call Li+ diffusion in Wadsley–Roth H-Nb2O5 surface-like diffusion, and these “internal surfaces” are distinguishable from physical surfaces as the pores are not large enough to allow even the smallest molecules like H2O to enter. The latter was proved by thermogravimetric analysis for wet WRO powders (Fig. S2, ESI†), where no weight loss occurred above 100 °C (surface water was mostly removed below 100 °C). Indeed, WROs have been reported as bulk intercalation pseudocapacitors, and the bulk Li+ diffusion kinetics share a similar dependence on the charge/discharge rate as the one observed for electric double-layer supercapacitors.33
From a crystallographic perspective, WROs are related to the ReO3 structure34 (Fig. 1(c), which differs from the parent perovskite structure ABO3, e.g., SrTiO3 in Fig. 1(b), by removing the A-site cation) by condensing some of the corner-sharing octahedra in ReO3 to edge-sharing ones on the boundaries of “blocks”.35 This results in better structural rigidity and d–d orbital coupling. (Compared to corner-sharing ones, edge-sharing octahedra give shorter metal–metal distance and suitable orbital orientations—dxy, dyz, and dxz point to edge centers—for d–d coupling). Meanwhile, there are still sufficient numbers of corner-sharing octahedra inside the “blocks”35 that form pores for Li+ surface-like diffusion. For cubic SrTiO3 and ReO3, one may estimate the pore size by the cation–cation distance (marked in Fig. 1(b)–(g)) divided by 21/2, thus being 2.79 Å for SrTiO3 and 2.69 Å for ReO3. A similar pore size is also noted in H-Nb2O5, for example, 2.70–2.71 Å for P6 (Fig. 1(d)), despite the lattice distortion and lower symmetry. Such pores allow for adatom-like Li+ storage and internal surface-like diffusion, and are even large enough for interstitial Na+ and K+ storage (but not Na+/K+ surface-like diffusion), as cubic NaNbO3 and KNbO3 have cage sizes of 2.84 Å and 2.87 Å (e.g., see atomic structures at materialsproject.org36), respectively. Therefore, the off-center Li+ storage and Li+ migration without steric hindrance are both verifiable, distinguishable features. Meanwhile, the framework oxygen can be replaced by other groups if large pore sizes are desired. The examples beyond LIB applications include replacing the O2− for corner-sharing octahedra by (CN)− in Fe(CN)3 (Fig. 1(e)) and other PBAs for Na+ battery cathodes37 and proton battery cathodes (H3O+ storage in the cage29), and by larger chain-molecules in mesoporous metal–organic frameworks such as IRMOF-1 (Fig. 1(f)) and IRMOF-16 (Fig. 1(g)), with varying pore sizes for gas storage and catalysis.34 A WRO is thus identified as a pre-zeolitic framework that does not have strong hygroscopy, but can take up a large amount of Li atoms electrochemically.
The calculations above rationalize the superfast Li+ transport in a model WRO. The insights into surface-like diffusion, as opposed to diffusion in tight-fitting channels of lithium intercalation oxides, should apply to all open pore structures with pre-zeolitic pore diameters 2.5 Å < d < 2.8 Å. We thus hypothesize that the Wadsley–Roth structure by itself, with a pore channel locally similar to ReO3, has already ensured facile bulk diffusion. Thus in real batteries, bulk diffusion in these compounds is likely not the bottleneck. The real challenge is in the boundary conditions, where side reactions and solid electrolyte interphases (SEIs) at the oxide surface build up impedance and degrade the battery during both early and prolonged cycles. As long as the 2.5 Å < d < 2.8 Å pores are maintained, we can tune the compositional space to optimize anode–electrolyte interactions to improve the cyclability. Finally, the large free volume gives rise to other structural and physical properties, such as anomalously low coefficient of thermal expansion (CTE) and formation of planar defects, which suggest soft phonon modes that could buffer strain and facilitate transport during electrochemical cycling. These shall be investigated in the following sections.
Rate parities: super-MIEC anodes with a large free volume and low CTE
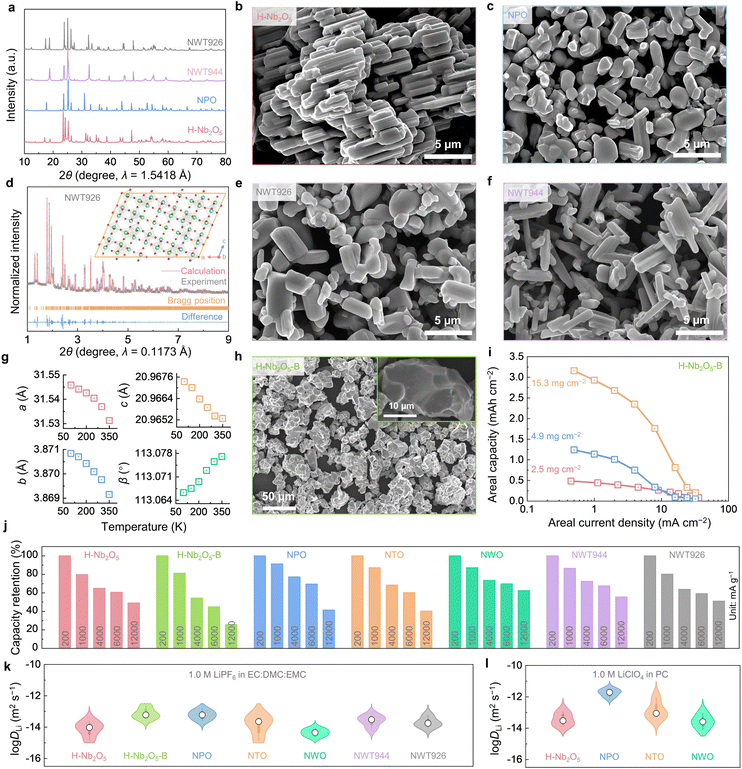
