Open Access Article
Open Access ArticleEnhanced two-step two-frequency upconversion luminescence in a core/shell/shell nanostructure†
Dongcheng
Han
 abc,
Shizhi
Yang
*ac,
Qiang
Zhao
*d,
Liangliang
Zhang
e and
Yan
Deng
d
abc,
Shizhi
Yang
*ac,
Qiang
Zhao
*d,
Liangliang
Zhang
e and
Yan
Deng
d
aKey Laboratory of Atmospheric Optics, Anhui Institute of Optics and Fine Mechanics, HFIPS, Chinese Academy of Sciences, Hefei 230031, China. E-mail: szyang@aiofm.ac.cn
bScience Island Branch of Graduate School, University of Science and Technology of China, Hefei, Anhui 230026, China
cAdvanced Laser Technology Laboratory of Anhui Province, Hefei 230037, China
dSchool of Environment and Energy Engineering, Anhui Jianzhu University, Hefei 230601, China. E-mail: rommel99@163.com
eAnhui Easpeed Technology Co. Ltd, Hefei, Anhui 230088, China
First published on 16th December 2022
Abstract
Hexagonal-phase NaYF4:Er@NaGdF4:Yb@NaYF4:Er core/shell/shell upconversion nanoparticles (UCNPs) were synthetized using the solvothermal method and first applied in enhancing two-step two-frequency upconversion luminescence (TSTF UCL). In comparison to NaYF4:0.5%Er UCNPs, the UCL intensities and contrast of NaYF4:0.5%Er@NaGdF4:2%Yb@NaYF4:1%Er UCNPs under 850 & 1550 nm excitation increase by about 3.4 and 2.8 times, respectively. By analyzing the TSTF UCL processes, the amplified UCL was mainly attributed to the reduction of surface quenching and the improvement of 1550 nm absorption. The designed nanostructure provides a novel strategy for studying the mechanism of TSTF UCL and would drive the adoption of UCNPs in a volumetric three-dimensional (3-D) display field.
Introduction
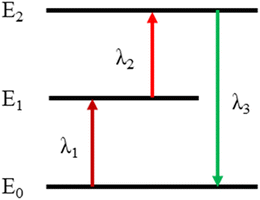
Rare-earth (RE)-doped two-step two-frequency upconversion (TSTF-UC) materials have attracted great interest owing to their unique process in which invisible near-infrared light at two different wavelengths is absorbed sequentially and afterwards converted to visible light1–4 (Fig. 1), which is now being explored for its potential use in 3-D displays,5–11 lasers,12–14 solar cells15–17 and super resolution fluorescence imaging.18 In particular, these materials with high transparency and brightness make them suitable for volumetric 3-D display.As early as the 1920s, TSTF UCL performance was first discovered by chance.1,2 However, owing to technique limitation,19–23 the TSTF-UC materials were difficult to apply to a 3-D display. Zito et al. were the first to achieve volumetric 3-D displays in a mercury vapor host material in 1962.24 Since then, diverse gaseous host materials have been demonstrated to be capable of 3-D displays.25–27 Compared with gaseous host materials, RE-doped transparent bulk materials could not only provide spatial voxels but also a real physical space, so people would like to achieve a volumetric 3-D display with these materials. In 1964, Brown et al.7 applied for the first volumetric 3-D display patent based on fluoride crystals doped with Tm3+, Yb3+, and Ho3+. Since then, numerous TSTF-UC fluoride crystals have been gradually used for volumetric 3-D displays.5,28–30 Particularly, Downing et al.6 successfully presented a three-color 3-D display derived from Er3+, Tm3+ and Pr3+ singly-doped fluoride glass under the simultaneous excitation of two intersecting laser beams, which was considered the greatest achievement of the physics in 1996. Afterwards, a research boom in RE-doped fluoride glass for 3-D displays has been established in China. For example, Hou, Chen and Sun et al. studied and optimized the TSTF UCL performances of RE-doped fluoride glasses and analyzed their application prospects in volumetric 3-D displays.31–33
However, fluoride glasses doped with RE3+ ions are always accompanied by deep colors and poor UCL contrast, which may lead to an impure 3-D color display and low resolution.34 In addition, restricted by the growth process, providing a large and perfect fluoride crystal is not easy.35 Compared with RE-doped fluoride glasses, transparent colorless fluoride nanoparticle solutions can be more easily prepared. To obtain larger and higher resolution images, nanoparticle solutions are the better choice for a volumetric 3-D display. With the maturity of the fluoride nanoparticle synthesis process, recently, RE-doped UC fluoride nanoparticles with designed multilayer core/shell structures were employed as a full-color 3-D display material with high resolution by Deng et al.36 The 3-D display was achieved by moving the overlapped focal point of the 808 nm C.W. beam and 980 nm pulse beam within the volume of the NaYF4:Nd/Yb@NaYF4:Yb/Tm@NaYF4@NaYF4:Yb/Ho/Ce@NaYF4 nanoparticles. They overcame the problem of the low resolution of 3-D images caused by using RE-doped fluoride glasses as the 3-D display media. However, Deng presented a 3-D display based on single-frequency multiphoton UCL, which cannot present dynamic and complex 3-D images. Therefore, it is necessary to develop RE-doped TSTF UCNPs as volumetric 3-D display materials to obtain better 3-D images.
