Open Access Article
Open Access ArticleConjugated polymer nanoparticles with tunable antibacterial photodynamic capability†
Anderson R. L.
Caires
 *,
Thalita H. N.
Lima
*,
Thalita H. N.
Lima
 and
Thais F.
Abelha
and
Thais F.
Abelha
 *
*
Federal University of Mato Grosso do Sul, Institute of Physics, Mato Grosso do Sul, Campo Grande 79070-900, Brazil. E-mail: thais.abelha@ufms.br; anderson.caires@ufms.br
First published on 22nd February 2023
Abstract
Conjugated polymers are versatile materials with promising applications as light-activated antibacterial agents. This work investigated the photoantimicrobial effect of conjugated polymer nanoparticles (CPNs) composed of poly(2.5-di(hexyloxy)cyanoterephthalylidene) (CN-PPV) prepared under different conditions via a nanoprecipitation method. The stabilization of CN-PPV CPNs with the surfactant polysorbate 20 (tween® 20) increased the CN-PPV product yields and resulted in the formation of nanoparticles of diminished size with slightly negative charge; which did not affect the cell viability of S. aureus and E. coli in the absence of light. Altering the ratio of the organic to aqueous phase from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to 2
10 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (tetrahydrofuran – THF:water) led to the formation of CPNs of smaller size that presented increased photostability compared to that of 1
10 (tetrahydrofuran – THF:water) led to the formation of CPNs of smaller size that presented increased photostability compared to that of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 counterparts. All CPNs were capable of generating reactive oxygen species (ROS) under illumination (450 nm). In addition, CPNs produced with a 2
10 counterparts. All CPNs were capable of generating reactive oxygen species (ROS) under illumination (450 nm). In addition, CPNs produced with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THF
10 THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio presented the highest photoantimicrobial activity against both Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria. Overall, by changing the CPN preparation conditions, CPNs of the same conjugated polymer having remarkably different properties, including greater photostability and more effective microorganism inactivation following photoexcitation, can be generated.
H2O ratio presented the highest photoantimicrobial activity against both Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria. Overall, by changing the CPN preparation conditions, CPNs of the same conjugated polymer having remarkably different properties, including greater photostability and more effective microorganism inactivation following photoexcitation, can be generated.
Introduction
Bacteria are abundant prokaryote microorganisms that colonize virtually all habitats of the planet.1,2 Amongst their great diversity, some strains cause diseases of varied severity and have become resistant to traditional antibiotic therapy, leading to a worldwide crisis of effective treatments.3,4 The consequences of the antibiotic resistance crisis include the increased cost for health care systems, higher mortality rates and the compromise of surgical procedures that rely on antibiotic efficacy.4,5 The increasing number of multidrug-resistant microorganisms is an ongoing problem that will continue worsening; thus there is an urgent need for the development of new effective treatments against pathogenic bacteria and their antibiotic-resistant strains.3,4,6 Besides the search for new antibiotics, photosensitizer (PS) agents, which are capable of tackling bacteria through the generation of reactive oxygen species (ROS) by light stimulation, have been used as an alternative and successful treatment.7 Although photodynamic therapy (PDT) was discovered over a hundred years ago, there is a recent interest in the development of new PSs with improved performance.8,9Conjugated polymers are versatile organic materials that have been broadly explored for biomedical applications.2,10–12 There has been a growing interest in applying their useful physicochemical properties for bacterial photothermal and photodynamic therapies, with promising results.2 For example, conjugated polymer nanoparticles (CPNs) presented light-activated cytotoxicity against both bacteria planktonic solutions and biofilms with good selectivity and biocompatibility in vivo.2 In addition, the conjugated polymer chemical structure can be refined to achieve greater ROS/heat generation capabilities and to enhance water dispersibility, while enabling greater targetability against microorganisms.2 The commercially available conjugated polymer poly(2.5-di(hexyloxy)cyanoterephthalylidene) (CN-PPV) has been successfully explored for fluorescence bioimaging13 and has potential applications as a photosensitizer14 due to its useful optical features. The optical properties of such materials are highly dependent not only on the environment in which they are dispersed (such as solvent polarity and nanoparticle forming agent composition), but also on the nanoparticle preparation settings.10,11,15,16 For example, nanoparticles of CN-PPV embedded into the poly(ethylene glycol) methyl ether-block-poly(lactide-co-glycolide) (PEG-PLGA) polymer exhibited redshifted emission compared to the conjugated polymer in tetrahydrofuran (THF) and variable fluorescence quantum yield in water (35–55% CN-PPV) depending on the manufacturing settings (microfluidics or bulk method).15
The influence of the method of nanoparticle preparation on the performance of conjugated polymers as PS agents is still open to investigation. CN-PPV present tunable optical properties15 that might be useful for inactivating bacteria, but such characteristics have not been explored yet in this scenario. Therefore, the study of the influence of preparation conditions on the CPN photoinactivation capability would enable the screening for optimized conditions to produce high-performance CPNs against bacteria. In this work, CN-PPV CPNs were prepared by varying the ratio of organic (CN-PPV/THF) and aqueous phases and the concentrations of polysorbate 20 in water (Fig. 1). The chosen surfactant (Tween® 20) is a broadly used colloid stabilizing agent and is present in many commercialized pharmaceutical formulations.17 Regarding the choice of microorganisms, Staphylococcus aureus and Escherichia coli have been used by most literature studies that evaluated the anti-bacterial performance of conjugated polymers; accordingly, they are a good model of both Gram-positive and negative bacteria, enabling comparison with the literature record.2Staphylococcus aureus is a Gram-positive bacteria that causes complicated skin infections and has developed resistant strains, especially to methicillin; its incidence is increasing, causing life-threatening illnesses (e.g., necrotizing pneumonia) that are not responsive to conventional treatments.18 It also thrives within the human flora without being harmful to healthy individuals, causing easy spread, which has already triggered a pandemic worldwide due to the lack of available treatment.18 As to the Gram-negative Escherichia coli, it is one of the most abundant microorganisms in the healthy human gut flora, but when pathogenic strains thrive, they may cause diarrhoea and extraintestinal conditions like urinary tract infections and sepsis/meningitis.19
 | ||
| Fig. 1 Illustration of CPN preparation conditions and representative images of the nanoparticles under ambient light and blue light illumination. | ||
Experimental
Nanoparticle preparation
CPNs of the commercially available conjugated polymer poly(2.5-di(hexyloxy)cyanoterephthalylidene) (CN-PPV) (Sigma-Aldrich Corporation, USA) were prepared by an adapted nanoprecipitation method.20 Briefly, an organic solution of CN-PPV in tetrahydrofuran (THF) was added dropwise to 10 mL of water containing varied concentrations (0; 0.3; 0.6 and 1.2 mg mL−1) of polysorbate 20 (tween® 20, Quimesp Química Ltda, Brazil). The CPNs were prepared with THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratios of 1
H2O ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 2
10 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. After stirring overnight, the water lost due to evaporation was replaced and the CN-PPV content (product yield) of each formulation was characterized as previously described,15 as the conjugated polymer attachment to the magnetic stirrer and vial walls was visually noticeable. The CN-PPV theoretical concentration in the end product was 45 μg mL−1.
10. After stirring overnight, the water lost due to evaporation was replaced and the CN-PPV content (product yield) of each formulation was characterized as previously described,15 as the conjugated polymer attachment to the magnetic stirrer and vial walls was visually noticeable. The CN-PPV theoretical concentration in the end product was 45 μg mL−1.
Instrumentation
Dynamic light scattering (DLS) (Zetasizer NanoZS, Malvern Instruments Ltd, UK) was used to characterize the hydrodynamic diameters (expressed as Z-average values obtained from the intensity distribution of particle size) and zeta potential of CPNs at 25 °C. CPNs stabilized with surfactants were characterized at concentrations below and above tween® 20 critical micellar concentration (CMC, 60 μg mL−1) shortly after dilution. For zeta potential analysis, NaCl was added to CPN samples at a final working concentration of 6 mM. Absorbance spectra were obtained between 250 and 800 nm using a UV-vis absorption spectrometer (Lambda 265, PerkinElmer, USA). Fluorescence measurements were performed at room temperature with a spectrofluorimeter (Scinco FS-2, Seoul, Korea) in a right-angle geometry with the aid of a quartz cuvette with four polished faces and 1 cm optical path length. The CPN fluorescence spectra were collected in the 470 to 800 nm range with 460 nm excitation. To irradiate samples, a previously described light emitting diode (LED) device was used.21 To assess the photostability of CPNs under blue light (450 nm) irradiation, absorbance and fluorescence spectra of the samples containing 20 μg mL−1 CN-PPV were measured prior to illumination (time 0) and after 30 and 60 minutes of light exposure at 27 mW cm−2. Fourier transform infrared spectroscopy (FTIR) was used to further assess the structural changes induced by light exposure. For FTIR characterization, CN-PPV powder was used as provided by the manufacturer and CPNs prepared at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 2
10 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THF
10 THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratios and with 0 and 1.2 mg mL−1 tween® 20 were dried on silicon wafers prior to light exposure and after 30 and 60 minutes of light irradiation. FTIR spectra were collected by using a spectrometer (Spectrum 100, PerkinElmer, USA) equipped with an ATR accessory. Acellular reactive oxygen species (ROS) produced by CNPs under blue light (450 nm) illumination at 27 mW cm−2 was assessed using a dihydroethidium (DHE) probe by monitoring the fluorescent oxidized products originated under illumination due to the interaction between the photoproduced ROS and DHE. Absorbance and fluorescence emission (λex.: 500 nm) spectra of CPNs at 5 μg mL−1 CN-PPV (2 mL) were assessed prior to the addition of 4 μL of DHE stock (5 mM) and at time 0 after DHE addition. In sequence, the spectra were acquired at 1-minute intervals for 10 min in the dark and then blue light was turned on and the spectra were collected every minute for 10 minutes under continuous illumination. Due to the intrinsic fluorescence of CN-PPV, absorbance (λ470 nm) and fluorescence (λ580 nm) measurements were investigated to characterize the formation of DHE oxidized products and the data were normalized at time 0 after DHE addition to CPN samples. GraphPad Prism (version 5.00 for Windows, GraphPad Software, San Diego California, USA) was used to perform statistical analysis (One-way ANOVA with Tukey post hoc test).