We have synthesized H-Nb2O5, Nb9PO25 (PNb9O25, NPO), a well-studied WRO Nb2TiO7 (TiNb2O7, NTO), a closely-related tungsten bronze structure oxide Nb18W16O93 (NWO, also with a large pore size), and two new ternary oxides Nb9W2Ti6O40.5 (NWT926) and Nb9W4Ti4O42.5 (NWT944) via solid-state synthesis.9,17,38,39 For all the samples, we heat-treated the powders at high temperatures (1100–1125 °C) to grow them into micron-sized single crystals. The powders obtained are phase-pure (shown by X-ray diffraction (XRD) in Fig. 2(a)) with crystal sizes shown by scanning electron microscopy (SEM) in Fig. 2(b), (c), (e) and (f). Using synchrotron powder XRD data (Fig. 2(d)), we analyzed the crystal structure of NWT926 and confirmed that it is a new phase with large pores and monoclinic symmetry (crystal structure in the inset of Fig. 2(d); data listed in Table S2, ESI†). To shed light on the free volume in the crystal lattice (critical for surface-like diffusion), we calculated the average atomic volume (defined as the supercell volume divided by the number of atoms in the supercell) of H-Nb2O5, NPO, NTO, NWO, and NWT926, which are in the range of 13.2–14.4 Å3. The average atomic volume has been shown to correlate well with the CTE, a phonon-controlled property, and could be abnormally low or even negative in framework materials like zeolites.40 The obtained values in our materials are close to the critical value that leads to a crossover from positive to a negative CTE (Fig. S3, ESI†). (Local average atomic volume at the pores of WROs can also be estimated from the geometry by neglecting lattice distortion and assuming a cubic ReO3-type local structure. For example, for P6 of H-Nb2O5 in Fig. 1(e), it is around 3.823/4 = 13.9 Å3.) We measured the CTEs of our powder samples using in situ XRD measurements (Fig. S4–S8, ESI†) conducted at 100–650 K, and the linear CTE α was obtained from the refined primary-cell volume V0(T). Compared to the CTE database for 260 compounds centered around ∼7 × 10−6 K−1 (Fig. S9 and Table S3, ESI†), H-Nb2O5, NPO, NTO, NWO, and NWT926 indeed all have negative or close-to-zero CTEs (Fig. S4–S8, ESI†). Of particular interest is NWT926 which has negative CTEs along all three lattice axes (Fig. 2(g)), which are rare and termed triaxial negative CTEs. The reason may be that the transverse vibrations of oxygen atoms in the M–O–M (M = Nb, Ti, W) with increasing temperature lead to the rotation of corner-sharing MO6 polyhedra, giving rise to the contraction.24,41,42 Such anomalously low CTEs, similar to other open frameworks such as zeolites, PBAs, and MOFs, indirectly support the notion of surface-like adsorption and diffusion in all WRO super-MIECs.We next measured the electrochemical performances of H-Nb2O5, NPO, NWT926, and NWT944 as LIB anodes in half cells and compared them with NTO and NWO references. By definition, super-MIEC materials should have high intrinsic electronic conductivity to assist electronic percolation. Therefore, we minimized the usage of conductive carbon in the composite electrode and tested all the anodes with >85 wt% active materials. At a low rate of 200 mA g−1 and a lower cutoff voltage of 1.0 V (vs. Li+/Li), H-Nb2O5, NPO, NTO, NWO, NWT926, and NWT944 have specific capacities of 192 mA h g−1, 210 mA h g−1, 236 mA h g−1, 180 mA h g−1, 187 mA h g−1, and 204 mA h g−1, respectively. They all have suitable average voltage (1.5–1.7 V vs. Li+/Li), high Coulombic efficiency (CE), and a stable charge–discharge profile upon cycling (Fig. S10–S12, ESI†). When tested at higher rates (for both charge and discharge) up to 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mA g−1 (roughly 200∼300C), we found all six materials have good capacity retention (for the capacity at 200 mA g−1, Fig. 2(j)). At 6000 mA g−1, the capacity retentions are >50%, offering 116 mA h g−1 capacity for H-Nb2O5, 146 mA h g−1 for NPO, 142 mA h g−1 for NTO, 125 mA h g−1 for NWO, 110 mA h g−1 for NWT926, and 138 mA h g−1 for NWT944. These correspond to ∼60C, which would satisfy many high-rate applications, shifting the rate-limiting consideration to the cathode or electrolyte in the full cells.
000 mA g−1 (roughly 200∼300C), we found all six materials have good capacity retention (for the capacity at 200 mA g−1, Fig. 2(j)). At 6000 mA g−1, the capacity retentions are >50%, offering 116 mA h g−1 capacity for H-Nb2O5, 146 mA h g−1 for NPO, 142 mA h g−1 for NTO, 125 mA h g−1 for NWO, 110 mA h g−1 for NWT926, and 138 mA h g−1 for NWT944. These correspond to ∼60C, which would satisfy many high-rate applications, shifting the rate-limiting consideration to the cathode or electrolyte in the full cells.
D Li is the composite of the Li+ ion and e− polaron diffusivities in ambipolar diffusion theory. First, the electronic conductivities of Nb2O5 and Li0.1Nb2O5 were calculated to be 1.0 × 10−6 S m−1, and 6.6 S m−1, respectively, by measuring the 2-probe electronic resistance, area, and thickness of the pellet samples. Therefore, slightly lithiated Nb2O5 can be regarded as a good electronic conductor. To enable facile kinetics at 60C for ∼1 μm single crystals, DLi needs to be ∼10−14 m2 s−1. To verify, galvanostatic intermittent titration technique (GITT) measurements (Fig. S13, ESI†) were conducted at different states of charge and at temperatures of 10–50 °C relevant for LIB operations. As shown by the violin plots in Fig. 2(k), DLi values from GITT measurements are within the range of 10−15–10−12 m2 s−1 for all the anodes. These values are comparable to the DLi values of 10−16–10−12 m2 s−1 in famous cathodes of LiCoO2 and LiNi0.33Co0.33Mn0.33O2 (Fig. S14, ESI,† similarly calculated from GITT measurements). The measurements above were from half cells using ethylene carbonate (EC)-based electrolytes. Interestingly, when we switched to propylene carbonate (PC)-based electrolytes, H-Nb2O5, NPO, NTO, and NWO showed 1–2 orders of magnitude higher DLi (Fig. 2(l) and Fig. S15, ESI†) than their respective values measured by the GITT in EC-based ones. It suggests that the measured diffusivities may not be the intrinsic ones in the crystal lattice but are likely to be constrained by SEIs formed during electrochemical tests. This inspires us to further modify the morphology and grow larger single crystals with less electrochemically active surfaces. We have made successful attempts to grow H-Nb2O5 in ∼20 μm-sized particles (abbreviated as H-Nb2O5-B; XRD in Fig. S16c, ESI;† SEM in Fig. 2(h) and a particle size analyzer in Fig. S16d, ESI† were used to obtain an agglomeration size of D50 = 49.0 μm) and again tested its electrochemical performance (216 mA h g−1 capacity at 200 mA g−1, Fig. S16b, ESI†). Remarkably, H-Nb2O5-B shows superior rate capability at different mass loadings (Fig. 2(i)), which is similar to H-Nb2O5 (Fig. 2(j)), despite ∼10 times larger grain size, and it can deliver an impressive capacity of 110 mA h g−1 at 6000 mA g−1 (∼60C). The DLi value of H-Nb2O5-B from GITT measurements is also >10 times larger than H-Nb2O5. Therefore, we conclude that WROs have high DLi in electrochemical cells and superior rate performance, which is relatively insensitive to oxide compositions but more sensitive to SEIs. The formation and growth of SEIs depend on the electro-chemo-mechanical interactions between active electrode materials and the electrolytes, which affect the rate capability and cycling stability of the anode and the full cell.
Cyclability disparities: towards the long cycle life of high-rate LIBs
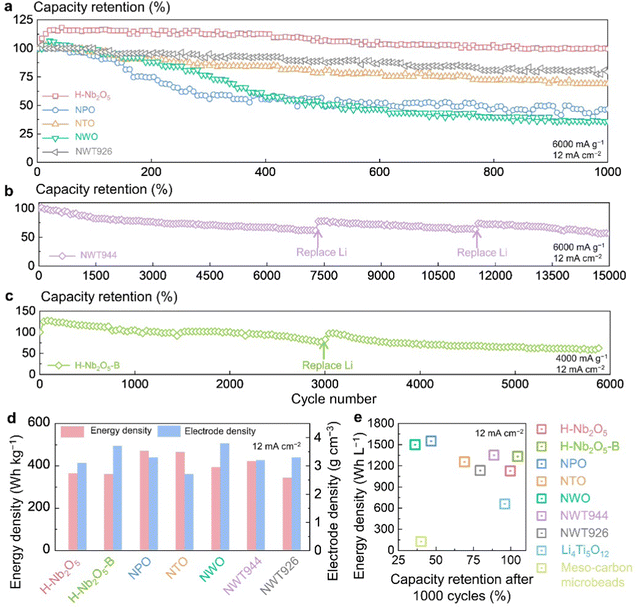
High-rate LIBs require an extended cycle life, and >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycle life has been demonstrated by the LiFePO4 cathode and Li4Ti5O12 anode. We therefore evaluated the cycling stability of the synthesized super-MIEC anodes in half cells (i.e., with excess electrolyte and Li reservoir). As shown in Fig. 3(a), when cycled at 6000 mA g−1 (12 mA cm−2, ∼60C), NTO, NPO, and NWO rapidly decay and have capacity retentions of 69%, 47%, and 36% after 1000 cycles, respectively. H-Nb2O5 and NWT926 have better cycling stability, with 100% and 80% capacity retentions after 1000 cycles. In comparison, NWT944 shows remarkably improved stability (Fig. 3(b)), with 62% capacity retention after 7000 cycles (5.4% decay per 1000 cycles). To exclude capacity decay drivers from the other battery components, we replaced the Li metal counter electrode and the electrolytes after 7300 and 11
000 cycle life has been demonstrated by the LiFePO4 cathode and Li4Ti5O12 anode. We therefore evaluated the cycling stability of the synthesized super-MIEC anodes in half cells (i.e., with excess electrolyte and Li reservoir). As shown in Fig. 3(a), when cycled at 6000 mA g−1 (12 mA cm−2, ∼60C), NTO, NPO, and NWO rapidly decay and have capacity retentions of 69%, 47%, and 36% after 1000 cycles, respectively. H-Nb2O5 and NWT926 have better cycling stability, with 100% and 80% capacity retentions after 1000 cycles. In comparison, NWT944 shows remarkably improved stability (Fig. 3(b)), with 62% capacity retention after 7000 cycles (5.4% decay per 1000 cycles). To exclude capacity decay drivers from the other battery components, we replaced the Li metal counter electrode and the electrolytes after 7300 and 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cycles (severe degradation of the Li metal electrode is shown in Fig. S17, ESI†). 56% capacity retention over 15
500 cycles (severe degradation of the Li metal electrode is shown in Fig. S17, ESI†). 56% capacity retention over 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles has thus been achieved in NWT944. On the other hand, by tailoring the morphology and increasing the particle size, we show in Fig. 3(c) that H-Nb2O5-B has 5% higher capacity after 1000 cycles than its initial capacity (attributed to the activation process occurring in electrochemical cycling43), and it also has capacity retentions of 77% after 3000 cycles, and 60% after 5800 cycles when cycled at 4000 mA g−1 (similar areal current density of 12 mA cm−2), representing improvements over H-Nb2O5. Therefore, our new composition (NWT944) and crystal size-coarsened H-Nb2O5-B both demonstrate a superior cycle life compared with previous WROs9,12,17–19,44–48 (Table S1, ESI†). With suitable coatings and electrolyte modifications, we believe extended cycle life can be realized in full cells in all WROs, as the lattice volume change is small. (Preliminary investigations have been conducted in coin-type full cells using the H-Nb2O5 anode paired with the LiNi0.5Mn1.5O4 or LiFePO4 cathode, and in pouch-type full cells using the H-Nb2O5 anode paired with the LiNi0.6Co0.2Mn0.2O2 cathode and the H-Nb2O5-B anode paired with the LiCoO2 cathode, as shown in Fig. S18, ESI†).
000 cycles has thus been achieved in NWT944. On the other hand, by tailoring the morphology and increasing the particle size, we show in Fig. 3(c) that H-Nb2O5-B has 5% higher capacity after 1000 cycles than its initial capacity (attributed to the activation process occurring in electrochemical cycling43), and it also has capacity retentions of 77% after 3000 cycles, and 60% after 5800 cycles when cycled at 4000 mA g−1 (similar areal current density of 12 mA cm−2), representing improvements over H-Nb2O5. Therefore, our new composition (NWT944) and crystal size-coarsened H-Nb2O5-B both demonstrate a superior cycle life compared with previous WROs9,12,17–19,44–48 (Table S1, ESI†). With suitable coatings and electrolyte modifications, we believe extended cycle life can be realized in full cells in all WROs, as the lattice volume change is small. (Preliminary investigations have been conducted in coin-type full cells using the H-Nb2O5 anode paired with the LiNi0.5Mn1.5O4 or LiFePO4 cathode, and in pouch-type full cells using the H-Nb2O5 anode paired with the LiNi0.6Co0.2Mn0.2O2 cathode and the H-Nb2O5-B anode paired with the LiCoO2 cathode, as shown in Fig. S18, ESI†).
Super-MIEC anodes compete with Li4Ti5O12 in high-rate applications. We compared the gravimetric energy density and electrode density of super-MIEC anodes in Fig. 3(d), which gives volumetric energy density in the range of 1128∼1550 W h L−1 (at 6000 mA g−1, Fig. 3(e)) with the rank of NPO > NWO > NWT944 > H-Nb2O5-B > NTO > NWT926 > H-Nb2O5. (More detailed comparisons on characterized particle size, electrode density, initial Coulombic efficiency, capacity, rate retention, average voltage, energy density, and cyclability are listed in Tables S4 and S5, ESI†.) These values are much higher than 658 W h L−1 for Li4Ti5O12 and 127 W h L−1 for meso-carbon microbeads, which are the commercially prevailing high-rate anodes. Through trial and error, it appears that Nb is the baseline element to form the Wadsley–Roth oxide structure, W is beneficial for increasing the crystal density and energy density, and Ti is beneficial for enhancing the structural stability. We note that in many applications, the cycle life is an important metric, which sets NWT944 and H-Nb2O5-B to be the best candidates among the super-MIEC anodes investigated.
Other lattice-structural and microstructural features
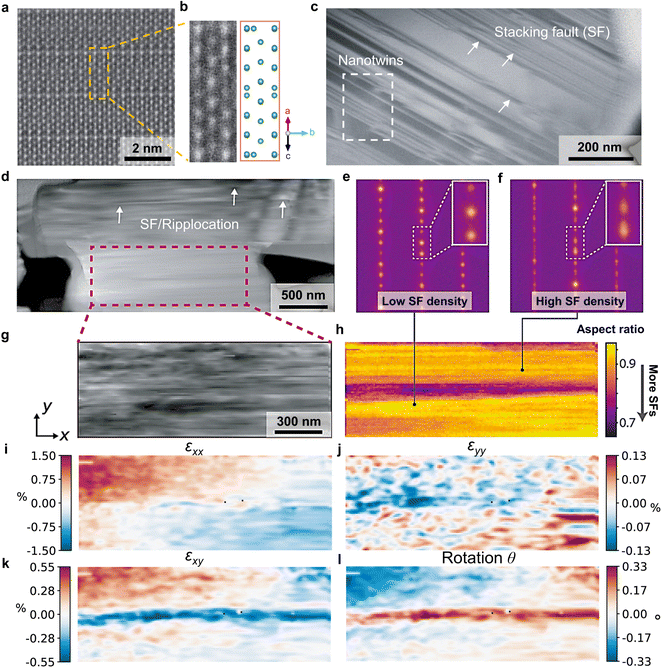
As an anode must host and disgorge a great amount of excess lithium reversibly, a robust atomic structure is required. As shown by the top surfaces and cross-sections of H-Nb2O5, NPO, and NWT944 electrodes (Fig. S19, ESI†), the single-crystal WRO particles did not fracture after cycling. This means the particles could survive the mechanical stresses and stress-corrosion cracking during cyclic electrochemical loading, which would benefit the long-term cycling stability. Indeed, the scanning transmission electron microscopy – high-angle annular dark-field (STEM-HAADF) image in Fig. 4(a) shows well-ordered crystal lattices without point defects (corresponding atomic structure for the Nb sublattice in Fig. 4(b)).However, over a larger length scale of a few hundred nanometers, extended defects including stacking faults, nano-twins, and ripplocations49 were found in the WRO single crystals (Fig. 4(c), (d) and Fig. S20, ESI†). Different levels of diffuse scattering exist in the nanobeam electron diffraction patterns (Fig. 4(e) and (f)) at different locations of Fig. 4(d), and mapping of stacking fault density in Fig. 4(g) and (h) further indicates spatial variations. As these planar defects are formed in pristine H-Nb2O5 synthesized from high-temperature heat treatment, the observations indicate their relatively low formation energies. It is in contrast with the high formation energy of point defects, but consistent with the fact that the large free volume and low CTE in WROs are a result of low polyhedral packing density and their collective twisting/relaxation. In addition, strain mapping by four-dimensional scanning transmission electron microscopy (4D-STEM) at 10 nm spatial resolution (Fig. 4(g)) visualizes the lattice at the mesoscale, with a standard deviation of 1.53% for εxx, 1.44% for εyy, 0.20% for εxy, and 0.12% for the rotation angle θ (Fig. 4(i)–(l)).