Many excellent studies on TSTF UCNPs have been reported.37–41 For example, Chen et al.37 demonstrated that enhanced UCL intensity in NaYF4:1%Er nanoparticles was achieved by 790 & 1520 nm excitation. Li et al.38 demonstrated that enhanced red emission in Gd2MoO6:Er/Yb nanoparticles co-doped WO66− and La3+ was achieved by 980 & 1550 nm excitation. Guo et al.39 showed that enhanced emission intensity in GdVO4:Yb/Er nanoparticles doped with Lu3+, Y3+ and PO43− ions was achieved by 980 & 1550 nm excitation. However, TSTF UCNPs often suffer from low luminescent efficiency,42–44 attributed to concentration quenching and surface defects,45–50 which partly restrict further applications in the 3-D display. To enhance UCL efficiency, many approaches, such as doping sensitizers, fabricating core/shell structures, and changing the hosts, have been widely studied.51–54 In particular, by designing core/shell structure, Chen et al.55 improved the UC quantum yield to 5% in LiLuF4:Yb/Er@LiLuF4 UCNPs. Chen et al.56 remarkably improved the UCL of Ln3+-doped nanoparticles in NaGdF4:Yb@NaYF4:Yb/Er@NaGdF4:Yb UCNPs.
In this study, a novel strategy is first described to improve TSTF UCL intensities and contrast via a designed core/shell/shell nanostructure. Er3+-doped NaYF4 was used as the luminescent core and outer luminescent shell, and Yb3+-doped NaGdF4 was used as the inner active shell. By characterization, we can conclude that controlling the distribution of Yb3+ and Er3+ ions can serve two purposes: (a) to protect the UCNPs from surface quenching and (b) to improve the absorption of 1550 nm. Through these two ways of enhancing TSTF UCL performances, clear volumetric 3-D images were achieved in NaYF4:Er@NaGdF4:Yb@NaYF4:Er UCNPs solution under 850 & 1550 nm excitation.
Experimental section
Materials
YCl3·6H2O (99.99%), GdCl3·xH2O (99.99%), YbCl3·6H2O (99.9%), ErCl3·6H2O (99.9%), NaOH (98%), NH4F (98%), oleic acid (OA, 90%), and 1-octadecene (ODE, 90%) were purchased from Sigma-Aldrich (Shanghai, China). Ethanol, cyclohexane, and chloroform were purchased from Sinopharm Chemical Reagent Co. (Shanghai, China). All the chemical reagents were used as received without further purification.Synthesis of TSTF core/shell/shell UCNPs
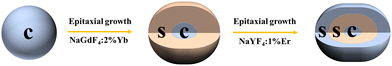
The UCNPs were prepared by applying a solvothermal method.57,58 The core/shell/shell UCNPs were prepared using a layer-by-layer epitaxial growth method.59,60 NaYF4:0.5%Er UCNPs were first prepared and then covered by NaGdF4:2%Yb and NaYF4:1%Er shell gradually (Fig. 2).Synthesis of NaYF4:0.5%Er UCNPs
In a typical experiment, ErCl3·6H2O (0.005 mmol), YCl3·6H2O (0.995 mmol), 6 mL OA and 15 mL ODE were mixed into a 50 mL flask. The mixture was heated at 150 °C under argon flow for 30 min. After cooling to 50 °C, 10 mL of methanol solution containing 4 mmol NH4F and 2.5 mmol NaOH was added, and the solution was vigorously stirred for 1 h. Then, the slurry was slowly heated and kept at 100 °C for 10 min to remove methanol. Next, the solution was heated to 305 °C and maintained for 1 h. The resulting UCNPs were precipitated out by the addition of ethanol, collected by centrifugation, washed with a mixture of cyclohexane and absolute ethanol solvent several times, and redispersed in 4 mL chloroform.Synthesis of NaYF4:0.5%Er@NaGdF4:2%Yb UCNPs
YbCl3·6H2O (0.02 × 0.5 mmol), GdCl3·6H2O (0.98 × 0.5 mmol), 6 mL OA and 15 mL ODE were mixed into a 50 mL flask. The mixture was heated at 150 °C under argon flow for 30 min. After cooling down to 50 °C, 10 mL methanol solution containing 4 mmol NH4F and 2.5 mmol NaOH was added along with the as-prepared NaYF4:0.5%Er UCNPs in 4 mL chloroform; the solution was vigorously stirred for 1 h. Then, the slurry was slowly heated and kept at 100 °C for 10 min to remove methanol. Next, the solution was heated to 305 °C and maintained for 1 h. The resulting core/shell UCNPs were precipitated out by the addition of ethanol, collected by centrifugation, washed with a mixture of cyclohexane and absolute ethanol solvent several times, and redispersed in 4 mL chloroform.Synthesis of NaYF4:0.5%Er@NaGdF4:2%Yb@NaYF4:1%Er UCNPs