H2O ratios and with 0 and 1.2 mg mL−1 tween® 20 were dried on silicon wafers prior to light exposure and after 30 and 60 minutes of light irradiation. FTIR spectra were collected by using a spectrometer (Spectrum 100, PerkinElmer, USA) equipped with an ATR accessory. Acellular reactive oxygen species (ROS) produced by CNPs under blue light (450 nm) illumination at 27 mW cm−2 was assessed using a dihydroethidium (DHE) probe by monitoring the fluorescent oxidized products originated under illumination due to the interaction between the photoproduced ROS and DHE. Absorbance and fluorescence emission (λex.: 500 nm) spectra of CPNs at 5 μg mL−1 CN-PPV (2 mL) were assessed prior to the addition of 4 μL of DHE stock (5 mM) and at time 0 after DHE addition. In sequence, the spectra were acquired at 1-minute intervals for 10 min in the dark and then blue light was turned on and the spectra were collected every minute for 10 minutes under continuous illumination. Due to the intrinsic fluorescence of CN-PPV, absorbance (λ470 nm) and fluorescence (λ580 nm) measurements were investigated to characterize the formation of DHE oxidized products and the data were normalized at time 0 after DHE addition to CPN samples. GraphPad Prism (version 5.00 for Windows, GraphPad Software, San Diego California, USA) was used to perform statistical analysis (One-way ANOVA with Tukey post hoc test).
Microbiological assays
Staphylococcus aureus (strain 25923) and Escherichia coli (strain 25922) were acquired from the American Type Culture Collection (ATCC). The bacterial strains were grown in sterilized brain heart infusion broth (BHIB, KASVI, Brazil) and, for biological assays, diluted in sterile phosphate buffer saline (PBS) to adjust the bacterial concentration to the 0.5 McFarland scale (1.5 × 108 bacteria mL−1, Probac do Brasil, Brazil). The cell metabolic activity was assessed using (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT) tetrazolium assay.22 Briefly, bacteria receiving CPN treatments (5 μg mL−1 CN-PPV working concentration) and controls (PBS and tween® 20 solution at 0.2 mg mL−1) were kept in the dark for 30 min. Then, 100 μL samples were transferred to a 96-well plate in triplicate and irradiated (450 nm at 27 mW cm−2) for 1 h. In sequence, 100 μL of the samples kept in the dark were transferred in triplicate to a 96-well plate and 10 μL of MTT stock solution (5 mg mL−1) was added to each well and the plate was incubated at 37 °C for 4h protecting from light. The formazan crystals were solubilized with isopropanol and the prevalence of its purple colour was visually inspected. The colony assay was performed similarly, except that after light exposure, the samples kept in the dark and under radiation were diluted gradually with PBS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, 1
200, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 and 1
200 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 fold) and 100 μL was transferred and spread onto agar plates (90 × 15 mm) in duplicate. After 18 h of incubation at 37 °C, the bacterial colonies present on the agar plates were counted and the colony-forming units (CFU) per mL was calculated taking into account the sample dilution factor and the volume transferred to the plates.
37 fold) and 100 μL was transferred and spread onto agar plates (90 × 15 mm) in duplicate. After 18 h of incubation at 37 °C, the bacterial colonies present on the agar plates were counted and the colony-forming units (CFU) per mL was calculated taking into account the sample dilution factor and the volume transferred to the plates.
Results and discussion
CN-PPV nanoparticles were generated with variable preparation settings that influenced the final product yield (Fig. 2). Although CN-PPV can self-assemble into nanoparticles in water by forming nanoaggregates due to the folding and collapsing of their hydrophobic chains,13,15 increasing tween® 20 concentration in the aqueous phase greatly contributed to high CN-PPV yields in the end product, whereas in the absence of tween® 20, the mean product yields were below 35%. In addition, a THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio of 2
H2O ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 led to higher mean product yields at higher tween® 20 concentrations. Overall, increasing the surfactant concentration and the THF
10 led to higher mean product yields at higher tween® 20 concentrations. Overall, increasing the surfactant concentration and the THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio improved the CN-PPV product yield and led to lower batch variability. The mean CN-PPV yield of CPNs prepared with 0.6 and 1.2 mg mL−1 tween® 20 was comparable with that of CPNs of the same conjugated polymer prepared with a bulk nanoprecipitation method, but stabilized with PEG-PLGA.15
H2O ratio improved the CN-PPV product yield and led to lower batch variability. The mean CN-PPV yield of CPNs prepared with 0.6 and 1.2 mg mL−1 tween® 20 was comparable with that of CPNs of the same conjugated polymer prepared with a bulk nanoprecipitation method, but stabilized with PEG-PLGA.15