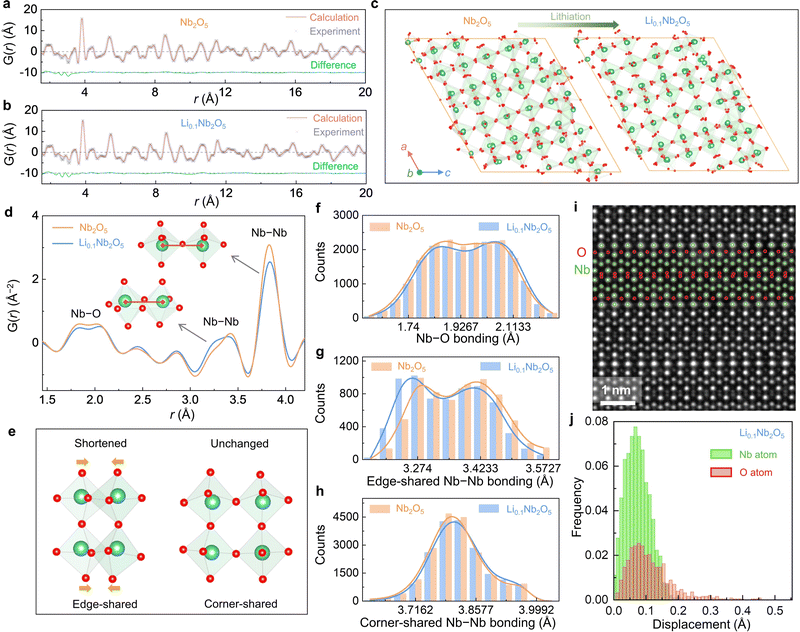
Pair distribution function (PDF) analysis was conducted on unlithiated (Nb2O5 in Fig. 5(a)) and slightly lithiated (Li0.1Nb2O5 in Fig. 5(b)) H-Nb2O5 powders using synchrotron X-ray total scattering. Experimentally, the raw total scattering data were collected and then transformed into the real-space PDF G(r).50,51 For Nb2O5, we noted the measured G(r) significantly deviates from the calculated one from the “perfect” H-Nb2O5 structure (Fig. 1(a)), especially at large r up to 20 Å. We thus conducted reverse Monte Carlo (RMC) simulations to fit the experimental data (calculated G(r) shown in Fig. 5(a), (b)) and to analyze the structure. As shown by simulated atomic structures (Fig. 5(c)) after converged RMC simulations, there are distortions at both Nb and O sublattices, even though O displacements (average value 0.06 Å for Nb2O5 and 0.05 Å for Li0.1Nb2O5) are larger than Nb displacements (average value 0.01 Å for Nb2O5 and 0.01 Å for Li0.1Nb2O5, Fig. S21, ESI†). To compare the structure before and after lithiation, we focused on G(r) data at 1.5∼4.0 Å (Fig. 5(d)), especially the ∼1.9 Å double peaks for nearest Nb–O bonds, the ∼3.3 Å double peaks for nearest Nb–Nb bonds for edge-sharing NbO6 octahedra, and the ∼3.8 Å peak for Nb–Nb bonds for corner-sharing NbO6 octahedra. When lithiating from Nb2O5 to Li0.1Nb2O5, we found minimum changes in ∼1.9 Å Nb–O bonds (Fig. 5(f)) and ∼3.8 Å Nb–Nb bonds (Fig. 5(h)) but shortened Nb–Nb bonds at ∼3.3 Å (Fig. 5(g)). It indicates the pore structure is relatively robust and does not involve much structural change upon lithiation. To confirm, we conducted STEM-HAADF on Li0.1Nb2O5, which provides contrasts for light-element O. As shown in Fig. 5(i), the lattice is again well ordered, yet slight distortions on the O sublattice are notable. Quantitative analysis of the atomic positions (Fig. 5(j)) shows 0.004 Å (with a standard deviation of 0.008 Å) displacements in the Nb sublattice and 0.02 Å (with a standard deviation of 0.02 Å) displacements in the O sublattice, and some O atoms are displaced further up to ∼0.45 Å. These results agree with the diffraction and RMC data.
Lastly, we used in situ XRD to measure the CTEs of lithiated H-Nb2O5 at 100–200 K (Fig. S22 and S23, ESI†). Interestingly, negative CTEs of −8.80 × 10−6 K−1 and −4.21 × 10−6 K−1 were obtained for Li0.2Nb2O5 and Li1.6Nb2O5, respectively, which are lower than that of −0.53 × 10−6 K−1 for non-lithiated H-Nb2O5. This again suggests that the large free volume holds even upon electrochemical lithiation.