The procedure for preparing core/shell/shell UCNPs is the same as that for core/shell. The as-prepared core/shell UCNPs in 4 mL chloroform are used as seeds to induce subsequent epitaxial growth of an additional shell provided by ErCl3·6H2O ((0.01 × 0.8 mmol) and YCl3·6H2O (0.99 × 0.8 mmol). The resulting core/shell/shell UCNPs were dispersed in 4 mL of chloroform before characterization.Characterization
The phase structure of the as-prepared UCNPs was investigated using an X-ray diffractometer (XRD; Bruker D8 Advance, LynxEye detector; Rigaku SmartLap, Japan) under Cu Kα radiation (λ = 1.5406 Å) performing at 40 kV and 40 mA. The morphology and size of the as-prepared UCNPs were characterized by applying a transmission electron microscope (TEM; Hitachi HT7700 Exalens, Tokyo Japan) and scanning electron microscope (SEM; Hitachi SU8010, Tokyo, Japan). The photoluminescence quantum yield of the as-prepared UCNPs was measured using the visible-near-infrared absolute photoluminescence quantum yield test system (PLQY; C9920-02, Hamamatsu Japan). UC emission spectra were measured by employing an Aurora4000 spectrofluorometer under excitation by 850 and 1550 nm laser sources. The 850 nm laser power is maintained at 0.2 W, and the 1550 nm laser power is maintained at 0.2–2.23 W. All spectral measurements were performed at room temperature.Results and discussion
A novel strategy of designed core/shell/shell nanostructure was employed to enhance the TSTF UCL intensities and contrast, as shown in Fig. S1 (ESI†). Er3+-doped hexagonal NaYF4 was chosen as the host lattice of the luminescent core and outer luminescent shell because of its low phonon energies (<400 cm−1), low non-radiative decay rates and high radiative emission rates, which can provide high UC efficiency.61 Yb3+-doped hexagonal NaGdF4 was chosen as the host lattice of the inner active shell for the following reasons: (a) confirmation of the successful formation of core/shell/shell structure easily. Owing to the difference in the atomic number between Gd3+ (Z = 64) and Y3+ (Z = 39), high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) can be adopted to identify bright inner shells using image contrast. Furthermore, Gd3+ ions can be detected using the energy dispersive spectrometer (EDS) and the electron energy loss spectrum (EELS) line scan to confirm the successful formation of core/shell/shell structure. (b) Decreasing the average crystallite size of core/shell UCNPs contributes to outer shell cladding, and promoting the phase transition from cubic to hexagonal enhances UCL intensities.62 To illustrate that the choice of NaGdF4 as the host lattice of the inner shell could improve UCL intensities, the UC emission spectra of NaYF4:Er@NaGdF4:Yb@NaYF4:Er and NaYF4:Er@NaYF4:Yb@NaYF4:Er UCNPs under 850 & 1550 nm and 1550 nm excitation were measured in Fig. S2 (ESI†). Compared with NaYF4:Er@NaYF4:Yb@NaYF4:Er, the UCL integral intensities of NaYF4:Er@NaGdF4:Yb@NaYF4:Er are greater owing to its purer hexagonal phase, which agrees with the result reported in the literature.62Fig. 3 shows the crystal phase of the as-prepared core, core/shell and core/shell/shell UCNPs by applying XRD. All the XRD patterns of these samples match well with the standard JCPDS No. 16-0334 pattern of β-NaYF4 and JCPDS No. 27-0699 pattern of β-NaGdF4, and no significant impurity peaks were detected, indicating that all samples belong to a hexagonal-phase structure.
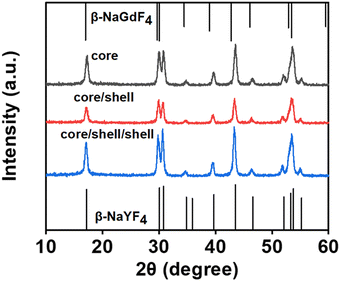 | ||
| Fig. 3 XRD patterns of NaYF4:0.5%Er, NaYF4:0.5%Er@NaGdF4:2%Yb, NaYF4:0.5%Er@NaGdF4:2%Yb@NaYF4:1%Er UCNPs and standard date of β-NaYF4 (JCPDS No. 16-0334) and β-NaGdF4 (JCPDS No. 27-0699). | ||
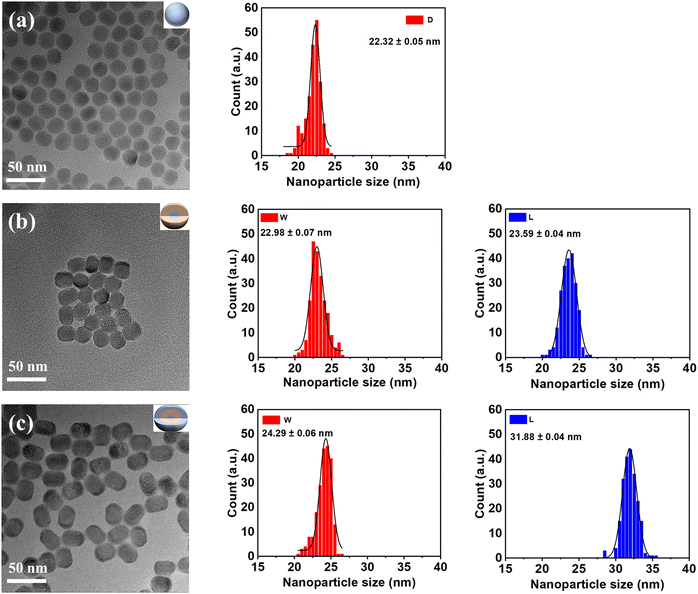
Fig. 4 shows the morphology and particle size of the as-prepared UCNPs by employing TEM. In Fig. 4a, NaYF4:0.5%Er UCNPs have a quasi-sphere morphology. These core UCNPs are monodisperse and uniform with a mean size of 22.32 ± 0.05 nm. In Fig. 4b, the core/shell UCNPs show a uniform distribution with a short rod-like shape with an average width of 22.98 ± 0.07 nm and a length of 23.59 ± 0.04 nm. As illustrated in Fig. 4c, the rod-like shape of the core/shell/shell UCNPs dispersed well and possessed a uniform width of 24.29 ± 0.06 nm and a length of 31.88 ± 0.04 nm. The increasing size of the above UCNPs indicates that NaGdF4:Yb and NaYF4:Er shells were possibly covered on the surface of NaYF4:1%Er UCNPs. Similar results regarding the shape of the core, core/shell and core/shell/shell UCNPs were also verified by applying SEM (Fig. S3, ESI†).
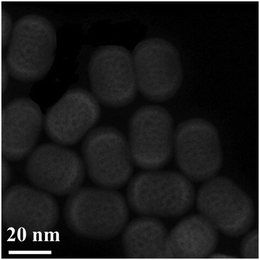
Considering the difference in atomic numbers between Y3+ (Z = 39) and Gd3+ (Z = 64) in NaYF4@NaGdF4@NaYF4 nanostructure, HAADF-STEM was used to identify the core, core/shell and core/shell/shell by image contrast. As shown in Fig. 5, compared with NaYF4, a bright NaGdF4 shell can be clearly observed, which demonstrates the successful formation of core/shell/shell nanostructure. The core/shell/shell UCNPs were analyzed by applying EDS and EELS line scans to confirm their compositions (Fig. S4, ESI†). The detected Gd3+ ions could also provide solid proof of this core/shell/shell nanostructure.
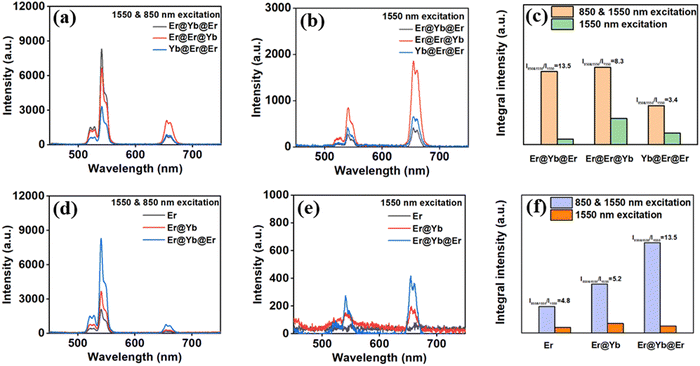
As depicted in Fig. 6, the UC emission spectra under 1550 nm and simultaneous 850 & 1550 nm excitation were shown, which was measured to compare UCL intensities (I) and contrast of as-prepared UCNPs. Here, the contrast is defined by I850&1550/I1550, where I850&1550 and I1550 represent the UCL integral intensities (from 500 to 700 nm). While maintaining the same doping concentration of Yb3+ and Er3+ ions and changing the distribution position of doping ions in different layers, we found that the UCL intensities I850&1550 and I1550 of these core/shell/shell UCNPs varied significantly (Fig. 6a and b). The TSTF UCL integral intensities I850&1550 are described as follows: Er@Er@Yb > Er@Yb@Er > Yb@Er@Er. The corresponding contrast of the above UCNPs is about 8.3, 13.5 and 3.4.
The possible reasons for the differences in the UCL intensities and contrasts of the aforementioned UCNPs are as follows. According to FRET theory, within a certain distance, the energy transfer efficiency from the luminescent center to the surface defect is inversely related to the distance between each other, namely the farther the distance is from the luminescent center to the surface defect, the less energy loss and the higher UCL intensity. Compared with the other two UCNPs, in Er@Er@Yb UCNPs, the distance from Er3+ ions located in the core and inner shell to the surface defect is the farthest, and the TSTF UCL intensity is the highest. In Yb@Er@Er UCNPs, the distance from Er3+ ions located in the inner and outer shell to the surface defect is the closest, and the TSTF UCL intensity is the weakest. At 1550 nm excitation, the single-frequency UCL of Er@Yb@Er UCNPs is mainly generated by NaYF4:0.5%Er core UCNPs, which caused a weak UCL intensity. However, the single-frequency UCL of Er@Er@Yb UCNPs is mainly generated by Er3+ ions in the core and inner shell, which caused a strong UCL intensity, but a decreased contrast.
The best UCL performances appeared in NaYF4:Er@NaGdF4:Yb@NaYF4:Er (Fig. 6c). By optimizing the doping ratio of Er3+ and Yb3+ ions in the above nanostructure, as shown in Fig. S5 (ESI†), UCNPs with 0.5 mol% Er3+ in the core, 2 mol% Yb3+ in the inner shell, and 1 mol% Er3+ in the outer shell were obtained, which exhibits the highest emission intensity and contrast (Fig. 6d and e). In the case of excitation at 850 & 1550 nm, the UCL intensity and contrast of NaYF4:0.5%Er@NaGdF4:2%Yb@NaYF4:1%Er UCNPs were about 3.4 and 2.8 times higher than those of NaYF4:0.5%Er, respectively (Fig. 6f). These results show that the designed core/shell/shell nanostructure significantly enhances TSTF UCL intensities and contrast.
To describe the good performance of the core/shell/shell nanostructure that we designed, a series of measurements were carried out. First, the emission spectra of NaYF4:Er and NaYF4:Yb,Er UCNPs were measured to compare their TSTF UCL intensities and contrasts. As shown in Fig. S6a–c (ESI†), with an increase in Yb3+ ion concentration, the UCNPs exhibited higher UCL intensity and lower contrast. In addition, the calculated quantum yields (QY) of core, core/shell and core/shell/shell UCNPs under 1550 nm excitation are 1.73 × 10−8, 1.11 × 10−7 and 4.58 × 10−8, respectively. The changed QY of the as-prepared UCNPs corresponds to the designed nanostructure. With NaGdF4:Yb shell coated, the enhanced UCL intensity in core/shell UCNPs maybe due to the reduction of surface defects and the improvement of absorption at 1550 nm. After NaYF4:Er shell coating, the enhanced UCL contrast in core/shell/shell UCNPs is mainly caused by improving 1550 nm absorption. Finally, we measured the UCL spectra of NaYF4:Er@NaGdF4:Yb@NaYF4:Er and NaYF4:Er@NaGdF4:Yb@NaYF4 UCNPs (Fig. S6d and e, ESI†). The result shows that inactive NaYF4 shell coated onto the NaYF4:Er@NaGdF4:Yb core/shell UCNPs could improve the UCL performance, and the designed nanostructure exhibits better performance. Designing the sensitizer and activator ions in separate layers obtained enhanced UCL intensity and improved contrast.
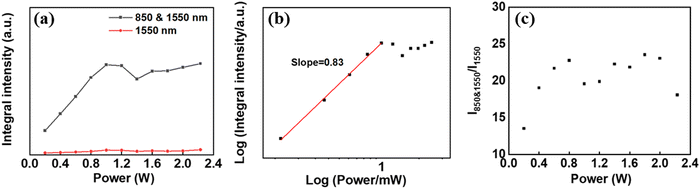
Under the excitation of an 850 nm laser, no single-frequency UCL was detected in the as-prepared UCNPs. Fixing the 850 nm excitation power at 0.2 W, the 1550 nm laser excitation power gradually increased from 0.2 W to 2.23 W. The TSTF UCL spectra were measured at different 1550 nm excitation powers (Fig. S7a–c, ESI†). As demonstrated in Fig. 7a, it could be observed that the UCL integral intensities I850&1550 of Er@Yb@Er UCNPs were enhanced with increasing pump power (P, 0.2–1 W). In addition, the UCL contrast was also enhanced with increasing pump power (0.2–0.8 W) (Fig. 7c). The result of I ∝ P0.83 suggests that the emissions under 1550 nm excitation were a single-photon process when the 850 nm excitation power was fixed (Fig. 7b). Therefore, the UC emission was a two-photon process at the excitation of 850 & 1550 nm.
Fig. 8 illustrates the possible energy transfer process of Er@Yb@Er UCNPs excited simultaneously by 850 & 1550 nm. There are two possible pathways for UC emission enhancement; in the pathway ①, Er3+ ions distributed in the core and outer shell are first promoted from the 4I15/2 state to 4I13/2 state by the 1550 nm excitation, then pumped to 4S3/2 state by 850 nm excitation, and finally returned to state 4I15/2, emitting green TSTF UCL. Additionally, the introduction of the inner shell reduces the surface defect concentration, decreases the energy transfer probability from Er3+ to surface defects and enhances the UCL intensity. In pathway ②, Yb3+ ions doped in the inner shell played a significant role in promoting the absorption of 1550 nm excitation. The new processes could be described as 4I11/2 (Er3+) + 2F7/2 (Yb3+) → 4I15/2 (Er3+) + 2F5/2 (Yb3+), 4I15/2 (Er3+) + 2F5/2 (Yb3+) → 4I11/2 (Er3+) + 2F7/2 (Yb3+), 4I11/2 (Er3+) → 4I13/2 (Er3+). These Er3+ → Yb3+ → Er3+ energy transfer processes increase the population of 4I13/2 (Er3+) level, thereby harvesting the absorption of 1550 nm.
 | ||
| Fig. 8 Schematic illustration of possible energy transfer processes in the core/shell/shell nanostructure. | ||
The emission time–decay curves of 542 nm UC emission for the C, C@S and C@S@S UCNPs were measured at the excitation of 1550 nm. (Fig. S8, ESI†). The corresponding lifetimes of possible energy transfer processes ① and ② are τ1 and τ2, respectively. With NaGdF4:Yb and NaYF4:Er shells covered, the lifetime τ1 and τ2 of 542 nm UC emission increased, which was mainly due to the reduction of energy transfer between Er3+ ions and surface defects. The doped Yb3+ ions harvest the absorption of 1550 nm (Fig. S9, ESI†). The results agreed well with the assumed transition processes.
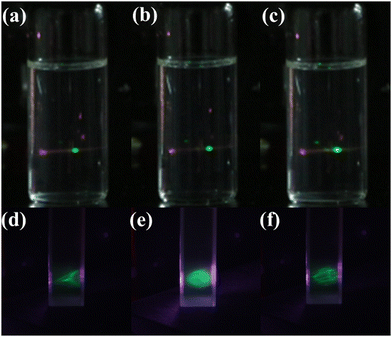
Fig. 9a, b and c show the TSTF UCL photographs of core/shell/shell UCNPs solution. Excited by 850 & 1550 nm, the green TSTF UC emission can be detected in NaYF4:0.5%Er UCNPs (Fig. 9a). After the NaGdF4:Yb shell is covered, the NaYF4:0.5%Er@NaGdF4:2%Yb UCNPs possess a higher UC emission (Fig. 9b). Inspiringly, the strongest UCL was observed in resulting NaYF4:0.5%Er@NaGdF4:2%Yb@NaYF4:1%Er UCNPs (Fig. 9c). The enhanced UCL of these UCNPs is consistent with their emission spectra shown in Fig. 6d, significantly supporting the strategy of enhancing TSTF UCL by core/shell/shell nanostructure.
For further application in a 3-D display, a confirmatory experiment was carried out. As shown in Fig. 9d–f, the triangular pyramid, sphere and hollow dodecahedron 3-D images with high resolution were successfully displayed in the as-prepared core/shell/shell UCNPs solution. Moreover, under the same experimental conditions, the Er@Yb@Er and Er@Er@Yb UCNPs with different contrasts were used to display the same 3-D cube (Fig. S10, ESI†). Compared with Er@Er@Yb UCNPs, the higher contrast Er@Yb@Er UCNPs had a better display effect, reasonably. The above 3-D images demonstrate the great prospects of core/shell/shell UCNPs in the volumetric 3-D display.
Conclusions
In conclusion, a designed core/shell/shell (Er@Yb@Er) nanostructure was employed to enhance TSTF UCL performance. After optimizing the doping ratio of Er3+ and Yb3+ ions in this nanostructure, UCNPs with 0.5 mol% Er3+ in the core, 2 mol% Yb3+ in the inner shell and 1 mol% Er3+ in the outer shell exhibited enhanced TSTF UCL performance. In the case of excitation at 850 & 1550 nm, the UCL intensity and contrast of core/shell/shell UCNPs were about 3.4 and 2.8 times higher than those of core UCNPs. This designed nanostructure protects the UCNPs from surface quenching and reduces the concentration quenching of Er3+ ions. In particular, the doped Yb3+ ions play a promoting role in raising the absorption of 1550 nm. This designed core/shell/shell nanostructure could provide new insights into enhancing TSTF UCL performance and could offer potential applications in the volumetric 3-D display.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (11704003).References
- C. Fuchtbauer, Z. Phys., 1920, 21, 635–638 CAS.
- R. W. Wood, Proc. R. Soc. London, 1924, 106, 679–694 CAS.
- D. R. Gamelin and H. U. Gudel, Top. Curr. Chem., 2001, 214, 1–56 CrossRef CAS.
- K. Langhans, C. Guill, E. Rieper, K. Oltmann and D. Bahr, Proc. SPIE, 2003, 5006, 161–174 CrossRef.
- J. D. Lewis, C. M. Verber and R. B. McGhee, IEEE Trans. Electron Devices, 1971, 18, 724–732 Search PubMed.
- E. Downing, L. Hesselink, J. Ralston and R. Macfalane, Science, 1996, 273, 1185–1189 CrossRef CAS.
- M. R. Brown and G. S. Waters, US Pat., US3474248A, 1969 Search PubMed.
- X. Chen and Z. Song, Sci. China, Ser. G: Phys., Mech. Astron., 2006, 49, 169–177 CrossRef CAS.
- H. H. Refai, J. Disp. Technol., 2009, 5, 391–397 Search PubMed.
- H. H. Refai, J. Soc. Inf. Disp., 2010, 18, 1065–1070 CrossRef.
- J. Dou and Y. Hou, Chin. J. Lumin., 2008, 29, 85–88 CAS.
- D. C. Nguyen, G. E. Faulkner and M. Dulick, Appl. Opt., 1989, 28, 3553–3555 CrossRef CAS PubMed.
- A. C. Tropper, J. N. Carter, R. Lauder, R. D. T. Lauder, D. C. Hanna, S. T. Davey and D. Szebesta, J. Opt. Soc. Am. B, 1994, 11, 886–893 CrossRef CAS.
- O. Henderson-Sapir, J. Munch and D. J. Ottaway, Opt. Lett., 2014, 39, 493–496 CrossRef PubMed.
- J.-C. G. Bünzli, Chem. Rev., 2010, 110, 2729–2755 CrossRef PubMed.
- Y. Chen, W. He, Y. Jiao, H. Wang, X. Hao, J. Lu and S. Yang, J. Lumin., 2012, 132, 2247–2250 CrossRef CAS.
- J. Zhou, J. Deng, H. Zhu, X. Chen, Y. Teng, H. Jia, S. Xu and J. Qiu, J. Mater. Chem. C, 2013, 1, 8023–8027 RSC.
- Y. Liu, Y. Lu, X. Yang, X. Zheng, S. Wen, F. Wang, X. Vidal, J. Zhao, D. Liu, Z. Zhou, C. Ma, J. Zhou, J. A. Piper, P. Xi and D. Jin, Nature, 2017, 543, 229–233 CrossRef CAS PubMed.
- A. C. Traub, Appl. Opt., 1967, 6, 1085–1087 CrossRef CAS PubMed.
- D. Perkins, Space/Aeronaut., 1963, 64–67 Search PubMed.
- J. L. Coddington and R. J. Schipper, SPIE MILESTONE SERIES MS, 2001, vol. 162, pp. 350–357 Search PubMed.
- A. W. Lohmann and D. Paris, Appl. Opt., 1967, 6, 1739–1748 CrossRef CAS PubMed.
- B. Lee, Phys. Today, 2013, 66, 36 CrossRef CAS.
- R. Zito Jr, J. Appl. Phys., 1963, 34, 1535–1543 CrossRef.
- R. Barnes, C. Moeller, J. Kircher and C. Verber, Appl. Phys. Lett., 1974, 24, 610–612 CrossRef CAS.
- E. A. Downing and B. Torres, US Pat., US4870485A, 1989 Search PubMed.
- I. I. Kim, E. Korevaar and H. Hakakha, Proc. SPIE., 1996, 2650, 274–284 CrossRef CAS.
- K. Langhans, C. Guill, E. Rieper, K. Oltmann and D. Bahr, Proc. SPIE, 2003, 161–174 CrossRef.
- J. Allain, M. Monerie and H. Poignant, Electron. Lett., 1991, 27, 189–191 CrossRef.
- M. A. Dugay, J. A. Giordmaine and P. M. Rentzepis, US Pat., US3541542, 1970 Search PubMed.
- X. Chen, Y. Feng, G. Zhang, M. Li, K. Li, F. Song, S. Bi, M. Shang, Z. Song, Y. Sun, S. Feng, J. Xiong, C. He, X. Liu and Z. Zheng, Proc. SPIE., 1998, 3560, 122–131 CrossRef CAS.
- X. Chen and Z. Song, Sci. China, Ser. G: Phys., Mech. Astron., 2006, 49, 169–177 CrossRef CAS.
- J. Dou and Y. Hou, Chinese J. Lumin., 2008, 29, 85–88 CAS.
- J. H. Cho, M. Bass and H. P. Jenssen, J. Soc. Inf. Disp., 2007, 15, 1029–1036 CrossRef CAS.
- M. Bass and H. Jennsen, US Pat., US06501590B2, 2001 Search PubMed.
- R. Deng, F. Qin, R. Chen, W. Huang, M. Hong and X. Liu, Nat. Nanotechnol., 2015, 10, 237–242 CrossRef CAS PubMed.
- P. Chen, S. Yu, B. Xu, J. Wang, X. Sang, X. Liu and J. Qiu, Mater. Lett., 2014, 128, 299–302 CrossRef CAS.
- Y. Li, Z. Li, L. Guo, B. Yang and T. Li, J. Alloys Compd., 2020, 847, 156399 CrossRef CAS.
- Y. Li, L. Guo and B. Yang, Dalton Trans., 2021, 50, 2112–2122 RSC.
- T. J. de Prinse, A. Karami, J. E. Moffatt, T. B. Payten, G. Tsiminis, L. Da Silva Teixeira, J. Bi, T. W. Kee, E. Klantsataya, C. J. Sumby and N. A. Spooner, Adv. Opt. Mater., 2021, 9, 2001903 CrossRef CAS.
- X. Zhao, Z. Wu, Z. Yang, X. Yang, Y. Zhang, M. Yuan, K. Han, C. Song, Z. Jiang, H. Wang, S. Li and X. Xu, Nanomaterials, 2020, 10, 1475 CrossRef CAS PubMed.
- M. Tan, M.-J. Monks, D. Huang, Y. Meng, X. Chen, Y. Zhou, S.-F. Lim, C. Würth, U. Resch-Genger and G. Chen, Nanoscale, 2020, 12, 10592–10599 RSC.
- M. Quintanilla, E. Hemmer, J. Marques-Hueso, S. Rohani, G. Lucchini, M. Wang, R. R. Zamani, V. Roddatis, A. Speghini, B. S. Richards and F. Vetrone, Nanoscale, 2022, 14, 1492–1504 RSC.
- Y. Zhang, X. Zhu and Y. Zhang, ACS Nano, 2021, 15, 3709–3735 CrossRef CAS PubMed.
- B. Zhou, L. Yan, J. Huang, X. Liu, L. Tao and Q. Zhang, Nat. Photonics, 2020, 14, 760–766 CrossRef CAS.
- R. A. Janjua, O. Iqbal, M. A. Ahmed, A. A. Al-Kahtani, S. Saeed, M. Imran and A. G. Wattoo, RSC Adv., 2021, 11, 20746–20751 RSC.
- X. Qin and X. Liu, Nanoscale, 2021, 13, 19561–19567 RSC.
- X. Zhu, H. Zhang and F. Zhang, ACS Mater. Lett., 2022, 4(9), 1815–1830 CrossRef CAS.
- N. Kang, J. Zhao, Y. Zhou, C. Ai, X. Wang and L. Ren, Nanotechnology, 2019, 30, 105701 CrossRef CAS PubMed.
- F. Zhang, R. Che, X. Li, C. Yao, J. Yang, D. Shen, P. Hu, W. Li and D. Zhao, Nano Lett., 2012, 12, 2852–2858 CrossRef CAS PubMed.
- B. Chen and F. Wang, Trends Chem., 2020, 2, 427–439 CrossRef CAS.
- N. Dubey and S. Chandra, J. Rare Earths, 2022, 40, 1343–1359 CrossRef CAS.
- B. Zheng, J. Fan, B. Chen, X. Qin, J. Wang, F. Wang, R. Deng and X. Liu, Chem. Rev., 2022, 122, 5519–5603 CrossRef CAS PubMed.
- B. Zhou, B. Tang, C. Zhang, C. Qin, Z. Gu, Y. Ma, T. Zhai and J. Yao, Nat. Commun., 2020, 11, 1174 CrossRef CAS PubMed.
- P. Huang, W. Zheng, S. Zhou, D. Tu, Z. Chen, H. Zhu, R. Li, E. Ma, M. Huang and X. Chen, Angew. Chem., Int. Ed., 2014, 53, 1252–1257 CrossRef CAS PubMed.
- M. G. Ding, D. Q. Chen, D. Y. Ma, J. N. Dai, Y. T. Lia and Z. G. Jia, J. Mater. Chem. C, 2016, 4, 2432–2437 RSC.
- D. Li, S. Wen, M. Kong, Y. Liu, W. Hu, B. Shi, X. Shi and D. Jin, Anal. Chem., 2020, 92, 10913–10919 CrossRef CAS PubMed.
- Y. Zhou, Y. Cheng, J. Xu, H. Lin and Y. Wang, Nanoscale, 2021, 13, 6569–6576 RSC.
- C. Lin, Z. Xia, L. Zhang, X. Chen, Q. Sun, M. Lu, Z. Yuan, X. Xie and L. Huang, ACS Appl. Mater. Interfaces, 2020, 12, 31783–31792 CrossRef CAS PubMed.
- S. Wen, D. Li, Y. Liu, C. Chen, F. Wang, J. Zhou, G. Bao, L. Zhang and D. Jin, J. Phys. Chem. Lett., 2022, 13, 5316–5323 CrossRef CAS PubMed.
- D. Chen, P. Huang, Y. Yu, F. Huang, A. Yang and Y. Wang, Chem. Commun., 2011, 47, 5801–5803 RSC.
- F. Wang, Y. Han, C. Lim, Y. Lu, J. Wang, J. Xu, H. Chen, C. Zhang, M. Hong and X. Li, Nature, 2010, 463, 1061–1065 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ma01040b |
| This journal is © The Royal Society of Chemistry 2023 |