Visualizing morphological structures of rice grains in precooked products using synchrotron radiation X-ray phase-contrast computed tomography
Hiromi
Miki
 *ab,
Akio
Yoneyama
*ab,
Akio
Yoneyama
 bc and
Keiichi
Hirano
bc and
Keiichi
Hirano
 ab
ab
aDepartment of Materials Structure Science, School of High Energy Accelerator Science, SOKENDAI (The Graduate University for Advanced Studies), Tsukuba, Ibaraki 305-0801, Japan. E-mail: hiromim@post.kek.jp
bInstitute of Materials Structure Science, High Energy Accelerator Research Organization, Tsukuba, Ibaraki 305-0801, Japan
cSAGA Light Source, Tosu, Saga 841-0005, Japan
First published on 6th December 2022
Abstract
Rice is a staple food eaten by more than half of the world's population. In recent years, various types of precooked rice products have become commercially available as more and more people, including the elderly, do not have time to prepare meals or are coping with eating disorders. To address this situation, analysis of the internal structure of rice is important to understand the phenomena that occur during cooking rice grains and to develop rice products for specific purpose with desired physical properties. Although the internal structure of food has been observed by many methods, it is difficult to image the internal structure of rice porridge or rice grains with sufficient spatial resolution in a nondestructive manner. In this study, we applied X-ray interferometer-based phase-contrast computed tomography for the nondestructive observation of precooked rice products. For comparison, X-ray absorption-contrast images were also observed. The samples consisted of packaged aseptic cooked rice, precooked rice porridge for the general public, and a texture-modified rice product for people with dysphagia. In the phase-contrast two-dimensional (2D) tomographic images, the central lines of the cooked rice grains were successfully observed in all samples. The changes in the peripheral regions and internal hollows of the cooked rice grains were also clearly observed. Meanwhile, rice grains were hardly discernible in the X-ray absorption images. These results indicate that X-ray interferometer-based phase-contrast imaging is a promising tool for analyzing the morphology and internal structure of cooked rice grains in various prepacked products such as rice porridge and texture-modified rice.
Introduction
Rice is a staple food eaten by more than half of the world's population.1 Until now, consumers have purchased raw rice grains and cooked them at home by soaking in water, steaming, or boiling.2,3 In recent years, however, the demand for precooked rice products has increased as lifestyles have become more diverse. In particular, with the aging of the population worldwide, there is a need for texture-modified precooked rice for people with dysphagia and/or dysmasesis who live at home rather than in hospitals where properly prepared meals are provided; consequently, daily meal preparation is a burden for them and their caregivers. The importance of precooked rice is also increasing for stockpiling food in preparation for disaster.To address this situation, analysis of the internal structure of rice grains is important to understand the phenomena that occur when rice grains are cooked and to develop rice products for specific purpose with the desired physical properties. The internal structure of rice grains undergoes physicochemical changes during the cooking process, and could affect palatability and texture.4,5 Precooked rice products are convenient because they are ready-to-eat or ready-to-heat. However, taste and flavor are also important when it comes to consumer preferences and food choices. Therefore, analysis of the internal structure of rice grains would be useful to improve the quality of cooked rice products. Changes in the internal and surface structures of cooked rice grains have been visually observed through various methods such as nuclear magnetic resonance (NMR) microimaging,6,7 light microscopy,2,8 scanning electron microscopy (SEM),2,8,9 stereomicroscopy with transmission and fluorescence modes,10 light transmittance photography,11 and X-ray absorption-contrast imaging.11 However, analysis by light microscopy, SEM, and stereomicroscopy has the disadvantage of requiring destructive pretreatments such as slicing, freezing, and staining. NMR microimaging, on the other hand, is a nondestructive test and allows observation of an intact sample without destructive pretreatment. However, NMR has inherently low sensitivity, and a strong magnetic field must be applied to achieve a high spatial resolution. To generate a higher magnetic field, the diameter of the RF coil in which a sample is set must be smaller, which limits the size of the sample. As for light transmission photography, although it can detect most internal hollows in rice, its resolution is limited to that of the naked eye. The detection of internal hollows by X-ray absorption-contrast imaging is inferior to that by light transmission photography.11 For analysis in X-ray absorption-contrast computed tomography (CT), it is difficult to detect rice and rice porridge without contrast agent.12 One of the most promising candidates for avoiding these problems is X-ray phase-contrast imaging, which has a high sensitivity, especially for samples composed of light elements, such as hydrogen, carbon, and oxygen, and can nondestructively observe the internal structure of large samples with sufficient spatial resolution.
Conventionally, contrast in X-ray imaging is generated by the absorption of X-rays by the sample, i.e., by the intensity of the beam. However, when X-rays go through a sample, they are subject to physical interactions other than absorption, such as refraction and scattering. Refraction is a change in the direction of X-ray propagation caused by a slight shift in the X-ray wavefront, and scattering is a slight broadening of the X-rays divergence angle. X-ray phase-contrast imaging uses refraction or scattering information, which have not been focused on so far, to compose image contrast. Compared to conventional X-ray absorption-contrast imaging, X-ray phase-contrast imaging has a much higher sensitivity, especially for objects composed of light elements.13,14 Thanks to this advantage, the applications for X-ray phase-contrast imaging have rapidly expanded in recent years. Currently, X-ray phase-contrast imaging is categorized into four methods: crystal-interferometer-based imaging, grating-interferometer-based imaging, analyzer-based imaging, and propagation-based imaging.14,15 Among these methods, crystal-interferometer-based imaging was used for this study because it has the highest sensitivity to light elements and is most suitable for observing rice products with small density differences without using a contrast agent.16 X-ray phase-contrast imaging method is not common in food science. However, the use of X-ray phase-contrast imaging is gradually spreading to the observation of air-free, frozen processed or cooked foods such as frozen tuna,17 frozen soybean curd,17 raw and cooked beef,18 and cooked vegetable soybean (edamame).19 In particular, such non-aerated, frozen or heat-treated soft foods show little contrast in conventional X-ray absorption images. Therefore, the X-ray phase-contrast imaging is considered promising. By understanding the differences in structure between the texture-modified rice product currently available on the market and generally commercial precooked rice, which cannot be visualized by conventional methods, we can gain some important features for developing foods with desirable physical properties and structure that are easy for people with dysphagia to eat. In this study, we compared the morphology and internal structure of various types of industrially cooked rice using X-ray phase-contrast imaging and verified the usefulness of this innovative method.
Materials and methods
Samples
The samples consisted of three types of industrially precooked rice products. Sample 1 (S1) was packaged aseptic cooked rice (Sato No Gohan Ginsyari, Sato Foods Co., Ltd, Niigata, Japan). The length and width of the 20 rice grains in S1 were measured using a caliper (MITUTOYO, Kanagawa Japan) and RStudio.20 Sample 2 (S2) was precooked rice porridge for the general public (Ajinomoto KK Okayu (Shirogayu), Ajinomoto Co., Inc., Tokyo, Japan). Sample 3 (S3) was a texture-modified rice product (Kewpie Yasashii Kondate Yawaraka Gohan, Kewpie Corporation, Tokyo, Japan). S3 is classified as ‘mashable with tongue’ under the Universal Design Food (UDF) criteria set by the Japan Care Food Conference. The UDF criteria are used to classify commercially available texture-modified foods into four categories based on texture, food properties, chewing power, and ease of swallowing. The UDF criteria can be applied to a wide range of diets, from daily meals to meals for people with swallowing difficulties.21 In accordance with the package instructions, S1 was heated for 2 min in a microwave oven. S2 and S3 did not need to be heated before eating. The samples were bought at local retail stores in Japan. All samples were made with nonglutinous rice. The package and appearance of each sample are shown in Fig. 1. The cultivars and growing conditions of the precooked rice selected as samples were not disclosed. Therefore, it was not possible to compare the raw rice grains with the samples used in this study.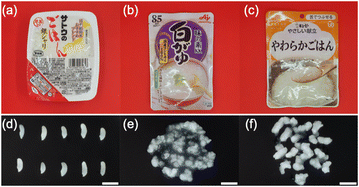 | ||
| Fig. 1 Sample package of (a) S1, (b) S2, and (c) S3. Appearance of (d) S1, (e) S2, and (f) S3. The scale bar is 10 mm. | ||
Synchrotron radiation X-ray phase-contrast imaging of rice products
Fig. 2(b) schematically shows the X-ray interferometer used in this study.22 The X-ray interferometer consists of two silicon crystal blocks with four half mirrors. First, the monochromatic incident X-ray beam is split into two coherent beams by the first half mirror. Then, the two coherent beams overlap and interfere with each other at the last half mirror. A sample is inserted in one of the two coherent beam paths. The interference patterns produced by the sample are observed by an X-ray area sensor.23 A phase-contrast projection image (phase map) of the sample can be obtained by the fringe-scanning method.13,24 In addition, three-dimensional phase-contrast images can be obtained by CT. For the CT measurement, the sample was mounted on a rotating stage.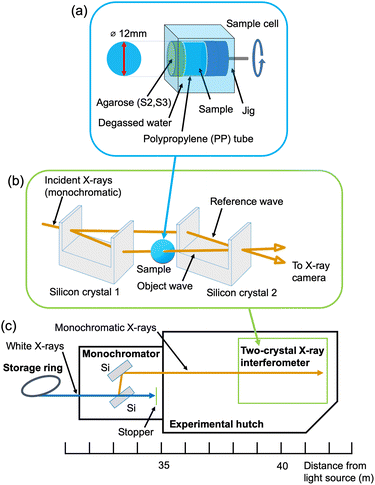 | ||
| Fig. 2 (a) Sample cell, (b) X-ray interferometer, and (c) layout of beamline BL-14C of the Photon Factory. | ||
The experiment was carried out at beamline BL-14C of the Photon Factory (Tsukuba, Japan), where the X-ray interferometer-based phase-contrast CT system is dedicated (Fig. 2(c)). The characteristics of the imaging system were as follows: fiber-coupled X-ray sCMOS imager (Zyla 5.5 HF, Andor Technology Ltd, Belfast UK); number of pixels, 2560 × 2160; pixel size, 6.5 μm; binning, 2 × 2; field of view, 16 mm × 13 mm; density resolution, < 0.5 mg cm−3; spatial resolution, 20–30 μm.25 The measurement parameters were as follows: X-ray energy, 17.8 keV; exposure time, 4.0 s per projection; number of projections, 500; total measurement time for each sample, about 3 hours. For comparison, X-ray absorption-contrast CT using synchrotron radiation was also performed at beamline BL-14B of the Photon Factory. The characteristics of the imaging system were as follows: X-ray CCD camera (FDI-VHR 16M 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Air-Cooled, Photonic Science, Saint Leonards-on-sea UK); number of pixels, 4872 × 3248; pixel size, 7.4 μm; field of view, 36.1 mm × 24 mm. The measurement parameters were as follows: X-ray energy, 17.8 keV; exposure time for S1, 300 ms per projection; exposure time for S2 and S3, 1.0 s per projection; number of projections, 251; total measurement time for each sample, about 20–30 minutes.
1 Air-Cooled, Photonic Science, Saint Leonards-on-sea UK); number of pixels, 4872 × 3248; pixel size, 7.4 μm; field of view, 36.1 mm × 24 mm. The measurement parameters were as follows: X-ray energy, 17.8 keV; exposure time for S1, 300 ms per projection; exposure time for S2 and S3, 1.0 s per projection; number of projections, 251; total measurement time for each sample, about 20–30 minutes.
Additionally, Fiji was employed to measure the size of rice grains.26
S1 was prepared by soaking in 1% agarose aqueous solution in a polyethylene bottle for 30 min. The purpose of this process was to fill the empty spaces within the grains that cause noise during the image reconstruction. During this soaking process, the polyethylene bottle was placed in 80 °C water in a stainless steel vacuum bottle to prevent the agarose solution from solidifying. S1 and the agarose solution were then placed together in a polypropylene (PP) tube. S2 and S3 were not immersed in agarose solution, but instead were placed directly into PP tubes, because they already contained liquid and there were no empty spaces within the grains. The PP tubes for S2 and S3 were covered with agarose to prevent the samples from leaking. The diameter of all the PP tubes was 12 mm. Note that no contrast agent was added to the samples because X-ray interferometer-based phase-contrast imaging has a much higher sensitivity to rice grains than conventional X-ray absorption-contrast imaging.
Fig. 2(a) schematically shows a sample cell. Each PP tube was placed in the cell filled with degassed water in order to reduce the large phase shift between the sample and the surroundings. The sample cell was inserted in one of the two coherent beam paths of the X-ray interferometer. For acquiring X-ray absorption-contrast images, the 12 mm diameter PP tube containing the sample was further set into a 15 mm diameter PP tube instead of the cells. The area between the tubes was filled with degassed water.
Results and discussion
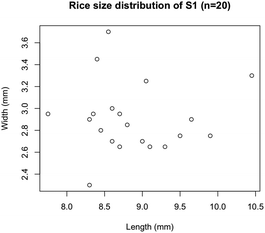
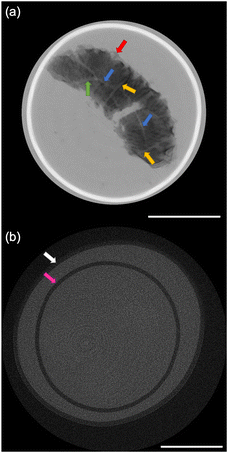
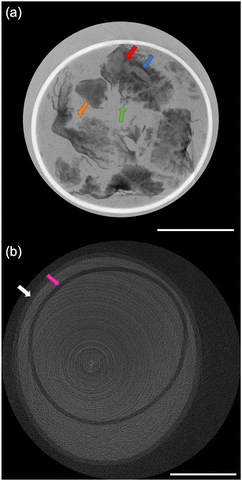
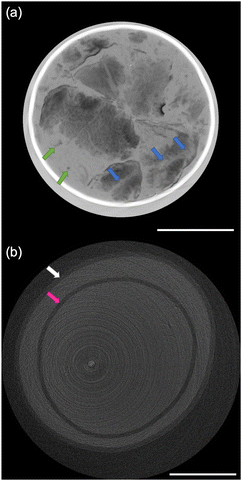
The size distribution of 20 rice grains in S1 is shown in Fig. 3. The average size of length and width of S1 are 8.87 ± 0.62 mm and 2.91 ± 0.31 mm, respectively. Note that S1 was blended with several varieties of Japanese-produced rice independently by the manufacturer27 and the varieties were not disclosed. The size of S1 appears to be slightly larger than the size of typical japonica rice. However, this is due to the possible effects of immersion in hot water in pretreatment, and the size of the cooked rice grains is similar to those produced in Japan by varieties such as ‘Hoshiyutaka’, ‘Nipponbare’, and ‘Milky Queen’.28Fig. 4–6 show the X-ray phase-contrast 2D tomographic images of the samples together with absorption-contrast images. In the phase-contrast images, the white circles around the rice grains indicate the PP tubes. In all samples, the central lines in the rice grains, indicated by blue arrows, were successfully observed. Horigane et al. reported that the central line and transverse cracks of rice grains during soaking process were observed with a spatial resolution of 65 × 65 × 130 μm3 and 65 × 65 × 65 μm3 using NMR.29 Thus, the spatial resolution of X-ray interferometer-based phase-contrast CT system was considered adequate to visualize the central line and the other structures in rice grains. On the other hand, the shape and structure of rice grains were hardly discernible in the X-ray absorption CT images, although PP tubes were visualized.
The central line is the last area where the embryo sac cavities are filled with endosperm cells; this area becomes the central point of the radial arrangement of endosperm cells when observing the cross-section of grain of rice.30 This result is significant for the research and development of rice products because the central lines inside the rice grains are closely related to water absorption. Water absorption plays an essential role in the change of the physicochemical properties and texture of the rice during the cooking process. It should also be noted that in Fig. 5(a) and 6(a), not only can the mixture of partially broken rice grains and liquid be clearly observed, but the fragments separated from the partially broken rice grains into the water, indicated by the green arrows, are also well visualized. The degree of mixture and fragments are considered to affect texture and eating quality.
Fig. 4(a) shows the 2D tomographic image of a whole cooked rice grain in S1. The length and width of the rice grain in S1 were measured to be 10.69 mm and 3.69 mm, respectively. The blue, yellow, and green arrows show the central lines, transverse hollows, and radial hollows, respectively, in the rice grain. Horigane et al. reported the results of NMR microimaging of the internal hollows of rice grains during cooking.6 The results of our study are consistent with those reported. Fig. 4(a) also shows a crack that opened in the middle part of the rice grain. This crack was caused by bursting along the transverse hollows during cooking in excess water. According to the report by Horigane et al., this kind of hollow has long been known.6 The indistinct structure in Fig. 4(a), indicated by the red arrow, shows the adherent layer on the rice grain surface formed by the accumulation of materials leached from rice grains during the cooking process.8,31 This change in the peripheral regions of the rice grains can be attributed not only to the general cooking process, but also to the extra soaking in S1 during the pretreatment. Some researchers have reported the leached materials affect the changes in the texture and eating quality of rice grains.31–33 This result is also important for the development and improvement of rice products.
In Fig. 5(a), the orange arrow indicates the laceration of a cooked rice grain in S2. It is due to the bursting of the central line of the rice grain caused by the pressure increase associated with the penetration of boiling water into the rice grains during the cooking process. The red arrow indicates the expanded central line on the verge of bursting. This feature contrasts with the fact that swelling and bursting of the central line was not observed in S1. This difference between S1 and S2 is considered to reflect the difference in the cooking process.
In Fig. 6(a), the rice grains of S3 are broken along transverse cracks or hollows, which is different from the laceration along the central line of the rice grains of S2 in Fig. 5(a). The rice grains in S3 also retain their shape to a relatively greater degree than those in S2. The difference between S2 and S3 may be due to the difference in the cooking method between rice porridge and texture-modified cooked rice. It is worth mentioning that the difference in the direction in which the rice grain is broken is considered to affect the ease of eating, texture, eating quality, and palatability.
Conclusions
X-ray interferometer-based phase-contrast computed tomography was applied for the nondestructive observation of the prepacked rice products. The central lines of the rice grains in the three types of precooked rice products were successfully visualized. For the packaged aseptic cooked rice product, the changes in the peripheral regions and internal hollows of the rice grains were also clearly observed. For the precooked rice porridge and texture-modified rice product, not only was the mixture of rice grains and liquid clearly observed, but the fragments separated from the rice grains into the water were also well visualized. On the other hand, the shape and structure of rice grains were hardly discernible in the X-ray absorption images. These results clearly show that X-ray interferometer-based phase-contrast imaging can visualize internal structure which are hardly discernible in X-ray absorption-contrast imaging, and indicate that X-ray phase-contrast imaging is a promising tool for nondestructive analysis of the morphology and internal structure of cooked rice grains in various prepacked rice products. Further study should reveal the relationship between the morphology and internal structure of cooked rice grains, the fragments separated from rice grains, the texture, and the eating quality for developing various types of precooked rice products.Author contributions
Hiromi Miki: conceptualization, data curation, formal analysis, investigation, project administration, resources, visualization, writing – original draft. Akio Yoneyama: investigation, methodology, software, visualization, writing – review & editing. Keiichi Hirano: supervision, validation, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Ms Kyoko Fukabori and Ms Hiroko Oshima, Public Relations Office, Institute of Materials Structure Science, Tsukuba, Japan, for taking the sample package and appearance picture.References
-
S. Pandey, D. Byerlee, D. Dawe, A. Dobermann, S. Mohanty, S. Rozelle and B. Hardy, Rice in the Global Economy: Strategic Research and Policy Issues for Food Security, International Rice Research Institute (IRRI), Los Baños, Philippines, 2010 Search PubMed
.
- Y. Ogawa, G. M. Glenn, W. J. Orts and D. F. Wood, Histological Structures of Cooked Rice Grain, J. Agric. Food Chem., 2003, 51, 7019–7023 CrossRef
.
- H. Tomita, M. Fukuoka, T. Takemori and N. Sakai, Development of the visualization and quantification method of the rice soaking process by using the digital microscope, J. Food Eng., 2019, 243, 33–38 CrossRef
.
- T. Sasahara, S. Katuyama and S. Tsunoda, Luster and Fine Surface Structure of Cooked Rice Grains in Relation to the Eating Quality, Jpn. J. Breed., 1980, 30, 58–64 CrossRef
.
- M. Tamura, T. Nagai, Y. Hidaka, T. Noda, M. Yokoe and Y. Ogawa, Changes in histological tissue structure and textural characteristics of rice grain during cooking process, Food Struct., 2014, 1, 164–170 CrossRef
.
- A. K. Horigane, H. Toyoshima, H. Hemmi, W. M. H. G. Engelaar, A. Okubo and T. Nagata, Internal Hollows in Cooked Rice Grains (Oryza sativa cv. Koshihikari) Observed by NMR Micro Imaging, J. Food Sci., 1999, 64, 1–5 CrossRef CAS
.
- A. K. Horigane, W. M. H. G. Engelaar, H. Toyoshima, H. Ono, M. Sakai, A. Okubo and T. Nagata, Differences in Hollow Volumes in Cooked Rice Grains with Various Amylose Contents as Determined by NMR Micro Imaging, J. Food Sci., 2000, 65, 408–412 CrossRef CAS
.
- R. Okuda, T. Ishimura and A. Kanatani, Study on Rice cooking, Part 1 : Effect of Solid Components in the Rice Cooking Liquid on the Rice Grain Surface, J. Cookery Sci. Jpn., 2009, 42, 394–403 Search PubMed
.
- O. Rewthong, S. Soponronnarit, C. Taechapairoj, P. Tungtrakul and S. Prachayawarakorn, Effects of cooking, drying and pretreatment methods on texture and starch digestibility of instant rice, J. Food Eng., 2011, 103, 258–264 CrossRef
.
- M. Tamura and Y. Ogawa, Visualization of the coated layer at the surface of rice grain cooked with varying amounts of cooking water, J. Cereal Sci., 2012, 56, 404–409 CrossRef
.
- M. Suzuki, A. K. Horigane, H. Toyoshima, X. Yan, H. Okadome and T. Nagata, Detection of Internal Hollows in Cooked Rice Using a Light Transmittance Method, J. Food Sci., 1999, 64, 1027–1028 CrossRef CAS
.
- Y. Iida, M. Matsuoka, I. Shimizu, T. Wakisaka and A. Katsumata, Micro-CT observation of test food materials for videofluoroscopic swallowing studies, Oral Radiol., 2012, 29, 56–63 CrossRef
.
- A. Momose, Demonstration of phase-contrast X-ray computed tomography using an X-ray interferometer, Nucl. Instrum. Methods Phys. Res., Sect. A, 1995, 352, 622–628 CrossRef CAS
.
- A. Momose, Recent Advances in X-ray Phase Imaging, Jpn. J. Appl. Phys., 2005, 44, 6355–6367 CrossRef CAS
.
- M. Endrizzi, X-ray phase-contrast imaging, Nucl. Instrum. Methods Phys. Res., Sect. A, 2018, 878, 88–98 CrossRef CAS
.
- A. Momose, T. Takeda, A. Yoneyama, I. Koyama and Y. Itai, Phase-Contrast X-Ray Imaging Using an X-Ray Interferometer for Biological Imaging, Anal. Sci., 2001, 17icas, i527–i530 Search PubMed
.
- M. Sato, K. Kajiwara and N. Sano, Non-destructive Three-dimensional Observation of Structure of Ice Grains in Frozen Food by X-ray Computed Tomography Using Synchrotron Radiation, Jpn. J. Food Eng., 2016, 17, 83–88 CrossRef
.
- R. Miklos, M. S. Nielsen, H. Einarsdottir, R. Feidenhans'l and R. Lametsch, Novel X-ray phase-contrast tomography method for quantitative studies of heat induced structural changes in meat, Meat Sci., 2015, 100, 217–221 CrossRef
.
- M. Hidaka, S. Miyashita, N. Yagi, M. Hoshino, Y. Kogasaka, T. Fujii and Y. Kanayama, High-Resolution X-ray Phase-Contrast Imaging and Sensory and Rheometer Tests in Cooked Edamame, Foods, 2022, 11, 730 CrossRef
.
-
RStudio Team, RStudio: Intergrated Development for R (Version 2021.9.0.351), RStudio, PBC, Boston, MA, 2020 Search PubMed
.
- Japan Care Food Conference, Universal Design Food to wa, https://www.udf.jp/outline/udf.html, (accessed September 2022).
- A. Yoneyama, A. Momose, I. Koyama, E. Seya, T. Takeda, Y. Itai, K. Hirano and K. Hyodo, Large-area phase-contrast X-ray imaging using a two-crystal X-ray interferometer, J. Synchrotron Radiat., 2002, 9, 277–281 CrossRef PubMed
.
- A. Momose, T. Takeda, Y. Itai and K. Hirano, Phase-contrast X-ray computed tomography for observing biological soft tissues, Nat. Med., 1996, 2, 473–475 CrossRef PubMed
.
- A. Momose, Phase-sentisive imaging and phase tomography using X-ray interferometers, Opt. Express, 2003, 11, 2303–2314 CrossRef PubMed
.
- Andor Technology, https://andor.oxinst.com/assets/uploads/products/andor/documents/High-Energy-Detection-Gatefold.pdf, (accessed September 2022).
- J. Schindelin, I. Arganda-Carreras, E. Frise, V. Kaynig, M. Longair, T. Pietzsch, S. Preibisch, C. Rueden, S. Saalfeld, B. Schmid, J.-Y. Tinevez, D. J. White, V. Hartenstein, K. Eliceiri, P. Tomancak and A. Cardona, Fiji: an open-source platform for biological-image analysis, Nat. Methods, 2012, 9, 676–682 CrossRef PubMed
.
- Sato Foods Co. Ltd., Ginsyari 200g, https://www.satosyokuhin.co.jp/products/products_rice_161.html, (accessed September 2022).
- M. Otahara, M. Kitahara, K. Ohishi and M. Kasai, Evaluation of Staling of Cooked Rice by Spectrophotometric and Image Analyses of Squashed Cooked Rice Grain, Nippon Shokuhin Kagaku Kogaku Kaishi, 2018, 65, 170–182 CrossRef
.
- A. K. Horigane, H. Takahashi, S. Maruyama, K. i. Ohtsubo and M. Yoshida, Water penetration into rice grains during soaking observed by gradient echo magnetic resonance imaging, J. Cereal Sci., 2006, 44, 307–316 CrossRef
.
-
K. Hoshikawa, in Science of the rice plant, ed. T. Matuo and K. Hoshikawa, Food and Agriculture Policy Reseach Center, Tokyo, 1993, ch. 2, pp. 339–376 Search PubMed
.
- J. Patindol, X. Gu and Y.-J. Wang, Chemometric analysis of cooked rice texture in relation to starch fine structure and leaching characteristics, Starch/Staerke, 2010, 62, 188–197 CrossRef
.
- I. Hanashiro, K. Ohta, C. Takeda, H. Mizukami and Y. Takeda, Leaching of Amylose and Amylopectin during Cooking of Rice Grains and Their Effect on Adhesiveness of Cooked Rice, J. Appl. Glycosci., 2004, 51, 349–354 CrossRef
.
- T. Wada, T. Umemoto, N. Aoki, M. Tsubone, T. Ogata and M. Kondo, Starch Eluted from Polished Rice during Soaking in Hot Water is Related to the Eating Quality of Cooked Rice, J. Appl. Glycosci., 2010, 58, 13–18 CrossRef
.
| This journal is © The Royal Society of Chemistry 2023 |