Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Aerosol-assisted chemical vapour deposition of highly efficient mixed anatase-rutile TiO2 for photoelectrochemical water splitting†
Thom R.
Harris-Lee
 ab,
Enrico
Della Gaspera
ab,
Enrico
Della Gaspera
 c,
Frank
Marken
c,
Frank
Marken
 a,
Jie
Zhang
a,
Jie
Zhang
 b,
Cameron L.
Bentley
b,
Cameron L.
Bentley
 b and
Andrew L.
Johnson
b and
Andrew L.
Johnson
 *a
*a
aDepartment of Chemistry, University of Bath, Claverton Down, Bath, BA2 7AY, UK. E-mail: a.l.hohnson@bath.ac.uk
bSchool of Chemistry, Monash University, Clayton, Vic 3800, Australia
cSchool of Science, RMIT University, Melbourne, VIC 3000, Australia
First published on 1st August 2023
Abstract
Aerosol-assisted chemical vapour deposition (AACVD) has been used in conjunction with new and bespoke precursors for the fabrication of highly nanostructured, mixed anatase-rutile phase TiO2. Precursor syntheses were carried out in two steps, reacting tetrakis(dimethylamino)titanium with a triethanolamine-based ligand complex, followed by reaction with the relevant dry solvent. Suitability for AACVD was assessed by thermogravimetric analysis, and the resulting TiO2 films were characterised by SEM, EDX, Raman, and PXRD, which was used to find relative ratios of anatase and rutile phase in the mixed-system, and respective grain sizes. The resulting TiO2 films show exceptional OH− oxidation performance under simulated solar irradiation, yielding photocurrent densities of 1.06 mA cm−2 at 1.23 V vs. RHE, and a peak incident photon conversion efficiency of 95.6% at 380 nm incident light, indicating their promise for use in photoanodes for water splitting application.
Introduction
Photoelectrochemical (PEC) water splitting is a promising method for efficient green hydrogen production, with most research focused on the rate limiting oxygen evolution reaction (OER) at the photoanode.1 One standout material for use in photoanodes is titanium dioxide (TiO2), owing to its low cost, non-toxicity, high photostability, and high catalytic activity with proper band edge positioning, however it is limited by its large bandgap (3–3.2 eV) and rapid recombination rate.2 It is therefore likely that TiO2 will be a key component in any future commercial PEC device, however it will need to be used in conjunction with other semiconductor layers and/or metal hybrids to overcome its limitations.3Crystalline TiO2 can exist in two photochemically active phases, anatase and rutile, with bandgaps of 3.2 and 3.0 eV respectively. While rutile is the more thermodynamically favoured structure, and possesses the narrowest bandgap, anatase is considered the more photochemically active form.4 In fact, the best performing un-doped TiO2 photoanodes have been reported to consist of both anatase and rutile phases, combining the best attributes of each material (increased photon absorption by virtue of the narrower rutile bandgap and an enhanced photochemical activity from anatase phase material). These features are further enhanced with the formation of a type II heterojunction, greatly improving charge separation, and reducing recombination.5
Aerosol-assisted chemical vapour deposition (AACVD) is a scalable and commonly used method for the fabrication of thin films for a diverse array of inorganic materials. In AACVD-based processes, chemical precursors need not be volatile, but merely soluble in any solvent from which the aerosol can be generated. As the requirement of volatility and thermal stability of precursors become less stringent, it is possible to produce high-quality CVD products at a lower cost. Compared to the conventional CVD method, AACVD presents some key advantages: (a) wider choice of precursors, (b) simplification of the delivery process with higher deposition rates, (c) flexible deposition parameters, (d) simplified production of multicomponent thin films with precise stoichiometric control. As such, AACVD lends itself to the use of chemically and thermally robust precursors which may be key to creating a commercially competitive device.6
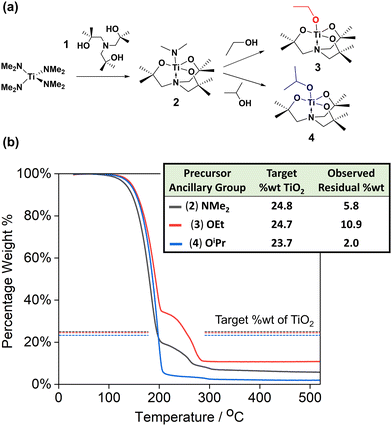
Herein, three new TiO2 precursors (Fig. 1a) have been designed and synthesised for use in AACVD, focusing on molecular structure that allows for (i) good solubility in ‘inert’ solvents such as toluene, (ii) controllable and clean thermal decomposition pathways at practical temperatures, (iii) deposition of high surface area morphologies, (iv) adaptable ligand structures applicable to alternative metal centres to produce compatible metal oxide precursors. The molecular design also makes them more user-friendly and easier to handle than alternative purchasable TiO2 precursors. Additionally, the resulting TiO2 thin films are shown to contain mixed anatase-rutile phase, likely to be a significant influence towards the exceptional PEC performances measured for the TiO2 films, and a rare property to obtain from a single-step deposition.