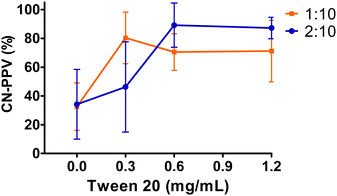 | ||
Fig. 2 CN-PPV product yield of CPNs prepared with variable preparation settings: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 2 10 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THF 10 THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratios and increasing surfactant concentration (0; 0.3; 0.6 and 1.2 mg mL−1). H2O ratios and increasing surfactant concentration (0; 0.3; 0.6 and 1.2 mg mL−1). | ||
CPNs prepared in water presented significantly (p ≤ 0.05) larger mean diameters than the counterparts generated with the same THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio, but containing tween® 20 (Fig. 3a). In addition, CPNs prepared with the 2
H2O ratio, but containing tween® 20 (Fig. 3a). In addition, CPNs prepared with the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THF
10 THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio presented significantly (p ≤ 0.05) smaller hydrodynamic diameters (< 130 nm) compared to the ones generated with the 1
H2O ratio presented significantly (p ≤ 0.05) smaller hydrodynamic diameters (< 130 nm) compared to the ones generated with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio (<220 nm), except for the ones containing 1.2 mg mL−1 tween® 20. The increasing content of the surfactant in the aqueous phase showed a significant (p ≤ 0.05) influence only on the nanoparticle size for CPNs prepared at the 1
10 ratio (<220 nm), except for the ones containing 1.2 mg mL−1 tween® 20. The increasing content of the surfactant in the aqueous phase showed a significant (p ≤ 0.05) influence only on the nanoparticle size for CPNs prepared at the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THF
10 THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio, as it led to a CPN size reduction similar to nanoparticles prepared with the 2
H2O ratio, as it led to a CPN size reduction similar to nanoparticles prepared with the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THF
10 THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio (ca. 118 nm for 1.2 mg mL−1 tween® 20). Overall, nanoparticles with smaller sizes were prepared with the 2
H2O ratio (ca. 118 nm for 1.2 mg mL−1 tween® 20). Overall, nanoparticles with smaller sizes were prepared with the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THF
10 THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio, regardless of the surfactant concentration, and with the 1
H2O ratio, regardless of the surfactant concentration, and with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THF
10 THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio with 1.2 mg mL−1 tween® 20. The polydispersity indexes of all CPNs were typically between 0.10–0.25, regardless of the production settings, and they slightly increased when CPNs were diluted below the CMC of tween® 20. CPNs prepared with tween® 20 presented a mild negative charge, while the zeta potential of bare CN-PPV nanoparticles was more electronegative (Fig. 3b), in agreement with the previous report.15 It was noteworthy that CPNs diluted below the CMC of tween® 20 presented more electronegative zeta potential values, which we attribute to micelle destabilization and exposure of the electronegative cyan groups of CN-PPV.
H2O ratio with 1.2 mg mL−1 tween® 20. The polydispersity indexes of all CPNs were typically between 0.10–0.25, regardless of the production settings, and they slightly increased when CPNs were diluted below the CMC of tween® 20. CPNs prepared with tween® 20 presented a mild negative charge, while the zeta potential of bare CN-PPV nanoparticles was more electronegative (Fig. 3b), in agreement with the previous report.15 It was noteworthy that CPNs diluted below the CMC of tween® 20 presented more electronegative zeta potential values, which we attribute to micelle destabilization and exposure of the electronegative cyan groups of CN-PPV.
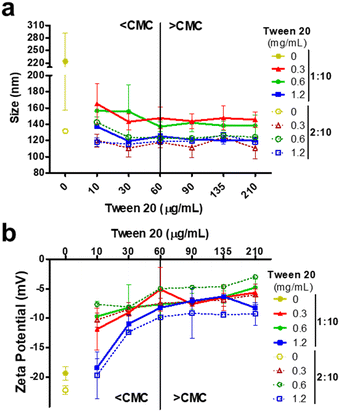 | ||
| Fig. 3 Hydrodynamic diameters (a) and zeta potential (b) of CPNs characterized above and below the CMC of tween® 20. The CPNs prepared without tween® 20 are denoted as 0 mg mL−1. | ||
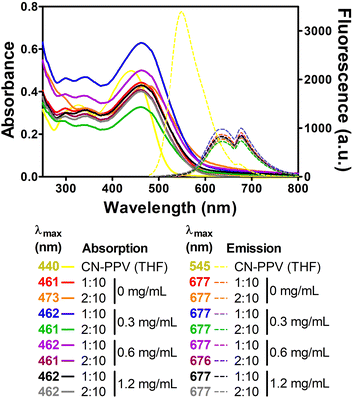
It is known that the optical properties of conjugated polymers are highly dependent on their chain conformation, which is influenced by nanoparticle preparation conditions.10,15,16 Under microfluidic conditions, different THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratios generated CN-PPV CPNs with distinctive optical properties,15 while in this work, all CPNs presented similar absorption and fluorescence emission spectra, independent of the preparation settings (Fig. 4). Compared to CN-PPV solution in THF, the maximum absorption of CPNs was approximately 20 nm red-shifted, while their maximum fluorescence emission wavelength presented a red-shift of approximately 132 nm, which is greater than that previously reported for CN-PPV nanoparticles prepared via a similar bulk method.15 Importantly, the red-shifted emission leads to a desirable large Stokes shift (decreasing the probability of self-absorption) and near-infrared emission that is more suitable for biomedical applications. Noteworthily, a distinct spectral shape of fluorescence emission compared to that of the conjugated polymer in organic solvent was observed. This was observed as a dual-band emission due to the self-arrangement and interactions of the confined CN-PPV polymers within the nanoparticle structure. The dual-band characteristic indicates the existence of aggregated states in the confined nanoparticle architecture, allowing emission originating from intrachain and interchain interactions, which may explain the observed red shift and dual-band emission feature of CN-PPV CNPs.23–30
H2O ratios generated CN-PPV CPNs with distinctive optical properties,15 while in this work, all CPNs presented similar absorption and fluorescence emission spectra, independent of the preparation settings (Fig. 4). Compared to CN-PPV solution in THF, the maximum absorption of CPNs was approximately 20 nm red-shifted, while their maximum fluorescence emission wavelength presented a red-shift of approximately 132 nm, which is greater than that previously reported for CN-PPV nanoparticles prepared via a similar bulk method.15 Importantly, the red-shifted emission leads to a desirable large Stokes shift (decreasing the probability of self-absorption) and near-infrared emission that is more suitable for biomedical applications. Noteworthily, a distinct spectral shape of fluorescence emission compared to that of the conjugated polymer in organic solvent was observed. This was observed as a dual-band emission due to the self-arrangement and interactions of the confined CN-PPV polymers within the nanoparticle structure. The dual-band characteristic indicates the existence of aggregated states in the confined nanoparticle architecture, allowing emission originating from intrachain and interchain interactions, which may explain the observed red shift and dual-band emission feature of CN-PPV CNPs.23–30
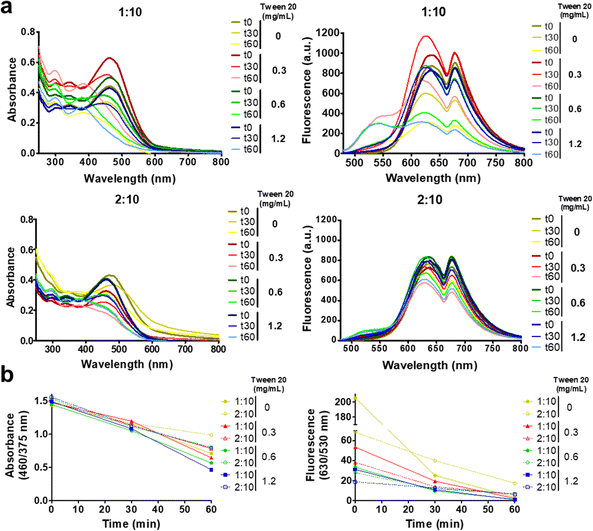
In order to study the CPN photostability under 450 nm light irradiation, the absorption and fluorescence emission spectra of nanoparticle solutions with the same CN-PPV content were monitored prior to illumination and after continuous irradiation for 30 and 60 minutes. Fig. 5a and Fig. S1 (ESI†) reveal that regardless of the tween® 20 concentrations, after 30 minutes of irradiation there was a decrease in absorbance intensity at 460 nm and an increase in absorption at 290 nm, which continued to change under 60 minute light exposure, reaching the maximum absorbance intensity at 290 nm with a second peak at 375 nm. The fluorescence emission spectra showed a blue-shifted spectra with increasing light exposure time, resulting in diminished intensities and the appearance of a shoulder at ∼530 nm after 60 minutes of light irradiation. It is noteworthy that CPNs prepared with different THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratios showed remarkedly different susceptibility to light irradiation, with 2
H2O ratios showed remarkedly different susceptibility to light irradiation, with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (THF
10 (THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) nanoparticles presenting a lower extent of spectral alterations than 1
H2O) nanoparticles presenting a lower extent of spectral alterations than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 CPNs, independent of the concentration of tween® 20 (Fig. 5b). We ascribed the spectroscopic alterations to the photodegradation of CN-PPV. Hence, to further characterize the effect of light exposure, the size, zeta potential and FTIR of CPNs prepared with 1
10 CPNs, independent of the concentration of tween® 20 (Fig. 5b). We ascribed the spectroscopic alterations to the photodegradation of CN-PPV. Hence, to further characterize the effect of light exposure, the size, zeta potential and FTIR of CPNs prepared with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 2
10 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THF: H2O ratios and with no stabilizing agent and with 1.2 mg mL−1 tween® 20 were investigated. While the FTIR analysis did not reveal any functional group alteration with increasing irradiation times (Fig. S2, ESI†), the DLS analysis of photostable samples (Fig. S2, ESI†) showed that both 1
10 THF: H2O ratios and with no stabilizing agent and with 1.2 mg mL−1 tween® 20 were investigated. While the FTIR analysis did not reveal any functional group alteration with increasing irradiation times (Fig. S2, ESI†), the DLS analysis of photostable samples (Fig. S2, ESI†) showed that both 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 2
10 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 CPNs without tween® presented a decrease in electronegativity from approximately −20 mV to −5 mM, suggesting an alteration in the sample composition after illumination.
10 CPNs without tween® presented a decrease in electronegativity from approximately −20 mV to −5 mM, suggesting an alteration in the sample composition after illumination.
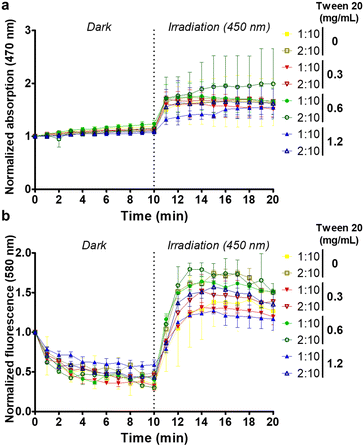
The light-activated ROS generation capability of CN-PPV CPNs was assessed using the DHE probe.31 Although DHE is non-fluorescent, ethidium is generated in the presence of ROS and its fluorescence emission (530 to 750 nm range) can be monitored.32 Due to the overlap between the intrinsic spectra of CN-PPV CPNs and ethidium, the ROS production was determined from the relative intensity variation as a function of the time after normalizing the fluorescence and absorption spectra at the initial time (i.e., after the addition of DHE to CPN samples at time 0). Fig. 6a and b reveals that upon light exposure there is a sharp increase in fluorescence and absorbance intensities for all CPN samples. Overall, the CPNs prepared with the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THF
10 THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio presented higher intensity values than the corresponding 1
H2O ratio presented higher intensity values than the corresponding 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 formulations.
10 formulations.
The light-activated antimicrobial activity of CN-PPV CPNs was evaluated in Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria, both widely used to study the photoinactivation capability of conjugated polymers.2 MTT tetrazolium assay was used to evaluate the influence of treatments and controls on the cell metabolic activity. Fig. S3 (ESI†) shows a reduction in the metabolic activity of both Gram-positive and Gram-negative bacteria exposed to light in the presence of CN-PPV nanoparticles, observed from lower staining intensity due to the reduced metabolism of MTT to the purple coloured tetrazolium.22,33 It is noteworthy that the nanoparticles prepared without a stabilizing agent reduced the metabolic activity of bacterial strains kept in the dark, while the nanoparticles prepared with tween® 20 were more compatible in the absence of irradiation and were activated only in the presence of light, which is a desirable characteristic for new materials investigated for photodynamic therapy.
To characterize the bacterial photoinactivation of CN-PPV nanoparticles, the CPNs prepared at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 2
10 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (THF
10 (THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) ratios, without the surfactant and with the highest concentration of tween® 20 (1.2 mg mL−1) were selected for the colony assay. It was observed that S. aureus and E. coli presented different susceptibility to the different irradiated treatments, with the Gram-negative bacteria showing a lower extent of reduction compared to the Gram-positive strain (Fig. 7 and Fig. S3, ESI†). The log10 CFU reduction of bacteria exposed to CPNs under light illumination was larger than that of the control, irrespective of the nanoparticle preparation conditions (Fig. 7c). It is noteworthy that the CPNs prepared at the 2
H2O) ratios, without the surfactant and with the highest concentration of tween® 20 (1.2 mg mL−1) were selected for the colony assay. It was observed that S. aureus and E. coli presented different susceptibility to the different irradiated treatments, with the Gram-negative bacteria showing a lower extent of reduction compared to the Gram-positive strain (Fig. 7 and Fig. S3, ESI†). The log10 CFU reduction of bacteria exposed to CPNs under light illumination was larger than that of the control, irrespective of the nanoparticle preparation conditions (Fig. 7c). It is noteworthy that the CPNs prepared at the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio (THF
10 ratio (THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) and without a stabilizing agent presented the greatest light-induced bacteria inactivation with approximately 3 and 5.5 Log10 CFU reduction for E. coli and S. aureus, respectively. In fact, S. aureus was more susceptible to CPNs prepared at the 2
H2O) and without a stabilizing agent presented the greatest light-induced bacteria inactivation with approximately 3 and 5.5 Log10 CFU reduction for E. coli and S. aureus, respectively. In fact, S. aureus was more susceptible to CPNs prepared at the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio (THF
10 ratio (THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O), independent of the presence of tween® 20. This could be related to the greater photostability of CPNs prepared with the 2
H2O), independent of the presence of tween® 20. This could be related to the greater photostability of CPNs prepared with the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio setting. In spite of the fact that the majority of novel photosensitizers are designed with a positive charge to facilitate their interaction with the bacterial anionic cell wall,2 electronegative CN-PPV nanoparticles were capable of inducing cytotoxicity triggered by light to both Gram-negative and Gram-positive bacteria.
10 ratio setting. In spite of the fact that the majority of novel photosensitizers are designed with a positive charge to facilitate their interaction with the bacterial anionic cell wall,2 electronegative CN-PPV nanoparticles were capable of inducing cytotoxicity triggered by light to both Gram-negative and Gram-positive bacteria.
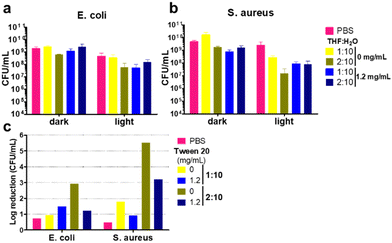 | ||
| Fig. 7 Charts representing mean CFU mL−1 of S. aureus (a) and E. coli (b). Log10 reduction calculated from mean values of bacterial strains kept in dark and exposed to light (c). | ||
Conclusions
In this work, we report the light-activated antibacterial capability of CN-PPV nanoparticles prepared with variable settings. We show that increasing concentrations of polysorbate 20 (tween® 20) in an aqueous phase led to the formation of smaller CPNs with higher CN-PPV yields in the final product and that the use of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THF
10 THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio led to the formation of CPNs of smaller sizes compared to their 1
H2O ratio led to the formation of CPNs of smaller sizes compared to their 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 counterparts. CPNs stabilized with tween® 20 presented mild negative charge, while the ones prepared without the surfactant were more electronegative. The preparation conditions studied did not affect the absorbance and emission spectra profile of CPNs, which were red-shifted and presented two emission peaks compared to CN-PPV in THF. All CPNs were capable of generating ROS under blue light irradiation, affecting the cell metabolic activity and photoinactivating both Gram-positive and Gram-negative bacteria. It was noteworthy that while CPNs presented similar absorbance and emission spectra, changing the organic and aqueous ratio from 1
10 counterparts. CPNs stabilized with tween® 20 presented mild negative charge, while the ones prepared without the surfactant were more electronegative. The preparation conditions studied did not affect the absorbance and emission spectra profile of CPNs, which were red-shifted and presented two emission peaks compared to CN-PPV in THF. All CPNs were capable of generating ROS under blue light irradiation, affecting the cell metabolic activity and photoinactivating both Gram-positive and Gram-negative bacteria. It was noteworthy that while CPNs presented similar absorbance and emission spectra, changing the organic and aqueous ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to 2
10 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 led to the formation of nanoparticles with diminished size, improved photostability and more efficiency toward bacterial inactivation. The most successful antibacterial photodynamic agent was prepared without tween® 20 and with a 2
10 led to the formation of nanoparticles with diminished size, improved photostability and more efficiency toward bacterial inactivation. The most successful antibacterial photodynamic agent was prepared without tween® 20 and with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THF
10 THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio; however such CPNs presented lower CN-PPV yield and affected the cell metabolic activity in the dark. Nonetheless, CPNs containing tween® 20 were capable of 3 log10 CFU reduction, while being inert in the dark. Overall, this work showed that CPNs prepared with commercially available products could show enhanced antibacterial performance by altering the nanoparticle production setting, without requiring complicated synthesis of novel materials.
H2O ratio; however such CPNs presented lower CN-PPV yield and affected the cell metabolic activity in the dark. Nonetheless, CPNs containing tween® 20 were capable of 3 log10 CFU reduction, while being inert in the dark. Overall, this work showed that CPNs prepared with commercially available products could show enhanced antibacterial performance by altering the nanoparticle production setting, without requiring complicated synthesis of novel materials.
Author contributions
TFA was responsible for the conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, supervision and writing (original draft). ARLC contributed with the conceptualization, funding acquisition, investigation, methodology and writing (review and editing). THNL participated in data curation, investigation, methodology and writing (review and editing).Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Federal University of Mato Grasso do Sul (UFMS). This study was financed in part by the Brazilian agency Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES) – CAPES/PrInt (grant number: 88887.507844/2020-00). TFA also thanks the National Council for Scientific and Technological Development – CNPq (grant number: 164249/2020-6). ARLC thanks the financial support provided by the National System of Photonics Laboratories – Sisfóton/MCTI (grant number: 440214/2021-1).References
- L. A. Cole, Biology of Life, Elsevier, 2016, pp. 93–99 Search PubMed.
- T. Fedatto Abelha and A. Rodrigues Lima Caires, Adv. NanoBiomed Res., 2021, 1, 2100012 CrossRef CAS.
- World Health Organisation, Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug resistant bacterial infections, including tuberculosis, Geneva, Switzerland, 2017.
- K. Bush, P. Courvalin, G. Dantas, J. Davies, B. Eisenstein, P. Huovinen, G. A. Jacoby, R. Kishony, B. N. Kreiswirth, E. Kutter, S. A. Lerner, S. Levy, K. Lewis, O. Lomovskaya, J. H. Miller, S. Mobashery, L. J. V. Piddock, S. Projan, C. M. Thomas, A. Tomasz, P. M. Tulkens, T. R. Walsh, J. D. Watson, J. Witkowski, W. Witte, G. Wright, P. Yeh and H. I. Zgurskaya, Nat. Rev. Microbiol., 2011, 9, 894–896 CrossRef CAS PubMed.
- J. O’neill, Tackling drug-resistantinfections globally: Finalreport and recommendations, 2016.
- World Health Organisation, Global Action Plan on Antimicrobial Resistance, 2015.
- Y. Liu, R. Qin, S. A. J. Zaat, E. Breukink and M. Heger, J. Clin. Transl. Res., 2015, 1, 140–167 Search PubMed.
- H. Hönigsmann, Photochem. Photobiol. Sci., 2013, 12, 16–21 CrossRef PubMed.
- S. Liu, G. Feng, B. Z. Tang and B. Liu, Chem. Sci., 2021, 12, 6488–6506 RSC.
- T. F. Abelha, C. A. Dreiss, M. A. Green and L. A. Dailey, J. Mater. Chem. B, 2020, 8, 592–606 RSC.
- T. F. Abelha, P. R. Neumann, J. Holthof, C. A. Dreiss, C. Alexander, M. Green and L. A. Dailey, J. Mater. Chem. B, 2019, 7, 5115–5124 RSC.
- R. A. Khanbeigi, Z. Hashim, T. F. Abelha, S. Pitchford, H. Collins, M. Green and L. A. Dailey, J. Mater. Chem. B, 2015, 3, 2463–2471 RSC.
- E. Kemal, T. F. Abelha, L. Urbano, R. Peters, D. M. Owen, P. Howes, M. Green and L. A. Dailey, RSC Adv., 2017, 7, 15255–15264 RSC.
- P. L. C. Feyen, B. F. E. Matarèse, L. Urbano, T. F. Abelha, H. Rahmoune, M. Green, L. A. Dailey, J. C. de Mello and F. Benfenati, Front. Bioeng. Biotechnol., 2022, 10, 932877 CrossRef PubMed.
- T. F. Abelha, T. W. Phillips, J. H. Bannock, A. M. Nightingale, C. A. Dreiss, E. Kemal, L. Urbano, J. C. de Mello, M. A. Green and L. A. Dailey, Nanoscale, 2017, 9, 2009–2019 RSC.
- T. F. Abelha, J. Calvo-Castro, S. M. Lima, J. R. Silva, L. H. da Cunha Andrade, J. C. de Mello, C. A. Dreiss, M. Green and L. A. Dailey, ACS Appl. Polym. Mater., 2022, 4, 6219–6228 CrossRef.
- A. Martos, W. Koch, W. Jiskoot, K. Wuchner, G. Winter, W. Friess and A. Hawe, J. Pharm. Sci., 2017, 106, 1722–1735 CrossRef CAS PubMed.
- H. F. Chambers and F. R. DeLeo, Nat. Rev. Microbiol., 2009, 7, 629–641 CrossRef CAS PubMed.
- J. B. Kaper, J. P. Nataro and H. L. T. Mobley, Nat. Rev. Microbiol., 2004, 2, 123–140 CrossRef CAS PubMed.
- L. Urbano, L. Clifton, H. K. Ku, H. Kendall-Troughton, K. A. Vandera, B. F. E. Matarese, T. Abelha, P. Li, T. Desai, C. A. Dreiss, R. D. Barker, M. A. Green, L. A. Dailey and R. D. Harvey, Langmuir, 2018, 34, 6125–6137 CrossRef CAS PubMed.
- C. M. Silva, A. R. Lima, T. F. Abelha, T. H. N. Lima, C. S. A. Caires, T. V. Acunha, E. J. Arruda, S. L. Oliveira, B. A. Iglesias and A. R. L. Caires, J. Photochem. Photobiol., B, 2021, 224, 112323 CrossRef CAS PubMed.
- E. Grela, J. Kozłowska and A. Grabowiecka, Acta Histochem., 2018, 120, 303–311 CrossRef CAS PubMed.
- C. Wu, B. Bull, C. Szymanski, K. Christensen and J. McNeill, ACS Nano, 2008, 2, 2415–2423 CrossRef CAS PubMed.
- B. J. Schwartz, Annu. Rev. Phys. Chem., 2003, 54, 141–172 CrossRef CAS PubMed.
- L. E. Ibarra, S. R. Martínez, R. A. Ponzio and R. E. Palacios, Advances in Photodynamic Therapy Research, Nova Science Publishers, 2020, pp. 65–92 Search PubMed.
- D. A. Vanden Bout, W.-T. Yip, D. Hu, D.-K. Fu, T. M. Swager and P. F. Barbara, Science, 1997, 277, 1074–1077 CrossRef CAS.
- J. Kim, Pure Appl. Chem., 2002, 74, 2031–2044 CrossRef CAS.
- F. C. Spano and C. Silva, Annu. Rev. Phys. Chem., 2014, 65, 477–500 CrossRef CAS PubMed.
- M. E. Ziffer, S. B. Jo, Y. Liu, H. Zhong, J. C. Mohammed, J. S. Harrison, A. K.-Y. Jen and D. S. Ginger, J. Phys. Chem. C, 2018, 122, 18860–18869 CrossRef CAS.
- K. Li and B. Liu, J. Mater. Chem., 2012, 22, 1257–1264 RSC.
- R. R. Nazarewicz, A. Bikineyeva and S. I. Dikalov, J. Biomol. Screen., 2013, 18, 498–503 CrossRef PubMed.
- D. P. Pedruzzi, L. O. Araujo, W. F. Falco, G. Machado, G. A. Casagrande, I. Colbeck, T. Lawson, S. L. Oliveira and A. R. L. Caires, NanoImpact, 2020, 19, 100246 CrossRef.
- T. Mosmann, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ma00970f |
| This journal is © The Royal Society of Chemistry 2023 |