Changes in optical properties and molecular composition of dissolved organic matter and formation of disinfection by-products during conventional water treatment processes†
Vitharuch
Yuthawong
 *a,
Chatyapha
Thongnueaha
a and
Phanwatt
Phungsai
*a,
Chatyapha
Thongnueaha
a and
Phanwatt
Phungsai
 bc
bc
aDepartment of Environmental Engineering, Faculty of Engineering, Kasetsart University, Bangkok 10900, Thailand. E-mail: vitharuch.y@ku.th; Tel: +660 2797 0999 ex 1009
bDepartment of Environmental Engineering, Faculty of Engineering and Khon Kaen University, Khon Kaen 40002, Thailand
cResearch Center for Environmental and Hazardous Substance Management, Khon Kaen University, Khon Kaen 40002, Thailand
First published on 15th November 2022
Abstract
Dissolved organic matter (DOM) is present ubiquitously in natural water environments. It is inevitable that DOM enters water treatment plants (WTPs) via raw water. When in a WTP, DOM introduces some optional issues and reacts with disinfectants forming harmful disinfection by-products (DBPs). Due to the complexity of DOM, specific portions of DOM are removed and/or transformed differently in treatment processes. In this study, DOM in raw waters and processed waters of two main WTPs, in Bangkok, Thailand, was investigated, namely, Bangkhen Water Treatment Plant (BK-WTP) and Maha Sawat Water Treatment Plant (MH-WTP). Treatment processes of both WTPs consisted of coagulation/flocculation followed by sedimentation, sand filtration, and disinfection. Changes in organic concentration, fluorometric properties, and molecular composition of DOM were illustrated. Our results showed that the treatment processes of both WTPs removed different portions of DOM, although the WTPs possessed identical treatment processes. Dissolved organic carbon (DOC) concentrations of BK-WTP decreased drastically from 6.3 mg C per L in raw water to 3.5 mg C per L after sedimentation and remained relatively stable throughout the treatment chain. In MH-WTP, DOC concentrations gradually decreased from 1.9 mg C per L in raw water to 1.2 mg C per L after disinfection. Raw water of BK-WTP showed exclusively terrestrial DOM features, while that of MH-WTP showed terrestrial DOM features with an addition of microbial DOM features. Sedimentation and filtration of BK-WTP removed those terrestrial DOM features characterized by humic acid- and fulvic acid-like FDOM and unsaturated molecular DOM features. The processes of MH-WTP indicated otherwise, wherein microbial FDOM features were selectively removed by sedimentation and filtration. As a result, FDOM and DOM components left to the disinfection process were different between the WTPs resulting in different DBP formations. Comparing before and after disinfection samples, we could extract a list of unknown DBPs and the fate of their putative precursors in the prior samples showing their treatability by the treatment processes.
Water impactDissolved organic matter (DOM) causes operational issues in water treatment processes. The fate of DOM and potential precursors of disinfection by-products were revealed via fluorometric and high-resolution mass spectrometric analyses. A deeper understanding of DOM during water treatment processes is useful for selection and modification treatment processes to cope with challenging water quality. |
1. Introduction
In Bangkok Metropolitan Area (BMA), drinking water is supplied by Metropolitan Waterworks Authority (MWA). MWA operates four water treatment plants (WTPs) including Bangkhen WTP, Samsen WTP, Thonburi WTP and Maha Sawat WTP. These WTPs source raw water from Chao Phraya River (Bangkhen, Samsen and Thonburi WTP) and Mae Klong River,1 two major rivers in the central region of Thailand. The rivers span over a long distance from the upper region of the central part of the country down to the intakes of the WTPs in the lower region and receive discharges from numerous activities along the rivers resulting in detrimental water quality. Reports showed the deteriorated water quality of the downstream portion of the rivers.1,2 As a result, downstream water is expected to contain various contaminants including naturally and anthropogenically occurring organic matter. It is inevitable that those organic compounds could enter the WTPs as parts of deteriorated raw water.Naturally occurring dissolved organic matter (DOM) is a complex mixture of known and unknown compounds. It originates from leaching of decomposed organisms on land, microbial activity in waterbodies,3 and anthropogenic activities.4 The presence of DOM in raw water of a WTP introduces operational problems and causes deteriorated water quality in product water. When in water treatment processes, DOM increases the chemical demand, clogs filters and membranes and reacts with disinfectants producing disinfection by-products (DBPs). Several studies pointed out the prominent role of DOM in chlorine-based disinfections.5 Various well-known DBPs such as trihalomethanes, haloacetic acids and haloacetronitriles have been widely regulated in various countries to control their concentration in tap water as it could promote adverse health effects.6 However, less than half of DBPs have been identified7 and their health impacts are currently unknown. Hence, controlling the formation of DBPs is an important challenge in water treatment processes.
To understand insight into changes and removal of DOM in water treatment, DOM characterization is a very important practice and has been done by several techniques.8 Characterization of DOM can be done in quantitative and qualitative ways. Organic carbon concentration is used to indicate the amount of DOM in water samples while the characteristics of DOM are omitted. Characterization of DOM can be done through several aspects of DOM including biological lability, optical properties, polarity, molecular weight and molecular structure.9 Simple spectrometric techniques can be used to demonstrate the aromaticity index, functional groups, hydrophobicity. Advanced fluorometric techniques classify DOM into groups of fluorescent components of DOM. However, these analytical techniques reveal parts of DOM while overlook another, e.g., non-light absorbing molecules, or non-fluorescent molecules. Recent research of DOM in water environments and water and wastewater treatment has been done using high resolution mass spectrometry (HRMS). Fourier Transform Ion Cyclotron Mass Spectrometry (FT ICR MS) and Orbitrap Mass Spectrometry (Orbitrap MS) are often used to analyze the molecular composition of DOM.10–17 Coupling with soft ionization sources, HRMS allows us to determine accurate masses of intact DOM molecules. The accurate mass data obtained from HRMS can be assigned to unambiguous molecular formulas which can be used to determine the molecular characteristics of DOM. Types of DOM in water environments including riverine DOM,16–18 lacustrine DOM,11,19 effluent DOM,20 and processed water DOM15,21 were extensively studied using HRMS proving the effectiveness of HRMS to study DOM. The molecular characteristics of DOM have been used to provide insight into molecular changes and mechanisms in several treatments15,22,23 including chlorination. In addition, this method can also assign or identify unknown DBPs and track its putative precursors in WTPs of various countries.21,24
In this study, we investigated changes in DOM fluorescence properties and molecular compositions in water treatment processes. Water samples from two WTPs in Bangkok, Thailand were collected, namely, Bangkhen WTP (BK-WTP) and Maha Sawat WTP (MH-WTP). Raw waters of the WTPs were taken from Chao Phraya River and Mae Klong River for BK-WTP and MH-WTP, respectively. The water quality of Mae Klong River was different with that of Chao Phraya River which was expected to have worse water quality.1,2 The samples included raw water, after sedimentation, after filtration, and after disinfection. The fluorometric properties of DOM in the samples were analyzed via a spectrofluorometer. Solid phase extracted DOM was analyzed via electrospray ionization Orbitrap Mass Spectrometry (ESI Orbitrap MS) to demonstrate the molecular composition.
2. Materials and methods
2.1 Water samplings
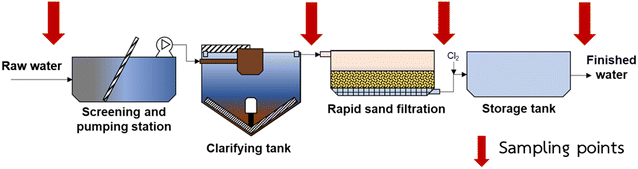
One-time samplings were conducted in June 2021 from two WTPs in Bangkok, Thailand, namely Bangkhen WTP (BK-WTP) and Maha Sawat WTP (MH-WTP). The WTPs are operated by MWA and intake raw water from Chao Phraya River and Mae Klong River for BK-WTP and MH-WTP, respectively. Raw water from the rivers is conveyed through the water canal (Klong Prapa) to the WTPs. BK-WTP and MH-WTP have over 4 Mm3 per day production capacity (3.6 Mm3 per day and 1.6 Mm3 per day for BK-WTP and MH-WTP, respectively). Treatment processes of both WTPs consist of coagulation/flocculation followed by sedimentation, rapid sand filtration and disinfection. Hydraulic retention time (HRT) was 90 min, 30 min and 120 min for sedimentation, rapid sand filtration, and holding tank after the disinfection process, respectively, for BK-WTP. The HRTs of the processes of MH-WTP were similar to those of BK-WTP with the exception of rapid sand filtration (15 min). Alum (28.89 mg L−1) and poly aluminum chloride (13.23 mg L−1) were used as coagulants in BK-WTP, whereas only alum (30.14 mg L−1) was used in MH-WTP. Disinfection in both WTPs was done using chlorine (total concentration of 6.54 mg L−1 for BK-WTP and 4.07 mg L−1 for MH-WTP) to maintain the concentration of 0.2 mg L−1 in tap water. Raw water (RW) from the intake of the WTPs and processed waters after sedimentation (AS), sand filtration (AF) and disinfection (AD) were taken from the WTPs (Fig. 1). The samples were immediately transported back to our laboratory at Kasetsart University, filtered with 0.3 μm glass fiber filters (GF-75, Advantec) and kept in a refrigerator (4 °C) until further analyses.2.2 Water quality analyses
Dissolved organic carbon (DOC) was measured for the samples filtered by a 0.3 μm pre-combusted glass fiber filter (RW, AS, AF, and AD) in non-purgeable organic carbon mode using an organic carbon analyzer (TOC-L, Shimadzu). Ultraviolet absorbance at 254 nm wavelength (UV254) was measured by a spectrophotometer (DR 5000, Hach Company) using filtered samples (RW, AS, AF, and AD) in a 1 cm cell. Specific UV absorbance (SUVA) was obtained using normalized UV254 values with the corresponding DOC concentrations. For trihalomethane formation potential (THMFP), the filtered samples (RW, AS, and AF) were spiked with phosphate buffer and chlorine (5–8 mg Cl2 per L), were kept under dark conditions at 25 °C, and were incubated for 7 days. Thereafter, NaSO3 was added to the incubated samples to cease the reaction. The incubated samples were sent to MWA for THM measurement according to Standard Method 5710b.2.3 Fluorescence excitation and emission matrix
The fluorescence properties of DOM were investigated via a spectrofluorometer (FP-8200, JASCO) using filtered water samples including RW, AS, AF, and AD. 3D excitation and emission matrix (EEM) spectra were retrieved in a range of excitation (ex) wavelengths of 200–500 nm and a range of emission (em) wavelengths of 200–500 nm. During the analyses, the increments of excitation wavelength and emission wavelength were set to 5 nm and 1 nm, respectively. The results of fluorometric analyses were interpreted using peak picking, regional integration and fluorescence indices. Prominent peaks in specific ranges of ex and em were picked (peak picking) according to previous research;25 Peak A (fulvic acid-like) ex: 260 nm and em: 400–460 nm, peak C (humic acid-like) ex: 320–360 nm and em: 420–460 nm; peak M (marine humic acid-like) ex: 290–310 nm and em: 370–410 nm; peak B (tyrosine-like) ex: 275 nm and em 305 nm and peak T (tryptophan-like) ex: 275 nm and em: 340 nm. Regions on EEM spectra were categorized according to Table 1.26 Indices were calculated based on emission intensities. The indices were the fluorescence index (FI), humification index (HIX) and freshness index (BIX) and were calculated according to previous research.13| Regions | Excitation wavelength (nm) | Emission wavelength (nm) |
|---|---|---|
| Region I: aromatic proteins I | <250 | <330 |
| Region II: aromatic proteins II | <250 | 330–380 |
| Region III: fulvic acid-like | <250 | >380 |
| Region IV: soluble microbial by-product-like | >250 | <380 |
| Region V: humic acid-like | >250 | >380 |
2.4 Unknown screening analysis
Dissolved organic matter in the pre-filtered samples was extracted via Solid Phase Extraction (SPE) using a PPL cartridge. The cartridges contained polystyrene–divinylbenzene modified with a nonpolar surface suitable for retaining polar and nonpolar compounds. The extraction procedures were modified from a previous study27 and can be found in our previous studies.10–12 In brief, the cartridges were rinsed with 10 mL MeOH and preconditioned with 10 mL of 0.01 M HCl. A thousand mL of filtered and pH-adjusted (pH 2) samples was then passed through the cartridges under vacuum conditions. Thereafter, the cartridges were rinsed with 10 mL of 0.01 M HCl and DOM retained on the cartridges was eluted with 10 mL of MeOH. The elution was kept in a refrigerator (−20 °C) until further analysis.Unknown screening analyses were done using a Q Exactive Plus (Orbitrap MS, Thermo Fisher Scientific, Waltham, MA, USA) coupled with an electrospray ionization (ESI) spectrometer at Synchrotron Light Research Institute (SLRI, Nakhon Ratchasima, Thailand). ESI was set in negative ionization mode to cover the primary functional groups of DOM (carboxyl and hydroxyl groups). Prior to the sample injection, the system was externally calibrated using a Piece™ ESI (Thermo Scientific, Walham, MA, USA) and internally calibrated using reported natural fatty acids.28 The extracts (2 μL) were introduced to the system using flow injection mode with a methanol mobile phase with a flow rate of 200 μL min−1. The mass-to-charge ratio (m/z) was retrieved in the range of m/z 100–1000. Most ions detected were singly charged; it implies that our analytical windows were roughly in the 100–1000 Da range. The mass data were processed by Xcalibur 2.0 (Thermo Fisher Scientific) and Compound Discoverer 2.1 (Thermo Fisher Scientific) for background subtraction, component extraction and molecular formula assignment. Mass peaks whose signal to noise ratios (S/N ratio) were greater than 3 were selected and assigned molecular formulas. The molecular formula assignment considered the following elements: C0–39, H0–72, O0–20, N0–2, S0–2 and Cl0–3 with a mass tolerance of 2 ppm. Additional criteria included seven golden rules; a DBE-O range of −10 to 10 was also applied to discard falsely assigned formulas. The isotopes of chlorine (37Cl135Cln−1) containing molecular formulas were checked and only the chlorinated formulas with the isotope were retained for further analysis.
Molecular formulas obtained from unknown screen analyses were used for interpretations of molecular characteristics of DOM. Molecular weight, elemental ratios, and unsaturation indices were calculated based on the number of elements present in the molecular formulas. Double bond equivalent (DBE) and DBE minus number of oxygen atoms (DBE-O) were calculated using eqn (1) and (2), respectively. The modified aromaticity index (AImod) was calculated according to a previous study29 as in eqn (3). The van Krevelen diagram was used to identify the possible chemical groups of DOM.30 The diagram is a plot between O/C in the abscissa and H/C in the ordinate.
