Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: DFT investigation of the oxygen reduction reaction over nitrogen (N) doped graphdiyne as an electrocatalyst: the importance of pre-adsorbed OH* and the solvation effect
Yuelin
Wang
a,
Thanh Ngoc
Pham
a,
Harry H.
Halim
a,
Likai
Yan
c and
Yoshitada
Morikawa
*ab
aDepartment of Precision Engineering, Graduate School of Engineering, Osaka University, 2-1, Yamada-oka, Osaka 565-0871, Suita, Japan. E-mail: morikawa@prec.eng.osaka-u.ac.jp
bResearch Center for Precision Engineering, Graduate School of Engineering, Osaka University, 2-1 Yamada-oka, Suita 565-0871, Osaka, Japan
cInstitute of Functional Material Chemistry, Key Laboratory of Polyoxometalate Science of Ministry of Education, Faculty of Chemistry, Northeast Normal University, Changchun, 130024, P. R. China
First published on 17th November 2023
Abstract
Correction for ‘DFT investigation of the oxygen reduction reaction over nitrogen (N) doped graphdiyne as an electrocatalyst: the importance of pre-adsorbed OH* and the solvation effect’ by Yuelin Wang et al., Mater. Adv., 2023, https://doi.org/10.1039/d3ma00502j.
The authors regret that on the 5th page of the article, in the three paragraphs from “Consequently, upon applying a limiting potential of 0.22 V…” to “…cannot be used as an ORR electrocatalyst.”, all instances of ‘sp-N1GDY(OH)/GDY’ and ‘sp-N2GDY(OH)/GDY’ were mislabelled. They should be read as ‘sp-N1GDY(OH)/G’ and ‘sp-N2GDY(OH)/G’, respectively.
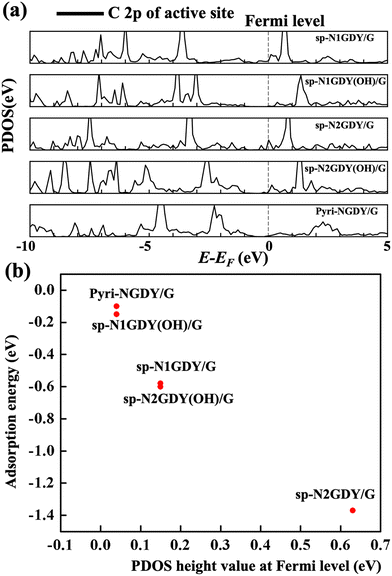
In Fig. 4, section (a), ‘sp-N1GDY/G (OH)’ and ‘sp-N2GDY/G (OH)’ were mislabelled. They should be read as ‘sp-N1GDY(OH)/G’ and ‘sp-N2GDY(OH)/G’, respectively. The correctly labelled Fig. 4 is given below.
In the section titled “The electronic structure of the active site relates to O2 activation”, the O2 dissociation barrier values for sp-N1GDY(OH)/G and Pyri-NGDY/G are given incorrectly. The correct text should be “…and O2 dissociation barrier is high (1.86 eV and 1.52 eV)”.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2023 |