Results and discussions
The triethanolamine ligand (1), synthesised using a method adapted from Mun et al.,1 and similar to work by Sharma et al.,2 has been used to produce three different TiO2 precursors by reaction of the pro-ligand 1 with Ti(NMe2)4 to yield the Ti(IV) amide complex 2 (Fig. 1a). Subsequent reaction of the monoamide complex 2 with one molar equivalent of either anhydrous ethanol or anhydrous isopropanol resulted in the formation of the Ti(IV) alkoxide complexes 3 and 4 respectively. During the course of our investigations, the bridged-oxo complex, 5, [{N(CH2CMe2)3Ti}2(μ-O)] was also isolated. Complexes 2–5 have been fully characterised by single crystal X-ray diffractometry and 1H and 13C{1H} NMR spectroscopy (see ESI,† Sections S2 and S6).Thermal characterisation of compounds 2, 3, and 4 was carried out using thermogravimetric analysis (TGA) (Fig. 1b). The onset decomposition temperatures (temperature after 1% mass loss) and end temperatures (after completion of a decomposition event) were recorded (Table S2, ESI†), as well as the TiO2 target and experimental residual weight percentages (wt%). All three compounds showed lower residual wt% than expected for conversion to TiO2, indicating a degree of volatility. However, it is interesting to note that the TGA plots of compounds 2 and 3 show secondary mass loss events starting at 210 and 227 °C respectively. This likely indicates both decomposition and volatilisation of the initial precursors are occurring simultaneously at temperatures below 210 (or 227) °C, while at higher temperatures only decomposition occurs. The final wt% of both 2 and 3 are above 0%, but significantly lower than the target residual mass corresponding to TiO2, suggesting some by-product may be produced that has a vaporization (and decomposition) temperature greater than 520 °C. The final wt% for compound 4 (2.0%) is significantly lower than that expected for the formation of TiO2 (23.7%), indicating a high degree of volatility. Based on TGA, all 3 compounds show suitability as AACVD precursors, however due to their increased air and moisture stability (Fig. S1, ESI†), complexes 3 and 4 were deemed to be the most promising precursors, and as such were used further in the AACVD of TiO2 thin films.
For clarity of reading, thin films are denoted as TiO2-X-Y, where X is the compound used as a precursor and Y is the deposition condition changed compared to the ‘standard deposition procedure’; a deposition temperature of 450 °C, precursor concentration of 100 mM, and deposition time of 120 min. For example, a TiO2 film deposited using 3 at 500 °C and otherwise standard conditions would be denoted as TiO2-3-500 °C, while the film obtained using ‘standard deposition procedure’ is denoted TiO2-3 (refer to Table S1, ESI† for the full sample list).
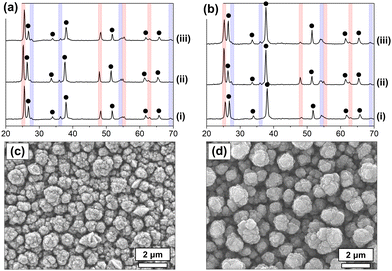
X-ray diffraction (XRD) patterns of TiO2 thin films deposited using compounds 3 (Fig. 2a and Fig. S4a, b, ESI†) and 4 (Fig. 2b and Fig. S4c, d, ESI†) show a mixture of anatase and rutile phases, despite the single-step deposition process. This is promising for the application of these films in PEC devices as TiO2 has been shown to have enhanced performance when both phases are present, as previously discussed. Anatase diffraction peaks are seen at approximately 2θ 25.3, 48.0, 55.1 and 62.8°, corresponding to (101), (200), (211) and (204) diffraction planes respectively, the (101) plane being the most intense, followed by (200). Rutile diffraction peaks are located at 2θ 27.4, 36.1, 54.3 and 69.0°, indexed to (110), (101), (211), (301) planes respectively, the (101) peak being the most intense, followed by (110).
Scanning electron microscopy (SEM) images (Fig. 2c and d and Fig. S2, ESI†) revealed all samples across both precursors had a similarly shaped nanostructure of tree-like rods extending perpendicular to the substrate surface. For compound 3, there was no variation in rod size with precursor concentration; both TiO2-3 and TiO2-3-80 mM contained rods with 0.7–1.1 μm diameter. Conversely, TiO2-4 and TiO2-4-80 mM consisted of rods with diameters of 1.0–1.3 μm and 0.5–0.9 μm respectively, evidencing the difference in deposition kinetics/pathway between the two precursors despite their similar molecular structures. All films were found to pass the ‘Scotch tape test’, and be strongly bound to the substrate, showing no obvious removal or damage.
Texture coefficients of the observed planes in the XRD spectra for both anatase and rutile, Table 1, were found using the Harris method3 and powder diffraction standards for anatase (ICDD-JCPDS 75-1537) and rutile (ICDD-JCPDS 75-1748). The (101) XRD peak intensities for anatase phase showed no significant orientation preference compared to standard powder TiO2, as indicated by texture coefficients shown in Table 1. The XRD (101) integrated peak area was therefore used to calculate anatase/rutile phase ratios. Volume average mean grain sizes were calculated (Fig. 3) using the most intense anatase and rutile peaks, (101), according to the equations:
| XA = [1 + K(IR/IA)]−1; XR = [1 − XA], | (1) |
τ = [κλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ] θ] | (2) |
| Anatase | TC(101) | TC(200) | TC(211) | TC(204) |
|---|---|---|---|---|
| TiO2-3-80 | 0.96 | 1.04 | 0.65 | 0.85 |
| TiO2-4 | 1.07 | 0.93 | 0.78 | 1.05 |
| Rutile | TC(110) | TC(101) | TC(211) | TC(301) |
| TiO2-3-80 | 0.41 | 1.29 | 1.00 | 1.42 |
| TiO2-4 | 0.62 | 0.74 | 1.43 | 1.21 |
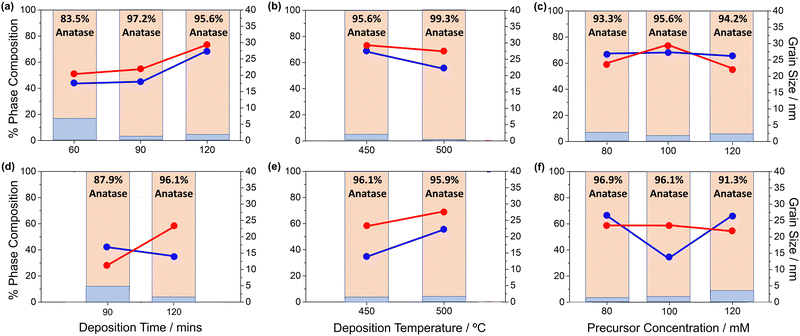
Fig. 3a illustrates that increasing the deposition time using complex 3 results in a larger grain size, minorly increasing for both anatase and rutile crystallites from 60 to 90 minutes, followed by a significant increase from 90 to 120 minute deposition. Unexpectedly, an increased deposition temperature of 500 °C showed smaller grain sizes for both phases (Fig. 3b), and a greater anatase percentage presence despite its metastability, usually transforming to rutile at high temperatures.10 Except for the anatase grain size using 100 mM precursor, 3 showed little variation in grain sizes or phase composition as a function of precursor concentration (Fig. 3c).
Compound 4 showed significantly increase anatase phase fraction and grain size with increased deposition time (Fig. 3d). The rutile grain size calculated after a 60-minute deposition has been omitted due to its anomalous value (79.91 nm).
Increasing the deposition temperature (Fig. 3e) resulted in little change to anatase phase percent, contrasting to the relationship seen for compound 3, however the crystallite grain sizes for both rutile and anatase phases appear to increase at higher deposition temperatures, consistent with previous observations. As can be seen from Fig. 3f, changes in the precursor concentration have little effect on phase composition of the resulting thin films for 80 mM and 100 mM, however there is an ∼5% increase in rutile percent with 120 mM precursor, interesting as the respective grain sizes remain similar for all, except for the 100 mM rutile grain size. This is unlike the anatase phase, which does not show a similar change in composition.
As previously discussed, anatase-rutile mixed phase TiO2 is consistently reported to improve PEC performance, however the optimum ratio can vary greatly between systems. UV/Vis spectroscopy was used to determine band gaps for TiO2-3-80 and TiO2-4 by Tauc analysis (Fig. S6, ESI†), resulting in a band gap of ∼3.08 eV, close to both anatase (∼3.2 eV) and rutile (∼3.0 eV),4 and consistent with both phases being present in the films. Given how close anatase and rutile band gaps are to one another, it is not expected to see two distinct transitions here. PEC analysis on all TiO2 films developed here was therefore required to determine the optimum AACVD deposition conditions, how grain size and phase fractions impact performance, and compare the precursors in application (Fig. S7 and S8, ESI†).
TiO2-3 showed higher photocurrent density compared to TiO2-3-500 °C, while TiO2-3-400 °C resulted in no visible deposition. It should be noted that these temperatures are not the exact temperature of the substrate due to the cooling effect of the argon and aerosol flow, however it was not possible to monitor the extent of this on the hot-wall AACVD setup.
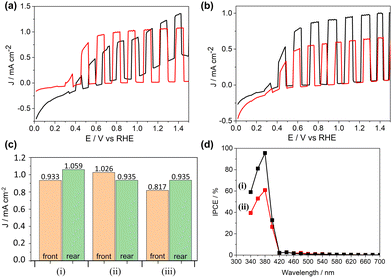
Photocurrent densities of samples using 3 suggest less TiO2 deposition gives enhanced performance, evidenced by TiO2-3-80 mM and TiO2-3-60 min (Fig. 4a and Fig. S7b, ESI†), the lowest precursor concentration and deposition time respectively, displaying the greatest photocurrent densities of all those tested. Interestingly, for both these films, the front and rear side illuminated values are similar, suggesting that there are no limitations due to bulk charge transport within the TiO2 material. Conversely, the photocurrent density of TiO2-3 has a greater rear-side illumination performance than front-side (0.822 and 0.672 mA cm−2 respectively) indicating that electron mobility is limiting the photocurrents in the thicker films only. An area of ongoing work with these electrodes is in cation doping to improve bulk charge transport, enhancing the performance of thicker films to equal or surpass the best performing thinner ones here. Films deposited using compound 3 show clear correlation between increasing photocurrent density and increasing fraction of rutile phase for both precursor concentration (j80 mM > j120 mM > j100 mM; 6.7, 5.8, 4.4% rutile respectively) and deposition time (j60 min > j120 min > j90 min; 16.5, 4.4, 2.8% respectively).
All films deposited using compound 4 had a greater rear-side performance than front, indicating poor bulk charge transport properties. Similarly thin films deposited using 3 at 450 °C were shown to have an improved performance compared to those deposited and 500 °C particularly with respect to rear illumination, however, deposition times of 120 min were superior to those deposited over 90 and 60 min respectively, which showed no visible evidence of deposition.
The differences in deposition between 3 and 4 is highlighted by opposing optimal precursor concentrations, 80 mM showing the best performance for 3, but 100 mM best for 4. The anatase/rutile percentage compositions are similar for both precursors at 100 mM, however the anatase/rutile grain sizes for TiO2-3 are significantly larger than TiO2-4 (29.25/27.44 nm and 23.32/13.69 nm respectively). Additionally, TiO2-4, the highest performing film for 4, possessed both a smaller rutile grain size relative to all other films, but also a large difference between anatase and rutile sizes. This matches previous literature which has reported the benefits of small rutile grain sizes in mixed systems, forming more intimate contact with anatase grains, allowing for more rapid electron injection across the interface, enhancing charge separation, and reducing recombination.5
Linking photocurrent density to anatase/rutile phase composition of the best performing samples (Fig. 4c), TiO2-3-80 mM and TiO2-3-60 min surprisingly had significantly different rutile percentages, 6.7 and 16.5% respectively, while TiO2-4 contained an even lower rutile fraction of 3.9%. Despite its notable impact, it is impossible to predict the phase ratios that will result from an untested deposition of these precursors, nor what the optimum ratio will be for a given precursor.
The onset potential (Von) for TiO2-3-80 mM was 0.05 V more positive than that for TiO2-4, indicating that the charge transport within the sample deposited using precursor 3 had worse charge separation properties, despite its greater photocurrent at 1.23 VRHE.
Incident photon-electron conversion efficiency (IPCE) measurements were carried out to further investigate photoelectronic properties of the films (Fig. 4d). The highest performing samples from 3 and 4, TiO2-3-80 mM and TiO2-4, were measured, with IPCE values peaking at 380 nm (95.6% and 60.9% respectively) in both cases, followed by a rapid drop to negligible values at, and beyond, 420 nm. While the trend matches what is expected for the relatively wide band gap of TiO2, peak conversion efficiencies are significantly higher than the 350 nm peak commonly seen. Reports of TiO2 with similarly high efficiencies have often been further modified post-deposition, either by hydrogen or vacuum annealing,5,6 or by formation of a second TiO2 phase in a separate deposition process to obtain mixed anatase-rutile composition.7
Chronoamperometry over a 3 hour period has been used to determine photoanode stability. The absence of significant performance decrease over this period, as shown in Fig. S9 (ESI†), indicated no discernible change in the sample that would photocurrent drop-off. We therefore conclude that photocurrent densities are a direct consequence of OER from oxidation of OH− species, rather than any potential self-oxidation of the photoanode (e.g. Ti(III) to Ti(IV) species).
A summary of the highest performing TiO2 thin films for PEC application from literature have been outlined in Table 1, alongside the highest performing sample reported here (TiO2-3-80 mM). Uniquely for TiO2 films, the IPCE value for both TiO2-3-80 mM and TiO2-4 peaked at 380 nm, compared to the usual 350 nm peak reported.
The highest performing samples that have been reported previously use TiO2 films that have been annealed in a reducing hydrogen environment. The next best samples commonly consist of mixed anatase-rutile phase TiO2, combining the best properties from each material (smaller rutile bandgap, higher anatase activity), and forming intrinsic heterojunctions to improve charge separation. For further context, while Degussa P25 TiO2 nanoparticles are commercially used in photocatalysis, we are unable to provide a direct comparison with these commercial materials as j and Von metrics for these materials are not readily available.
All samples reported in this article were annealed in air at a relatively low temperature of 550 °C, therefore despite the hydrogen-annealed samples in Table 2 showing higher photocurrent densities, the films outlined here far outperformed those same samples when air annealed instead. The effect of H-annealing, as well as previously studied vacuum annealing,6,14 will be investigated as part of ongoing work on these films, and other metal oxide films developed in a similar manner.
| Synthetic route | TiO2 phase | J /mA cm−2 | IPCEb | V on /VRHE | Additional notes | Ref. |
|---|---|---|---|---|---|---|
| a Photocurrent density measured at 0 V vs. RHE under 1 sun (100 mW cm−2, AM 1.5G) solar simulation. b IPCE measured at 350 nm incident photons, unless stated otherwise. c Photocurrent onset potential, measured versus RHE. | ||||||
| AACVD | 93.3% A, 6.7% R | 1.06 | 70% 95.6% (380 nm peak) | 0.20 | New precursor | Here |
| Anodization | 100% R | H anneal: 2.50 | 98% | 0.10 | H annealed | 8 |
| Air anneal: 0.60 | 2% | 0.15 | ||||
| Nanowire dropcast | 80% A, 20% R | 2.60 | 90% | n/a | H annealed | 9 |
| PP-MOCVD | 91% A, 9% R | 1.20 | 85% | 0.1 | 7 | |
| Hydrothermal | A/R layered | 1.10 | 57% | 0.0 | R rods with A branches | 10 |
| Anodization | 100% A | 1.59 | n/a | 0.25 | 11 | |
| Hydrothermal | 100% R | H anneal: 1.25 | 22.5% | 0.10 | H annealed | 12 |
| Air anneal: 0.65 | 10% | |||||
| AACVD | 100% A | 0.20 | n/a | n/a | 13 | |
| AACVD | 100% A | 0.67 | 100% | 0.05 | New precursor | 4 |
It is interesting that the rutile phase ratio for TiO2-3-80 mM (6.7% rutile) was very similar to that reported by Krumdiek et al.,7 another mixed phase film deposited using a CVD technique. The ability to fabricate competitive films using CVD, particularly AACVD, is vital for scale-up and commercialisation of such devices due to the scalability, versatility, and cost-effectiveness of the technique, especially when deposited in a single deposition step as is achieved here.
Conclusions
In summary, high performance, highly nanostructured, mixed anatase-rutile phase TiO2 films have been produced using new precursors tailor-made for AACVD application in a single-step deposition procedure, outlining the importance of molecular precursor design in the development of photoelectrochemical materials and devices. The differences in performance, phase composition, grain sizes, and photoelectrochemical properties between the films deposited using different precursors evidenced the significant impact of small structural differences in precursor molecular design. These precursors (3 and 4) both had the valuable property of depositing mixed phase anatase-rutile films in a single deposition step, enhancing both PEC performance, and scale-up and commercial viability. This is important work in the development of a ‘toolbox’ of bespoke metal oxide precursors systematically designed for AACVD that can be used to produce high performance metal oxide films and are also cross-compatible for the fabrication of mixed-metal and doped photoelectrodes in single-step depositions.Experimental
Full experimental details for the synthesis and characterisation of the pro-ligand, 1, as well as the Ti(IV) complexes 2, 3, 4 and 5 are included in the electronic supplementary information associated with this manuscript. Characterisation data includes 1H and 13C NMR data for 1–5 and single-crystal X-ray data for complexes 2–5. Experimental details relating to the single-crystal X-ray crystallographic studies for compounds 2–5 are summarised in the ESI.†Experimental procedure and thermogravimetric data for the complexes 2–4 is included in the ESI.† The ESI† also includes a comprehensive table of deposition conditions and sample notation, along with experimental procedure and results for the thin film characterisation, including SEM, EDX spectroscopy, PXRD, Raman spectroscopy, optical spectroscopy, photoelectrochemical characterisation of the thin TiO2 thin films (including photocurrent density data).
Author contributions
T. R. Harris-Lee and A. L. Johnson prepared the original draft; F. Marken, J. Zhang, C.L. Bentley and A. L. Johnson reviewed and edited the article; T. R. Harris-Lee carried out the precursor synthesis, deposition experiments and photoelectrochemical characterisation; A. L. Johnson collected and processed all single crystal X-ray data. E. Della Gaspera conducted UV-Vis measurements and determined the band gap values. All authors approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been supported by the Institute for Sustainability, University of Bath, and Monash University, both of which are thanked for the provision of a joint Bath-Monash Global PhD studentship to T. R. H-L. C. L. B. is the recipient of an Australian Research Council (ARC) Discovery Early Career Researcher Award (DECRA, project number DE200101076), funded by the Australian Government. The authors acknowledge the use of the instruments and scientific and technical assistance at the Monash Centre for Electron Microscopy, a Node of Microscopy Australia. The authors gratefully acknowledge the Material and Chemical Characterisation Facility (MC2) at the University of Bath, http://doi.org/10.15125/mx6j-3r54, for technical support and assistance in this work.Notes and references
- S. Deok Mun, J. Lee, S. H. Kim, Y. Hong, Y. Ho Ko, Y. K. Shin, J. H. Lim, C. S. Hong, Y. Do and Y. Kim, J. Organomet. Chem., 2007, 692, 3519–3525 CrossRef.
- P. Sharma, S. K. Tripathi and P. Jaiswal, IOP Conf. Ser.: Mater. Sci. Eng., 2021, 1136, 012054 CrossRef CAS.
- G. B. Harris, Philos. Mag. A J. Theor. Exp. Appl. Phys., 1951, 43, 113–123 Search PubMed.
- M. Regue, S. Sibby, I. Y. Ahmet, D. Friedrich, F. F. Abdi, A. L. Johnson and S. Eslava, J. Mater. Chem. A, 2019, 7, 19161–19172 RSC.
- G. Wang, H. Wang, Y. Ling, Y. Tang, X. Yang, R. C. Fitzmorris, C. Wang, J. Z. Zhang and Y. Li, Nano Lett., 2011, 11, 3026–3033 CrossRef CAS PubMed.
- T. R. Harris-Lee, Y. Zhang, C. R. Bowen, P. J. Fletcher, Y. Zhao, Z. Guo, J. W. F. Innocent, S. A. L. Johnson and F. Marken, Electrocatalysis, 2021, 12, 65–77 CrossRef CAS.
- A. J. Gardecka, C. Bishop, D. Lee, S. Corby, I. P. Parkin, A. Kafizas and S. Krumdieck, Appl. Catal., B, 2018, 224, 904–911 CrossRef CAS.
- G. Wang, H. Wang, Y. Ling, Y. Tang, X. Yang, R. C. Fitzmorris, C. Wang, J. Z. Zhang and Y. Li, Nano Lett., 2011, 11, 3026–3033 CrossRef CAS PubMed.
- M. Liu, N. De Leon Snapp and H. Park, Chem. Sci., 2011, 2, 80–87 RSC.
- J. Liu, X. Yu, Q. Liu, R. Liu, X. Shang, S. Zhang, W. Li, W. Zheng, G. Zhang, H. Cao and Z. Gu, Appl. Catal., B, 2014, 158–159, 296–300 CrossRef CAS.
- Z. Zhang and P. Wang, Energy Environ. Sci., 2012, 5, 6506–6512 RSC.
- J. Park, S. Lee, T. H. Lee, C. Kim, S. E. Jun, J. H. Baek, J. Y. Kim, M. G. Lee, S. H. Ahn and H. W. Jang, Nano Convergence, 2022, 9, 1–14 CrossRef PubMed.
- A. A. Tahir, T. A. N. Peiris and K. G. U. Wijayantha, Chem. Vap. Deposition, 2012, 18, 107–111 CrossRef CAS.
- T. R. Harris-Lee, S. A. L. Johnson, L. Wang, P. J. Fletcher, J. Zhang, C. Bentley, C. R. Bowen and F. Marken, New J. Chem., 2022, 46, 8385–8392 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: The experimental methods, detailed crystallographic discussion, TGA discussion, SEM, EDX, Raman, optical spectroscopy data, X-ray crystallography data, detailed grain size and phase ratio data, photoelectrochemical data. CCDC 2260244–2260247. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ma00364g |
| This journal is © The Royal Society of Chemistry 2023 |