| DBE = C + 1 − 0.5(H + Cl–N) | (1) |
| DBE-O = C + 1 − 0.5(H + Cl–N)–O | (2) |
| AImod = [1 + C–O–S − 0.5(H + Cl)]/[C–O–S–N] | (3) |
Disinfection by-products were assigned to newly formed components and components whose intensity increased for more than 30% of the original intensity. Putative precursors of disinfection by-products detected in this study were assigned to DOM components in the samples prior to disinfection (RW, AS, and AF) based on chlorine reactions with organic compounds namely, electrophilic substitution and addition reaction.22
2.5 Statistical analysis
Average values of molecular characteristics including H/C, O/C, DBE, DBE-O, AImod, and molecular weight of DOM components in different samples in the water treatment processes were determined by analysis of variance using one-way ANOVA (Microsoft Excel 2016). Sets of values which rejected null hypothesis (significant different, p < 0.05) were further analyzed by post hoc analysis (pairwise comparison) to quantitatively define the differences.3. Results and discussion
3.1 Changes in DOM parameters and optical properties
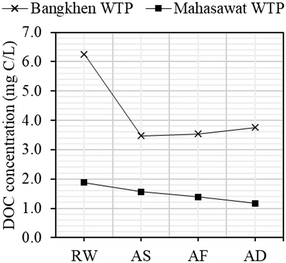
Organic carbon, specific UV absorbance at 254 nm (SUVA), and total trihalomethanes (TTHM) were analyzed as basic water parameters following Standard Method 5310B, 5910, and 5710B, respectively. Dissolved organic carbon (DOC) concentrations of samples collected from the treatment processes of the WTPs in June 2021 are shown in Fig. 2. DOC values of raw water samples (RW) were 6.3 mg C per L and 1.9 mg C per L for BK-WTP and MH-WTP, respectively. The concentration of RW of BK-WTP was considerably high, whereas the RW of MH-WTP was fairly low when compared to previous studies. A study31 reported an average value of DOC of the water canal and intake of BK-WTP in 2017 to be 1.93 mg C per L during the March–June period. Several studies also reported the DOC of different types of raw water of other WTPs including Phong River (6.4 mg C per L in June 2019)16 and Sri-Trang reservoir (4.3 mg C per L).32 For BK-WTP, substantial reduction of DOC was observed after sedimentation as DOC was reduced to 3.5 mg C per L. Thereafter, DOC concentrations were relatively constant with an average value of 3.6 ± 0.15 mg C per L. For MH-WTP, DOC concentrations were gradually decreased from 1.9 mg C per L to 1.6 mg C per L, 1.4 mg C per L, and 1.2 mg C per L in AS, AF, and AD, respectively. For both WTPs, the pH remained relatively stable with an average value of 7.3 ± 0.13 and 7.7 ± 0.37 for BK-WTP and MH-WTP, respectively.Specific UV absorbance at 254 nm (SUVA) of RW samples of the WTPs was 1.47 L mg−1 m−1 and 2.50 L mg−1 m−1 for BK-WTP and MH-WTP, respectively. These values lay below average and in the average range compared to 1.7–3.1 L mg−1 m−1 in natural water sources in Thailand.32 During the treatment processes, SUVA values were slightly changed for BK-WTP, remaining at an average value of 1.58 ± 0.278 L mg−1 m−1. However, SUVA values of samples of MH-WTP were considerably changed. The SUVA was 2.50 L mg−1 m−1, 1.41 L mg−1 m−1, 1.72 L mg−1 m−1, and 3.67 L mg−1 m−1 for RW, AS, AF, and AD, respectively. After sedimentation, a considerable decrease of SUVA was observed, indicating that aromatic substances were selectively removed in the process. Reports showed that the coagulation/flocculation removed high molecular weight and aromatic components of DOM13,33 which was consistent with our results observed for MH-WTP. After disinfection, the SUVA increased from 1.41 L mg−1 m−1 in AF to 3.67 L mg−1 m−1. The result suggests that aliphatic components of DOM were selectively removed during chlorination. Such changes suggested that aliphatic parts of DOM in the sample were more reactive towards chlorine. It was possible that aliphatic moieties in our sample were less oxygenated than aromatic moieties and thus were more reactive. It was also reported that chlorine could oxidize hydroquinone to quinone moieties in DOM resulting in increasing UV254 absorption.34
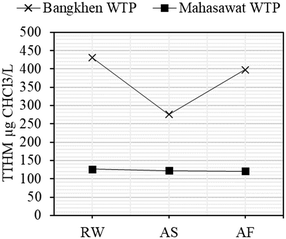
Trihalomethane formation potential (THMFP) was analyzed for the chlorinated samples (RW, AS, and AF) and are shown in Fig. 3. The concentrations of THMFP of the samples in BK-WTP were 430.22 μg CHCl3 per L, 275.81 μg CHCl3 per L and 398.01 μg CHCl3 per L for RW, AS and AF, respectively. For MH-WTP, the concentrations were 125.96 μg CHCl3 per L, 122.12 μg CHCl3 per L and 120.96 μg CHCl3 per L for RW, AS and AF, respectively. The levels of THMFP of BK-WTP samples were similar to those found in U-Tapao Canal (425 μg L−1).35 The sedimentation process of BK-WTP seemed to be effective in the removal of THM precursors as indicated by the 35.9% reduction of THMFP. This was also found in a study36 which showed the reduction of THM after an enhanced coagulation process. Coagulation and flocculation followed by sedimentation have been known to preferentially remove the hydrophobic fraction of DOM37 and humic and fulvic acid.38 Those compounds were also reported to be highly correlated with THM formation.39 On the other hand, those compounds were not so effectively removed by rapid sand filtration37 which could be the reason why THMFP remained stable after the process. For MH-WTP, relatively unchanged FDOM and DOM (see sections 3.2 and 3.3) could be the reason why THMFP remained relatively stable during treatment processes.
3.2 Changes in fluorescence properties of DOM
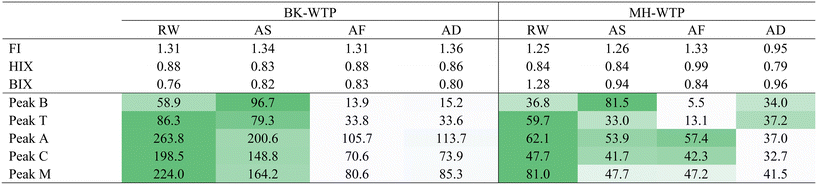
The general EEM spectra of FDOM in the samples are illustrated in Fig. S1 (ESI† data). Based on the EEM spectra, peaks and fluorescence indices were calculated and are shown in Table 2. Overall, prominent peaks were found in the regions depicted as fulvic acid-like (region III) and humic acid-like (region V) components in RW samples of the WTPs. The presence of this FDOM signature was expected since RW samples of both BK-WTP and MH-WTP were sourced from rivers (Chao Phraya River and Mae Klong River, respectively). Humic substances were shown to be one of the major parts of allochthonous dissolved organic matter in freshwater environments.40 The dominance of humic acid-like and fulvic acid-like components was similar to the observation found in the same water in summer 2016 (ref. 31) and another study.41 Moreover, the emission wavelength of peak C which indicates the hydrophobicity of DOM (420 nm for both WTPs) was quite similar to those of river samples in a previous study.42 However, for MH-WTP, prominent peaks were observed in the shorter emission wavelength (region II and IV) than those observed in BK-WTP which represent aromatic protein-like and soluble microbial by-products (SMPs). The presence of aromatic protein-like components might indicate the higher degree of urbanization and pollution from various sources such as industrial effluent and biologically treated effluent.43Based on fluorescence indices, low FI values (1.25–1.31) suggested that FDOM of both WTPs originated from a terrestrial source. They both had high HIX (0.88 and 0.84 for BK-WTP and MH-WTP, respectively) which indicated that FDOM was highly humified. However, BIX values of RW samples of the WTPs were different. The BIX value was 0.76 for BK-WTP, whereas that of MH-WTP was 1.28. BIX is an indicator associated with freshly produced DOM of autochthonous origin.44 During the treatment processes, only BIX values found in MH-WTP samples changed considerably from 1.28 in the RW sample to 0.94 in the AS sample.
Along the treatment processes, the overall emission intensity of samples in both WTPs decreased drastically indicating the removal of FDOM (Fig. S1†). For BK-WTP, significant changes were observed in AS and AF samples where regions III and V were substantial decreased in emission intensity. After disinfection, no significant change was observed. For MH-WTP, changes were clearly observed in every process. Sedimentation caused the reduction of overall emission intensity. Both sedimentation and sand filtration seemed to selectively remove SMP-like components (region IV). Disinfection also drastically changed the spectrum by removal of a large portion of humic acid-like components (region V).
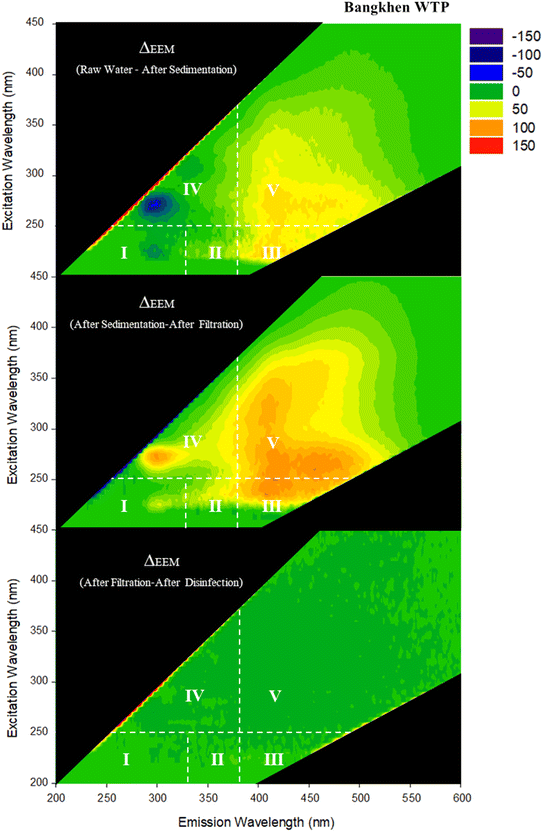
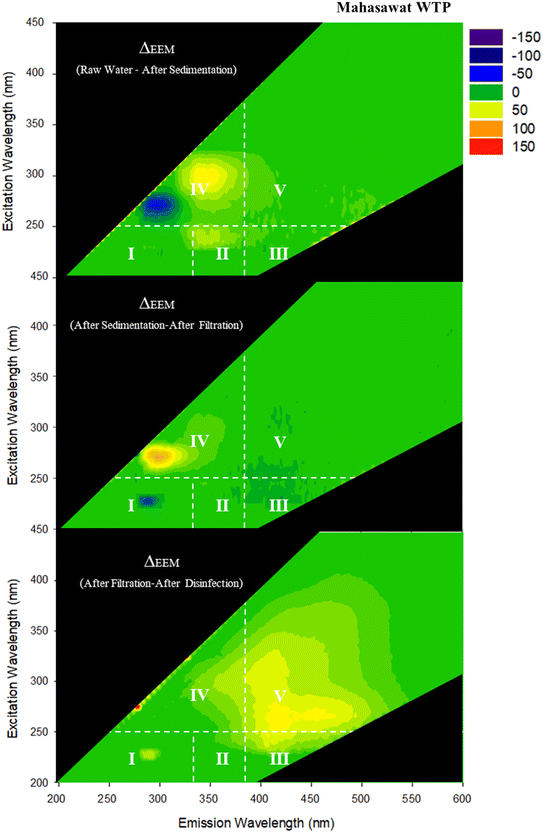
To clearly demonstrate the changes done by water treatment processes, changes during water treatment processes were illustrated by comparison between adjacent samples (i.e., RW and AS, AS and AF, AF, and AD). The fluorescence intensities (Z-axis) of the latter sample were deducted from those of the former sample to represent relative changes of FDOM done by the treatment processes. The relative changes of FDOM during water treatment processes are shown in Fig. 4 and 5 for BK-WTP and MH-WTP, respectively.
The sedimentation process of the WTPs resulted in different portions of FDOM being removed from the samples. For BK-WTP, the majority of components located in regions III and V which represented fulvic acid-like and humic acid-like components, respectively, reduced in fluorescent intensity (Fig. 4). For MH-WTP, the reduction in fluorescent intensities was exclusively in region IV (Fig. 5) which represented aromatic proteins. Previous studies reported the removal DOM associated with terrestrial sources by the coagulation process.13,38,42,45,46 This was consistent with our observation after sedimentation in BK-WTP while this was inconsistent with those in MH-WTP. It was possible that different operational conditions, types of coagulants and characteristics of FDOM resulted in different removal of different portions of FDOM in the WTPs.47–49 High molecular weight proteins were shown to be removed by the coagulation/flocculation process; moreover, the molecular size was shown to have an effect on the removal efficiency of protein components by the coagulation and flocculation process.50 It was possible that the protein-like fraction in RW of MH-WTP consisted of high molecular weight molecules and they were selectively removed during the coagulation and flocculation process.
Broad changes were observed for FDOM after the filtration process of BK-WTP. Prominently reduced peaks were observed in regions III (fulvic acid-like), IV (soluble microbial by-product-like) and V (humic acid-like components). On the other hand, the filtration process of MH-WTP exclusively removed FDOM in region IV. Generally, sand filtration has limited effect on FDOM.49 However, both humic-like and protein-like were reported to be removed by the process. Previous studies51,52 reported the removal of humic-like and protein-like FDOM components by sand filtration. A study13 found the intensity reduction of the shorter emission wavelength FDOM components (region I, II, and IV) after slow sand filtration. The different observations in BK-WTP and MH-WTP could be a result of several factors including operational conditions, filter media, and FDOM components originally existing in the influent of sand filtration.
Effects of the disinfection process on FDOM compositions in our study were quite specific to each WTP. For BK-WTP, FDOM was relatively unchanged after disinfection indicating that FDOM components left after sand filtration were not reactive towards the disinfectant. A previous study13 also could not find significant changes in fluorometric intensity done by the disinfection process. They discussed that FDOM components which were reactive to chlorine were also reactive in the sand filtration process; thus, they were removed prior to disinfection. In our study, substantial removal of FDOM was also observed in AF and it was possible that the filtration process removed FDOM components which were reactive to disinfection. On the other hand, for MH-WTP, FDOM components in region V (humic acid-like components) were particularly removed by the disinfection process. Our results were consistent with several previous studies.45,53,54 One of the studies45 stated that disinfection rather modified FDOM molecules targeting double bonds and conjugated bonds which were known to be molecular features of humic substances.
3.3 Changes in molecular-level compositions
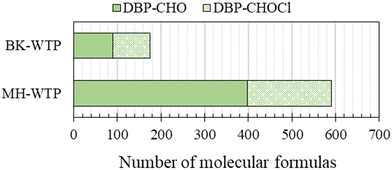
The total number and types of molecular formulas that satisfied the assignment criteria are shown in Fig. S2 (ESI† data). For simplification, only components comprising carbon, oxygen and hydrogen (CHO) and chlorine containing CHO (CHOCl) were considered in the following discussion. For BK-WTP, the number of CHO and CHOCl was 578 (56.0%) and 455 (44.0%) components, respectively. For MH-WTP, the number of CHO and CHOCl were 696 (63.4%) and 401 (36.6%) components, respectively. Based on classifications of chemical families of van Krevelen diagrams (Fig. S3, ESI† data), the majority of CHO components of the WTPs had elemental ratios similar to those of the lignin group. This was consistent with other studies focusing on riverine DOM.10,14,16,18,55 CHOCl components were also clustered in the lignin region with an additional cluster on the lipid region. Naturally occurring chlorinated phenolic compounds were reported.56 Therefore, it was possible that chlorine incorporated into the phenolic structure of humic substances naturally present in the samples.Although significant changes were not observed in DOC concentrations before and after disinfection, the optical properties and molecular-level information indicated otherwise. This implies the modification rather than removal of DOM. The modifications of DOM are demonstrated here by the changes of intensities during the treatment processes. Changes of DOM components after passing through the water treatment processes were evaluated in semi-quantitative comparisons between intensities of the same components in two adjacent treatment processes. According to the changes of intensity, DOM components were classified into newly formed (NF, absent in the before sample and present in the after sample), increased (IC, components whose intensities increase more than 30% of the intensities in the before sample), constant (CS, components whose intensity remains within ±30% of the before sample), decreased (DC, components whose intensity decreased by more than 30% of the intensities in the before sample), and disappeared (DP, components were present in the before sample and absent in the after sample). The number of each classification can be seen in Fig. 6.
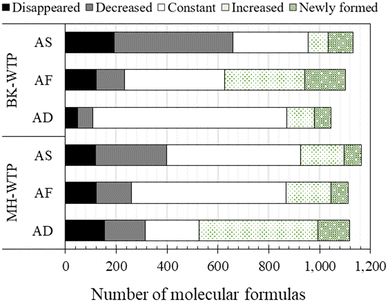 | ||
| Fig. 6 Number of CHO components classified into different changes between two consecutive processes. | ||
Consideration of the molecular characteristics of components in each category revealed unique features. Number-average values of characteristics of DOM components classified into NF, IC, CS, DC, and DP are shown in Table S1 (ESI† data) for BK-WTP. The sedimentation process of BK-WTP tended to remove components with high AI and MW. DP components had significantly higher Al (0.60) as compared to DC (0.23), CS (0.23), and IC (0.13). Additionally, DP components also had higher molecular weight (327.07 Da) than others (294.15–304.87 Da). The results were consistent with the fluorescence results that humic acid-like and fulvic acid-like FDOM were removed after sedimentation (Fig. 4).
The filtration process of BK-WTP seemed to be selective towards high molecular weight and saturated components and introduced components with relatively lower molecular weight and less saturated characteristics. DC components had a significantly higher H/C ratio (1.35) than IC components (1.21). DP and DC components were significantly larger in terms of molecular weight (307.78 and 326.30 Da, for DP and DC, respectively) than IC (282.69 Da).
For the disinfection process of BK-WTP, the only molecular characteristic which differs among categories was molecular weight. DP (313.84 Da) and DC (286.52 Da) components had significantly higher molecular weight than CS (236.64 Da). This minor change was consistent with limited changes observed in fluorescence results.
Based on the number-average values of characteristics of DOM components (Table S2, ESI† data), sedimentation of MH-WTP removed higher molecular weight and relatively less saturated components. The DP (382.39 Da) and DC (339.24 Da) components were relatively larger than others (243.41–269.86 Da). Moreover, DP (6.93) and DC (6.64) also had high DBE than others (4.80–5.77).
Generally, the rapid sand filtration process in MH-WTP had limited effects on DOM as indicated by large portions of DOM being classified as CS (54.5%, Fig. 6). The process preferentially removed oxygenated, high molecular weight, and unsaturated components as indicated by the higher O/C ratio (0.52), molecular weight (325.45 Da), and DBE (6.62) of DP than those of CS (O/C = 0.44, MW = 298.04 Da, and DBE = 5.69).
The disinfection process of MH-WTP tended to remove components with relatively larger, more saturated and less oxygenated characteristics. Our results showed that DP and DC components shared similar characteristics including a relatively low O/C ratio (0.40 and 0.38), high H/C ratio (1.40 and 1.43), and high molecular weight (318.59 Da and 317.51 Da) as compared to other categories.
Rapid sand filtration of both WTPs removed substantially smaller number of components (DC and DP). Such small changes were expected in DOM in our study partly because of the limited analytical windows of Orbitrap MS (∼100–1000 Da).
Based on results of both WTPs, chlorination was selective to large and less oxygenated components of DOM. This was quite reasonable since chlorination is an oxidation process which preferentially reacts with unsaturated bonds and less oxygenated compounds. The reactions of chlorine and organic compounds included 1) oxidation, 2) addition to unsaturated bonds and 3) electrophilic substitution.22 Several reports showed the preferential oxidation of chlorine to unsaturated moieties and oxygen deficient molecules.34,62
3.4 Formation of disinfection by-products in the water treatment plants
Components whose intensities increased (IC) and components newly formed (NF) after the disinfection process were collectively regarded as DBPs. Table S3 (ESI† data) shows the full list of DBPs detected in this study. Fig. 7 shows the number of the said components classified as CHO components (DBP-CHO) and chlorine-containing CHO components (DBP-CHOCl). Overall, 174 and 591 components were classified as DBPs for BK-WTP and MH-WTP, respectively. About half (85, 48.8%) of the DBPs in BK-WTP were DBP-CHOCl, while about one-third (193, 32.6%) of the DBPs of MH-WTP were DBP-CHOCl. These DBPs might potentially be intermediates during the THM or other DBP formation. This indicated that these non-volatile DBP components were overlooked in the actual treatment plants.Collectively, the number of total DBP-CHOCl observed in two WTPs was 252 components. Out of the total DBPs, 26 of them were detected in both WTPs, 59 of them were specifically detected in BK-WTP, and 167 of them were specifically detected in MH-WTP. Although the water treatment processes of the WTPs were quite similar, large portions of DBPs detected in this study were unique in each WTP. From the available data, 138 DBPs detected in this study were previously reported via Orbitrap MS and FT ICR MS,15,21,22,63 while 114 DPBs were not found in those studies. Those studies reported DBP formation during water treatment processes with different water sources, for example: Ara River and Edo River, Japan;15 Stångån River21 and other rivers13,22 in Sweden; Suwannee River fulvic acid.63 This indicates that the formation of DBPs could be partly dependent on the source waters of individual water treatment systems. In this study, raw water of BK-WTP and MH-WTP was taken from downstream Chao Phraya River and Mae Klong River, respectively. The rivers had different water qualities1,2 suggesting the differences in DOM composition. As a result, unique DBPs were detected in our study.
DBP-CHOCl components whose potential precursors could be found in the former treatment processes, namely, raw water, sedimentation, and filtration, were investigated by determining the electrophilic substitution and addition reaction. The potential precursors for the DBP-CHOCl are listed in Tables 3 and 4 for BK-WTP and MH-WTP, respectively. The tables also indicate the fate of the precursors in the treatment processes.
| Potential precursor | Absolute intensity of the precursor in water treatment plant | DBP-CHOCl | Mechanismsa | ||
|---|---|---|---|---|---|
| AS | AF | AD | |||
| a ES: electrophilic substitution, which replaced H atom(s) with Cl (s); AR: addition reaction which is addition of HOCl (s). | |||||
| C8H12O | Decreased | Constant | Constant | C8H9OCl3 | ES |
| C6H6O5 | Decreased | Constant | Increased | C6H3O5Cl3 | ES |
| C11H10O4 | Decreased | Constant | Constant | C11H8O4Cl2 | ES |
| C14H14O4 | Decreased | Increased | Decreased | C14H13O4Cl | ES |
| C13H18O5 | Decreased | Constant | Constant | C13H17O5Cl | ES |
| C12H18O6 | Decreased | Constant | Constant | C12H17O6Cl | ES |
| C10H10O6 | Increased | Decreased | Constant | C10H8O6Cl2 | ES |
| C15H24O5 | Constant | Constant | Decreased | C15H23O5Cl | ES |
| C16H22O5 | Decreased | Constant | Constant | C16H21O5Cl | ES |
| C10H20O10 | Decreased | Increased | Constant | C10H19O10Cl | ES |
| C11H12O8 | Decreased | Constant | Decreased | C11H10O8Cl2 | ES |
| C14H20O5 | Increased | Disappeared | Newly formed | C14H17O5Cl3 | ES |
| C17H28O5 | Constant | Constant | Constant | C17H25O5Cl3 | ES |
| C5H6O3 | Decreased | Constant | Decreased | C5H6O3Cl2 | AR |
| C11H8O4 | Constant | Increased | Constant | C11H8O4Cl2 | AR |
| C10H8O6 | Decreased | Increased | Constant | C10H8O6Cl2 | AR |
| C10H8O8 | Disappeared | Newly formed | Decreased | C10H8O8Cl2 | AR |
| C15H20O4 | Constant | Constant | Constant | C15H20O4Cl2 | AR |
| C11H10O8 | Decreased | Increased | Constant | C11H10O8Cl2 | AR |
| Potential precursor | Absolute intensity of the precursor in water treatment plant | DBP-CHOCl | Mechanismsa | ||
|---|---|---|---|---|---|
| AS | AF | AD | |||
| a ES: electrophilic substitution, which replaced H atom(s) with Cl (s); AR: addition reaction which is addition of HOCl (s). | |||||
| C5H6O3 | Increased | Increased | Increased | C5H5O3Cl | ES |
| C6H6O3 | Constant | Constant | Increased | C6H5O3Cl | ES |
| C7H8O4 | Constant | Increased | Increased | C7H7O4Cl | ES |
| C8H6O5 | Decreased | Decreased | Increased | C8H5O5Cl | ES |
| C10H6O4 | Increased | Constant | Constant | C10H5O4Cl | ES |
| C5H6O4 | Increased | Constant | Increased | C5H3O4Cl3 | ES |
| C9H12O5 | Increased | Constant | Increased | C9H11O5Cl | ES |
| C8H10O5 | Constant | Decreased | Increased | C8H8O5Cl2 | ES |
| C10H8O4 | Constant | Disappeared | Newly formed | C10H6O4Cl2 | ES |
| C6H10O5 | Increased | Constant | Disappeared | C6H7O5Cl3 | ES |
| C9H14O7 | Constant | Disappeared | Newly formed | C9H13O7Cl | ES |
| C10H14O7 | Decreased | Increased | Increased | C10H13O7Cl | ES |
| C13H14O5 | Decreased | Decreased | Increased | C13H13O5Cl | ES |
| C16H18O3 | Constant | Constant | Decreased | C16H17O3Cl | ES |
| C10H10O6 | Increased | Decreased | Increased | C10H8O6Cl2 | ES |
| C11H12O8 | Constant | Constant | Increased | C11H11O8Cl | ES |
| C10H14O7 | Decreased | Increased | Increased | C10H12O7Cl2 | ES |
| C10H14O5 | Increased | Constant | Disappeared | C10H11O5Cl3 | ES |
| C11H6O7 | Increased | Constant | Increased | C11H4O7Cl2 | ES |
| C12H12O8 | Constant | Increased | Increased | C12H11O8Cl | ES |
| C12H14O8 | Constant | Decreased | Constant | C12H13O8Cl | ES |
| C12H18O8 | Increased | Constant | Increased | C12H17O8Cl | ES |
| C12H8O5 | Decreased | Decreased | Increased | C12H5O5Cl3 | ES |
| C11H12O8 | Constant | Constant | Increased | C11H10O8Cl2 | ES |
| C16H20O6 | Constant | Constant | Increased | C16H19O6Cl | ES |
| C11H8O7 | Increased | Constant | Increased | C11H5O7Cl3 | ES |
| C12H16O8 | Constant | Increased | Increased | C12H14O8Cl2 | ES |
| C18H18O6 | Constant | Decreased | Increased | C18H17O6Cl | ES |
| C14H20O9 | Constant | Constant | Increased | C14H19O9Cl | ES |
| C17H18O7 | Constant | Constant | Disappeared | C17H17O7Cl | ES |
| C16H24O8 | Constant | Constant | Increased | C16H23O8Cl | ES |
| C17H28O8 | Newly formed | Disappeared | Disappeared | C17H27O8Cl | ES |
| C12H8O9 | Decreased | Disappeared | Newly formed | C12H5O9Cl3 | ES |
| C14H28O11 | Disappeared | Newly formed | Increased | C14H27O11Cl | ES |
| C17H28O5 | Constant | Constant | Increased | C17H25O5Cl3 | ES |
| C5H6O4 | Increased | Constant | Increased | C5H6O4Cl2 | AR |
| C8H6O2 | Increased | Constant | Decreased | C8H6O2Cl2 | AR |
| C6H6O5 | Constant | Constant | Increased | C6H6O5Cl2 | AR |
| C7H10O4 | Increased | Increased | Increased | C7H10O4Cl2 | AR |
| C10H6O4 | Increased | Constant | Constant | C10H6O4Cl2 | AR |
| C10H8O6 | Increased | Increased | Increased | C10H8O6Cl2 | AR |
| C10H12O7 | Constant | Decreased | Increased | C10H12O7Cl2 | AR |
| C11H10O8 | Constant | Constant | Increased | C11H10O8Cl2 | AR |
| C12H14O8 | Constant | Decreased | Constant | C12H14O8Cl2 | AR |
For BK-WTP, there were 19 DBP-CHOCl components whose precursors could be traced in the prior processes. The majority of them were removed in the sedimentation process as indicated by 12 decreased components and 1 disappeared component. This indicated the capability of the process to decrease DBP formation. On the other hand, the filtration process seemed not to be so effective in removing the precursors. Most of the precursors remained relatively constant and only 2 components were removed (1 decreased and 1 disappeared component). However, there was 1 newly formed component and 5 components whose intensities increased after the filtration process.
For MH-WTP, 44 DBP-CHOCl components could be traced back to their potential precursors. The sedimentation process did not yield the same results as those of BK-WTP. About half of the precursors (21 components) remained constant and another half (15 components) increased in intensities after the process. On the other hand, only 6 components were decreased and only 1 component completely disappeared. These results showed the exact opposite to the results we found in BK-WTP. The following process, filtration, did not show an apparent trend. Most components (22 components) remained constant. The process also removed (9 decreased and 4 disappeared components) and introduced (9 increased and 1 newly formed component) approximately the same number of components.
Conclusions
Dissolved organic matter in raw water and processed water from BK-WTP and MH-WTP in June 2021 was investigated in terms of fluorescence properties and molecular compositions. Fluorescence results showed that FDOM in both WTPs had a terrestrial signature and a specific microbial signature in MH-WTP. Molecular compositions of DOM in both WTPs revealed that the original samples comprised CHO, CHON, CHOS, CHONS and chlorinated components. Focusing on CHO and chlorinated CHO components, the majority of components were classed as lignin and lipid.Water treatment processes had different effects on FDOM and DOM composition depending on WTPs. Changes of FDOM and DOM in BK-WTP were substantial during sedimentation and filtration processes where humic acid-like and fulvic acid-like FDOM components were selectively removed from the water. Changes in FDOM after disinfection in BK-WTP could not be observed. The opposite effects were observed for MH-WTP. Small changes were done by sedimentation and filtration processes where protein-like FDOM components were preferentially removed. The chlorination process of MH-WTP removed humic-like and fulvic-like FDOM components. Unlike FDOM, changes in molecular characteristics of DOM during the treatment process were consistent between both WTPs. Sedimentation preferentially removed relatively large and less saturated components. Filtration had limited effects on DOM components. Disinfection was selective to large and less oxygenated components of DOM which could potentially be precursors of DBPs.
Comparison of samples between before and after disinfection processes in the WTPs, potential DBPs and their precursors were extracted from our data set. In total, 252 components were assigned as DBPs in our study. The DBPs were both common in the WTPs and specific to the WTPs. 138 of them were previously reported while the remaining 114 components were newly found. The putative precursors of those DBPs could also be traced back through electrophilic and addition reaction in the earlier treatment processes prior to disinfection. Semi-quantitative changes in intensity of the precursors revealed the treatability in the treatment process.
The fact that the formation of DBPs was specific in both our study and others indicates that the characteristics of DOM in raw water of the WTPs are crucial aspects to control DBP formation. Our study clearly shows that different raw water sources resulted in unique DBPs formed after the disinfection process which could potentially be the result of the fate of different precursors in the prior treatment processes. The fate of the precursors could reflect their treatability in the current treatment processes which can be used to improve the removal efficiency of the precursors and the formation of DBPs.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This research was funded by Kasetsart University Research and Development Institute, KURDI, FF(KU)13.63.References
- K. Kruawal, F. Sacher, A. Werner, J. Muller and T. P. Knepper, Chemical water quality in Thailand and its impacts on the drinking water production in Thailand, Sci. Total Environ., 2005, 340, 57–70 CrossRef CAS PubMed.
- W. Simachaya, A decade of water quality monitoring in Thailand’s four major rivers: the results and the implications for management, in the 6th International Conference on the Environmental Management of Enclosed Coastal Seas, 2003 Nov 18–21, Bangkok, Thailand Search PubMed.
- M. Filella, Freshwaters: which NOM matters?, Environ. Chem. Lett., 2008, 7, 21–35 CrossRef.
- P. Li, C. Zhao, K. Liu, X. Xiao, Y. Wang, Y. Wang and D. He, Anthropogenic Influences on Dissolved Organic Matter in Three Coastal Bays, North China, Front. Earth Sci., 2021, 9, 697758 CrossRef.
- L. F. Yee, M. P. Abdullah, S. Ata and B. Ishak, Dissolved organic matter and its impact on the chlorine demand of treated water, Malaysian J. Anal. Sci., 2006, 10, 243–250 Search PubMed.
- A. F. Gilca, C. Teodosiu, S. Fiore and C. P. Musteret, Emerging disinfection byproducts: A review on their occurrence and control in drinking water treatment processes, Chemosphere, 2020, 259, 127476 CrossRef CAS.
- A. A. Cuthbertson, S. Y. Kimura, H. K. Liberatore, R. S. Summers, D. R. Knappe, B. D. Stanford, J. C. Maness, R. E. Mulhern, M. Selbes and S. D. Richardson, Does granular activated carbon with chlorination produce safer drinking water? From disinfection byproducts and total organic halogen to calculated toxicity, Environ. Sci. Technol., 2019, 53, 5987–5999 CrossRef CAS PubMed.
- A. Matilainen, E. T. Gjessing, T. Lahtinen, L. Hed, A. Bhatnagar and M. Sillanpaa, An overview of the methods used in the characterisation of natural organic matter (NOM) in relation to drinking water treatment, Chemosphere, 2011, 83, 1431–1442 CrossRef CAS PubMed.
- J. A. Leenheer and J.-P. Croué, Peer reviewed: characterizing aquatic dissolved organic matter, Environ. Sci. Technol., 2003, 37, 18A–26A CrossRef CAS.
- V. Yuthawong, I. Kasuga, F. Kurisu and H. Furumai, Comparison of Low Molecular Weight Dissolved Organic Matter Compositions in Lake Inba and Kashima River by Orbitrap Mass Spectrometry, J. Water Environ. Technol., 2017, 15, 12–21 CrossRef.
- V. Yuthawong, I. Kasuga, F. Kurisu and H. Furumai, Molecular-Level Changes in Dissolved Organic Matter Compositions in Lake Inba Water during KMnO4 Oxidation: Assessment by Orbitrap Mass Spectrometry, J. Water Environ. Technol., 2019, 17, 27–39 CrossRef.
- V. Yuthawong, I. Kasuga, F. Kurisu and H. Furumai, Application of Orbitrap Mass Spectrometry to Investigate Seasonal Variations of Dissolved Organic Matter Composition in a Eutrophic Lake in Japan, Environ. Sci.: Water Res. Technol., 2020, 6, 1816–1827 RSC.
- E. E. Lavonen, D. N. Kothawala, L. J. Tranvik, M. Gonsior, P. Schmitt-Kopplin and S. J. Kohler, Tracking changes in the optical properties and molecular composition of dissolved organic matter during drinking water production, Water Res., 2015, 85, 286–294 CrossRef CAS PubMed.
- W. N. Phatthalung, O. Suttinun, P. Phungsai, I. Kasuga, F. Kurisu, H. Furumai and C. Musikavong, Non-target screening of dissolved organic matter in raw water, coagulated water, and chlorinated water by Orbitrap mass spectrometry, Chemosphere, 2021, 264, 128437 CrossRef CAS PubMed.
- P. Phungsai, F. Kurisu, I. Kasuga and H. Furumai, Changes in Dissolved Organic Matter Composition and Disinfection Byproduct Precursors in Advanced Drinking Water Treatment Processes, Environ. Sci. Technol., 2018, 52, 3392–3401 CrossRef CAS.
- T. Prasert, Y. Ishii, F. Kurisu, C. Musikavong and P. Phungsai, Characterization of lower Phong river dissolved organic matters and formations of unknown chlorine dioxide and chlorine disinfection by-products by Orbitrap mass spectrometry, Chemosphere, 2021, 265, 128653 CrossRef CAS.
- S. Kim, L. A. Kaplan and P. G. Hatcher, Biodegradable dissolved organic matter in a temperate and a tropical stream determined from ultra-high resolution mass spectrometry, Limnol. Oceanogr., 2006, 51, 1054–1063 CrossRef CAS.
- M. Urai, I. Kasuga, F. Kurisu and H. Furumai, Molecular characterization of dissolved organic matter in various urban water resources using Orbitrap Fourier transform mass spectrometry, Water Sci. Technol.: Water Supply, 2014, 14, 547 CAS.
- E. C. Minor, C. J. Steinbring, K. Longnecker and E. B. Kujawinski, Characterization of dissolved organic matter in Lake Superior and its watershed using ultrahigh resolution mass spectrometry, Org. Geochem., 2012, 43, 1–11 CrossRef CAS.
- M. Gonsior, M. Zwartjes, W. J. Cooper, W. Song, K. P. Ishida, L. Y. Tseng, M. K. Jeung, D. Rosso, N. Hertkorn and P. Schmitt-Kopplin, Molecular characterization of effluent organic matter identified by ultrahigh resolution mass spectrometry, Water Res., 2011, 45, 2943–2953 CrossRef CAS PubMed.
- M. Gonsior, P. Schmitt-Kopplin, H. Stavklint, S. D. Richardson, N. Hertkorn and D. Bastviken, Changes in dissolved organic matter during the treatment processes of a drinking water plant in Sweden and formation of previously unknown disinfection byproducts, Environ. Sci. Technol., 2014, 48, 12714–12722 CrossRef CAS.
- E. E. Lavonen, M. Gonsior, L. J. Tranvik, P. Schmitt-Kopplin and S. J. Kohler, Selective chlorination of natural organic matter: identification of previously unknown disinfection byproducts, Environ. Sci. Technol., 2013, 47, 2264–2271 CrossRef CAS PubMed.
- C. K. Remucal, E. Salhi, N. Walpen and U. von Gunten, Molecular-level transformation of dissolved organic matter during oxidation by ozone and hydroxyl radical, Environ. Sci. Technol., 2020, 54, 10351–10360 CrossRef CAS PubMed.
- R. P. Milstead and C. K. Remucal, Molecular-level insights into the formation of traditional and novel halogenated disinfection byproducts, ACS ES&T Water, 2021, 1, 1966–1974 Search PubMed.
- P. G. Coble, Marine Optical Biogeochemistry: The Chemistry of Ocean Color, Chem. Rev., 2007, 107, 402–418 CrossRef CAS.
- W. Chen, P. Westerhoff, J. A. Leenheer and K. Booksh, Fluorescence excitation− emission matrix regional integration to quantify spectra for dissolved organic matter, Environ. Sci. Technol., 2003, 37, 5701–5710 CrossRef CAS PubMed.
- T. Dittmar, B. Koch, N. Hertkorn and G. Kattner, A simple and efficient method for the solid-phase extraction of dissolved organic matter (SPE-DOM) from seawater, Limnol. Oceanogr.: Methods, 2008, 6, 230–235 CrossRef CAS.
- R. L. Sleighter and P. G. Hatcher, Molecular characterization of dissolved organic matter (DOM) along a river to ocean transect of the lower Chesapeake Bay by ultrahigh resolution electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry, Mar. Chem., 2008, 110, 140–152 CrossRef CAS.
- B. Koch and T. Dittmar, From mass to structure: An aromaticity index for high-resolution mass data of natural organic matter, Rapid Commun. Mass Spectrom., 2006, 20, 926–932 CrossRef CAS.
- S. Kim, R. W. Kramer and P. G. Hatcher, Graphical method for analysis of ultrahigh-resolution broadband mass spectra of natural organic matter, the van Krevelen diagram, Anal. Chem., 2003, 75, 5336–5344 CrossRef CAS PubMed.
- K. Tungsudjawong, S. Leungprasert and P. Peansawang, Investigation of humic acids concentration in different seasons in a raw water canal, Bangkok, Thailand, Water Sci. Technol.: Water Supply, 2017, 18, 1727–1738 Search PubMed.
- T. Kueseng, T. T. Suksaroj, C. Musikavong and C. Suksaroj, Enhanced coagulation for removal of dissolved organic matter and trihalomethane formation potential from raw water supply in Sri-Trang Reservoir, Thailand, Water Pract. Technol., 2011, 6, wpt2011002 CrossRef.
- S. A. Baghoth, S. K. Sharma, M. Guitard, V. Heim, J. P. Croué and G. L. Amy, Removal of NOM-constituents as characterized by LC-OCD and F-EEM during drinking water treatment, J. Water Supply: Res. Technol.--AQUA, 2011, 60, 412–424 CrossRef CAS.
- J. Wenk, M. Aeschbacher, E. Salhi, S. Canonica, U. Von Gunten and M. Sander, Chemical oxidation of dissolved organic matter by chlorine dioxide, chlorine, and ozone: effects on its optical and antioxidant properties, Environ. Sci. Technol., 2013, 47, 11147–11156 CrossRef CAS PubMed.
- K. Sitham, S. Sinyoung and C. Musikavong, DBPs Formation by Chlorination and Chloramination of Water and Treated Water at Short and Long Reaction Times, Thai Environ. Eng. J., 2022, 36, 69–77 Search PubMed.
- A. Teksoy, U. Alkan and H. S. Başkaya, Influence of the treatment process combinations on the formation of THM species in water, Sep. Purif. Technol., 2008, 61, 447–454 CrossRef CAS.
- B. Ramavandi, S. Farjadfard, M. Ardjmand and S. Dobaradaran, Effect of water quality and operational parameters on trihalomethanes formation potential in Dez River water, Iran, Water Resour. Ind., 2015, 11, 1–12 CrossRef.
- D. L. Gone, J.-L. Seidel, C. Batiot, K. Bamory, R. Ligban and J. Biemi, Using fluorescence spectroscopy EEM to evaluate the efficiency of organic matter removal during coagulation–flocculation of a tropical surface water (Agbo reservoir), J. Hazard. Mater., 2009, 172, 693–699 CrossRef CAS PubMed.
- L. Yang, D. Kim, H. Uzun, T. Karanfil and J. Hur, Assessing trihalomethanes (THMs) and N-nitrosodimethylamine (NDMA) formation potentials in drinking water treatment plants using fluorescence spectroscopy and parallel factor analysis, Chemosphere, 2015, 121, 84–91 CrossRef CAS PubMed.
- K. M. Mostofa, C.-Q. Liu, M. A. Mottaleb, G. Wan, H. Ogawa, D. Vione, T. Yoshioka and F. Wu, in Photobiogeochemistry of organic matter, Springer, 2013, pp. 1–137 Search PubMed.
- C. L. Osburn, L. T. Handsel, M. P. Mikan, H. W. Paerl and M. T. Montgomery, Fluorescence tracking of dissolved and particulate organic matter quality in a river-dominated estuary, Environ. Sci. Technol., 2012, 46, 8628–8636 CrossRef CAS.
- M. Bieroza, A. Baker and J. Bridgeman, Relating freshwater organic matter fluorescence to organic carbon removal efficiency in drinking water treatment, Sci. Total Environ., 2009, 407, 1765–1774 CrossRef CAS PubMed.
- N. Hudson, A. Baker and D. Reynolds, Fluorescence analysis of dissolved organic matter in natural, waste and polluted waters—a review, River Res. Appl., 2007, 23, 631–649 CrossRef.
- A. Huguet, L. Vacher, S. Relexans, S. Saubusse, J.-M. Froidefond and E. Parlanti, Properties of fluorescent dissolved organic matter in the Gironde Estuary, Org. Geochem., 2009, 40, 706–719 CrossRef CAS.
- W. Moyo, N. Chaukura, T. A. Msagati, B. B. Mamba, S. G. Heijman and T. T. Nkambule, The properties and removal efficacies of natural organic matter fractions by South African drinking water treatment plants, J. Environ. Chem. Eng., 2019, 7, 103101 CrossRef CAS.
- Y. Shutova, A. Baker, J. Bridgeman and R. K. Henderson, Spectroscopic characterisation of dissolved organic matter changes in drinking water treatment: From PARAFAC analysis to online monitoring wavelengths, Water Res., 2014, 54, 159–169 CrossRef CAS PubMed.
- P. Wang, S. Ding, G. An, R. Qu, X. Liu, C. Fang and W. Chu, Removal of disinfection by-product precursors by Al-based coagulants: A comparative study on coagulation performance, J. Hazard. Mater., 2021, 420, 126558 CrossRef CAS PubMed.
- P. Krzeminski, C. Vogelsang, T. Meyn, S. Köhler, H. Poutanen, H. de Wit and W. Uhl, Natural organic matter fractions and their removal in full-scale drinking water treatment under cold climate conditions in Nordic capitals, J. Environ. Manage., 2019, 241, 427–438 CrossRef CAS PubMed.
- L. Yang, J. Hur and W. Zhuang, Occurrence and behaviors of fluorescence EEM-PARAFAC components in drinking water and wastewater treatment systems and their applications: a review, Environ. Sci. Pollut. Res., 2015, 22, 6500–6510 CrossRef CAS PubMed.
- Q. V. Ly, M.-H. Lee and J. Hur, Using fluorescence surrogates to track algogenic dissolved organic matter (AOM) during growth and coagulation/flocculation processes of green algae, J. Environ. Sci., 2019, 79, 311–320 CrossRef CAS.
- D. Xu, L. Bai, X. Tang, D. Niu, X. Luo, X. Zhu, G. Li and H. Liang, A comparison study of sand filtration and ultrafiltration in drinking water treatment: Removal of organic foulants and disinfection by-product formation, Sci. Total Environ., 2019, 691, 322–331 CrossRef CAS.
- E. N. Hidayah, Y. N. Pramitasari, Y. H. Hsien and O. H. Cahyonugroho, Fluorescence organic carbon components and its correlation with bulk organic parameters in water treatment processes, J. Appl. Sci. Eng., 2020, 23, 391–396 Search PubMed.
- T. E. Kraus, C. A. Anderson, K. Morgenstern, B. D. Downing, B. A. Pellerin and B. A. Bergamaschi, Determining sources of dissolved organic carbon and disinfection byproduct precursors to the McKenzie River, Oregon, J. Environ. Qual., 2010, 39, 2100–2112 CrossRef CAS PubMed.
- J. Hur, M.-H. Lee, H. Song and M. A. Schlatman, Microbial transformation of dissolved organic matter from different sources and its influence on disinfection byproduct formation potentials, Environ. Sci. Pollut. Res., 2013, 20, 4176–4187 CrossRef CAS PubMed.
- J. J. Melendez-Perez, M. J. Martínez-Mejía, A. T. Awan, P. S. Fadini, A. A. Mozeto and M. N. Eberlin, Characterization and comparison of riverine, lacustrine, marine and estuarine dissolved organic matter by ultra-high resolution and accuracy Fourier transform mass spectrometry, Org. Geochem., 2016, 101, 99–107 CrossRef CAS.
- J. A. Field, in Organohalide-respiring bacteria, Springer, 2016, pp. 7–29 Search PubMed.
- Y. Qian, Y. Chen, D. Hanigan, Y. Shi, S. Sun, Y. Hu and D. An, pH adjustment improves the removal of disinfection byproduct precursors from sedimentation sludge water, Resour., Conserv. Recycl., 2022, 179, 106135 CrossRef CAS.
- Z. Yuan, C. He, Q. Shi, C. Xu, Z. Li, C. Wang, H. Zhao and J. Ni, Molecular insights into the transformation of dissolved organic matter in landfill leachate concentrate during biodegradation and coagulation processes using ESI FT-ICR MS, Environ. Sci. Technol., 2017, 51, 8110–8118 CrossRef CAS PubMed.
- C. W. Chow, R. Fabris, J. V. Leeuwen, D. Wang and M. Drikas, Assessing natural organic matter treatability using high performance size exclusion chromatography, Environ. Sci. Technol., 2008, 42, 6683–6689 CrossRef CAS PubMed.
- M. Gonsior, P. Schmitt-Kopplin, H. Stavklint, S. D. Richardson, N. Hertkorn and D. Bastviken, Changes in dissolved organic matter during the treatment processes of a drinking water plant in Sweden and formation of previously unknown disinfection byproducts, Environ. Sci. Technol., 2014, 48, 12714–12722 CrossRef CAS.
- G. Hua and D. A. Reckhow, Characterization of disinfection byproduct precursors based on hydrophobicity and molecular size, Environ. Sci. Technol., 2007, 41, 3309–3315 CrossRef CAS.
- H. Zhang, Y. Zhang, Q. Shi, S. Ren, J. Yu, F. Ji, W. Luo and M. Yang, Characterization of low molecular weight dissolved natural organic matter along the treatment trait of a waterworks using Fourier transform ion cyclotron resonance mass spectrometry, Water Res., 2012, 46, 5197–5204 CrossRef CAS PubMed.
- H. Zhang, Y. Zhang, Q. Shi, H. Zheng and M. Yang, Characterization of unknown brominated disinfection byproducts during chlorination using ultrahigh resolution mass spectrometry, Environ. Sci. Technol., 2014, 48, 3112–3119 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ew00609j |
| This journal is © The Royal Society of Chemistry 2023 |