Conclusions and outlook
We have explained why the WROs have fast Li+ diffusion ability and abnormally low CTEs. The low topological constraints per atom and large free volume in the crystal lattice should be the fundamental cause of soft phonons, low CTE, low-coordination-number Li+ storage, and surface-like diffusion, similar to other open frameworks such as zeolites, PBAs, and MOFs. But unlike those frameworks that take up molecules, the WRO frameworks with 2.5 Å < d < 2.8 Å can only take up atoms like Li. The phonon anomalies are expected to influence the pre-exponential factor of the Arrhenius-type diffusivity. There is literature on DLi measured by pulsed-field-gradient nuclear magnetic resonance (PFG NMR) spectroscopy, which is a bulk measurement and not sensitive to SEIs. In Fig. S2a, ESI† of ref. 9, in LixNbyWzO(5y+6z)/2, we note that larger activation energy gives higher DLi values, because a large pre-exponential term not only compensates but also dominates (Table S6, ESI†), signifying perhaps collective Li motion in pores.Another question is why Nb (group-5, period-5) is essential in forming WRO super-MIEC anodes. Turning back to the parent structure ReO3, partial condensation of the corner-sharing octahedra to edge-sharing ones is necessary to enhance the structural rigidity (to suppress extensive phase transitions during electrochemical cycling; unalloyed ReO3 has multiple phase transitions upon lithiation, which leads to slow kinetics, voltage hysteresis, and poor cycling52) and d–d coupling for better electron transport. Therefore, an ACR around 2.5 is expected, which requires a +5 average cation valence, and thus group-5 elements (Zr/Hf likes to be +4, Mo/W likes to be +6). Meanwhile, as the octahedron majority should be maintained, the fact—that V5+ is so small that it prefers to be coordinated by four neighboring O2− as is the case for various V2O5 polymorphs; while Ta5+ is so large that it prefers mixed TaO6/TaO7 occupancy as is the case for L- and H-Ta2O5—sets group-5 and period-5 Nb5+ to be the best candidate for the major cation. Other elements (Ti, W) can be alloyed into the lattice, to tune the bulk redox and surface stability.
Finally, we provide some guidance on the search for other candidates in the multi-element compositional space. As Roth and Wadsley categorized, the “block” structured oxides MxOy can be represented by a series of chemical formulas with one single integer variable n, including M3nO8n−3 for Group A, M3n+1O8n−2 (n odd) for Group B, M3n+1O8n−2 (n even) for Group C, M3n+1O8n+1 for Group D, M4n+1O11n for Group E, and M5n+1O14n−1 for Group F.35,53 Taking the limiting cases as n → ∞ and known small n compositions (e.g., n = 3 for Nb2TiO7 in Group A, n = 7 for Nb22O54 in Group B, n = 8 for Nb24TiO62 in Group C, n = 3 for Nb9TPO25 in Group D, n = 3 for Nb12WO33 in Group E, and n = 4 for Nb16W5O55 in Group F), we obtained the O/M ratio in the range of 2.33–2.8. Applying such boundaries to the NbO2.5–WO3–TiO2 ternary phase diagram offers the potential compositional ranges to synthesize new WROs (Fig. S24, ESI†) to optimize bulk redox and SEIs. Indeed, all the known materials within this category fall into the colored compositional space, including our newly synthesized NWT944 and NWT926. The O/M = 2.5 tie-line is of particular interest, which is achieved by alloying WO3 and TiO2 with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio into the NbO2.5 matrix. This, together with the coarsened crystal size and suitable coatings, enables the prolonged cycle lives of super-MIEC anodes in high-rate LIBs.
1 molar ratio into the NbO2.5 matrix. This, together with the coarsened crystal size and suitable coatings, enables the prolonged cycle lives of super-MIEC anodes in high-rate LIBs.
Author contributions
S. W., L. Z., Y. D., J. Z., Z. T., and J. L. conceived the project. L. Z. synthesized the materials and conducted electrochemical testing. H. Z. and H. X. contributed to the PDF analysis and RMC simulation. H. W. and Y. D. conducted the bond-valence sum simulations and first-principles calculations. Y. Yang, B. W., Y. S., and A. M. contributed to the FIB sample preparation, spherical aberration TEM and atomic resolution STEM-HAADF imaging, and 4D-STEM characterization and stacking fault mapping analysis. Y. Yuan and Q. H. contributed to the displacement analysis of the HAADF image. S. W. and Y. H. analyzed the GITT data. H. Z., Q. L., Y. R., C. S., and Q. L. contributed to the synchrotron measurement for structural analysis and CTE analysis. R. F. contributed to Li nuclear magnetic resonance measurements confirming ultrafast Li+ diffusion. X. H., Y. C., X. X., and W. L. contributed to the X-ray scanning nano-diffraction measurements. W. Q., Y. L., Z. X., and Z. Z. contributed to the pouch cells assembling and measurement. S. W., Y. D., and J. L. wrote the paper. All authors discussed and contributed to the writing.Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
J. Z. acknowledges the support from the State Key Laboratory of New Ceramic and Fine Processing, Tsinghua University (No. KF201801). Z. T. acknowledges support from the National Natural Science Foundation of China (No. 51772163, 52172210). J. L. acknowledges the support from Honda Research Institute USA, Inc. Y. Y. was supported by the Director, Office of Science, Office of Basic Energy Sciences, Materials Sciences and Engineering Division, the U.S. Department of Energy under contract no. DE-AC02-05-CH11231 within the Mechanical Behavior of Materials (KC 13) program at the Lawrence Berkeley National Laboratory. A. M. was supported by the Toyota Research Institute. The authors acknowledge support from the Molecular Foundry at Lawrence Berkeley National Laboratory, which is supported by the U.S. Department of Energy under contract no. DE-AC02-05-CH11231. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. Q. L. acknowledges the Shenzhen Science and Technology Innovation Commission under grant SGDX2019081623240948. X. H acknowledges the support from the U.S. Department of Energy under contract no. DE-SC0012704. R. F. acknowledges the support from the NSF Cooperative Agreement DMP-1644779 and the State of Florida.References
- Y. Chen, Z. Wang, X. Li, X. Yao, C. Wang, Y. Li, W. Xue, D. Yu, S. Y. Kim, F. Yang, A. Kushima, G. Zhang, H. Huang, N. Wu, Y. W. Mai, J. B. Goodenough and J. Li, Nature, 2020, 578, 251–255 CrossRef CAS PubMed.
- A. Sood, A. D. Poletayev, D. A. Cogswell, P. M. Csernica, J. T. Mefford, D. Fraggedakis, M. F. Toney, A. M. Lindenberg, M. Z. Bazant and W. C. Chueh, Nat. Rev. Mater., 2021, 6, 847–867 CrossRef CAS.
- P. C. Harikesh, A. Surendran, B. Ghosh, R. A. John, A. Moorthy, N. Yantara, T. Salim, K. Thirumal, W. L. Leong, S. Mhaisalkar and N. Mathews, Adv. Mater., 2020, 32, 1906976 CrossRef CAS PubMed.
- I. Riess, Solid State Ionics, 2003, 157, 1–17 CrossRef CAS.
- H. Sun, L. Mei, J. Liang, Z. Zhao, C. Lee, H. Fei, M. Ding, J. Lau, M. Li, C. Wang, X. Xu, G. Hao, B. Papandrea, I. Shakir, B. Dunn, Y. Huang and X. Duan, Science, 2017, 356, 599–604 CAS.
- Y. Liu, Y. Zhu and Y. Cui, Nat. Energy, 2019, 4, 540–550 CrossRef.
- H. Liu, Z. Zhu, Q. Yan, S. Yu, X. He, Y. Chen, R. Zhang, L. Ma, T. Liu, M. Li, R. Lin, Y. Chen, Y. Li, X. Xing, Y. Choi, L. Gao, H. S.-Y. Cho, K. An, J. Feng, R. Kostecki, K. Amine, T. Wu, J. Lu, H. L. Xin, S. P. Ong and P. Liu, Nature, 2020, 585, 63–67 CrossRef CAS PubMed.
- W. Zhang, D. H. Seo, T. Chen, L. Wu, M. Topsakal, Y. Zhu, D. Lu, G. Ceder and F. Wang, Science, 2020, 367, 1030–1034 CrossRef CAS PubMed.
- K. J. Griffith, K. M. Wiaderek, G. Cibin, L. E. Marbella and C. P. Grey, Nature, 2018, 559, 556–563 CrossRef CAS PubMed.
- Y. Li, S. Wang, Y. Dong, Y. Yang, Z. Zhang and Z. Tang, Adv. Energy Mater., 2019, 10, 1903411 CrossRef.
- S. T. Wang, Y. H. Dong, F. J. Cao, Y. T. Li, Z. T. Zhang and Z. L. Tang, Adv. Funct. Mater., 2020, 30, 2001026 CrossRef CAS.
- B. Babu, P. Simon and A. Balducci, Adv. Energy Mater., 2020, 10, 2001128 CrossRef CAS.
- Q. Ai, Q. Fang, J. Liang, X. Xu, T. Zhai, G. Gao, H. Guo, G. Han, L. Ci and J. Lou, Nano Energy, 2020, 72, 104657 CrossRef CAS.
- S. Wang, W. Quan, Z. Zhu, Y. Yang, Q. Liu, Y. Ren, X. Zhang, R. Xu, Y. Hong, Z. Zhang, K. Amine, Z. Tang, J. Lu and J. Li, Nat. Commun., 2017, 8, 627 CrossRef PubMed.
- C. Choi, D. S. Ashby, D. M. Butts, R. H. DeBlock, Q. Wei, J. Lau and B. Dunn, Nat. Rev. Mater., 2019, 5, 5–19 CrossRef.
- T. Yuan, Z. Tan, C. Ma, J. Yang, Z.-F. Ma and S. Zheng, Adv. Energy Mater., 2017, 7, 1601625 CrossRef.
- Y. Yang and J. Zhao, Adv. Sci., 2021, 8, 2004855 CrossRef CAS PubMed.
- Q. Deng, Y. Fu, C. Zhu and Y. Yu, Small, 2019, 15, 1804884 CrossRef PubMed.
- K. J. Griffith, Y. Harada, S. Egusa, R. M. Ribas, R. S. Monteiro, R. B. Von Dreele, A. K. Cheetham, R. J. Cava, C. P. Grey and J. B. Goodenough, Chem. Mater., 2020, 33, 4–18 CrossRef.
- Z. Liu and X. Huang, Solid State Ionics, 2010, 181, 907–913 CrossRef CAS.
- N. Rangnekar, N. Mittal, B. Elyassi, J. Caro and M. Tsapatsis, Chem. Soc. Rev., 2015, 44, 7128–7154 RSC.
- C. J. Jacobsen, C. Madsen, J. Houzvicka, I. Schmidt and A. Carlsson, J. Am. Chem. Soc., 2000, 122, 7116–7117 CrossRef CAS.
- T. Carey, C. C. Tang, J. A. Hriljac and P. A. Anderson, Chem. Mater., 2014, 26, 1561–1566 CrossRef CAS.
- Q. Gao, J. Chen, Q. Sun, D. Chang, Q. Huang, H. Wu, A. Sanson, R. Milazzo, H. Zhu, Q. Li, Z. Liu, J. Deng and X. Xing, Angew. Chem., Int. Ed., 2017, 56, 9023–9028 CrossRef CAS PubMed.
- Z. Chang, D. H. Yang, J. Xu, T. L. Hu and X. H. Bu, Adv. Mater., 2015, 27, 5432–5441 CrossRef CAS PubMed.
- J. Li, P. M. Bhatt, J. Li, M. Eddaoudi and Y. Liu, Adv. Mater., 2020, 32, 2002563 CrossRef CAS PubMed.
- L. S. Xie, G. Skorupskii and M. Dinca, Chem. Rev., 2020, 120, 8536–8580 CrossRef CAS PubMed.
- K. Hurlbutt, S. Wheeler, I. Capone and M. Pasta, Joule, 2018, 2, 1950–1960 CrossRef CAS.
- X. Y. Wu, J. J. Hong, W. Shin, L. Ma, T. C. Liu, X. X. Bi, Y. F. Yuan, Y. T. Qi, T. W. Surta, W. X. Huang, J. Neuefeind, T. P. Wu, P. A. Greaney, J. Lu and X. L. Ji, Nat. Energy, 2019, 4, 123–130 CrossRef CAS.
- R. J. Cava, A. Santoro, D. W. Murphy, S. M. Zahurak and R. S. Roth, J. Solid State Chem., 1983, 50, 121–128 CrossRef CAS.
- K. J. Griffith, I. D. Seymour, M. A. Hope, M. M. Butala, L. K. Lamontagne, M. B. Preefer, C. P. Kocer, G. Henkelman, A. J. Morris, M. J. Cliffe, S. E. Dutton and C. P. Grey, J. Am. Chem. Soc., 2019, 141, 16706–16725 CrossRef CAS PubMed.
- C. P. Kocer, K. J. Griffith, C. P. Grey and A. J. Morris, Chem. Mater., 2020, 32, 3980–3989 CrossRef CAS PubMed.
- Y. Liu, S. P. Jiang and Z. Shao, Mater. Today Adv., 2020, 7, 100072 CrossRef.
- H. A. Evans, Y. Wu, R. Seshadri and A. K. Cheetham, Nat. Rev. Mater., 2020, 5, 196–213 CrossRef CAS.
- R. Roth and A. Wadsley, Acta Crystallogr., 1965, 19, 42–47 CrossRef CAS.
- S. P. Ong, A. Jain, G. Hautier, B. Kang and G. Ceder, Electrochem. Commun., 2010, 12, 427–430 CrossRef CAS.
- Q. N. Liu, Z. Hu, M. Z. Chen, C. Zou, H. L. Jin, S. Wang, S. L. Chou, Y. Liu and S. X. Dou, Adv. Funct. Mater., 2020, 30, 1909530 CrossRef CAS.
- L. Zhao, S. Wang, Y. Dong, W. Quan, F. Han, Y. Huang, Y. Li, X. Liu, M. Li, Z. Zhang, J. Zhang, Z. Tang and J. Li, Energy Storage Mater., 2021, 34, 574–581 CrossRef.
- T. Li, G. Nam, K. Liu, J.-H. Wang, B. Zhao, Y. Ding, L. Soule, M. Avdeev, Z. Luo, W. Zhang, T. Yuan, P. Jing, M. G. Kim, Y. Song and M. Liu, Energy Environ. Sci., 2022, 15, 254–264 RSC.
- Q. Gao, J. Wang, A. Sanson, Q. Sun, E. Liang, X. Xing and J. Chen, J. Am. Chem. Soc., 2020, 142, 6935–6939 CrossRef CAS PubMed.
- Z. Liu, Q. Li, H. Zhu, K. Lin, J. Deng, J. Chen and X. Xing, Chem. Commun., 2018, 54, 5712–5715 RSC.
- Q. Gao, N. Shi, Q. Sun, A. Sanson, R. Milazzo, A. Carnera, H. Zhu, S. H. Lapidus, Y. Ren, Q. Huang, J. Chen and X. Xing, Inorg. Chem., 2018, 57, 10918–10924 CrossRef CAS PubMed.
- Z. Song, H. Li, W. Liu, H. Zhang, J. Yan, Y. Tang, J. Huang, H. Zhang and X. Li, Adv. Mater., 2020, 32, 2001001 CrossRef CAS PubMed.
- F. Shen, Z. Sun, Q. He, J. Sun, R. B. Kaner and Y. Shao, Mater. Horiz., 2021, 8, 1130–1152 RSC.
- X. Han, Q. Meng, X. Wan, B. Y. Sun, Y. Zhang, B. C. Shen, J. L. Gao, Y. L. Ma, P. J. Zuo, S. F. Lou and G. P. Yin, Nano Energy, 2021, 81, 105635 CrossRef CAS.
- T. F. Yi, H. M. K. Sari, X. Z. Li, F. F. Wang, Y. R. Zhu, J. H. Hu, J. J. Zhang and X. F. Li, Nano Energy, 2021, 85, 105955 CrossRef CAS.
- J.-T. Han and J. B. Goodenough, Chem. Mater., 2011, 23, 3404–3407 CrossRef CAS.
- Y. Yang, H. Zhu, J. Xiao, H. Geng, Y. Zhang, J. Zhao, G. Li, X. L. Wang, C. C. Li and Q. Liu, Adv. Mater., 2020, 32, 1905295 CrossRef CAS PubMed.
- A. Kushima, X. Qian, P. Zhao, S. Zhang and J. Li, Nano Lett., 2015, 15, 1302–1308 CrossRef CAS PubMed.
- H. Zhu, Y. Huang, J. Ren, B. Zhang, Y. Ke, A. K. Jen, Q. Zhang, X. L. Wang and Q. Liu, Adv. Sci., 2021, 8, 2003534 CrossRef CAS PubMed.
- Q. Li, H. Zhu, L. Zheng, L. Fan, N. Wang, Y. Rong, Y. Ren, J. Chen, J. Deng and X. Xing, Nano Lett., 2017, 17, 7892–7896 CrossRef CAS PubMed.
- N. H. Bashian, S. Zhou, M. Zuba, A. M. Ganose, J. W. Stiles, A. Ee, D. S. Ashby, D. O. Scanlon, L. F. J. Piper, B. Dunn and B. C. Melot, ACS Energy Lett., 2018, 3, 2513–2519 CrossRef CAS.
- A. Wadsley, S. Andersson, J. Dunitz and J. Ibers, Perspectives in Structural Chemistry, Wiley, New York, 1970, vol. 3, pp. 1–58 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental and computational details, supplementary figures, supplementary tables, and supplementary references. See DOI: https://doi.org/10.1039/d2ee02918a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |