Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A review on 2D-ZnO nanostructure based biosensors: from materials to devices
M. Sankush
Krishna
a,
Sangeeta
Singh
*a,
Maria
Batool
bc,
Heba Mohamed
Fahmy
*d,
Kondaiah
Seku
 e,
Ahmed Esmail
Shalan
e,
Ahmed Esmail
Shalan
 fg,
Senentxu
Lanceros-Mendez
fg,
Senentxu
Lanceros-Mendez
 fh and
Muhammad Nadeem
Zafar
fh and
Muhammad Nadeem
Zafar
 *b
*b
aMicroelectronics & VLSI lab, National Institute of Technology, Patna 800005, India. E-mail: sangeeta.singh@nitp.ac.in
bDepartment of Chemistry, University of Gujrat, Gujrat 50700, Pakistan. E-mail: znadeempk@gmail.com; nadeem.zafar@uog.edu.pk
cDepartment of Chemistry, University of Management and Technology, Sialkot Campus, Sialkot, 51310, Pakistan
dBiophysics Department, Faculty of Science, Cairo University, 12613, Egypt. E-mail: hfahmy@sci.cu.edu.eg
eEngineering Department, Civil Section (Applied Sciences – Chemistry), University of Technology and Applied Sciences, Shinas, Sultanate of Oman
fBCMaterials, Basque Center for Materials, Applications and Nanostructures, Martina Casiano, UPV/EHU Science Park, Barrio Sarriena s/n, Leioa 48940, Spain
gCentral Metallurgical Research and Development Institute (CMRDI), P. O. Box 87, Helwan, Cairo 11421, Egypt
hIKERBASQUE, Basque Foundation for Science, 48009 Bilbao, Spain
First published on 11th November 2022
Abstract
During the COVID’19 outbreak, biosensing devices won increasing relevance, demonstrating their potential in the medical diagnostic field. Hence, the present review reports on the main advances in 2D-ZnO nanostructure-based biosensors. So far, bulk ZnO has shown potential for biosensing, optical, and power electronic applications, mainly based on its wide band gap. In the post graphene era, its 2-D allotropes like ZnO sheets and ZnO nanoribbons have outperformed the bulk ZnO structures for specific applications. ZnO demonstrates various stable and feasible morphologies: nanotubes, nanowires, nanorods, nanosheets, nanoparticles, and nanobelts. As a matrix layer in biosensing applications, ZnO strongly binds to biomolecules due to its high isoelectric point (IEP) and shows a strong sensitivity due to the high surface-to-volume ratio. Further, ZnO nanostructures used as a matrix layer play an important role in inhibiting specific biological interactions and hence improve the sensitivity of sensing devices. Further, bioselective layers are typically immobilized onto ZnO either by direct adsorption or by covalent binding. ZnO based biosensors are categorized into optical, piezoelectric, and electrochemical biosensors, among others, based on their biosensing mechanism. In particular, electrochemical sensors produce signals via an electrical pathway for detecting and monitoring the target molecules. Optical sensors produce signals based on luminescence or reflectance, among others. Piezoelectric biosensors produce signals by mass loading of the piezoelectric material. ZnO-based FET biosensors are also reported, showing sensing application by the change in the channel's conductance. Further, recent literature on the detection of COVID-19 using ZnO nanostructures is presented.
1. Introduction
The global spread of COVID-19 has been labelled as pandemic by the World Health Organization (WHO). Among the best measures to properly address this issue has been the early diagnosis for the detection of the virus and precautionary measures. The limited availability of testing kits and professional operators made the rapid detection of the COVID-19 virus a challenge. COVID-19 carries a unique RNA sequence, and it can be detected by nucleic acid amplification tests (NAAT) or real-time reverse-transcription polymerase chain reaction (rRT-PCR).1,2 The second detection approach for the SARS-CoV-2 virus is the Enzyme-Linked Immunosorbent Assay (ELISA) IgG antibody test.3 The issue with the above methods is the cost of the testing kits and their commercial fabrication.Further, these methods are time-consuming, and need skilled staff with a possible risk of false positives and false negatives.4 Hence the fabrication of biosensors with low-cost for the selection of SARS-CoV-2 and other related viruses could prove to be very helpful. Over conventional testing equipment, biosensors are more reliable for COVID-19 detection due to their high specificity and sensitivity at low sample volumes.5 Advancements in nanotechnology introduced nanostructures in the construction of biosensors. Easy tailoring of nanostructures because of their high surface-to-volume ratio made it suitable to manufacture sensitive biosensors. The urgent need for diagnosis of COVID-19 stressed the development of point-of-care (POC) biosensor devices as they are easy to use, fast, cost-effective, portable, and user-friendly.6,7 POC devices involve spot testing and provide rapid access to the needed information. Thus POC testing is becoming of paramount importance in the biomedical field, particularly when resources are limited. An ideal POC device is generally obtained by designing the biosensors with the “sample-in-answer-out” mechanism, some of them being based on the detection of nucleic acids/proteins from respiratory samples/blood samples. To satisfy the increasing need for POC devices, device miniaturization is of paramount importance.8 Several distinct types of point-of-care (POC) devices have been developed over the past several years, each one based on a different type of portable device used to measure a different type of classical physical parameter. The microfluidic technology allows the development of small and portable systems for POC testing applications. Miniaturized biosensors rely on target identification to determine sensor specificity and enable detection of targets using a portable device or microfluidic platform. An effective tiny biosensor frequently has to be capable of integration, automation, and/or multiplex detection to be used in places where well-trained workers may not be readily available, in addition to the need for high sensitivity and specificity. Miniaturized biosensors for point-of-care use are becoming a commercial reality, and the research and development of portable, integrated, and automated biosensing technologies is a promising new field that could significantly improve the efficiency and quality of care for people living in low-resource areas. They are increasingly required in developing countries where medical facilities are not yet fully available.9
Among different nanomaterials for POC and biosensor development, metal oxide nanomaterials such as zinc, magnesium, tin, zirconium, and iron oxides, among others, have grabbed significant attention because of their functional properties, biocompatibility, diverse morphology, and catalytic properties.10 In particular, ZnO is being used for decades for biosensing applications as a matrix layer that strongly binds the biomolecules due to its high isoelectric point (IEP).
ZnO is being studied a lot for the design of new transducer platforms. This is one of the many nanostructured materials that are being used. Zinc oxide (ZnO) is a good metal–oxide semiconductor that can be used as an intracellular sensor or transducer. ZnO is a fantastic main transducer for generating electrical signals because its dimensions may be tailored to the size of the biological species being detected. Also, ZnO is a good material for designing biosensors because it is non-toxic, doesn't cost much and simpler to synthesize. People have also pointed out that ZnO is biocompatible and that it can have many different shapes that can be easily altered in a controlled manner to increase the surface area. Also, surface properties become more important at the nanoscale, so having varied morphologies and, therefore, varied surface conditions can make its properties change in ways that can be controlled and may be useful for sensing. ZnO has a strong adsorption efficiency with a high IEP of about 9.5.11 Since antibodies and enzymes have a lower isoelectric point than ZnO and prefer electrostatic interactions, the high IEP aids in immobilising them.
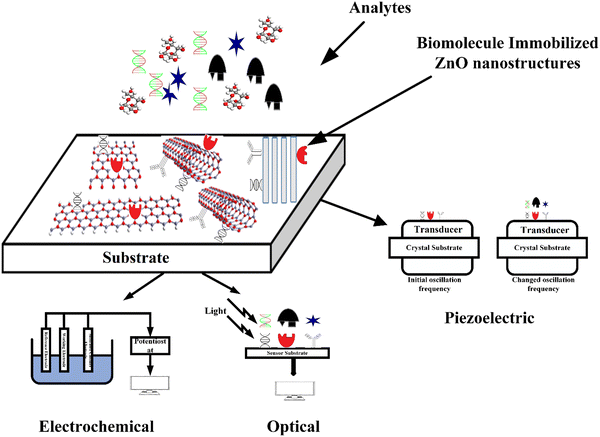
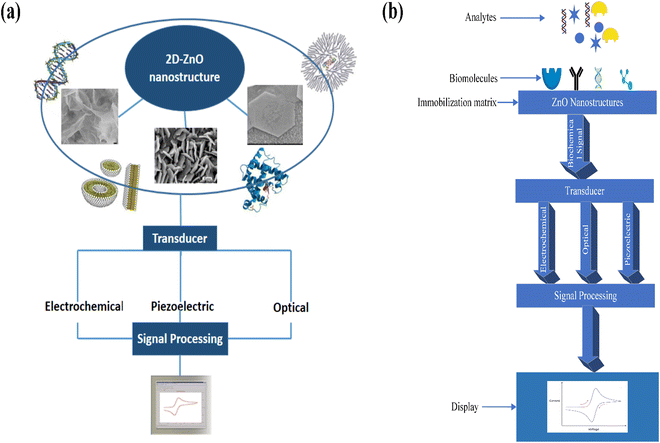
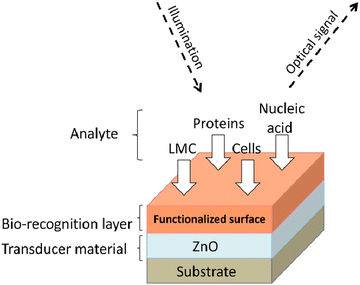
Further, its 2-D allotropes, including ZnO sheets and ZnO nanoribbons, outperform bulk ZnO structures for biosensing applications as they provide higher surface-to-volume ratio.12 The basic process of a biosensor and the state-of-the-art sensing mechanism used are shown in Fig. 1, with the biomolecules being immobilized on ZnO nanostructures which act as a matrix layer. When these biomolecules contact target analytes, they undergo reactions and produce signals which are sent to a transducer and read on a display. The biomolecules could be enzymes, proteins, DNA, and antibodies.
ZnO nanostructures have gained much attention for their application in sensors and actuators.13–15 ZnO is a semiconductor compound belonging to group II and VI metal oxides. It has a wide bandgap of about 3.37 eV and a large exciton emission energy of about 60 meV. Due to the wide band gap it is widely employed in optical, electrical, and optoelectronics applications.16 ZnO has a wide variety of nanostructures: zero dimensional nanostructures such as quantum dots,17 one-dimensional structures like nanorods,18 nanowires,19 and nanotubes,20 and two-dimensional structures like nanodiscs,21 nanobelts,22 and nanosheets.23 ZnO is the preferred candidate to be used as a matrix for biosensing because of its semiconducting properties and variety of morphologies. It can provide an effective channel for transportation of the carriers.
Further, its low cost, non-toxicity, ease of fabrication, and biocompatibility are key factors for selecting this material. 2D-ZnO, with its large surface-to-volume ratio, increases the active surface area of the sensor for biosensing.24 Certain enzymes are immobilized on biosensors for binding of specific target analytes like glucose oxidase (GOx) for glucose sensors25 and cholesterol oxidase (ChOx) for cholesterol sensors.26 The surface properties of the matrix layer influence enzyme immobilization.11 Nanoporous structures provide higher enzyme loading due to their high surface area.27,28 The enzyme can be directly immobilized through adsorption techniques or through cross-linking polymers.29 Different types of biosensors are gaining attention based on the sensing mechanism, with ZnO nanostructures being strong candidates for designing biosensors and POC devices requiring low sample volumes and low operation power.30
The present work provides a comprehensive review of ZnO-based biosensors with different types of sensing mechanisms. Immobilization criteria of biomolecules, both based on direct adsorption and through cross-linking agents, are discussed together with biosensor applications of different ZnO nanostructures. The different types of ZnO based biosensors are presented and discussed based on the detection method. Finally, ZnO-based biosensors for COVID-19 detection are presented.
2. Synthesis methods of ZnO nanostructures
Various approaches have been implemented for nanostructure synthesis which can be categorized into top-down and bottom-up methods.31 In the top-down approach, the process starts with the bulk material, and it is broken/exfoliated to nanosized structures, whereas in the bottom-up approach, the process starts at the atomic level, with the aggregation of atomic level components to produce the nanostructures. The main methods used to develop ZnO nanostructures are presented in the following.2.1. Pulsed laser deposition
In the pulsed laser deposition (PLD) method, the nanostructures are deposited by focusing a high-energy laser on the bulk crystal. Upon focusing the high-energy laser, the target ablates and deposits as the substrate's nanostructures are placed at a small distance away from the target. Kawakami et al.32 synthesized a 99% pure target of ZnO nanorods with sapphire as the substrate. Nanorods are produced at a substrate temperature of 400–700 °C and 1–10 torr oxygen pressure. The deposition time allows control of the length of rods, and 6 μm long rods with 300 nm diameter are produced after 30 minute deposition. Bae et al.33 synthesized nanocones at 580 °C temperature, 90–100 mJ per pulse energy, and 10 mTorr O2 atmosphere on a substrate. The PLD method ensures the stoichiometry and good quality of the nanostructures but demonstrates a limited area of nanostructure deposition.2.2. Sputtering
Sputtering enables flexibility in the growth of nanostructures over a wide area of substrates. In sputtering, Zn/ZnO is the target material and is bombarded with energetic ions like Ar. The collision causes the removal of target atoms that condense on the substrate. In the radio frequency (RF) magnetron sputtering process, a large magnetic field is developed at the target, allowing confinement of the plasma near the substrate. Liu et al.34 synthesized nanotubes by RF reactive sputtering with 99.99% pure Zn as the target material and PVP (polyvinyl pyrrolidone) fibers as the substrate. The tubes were deposited for 20 min with 7 × 10−4 Pa pressure followed by annealing at 600 °C. Chiou et al.35 synthesized nanowires on Cu/Ti/Si wafers at 0.05 torr pressure. Nanowires presented a diameter of around 45 nm with 5 min deposition and 55 nm with 30 min deposition. Nanorods with preferential growth in the (002) direction have been deposited by Venkatesh et al.36 with Ar sputtering at 0.01 mbar pressure and at a substrate temperature of 650 °C with 60 min deposition. Sputtering allows large area deposition, but the quality of the films is compromised relative to PLD.2.3. Chemical vapor deposition
Chemical vapor deposition (CVD) allow the synthesis of solid nanostructures in a gaseous phase. The chemical reactants/precursors are first vaporized and then deposited as solid structures on a substrate. There are different types of CVD processes like atmospheric CVD, metal–organic CVD (MOCVD), and plasma enhanced CVD (PECVD). Wu et al.37 synthesized ZnO nanorods with Zn acetylacetonate vaporized in a tube furnace at 30–140 °C with N2/O2 as carrier gases. Nanorods are deposited with (002) orientation at a substrate temperature of 500 °C with 60–80 nm diameter. Bae et al.38 synthesized sulfur-doped nanowires with Au as the catalyst. The sulfur powder is placed at the beginning of the tube, whereas the Zn powder is placed at the center. Structures grown by MOCVD present good crystallinity with weak deep-level emissions. Kim et al.39 synthesized nanowires with N2O gas flow rate at 1250 μmol min−1 and diethyl zinc (DEZ) as the precursor. Wu et al.40 reported nanotubes with DEZ and O2. The nanotubes were oriented along the (001) direction with 50–150 nm diameter and a few micrometers of length. Further, Liu et al.41 synthesized nanorods with the PECVD process. DEZ and O2 as precursors were placed in a bubbler at −26 °C and conveyed to reaction chambers with He gas. Oxygen gas was separately introduced, and microwave plasma at 3–20 mTorr was maintained in the chamber. Later, the substrates were heated to 700 °C, and the nucleation process by pulsing DEZ vapor into the chamber was carried out.Though CVD has the advantage of good quality nanostructures and high deposition rates, it still has a few drawbacks. The precursors used in CVD could be costly and toxic, especially the ones used in MOCVD. Moreover, by-products of the process are hazardous, and high temperature is needed for deposition, limiting substrate availability.42
2.4. Sol–gel
Compared to physical vapor deposition techniques, sol–gel is a low-cost and large area deposition technique, also compatible with high deposition rates,43 while maintaining excellent control of the morphologies of the nanostructures. Sol–gel methods mainly involve three steps. Firstly, preparation of the sol from starting materials. Secondly, deposition of the sol onto the substrate with a suitable deposition method and then a final heat treatment of the xerogel film. Ahn et al.44 synthesized ZnO nanorods with the sol prepared from Zn(NO3)2·6H2O and hexamethylenetetramine (HMTA) in equal proportion. The temperature should be around 95 °C, and crystallite ZnO nanorods with hexagonal wurtzite structures are deposited on the SiO2/Si substrate. The diameter of the nanorods can be varied by varying the concentration of the starting materials. For the nanotubes and nanowires grown by Wu et al.,45 an aqueous solution of Zn nitrate hexahydrate dissolved in deionized water with urea was used for sol preparation. Under the same growth conditions, nanowires were obtained after 24 hours of deposition and nanotubes after 48 h. Several precursors are used, such as Zn nitrates, chlorides, alkoxides, and Zn acetate dihydrate. Alkoxides are not preferred due to their insolubility in alcohols, inorganic salts like nitrates lead to difficulty in removing the anionic species in the final product,46 whereas using acetate dihydrate produces volatile by-products. A brief review of the sol–gel process is provided by Lamia Znaidi et al.472.5. Spray pyrolysis
Spray pyrolysis is a powerful tool to obtain high-quality ZnO nanostructures at a low cost. The morphology and stoichiometry can be controlled effectively by properly determining the growth conditions and the ratio of starting materials. The spray pyrolysis method involves preparation of the solution containing the precursor, followed by the generation of mist from it and then transfer into the furnace tube by passing conveyer gas. Finally, some materials are deposited on the substrate, and others are vaporized. The deposition temperature must be selected such that all other materials except the desired ones are volatile. Htay et al.48 prepared ZnO nanowire structures on glass substrates with zinc acetate and deionized water as starting solutions. The pH is adjusted with ammonium acetate. The structures were deposited at 250–400 °C temperature. Karber et al.49 synthesized nanorods using ZnCl2 as a precursor with soda-lime glass and indium-coated tin oxide (ITO) glass substrates. The structures grown at 550 °C are found to have good optical quality. However, spray pyrolysis still demonstrates a low yield.2.6. Molecular beam epitaxy
In molecular beam epitaxy (MBE), the crystals are grown by the interactions of the beam of atoms or molecules with the substrate, with sublimation of source materials producing the beam of molecules. Tien et al.50 synthesized ZnO nanorods using metallic Zn, and O3/O2 plasma as precursors. Ag islands were developed on SiO2 and SiNx in the range of 8–30 and 10–65 nm respectively. The base pressure was 5 × 10−8 mbar, and O3/O2 pressure varied from 5 × 10−6 to 5 × 10−4 mbar. ZnO nanorods were developed selectively on Ag islands at 600 °C. Heo et al.51 reported nanowires on a Ag coated Al2O3 substrate with 30–150 nm diameter and 14 μm length. Feng et al.52 synthesized nanotubes with a plasma-assisted MBE process, with Si being used as the substrate material. MBE is particularly suitable for synthesizing nanostructures with good quality, but it is a slow process and not preferred for large-scale production.2.7. Electrochemical deposition
In this process, the nanostructures are deposited onto the substrate surface by simple electrolysis of the solution containing the desired material and its complexes. Precise control of the dimensions is possible in this method, and it is low cost and simple, operates at low temperatures, and large area deposition is possible. Hames et al.53 synthesized nanorods by using three-electrode systems. The structures are deposited on both ITO and ZnO-coated ITO substrates. By using a glass/SnO2:F working electrode, a saturated calomel electrode (SCE) as a reference electrode, and a Pt wire counter electrode, Elias et al.54 were able to calibrate their measurements. The electrolyte was 5 mM KCl and 0.1 M ZnCl2 solution. In the first step, the working electrode is coated with a ZnO buffer layer, and then nanorods are deposited on it. The diameter of the rods can be varied by varying the ZnCl2 concentration. Tang et al.55 synthesized nanotubes with a similar setup.2.8. Thermal evaporation
In the thermal evaporation process, the source material is heated at a higher temperature, and the evaporated material gets deposited on the substrate. Umar et al.56 synthesized ZnO nanorods with ZnO powder as the source and Ni coated Si as the substrate. N2 and O2 gases were pumped at 5–15 and 10–30 sccm, respectively, and the deposition time was 30–120 minutes. 4–5 μm long ZnO nanorods with typical diameters of 300–350 nm were grown at 500–550 °C temperature. Bae et al.57 synthesized nanowires with temperature in the range of 800–1000 °C at 500 sccm of Ar gas. Ga was doped by placing Ga powder along with ZnO powder. Tin (Sn) and indium (In) can be doped similarly by placing Sn and In powders along with ZnO powder. Nanotubes with an outer radius of 200 nm and (001) preferential growth were synthesized at 475 °C by Zhang et al.58 Here Zn powder was the source material and Si wafer was the substrate. Argon was pumped into the tube furnace, and O2 and O3 acted as the source of oxidation for Zn to form ZnO. Thermal evaporation does not use any catalyst, which avoids unintentional contaminants. However, high temperature limits the available substrates for the synthesis of ZnO nanostructures.2.9. Hydrothermal method
The hydrothermal method is often preferred over other methods as it provides nanostructures with controlled morphology and composition. ZnO nanostructures can be deposited at low temperatures, and the method is simple and catalyst-free. Since it is catalyst-free, the purity of the nanostructures can be high. In the hydrothermal process, zinc nitrate hexahydrate is used as a precursor with other materials like hexamethylene tetramine (HMTA). Ammonium compounds can be used to synthesize nanostructures. Wang et al.59 synthesized ZnO nanowires. 1 M (NH4)2CO3 and 0.04% PEG dissolved in distilled water with Zn nitrate were used as precursors. Zinc nitrate was gradually dropped into the solution. The solution was spin-coated on a substrate, and the process was carried out at 200 °C for 10 h. Tam et al.60 synthesized ZnO nanorods. Zinc nitrate hexahydrate and HMTA were used as starting materials and polyethylene was added to enhance the aspect ratio. The obtained rods were annealed at 200 °C, 400 °C, and 600 °C, and the deposited structures showed (001) crystallographic orientation. Wei et al.61 synthesized ZnO nanotubes with ZnCl2 and ammonia as starting materials. Nanotubes produced at 95 °C presented 500 nm diameter and 3 μm length. A brief review of the hydrothermal method for the growth of different nanostructures is given by Djurisic et al.,62 as the growing conditions have a considerable influence on the morphology of the nanostructures. Since the hydrothermal method is a wet chemical approach, the high aspect ratios of the synthesized structures cannot be expected, and the crystallinity of the structure is not high. The crystallinity can be improved by using the ZnO seed layer and annealing techniques. The prepared structures present many defects, leading to poor UV emission and enhancement in deep-level emission (DLE), thereby indicating poor optical quality.All the methods specified above have their advantages and drawbacks. A particular method is typically selected based on the structure to be deposited and the field of application. Table 1 summarizes representative examples for the various methods to synthesize ZnO nanostructures along with their structural dimensions and starting materials.
| Method | Starting materials | Substrates | Structure | Dimensions and orientation | Ref. |
|---|---|---|---|---|---|
| PLD | 99.99% pure Zn and O2 | Sapphire | Nanorods | D = 300 nm, L = 6 μm, O: [0006] | 63 |
| Zn disc and O2 | Silicon | Nanocones | L = 1 μm, D = 100–200 nm, O: [0002] | 64 | |
| ZnO bulk | Ag | Monolayer | Two mono-layer thick | 65 | |
| Sputtering | 99.99% Zn, O2 | Si | Nanotubes | R (outer) = 200 nm | 66 |
| ZnO, O2 | Cu/Ti/Si | Nanowires | D = 45–55 nm, O: [0 0 2] | 67 | |
| Pure Zn | n-Type Si | Nanorods | D = 125 nm, L = 675 nm | 36 | |
| CVD | Zn acetyl-acetonate hydrate | Fused silica or Si | Nanorods | D = 60–80 nm, O: [0002], [0004] | 37 |
| DEZ, NO2 | Si/SiO2 | Nanowires | D = 20–60 nm, L = 5–15 μm | 39 | |
| DEZ, O2 | Sapphire | Nanotubes | D = 50–150 nm, L = few μm, O: [001] | 40 | |
| Sol–gel | Zn acetate hexahydrate, methenamine | SiO2/Si | Nanorods | D = 170 nm | 44 |
| Zn acetate hexahydrate, methenamine, DI water, urea | PAA templates | Nanowires | D = 70 nm | 45 | |
| Zn acetate hexahydrate, methenamine, DI water, urea | PAA templates | Nanotubes | D = 50–80 nm | 45 | |
| Spray pyrolysis | ZnCl2 | SLG | Nanorods | D = 0.1–0.2 μm, L = 0.7–0.8 μm, O: [002] | 49 |
| Zn acetate, DI water | ITO glass or SLG | Nanowires | O: [0001], [1010], [1120] | 68 | |
| MBE | Metal Zn, O3/O2 plasma | SiO2 | Nanorods | D = 20–150 nm, L = 5–15 μm | 50 |
| Metal Zn, O3/O2 plasma | SiO2 | Nanowires | D = 30–150 nm, L = 14 μm | 51 | |
| ZnO thin layer on a substrate, atomic O2 by EIT | Si | Nanotubes | D = 10–90 nm; O: [0002] | 52 | |
| ED | KCl, ZnCl2 | ITO | Nanorods | D = 250–300 nm, L = few μm | 53 |
| KCl, ZnCl2 | Conducting glass/SnO2:F | Nanowires | D = 25–80 nm, L = 0.5–1.8 μm, O: [0001] | 69 | |
| KCl, ZnCl2 | Conducting glass/SnO2:F | Nanotubes | D = 60–200 nm, O: [0001] | 55 | |
| Thermal evaporation | ZnO powder | Ni coated Si | Nanorods | D = 300–500 nm; L = 4 μm | 70 |
| ZnO powder | Si | Nanowires | D = 80 nm; L = 10 μm, O: [0001] | 57 | |
| ZnO powder | Si | Nanotubes | D = 200 nm; O: [0001] | 58 | |
| Hydrothermal | (NH4)2CO3, PEG | Nanowires | D = 50–80 nm; L = 6 μm | 59 | |
| HMTA Zn nitrate hexahydrate | Si | Nanorods | O: [0001], D: 55–70 nm, L: 800 nm | 60 | |
| Ammonia and Zn chloride | Cu plate | Nanotubes | D = 500 nm; L = 3 μm, T: 50 nm, O: [0002] | 61 | |
| HMTA Zn nitrate hexahydrate, sodium citrate | — | Monolayer | O: [0001] | 71 | |
3. Biomolecule immobilization on ZnO nanostructures
Conventional ZnO is a safe and non-toxic substance, and it is essential to have details of the toxicity of ZnO nanostructures for their application in biosensing. More complexity has been added by the current trend toward smaller sizes. Nanomaterials have greater surface area and reactivity than their bulk counterparts, which can allow them to translocate across cell membranes, bind molecular species effectively, and catalyse chemical processes with greater ease.72 For example, ZnO tetrapods (ZnO-T) have been put to use in a number of biological contexts because they are biocompatible, nontoxic, and harmless to normal cells.73 The synthesis of nano-ZnO was described by Bhall et al.74 using a surfactant-polyol assembly as a caging agent to keep the ZnO crystallite size down to nano-regime proportions. The surfactant-polyol-assembly acts as the agent for improving the biocompatibility of ZnO structures.Applications in living organisms require the nanomaterial of interest to be biocompatible and to have less harmful by-products from its production process. Mouse positron emission tomography utilising ZnO NWs as an optical agent is described by Hong et al.75 To increase bio-compatibility and decrease cellular toxicity, the NW conjugate was peptide-functionalized (NW-PEG-DOTA). In HeLa and L-929 cell lines, Li et al.76 used MTT tests (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) to demonstrate ZnO NWs' good biocompatibility at concentrations below 100 mg mL−1.
3.1. Criteria for biomolecule immobilization
A biosensor (Fig. 1) is an analytical device that contains a sensitive layer to detect a specific analyte coupled with a transducer which generates a quantifiable signal in proportionate to the analyte concentration.77 Biosensors generally contain a sensitive layer that interacts with a specific target analyte and produces a signal due to proton exchange, heat or light emission, absorption or reflectance, the release of gases or ions, or any other kind of mechanism. The objective of a transducer is to transform the signal produced to a quantifiable signal that can be monitored.78 A good biosensor should fulfill various factors such as stability under normal storage conditions and the ability to retain the response for longer days. The sensor must be highly selective and provide results with high reproducibility, sensitivity, and accuracy over a wide linear range of concentrations. The lower detection limit (LOD) must be as low as possible and the shelf-life as high as possible.The main key factor for the development of biosensing devices is the immobilization of the biomolecule onto the transducer sensing area. The immobilization techniques must be such that the biomolecule should be strongly attached to the transducer sensing area and specific to detecting required materials or substances.77,79 The immobilization of the biomolecule must follow a few necessary criteria such as less loss of bioactivity after attachment to the sensor surface area, must be adhered to the sensor surface for a long term with high stability and durability, and must be active to only certain selected substances.77 Other factors include resistance to pH variations, temperature, and chemical compositions. Also, unnecessary interferents should not influence the sensor activity. Various methods for immobilizing biomolecules include physical/chemical adsorption, covalent binding, and cross-linking between molecules.80 The formation of a stable bioselective layer is an important task. For this process, ZnO is the best due to its high IEP at a pH of 9.5 as it attracts substances with low IEPs such as proteins or DNA, among others. The structural properties of ZnO play an important role in the immobilization of the bioselective layer. Two main methodologies of biomolecule immobilization are direct adsorption and covalent binding.
Silanization through aminopropyl-triethoxysilane (APTES) and mercaptopropyl trimethoxysilane (MPTMS) has been reported. Sanguino et al.88 reported ZnO nanorods for interdigitated immunosensors. Sulfo-MBS, which comprises N-hydroxysuccinimide (NHS) ester and maleimide groups, cross-links antibodies to amino acid side chains containing amine and sulfhydryl groups. Protein detection using ZnO nanostructures immobilized with immunoglobin has been reported by Sang et al.89 The substrate was immersed in 2% (v/v) 3-APTES in deionized water. Later, after washing, the substrate was immersed in disuccinimidyl suberate (DSS) and in dimethyl sulfoxide (DMSO). Two cross-linking polymers for ZnO biosensors are reported by Munje et al.90 Dithiobis succinimidyl-propionate (DSP) is a homobifunctional molecule that contains N-hydroxysuccinimide (NHS). The NHS esters are responsible for the formation of stable bonds in proteins when reacting with amines. Thiol groups form stable bonds with positive zinc ions and preferentially bind to Zn terminated surfaces. APTES silanizes by forming bonds with hydroxyl groups attached to the oxygen atoms of ZnO surfaces. The substrates are first sonicated in APTES solution with 2% ethanol and then dip-coated in solution. Baking the substrates in an atmosphere devoid of oxygen helps prevent silanization of APTES, which can be caused by exposure to ambient oxygen. Improved performance is obtained by thiol–ZnO interaction using DSP. ZnO nanoparticles for detection of troponin I are reported by Tan et al.91 The ZnO surface is immobilized with an anti-cTnI monoclonal antibody (MAb). APTES with deionized water is dropped onto the ZnO surface. APTES binds with the ZnO surface by forming bonds with hydroxyl groups on oxygen atoms. Later, bifunctional linker glutaraldehyde in deionized water is dropped on the ZnO transducer. Similarly, surface functionalization of ZnO-FET biosensors for cardiac troponin I detection has been reported by Fathil et al.92
Biomolecule immobilization with DSP molecules and DMSO has been also reported by Jacobs et al.93 Nanostructures of ZnO produced under oxygen-free conditions manifested as nanotextured films with columnar growth. Anti-troponin-T is immobilized onto the surface by first immobilizing the cross-linker molecule. DSP dissolved in DMSO is first dropped onto the surface, followed by anti-troponin T dissolved in phosphate-buffered solution (PBS). The superblock is then inserted to ensure that no DSP molecule has available N-hydroxysuccinimide sites. ZnO samples grown without the presence of oxygen displayed enhanced performance compared to samples grown with oxygen. ZnO samples grown without oxygen have mostly Zn terminated surfaces, and thiols bind to the positive Zn ions with higher coverage leading to a higher electrical biosensor response.
Self-assembled monolayers (SAMs) can be effective for surface functionalization due to the formation of a stable sensing surface area. Zhang et al.94 reported surface functionalization of ZnO self-assembled monolayers. Synthesized ZnO nanowires and wafers were submerged in 3-PPA. The SAMs on their surfaces were formed using either an aqueous solution or a 10-phosphonodecanoic acid (10-PDA) methanol solution. ZnO wafers containing SAMs were first put into NHS and 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC) solution in 2-(N-morpholine)-ethane sulfonic acid (MES). Submerging the modified wafers in an IgG solution in MES buffer was the next step. Atomic force microscopy (AFM) images indicated island-like structures for 10-PDA molecule functionalized ZnO. Biomolecule immobilization with phosphoric cross-linking molecules has been reported by Dembereldorj et al.95 16-Phosphonohexadecanoic acid (16-PHDA) assembled ZnO nanoparticle solution was mixed with the mixture of BSA (or transferrin (Tf)) and EDC. Using 16-PHDA, the proteins were stabilised. Due to the presence of the phosphonic acid group, 16-PHDA was predicted to adsorb onto the ZnO surfaces. By means of an EDC coupling process, the carboxylic groups of 16-PHDA on ZnO's surface reacted with the amino groups of the proteins. Functional groups of enzymes or proteins lead to covalent binding to a solid surface. The advantage of covalent binding is that the biomolecules are strongly immobilized onto the surface and are less likely to detach. This method provides minimal loss of biological activity. Moreover, the availability of several functional groups for covalent immobilisation makes it possible to avoid the active site of the binding process.
4. Classification of biosensors
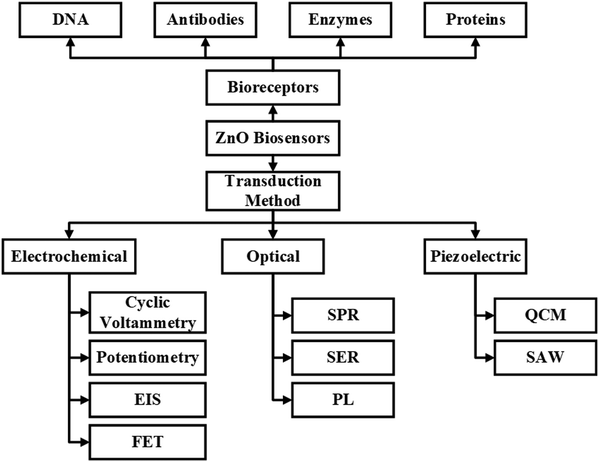
The basic working principle of biosensors is shown in Fig. 2. ZnO biosensors can be classified based on the transduction method and the bioreceptor.96 In the case of bioreceptors, ZnO biosensors are classified depending on the type of sensing molecule used. The sensing molecule could be an enzyme, antibody, DNA, and protein.Enzymes are used to detect specific molecules, which upon interaction with analytes, produce a product that can be detected by one of the transduction methods. Enzymatic biosensors are affected by factors like pH and temperature. A specific antigen binds to the antibody in a highly specific way for antibody-based sensors, making them essential for immunosensors. DNA-based biosensors use complementary nucleotides as the recognition element. For known sequences of DNA, a complementary sequence called a DNA probe is synthesized, labeled, and hybridized to detect the target biomolecule. In detecting proteins, selecting a required protein by neglecting a wide range of other proteins depends on the biocomponent, which serves as a molecular recognition tool in binding the required protein. The specific protein detection requires sensing areas immobilized with antibodies or aptamers that are connected to transduction systems.
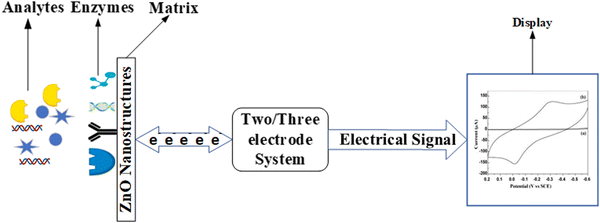
ZnO based biosensors are typically categorized into electrochemical, optical, and piezoelectric biosensors based on the transduction method.97 In electrochemical biosensors,98 the biomolecule and the target analyte react to produce or consume carriers that produce an electrical signal. Three different methods are used in electrochemical transduction: potentiometry, voltammetry, and amperometry. Amperometry is widely used for biosensor transduction mechanisms. In this method, the sensor is placed at a fixed potential which acts as a driving force for carriers, and the target analyte is added in steps. A change in current occurs with the change in the target analyte concentration, which allows monitoring the amount of the target analyte. The potential is swept between two values between the two electrodes for cyclic voltammetry methods, and the current is plotted against the potential.
The reduction and oxidation peaks appear in the plotting cycle based on the reduction and oxidation reactions. The current response per concentration and area of electrode gives the sensitivity of the biosensor. In the potentiometric method, the charge accumulated at the surface of the electrode causes a change in the potential of the working electrode with respect to the reference electrode. The current produced is plotted against the logarithmic concentration of the analyte to monitor the amount of the target analyte. The slope of the plotted curve provides the sensitivity. In impedimetric biosensors, the production of carriers changes the resistance of the solution, and this measurable parameter is used for the detection and monitoring of the target analyte. Because the relationship between potential and analyte concentration in potentiometric biosensors is logarithmic, the sensors are less sensitive.
Surface plasmon resonance biosensors are used in optical biosensors to detect changes in the refractive index caused by the interaction of analytes with bioreceptors.99 Variation in density is quantified by observing the angle at which reflected light shifts. The interaction of analytes with adsorbed receptors on the transducer's surface area causes a change in the Raman signal, which is measured by surface enhanced Raman spectroscopy (SERS). This occurs because carriers are transferred between ZnO and the electrodes. For the photoluminescence (PL) technique, the change in the PL spectra is observed before and after adsorption of the bioreceptor and after the interaction with the target analyte. The change in the PL band allows the detection of the target analyte.
The third category of the biosensor transduction method is the piezoelectric method.100 Piezoelectric biosensors use a piezoelectric material that oscillates at a specific frequency upon giving an electrical signal of a specific voltage, and the frequency of oscillation changes when the mass of the crystal changes or the electric signal changes. The change in the mass on the surface of piezoelectric material upon binding with biomolecules changes the oscillation frequency. A quartz crystal is placed between two electrodes in a quartz crystal microbalance (QCM) device, with the crystal's oscillation frequency being affected by a change in mass. FETs are also explored for biosensor applications. The ZnO nanostructures are used as the channel, and when the target molecules interact with the biorecognition element, the conductance of the channel changes. The change in the channel's conductance is used as the measuring parameter for detecting target molecules. Further detailed information can be obtained from the reviews reported.101–103 The detailed classification of biosensors based on bioreceptors and transduction methods is shown in Fig. 3.
4.1. Dimension based classification for ZnO biosensing applications
The structure of ZnO nanoflakes is directed towards a nano-honeycomb structure when grown on an Al coated glass capillary.109 The ZnO nanoflakes can be immobilized with GOx and then coated with Nafion for higher sensitivity. Glucose concentrations can be measured from human adipocytes and frog oocytes. The sensors showed a linear response from 500 nM to 10 mM with a fast response within 4 s. ZnO nanoflakes for uric acid detection are reported by Ali et al.110 The SEM images are depicted in Fig. 4(a). Uricase along with Nafion coating is dropped on ZnO nanoflakes for detection of uric acid. Uric acid is oxidized to allantoin and carbon dioxide in the presence of uricase. Allantoin is converted to allantoinium ions in water, which causes a potential change at the ZnO electrode. The potentiometric results show a wide linear response from 500 nM to 1.5 mM with 66 mV dec−1 sensitivity, with the sensors being inert toward ascorbic acid and glucose. Chauhan et al.111 reported the detection of Helicobacter pylori using the ZnO tetrapods grown on screen printed electrodes. The designed electrochemical immunosensor showed a good linearity in the range of 0.2 0.2 ng mL−1 to 50 ng mL−1 with a LOD of 0.2 ng mL−1. Also, ZnO–Ag2O composite nanoflowers are proposed for the detection of dinitrotoluene in water.112 The composite ZnO modified Ag electrode showed enhanced sensor performance compared to ZnO or Ag2O electrodes. The sensor exhibited a sensitivity of 5 μA μM−1 cm−2 with an LOD of 13 nM in a linear range from 0.4 μM to 40 μM. Psychoyios et al.113 reported biosensors for cholesterol by using ZnO nanowalls immobilized with ChOx. ZnO nanowalls facilitate electron transport between the enzyme and the electrode by providing a high surface area for enzyme loading. The lipid coating not only aids in the preservation of the enzyme's function, but it also improves biocompatibility. The sensor is highly sensitive with 57 mV dec−1 sensitivity in a linear concentration range from 1 × 10−6 M to 1 × 10−3 M with a response time of around 5 s. MoS2 assisted the growth of ZnO nanostructures for biosensing as reported by Yang et al.106 Thus DNA biosensors were fabricated through ZnO nanowalls grown on 2D MoS2 (Fig. 4(b)). Promyelocytic leukaemia and retinoic acid receptor alpha (PML/RARA) fusion genes were closely monitored with the sensor. The ZnO nanostructures are vertically aligned on the MoS2 layer and show higher hybridization efficiencies than individual structures. Porous structures provide a higher surface area for bioreceptor immobilization. Similarly, nanoporous ZnO films for uric acid detection were reported by Mozaffari et al.28 The cavities in the nanoporous films increase the surface area for enzyme loading, thus reducing the diffusion distance for the substrate to access the enzyme. The sensors present good sensitivity with linear detection from 0.83 to 23.24 mM and a LOD of about 0.40 mM.
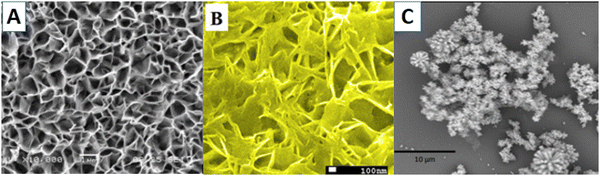 | ||
| Fig. 4 ZnO 2D nanostructures: (a) as-fabricated ZnO-nanofiber arrays,110 (b) ZnO/MoS2 nanowalls106 and (c) ZnO nanoflowers grown on an activated glass surface.114 | ||
ZnO nanoflower-based biosensors for amyloid detection have been reported by Akhtar et al.114 ZnO nanoflowers with thioflavin were grown on a silver film-coated glass slide (Fig. 4(c)). This sensor worked by enhancing the fluorescence of thioflavin T bound insulin due to the waveguiding capacity of ZnO nanoflowers, thus acting as a reflecting mirror in a Fabry–Perot resonator. ZnO's rough surface provided a larger surface area for sensor activity, as Saha et al.115 reported. High sputtering pressure introduced Zn interstitial defects, which increased the electrical conductivity and electron transferability. Though 2-D ZnO nanostructures do not have a particularly high surface area, as shown in Fig. 3, their high surface charge density proves to be a good aspect for biosensor applications. Also, the synthesis procedures for 2-D ZnO nanostructures are simpler than for 1-D nanostructures. Owing to their planar structure, they can provide a higher density of bioreceptor immobilization, thereby improving the sensitivity of the biosensor. Furthermore, for a particular structure, the biosensitivity can be more efficient for certain biomolecule targets.
Shukla et al.119 reported that higher aspect ratios support to improve enzyme immobilization (Fig. 5). Also, the charge transfer resistance decreases, which further enhances the electron transferability. Therefore, higher sensitivity is recorded for ZnO structures having a higher aspect ratio with a response time of around 5 s. Lee et al.120 reported that nanorods with higher deposition times have better efficiency for detection of DNA oligonucleotides. To detect streptavidin, Sang et al.121 reported rod nanowires, hexahedral puncheons, and sharp ZnO nanowires. Sharp ZnO nanowires showed a better response as they present a high aspect ratio and higher binding sites on the surface than rod nanowires and hexahedral puncheons. Pradhan et al.122 reported ZnO nanowires for glucose biosensors. GOx immobilized ZnO nanowires on Au-coated polyester exhibited a high sensitivity of 19.5 μA cm−2 m−2 M−1, with a response time of less than 5 s.
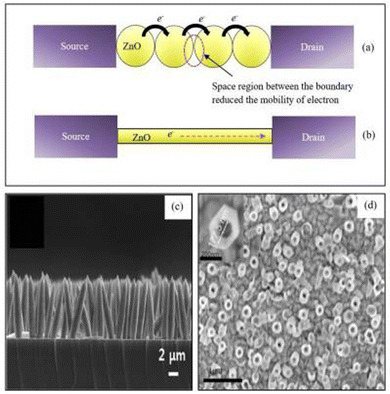 | ||
| Fig. 5 The schematic diagram of electron flow through ZnO nanostructure bridges between source and drain for (a) nanoparticles and (b) a single lateral nanowire. (c) Cross-sectional image of ZnO nanorods. (d) Top view FESEM image of ZnO nanotubes.127 | ||
Uric acid biosensors involving ZnO nanorods grown on glassy carbon electrodes have been reported by Zhang et al.123 The surface area of ZnO nanorods facilitates direct electron transfer between uricase and the electrode. The amperometric measurements also showed a linear response for the concentration range 5.0 × 10−6 to 1.0 × 10−3 mol L−1 with a LOD of 2.0 × 10−6 mol L−1. Kong et al.124 reported glucose biosensors based on ZnO nanotubes immobilized with GOx. The sensor demonstrated a detection range of 50 μM to 12 mM with a LOD of 1 μM and a sensitivity of 21.7 μA cm−2 mM−1. Copper-doped ZnO nanofibers have been implemented for malaria detection by Paul et al.125 The copper-doped ZnO nanofibers functionalized with mercaptopropylphosphonic acid are used for the detection process. Mercaptopropylphosphonic acid enhanced the ability of functional groups for enzyme immobilization. In this system, copper enhanced the electrical conductivity, and the electric field created at the copper–ZnO junction brought the target closer to the mercaptopropylphosphonic acid-treated nanofiber. Ag doping is found to improve biosensor performance, as reported by Zhou et al.126 The ZnO nanorods doped with Ag and immobilized with GOx are used for the detection of glucose. The Ag doping makes the surface of ZnO nanorods smoother and reduces the contact area of the solid–liquid interface. Also, it allows reduction of the charge transfer resistance and enhancement of the electron transferability of the ZnO nanorods. Moreover, the catalytic activity of GOx is also improved. Due to the above factors, the glucose biosensor performance is enhanced, and the optimum doping of Ag is around 2 mM, with higher Ag doping leading to the formation of Ag2O, which reduces the performance of the biosensor. The linear range of the sensor is from 1.5 × 10−3 to 6.5 mM, and its sensitivity is 85 μA mM−1 cm−2 with an LOD of 1.5. μM.
ZnO biosensor performance also depends on the dimensions of the nanostructures. Kim et al.128 reported glucose biosensor performance based on ZnO dimensions. The ZnO nanorods grown on the substrate are classified into three groups based on dimensions. The ZnO nanorods with lower diameters and high aspect ratios are densely packed and found to have the highest sensitivity of 69.8 nA μM−1 cm−2. The large surface area of ZnO nanorods has a larger amount of GOx and hence improved performance of the sensor. 1-D nanostructures provide a higher surface area for biomolecule immobilization and also better electrical conductivity. The high aspect ratio of the nanostructures also improves their sensitivity as it allows higher biomolecule immobilization. Densely packed structures can also provide improved results. Small signal strength, difficulties in creating electrical connections, incompatibility with the CMOS process, and the typical nanostructure production procedure, which normally needs high temperature, are all limitations of 1-D ZnO in the context of the CMOS process for biosensors.129
Hybrid ZnO nanostructures with carbon nanotubes (CNTs) are also explored due to the good catalytic activity of CNTs. Hayat et al.134 reported cholesterol biosensors constructed by ZnO nanoparticles incorporated onto carbon nanotubes immobilized with ChOx. The cholesterol reacts with ChOx and produces H2O2. The H2O2 is then used to oxidize 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), thus producing a green-colored product that helps in quantizing the cholesterol content through calorimetric analysis. The nanocomposite structure of ZnO and CNTs catalyzes the oxidation of ABTS. Fidal et al.,135 on the other hand, reported that Al-doped ZnO immobilized with GOx showed a better response in terms of sensitivity, linearity, and LOD compared to the bare ZnO structure immobilized with GOx. GOx bioactivity can be improved by avoiding leakage with poly diallyl dimethylammonium chloride (PDDA) layer coating. Wang et al.136 reported a multi-layer structure of biosensors for glucose detection. The nanocomposite of ZnO nanoparticles and multi-walled carbon nanotubes (ZnO/MWCNTs) immobilized with GOx was reported to be coated with the PDDA layer. The PDDA layer avoids GOx leakage, thereby improving the bioactivity of GOx. The electrocatalytic response towards H2O2 indicated that ZnO nanoparticles do not influence the MWCNTs. The sensor had a LOD of 250 nM for 2.0 U of GOx and retained 90% of the initial response after 160 days.
Aini et al.137 reported electrochemical glucose biosensors based on ZnO nanoparticles immobilized with GOx, ionic liquid, and an eggshell membrane onto a glassy carbon electrode (Fig. 6). Methylene blue was used as a redox indicator. The ZnO nanoparticles in the egg shell membrane and ionic liquid increase the surface area of the sensor. The sensor with the ESM showed a better response than the one with a chitosan (CHIT) membrane. The LOD was 10−13 M with a linear detection range from 1 × 10−12 to 6 mM. The novel method of ZnO synthesis from leaf extract is reported by Dayakar et al.138 Glucose detection is done by utilising ZnO nanoparticles generated by a bio-mediated approach employing Ocimum tenuiflorum leaf extract. The sensor has a linear range of 1 to 8.6 mM, a LOD of 0.043 mM, and a sensitivity of 631.30 μA mM−1 cm−2.
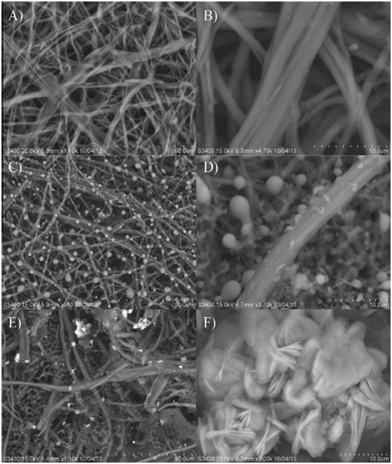 | ||
| Fig. 6 A scanning electron micrograph of the (A) and (B) eggshell membrane [ESM], (C) and (D) GOx immobilized ESM [GOx/ESM] and (E) and (F) ZnO and GOx immobilized ESM [GOx/ZnONPs-[EMIM][Otf]/ESM].137 | ||
Furthermore, a biosensor designed by using doped ZnO nanostructures was presented by Mahmoud et al.139 A non-enzymatic glucose biosensor was designed by using Cu doped ZnO. Cu doping is found to increase the electrochemical properties of the sensor. The nanocomposites with their catalytic features exhibit enhanced activity.140 ZnO quantum dots are reliable candidates for the design of the biosensor. The major aspect of ZnO quantum dots is the size of the nanoparticles. Reduction in the nanoparticle size increases the surface area for higher enzyme loading, thereby enhancing the biosensor's performance. One of the drawbacks of quantum dots is their degraded mobility with increasing grain boundaries.141
4.2. Types of ZnO biosensors
ZnO–rGO nanocomposites have been functionalized with tyrosinase for the detection of dopamine.143 The sensor's LOD was as low as 8.75 ± 0.64 pM, and its linear range was as large as 0.1–1500 pM. Its sensitivity was 39.56 ± 0.41 μA nM−1, which is rather impressive. The sensor had a response time of 0.34 ± 0.09 s and showed excellent selectivity for DA in a variety of blood serum components. ZnO nanoarchitecture decorated rGO is reported for the non-enzymatic detection of uric acid and glucose.144 The sensor was sensitive to uric acid in the concentration range from 0.02 × 10−3 to 7.2 × 10−3 mM with a LOD of about 0.012 mM whereas for glucose the concentration range was 0.02 × 10−3–18 × 10−3 mM with a LOD of 0.008 mM. The biosensor was shown to have a sensitivity of 682.8 mA mM−1 cm−2 for urea, while for glucose the sensitivity was only 481 mA mM−1 cm−2.
Reports of Ag/ZnO with a variety of ZnO nanostructures for non-enzymatic urea detection have been made.145 By combining a sputter deposition and solution growth technique, ZnO nanorods and nanoflakes with varied crystallographic orientations were produced. The Ag/ZnO nanorods on carbon electrodes are found to exhibit better characteristics than ZnO nanoflakes due to the larger surface area. Another non-enzymatic biosensor based on ZnO nanorods with an Au coating has been described for the detection of glucose. The Au–ZnO nanorods allowed for a high sensitivity of 4416 μA mM−1 cm−2, a low LOD of 0.12 μM, and a linear range of up to 15 mM. Baruah et al.146 reported Co and Fe codoped ZnO for glucose sensing. The PL intensity of the doped ZnO was reduced and it showed better conductivity than the pristine ZnO. The doped ZnO was modified with GOx for the detection of glucose. The sensor presented 2-fold improved sensitivity compared to pristine ZnO of about 32.2 μA mM−1 cm−2 with a linear range from 0 to 4 mM. Fig. 8 depicts the merits of various ZnO nanostructures with different morphologies.
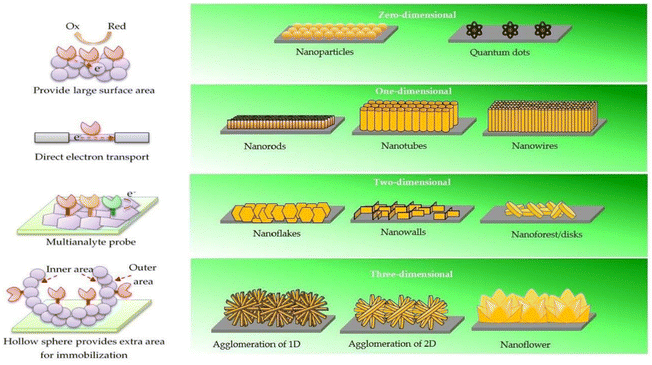 | ||
| Fig. 8 Size-dependent benefits of ZnO nanostructures. 0-D nanostructures have a higher surface area. Electron transport in 1-D nanostructures is both stable and direct. Two-dimensional nanostructures provide specific planes for the immobilisation process, allowing for the multiplexed detection of analytes. Extra outer and inner surface area of 3-D nanostructures means additional immobilisation sites.147 | ||
Israr et al.148 reported the detection of cholesterol by ZnO nanorods. Ag/AgCl was used as a reference electrode, and the ZnO/Ag electrode with dip-coated ChOx served as the working electrode. The variation in the concentration of the cholesterol electrolyte solution causes potential difference between the electrodes, which is due to a change in cholesterol concentration. The reference electrode exhibits constant potential, but the working electrode causes a change in emf due to the reactions between the electrolyte solution and ChOx. Recently Supraja et al.149 reported a CNT embedded ZnO nanofibre-based biosensor. The nanofibre modified the electrode and immobilized it with an antiatrazine antibody for atrazine detection. The sensor showed good electrical conductivity and exhibited a sensitivity of 21.61 kΩ μg−1 mL−1. Potentiometric results of the bioelectrode are plotted against the logarithmic concentration of electrolytes.
Ali et al.150 reported the detection of uric acid by using ZnO nanowires immobilized with uricase. The uric acid solution was used as the electrolyte, Ag/AgCl as the reference electrode and the uricase immobilized ZnO nanowire electrode as the working electrode. The potential response against concentration showed a linear response from 1 μM to 1 mM on the log scale. In another work, Israr et al.151 reported the detection of cholesterol by using ZnO nanowall structures immobilized with ChOx. Detection of C-reactive protein (CRP) by using ZnO nanotubes was reported by Ibupoto et al.152 ZnO nanotubes were immobilized with monoclonal anti-c-reactive protein and glutaraldehyde using the physical adsorption method. The sensor showed a linear response for CRP in the concentration range 1 × 10−5 mg L−1 to 1 × 100 mg L−1 with a sensitivity of approximately 13.17 mV per decade. The sensor has a lifetime of three days, and hence it can be used as a disposable sensor. The sensor electrode showed good reproducibility with a relative standard deviation of less than 5%. Further, when tested with human blood serum, which contains interferents like glucose, sodium and potassium ions, uric acid, etc., the sensor shows good selectivity.
The same type of biosensor was reported for L-lactic acid detection.153 The sensor was constructed by using ZnO nanorods immobilized with lactate oxidase along with glutaraldehyde. The potentiometric analysis of the sensor indicated a linear range of detection within the concentration range from 1 × 10−4 to 1 × 100 mg L−1 with 41.33 1.58 mV dec−1 sensitivity. The sensor showed good selectivity in the presence of interferents such as glucose, ascorbic acid, galactose, magnesium ions, and calcium ions. The sensor had a stability of about three weeks. ZnO nanoflakes immobilized with GOx were reported for detection of glucose by Fulati et al.154 The potentiometric response showed linear response within the concentration range 500 nM to 10 mM. The sensor had a fast response time of about 4 s. Improved glucose biosensing performance was reported by incorporating graphene nanoplates beneath ZnO nanowires by Rafiee et al.155 The sensor reported a linear range from 0.003 to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg dL−1 with a response time of 5 s.
000 mg dL−1 with a response time of 5 s.
ZnO nanorods were used by Ibupoto et al.156 to detect penicillin by an aqueous chemical growth method. Nanorods were immobilized with penicillinase along with N-5-azido-2-nitrobenzoyloxysuccinimide as a cross-linking polymer. The potentiometric analysis revealed good sensor selectivity with negligible response to sucrose, glucose, ascorbic acid, uric acid, etc., with a good sensitivity of 121 mV dec−1. The sensor had a linear response for concentration ranging from 100 μM to 100 mM with a response time of 5 s. Indirect detection of mercury ions by a glucose biosensor was reported by Chey et al.157 The ZnO nanorods were observed to be immobilized with GOx along with the CHIT membrane and glutaraldehyde. The sensor showed inhibition activity from 0.5 × 10−6 mM to 0.5 × 10−4 mM and from 0.5 × 10−4 mM to 20 mM of mercury ion for 1 mM glucose. The inhibition was further reduced for 10 mM glucose concentration. The sensor showed good selectivity with a response time of 8 s and 90% sensitivity even after three weeks.
Furthermore, Ali et al.158 reported a glucose biosensor based on ZnO nanotubes immobilized with GOx along with Nafion coating. The sensor had a linear response over the concentration range of 0.5 × 10−6 to 12 × 10−3 M with a response time of 4 s and a sensitivity of 69.12 mV dec−1. The sensor was found to be less influenced by interferents like uric acid and ascorbic acid. Later, Khun et al.159 reported the detection of galactose by using a ZnO nanorod-based biosensor, immobilized with galactose oxidase and glutaraldehyde as a cross-linker. Potentiometric analysis of the sensor demonstrated its linear response throughout the concentration range of 10 mM to 200 mM, with a sensitivity of 89.10 ± 1.23 mV dec−1 and a reaction time of less than 10 s. The sensor can be used for about four weeks which is its shelf-life. In order to detect the BCR/ABL fusion gene, Xia et al.160 built a new biosensor based on ZnO nanorods/Pt. The linear detection range for this sensor was between 1 pM L−1 and 1 nM L−1, and the LOD was 2.75 × 10−13 mol L−1.
In another work, Singh et al.161 reported the detection of cholesterol using nanoporous ZnO immobilized with ChOx. The cyclic voltammetry measurements indicate the oxidation peak at 0.5 V and the current at 0.5 V, which increased further by an increase in cholesterol concentration from 25 to 500 mg dl−1. The sensor had a response time of 15 s. Its oxidation peak at lower cholesterol concentration indicated an enhanced electron transfer rate. DNA based detection of bacterial meningitis was presented by Tak et al.162 using flowerlike ZnO nanostructures. The cyclic voltammetry measurements were performed on a Pt/Si electrode, ZnO/Pt/Si electrode, single-stranded thiolated DNA probe modified ZnO/Pt/Si electrode, and double-stranded DNA probe modified electrode. The redox peaks were high for Pt/Si electrodes, then reduced due to ZnO's semiconducting nature. DNA probe modified electrodes have shown further reduced peaks due to repulsion between the phosphate backbone of DNA and PBS solution containing [Fe(CN)6]3−/4− but for the double-stranded DNA modified electrode, the redox peaks are barely visible.
An amperometric biosensor for glucose detection was reported by Liu et al.163 The cyclic voltammetry curves for Nafion coated ZnO nanorod film/ITO glass exhibited the typical capacitive squared curves, thus indicating its electrochemical inactivity in the potential window from 0.2 to −0.6 V. The GOx immobilized electrode showed well-defined peaks at −300 and −20 mV. Later, amperometric measurements were carried out by adding glucose aliquots. For each successive glucose injection, the current increases, and the response time is noticed to be less than 5 s. ZnO/Co-based nanoclusters with high specific surface area and better electrocatalytic activity for a glucose biosensor were investigated by Zhao et al.164 The cyclic voltammetry curves indicated a sharp increase in current in the presence of glucose at 0.3 V. The amperometric tests were performed by adding glucose stepwise. The current increased for each addition of glucose, and the steady-state currents were attained within 8 s. The sensor has a LOD of 20 μM and a sensitivity of 13.3 μA mM−1 cm−2. MWCNTs and AuNPs were employed to improve biosensor sensitivity, as reported by Wang et al.165 A DNA biosensor was fabricated from carbon nanotubes, ZnO nanowires, and gold nanoparticles (AuNPs). Then a working electrode was designed via ZnO nanowires and MWCNTs immobilization with AuNPs. The differential pulse voltammetry (DPV) measurements were performed by dipping the electrode in Tris–HCl solution containing 1.5 × 10−4 M[Ru (NH3)6]3+. The reduction peak in RuHEX increased for MWCNT/ZnO nanowires, then an increase in the reduction peak was observed for the AuNP electrodeposited electrode. Finally, enzyme immobilization further increased the redox current. The sensor showed a detection range for DNA of 1.0 × 10−13 to 1.0 × 10−7 M with a LOD of 3.5 × 10−14 M. Also, Liu et al.166 reported a DNA biosensor based on ZnO and a modified glassy carbon electrode. Differential pulse voltammetry (DPV) measurements were performed using methylene blue (MB) as an indicator. The ssDNA probe was used to hybridize the target DNA sequences of different concentrations. Linear reduction peak currents were noticed against logarithmic DNA concentration from 3.57 × 10−11 mol L−1 to 3.57 × 10−7 mol L−1 with a LOD of 1.09 × 10−11 mol L−1.
Dispersing nanoparticles on chitosan (CHIT) helps better immobilization of enzymes due to their biocompatibility and film-forming ability. Khan et al.167 reported a cholesterol biosensor based on a ZnO/CHIT nanocomposite film. The working electrode was designed by immobilizing ChOx on the nano ZnO–CHIT/ITO electrode. The electrochemical impedance spectrum was measured for CHIT/ITO, nano ZnO–CHIT/ITO, and ChOx/nano ZnO–CHIT/ITO electrodes. The charge transfer resistance was measured to be 6.68 × 102, 4.47 × 102, and 9.38 × 102 Ω, respectively, for the three electrodes. The increased resistance of the ChOx immobilized electrode was due to the hindrance of electron transfer by ChOx. Xiang et al.168 reported direct electrochemistry of horseradish peroxidase (HRP) based on a flower-like ZnO/Au/Nafion-based nanocomposite. The HRP immobilized ZnO/GNPs/Nafion glassy carbon electrode showed well-defined peaks in cyclic voltammograms indicating direct electron transfer between protein and respective electrodes. The electrolytic activity of the ZnO/GNPs/Nafion/HRP/glassy carbon electrode showed reduced oxidation currents and increased reduction currents upon the addition of H2O2, indicating the reduction of H2O2. The resulting biosensor showed a wide linear range of 1.5 × 10−5 to 1.1 × 10−3 M with a low LOD of 9.0 × 10−6.
It was also discovered that tetragonal pyramid-shaped porous ZnO nanostructures (TPSP–ZnO) could provide better biosensing abilities due to their large specific surface area and shape. TPSP–ZnO immobilized with GOx was used for glucose detection by Dai et al.169 The GOx immobilized TPSP–ZnO/Nafion electrode showed stable redox peaks in cyclic voltammetry plots. This was due to the electron transfer between the electroactive center of GOx and bioelectrode. The sensor showed the highest redox peaks when the solution was at a pH of 7.0. The interferents were observed to not affect the sensor response except for ascorbic acid, which reduced the peaks by 2.1%. The sensor has a linear response within the concentration range of 0.05 to 8.2 mM with a LOD of 0.01 mM. Similar detection of glucose was reported by using porous ZnO nanostructures by Fatemi et al.170 The sensor has a LOD of 10 μM and a response time of 7 s with 23.4 mA mM−1 cm−2 sensitivity. The skin template ZnO was found to enhance the direct electron transfer between GOx and electrode. High sensitivity and faster response time were due to the smaller crystallite size and more mesopores. A ZnO/CuO nanocomposite was used for dopamine detection, as reported by Khun et al.171 The cyclic voltammetry results showed a wide linear response over the range of 10−3–8 mM with a sensitivity of 90.9 μA mM−1 cm−2. The sensor showed a good response time of about 10 seconds and good selectivity over interferents like ascorbic acid, uric acid, and glucose. Direct electron transfer was reported in a GOx immobilized ZnO nanorods/graphene heterostructure by Zhao et al.172 The ZnO nanorods helped to immobilize GOx as well as reduce the distance between the active redox center of GOx and electrode, thus facilitating the direct electron transfer process. The sensor had a linear response for amperometric calibration of glucose from 0.2 to 1.6 mM. The sensitivity was measured to be around 89.84 μA mM−1 cm−2.
Energy-saving, environment-friendly, green, and cost-efficient methods for the synthesis of NPs have emerged in response to rising environmental concerns. For electrochemical detection and removal of 4-nitrophenol, Chakraborty et al.173 reported microwave-assembled Ag2O–ZnO composite nanocones. A quick, cheap, and energy-efficient biosensor was designed using microwave-assisted Ag2O–ZnO composite nanocones synthesised using a green aqueous solvent. Electrochemical sensing and photocatalytic degradation of hazardous 4-NP were achieved with these Ag2O–ZnO composite nanocones. The improved sensor outperformed the pure ZnO modified electrode at neutral pH with an excellent sensitivity of 1.6 μA μM−1 cm−2 s and a very low LOD of 23 nM, substantially lower than the permissible limit of 0.43 μM in drinkable water. The sensor also selected 4-NP above other possible interferents. Sharma et al.174 reported green ZnO nanoparticle production utilising plant extracts. A systematic strategy was employed to green synthesise zinc oxide nanoparticles (ZnO NPs) utilising Carica papaya seed extract. The generated ZnO NPs were integrated with multiwalled carbon nanotubes (MWCNTs) on the GCE to evaluate silymarin sensing. MWCNTs/ZnONPs/GCE had 2-fold stronger electrochemical signals than MWCNTs/GCE and bare GCE. Electrochemical detection employing our methodology and the MWCNTs/ZnO NPs composite could detect 122 mg of silymarin in the 160 mg commercial Milk Thistle pill, indicating a detection efficiency of 76%.
For the sensors based on direct electron transfer, the electrode and the enzyme both work in the redox potential window of the enzyme. This is an advantage as it provides superior selectivity and make them less prone to interferents. The voltammetry analysis provides information about an analyte based on the current obtained by varying the potential. The voltage is measured between two electrons when the potential is swept between two values. The amperometric analysis includes the measurement of current with an increase in the concentration at a fixed potential. Amperometric sensors are found to have better sensitivity as compared to potentiometric sensors. In potentiometric analysis, the accumulation of charge potential is measured at the working electrode in comparison with the reference electrode. The potentiometric analysis would be useful for measuring lower concentrations in small sample volumes.
ZnO's unique properties make it a promising biosensing material. These include a high exciton binding energy (60 meV) and a broad band gap (3.37 eV), as well as improved electron mobility. Owing to its other merits such as biocompatibility, ease of synthesis, and cost-effectiveness, it can be employed in the design of electrochemical biosensors.147,175
 | ||
| Fig. 9 Optical biosensing mechanism. Reproduced with permission from ref. 178. | ||
Galdamez et al.179 studied different orientations of ZnO/Au nanowires for biosensing applications, when functionalized with the thiolated oligonucleotide probe labeled by cyt5. The SERS signal for random-standing nanowires was found to be amplified due to chemical and electromagnetic enhancement. The PL of ZnO nanowires functionalized with DNA revealed surface modifications prior to and after DNA immobilisation, with results favouring the zigzag orientation of ZnO nanowires. ZnO nanorods targeted by MAbs anti-CD5 could be employed to construct a biosensing platform for detecting human leukemic T-cells.180 T-Lymphoblast cells were found to bind with CD5-targeted ZnO nanorod platforms having great selectivity, and the PL signal was much higher than that of IgG2a-targeted platforms. Human MOLT-4 cells conjugated with anti-CD5 MAbs were detected using the ZnO nanorod platform at concentrations as low as 3–128 cells in a 1.0 mm2 well. Myndrul et al.181 reported aflatoxin detection using polyacrylonitrile/zinc oxide nanofibers. The nanofibers were then modified with APTES, glutaraldehyde, bovine serum albumin and MAbs (anti-AFB1). The alteration in PL developed by the AFB1/anti-AFB1 complex was investigated. When tested, the sensor's sensitivity range was determined to be between 0.1 and 20 ng mL−1, and its PL rose in proportion to the concentration of the analyte. The LOD was around 39 pg mL−1.
Sodzel et al.182 reported the detection of H2O2 and glucose using ZnO nanoparticles based on UV and visible luminescence. Fig. 10 represents the schematic of the ZnO nanoparticles’ PL sensitivity for H2O2. The ZnO nanoparticles showed two peaks corresponding to near band edge (NBE) emission and dry low emission (DLE) emission. After immobilization of GOx, the structure was tested for the detection of hydrogen peroxide. Reduction in PL spectral peaks was observed after adding H2O2 to the solution in the concentration range from 0.05 mM to 100 mM. This PL peak reduction occurred exponentially. The sensitivity of low concentrations of H2O2 about 0.05 mM was attributed to the large surface area. The same structure was used to detect glucose concentration in solution as GOx oxidizes glucose and produces H2O2, reducing the PL intensity. The detection range of glucose levels was from 10 mM to 130 mM.
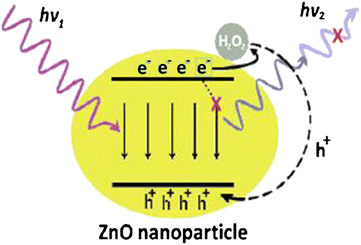 | ||
| Fig. 10 Mechanism of ZnO nanoparticles’ PL sensitivity for H2O2.183 | ||
A ZnO nanorod-based non-enzymatic glucose biosensor was reported by Sarangi et al.184 The PL intensity decreased upon adding glucose, which continued to decrease with increased glucose concentration. The production of H2O2 happened due to glucose, being responsible for PL quenching. The sensor had a sensitivity of about 1.4%/mM with a linear concentration range from 0.5 to 30 mM. The sensor was unaffected by interferents like amino acids, uric acid, and BSA, generally present in human blood samples.
Furthermore, Viter et al.185 reported ZnO nanorods for the detection of salmonella. The PL spectrum of ZnO nanorods showed NBE emission at 380 nm and DLE emission at 590 nm. Anti-salmonella antibodies were immobilized to detect salmonella antigens, and the BSA blocking agent was also added for specific binding. The PL intensity increased after immobilization of antibodies, and the BSA coating further increased the PL peak intensity. After interaction with the antigens, the PL intensity was reduced. The optimal response was observed for the concentration range from 102 to 106 cells per mL. The ZnO nanorods and the antigens interact by hydrophobic and van der Waals bonds. Dengue virus detection was accomplished by using ZnO thin films by Iyer et al.186 ZnO thin films were immobilized with sequence-specific probe strands for the detection of dengue virus DNA. ZnO has strong adsorption efficiency towards negatively charged DNA.
Liu et al.187 reported bi-functional ZnO nanorod arrays. The PL spectra of ZnO nanorods increased with the immobilization of dimercaptosuccinic acid (DMSA) and BSA. BSA immobilization reported a higher increase in the PL spectral intensity. Moreover, upon adding HSA to BSA immobilized ZnO nanorods, a twice increase in PL intensity was observed. Also, Chang et al.188 reported the detection of carbohydrate antigen CA 15-3 by using an Au/ZnO thin film. The proposed structure was found to have better SPR intensity than the Au/Cr structure. The detection of antigen was done by recording the phase intensity. The SPR intensity was observed to increase with an increase in antigen CA 15-3. A linear response was obtained for antigen concentrations from 1 to 40 U mL−1 above which quantification was impossible. The phase intensity increased by two-fold for the Au/ZnO thin film compared to Au/Cr, and it increased by three-fold for lower concentrations such as 0.1 and 0.025 U mL−1. The LOD decreased four-fold to 0.025 U mL−1.
Later, Kim et al.189 reported glucose biosensors based on ZnO nanostructures. ZnO nanostructures were reported to be synthesized by using the mercaptoundecanoic acid (MUA) surfactant, which provides water solubility and biocompatibility. GOx was immobilized on ZnO nanocrystals for the detection of glucose. The PL peak intensities were measured by adding glucose to the ZnO/MUA/ GOx electrode. The PL peak intensity was reduced with an increase in the concentration of glucose from 1.6 to 33.3 mM, covering the general physiological glucose level. The sensor response time was less than 5 s with a LOD of about 0.33 mM. The sensor also showed specificity to cholesterol. A cholesterol biosensor by using ZnO thin films was reported by Kaur et al.190 Three cases of Au/prism, ZnO/Au/prism, and ChOx/ZnO/Au/prism were investigated. The resonance angle for Au/prism was 43.54°, which increased drastically upon adding the ZnO layer due to its dielectric properties.
Upon addition of ChOx the resonance angle again decreased to 45.37° as the cholesterol concentration had increased the resonance angle to higher values. The sensor is characterized by a LOD of 0.12 mM with a detection range from 0.12 to 10.23 mM.
Further, ZnO–Au nanocomposites can be used as active SERS substrates, as reported by Sun et al.191 Simple hydrothermally synthesized ZnO Au nanocomposites (hybrid A) and white light-emitting ZnO–Au nanocomposites were synthesized by treating hybrid A materials with HCL. These substrates showed greater enhancement in their ability and then showed a reduction in Au thickness. The lower thickness of Au showed a weaker SERS signal and vice versa. Nevertheless, for very high thickness, the SERS signal was again observed to be weaker.
Malachite green (MG) detection using Ag nanocomposites on ZnO nanodomes was reported by Sivashanmugan et al.192 The Ag nanocomposites on ZnO nanodome hybrid nanosystems were prepared by the coupling of relatively flat and smooth ZnO nanodomes with Ag nanocomposites. A high SERS enhancement factor (EF) was obtained for the Ag/ZnO nanocomposite for sensing the crystal violet molecular probe and MG at low concentrations. The enhanced effect of SERS was attributed to the generation of strong local electromagnetic fields induced by Ag nanoparticles on ZnO nanodomes and intra nanocomposite interactions. More hotspots were anticipated to be produced due to the existence of metal–semiconductor-induced gap states. Glucose detection by using a ZnO modified gold disc was reported by Singh et al.193 Efficient glucose sensing was observed with a LOD for glucose of about 0.01 mM. The sensor showed linear sensitivity for glucose concentrations up to 250 ng mL−1, and the sensitivity saturated beyond 250 ng mL−1. Electromagnetic fields on a gold surface induce electron transfer from Au to ZnO at resonance, thus resulting in good sensitivity. The presence of oxygen defects also enhanced the sensitivity by providing electrons to the active layer.
Later, Tao et al.194 reported the growth of ZnO nanorods on Si wafer and considered them a SERS substrate for detecting rhodamine. With Ag decoration, they were found to form scaffold-like structures. The Raman enhancement factor (EF) was measured for increased Ag deposition. As the Ag deposition time was increased, the EF was also seen to increase by four times. For this, the EF was calculated with different concentrations of rhodamine; for the concentration of 10−8 M, an EF of about 107 was noticed, which indicated its sensitivity towards a lower rhodamine concentration. For UV irradiated substrates, the detection of rhodamine was weakened, and no Raman signal was observed for substrates irradiated with UV for 2 hours. In another work, Yang et al.195 reported the detection of cancer cells by using ZnO nanorods. The ZnO nanorods were connected with the epidermal growth factor receptor (EGFR) of squamous cell carcinoma (SCC) cells. The ZnO/EGFR antibody probes were tested for SCC, epithelial cancer that arises in multiple organs. The PL spectra displayed a purple light upon UV light excitation, which indicates cancer cells' presence. The cancer cells can also be identified by the peak intensity ratio of UV and green light. Dorfman et al.196 reported protein–protein interactions existence with ZnO nanoscale structures. ZnO nanorods grown on Si wafers were investigated. Neither as-synthesized nor protein G-treated ZnO nanorods showed any fluorescence.
Further, a clear green emission indicated protein–protein interactions when reacting with fluorescein-conjugated anti-bovine IgG (FITC-antiIgG) to detect protein G adsorbed nanorods. Later, ZnO strips of 20 μm width were studied with repeated structures. Two chambers were placed on ZnO, and proteins fibronectin and IgG were introduced into one chamber. Further upon interaction with FTIC anti-IgG, strong fluorescence was observed from the chamber containing the IgG protein, indicating protein interactions. Also, Dorfamn et al.197 reported the detection of DNA molecules by using ZnO nanoscale structures. Two oligonucleotides were designed to correspond to B. anthracis (bas) and Bacillus cereus (bce). A 6-carboxyfluorescein modified oligonucleotide (basr) complementary to bas was also designed. ZnO nanoplatforms with two DNA strands of bce and basr did not show any fluorescence, while the nanostructure with bas and basr showed fluorescence, indicating the formation of a DNA complex. Optical biosensors detect biomolecules based on fluorescence, photoluminescence, SPR, and SERS techniques. Amongst the methods mentioned above, SERS, SPR, and fluorescence are costly. Though photoluminescence is cost-effective, the sensitivity will be compromised. It is also an easier and more prospective method.
For optical biosensors, the biosensor platform requires specific materials with advanced structural, electrical and optical properties for the effective transformation of biological interaction into physical signals. Metal oxides are quite attractive for biosensor applications as they possess all required physical properties (conductivity, luminescence and absorbance) as well as biocompatibility. Moreover, ZnO is an n-type semiconductor with wide band gap (3.37 eV), high isoelectric point (pH = 9–9.5) and intense room temperature photoluminescence.177 Owing to their peculiar optical properties, ZnO nanostructures can be potentially deployed for the design of optical biosensors.
 | ||
| Fig. 11 Nano-QCM based piezoelectric biosensor. Reproduced with permission from ref. 200. | ||
A self-powered creatinine biosensor was reported by Wang et al.199 The biosensor can detect creatinine concentration based on the piezo-enzymatic reaction of the ZnO nanowires. The detection range of the creatinine biosensor was measured to be around 1 × 10−5–1 × 10−1 mM and the sensitivity was 0.0229 V mM−1.
Reyes et al.200 reported the detection of DNA oligonucleotide molecules using ZnO nanostructures based on the QCM device. The ZnO nanotips were grown on to the nano QCM sensing area. The sensing area was made superhydrophilic by exposure to UV light, increasing its sensitivity ten-fold compared to the standard QCM and reducing the required sample volume. ZnO nanotips were functionalized for DNA in three steps. Firstly, the sensing area of the QCM was immersed in a linker solution, and then DNA incubation followed by hybridization was carried out. Following the above three step, the ZnO-based QCM exhibited an increase in frequency shift, indicating the uniform distribution of immobilized and hybridized DNA molecules. Later, only QCM was tested for DNA molecules, and no shift in the frequency was observed, thus indicating that ZnO nanotips aid in detecting DNA molecules.
Similar detection of DNA molecules was also reported by Lee et al.201via ZnO nanorods. The QCM with ZnO nanorods was coated with titanium and gold films. The device was then tested for mercaptohexanol solution. Compared to the QCM without ZnO, the QCM with ZnO nanorods coated with gold showed greater frequency shifts. The same phenomenon was observed for the ZnO nanorod QCM over the bare QCM coated with gold for DNA oligonucleotide molecules. Wang et al.202 reported ZnO nanorods coated with QCM to detect CA 15-3 antigens. For strong binding, the QCM sensors coated with ZnO nanorods were immobilized with anti-CA 15-3 using APTES and glutaraldehyde. Time-dependent frequency shifts of ZnO with anti-CA 15-3 are nearly 1 Hz and even less. 10 QCM sensors with ZnO nanorods and anti-CA 15-3 were designed and tested for different concentrations of CA 15-3 from 0.5 to 30 U mL−1. A linear response was noticed within the concentration range 0.5–26 U mL−1 with a sensitivity of 25.34 ± 0.67 Hz per scale and a regression coefficient of about 0.99. To check reproducibility, 20 sensors were designed for CA 15-3, showing a reproducibility with a relative standard deviation of 2%.
A surface acoustic wave (SAW) is another type of biosensing mechanism under the piezoelectric category. Krishnamoorthy et al.203 reported the detection of interleukin by a ZnO surface acoustic wave (SAW) based biosensor. The ZnO/SiO2/Si guided shear horizontal surface acoustic wave (SH SAW) based biosensor detected IL-6. The IL-6 attached to ZnO by a direct adsorption process was not efficient compared to immobilization through BSA and MAbs. The larger sensing area was found to have better sensitivity for higher frequency devices. A similar structure was reported by Luo et al.204 for the detection of glucose. The glucose detection was carried out without the use of disposable and expensive glucose test strips, as glucose was detected by mass load change of the sensitive area, which resulted from the change of pH of the testing solution. The sensor was also tested for pH changes due to interferents like ascorbic acid, lactic acid, and uric acid. The change in pH due to these interferents was not significant, indicating the sensor efficiency for application in glucose detection.
Mao et al.205 reported the self-powered piezoelectric biosensing textiles for physiological monitoring and time-motion analysis. Depending on the piezoelectric effect developed due to the interaction between the lactate and lactate oxidase, the moving speed, joint angle, frequency, and sweat lactate concentration of an athlete in real-time can be monitored. The entire process of monitoring and analysis uses no batteries. Using this method, various things (people) in motion can gauge the requirements of their motion and their own physiology. Fig. 12 represents the working mechanism of the self-powered piezoelectric biosensor.
 | ||
| Fig. 12 Working mechanism of self-powered piezoelectric-biosensing textiles for sweat-lactate analysis.205 | ||
In summary, for piezoelectric biosensors, the detection depends upon the mechanism of mass loading. The change in mass on the surface is recorded as the change in frequency. For piezoelectric sensors, higher mass loading provides higher sensitivity, and low mass loading on the sensor surface could be a limitation. SAW biosensors were observed to function at higher frequencies and provide higher sensitivity as compared to QCMs. Although QCM biosensors present lower sensitivity, they are simpler. The piezoelectric effect harvests mechanical energy from ZnO nanostructures and outputs voltage/current signal. After enzyme or antibody surface modification on ZnO nanowires, the piezoelectric output is a biosensing signal dependent on surface biological processes. Since ZnO nanostructures exhibit excellent piezoelectric properties in addition to other merits they can be considered as a potential candidate for the piezoelectric biosensor design.205
Phosphate detection was reported by Ahmad et al.207 using a ZnO nanorod based FET. The ZnO nanorods were grown on a SiO2/Si substrate and immobilized with pyruvate oxidase. The as-fabricated FET and ZnO nanorod-based FET were analyzed for phosphate detection. The ZnO nanorod-based FET showed enhanced current response compared to the as-fabricated FET biosensor. The better response is attributed to ZnO nanorods' high specific surface area and better electron transfer ability during electrocatalytic reactions. The sensors showed better reproducibility with a standard deviation of about 4.3%. The FET biosensor showed a linear concentration range from 0.1 μM to 7.0 mM with a sensitivity of 80.57 mA cm−2 mM−1 and a LOD of 50 nM.
Furthermore, Liu et al.208 reported ZnO nanowire and thin film-based FET biosensors. The sensor showed a change in conductance which was higher in the case of streptavidin due to specific binding between biotin and streptavidin, and a lower change was observed in conductance for common protein IgG non-specific binding. Also, Liu et al.209 reported a ZnO nanowire-based FET biosensor for uric acid detection. The uric acid reaction with uricase produces two hydrogens that change the surface potential, hence the conductance of the biosensor. The sensor showed a linear response for the concentration range from 1 pM to 0.5 mM. The sensor could detect uric acid at a concentration of 1 pM with a 14.7 nS change in conductance. The sensor response time was in the order of a millisecond.
Later, Zong et al.210 reported FET biosensors based on ZnO nanorods immobilized with GOx. A detection scheme of frequency mixing was used for glucose detection with better sensitivity and reduction in the complexity of fabrication and cost. The glucose detection was carried out based on a change in the resulting currents. The sensor showed a good sensitivity of about 1.6 mA μM−1 cm−2 with a LOD of 1 μM. A non-enzymatic ZnO nanorod-based FET for glucose detection was reported by Ahmad et al.211 NiO quantum dot modified ZnO nanorods were used for sensing purposes. The surface turned to the hydroxide phase upon water adsorption, and in the presence of glucose, it turned to Ni(OH)2, thus producing H2O2. The electrooxidation current of H2O2 was used for the detection of glucose. The sensor showed two linear current responses for glucose concentrations in the range of 0.001–10 mM and 10–50 mM with respective sensitivity of 13.14 μA cm−2 mM−1 and 7.31 μA cm−2 mM−1. Good selectivity in the presence of interferents was obtained and 97% of the initial response was retained after 8 weeks, indicating its stability. The tests carried out in the whole blood sample and human serum sample indicated that the sensor response was decreased a little in the whole blood sample due to the presence of blood cells and protein fragments.
Ahmad et al.212 also reported vertically aligned ZnO nanorods based on a FET glucose biosensor. The ZnO nanorods were modified by Fe2O3, which helped in the catalytic electro-oxidation of glucose. The FET biosensor without Fe2O3 showed a poor response in the presence of glucose, and with Fe2O3, it showed an enhanced response. The current was seen to increase with an increase in glucose concentration with a linear response from 0.05 to 18 mM. The sensor showed a decreased response in the whole blood sample and good selectivity in the presence of interferents and competing sugars. The sensor had a sensitivity of 105.75 μA mM−1 cm−2, 12 μM LOD, and 10 s response time, and retained 97.5% of the initial response after 10 weeks. A biosensor based on a ZnO nanorod FET immobilized with GOx for glucose detection was reported by Fathollazadeh et al.213 In this, ZnO nanorods served as the conducting channel for the electrolyte gated FET. The sensor showed good sensitivity towards glucose in positive bias. The change in channel conductivity was due to the hydronium ions produced due to the reaction between GOx and glucose. The LOD of the sensor was 3.8 μM. Recently, a brief review of ZnO nanorod-based FET biosensors was provided by Karim et al.214 In the FET based ZnO biosensor, the sensing mechanism was based on the change in conductance of the channel formed by ZnO nanorods. The nanorod-based FET provides reduced fabrication complexity and costs over conventional three-electrode systems. The FET-based biosensors have high detection potential and higher sensitivity. They have attracted major attention due to their low cost, portability, faster detection, and compatibility for integration on-chip.
4.3. Detection of specific biomolecules
ZnO biosensors have been used for the detection of a variety of biomolecules. Wei et al.215 reported the detection of glucose by hydrothermally synthesized ZnO nanorods. The ZnO nanorods are synthesized hydrothermally, and GOx is immobilized on the surface of ZnO nanorods. A three electrode system is used to perform electrochemical experiments. Cyclic voltammetry measurements show a strong change in the current upon the addition of glucose. Amperometric measurements show that the LOD of the sensor is 0.01 mM with a response time of about 5 s. Cholesterol biosensors with ChOx immobilized ZnO nanoparticles are reported by Umar et al.216 A gold electrode with Nafion-coated ChOx immobilized ZnO nanoparticles is used as the working electrode while Ag/AgCl is used as the reference electrode. The cyclic voltammetry curves showed a peak at 0.355 V in the presence of 0.1 mM cholesterol in 0.1 M PBS buffer solution. When the cholesterol concentration is increased gradually, the currents are found to increase. The sensor had a LOD of 0.37 nM and a sensitivity of 23.7 μA mM−1 cm−2. The response time of the sensor was less than 5 s. A ZnO nanorod field-effect transistor (FET) based biosensor for protein detection was reported by Kim et al.217 Biotin-modified ZnO nanorods were used for the study. The ZnO nanorod FET system provides a considerable increase in the current upon exposure to streptavidin, thus indicating its efficiency for streptavidin detection. Later, Sang et al.218 reported the detection of proteins by using ZnO nanowires. The surface was modified by using 3-APTES and biotin-N-hydroxysuccinimide ester (NHS-biotin). Streptavidin of varying concentration is injected simultaneously through the microfluidic second channel. Fluorescence intensity indicated the detection of streptavidin. Furthermore, Liu et al.219 reported streptavidin detection using a ZnO nanowire/thin-film biosensor. Recently dengue serotype 2 DNA detection has been reported by Al-Douri et al.220 using sol–gel synthesized aluminum-doped ZnO nanostructures. The nanostructures are dispersed over a p-type Si wafer. An inverse relationship is noticed with current magnitudes decreasing with increasing DNA concentration.A variety of biosensors for DNA detection have been also reported. Kaur et al.221 reported the detection of Neisseria meningitidis DNA by using ZnO thin films. The sputtering method allowed the preparation of ZnO thin films on gold-coated glass prisms and immobilized through a single-stranded DNA probe. The biosensor showed linearity towards DNA over a wide range of concentrations of 10–180 ng μL−1 and a good sensitivity of 0.03° ng−1 μL−1. Later, Gerbreders et al.222 reported the detection of the Trichinella DNA sequence using ZnO nanostructures. DNA primers were developed on the ZnO nanostructures and employed as a working electrode. It is noted that nanotubes lead to higher sensitivity than thin films and nanorods due to their porous structure. Gasparotto et al.223 reported detection of ovarian cancer antigen by using a ZnO nanorod and Au nanoparticle hybrid structure. The structure is synthesized via the hydrothermal method. The ZnO nanorod coated with Au nanoparticles efficiently immobilizes ovarian antigen via binding with cystamine and glutaraldehyde. Also, Liang et al.224 reported a Au/ZnO thin film to detect breast cancer cells. The surface plasmon resonance (SPR) of the Au/ZnO thin film was compared with the Biocare SPR system. The Biocare SPR system showed good selectivity for higher concentrations of saliva and reduced sensitivity for lower concentrations. The Au/ZnO thin film SPR system displayed linear response for saliva concentrations within 20 U mL−1 covering all relevant CA 15-3 concentrations from healthy to affected people. Dopamine detection using APTES capped ZnO quantum dots was reported by Zhao et al.225 The ZnO quantum dots are found to quench fluorescence upon the addition of dopamine. The quantum dots showed quenched fluorescence ability with and without APTES. Better fluorescence quenching without a clear shifting of the peak is observed for APTES capped ZnO quantum dots. The intensity is quenched to 11% for APTES capped and 67% for non-capped quantum dots. The quenching phenomenon is noticed due to the electron transfer mechanism between the quantum dots and dopamine. Rui et al.226 reported the detection of H2O2 using cytochrome c (Cyt c) modified ZnO nanosheets. The nanosheets observed fewer interferences from O2. The ZnO nanosheets with Cyt c showed linear response for H2O2 in amperometric calculations compared to bare ZnO nanosheets. The same was used for the detection of extracellular H2O2 from living hepatoma cells. The ZnO nanosheets with Cyt c showed increased cathodic currents with the addition of phorbol 12-myristate 13-acetate for H2O2. Phenolic compound detection was reported by Li et al.227 ZnO nanoparticles immobilized with tyrosinase proved to detect catechol without any mediator. The biosensor showed good sensitivity and stability, and can be used to detect phenolic compounds without any mediator. Salmonella detection based on optical intensity was reported by Viter et al.228 Anti-salmonella antibodies were coated on ZnO nanorods for the detection of salmonella agents. The BSA blocking agent was also coated to avoid unnecessary binding. ZnO nanorods with anti-salmonella antibodies had increased near band edge emission, which further increased with BSA coating. When exposed to salmonella antigens, the PL intensity decreased proportionally to Ag concentration.
In another work, Narang et al.229 reported a CHIT–ZnO nanocomposite film-based biosensor to detect triglyceraldehyde. ZnO–CHIT composites are found to provide a biocompatible environment for enzyme detection. ZnO–CHIT nanocomposites are found to have better currents for triglyceraldehyde with an optimized ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CHIT composite ratio. Too low concentrations of ZnO nanoparticles provide lower enzyme adsorption, thus reducing the sensitivity, and a higher concentration of ZnO nanoparticles increases the obstruction for species diffusion. Devi et al.230 reported xanthine detection using a ZnO nanoparticle and polypyrrole (PPy) composite film. The film was immobilized with xanthine oxidase (XOD) for the detection of xanthine. Cyclic voltammetry results for the XOD/ZnONP/PPy/Pt electrode reported linear currents for the concentration range between 8 μM and 40 μM. ZnO–Au nanocomposites for the detection of rabbit IgG were reported by Wang et al.231 The biosensor based on the ZnO–Au nanocomposite was shown to present better sensitivity than the biosensor based on Au film and Au nanoparticles. The ZnO–Au-based biosensor showed a sensitivity 16 times greater than that of the sensor based on Au films. Recently, a ZnO nanorods–AuNPs electrochemical biosensor was reported by Biasotto et al.232 The sensor was immobilized with an anti-hepatitis C virus antibody to detect the hepatitis virus. AuNPs improved the device's sensitivity, repeatability, and reliability with a LOD of about 0.25 μg μL−1. Also, tellurium doped ZnO nanowires were reported by Khosravi-Nejad et al.233 for label-free detection of hepatitis virus. The sensor had a LOD of 1 pM with a linear detection range from 1 pM to 1 μM. From the above discussion, it can be concluded that ZnO nanostructures can be an effective material for detecting various types of biomolecules because of their high IEP, biocompatibility, chemical activity, and electron mobility. The performance of biosensors depends on their components, especially the matrix material, i.e., the layer between the recognition layer of biomolecule and transducer, as it plays a crucial role in defining the stability, sensitivity, and shelf-life of a biosensor. Table 2 briefly describes various biomolecules that have been sensed by the ZnO nanostructures, the transduction method, and the biosensitive layer used along with the sensor performance.
CHIT composite ratio. Too low concentrations of ZnO nanoparticles provide lower enzyme adsorption, thus reducing the sensitivity, and a higher concentration of ZnO nanoparticles increases the obstruction for species diffusion. Devi et al.230 reported xanthine detection using a ZnO nanoparticle and polypyrrole (PPy) composite film. The film was immobilized with xanthine oxidase (XOD) for the detection of xanthine. Cyclic voltammetry results for the XOD/ZnONP/PPy/Pt electrode reported linear currents for the concentration range between 8 μM and 40 μM. ZnO–Au nanocomposites for the detection of rabbit IgG were reported by Wang et al.231 The biosensor based on the ZnO–Au nanocomposite was shown to present better sensitivity than the biosensor based on Au film and Au nanoparticles. The ZnO–Au-based biosensor showed a sensitivity 16 times greater than that of the sensor based on Au films. Recently, a ZnO nanorods–AuNPs electrochemical biosensor was reported by Biasotto et al.232 The sensor was immobilized with an anti-hepatitis C virus antibody to detect the hepatitis virus. AuNPs improved the device's sensitivity, repeatability, and reliability with a LOD of about 0.25 μg μL−1. Also, tellurium doped ZnO nanowires were reported by Khosravi-Nejad et al.233 for label-free detection of hepatitis virus. The sensor had a LOD of 1 pM with a linear detection range from 1 pM to 1 μM. From the above discussion, it can be concluded that ZnO nanostructures can be an effective material for detecting various types of biomolecules because of their high IEP, biocompatibility, chemical activity, and electron mobility. The performance of biosensors depends on their components, especially the matrix material, i.e., the layer between the recognition layer of biomolecule and transducer, as it plays a crucial role in defining the stability, sensitivity, and shelf-life of a biosensor. Table 2 briefly describes various biomolecules that have been sensed by the ZnO nanostructures, the transduction method, and the biosensitive layer used along with the sensor performance.
| Target analyte | Biomolecule immobilized on the matrix layer | Matrix layer | Detection method | Sensitivity (S) | LOD | Linear response (L) | Ref. |
|---|---|---|---|---|---|---|---|
| Glucose | GOx | ZnO nanotubes | Electrochemical | 21.7 μA mM−1 cm−2 | 1 μM | 50 μM–12 mM | 234 |
| Uric acid | Uricase | ZnO nanosheets | Electrochemical | 129.81 μA mM−1 cm−2 | 0.019 μM | 0.05–2 mM | 235 |
| Cholesterol | ChOx | ZnO nanorods | Electrochemical | 35.2 mV dec−1 | NR | 10−6–10−2 M | 236 |
| CA 19-9 | CA 19-9 antibody | ZnO quantum dots | Photoluminescence | 0.47 μA U−1 mL−1 | 0.025 U mL−1 | 1–180 U mL−1 | 237 |
| Streptavidin | NHS biotin | ZnO nanowires | Fluorescence | NR | 417 fM | 417 fM–41.7 nM | 218 |
| DENV non-structural protein 1 | DNA probe | ZnO/Pt–Pd nanocomposite | Electrochemical | NR | 4.3 × 10−5 M | 10−6–10−4 M | 238 |
| Neisseria meningitidis | ssDNA probe | ZnO thin film | SPR | 0.03° ng−1 μL−1 | 5 ng μL−1 | 10–180 ng μL−1 | 221 |
| CA 125 | Anti-CA 125 | ZnO nanorod–AuNP nanohybrid | Electrochemical | NR | 2.5 ng μL−1 | NR | 223 |
| Dopamine | Non-enzymatic | ZnO–CuO composite | Electrochemical | 90.9 μA mM−1 cm−2 | 10−4 mM | 10−3–8 mM | 239 |
| H2O2 | Cytochrome c | ZnO nanosheet | Electrochemical | 2 ± 0.1 μA mM−1 cm−2 | 0.8 μM | 1–1000 μM | 226 |
| Salmonella | Anti-salmonella | ZnO nanorods | Photoluminescence | NR | NR | 102–106 cells per mL | 228 |
| Goat IgG | Non-enzymatic | ZnO–Au nanocomposite | SPR | NR | 0.15 μg mL−1 | 0.15–2 μg mL−1 | 231 |
| Xanthine | XOD | ZnO nanoparticles | Electrochemical | NR | 0.8 μM | 8–40 μM | 230 |
| Penicillin | Penicillinase | ZnO nanorods | Electrochemical | 121 mV dec−1 | NR | 100 μM–100 mM | 240 |
| H2O2 | HRP | Flower ZnO–AuNPs | Electrochemical | NR | 9 × 10−6 M | 1.5 × 10−5–1.1 × 10−3 | 241 |
4.4. Recent works on ZnO nanostructure-based sensors
Haque et al.242 reported ZnO nanoparticles doped with Cu nanoparticles for the detection of myoglobin. The nanocomposite was deposited on a gold plated electrode. The electrochemical sensor was tested with the myoglobin in the concentration range of 3–15 nM. The highest sensitivity recorded was about 10.14 μA nM−1 cm−2 with a LOD of about 0.46 nM. Naik et al.243 reported the modified carbon paste electrode with Co doped ZnO nanoparticles for the detection of uric acid. The modified electrode under optimal conditions of the influencing parameters exhibited a LOD of about 3.37 μM through a diffusion controlled process. The modified electrode also exhibited two different oxidation peaks for the detection of uric acid and adrenaline separately. Kamaci et al.244 reported the detection of cysteine in tap water and BSA using the ZnO quantum dots fluorescent probe. The LOD value of the biosensor was found to be 0.642 μM, and the linear range was determined to be in the range of 0.1–600 μM. The interference of other analytes such as cations, amino acids, and anions was negligible. Dairy et al.245 reported ZnO nanorods sandwiched between two graphene layers for the detection of glucose based on the piezoelectric effect. The lower graphene layer was fixed at the lower surface and used for the detection of glucose concentration. The number of molecules connected to the upper graphene layer increased as the GOx concentration increased (from 0 to 0.01 M), resulting in an increase in mechanical stress and strain in the ZnO-NRs. Deformation in the crystal structure of ZnO-NRs causes displacement of the negative and positive charge centres, resulting in electric potential and piezoelectric polarisation in ZnO-NRs due to the piezoelectric effect. The sensitivity of the designed sensors was in the range of 0.12 to 0.28 mV mM−1 for a biosensor with a single ZnO-NR and 0.035 to 0.135 mV mM−1 for GR/ZnO-NRs/GR.Au coated ZnO nanorods immobilized with phenylalanine hydroxylase are reported for the detection of phenylalanine.246 Enzyme immobilization is achieved by dropping Au–ZnO and phenylalanine hydroxylase onto a paper disc on the graphene screen printed electrodes. Under optimal conditions, the sensor exhibited a linear range of 5.0 nM to 100 μM with a LOD (S/N = 3) and a limit of quantitation of 3.0 nM and 10.0 nM, respectively. A ZnO nanorod-based pH sensor was manufactured by doping magnesium into the sensing membrane, thus leading to an innovative electrolyte insulator semiconductor for pH sensing. The results showed high hydrogen sensitivity, linearity, and drift with 3% magnesium due to enhanced crystalline quality. This sensor can be explored further as a biosensor in the future.247 Multiple drug sensing was accomplished via layers of an acid–base functionalized carbon nanotube and zinc oxide nanocomposite (COOH-CNTs/ZnO/NH2-CNTs) to detect paracetamol, diclofenac and orphenadrine (PAR, DIC, and ORP) drugs with highly efficient sensitivity and selectivity. Similarly, the FCNTs/ZnO/fCNTs/GCE based sensor detects PAR, DIC, and ORP drugs with femtomolar limits of 46.8, 78, and 60 fM, respectively. This sensor proved six-fold more efficient than bare glassy carbon electrodes due to the increased surface area.248 This sensor can also be investigated further for detection as a biosensor. Complementary information to the one presented in the present work can be found in literature reports on ZnO based biosensors. ZnO nanowire based FET biosensing was reported by Ditshego et al.249 The review specifically covers the ZnO nanowire based FET biosensors. Other review works based on electrochemical biosensors, SPR biosensors, and metal–oxide modified ZnO nanomaterial based biosensors are reported.250–253 Further, different mechanisms of biosensing apart from electrochemical sensing and different nanostructure based biosensors are reported.254 Shetti et al.255 have reported a review of ZnO-based biosensors for electrochemical detection. The other review reported by Xu et al.256 confined the biosensor discussion to quantum dot and 1-D based ZnO biosensors. Thin films for biosensing applications are reported by Arya et al.257 Other reviews are previously reported with discussion specific to cardio biomarker detection,258 surface plasmon resonance detection,259 optical biosensors,260 and enzymatic biosensors.261 Tripathy et al.262 presented a review of ZnO nanosheet and ZnO based FET biosensors. However, in our manuscript, all the above mentioned topics are discussed in a comprehensive way enabling the readers a wide scope of knowledge. It will give us a bird's eye view of how ZnO based nanostructures can be employed for potential ZNO biosensors.
5. ZnO based POC biosensors
In order to facilitate point-of-care testing, miniature biosensors have been created in recent years that are based on already available handheld devices or microfluidic systems. The measurement of classical physical parameters has seen widespread use of several simple and portable instruments, including thermometers, pressure metres, and pH metres. Efforts have been made to develop novel signalling systems in conjunction with the aforementioned miniaturised devices to provide easy and portable POC testing without the need for cumbersome external instrumentation. As point-of-care (POC) devices, biosensors provide benefits such as quick detection, user friendliness, accuracy, portability, cost-effectiveness, and straightforward on-site detection in the field.263Several groups of researchers have been working on a biosensor based on ZnO nanostructures with the goal of using it for point-of-care diagnostics. Point-of-care testing using a ZnO-based substrate-gate coupled biosensor was described by Fathil et al.264 The device's primary purpose is to analyse cardiac troponin, a biomarker for heart disease. An antibody against cardiac troponin, a biomarker, has been covalently immobilised on a ZnO nanoparticle thin film. Between the two p-type regions, the device incorporates a thin coating of ZnO nanoparticles. ID in the channel drops when a biomolecule with a strong positive charge, like cardiac troponin, is detected. The detection of cardiac biomarker is done with a LOD of about 3.24 pg mL−1. POC biosensors for pesticide sensing are reviewed by Kalyani et al.265 The review comprises a brief discussion on different types of sensing mechanisms for POC biosensors. Xia et al.266 reported the smartphone based avian influenza biosensor. The virus catching antibodies immobilized on the 3D nanostructures made of polydimethylsiloxane structures with ZnO nanorod templates. The on-chip gold electrodes allow the calorimetric reaction when the virus comes in contact with the sensor. Further, with smartphone imaging and calorimetric reaction a very low LOD of about 8 × 103 EID50 per mL is possible.
Zika virus detection using an electrochemical immunosensor is reported by Macedo et al.267 The biosensor is created by manufacturing ZnO nanostructures on a printed circuit board via chemical bath deposition, and then immobilising antibodies using cystamine and glutaraldehyde. Evaluation of sensor responses using cyclic voltammetry measured a detection range between 0.1 ng mL−1 and 100 ng mL−1, with a very low LOD of 1.0 pg mL−1. In the graphene–ZnO nanorod heterostructure, an electric field enhances the mass transit of the analyte to the sensor surface.268 For PoC applications, these hybrid nanostructures have been placed on flexible polyethylene terephthalate substrates with screen printed electrodes. ZnO nanorods have been functionalized with aptamers and combined with a smartphone-interfaced low-cost potentiostat. Electrochemical impedance spectroscopy and commercial ELISA kits have confirmed the system's performance with 50 μL analyte. The limit of detection of 1 fg mL−1 in human serum with 6.5% coefficient of variation is three orders of magnitude lower than that of PoC devices.
Chaudary et al.269 reported a review on the design of next generation sensor systems based on nanomaterials. The merits of using nanostructure materials in the design of 5th generation sensors include high specific surface area with excellent porosity, mechanical stability, flexibility, diverse surface chemistries and customizable electrical and optical characteristics; tunable surface terminals, high dispersibility, mechanical and thermal durability, and hydrophilicity which allow machine processing; and degradability, biocompatibility, energy efficiency, and cost-effectiveness.
6. ZnO based systems for COVID-19 detection
The last few decades have been plagued by viral outbreaks that present some of the biggest challenges to public safety. The current coronavirus (COVID-19) disease pandemic has exponentiated society concerns in these issues. Increased research on diagnostic tools is currently being implemented in order to assist with rapid identification of the virus, as mass diagnosis and containment is the best way to prevent the outbreak of the virus.254 Accurate, rapid, and low-cost molecular diagnostics are essential in managing outbreaks of infectious diseases.270 The main complication with some repurposed drugs is the delivery process, which can promote secondary effects. In light of the considerations quoted above, the use of ZnO nanoparticles (ZnO-NPs) has been reported in state-of-the-art nano-compounds as a system for drug delivery. In addition, these kinds of systems have been used as a nano-carrier of antibiotics.271Lateral flow assay (LFA)-based qualitative diagnostics of COVID-19 were beneficial for laboratory testing and POCT due to their simple scaling-up capability. Because of their poor technical performance, including detection limit and false-positive and false-negative interferences, such kits could only be used for preliminary screening of large populations. So, LFA-based COVID-19 diagnostic kits have scaling-up capabilities, but the isolation advantage in identifying infectious SARS-CoV-2 should be the main benefit. The detection specificity issue might cause false-positive (other viral interference) and false-negative findings (likely due to mutation).272
Due to the lack of effective treatments, simple human-to-human transmission, severe respiratory infections and organ damage, the presence of SARS-CoV-2 throughout the life cycle (water, animals, food, air), unavoidable and frequent viral mutations, etc., COVID-19 monitoring was always the top priority. The finest R&D recommendations thus far have concentrated on re-engineering vaccinations, virus-free indoor air, antimicrobial coating on personal protective equipment, and nanostructure-based biosensors (optical, electrical, and magnetic) for quantitative detection of SARS-CoV-2 at low levels.272
In this scope, ZnO based systems are supporting those efforts. Paper based EIS biosensors are designed based on the ZNON NWs for SARS-CoV-2 detection.270 The ZnO nanowires are directly grown on the working electrodes and are immobilized with the SARS-CoV-2 S-protein receptor-binding domain (RBD) specific to COVID-19. To prepare human serum samples for testing, recombinant IgG antibody (CR3022) to SARS-CoV-2 spike glycoprotein S1 was spiked at different concentrations in human serum to mimic the real patient samples. The employed EIS sensors were able to differentiate the human serum sampled at different concentrations of cr3022 antibody indicating their feasibility for covid diagnosis. For asymptomatic patients, a serological assay with ZnO nanowires is reported for early detection of the virus.273 A microplate coated with hydrothermally synthesized ZnO nanowires is developed. This plate is coated with SARS-CoV-2 and used for fluorescence immunoassay (FIA) to detect antibodies specific for SARS-CoV-2. The ZnO nanowire microplate is reported to be more sensitive than commercial immunoassay. Cervantes et al.271 reported a theoretical study of nanostructured ZnO as a carrier of drugs. ZnO is coupled with three drugs chloroquine, dipyridamole, and lopinavir. Lopinavir is found to have the highest adsorption energy. From the docking tests, observing the interaction of free medications and composites with the SARS-CoV-2 major protease, it is discovered that the composites have higher coupling energy than free drugs. As a result, ZnO nanoparticles are found to regulate medication dosage on the SARS-CoV-2 target. Later Sportelli et al.274 reported the experimental approach for the ZnO nanoparticles to lower the antigen of SARS-CoV-2 by about 90%. The ZnO nanoparticles are synthesized with the galvanostatic approach in the presence of different stabilizers. Preliminary studies on ZnO nanoparticles and poly ethylene oxide composites showed that ZnO nanoparticles are highly successful as a self-cleaning coating for hard surfaces subjected to SARS-CoV-2 infection. The aforementioned composites could be easily brushed on routinely touched surfaces and let to dry due to their nontoxic and straightforward handy nature. Haghayegh et al.275 reported on carbon screen printed electrodes modified with ZnO nanoparticles in conjugation with reduced graphene oxide nanosheets dispersed in buffer (bbZnO/rGO) for the detection of the nucleocapsid protein antigen (SARS-CoV-2 N-protein antigen). The reported biosensor can yield acceptable sensitivity. For detection of N-protein in spiked samples, the immuno-biosensor has a LOD of 21 fg mL−1 over a linear range of 1–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 pg mL−1 and a sensitivity of 32.07 Ω mL pg−1 mm−2. The N-protein biosensor can distinguish between positive and negative clinical samples in 15 minutes. In another work, Hamdi et al.276 reported the ZnO nanoparticles with hexagonal wurtzite P63mc crystal structure. The ZnO nanoparticles are tested for possible interaction with the ACE2 receptor as the possible target for COVID-19 using an in silico docking approach. Furthermore, an enhanced dose-dependent cellular uptake was demonstrated.
000 pg mL−1 and a sensitivity of 32.07 Ω mL pg−1 mm−2. The N-protein biosensor can distinguish between positive and negative clinical samples in 15 minutes. In another work, Hamdi et al.276 reported the ZnO nanoparticles with hexagonal wurtzite P63mc crystal structure. The ZnO nanoparticles are tested for possible interaction with the ACE2 receptor as the possible target for COVID-19 using an in silico docking approach. Furthermore, an enhanced dose-dependent cellular uptake was demonstrated.
When rapid and early detection is of interest, strategies based on specific antibody/antigen viral biomarkers have been adopted. The structural proteins of SARS-CoV-2 can be advantageous to employ specific MAb immunoassays. Infected patients' blood, saliva, serum, and nasopharyngeal (NP) swab samples include nucleocapsid protein (N-protein), which has been found to be the most predictive for COVID-19 detection in the early stages of infection, with low vulnerability to mutation. When employed for rapid antigen testing, it has proven to be a dependable disease indicator.275
7. Conclusions and future perspectives
The need for biosensors could be understood clearly during this COVID pandemic which requires rapid, effective and delocalized diagnosis. Due to their simplicity, faster detection, and facility to test at the victim's site biosensors can be preferred over conventional testing equipment, which is costly and requires a skilled operator for testing. Research is still progressing for more reliable, accurate, sensitive, and miniaturized biosensor design.ZnO is a wide bandgap semiconductor with a bandgap of 3.37 eV and a large exciton emission of about 60 meV. ZnO is thus a suitable candidate for biosensors due to its low cost, non-toxicity and ease of fabrication in a variety of morphologies and dimensionalities.
ZnO nanostructure-based biosensors have been reviewed in this document. For biosensors, the bioselective layer plays an important role in the selection of specific targets and sensitivity. The methods of biomolecule adsorption, both direct adsorption and covalent binding, have been presented here. Direct adsorption binds the biomolecule to ZnO by weak van der Waals forces, hydrophobic bonds or ionic bonding. The direct adsorption process is simple, but the biomolecules are not tightly bound to the surface. Covalent binding binds the biomolecules through cross-linking polymers. The molecules are tightly bound to a matrix and retain the bioactivity of the biomolecule. Different ZnO nanostructures for ZnO biosensors have been also discussed. However, there are limitations to using 0-D ZnO nanostructures, despite their improved sensing properties. The main problem is that the nanoparticles have little mobility because of the abundance of grain boundaries. The carrier mobility decreases when the electrons encounter a larger area of space charge as they go from terminal to terminal. One of the main advantages of 1-D ZnO over 0-D ZnO is its superior sensing capabilities. One-dimensional ZnO has a high surface-to-volume ratio and facilitates fast electron transport via a direct and stable route. The larger surface-to-volume ratio enables higher enzyme loading capacity improving the biosensor performance. A high aspect ratio is a useful property of 1-D ZnO for biosensors. Enzymes may be loaded onto the vast surface area of 2-D nanostructures. For biochemical sensing applications, 2D materials are advantageous due to their ability to deliver a high density of active surface sites across a vast region. The carrier mobility of 2D materials is also quite high. The electrochemical biosensors produce electrical signals via an electrical pathway for monitoring the amount of the target analyte. The amount of the target analyte is monitored by either cyclic voltammetry, potentiometry or amperometry methods. In optical biosensors, the optical transducer produces signals based on absorption, reflectance, and luminescence. Piezoelectric biosensors detect the target biomolecules based on the surface mass loading of the piezoelectric material.
ZnO-based FET biosensors are also reported. In FET-based biosensors, the change in the channel's conductance upon interaction with target analytes is used to determine the detection process. In particular, due to its unique structure and properties, 2D ZnO has been used for the design of a large variety of biosensors. 2D structures offer good conductivity, mechanical stability, and ease of functionalization. Improved amount of binding sites can be observed for the 2D structures due to their higher specific surface area. They can be employed as good flat templates for conjugation with biomolecules and other nanomaterials to improve the sensitivity and selectivity of the designed biosensor. Generally, 2D materials exhibit a large surface area and a high surface to volume ratio while their distinctive electronic structures and atomically thin layers are highly appealing owing to the extraordinary material properties which cannot be achieved using traditional bulk structures. A considerable advantage of nanoscale biosensors is their detecting ability and sensitivity at very small volumes of samples. ZnO-based biosensors feature high IEP, non-toxicity, ease of fabrication, biocompatibility, and low cost. Thus, biosensors for different biomolecules such as glucose, cholesterol, uric acid, DNA, and proteins have been developed based on various transduction mechanisms such as electrochemical, piezoelectric, and optical mechanisms. Also, FET based ZnO nanostructure biosensors are also reported for biosensing applications.
Despite the interesting results obtained, it is still critical to select the specific properties of the nanomaterial for biosensor design. Variation in the properties of ZnO by surface engineering helps in obtaining biosensors with better efficiency. Though there are various methods for synthesizing ZnO nanostructures, obtaining uniform shaped ZnO nanostructures with repeatability is a challenge for biosensing and obtaining high reproducibility is a concern that would help design good point-of-care biosensor devices. As a result, advances in material synthesis, enzyme/protein engineering, and immobilization/conjugation methods will continue to provide innovative nano-engineered segments with enhanced functionality. The ZnO FET biosensors may replace the electrochemical biosensors in the future for miniaturized devices. The medical applications of these devices must be widely researched, as it may represent a huge step in terms of disease diagnosis and control. The practical application of biosensors in the medical diagnosis field is still way ahead, and the research is progressing to combine the electronic and biological systems to design faster, smaller, and cheaper ZnO biosensors.
Abbreviations
| ABTS | 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) |
| AFM | Atomic force microscopy |
| APTES | Aminopropyl-triethoxysilane |
| BSA | Bovine serum albumin |
| CHIT | Chitosan |
| ChOx | Cholesterol oxidase |
| CMOS | Complementary metal–oxide–semiconductor |
| CNTs | Carbon nanotubes |
| CRP | C-reactive protein |
| CVD | Chemical vapor deposition |
| Cyt c | Cytochrome c |
| DEZ | Diethyl zinc |
| DLE | Deep level emission |
| DMSA | Dimercaptosuccinic acid |
| DMSO | Dimethyl sulfoxide |
| DPV | Differential pulse voltammetry |
| DSP | Dithiobis succinimidyl-propionate |
| DSS | Disuccinimidyl suberate |
| EDC | 1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride |
| EF | Enhancement factor |
| EIS | Electrochemical impedance spectroscopy |
| ELISA | Enzyme-linked immunosorbent assay |
| EP | Epinephrine |
| FET | Field effect transistor |
| FITC-antiIgG | Fluorescein-conjugated anti-bovine IgG |
| GOx | Glucose oxidase |
| H2O2 | Hydrogen peroxide |
| HMTA | Hexamethylenetetramine |
| HRP | Horseradish peroxidase |
| HRTEM | High-resolution transmission electron microscopy |
| IEP | Isoelectric point |
| ITO | Indium tin oxide |
| LDH | Lactate dehydrogenase |
| LOD | Limit of detection |
| MAb | Monoclonal antibody |
| MBE | Molecular beam epitaxy |
| MG | Malachite green |
| MOCVD | Metal–organic CVD |
| MPTMS | Mercaptopropyl trimethoxysilane |
| MWCNTs | Multi-walled carbon nanotubes |
| NAAT | Nucleic acid amplification tests |
| NBE | Near band edge emission |
| NHS | N-Hydroxysuccinimide |
| NHS-biotin | Biotin-N-hydroxysuccinimide ester |
| PBS | Phosphate buffer solution |
| PDA | Phosphonodecanoic acid |
| PDA | Polydopamine |
| PECVD | Plasma enhanced CVD |
| PET | Polyethylene terephthalate |
| PHDA | Phosphonohexadecanoic acid |
| PL | Photoluminescence |
| PLD | Pulsed laser deposition |
| POC | Point of care |
| PtNDs | Platinum nanodendrites |
| PVB | Polyvinyl butyral |
| PVP | Polyvinyl pyrrolidone |
| QCM | Quartz crystal microbalance |
| RBD | Receptor-binding domain |
| rRT-PCR | Real-time reverse-transcription polymerase chain reaction |
| SAW | Surface acoustic wave |
| SCC | Squamous cell carcinoma |
| SCE | Saturated calomel electrode |
| SERS | Surface enhanced Raman spectroscopy |
| SHSAW | Shear horizontal surface acoustic wave |
| SPE | Screen-printed electrodes |
| SPR | Surface plasmon resonance |
| TPSP | Tetragonal pyramid-shaped porous |
| WHO | World Health Organization |
| XOD | Xanthine oxidase |
Author contributions
M. Sankush Krishna: writing – original draft (lead). Sangeeta Singh: conceptualization (equal), writing – original draft (supporting). Maria Batool: writing – review and editing (equal). Heba Mohamed Fahmy: conceptualization (equal), supervision (equal). Kondaiah Seku: software (lead), formal analysis (equal). Ahmed Esmail Shalan: writing, review, editing (equal). Senentxu Lanceros-Mendez: writing – review, editing (equal), technical support. Muhammad Nadeem Zafar: conceptualization (equal), supervision (lead).Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
AES acknowledges the National Research grants from MINECO, Spain, “Juan de la Cierva” [FJCI-2018-037717].References
- J. D. Whitman, et al., Evaluation of SARS-CoV-2 serology assays reveals a range of test performance, Nat. Biotechnol., 2020, 38(10), 1174–1183 CrossRef CAS PubMed.
- C. Xie, et al., Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests, Int. J. Infect. Dis., 2020, 93, 264–267 CrossRef CAS PubMed.
- L. Guo, et al., Profiling early humoral response to diagnose novel coronavirus disease (COVID-19), Clin. Infect. Dis., 2020, 71(15), 778–785 CrossRef CAS PubMed.
- M. Srivastava, N. Srivastava, P. K. Mishra and B. D. Malhotra, Prospects of nanomaterials-enabled biosensors for COVID-19 detection, Sci. Total Environ., 2020, 754, 142363 CrossRef PubMed.
- S. A. Abid, et al., Biosensors as a future diagnostic approach for COVID-19, Life Sci., 2021, 273, 119117 CrossRef CAS PubMed.
- J.-L. He, et al., Diagnostic performance between CT and initial real-time RT-PCR for clinically suspected 2019 coronavirus disease (COVID-19) patients outside Wuhan, China, Respir. Med., 2020, 168, 105980 CrossRef PubMed.
- Z. Li, et al., Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis, J. Med. Virol., 2020, 92(9), 1518–1524 CrossRef CAS PubMed.
- D. Liu, et al., Trends in miniaturized biosensors for point-of-care testing, TrAC, Trends Anal. Chem., 2020, 122, 115701 CrossRef CAS.
- Y. Rasmi, X. Li, J. Khan, T. Ozer and J. R. Choi, Emerging point-of-care biosensors for rapid diagnosis of COVID-19: current progress, challenges, and future prospects, Anal. Bioanal. Chem., 2021, 413(16), 4137–4159 CrossRef CAS PubMed.
- P. R. Solanki, A. Kaushik, V. V. Agrawal and B. D. Malhotra, Nanostructured metal oxide-based biosensors, NPG Asia Mater., 2011, 3(1), 17–24 CrossRef.
- Y. Zhang, Z. Kang, X. Yan and Q. Liao, ZnO nanostructures in enzyme biosensors, Sci. China Mater., 2015, 58(1), 60–76 CrossRef CAS.
- M. L. M. Napi, S. M. Sultan, R. Ismail, K. W. How and M. K. Ahmad, Electrochemical-based biosensors on different zinc oxide nanostructures: A review, Materials, 2019, 12(18), 2985 CrossRef CAS PubMed.
- T. Shibata, K. Unno, E. Makino, Y. Ito and S. Shimada, Characterization of sputtered ZnO thin film as sensor and actuator for diamond AFM probe, Sens. Actuators, A, 2002, 102(1–2), 106–113 CrossRef CAS.
- M. Que, C. Lin, J. Sun, L. Chen, X. Sun and Y. Sun, Progress in ZnO Nanosensors, Sensors, 2021, 21(16), 5502 CrossRef CAS PubMed.
- D. Gedamu, et al., Rapid fabrication technique for interpenetrated ZnO nanotetrapod networks for fast UV sensors, Adv. Mater., 2014, 26(10), 1541–1550 CrossRef CAS PubMed.
- D. Panda and T.-Y. Tseng, One-dimensional ZnO nanostructures: fabrication, optoelectronic properties, and device applications, J. Mater. Sci., 2013, 48(20), 6849–6877 CrossRef CAS.
- K.-F. Lin, H.-M. Cheng, H.-C. Hsu, L.-J. Lin and W.-F. Hsieh, Band gap variation of size-controlled ZnO quantum dots synthesized by sol–gel method, Chem. Phys. Lett., 2005, 409(4–6), 208–211 CrossRef CAS.
- G.-C. Yi, C. Wang and W. Il Park, ZnO nanorods: synthesis, characterization and applications, Semicond. Sci. Technol., 2005, 20(4), S22 CrossRef CAS.
- P. Yang, et al., Controlled growth of ZnO nanowires and their optical properties, Adv. Funct. Mater., 2002, 12(5), 323–331 CrossRef CAS.
- Y. J. Xing, et al., Optical properties of the ZnO nanotubes synthesized via vapor phase growth, Appl. Phys. Lett., 2003, 83(9), 1689–1691 CrossRef CAS.
- P. K. Samanta and S. Mishra, Wet chemical growth and optical property of ZnO nanodiscs, Optik, 2013, 124(17), 2871–2873 CrossRef CAS.
- W.-Z. Wang, et al., Aligned ultralong ZnO nanobelts and their enhanced field emission, Adv. Mater., 2006, 18(24), 3275–3278 CrossRef CAS.
- S. J. Chen, et al., Structural and optical properties of uniform ZnO nanosheets, Adv. Mater., 2005, 17(5), 586–590 CrossRef CAS.
- M. L. M. Napi, S. M. Sultan, R. Ismail, K. W. How and M. K. Ahmad, Electrochemical-based biosensors on different zinc oxide nanostructures: A review, Materials, 2019, 12(18), 2985 CrossRef CAS PubMed.
- B. N. Aini, S. Siddiquee, K. Ampon, K. F. Rodrigues and S. Suryani, Development of glucose biosensor based on ZnO nanoparticles film and glucose oxidase-immobilized eggshell membrane, Sens. Biosens. Res., 2015, 4, 46–56 Search PubMed.
- R. Ahmad, N. Tripathy, S. H. Kim, A. Umar, A. Al-Hajry and Y.-B. Hahn, High performance cholesterol sensor based on ZnO nanotubes grown on Si/Ag electrodes, Electrochem. Commun., 2014, 38, 4–7 CrossRef CAS.
- Z. Dai, G. Shao, J. Hong, J. Bao and J. Shen, Immobilization and direct electrochemistry of glucose oxidase on a tetragonal pyramid-shaped porous ZnO nanostructure for a glucose biosensor, Biosens. Bioelectron., 2009, 24(5), 1286–1291 CrossRef CAS PubMed.
- S. A. Mozaffari, R. Rahmanian, M. Abedi and H. S. Amoli, Urea impedimetric biosensor based on reactive RF magnetron sputtered zinc oxide nanoporous transducer, Electrochim. Acta, 2014, 146, 538–547 CrossRef CAS.
- B. Prieto-Simon, M. Campas and J.-L. Marty, Biomolecule immobilization in biosensor development: tailored strategies based on affinity interactions, Protein Pept. Lett., 2008, 15(8), 757–763 CrossRef CAS PubMed.
- N. R. Shanmugam, S. Muthukumar and S. Prasad, A review on ZnO-based electrical biosensors for cardiac biomarker detection, Future Sci. OA, 2017, 3(4), FSO196 CrossRef CAS PubMed.
- N. Abid, et al., Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review, Adv. Colloid Interface Sci., 2021, 102597 Search PubMed.
- M. Kawakami, A. B. Hartanto, Y. Nakata and T. Okada, Synthesis of ZnO nanorods by nanoparticle assisted pulsed-laser deposition, Jpn. J. Appl. Phys., 2003, 42(1A), L33 CrossRef CAS.
- J. Bae, J.-I. Hong, W. H. Han, Y. J. Choi and R. L. Snyder, Superior field emission properties of ZnO nanocones synthesized by pulsed laser deposition, Chem. Phys. Lett., 2009, 475(4–6), 260–263 CrossRef CAS.
- Y. Liu, et al., Synthesis and H2 sensing properties of aligned ZnO nanotubes, Appl. Surf. Sci., 2011, 257(6), 2264–2268 CrossRef CAS.
- W.-T. Chiou, W.-Y. Wu and J.-M. Ting, Growth of single crystal ZnO nanowires using sputter deposition, Diamond Relat. Mater., 2003, 12(10–11), 1841–1844 CrossRef CAS.
- P. S. Venkatesh, S. Balakumar and K. Jeganathan, Post-annealing effects on the structural and optical properties of vertically aligned undoped ZnO nanorods grown by radio frequency magnetron sputtering, RSC Adv., 2014, 4(10), 5030–5035 RSC.
- J.-J. Wu and S.-C. Liu, Low-temperature growth of well-aligned ZnO nanorods by chemical vapor deposition, Adv. Mater., 2002, 14(3), 215–218 CrossRef CAS.
- S. Y. Bae, H. W. Seo and J. Park, Vertically aligned sulfur-doped ZnO nanowires synthesized via chemical vapor deposition, J. Phys. Chem. B, 2004, 108(17), 5206–5210 CrossRef CAS.
- S.-W. Kim, S. Fujita and S. Fujita, ZnO nanowires with high aspect ratios grown by metalorganic chemical vapor deposition using gold nanoparticles, Appl. Phys. Lett., 2005, 86(15), 153119 CrossRef.
- C. C. Wu, D. S. Wuu, P. R. Lin, T. N. Chen and R. H. Horng, Three-step growth of well-aligned ZnO nanotube arrays by self-catalyzed metalorganic chemical vapor deposition method, Cryst. Growth Des., 2009, 9(10), 4555–4561 CrossRef CAS.
- X. Liu, X. Wu, H. Cao and R. P. H. Chang, Growth mechanism and properties of ZnO nanorods synthesized by plasma-enhanced chemical vapor deposition, J. Appl. Phys., 2004, 95(6), 3141–3147 CrossRef CAS.
- J. R. Creighton and P. Ho, Introduction to chemical vapor deposition (CVD), Chem. Vap. Deposition, 2001, 2, 1–22 CAS.
- M. N. Kamalasanan and S. Chandra, Sol–gel synthesis of ZnO thin films, Thin Solid Films, 1996, 288(1–2), 112–115 CrossRef CAS.
- S. E. Ahn, et al., Photoresponse of sol–gel-synthesized ZnO nanorods, Appl. Phys. Lett., 2004, 84(24), 5022–5024 CrossRef CAS.
- G. S. Wu, et al., Controlled synthesis of ZnO nanowires or nanotubes via sol–gel template process, Solid State Commun., 2005, 134(7), 485–489 CrossRef CAS.
- M. Guglielmi and G. Carturan, Precursors for sol–gel preparations, J. Non Cryst. Solids, 1988, 100(1–3), 16–30 CrossRef CAS.
- L. Znaidi, Sol–gel-deposited ZnO thin films: A review, Mater. Sci. Eng., B, 2010, 174(1–3), 18–30 CrossRef CAS.
- M. T. Htay, Y. Hashimoto, N. Momose and K. Ito, Position-selective growth of ZnO nanowires by ultrasonic spray pyrolysis, J. Cryst. Growth, 2009, 311(20), 4499–4504 CrossRef.
- E. Kärber, et al., Photoluminescence of spray pyrolysis deposited ZnO nanorods, Nanoscale Res. Lett., 2011, 6(1), 1–7 CrossRef PubMed.
- L. C. Tien, D. P. Norton, S. J. Pearton, H.-T. Wang and F. Ren, Nucleation control for ZnO nanorods grown by catalyst-driven molecular beam epitaxy, Appl. Surf. Sci., 2007, 253(10), 4620–4625 CrossRef CAS.
- Y. W. Heo, et al., Depletion-mode ZnO nanowire field-effect transistor, Appl. Phys. Lett., 2004, 85(12), 2274–2276 CrossRef CAS.
- Y. Jian-Feng, et al., Growth and properties of ZnO nanotubes grown on Si (1 1 1) substrate by plasma-assisted molecular beam epitaxy, J. Cryst. Growth, 2005, 280(1–2), 206–211 CrossRef.
- Y. Hames, Z. Alpaslan, A. Kösemen, S. E. San and Y. Yerli, Electrochemically grown ZnO nanorods for hybrid solar cell applications, Solar Energy, 2010, 84(3), 426–431 CrossRef CAS.
- J. Elias, R. Tena-Zaera and C. Lévy-Clément, Electrochemical deposition of ZnO nanowire arrays with tailored dimensions, J. Electroanal. Chem., 2008, 621(2), 171–177 CrossRef CAS.
- Y. Tang, et al., Electrodeposition of ZnO nanotube arrays on TCO glass substrates, Electrochem. Commun., 2007, 9(2), 289–292 CrossRef CAS.
- A. Umar, B. Karunagaran, E. K. Suh and Y. B. Hahn, Structural and optical properties of single-crystalline ZnO nanorods grown on silicon by thermal evaporation, Nanotechnology, 2006, 17(16), 4072 CrossRef CAS PubMed.
- S. Y. Bae, C. W. Na, J. H. Kang and J. Park, Comparative structure and optical properties of Ga-, In-, and Sn-doped ZnO nanowires synthesized via thermal evaporation, J. Phys. Chem. B, 2005, 109(7), 2526–2531 CrossRef CAS PubMed.
- X. Zhang, et al., Peculiar ZnO nanopushpins and nanotubes synthesized via simple thermal evaporation, Appl. Phys. Lett., 2005, 87(12), 123111 CrossRef.
- J. Wang and L. Gao, Hydrothermal synthesis and photoluminescence properties of ZnO nanowires, Solid State Commun., 2004, 132(3–4), 269–271 CrossRef CAS.
- K. H. Tam, et al., Defects in ZnO nanorods prepared by a hydrothermal method, J. Phys. Chem. B, 2006, 110(42), 20865–20871 CrossRef CAS PubMed.
- A. Wei, X. W. Sun, C. X. Xu, Z. L. Dong, M. B. Yu and W. Huang, Stable field emission from hydrothermally grown ZnO nanotubes, Appl. Phys. Lett., 2006, 88(21), 213102 CrossRef.
- A. B. Djurisic, X. Y. Chen and Y. H. Leung, Recent progress in hydrothermal synthesis of zinc oxide nanomaterials, Recent Pat. Nanotechnol., 2012, 6(2), 124–134 CrossRef CAS PubMed.
- M. Kawakami, A. B. Hartanto, Y. Nakata and T. Okada, Synthesis of ZnO nanorods by nanoparticle assisted pulsed-laser deposition, Jpn. J. Appl. Phys., 2003, 42(1A), L33 CrossRef CAS.
- J. Bae, J.-I. Hong, W. H. Han, Y. J. Choi and R. L. Snyder, Superior field emission properties of ZnO nanocones synthesized by pulsed laser deposition, Chem. Phys. Lett., 2009, 475(4–6), 260–263 CrossRef CAS.
- C. Tusche, H. L. Meyerheim and J. Kirschner, Observation of depolarized ZnO (0001) monolayers: formation of unreconstructed planar sheets, Phys. Rev. Lett., 2007, 99(2), 26102 CrossRef CAS PubMed.
- Y. Liu, et al., Synthesis and H2 sensing properties of aligned ZnO nanotubes, Appl. Surf. Sci., 2011, 257(6), 2264–2268 CrossRef CAS.
- W.-T. Chiou, W.-Y. Wu and J.-M. Ting, Growth of single crystal ZnO nanowires using sputter deposition, Diamond Relat. Mater., 2003, 12(10–11), 1841–1844 CrossRef CAS.
- M. T. Htay, Y. Hashimoto, N. Momose and K. Ito, Position-selective growth of ZnO nanowires by ultrasonic spray pyrolysis, J. Cryst. Growth, 2009, 311(20), 4499–4504 CrossRef.
- J. Elias, R. Tena-Zaera and C. Lévy-Clément, Electrochemical deposition of ZnO nanowire arrays with tailored dimensions, J. Electroanal. Chem., 2008, 621(2), 171–177 CrossRef CAS.
- S. J. Chen, et al., Structural and optical properties of uniform ZnO nanosheets, Adv. Mater., 2005, 17(5), 586–590 CrossRef CAS.
- T. Sahoo, S. K. Nayak, P. Chelliah, M. K. Rath and B. Parida, Observations of two-dimensional monolayer zinc oxide, Mater. Res. Bull., 2016, 75, 134–138 CrossRef CAS.
- R. Yakimova, L. Selegard, V. Khranovskyy, R. Pearce, A. L. Spetz and K. Uvdal, ZnO materials and surface tailoring for biosensing, Front. Biosci.-Elite, 2012, 4(1), 254–278 CrossRef PubMed.
- B. Ortiz-Casas, et al., Bio-acceptable 0D and 1D ZnO nanostructures for cancer diagnostics and treatment, Mater. Today, 2021, 50, 533–569 CrossRef CAS.
- N. Bhalla, et al., A facile approach to fabricate and embed multifunctional nano ZnO into soap matrix and liquid cleansing products for enhanced antibacterial and photostability for health and hygiene applications, Arabian J. Chem., 2022, 15(6), 103862 CrossRef CAS.
- H. Hong, et al., Cancer-targeted optical imaging with fluorescent zinc oxide nanowires, Nano Lett., 2011, 11(9), 3744–3750 CrossRef CAS PubMed.
- Z. Li, R. Yang, M. Yu, F. Bai, C. Li and Z. L. Wang, Cellular level biocompatibility and biosafety of ZnO nanowires, J. Phys. Chem. C, 2008, 112(51), 20114–20117 CrossRef CAS.
- S. K. Arya, S. Saha, J. E. Ramirez-Vick, V. Gupta, S. Bhansali and S. P. Singh, Recent advances in ZnO nanostructures and thin films for biosensor applications, Anal. Chim. Acta, 2012, 737, 1–21 CrossRef CAS.
- Y. Zhang, Z. Kang, X. Yan and Q. Liao, ZnO nanostructures in enzyme biosensors, Sci. China Mater., 2015, 58(1), 60–76 CrossRef CAS.
- A. Tereshchenko, et al., Optical biosensors based on ZnO nanostructures: advantages and perspectives. A review, Sens. Actuators, B, 2016, 229, 664–677 CrossRef CAS.
- B. Prieto-Simon, M. Campas and J.-L. Marty, Biomolecule immobilization in biosensor development: tailored strategies based on affinity interactions, Protein Pept. Lett., 2008, 15(8), 757–763 CrossRef CAS PubMed.
- N. P. Sasidharan, P. Chandran and S. S. Khan, Interaction of colloidal zinc oxide nanoparticles with bovine serum albumin and its adsorption isotherms and kinetics, Colloids Surf., B, 2013, 102, 195–201 CrossRef CAS PubMed.
- E. da Silva, Y. Kembouche, U. Tegner, A. Baun and K. A. Jensen, Interaction of biologically relevant proteins with ZnO nanomaterials: A confounding factor for in vitro toxicity endpoints, Toxicol. In Vitro, 2019, 56, 41–51 CrossRef CAS PubMed.
- T. Xie, et al., Low-index ZnO crystal plane-specific binding behavior of whole Immunoglobulin G proteins, Langmuir, 2015, 31(38), 10493–10499 CrossRef CAS PubMed.
- R. Ahmad, N. Tripathy, N. K. Jang, G. Khang and Y.-B. Hahn, Fabrication of highly sensitive uric acid biosensor based on directly grown ZnO nanosheets on electrode surface, Sens. Actuators, B, 2015, 206, 146–151 CrossRef CAS.
- M. Klaumünzer, U. Weichsel, M. Mačković, E. Spiecker, W. Peukert and C. Kryschi, Transmission electron microscopy and time resolved optical spectroscopy study of the electronic and structural interactions of ZnO nanorods with bovine serum albumin, J. Phys. Chem. B, 2013, 117(33), 9683–9689 CrossRef PubMed.
- A. Bhogale, et al., Systematic investigation on the interaction of bovine serum albumin with ZnO nanoparticles using fluorescence spectroscopy, Colloids Surf., B, 2013, 102, 257–264 CrossRef CAS PubMed.
- Y. Wang, et al., Research of protein adsorption on the different surface topography of the zinc oxide, Surf. Interface Anal., 2015, 47(2), 245–252 CrossRef CAS.
- P. Sanguino, T. Monteiro, S. R. Bhattacharyya, C. J. Dias, R. Igreja and R. Franco, ZnO nanorods as immobilization layers for interdigitated capacitive immunosensors, Sens. Actuators, B, 2014, 204, 211–217 CrossRef CAS.
- C.-H. Sang, S.-J. Chou, F.-M. Pan and J.-T. Sheu, Fluorescence enhancement and multiple protein detection in ZnO nanostructure microfluidic devices, Biosens. Bioelectron., 2016, 75, 285–292 CrossRef CAS PubMed.
- R. D. Munje, M. Jacobs, S. Muthukumar, B. Quadri, N. R. Shanmugam and S. Prasad, A novel approach for electrical tuning of nano-textured zinc oxide surfaces for ultra-sensitive troponin-T detection, Anal. Methods, 2015, 7(24), 10136–10144 RSC.
- C. M. Tan, et al., Interdigitated Electrodes integrated with zinc oxide nanoparticles for Cardiac Troponin I biomarker detection, in 2016 IEEE International Conference on Semiconductor Electronics (ICSE), 2016, pp. 220–223.
- M. F. M. Fathil, et al., Substrate-gate coupling in ZnO-FET biosensor for cardiac troponin I detection, Sens. Actuators, B, 2017, 242, 1142–1154 CrossRef CAS.
- M. Jacobs, S. Muthukumar, A. P. Selvam, J. E. Craven and S. Prasad, Ultra-sensitive electrical immunoassay biosensors using nanotextured zinc oxide thin films on printed circuit board platforms, Biosens. Bioelectron., 2014, 55, 7–13 CrossRef CAS PubMed.
- B. Zhang, T. Kong, W. Xu, R. Su, Y. Gao and G. Cheng, Surface functionalization of zinc oxide by carboxyalkylphosphonic acid self-assembled monolayers, Langmuir, 2010, 26(6), 4514–4522 CrossRef CAS PubMed.
- U. Dembereldorj, E.-O. Ganbold, J.-H. Seo, S. Y. Lee, S. I. Yang and S.-W. Joo, Conformational changes of proteins adsorbed onto ZnO nanoparticle surfaces investigated by concentration-dependent infrared spectroscopy, Vib. Spectrosc., 2012, 59, 23–28 CrossRef CAS.
- H. Liu, J. Ge, E. Ma and L. Yang, Advanced biomaterials for biosensor and theranostics, Biomaterials in translational medicine, Elsevier, 2019, pp. 213–255 Search PubMed.
- S. K. Arya, S. Saha, J. E. Ramirez-Vick, V. Gupta, S. Bhansali and S. P. Singh, Recent advances in ZnO nanostructures and thin films for biosensor applications, Anal. Chim. Acta, 2012, 737, 1–21 CrossRef CAS PubMed.
- M. L. M. Napi, S. M. Sultan, R. Ismail, K. W. How and M. K. Ahmad, Electrochemical-based biosensors on different zinc oxide nanostructures: A review, Materials, 2019, 12(18), 2985 CrossRef CAS PubMed.
- A. Tereshchenko, et al., Optical biosensors based on ZnO nanostructures: advantages and perspectives. A review, Sens. Actuators, B, 2016, 229, 664–677 CrossRef CAS.
- P. Skládal, Piezoelectric biosensors, TrAC, Trends Anal. Chem., 2016, 79, 127–133 CrossRef.
- R. Monosik, M. Stredansky and E. Sturdik, Biosensors—classification, characterization and new trends, Acta Chim. Slovaca, 2012, 5(1), 109–120 Search PubMed.
- B. Rezaei and N. Irannejad, Electrochemical detection techniques in biosensor applications, Electrochemical Biosensors, Elsevier, 2019, pp. 11–43 Search PubMed.
- V. Velusamy, K. Arshak, O. Korostynska, K. Oliwa and C. Adley, An overview of foodborne pathogen detection: In the perspective of biosensors, Biotechnol. Adv., 2010, 28(2), 232–254 CrossRef CAS.
- S.-J. Choi and I.-D. Kim, Recent developments in 2D nanomaterials for chemiresistive-type gas sensors, Electron. Mater. Lett., 2018, 14(3), 221–260 CrossRef CAS.
- F. Alam, A. H. Jalal, S. Forouzanfar, M. Karabiyik, A. R. Baboukani and N. Pala, Flexible and linker-free enzymatic sensors based on zinc oxide nanoflakes for noninvasive L-lactate sensing in sweat, IEEE Sens. J., 2020, 20(10), 5102–5109 CAS.
- T. Yang, M. Chen, Q. Kong, X. Luo and K. Jiao, Toward DNA electrochemical sensing by free-standing ZnO nanosheets grown on 2D thin-layered MoS2, Biosens. Bioelectron., 2017, 89, 538–544 CrossRef CAS PubMed.
- D. Zhu, et al., Hierarchical flower-like zinc oxide nanosheets in-situ growth on three-dimensional ferrocene-functionalized graphene framework for sensitive determination of epinephrine and its oxidation derivative, Appl. Surf. Sci., 2020, 526, 146721 CrossRef CAS.
- M. Eryiğit, B. K. Urhan, H. Ö. Doğan, T. Ö. Özer and Ü. Demir, ZnO Nanosheets-Decorated ERGO Layers: An Efficient Electrochemical Sensor for Non-Enzymatic Uric Acid Detection, IEEE Sens. J., 2022, 22(6), 5555–5561 Search PubMed.
- A. Fulati, et al., An intracellular glucose biosensor based on nanoflake ZnO, Sens. Actuators, B, 2010, 150(2), 673–680 CrossRef CAS.
- S. M. U. Ali, Z. H. Ibupoto, M. Kashif, U. Hashim and M. Willander, A potentiometric indirect uric acid sensor based on ZnO nanoflakes and immobilized uricase, Sensors, 2012, 12(3), 2787–2797 CrossRef PubMed.
- N. Chauhan, S. Gupta, D. K. Avasthi, R. Adelung, Y. K. Mishra and U. Jain, Zinc oxide tetrapods based biohybrid interface for voltammetric sensing of Helicobacter pylori, ACS Appl. Mater. Interfaces, 2018, 10(36), 30631–30639 CrossRef CAS.
- U. Chakraborty, et al., A flower-like ZnO–Ag2O nanocomposite for label and mediator free direct sensing of dinitrotoluene, RSC Adv., 2020, 10(46), 27764–27774 RSC.
- V. N. Psychoyios, et al., Potentiometric cholesterol biosensor based on ZnO nanowalls and stabilized polymerized lipid film, Electroanalysis, 2013, 25(2), 367–372 CrossRef CAS.
- N. Akhtar, S. K. Metkar, A. Girigoswami and K. Girigoswami, ZnO nanoflower based sensitive nano-biosensor for amyloid detection, Mater. Sci. Eng., C, 2017, 78, 960–968 CrossRef CAS PubMed.
- S. Saha and V. Gupta, Influence of surface defects in ZnO thin films on its biosensing response characteristic, J. Appl. Phys., 2011, 110(6), 64904 CrossRef.
- F. Zhou, et al., Electrodeposition of gold nanoparticles on ZnO nanorods for improved performance of enzymatic glucose sensors, Mater. Sci. Semicond. Process., 2020, 105, 104708 CrossRef CAS.
- N. S. Ridhuan, N. Mohamad Nor, K. Abdul Razak, Z. Lockman and N. D. Zakaria, ITO electrode modified with Pt nanodendrites-decorated ZnO nanorods for enzymatic glucose sensor, J. Solid State Electrochem., 2021, 25(3), 1065–1072 CrossRef CAS.
- V. Fedorenko, et al., Application of polydopamine functionalized zinc oxide for glucose biosensor design, Polymers, 2021, 13(17), 2918 CrossRef CAS PubMed.
- M. Shukla, T. Dixit, R. Prakash, I. A. Palani and V. Singh, et al., Influence of aspect ratio and surface defect density on hydrothermally grown ZnO nanorods towards amperometric glucose biosensing applications,”, Appl. Surf. Sci., 2017, 422, 798–808 CrossRef CAS.
- D. Lee, et al., Enhanced mass sensitivity of ZnO nanorod-grown quartz crystal microbalances, Sens. Actuators, B, 2009, 135(2), 444–448 CrossRef CAS.
- C.-H. Sang, S.-J. Chou, F.-M. Pan and J.-T. Sheu, Fluorescence enhancement and multiple protein detection in ZnO nanostructure microfluidic devices, Biosens. Bioelectron., 2016, 75, 285–292 CrossRef CAS PubMed.
- D. Pradhan, F. Niroui and K. T. Leung, High-performance, flexible enzymatic glucose biosensor based on ZnO nanowires supported on a gold-coated polyester substrate, ACS Appl. Mater. Interfaces, 2010, 2(8), 2409–2412 CrossRef CAS PubMed.
- F. Zhang, et al., Immobilization of uricase on ZnO nanorods for a reagentless uric acid biosensor, Anal. Chim. Acta, 2004, 519(2), 155–160 CrossRef CAS.
- T. Kong, Y. Chen, Y. Ye, K. Zhang, Z. Wang and X. Wang, An amperometric glucose biosensor based on the immobilization of glucose oxidase on the ZnO nanotubes, Sens. Actuators, B, 2009, 138(1), 344–350 CrossRef CAS.
- K. Brince Paul, S. Kumar, S. Tripathy, S. R. K. Vanjari, V. Singh and S. G. Singh, A highly sensitive self assembled monolayer modified copper doped zinc oxide nanofiber interface for detection of Plasmodium falciparum histidine-rich protein-2: Targeted towards rapid, early diagnosis of malaria,”, Biosens. Bioelectron., 2016, 80, 39–46, DOI:10.1016/j.bios.2016.01.036.
- F. Zhou, W. Jing, P. Liu, D. Han, Z. Jiang and Z. Wei, Doping Ag in ZnO nanorods to improve the performance of related enzymatic glucose sensors, Sensors, 2017, 17(10), 2214, DOI:10.3390/s17102214.
- M. L. M. Napi, S. M. Sultan, R. Ismail, K. W. How and M. K. Ahmad, Electrochemical-based biosensors on different zinc oxide nanostructures: A review, Materials, 2019, 12(18), 2985 CrossRef CAS PubMed.
- J. Y. Kim, S. Y. Jo, G. J. Sun, A. Katoch, S. W. Choi and S. S. Kim, Tailoring the surface area of ZnO nanorods for improved performance in glucose sensors, Sens. Actuators, B, 2014, 192, 216–220, DOI:10.1016/j.snb.2013.10.113.
- S. Xu and Z. L. Wang, One-dimensional ZnO nanostructures: solution growth and functional properties, Nano Res., 2011, 4(11), 1013–1098 CrossRef CAS.
- W.-J. Wu, Q. Zhao, R. Zhou, Y.-C. Liang, W.-B. Zhao and C.-X. Shan, Ratiometric fluorescence sensor based on europium-grafted ZnO quantum dots for visual and colorimetric detection of tetracycline, Spectrochim. Acta, Part A, 2021, 259, 119901 CrossRef CAS PubMed.
- M. Ali, I. Shah, S. W. Kim, M. Sajid, J. H. Lim and K. H. Choi, Quantitative detection of uric acid through ZnO quantum dots based highly sensitive electrochemical biosensor,”, Sens. Actuators, A, 2018, 283, 282–290 CrossRef CAS.
- X. Ren, et al., Zinc oxide nanoparticles/glucose oxidase photoelectrochemical system for the fabrication of biosensor, J. Colloid Interface Sci., 2009, 334(2), 183–187 CrossRef CAS PubMed.
- D. Zhao, H. Song, L. Hao, X. Liu, L. Zhang and Y. Lv, Luminescent ZnO quantum dots for sensitive and selective detection of dopamine, Talanta, 2013, 107, 133–139 CrossRef CAS.
- A. Hayat, W. Haider, Y. Raza and J. L. Marty, Colorimetric cholesterol sensor based on peroxidase like activity of zinc oxide nanoparticles incorporated carbon nanotubes, Talanta, 2015, 143, 157–161 CrossRef CAS PubMed.
- V. Fidal, S. Inguva, S. Krishnamurthy, E. Marsili, J.-P. Mosnier and T. S. Chandra, Mediator-free interaction of glucose oxidase, as model enzyme for immobilization, with Al-doped and undoped ZnO thin films laser-deposited on polycarbonate supports, Enzyme Microb. Technol., 2017, 96, 67–74 CrossRef PubMed.
- Y.-T. Wang, L. Yu, Z.-Q. Zhu, J. Zhang, J.-Z. Zhu and C. Fan, Improved enzyme immobilization for enhanced bioelectrocatalytic activity of glucose sensor, Sens. Actuators, B, 2009, 136(2), 332–337 CrossRef CAS.
- B. N. Aini, S. Siddiquee, K. Ampon, K. F. Rodrigues and S. Suryani, Development of glucose biosensor based on ZnO nanoparticles film and glucose oxidase-immobilized eggshell membrane, Sens. Biosens. Res., 2015, 4, 46–56 Search PubMed.
- T. Dayakar, K. V. Rao, K. Bikshalu, V. Rajendar and S.-H. Park, Novel synthesis and structural analysis of zinc oxide nanoparticles for the non enzymatic glucose biosensor, Mater. Sci. Eng., C, 2017, 75, 1472–1479 CrossRef CAS PubMed.
- A. Mahmoud, M. Echabaane, K. Omri, L. el Mir and R. ben Chaabane, Development of an impedimetric non enzymatic sensor based on ZnO and Cu doped ZnO nanoparticles for the detection of glucose, J. Alloys Compd., 2019, 786, 960–968 CrossRef CAS.
- H. Mirzaei and M. Darroudi, Zinc oxide nanoparticles: Biological synthesis and biomedical applications, Ceram. Int., 2017, 43(1), 907–914 CrossRef CAS.
- M. L. M. Napi, S. M. Sultan, R. Ismail, K. W. How and M. K. Ahmad, Electrochemical-based biosensors on different zinc oxide nanostructures: A review, Materials, 2019, 12(18), 2985 CrossRef CAS PubMed.
- S. Zhang, G. Wright and Y. Yang, Materials and techniques for electrochemical biosensor design and construction, Biosens. Bioelectron., 2000, 15(5–6), 273–282 CrossRef CAS PubMed.
- S. Verma, et al., ZnO-rGO nanocomposite based bioelectrode for sensitive and ultrafast detection of dopamine in human serum, Biosens. Bioelectron., 2020, 165, 112347 CrossRef CAS PubMed.
- K. B. Babitha, P. S. Soorya, A. P. Mohamed, R. B. Rakhi and S. Ananthakumar, Development of ZnO@ rGO nanocomposites for the enzyme free electrochemical detection of urea and glucose, Mater. Adv., 2020, 1(6), 1939–1951 RSC.
- J. Yoon, D. Lee, E. Lee, Y. S. Yoon and D.-J. Kim, Ag/ZnO catalysts with different ZnO nanostructures for non-enzymatic detection of urea, Electroanalysis, 2019, 31(1), 17–21 CrossRef CAS.
- S. Baruah, B. Maibam, C. K. Borah, T. Agarkar, A. Kumar and S. Kumar, A highly receptive ZnO-based enzymatic electrochemical sensor for glucose sensing, IEEE Sens. J., 2021, 21(13), 14601–14608 CAS.
- M. L. M. Napi, S. M. Sultan, R. Ismail, K. W. How and M. K. Ahmad, Electrochemical-based biosensors on different zinc oxide nanostructures: A review, Materials, 2019, 12(18), 2985 CrossRef CAS PubMed.
- M. Q. Israr, J. R. Sadaf, M. H. Asif, O. Nur, M. Willander and B. Danielsson, Potentiometric cholesterol biosensor based on ZnO nanorods chemically grown on Ag wire, Thin Solid Films, 2010, 519(3), 1106–1109 CrossRef CAS.
- P. Supraja, V. Singh, S. R. K. Vanjari and S. Govind, Singh, “Electrospun CNT embedded ZnO nanofiber based biosensor for electrochemical detection of Atrazine: a step closure to single molecule detection, Microsyst. Nanoeng., 2020, 6(1), 1–10 CrossRef PubMed.
- S. M. U. Ali, N. H. Alvi, Z. Ibupoto, O. Nur, M. Willander and B. Danielsson, Selective potentiometric determination of uric acid with uricase immobilized on ZnO nanowires, Sens. Actuators, B, 2011, 152(2), 241–247 CrossRef.
- M. Q. Israr, J. R. Sadaf, O. Nur, M. Willander, S. Salman and B. Danielsson, Chemically fashioned ZnO nanowalls and their potential application for potentiometric cholesterol biosensor, Appl. Phys. Lett., 2011, 98(25), 253705 CrossRef.
- Z. H. Ibupoto, N. Jamal, K. Khun and M. Willander, Development of a disposable potentiometric antibody immobilized ZnO nanotubes based sensor for the detection of C-reactive protein, Sens. Actuators, B, 2012, 166, 809–814 CrossRef.
- Z. H. Ibupoto, S. M. U. A. Shah, K. Khun and M. Willander, Electrochemical L-lactic acid sensor based on immobilized ZnO nanorods with lactate oxidase, Sensors, 2012, 12(3), 2456–2466 CrossRef CAS PubMed.
- A. Fulati, et al., An intracellular glucose biosensor based on nanoflake ZnO, Sens. Actuators, B, 2010, 150(2), 673–680 CrossRef CAS.
- Z. Rafiee, A. Mosahebfard and M. H. Sheikhi, High-performance ZnO nanowires-based glucose biosensor modified by graphene nanoplates, Mater. Sci. Semicond. Process., 2020, 115, 105116 CrossRef CAS.
- Z. H. Ibupoto, S. M. U. Ali, K. Khun, C. O. Chey, O. Nur and M. Willander, ZnO nanorods based enzymatic biosensor for selective determination of penicillin, Biosensors, 2011, 1(4), 153–163 CrossRef CAS PubMed.
- C. O. Chey, Z. H. Ibupoto, K. Khun, O. Nur and M. Willander, Indirect determination of mercury ion by inhibition of a glucose biosensor based on ZnO nanorods, Sensors, 2012, 12(11), 15063–15077 CrossRef CAS PubMed.
- S. M. U. Ali, M. Kashif, Z. H. Ibupoto, M. Fakhar-e-Alam, U. Hashim and M. Willander, Functionalised zinc oxide nanotube arrays as electrochemical sensors for the selective determination of glucose, Micro Nano Lett., 2011, 6(8), 609–613 CrossRef CAS.
- K. Khun, Z. H. Ibupoto, O. Nur and M. Willander, Development of galactose biosensor based on functionalized ZnO nanorods with galactose oxidase, J. Sens., 2012, 2012, 696247 Search PubMed.
- J. Xia, J. Qing and J. Liu, A sensitive electrochemical impedance DNA biosensor based on ZnO nanorod electrodes for BCR/ABL fusion gene detection, Int. J. Electrochem. Sci, 2019, 14, 4271–4279 CrossRef CAS.
- S. P. Singh, et al., Cholesterol biosensor based on rf sputtered zinc oxide nanoporous thin film, Appl. Phys. Lett., 2007, 91(6), 63901 CrossRef.
- M. Tak, V. Gupta and M. Tomar, Flower-like ZnO nanostructure based electrochemical DNA biosensor for bacterial meningitis detection, Biosens. Bioelectron., 2014, 59, 200–207 CrossRef CAS PubMed.
- X. Liu, Q. Hu, Q. Wu, W. Zhang, Z. Fang and Q. Xie, Aligned ZnO nanorods: a useful film to fabricate amperometric glucose biosensor, Colloids Surf., B, 2009, 74(1), 154–158 CrossRef CAS PubMed.
- Z. W. Zhao, X. J. Chen, B. K. Tay, J. S. Chen, Z. J. Han and K. A. Khor, A novel amperometric biosensor based on ZnO: Co nanoclusters for biosensing glucose, Biosens. Bioelectron., 2007, 23(1), 135–139 CrossRef CAS PubMed.
- J. Wang, S. Li and Y. Zhang, A sensitive DNA biosensor fabricated from gold nanoparticles, carbon nanotubes, and zinc oxide nanowires on a glassy carbon electrode, Electrochim. Acta, 2010, 55(15), 4436–4440 CrossRef CAS.
- Z.-M. Liu, Y.-L. Liu, G.-L. Shen and R.-Q. Yu, Nano-ZnO/chitosan composite film modified electrode for voltammetric detection of DNA hybridization, Anal. Lett., 2008, 41(6), 1083–1095 CrossRef CAS.
- R. Khan, A. Kaushik, P. R. Solanki, A. A. Ansari, M. K. Pandey and B. D. Malhotra, Zinc oxide nanoparticles-chitosan composite film for cholesterol biosensor, Anal. Chim. Acta, 2008, 616(2), 207–213 CrossRef CAS PubMed.
- C. Xiang, Y. Zou, L.-X. Sun and F. Xu, Direct electrochemistry and enhanced electrocatalysis of horseradish peroxidase based on flowerlike ZnO–gold nanoparticle–Nafion nanocomposite, Sens. Actuators, B, 2009, 136(1), 158–162 CrossRef CAS.
- Z. Dai, G. Shao, J. Hong, J. Bao and J. Shen, Immobilization and direct electrochemistry of glucose oxidase on a tetragonal pyramid-shaped porous ZnO nanostructure for a glucose biosensor, Biosens. Bioelectron., 2009, 24(5), 1286–1291 CrossRef CAS PubMed.
- H. Fatemi, A. A. Khodadadi, A. A. Firooz and Y. Mortazavi, Apple–biomorphic synthesis of porous ZnO nanostructures for glucose direct electrochemical biosensor, Curr. Appl. Phys., 2012, 12(4), 1033–1038 CrossRef.
- K. Khun, et al., An electrochemical dopamine sensor based on the ZnO/CuO nanohybrid structures, J. Nanosci. Nanotechnol., 2014, 14(9), 6646–6652 CrossRef CAS PubMed.
- Y. Zhao, et al., ZnO-nanorods/graphene heterostructure: a direct electron transfer glucose biosensor, Sci. Rep., 2016, 6, 32327 CrossRef CAS PubMed.
- U. Chakraborty, et al., Microwave-assisted assembly of Ag2O-ZnO composite nanocones for electrochemical detection of 4-Nitrophenol and assessment of their photocatalytic activity towards degradation of 4-Nitrophenol and Methylene blue dye, J. Hazard. Mater., 2021, 416, 125771 CrossRef CAS PubMed.
- D. Sharma, M. I. Sabela, S. Kanchi, K. Bisetty, A. A. Skelton and B. Honarparvar, Green synthesis, characterization and electrochemical sensing of silymarin by ZnO nanoparticles: experimental and DFT studies, J. Electroanal. Chem., 2018, 808, 160–172 CrossRef CAS.
- N. Tripathy and D.-H. Kim, Metal oxide modified ZnO nanomaterials for biosensor applications, Nano Convergence, 2018, 5(1), 1–10 CrossRef PubMed.
- P. Damborský, J. Švitel and J. Katrlík, Optical biosensors, Essays Biochem., 2016, 60(1), 91–100 CrossRef PubMed.
- A. Tereshchenko, et al., Optical biosensors based on ZnO nanostructures: advantages and perspectives. A review, Sens. Actuators, B, 2016, 229, 664–677 CrossRef CAS.
- A. Tereshchenko, et al., Optical biosensors based on ZnO nanostructures: advantages and perspectives. A review, Sens. Actuators, B, 2016, 229, 664–677 CrossRef CAS.
- A. Galdamez, et al., DNA probe functionalization on different morphologies of ZnO/Au nanowire for bio-sensing applications, Mater. Lett., 2019, 235, 250–253 CrossRef CAS.
- A. Tamashevski, Y. Harmaza, E. Slobozhanina, R. Viter and I. Iatsunskyi, Photoluminescent detection of human T-lymphoblastic cells by ZnO nanorods, Molecules, 2020, 25(14), 3168 CrossRef CAS PubMed.
- V. Myndrul, E. Coy, M. Bechelany and I. Iatsunskyi, Photoluminescence label-free immunosensor for the detection of Aflatoxin B1 using polyacrylonitrile/zinc oxide nanofibers, Mater. Sci. Eng., C, 2021, 118, 111401 CrossRef CAS PubMed.
- D. Sodzel, et al., Continuous sensing of hydrogen peroxide and glucose via quenching of the UV and visible luminescence of ZnO nanoparticles, Microchim. Acta, 2015, 182(9–10), 1819–1826 CrossRef CAS.
- D. Sodzel, et al., Continuous sensing of hydrogen peroxide and glucose via quenching of the UV and visible luminescence of ZnO nanoparticles, Microchim. Acta, 2015, 182(9–10), 1819–1826 CrossRef CAS.
- S. N. Sarangi, S. Nozaki and S. N. Sahu, ZnO nanorod-based non-enzymatic optical glucose biosensor, J. Biomed. Nanotechnol., 2015, 11(6), 988–996 CrossRef CAS PubMed.
- R. Viter, et al., Application of room temperature photoluminescence from ZnO nanorods for salmonella detection, IEEE Sens. J., 2014, 14(6), 2028–2034 CAS.
- M. A. Iyer, et al., Scanning fluorescence-based ultrasensitive detection of dengue viral DNA on ZnO thin films, Sens. Actuators, B, 2014, 202, 1338–1348 CrossRef CAS.
- T.-Y. Liu, H.-C. Liao, C.-C. Lin, S.-H. Hu and S.-Y. Chen, Biofunctional ZnO nanorod arrays grown on flexible substrates, Langmuir, 2006, 22(13), 5804–5809 CrossRef CAS PubMed.
- C.-C. Chang, N.-F. Chiu, D. S. Lin, Y. Chu-Su, Y.-H. Liang and C.-W. Lin, High-sensitivity detection of carbohydrate antigen 15-3 using a gold/zinc oxide thin film surface plasmon resonance-based biosensor, Anal. Chem., 2010, 82(4), 1207–1212 CrossRef CAS PubMed.
- K.-E. Kim, T. G. Kim and Y.-M. Sung, Enzyme-conjugated ZnO nanocrystals for collisional quenching-based glucose sensing, CrystEngComm, 2012, 14(8), 2859–2865 RSC.
- G. Kaur, M. Tomar and V. Gupta, Nanostructured zinc oxide thin film for application to surface plasmon resonance based cholesterol biosensor, International Workshop on Thin Films for Electronics, Electro-Optics, Energy, and Sensors, 2015, vol. 9667, p. 966706 Search PubMed.
- L. Sun, et al., A white-emitting ZnO–Au nanocomposite and its SERS applications, Appl. Surf. Sci., 2012, 258(20), 7813–7819 CrossRef CAS.
- K. Sivashanmugan, J.-D. Liao, B. H. Liu, C.-K. Yao and S.-C. Luo, Ag nanoclusters on ZnO nanodome array as hybrid SERS-active substrate for trace detection of malachite green, Sens. Actuators, B, 2015, 207, 430–436 CrossRef CAS.
- N. K. Singh, B. Jain and S. Annapoorni, ZnO modified gold disc: A new route to efficient glucose sensing, Sens. Actuators, B, 2011, 156(1), 383–387 CrossRef CAS.
- Q. Tao, et al., “Controlled growth of ZnO nanorods on textured silicon wafer and the application for highly effective and recyclable SERS substrate by decorating Ag nanoparticles, Mater. Res. Bull., 2014, 54, 6–12 CrossRef CAS.
- S.-C. Yang, Y.-C. Shen, T.-C. Lu, T.-L. Yang and J.-J. Huang, Tumor detection strategy using ZnO light-emitting nanoprobes, Nanotechnology, 2012, 23(5), 55202 CrossRef PubMed.
- A. Dorfman, N. Kumar and J. Hahm, Nanoscale ZnO-enhanced fluorescence detection of protein interactions, Adv. Mater., 2006, 18(20), 2685–2690 CrossRef CAS.
- A. Dorfman, N. Kumar and J. Hahm, Highly sensitive biomolecular fluorescence detection using nanoscale ZnO platforms, Langmuir, 2006, 22(11), 4890–4895 CrossRef CAS PubMed.
- P. Tetyana, P. M. Shumbula and Z. Njengele-Tetyana, Biosensors: Design, Development and Applications, Nanopores, IntechOpen, 2021 Search PubMed.
- M. Wang, et al., Self-Powered Biosensor for Specifically Detecting Creatinine in Real Time Based on the Piezo-Enzymatic-Reaction Effect of Enzyme-Modified ZnO Nanowires, Biosensors, 2021, 11(9), 342 CrossRef CAS PubMed.
- P. I. Reyes, Z. Duan, Y. Lu, D. Khavulya and N. Boustany, ZnO nanostructure-modified QCM for dynamic monitoring of cell adhesion and proliferation, Biosens. Bioelectron., 2013, 41, 84–89 CrossRef CAS.
- D. Lee, et al., Enhanced mass sensitivity of ZnO nanorod-grown quartz crystal microbalances, Sens. Actuators, B, 2009, 135(2), 444–448 CrossRef CAS.
- X. Wang, H. Yu, D. Lu, J. Zhang and W. Deng, Label free detection of the breast cancer biomarker CA15. 3 using ZnO nanorods coated quartz crystal microbalance, Sens. Actuators, B, 2014, 195, 630–634 CrossRef CAS.
- S. Krishnamoorthy, A. A. Iliadis, T. Bei and G. P. Chrousos, An interleukin-6 ZnO/SiO2/Si surface acoustic wave biosensor, Biosens. Bioelectron., 2008, 24(2), 313–318 CrossRef CAS PubMed.
- J. Luo, M. Xie, P. Luo, B. Zhao, K. Du and P. Fan, A sensitive glucose biosensor without using glucose test strips based on ZnO/SiO2/Si surface acoustic wave device, Mater. Lett., 2014, 130, 14–16 CrossRef CAS.
- Y. Mao, M. Shen, B. Liu, L. Xing, S. Chen and X. Xue, Self-powered piezoelectric-biosensing textiles for the physiological monitoring and time-motion analysis of individual sports, Sensors, 2019, 19(15), 3310 CrossRef CAS PubMed.
- R. Ahmad, N. Tripathy and Y.-B. Hahn, High-performance cholesterol sensor based on the solution-gated field effect transistor fabricated with ZnO nanorods, Biosens. Bioelectron., 2013, 45, 281–286 CrossRef CAS PubMed.
- R. Ahmad, M.-S. Ahn and Y.-B. Hahn, ZnO nanorods array based field-effect transistor biosensor for phosphate detection, J. Colloid Interface Sci., 2017, 498, 292–297 CrossRef CAS PubMed.
- J. Liu, J. Goud, P. M. Raj, M. Iyer, Z. L. Wang and R. R. Tummala, Real-time protein detection using ZnO nanowire/thin film bio-sensor integrated with microfluidic system, in 2008 58th Electronic Components and Technology Conference, 2008, pp. 1317–1322.
- X. Liu, et al., Enzyme-coated single ZnO nanowire FET biosensor for detection of uric acid, Sens. Actuators, B, 2013, 176, 22–27 CrossRef CAS.
- X. Zong and R. Zhu, ZnO nanorod-based FET biosensor for continuous glucose monitoring, Sens. Actuators, B, 2018, 255, 2448–2453 CrossRef CAS.
- R. Ahmad and Y.-B. Hahn, et al., Nonenzymatic flexible field-effect transistor based glucose sensor fabricated using NiO quantum dots modified ZnO nanorods, J. Colloid Interface Sci., 2018, 512, 21–28 CrossRef.
- R. Ahmad, M.-S. Ahn and Y.-B. Hahn, Fabrication of a non-enzymatic glucose sensor field-effect transistor based on vertically-oriented ZnO nanorods modified with Fe2O3, Electrochem. Commun., 2017, 77, 107–111 CrossRef CAS.
- M. Fathollahzadeh, et al., Immobilization of glucose oxidase on ZnO nanorods decorated electrolyte-gated field effect transistor for glucose detection, J. Solid State Electrochem., 2018, 22(1), 61–67 CrossRef CAS.
- S. S. A. Karim, C.-F. Dee, B. Y. Majlis and M. A. Mohamed, Recent progress on fabrication of zinc oxide nanorod-based field effect transistor biosensors, Sains Malays., 2019, 48(6), 1301–1310 CrossRef CAS.
- A. Wei, et al., Enzymatic glucose biosensor based on ZnO nanorod array grown by hydrothermal decomposition, Appl. Phys. Lett., 2006, 89(12), 123902 CrossRef.
- A. Umar, M. M. Rahman, M. Vaseem and Y.-B. Hahn, Ultra-sensitive cholesterol biosensor based on low-temperature grown ZnO nanoparticles, Electrochem. Commun., 2009, 11(1), 118–121 CrossRef CAS.
- J. S. Kim, W. Il Park, C.-H. Lee and G.-C. Yi, ZnO nanorod biosensor for highly sensitive detection of specific protein binding, J. Korean Phys. Soc., 2006, 49, 1635–1639 CAS.
- C.-H. Sang, S.-J. Chou, F.-M. Pan and J.-T. Sheu, Fluorescence enhancement and multiple protein detection in ZnO nanostructure microfluidic devices, Biosens. Bioelectron., 2016, 75, 285–292 CrossRef CAS PubMed.
- J. Liu, J. Goud, P. M. Raj, M. Iyer, Z. L. Wang and R. R. Tummala, Real-time protein detection using ZnO nanowire/thin film bio-sensor integrated with microfluidic system, in 2008 58th Electronic Components and Technology Conference, 2008, pp. 1317–1322.
- Y. Al-Douri, K. Gherab, K. M. Batoo and E. H. Raslan, Detecting the DNA of dengue serotype 2 using aluminium nanoparticle doped zinc oxide nanostructure: synthesis, analysis and characterization, J. Mater. Res. Technol., 2020, 9(3), 5515–5523 CrossRef CAS.
- G. Kaur, A. Paliwal, M. Tomar and V. Gupta, Detection of Neisseria meningitidis using surface plasmon resonance based DNA biosensor, Biosens. Bioelectron., 2016, 78, 106–110 CrossRef CAS PubMed.
- V. Gerbreders, et al., ZnO nanostructure-based electrochemical biosensor for Trichinella DNA detection, Sens. Biosens. Res., 2019, 23, 100276 Search PubMed.
- G. Gasparotto, J. P. C. Costa, P. I. Costa, M. A. Zaghete and T. Mazon, Electrochemical immunosensor based on ZnO nanorods-Au nanoparticles nanohybrids for ovarian cancer antigen CA-125 detection, Mater. Sci. Eng., C, 2017, 76, 1240–1247 CrossRef CAS PubMed.
- Y.-H. Liang, C.-C. Chang, C.-C. Chen, Y. Chu-Su and C.-W. Lin, Development of an Au/ZnO thin film surface plasmon resonance-based biosensor immunoassay for the detection of carbohydrate antigen 15-3 in human saliva, Clin. Biochem., 2012, 45(18), 1689–1693 CrossRef CAS PubMed.
- D. Zhao, H. Song, L. Hao, X. Liu, L. Zhang and Y. Lv, Luminescent ZnO quantum dots for sensitive and selective detection of dopamine, Talanta, 2013, 107, 133–139 CrossRef CAS PubMed.
- Q. Rui, K. Komori, Y. Tian, H. Liu, Y. Luo and Y. Sakai, Electrochemical biosensor for the detection of H2O2 from living cancer cells based on ZnO nanosheets, Anal. Chim. Acta, 2010, 670(1–2), 57–62 CrossRef CAS PubMed.
- Y.-F. Li, Z.-M. Liu, Y.-L. Liu, Y.-H. Yang, G.-L. Shen and R.-Q. Yu, A mediator-free phenol biosensor based on immobilizing tyrosinase to ZnO nanoparticles, Anal. Biochem., 2006, 349(1), 33–40 CrossRef CAS PubMed.
- R. Viter, et al., Application of room temperature photoluminescence from ZnO nanorods for salmonella detection, IEEE Sens. J., 2014, 14(6), 2028–2034 CAS.
- J. Narang and C. S. Pundir, Construction of a triglyceride amperometric biosensor based on chitosan–ZnO nanocomposite film, Int. J. Biol. Macromol., 2011, 49(4), 707–715 CrossRef CAS PubMed.
- R. Devi, M. Thakur and C. S. Pundir, Construction and application of an amperometric xanthine biosensor based on zinc oxide nanoparticles–polypyrrole composite film, Biosens. Bioelectron., 2011, 26(8), 3420–3426 CrossRef CAS PubMed.
- L. Wang, et al., Water-soluble ZnO–Au nanocomposite-based probe for enhanced protein detection in a SPR biosensor system, J. Colloid Interface Sci., 2010, 351(2), 392–397 CrossRef CAS PubMed.
- G. Biasotto, J. P. C. Costa, P. I. Costa and M. A. Zaghete, ZnO nanorods-gold nanoparticle-based biosensor for detecting hepatitis C, Appl. Phys. A: Mater. Sci. Process., 2019, 125(12), 1–7 CrossRef.
- F. Khosravi-Nejad, M. Teimouri, S. Jafari Marandi and M. Shariati, The highly sensitive impedimetric biosensor in label free approach for hepatitis B virus DNA detection based on tellurium doped ZnO nanowires, Appl. Phys. A: Mater. Sci. Process., 2019, 125(9), 1–8 CrossRef CAS.
- T. Kong, Y. Chen, Y. Ye, K. Zhang, Z. Wang and X. Wang, An amperometric glucose biosensor based on the immobilization of glucose oxidase on the ZnO nanotubes, Sens. Actuators, B, 2009, 138(1), 344–350 CrossRef CAS.
- R. Ahmad, N. Tripathy, N. K. Jang, G. Khang and Y.-B. Hahn, Fabrication of highly sensitive uric acid biosensor based on directly grown ZnO nanosheets on electrode surface, Sens. Actuators, B, 2015, 206, 146–151 CrossRef CAS.
- M. Q. Israr, J. R. Sadaf, M. H. Asif, O. Nur, M. Willander and B. Danielsson, Potentiometric cholesterol biosensor based on ZnO nanorods chemically grown on Ag wire, Thin Solid Films, 2010, 519(3), 1106–1109 CrossRef CAS.
- B. Gu, C. Xu, C. Yang, S. Liu and M. Wang, ZnO quantum dot labeled immunosensor for carbohydrate antigen 19-9, Biosens. Bioelectron., 2011, 26(5), 2720–2723 CrossRef CAS PubMed.
- C. Singhal, C. S. Pundir and J. Narang, A genosensor for detection of consensus DNA sequence of Dengue virus using ZnO/Pt-Pd nanocomposites, Biosens. Bioelectron., 2017, 97, 75–82 CrossRef CAS PubMed.
- K. Khun, et al., An electrochemical dopamine sensor based on the ZnO/CuO nanohybrid structures, J. Nanosci. Nanotechnol., 2014, 14(9), 6646–6652 CrossRef CAS PubMed.
- Z. H. Ibupoto, S. M. U. Ali, K. Khun, C. O. Chey, O. Nur and M. Willander, ZnO nanorods based enzymatic biosensor for selective determination of penicillin, Biosensors, 2011, 1(4), 153–163 CrossRef CAS PubMed.
- C. Xiang, Y. Zou, L.-X. Sun and F. Xu, Direct electrochemistry and enhanced electrocatalysis of horseradish peroxidase based on flowerlike ZnO–gold nanoparticle–Nafion nanocomposite, Sens. Actuators, B, 2009, 136(1), 158–162 CrossRef CAS.
- M. Haque, H. Fouad, H.-K. Seo, O. Y. Alothman and Z. A. Ansari, Cu-doped ZnO nanoparticles as an electrochemical sensing electrode for cardiac biomarker myoglobin detection, IEEE Sens. J., 2020, 20(15), 8820–8832 CAS.
- E. I. Naik, H. S. B. Naik, M. S. Sarvajith and E. Pradeepa, Co-precipitation synthesis of cobalt doped ZnO nanoparticles: Characterization and their applications for biosensing and antibacterial studies, Inorg. Chem. Commun., 2021, 130, 108678 CrossRef CAS.
- U. D. Kamaci and M. Kamaci, Selective and sensitive zno quantum dots based fluorescent biosensor for detection of cysteine, J. Fluoresc., 2021, 31(2), 401–414 CrossRef CAS PubMed.
- A. R. Al-Dairy, B. Albiss and A. A. Jaradat, Computational Modeling of ZnO-NRs and Graphene Nanostructure as a Glucose Biosensor, Sens. Imaging, 2021, 22(1), 1–13 CrossRef.
- M. Rahimi-Mohseni, J. B. Raoof, T. A. Aghajanzadeh and R. Ojani, Phenylketonuria monitoring in human blood serum by mosses extract/ZnO@ Au nanoarrays-loaded filter paper as a novel electrochemical biosensor, Microchem. J., 2021, 160, 105739 CrossRef CAS.
- E. M. Al-Khalqi, M. A. Abdul Hamid, N. H. Al-Hardan and L. K. Keng, Highly sensitive magnesium-doped ZnO nanorod pH sensors based on electrolyte–insulator–semiconductor (EIS) Sensors, Sensors, 2021, 21(6), 2110 CrossRef CAS PubMed.
- T. Kokab, et al., Simultaneous femtomolar detection of paracetamol, diclofenac, and orphenadrine using a carbon nanotube/zinc oxide nanoparticle-based electrochemical sensor, ACS Appl. Nano. Mater., 2021, 4(5), 4699–4712 CrossRef CAS.
- N. M. J. Ditshego, ZnO nanowire field effect transistor for biosensing: A review, J. Nano Res., 2019, 60, 94–112 CAS.
- N. Tripathy and D.-H. Kim, Metal oxide modified ZnO nanomaterials for biosensor applications, Nano Convergence, 2018, 5(1), 1–10 CrossRef PubMed.
- G. S. Mei, P. S. Menon and G. Hegde, ZnO for performance enhancement of surface plasmon resonance biosensor: a review, Mater. Res. Express, 2020, 7(1), 12003 CrossRef CAS.
- N. P. Shetti, S. D. Bukkitgar, K. R. Reddy, C. V. Reddy and T. M. Aminabhavi, ZnO-based nanostructured electrodes for electrochemical sensors and biosensors in biomedical applications, Biosens. Bioelectron., 2019, 141, 111417 CrossRef CAS PubMed.
- H. Beitollahi, S. Tajik, F. G. Nejad and M. Safaei, Recent advances in ZnO nanostructure-based electrochemical sensors and biosensors, J. Mater. Chem. B, 2020, 8(27), 5826–5844 RSC.
- S. Imran, S. Ahmadi and K. Kerman, Electrochemical biosensors for the detection of SARS-CoV-2 and other viruses, Micromachines, 2021, 12(2), 174 CrossRef PubMed.
- N. P. Shetti, S. D. Bukkitgar, K. R. Reddy, C. V. Reddy and T. M. Aminabhavi, ZnO-based nanostructured electrodes for electrochemical sensors and biosensors in biomedical applications, Biosens. Bioelectron., 2019, 141, 111417 CrossRef CAS PubMed.
- C. Xu, C. Yang, B. Gu and S. Fang, Nanostructured ZnO for biosensing applications, Chin. Sci. Bull., 2013, 58(21), 2563–2566 CrossRef CAS.
- S. K. Arya, S. Saha, J. E. Ramirez-Vick, V. Gupta, S. Bhansali and S. P. Singh, Recent advances in ZnO nanostructures and thin films for biosensor applications, Anal. Chim. Acta, 2012, 737, 1–21 CrossRef CAS PubMed.
- N. R. Shanmugam, S. Muthukumar and S. Prasad, Ultrasensitive and low-volume point-of-care diagnostics on flexible strips–a study with cardiac troponin biomarkers, Sci. Rep., 2016, 6(1), 1–10 CrossRef PubMed.
- G. S. Mei, P. S. Menon and G. Hegde, ZnO for performance enhancement of surface plasmon resonance biosensor: a review, Mater. Res. Express, 2020, 7(1), 12003 CrossRef CAS.
- A. Tereshchenko, et al., Optical biosensors based on ZnO nanostructures: advantages and perspectives. A review, Sens. Actuators, B, 2016, 229, 664–677 CrossRef CAS.
- Y. Zhang, Z. Kang, X. Yan and Q. Liao, ZnO nanostructures in enzyme biosensors, Sci. China Mater., 2015, 58(1), 60–76 CrossRef CAS.
- N. Tripathy and D.-H. Kim, Metal oxide modified ZnO nanomaterials for biosensor applications, Nano Convergence, 2018, 5(1), 1–10 CrossRef PubMed.
- D. Liu, et al., Trends in miniaturized biosensors for point-of-care testing, TrAC, Trends Anal. Chem., 2020, 122, 115701 CrossRef CAS.
- M. F. M. Fathil, et al., Substrate-gate coupling in ZnO-FET biosensor for cardiac troponin I detection, Sens. Actuators, B, 2017, 242, 1142–1154 CrossRef CAS.
- N. Kalyani, S. Goel and S. Jaiswal, On-site sensing of pesticides using point-of-care biosensors: a review, Environ. Chem. Lett., 2021, 19(1), 345–354 CrossRef CAS.
- Y. Xia, et al., Smartphone-based point-of-care microfluidic platform fabricated with a ZnO nanorod template for colorimetric virus detection, ACS Sens., 2019, 4(12), 3298–3307 CrossRef CAS PubMed.
- A. M. Faria and T. Mazon, Early diagnosis of Zika infection using a ZnO nanostructures-based rapid electrochemical biosensor, Talanta, 2019, 203, 153–160 CrossRef CAS PubMed.
- B. Chakraborty, A. Das, N. Mandal, N. Samanta, N. Das and C. R. Chaudhuri, Label free, electric field mediated ultrasensitive electrochemical point-of-care device for CEA detection, Sci. Rep., 2021, 11(1), 1–12 CrossRef PubMed.
- V. Chaudhary, A. K. Kaushik, H. Furukawa and A. Khosla, Review—Towards 5th generation AI and IoT driven sustainable intelligent sensors based on 2D mxenes and borophene, ECS Sens. Plus, 2022, 1, 013601 CrossRef.
- X. Li, et al., Enhancing the performance of paper-based electrochemical impedance spectroscopy nanobiosensors: An experimental approach, Biosens. Bioelectron., 2021, 177, 112672 CrossRef CAS PubMed.
- E. Díaz-Cervantes, C. Zenteno-Zúñiga, V. Rodríguez-González and F. Aguilera-Granja, Design of ZnO-Drug Nanocarriers against the Main Protease of SARS-CoV-2 (COVID-19): An In Silico Assay, Appl. Nano, 2021, 2(3), 257–266 CrossRef.
- A. Kaushik and E. Mostafavi, To manage long COVID by selective SARS-CoV-2 infection biosensing, The Innovation, 2022, 3(5), 100303 CrossRef CAS PubMed.
- J. Kim, et al., ZnO Nanowire-Based Early Detection of SARS-CoV-2 Antibody Responses in Asymptomatic Patients with COVID-19, Adv. Mater. Interfaces, 2022, 2102046 CrossRef CAS PubMed.
- M. C. Sportelli, et al., On the Efficacy of ZnO Nanostructures against SARS-CoV-2, Int. J. Mol. Sci., 2022, 23(6), 3040 CrossRef CAS PubMed.
- F. Haghayegh, R. Salahandish, M. Hassani and A. Sanati-Nezhad, Highly Stable Buffer-Based Zinc Oxide/Reduced Graphene Oxide Nanosurface Chemistry for Rapid Immunosensing of SARS-CoV-2 Antigens, ACS Appl. Mater. Interfaces, 2022, 14(8), 10844–10855 CrossRef CAS PubMed.
- M. Hamdi, H. M. Abdel-Bar, E. Elmowafy, A. El-Khouly, M. Mansour and G. A. S. Awad, Investigating the internalization and COVID-19 antiviral computational analysis of optimized nanoscale zinc oxide, ACS Omega, 2021, 6(10), 6848–6860 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |