Open Access Article
Open Access ArticleRhenium anchored Ti3C2Tx (MXene) nanosheets for electrocatalytic hydrogen production†
Selengesuren
Suragtkhuu
 ab,
Suvdanchimeg
Sunderiya
a,
Solongo
Purevdorj
a,
Munkhjargal
Bat-Erdene
c,
Batjargal
Sainbileg
ab,
Suvdanchimeg
Sunderiya
a,
Solongo
Purevdorj
a,
Munkhjargal
Bat-Erdene
c,
Batjargal
Sainbileg
 d,
Michitoshi
Hayashi
d,
Michitoshi
Hayashi
 d,
Abdulaziz S. R.
Bati
e,
Joseph G.
Shapter
d,
Abdulaziz S. R.
Bati
e,
Joseph G.
Shapter
 c,
Sarangerel
Davaasambuu
*a and
Munkhbayar
Batmunkh
c,
Sarangerel
Davaasambuu
*a and
Munkhbayar
Batmunkh
 *b
*b
aDepartment of Chemistry, Division of Natural Sciences, School of Arts and Sciences, National University of Mongolia, Ulaanbaatar, 14200, Mongolia. E-mail: sarangerel@num.edu.mn
bQueensland Micro- and Nanotechnology Centre, School of Environment and Science, Griffith University, Nathan, Queensland 4111, Australia. E-mail: m.batmunkh@griffith.edu.au
cAustralian Institute for Bioengineering and Nanotechnology, The University of Queensland, Brisbane, Queensland 4072, Australia
dCenter for Condensed Matter Sciences, Center of Atomic Initiative for New Materials, National Taiwan University, Taipei, 106, Taiwan
eCentre for Organic Photonics & Electronics, School of Chemistry and Molecular Biosciences, The University of Queensland, Brisbane, Queensland 4072, Australia
First published on 5th December 2022
Abstract
Atomically thin Ti3C2Tx (MXene) nanosheets with rich termination groups, acting as active sites for effective functionalization, are used as an efficient solid support to host rhenium (Re) nanoparticles for the electrocatalytic hydrogen evolution reaction (HER). The newly designed electrocatalyst – Re nanoparticles anchored on Ti3C2Tx MXene nanosheets (Re@Ti3C2Tx) – exhibited promising catalytic activity with a low overpotential of 298 mV to achieve a current density of 10 mV cm−2, while displaying excellent stability. In comparison, the pristine Ti3C2Tx MXene requires higher overpotential of 584 mV to obtain the same current density. After being stored under ambient conditions for 30 days, Re@Ti3C2Tx retained 100% of its initial catalytic activity for the HER, while the pristine Ti3C2Tx retained only 74.8% of its initial value. According to our theoretical calculations using density functional theory, dual Re anchored MXene (Re@Ti3C2Tx) exhibits a near-zero value of Gibbs free energy (ΔGH* = −0.06 eV) for the HER, demonstrating that the presence of Re significantly enhances the electrocatalytic activity of MXene nanosheets. This work introduces a facile strategy to develop an effective electrocatalyst for electrocatalytic hydrogen production.
Introduction
Hydrogen (H2) has received broad attention as part of the future energy solution to help deal with the growing energy storage problems and environmental pollution. H2 energy has several advantages, including high energy density, zero pollution emission, no greenhouse gas emission, recyclability and others. The key approach to produce H2 relies strongly on burning fossil fuels and/or biomass feedstock, leading to significant issues associated with the price and environment.1 Promisingly, the hydrogen evolution reaction (HER) through electrocatalytic reactions is the most economic and environmentally-friendly path to the future energy transition.2 To reduce the energy consumption and lower the overpotential for water splitting,3,4 catalysts have been the main subject of interest for the HER. To date, platinum (Pt) is widely known as the best performing catalyst; yet it has limited applicability because of its scarcity and high cost.5 This has led to increasing efforts focused on developing alternative electrocatalysts to the traditional Pt for the HER.In this regard, two-dimensional (2D) layered materials with their fascinating chemical and catalytic properties have garnered much attention as an alternative HER catalyst.3,6,7 The classic examples of 2D electrocatalyst materials include graphene, MoS2 and black phosphorus, all of which deliver outstanding HER activities with remarkable stabilities.8,9 As an emerging class of 2D materials, transition metal carbides/nitrides (MXenes) and their derivatives are regarded as promising alternatives to Pt.10,11 MXenes have a general structural formula of Mn + 1XnTx, where M is a transition metal (e.g., Ti, Mo, and Zr), X represents carbon (C) and/or nitrogen (N), and Tx symbolizes the termination groups such as –OH, –F and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (n = 1, 2, 3 or 4). Mono- or few-layer MXene nanosheets can be obtained from ceramic MAX phases by removing the A element (generally Al), which has a strong bonding with the transition metal through the etching process, followed by gentle exfoliation.12–14 2D MXenes have several extraordinary properties, such as high electronic conductivity (up to 10
O (n = 1, 2, 3 or 4). Mono- or few-layer MXene nanosheets can be obtained from ceramic MAX phases by removing the A element (generally Al), which has a strong bonding with the transition metal through the etching process, followed by gentle exfoliation.12–14 2D MXenes have several extraordinary properties, such as high electronic conductivity (up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 S cm−1), large surface area and strong affinity with water.15 Despite their recent discovery, MXenes have found applications in different areas including capacitors,16 batteries,17,18 solar cells,19 and catalysis reactions.20–23
000 S cm−1), large surface area and strong affinity with water.15 Despite their recent discovery, MXenes have found applications in different areas including capacitors,16 batteries,17,18 solar cells,19 and catalysis reactions.20–23
In general, anchoring single metal atoms and/or metallic nanoparticles on a solid support such as graphene and MXenes not only enhances the catalytic activity of electrocatalysts, but also enables reduction of the catalyst costs.24 Of particular importance in this research area is the functionalities of the support materials. Indeed, the rich termination groups of MXene nanosheets have opened vital avenues for research in designing 2D functional materials for various applications including the electrocatalytic HER. For instance, Zhang et al. developed an efficient catalyst using single platinum atoms immobilized on 2D MXene nanosheets for the HER.25 This novel catalyst displayed an impressive catalytic activity with a low overpotential of 77 mV to achieve 100 mA cm−2, while showing about 40 times greater mass activity than the commercial Pt@C catalyst. Recently, Bat-Erdene et al.22 designed boron-doped MXenes with highly dispersed ruthenium (Ru) nanoparticles (Ru@B-Ti3C2Tx), exhibiting outstanding catalytic activity for the HER with a low overpotential of 62.9 mV to reach 10 mA cm−2 in acidic media. Despite these excellent efforts, the search for novel HER electrocatalysts is still an active area of research, while the availability of many other low-cost metal based materials provide great opportunities to advance this field.26,27 In this regard, rhenium (Re) has recently attracted increasing attention as a promising catalyst for the HER due to several reasons. Re exhibits an optimal binding energy for adsorption and desorption of protons as well as an excellent exchange current density (comparable to Pt) for the HER.26,28 It is also about an order of magnitude less expensive than Pt (1/10 the price of Pt and 3/10 the price of Ru). Moreover, Re has great potential to overcome the shortcomings of Pt, and thus deserves exploration for the catalytic HER. Importantly, it was reported that the bulk state of metallic Re is not an appealing candidate for the HER and therefore recommended to downsize it to a nanostructure or more.29
Herein, we prepared Re ultrasmall nanoparticles (1–3 nm in diameter) uniformly anchored onto Ti3C2Tx (MXene) nanosheets and explored their electrocatalytic activity for the HER in acidic media. In addition to the excellent cycling stability, the newly developed electrocatalyst (Re@Ti3C2Tx) displayed promising catalytic activity for the HER with an overpotential of 298 mV to achieve 10 mA cm−2, which is significantly better than that of the pure MXene. A combination of theoretical calculations and experimental analysis was used to understand the catalytic kinetics of Re anchored onto MXenes.
Results and discussion
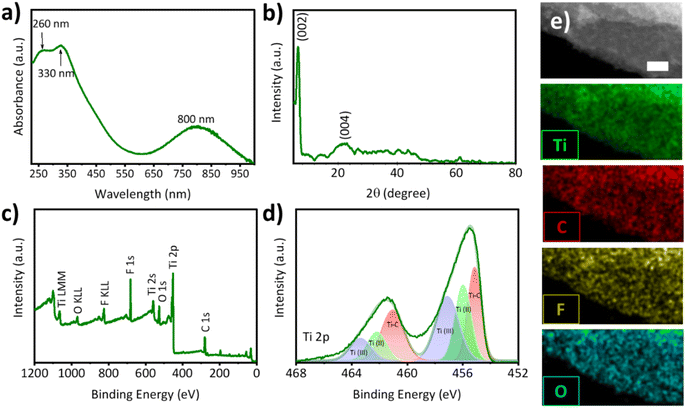
The as-prepared Ti3C2Tx dispersion was characterized using UV-vis spectroscopy. As shown in Fig. 1a, three characteristic peaks of Ti3C2Tx can be observed at 260, 330 and 800 nm wavelengths, showing excellent consistency with previous studies.22,30,31 The crystallographic structure of the Ti3C2Tx flakes was studied by X-ray diffraction (XRD) analysis. As illustrated in Fig. 1b, two main peaks appearing at around 6.5° and 20.7° can be assigned to the (002) and (004), respectively.22,30,31 Two main changes were observed after etching and exfoliating the MAX phase. First, no residual peak of Ti3AlC2 (104) was seen in the Ti3C2Tx sample (Fig. S1†). Secondly, the (002) peak shifted from 9.61° to 6.5° due to the increase in d-spacing, suggesting the successful synthesis of Ti3C2Tx surface termination groups.31,32X-ray photoelectron spectroscopy (XPS) was carried out to determine the chemical and electronic states of Ti3C2Tx. As depicted in Fig. 1c, the XPS survey scan of Ti3C2Tx revealed the presence of all the expected elements, namely Ti, C, O, and F, further confirming the successful preparation of Ti3C2Tx. The appearance of F with high intensity suggests the successful introduction of rich termination groups on the surface of Ti3C2Tx, which is in agreement with the XRD result. Fig. 1d shows the high resolution (HR) Ti 2p spectrum of the as-prepared Ti3C2Tx, confirming the two asymmetric peaks typically observed in the fresh MXene.31 In addition to the strong Ti–C bonding (see the HR C 1s spectrum in Fig. S2†), the absence of a peak at around 459 eV binding energy suggests that the Ti3C2Tx sample is not oxidized and further confirms the successful preparation of high quality Ti3C2Tx flakes. Fig. 1e depicts the high-angle annular dark-field scanning transmission electron microscope (HAADF-STEM) and energy dispersive X-ray (EDX) elemental mapping images of the Ti3C2Tx nanosheets. The presence of Ti, C, F and O with highly uniform dispersion suggests that Ti3C2Tx flakes with rich O- and F-containing termination groups were successfully prepared.
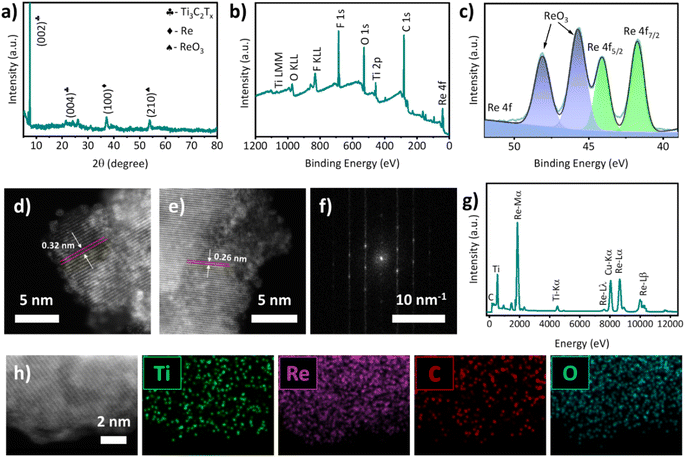
Further, the as-prepared Ti3C2Tx flakes were mixed with Re2O7 at a ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (wt%). After freeze-drying, the mixture was annealed at 500 °C for 3 h in a quartz tube under an Ar gas flow. During the annealing process, Re2O7 is thermally reduced to Re nanoparticles, anchoring uniformly on the surface of MXene. First, XRD was used to analyze the crystal structure of our Re@Ti3C2Tx as presented in Fig. 2a. It can be seen that the XRD pattern of Re@Ti3C2Tx was similar to that of Ti3C2Tx ((002) and (004 peaks)), suggesting that the introduction of Re nanoparticles did not alter the crystal structure of the MXene. Interestingly, two new characteristic peaks at 37.2° and 53.8° were observed and can be assigned to the (100) peak of Re and (210) peak of ReO3, respectively.29,33 Then, XPS was employed to analyze the chemical bond formation of our Re@Ti3C2Tx. In Fig. 2b, the XPS survey scan of Re@Ti3C2Tx shows the existence of Ti, C, O, F and Re confirming the successful synthesis of Re@Ti3C2Tx. To analyze the oxidation states of Re based on the binding energies HR XPS scans were recorded (see Fig. 2c). The dominant peaks at 41.7 eV and 44.1 eV correspond to the fully reduced Re (0) species, while the peaks at 45.8 eV and 48.2 eV correspond to the oxidized species of Re (ReO3). The estimated percentages of Re (0) and ReO3 from high-resolution 4f Re spectra (Fig. 2c) were 48.7% and 51.3%, respectively, in the sample.
1 (wt%). After freeze-drying, the mixture was annealed at 500 °C for 3 h in a quartz tube under an Ar gas flow. During the annealing process, Re2O7 is thermally reduced to Re nanoparticles, anchoring uniformly on the surface of MXene. First, XRD was used to analyze the crystal structure of our Re@Ti3C2Tx as presented in Fig. 2a. It can be seen that the XRD pattern of Re@Ti3C2Tx was similar to that of Ti3C2Tx ((002) and (004 peaks)), suggesting that the introduction of Re nanoparticles did not alter the crystal structure of the MXene. Interestingly, two new characteristic peaks at 37.2° and 53.8° were observed and can be assigned to the (100) peak of Re and (210) peak of ReO3, respectively.29,33 Then, XPS was employed to analyze the chemical bond formation of our Re@Ti3C2Tx. In Fig. 2b, the XPS survey scan of Re@Ti3C2Tx shows the existence of Ti, C, O, F and Re confirming the successful synthesis of Re@Ti3C2Tx. To analyze the oxidation states of Re based on the binding energies HR XPS scans were recorded (see Fig. 2c). The dominant peaks at 41.7 eV and 44.1 eV correspond to the fully reduced Re (0) species, while the peaks at 45.8 eV and 48.2 eV correspond to the oxidized species of Re (ReO3). The estimated percentages of Re (0) and ReO3 from high-resolution 4f Re spectra (Fig. 2c) were 48.7% and 51.3%, respectively, in the sample.
The spin–orbit split doublets with a splitting of 2.4 eV for Re were highly consistent with the XPS handbook. These results are not only consistent with previous literature,29,33,34 but also in excellent agreement with our XRD analysis. Notably, recent work showed that a combination of metallic and partially oxidized Ru nanoparticles is beneficial for overall water splitting reactions including the HER.35 Therefore, it is reasonable to expect that the presence of both metallic and oxidized Re would be valuable to enhance the catalytic activity of the catalyst.
Fig. 2d and e show the HR transmission electron microscopy (HRTEM) image of Re@Ti3C2Tx. Two lattice fringe values of 0.32 nm and 0.26 nm can be measured from the HRTEM. While the lattice fringe of 0.26 nm is consistent with previous studies,36 the spacing of 0.32 nm is slightly higher than that of the typical Ti3C2Tx, suggesting that the introduction of Re species may be responsible for this enlargement of lattice spacing.37 Moreover, it can be clearly observed from Fig. 2d that ultrasmall (Re) nanoparticles with an excellent lattice structure are dispersed on the edge of the MXene sheets. The particle size of the Re can be measured to be 1–3 nm. As shown in Fig. 2f, the selected area electron diffraction (SAED) pattern demonstrates that our Re@Ti3C2Tx is highly crystalline although the sample was prepared using high annealing temperatures. To extend the proof of successful preparation, the chemical composition of Re@Ti3C2Tx was studied using EDX (Fig. 2g), and HAADF-STEM coupled with elemental mapping (Fig. 2h). As illustrated in Fig. 2g, Ti, C, Re and Cu elements were mainly detected with Cu being from the grid for TEM. This is consistent with the XPS survey scan of our Re@Ti3C2Tx. Moreover, all expected elements including Ti, Re, C and O can be observed with excellent distribution from the EDX elemental mapping images (see Fig. 2h). In addition, we carried out inductively coupled plasma-optical emission spectrometry (ICP-OES) to determine the content of both Ti and Re in the sample. The measured Ti and Re content was 299 ppm and 17.63 ppm, respectively, leading to a mass ratio of 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Ti
1 (Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Re), which was different from the content that can be measured from the XPS analysis shown in Fig. 2b. This difference is due to the fact that XPS is an effective tool to analyze the elemental and chemical composition of the very top surface (1–10 nm) of the sample.
Re), which was different from the content that can be measured from the XPS analysis shown in Fig. 2b. This difference is due to the fact that XPS is an effective tool to analyze the elemental and chemical composition of the very top surface (1–10 nm) of the sample.
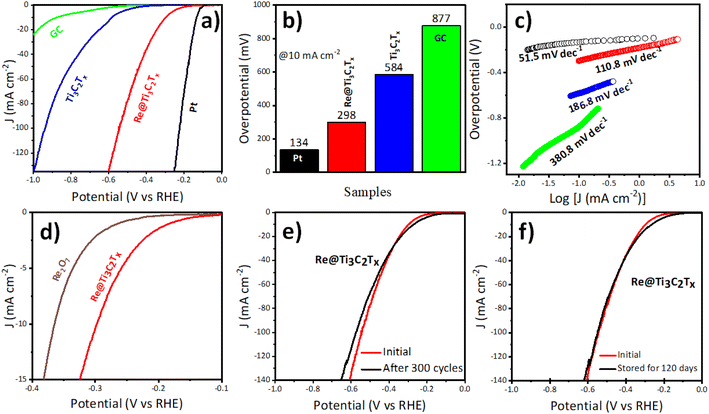
The electrocatalytic HER activities of the samples including Ti3C2Tx, Re@Ti3C2Tx and Pt catalysts were studied in an acidic medium (0.5 M H2SO4) with a three-electrode system. Fig. 3a displays the linear scan voltammetry (LSV) curves of the catalysts obtained by applying potentials from −1 V to 0 V (vs. RHE, reversible hydrogen electrode). At a constant potential of 500 mV, the measured current densities reveal that Pt exhibits remarkable catalytic activity (achieved 235.1 mA cm−2) while a glassy carbon (GC) electrode is inactive for the HER (Fig. S3†). Moreover, it can be seen that the pure MXene (Ti3C2Tx) showed poor catalytic performance which is in agreement with recent work.38 Indeed, our Re@Ti3C2Tx catalyst exhibited much improved electrocatalytic performance, achieving a current density of 81.3 mA cm−2 at 500 mV. This result indicates that the introduction of Re and ReO3 significantly improves the electrocatalytic activity of the MXene for the HER. Similarly, we compared the overpotential values of GC, pure Ti3C2Tx, Re@Ti3C2Tx and Pt to obtain 10 mA cm−2 (see Fig. 3b). It is well established that a current density of 10 mA cm−2 has become the benchmark value used to evaluate the activity of HER electrocatalysts.39 The required potential to achieve a current density of 10 mA cm−2 for Re@Ti3C2Tx was 298 mV, while the pure MXene catalyst needed a much higher potential (584 mV), demonstrating the excellent catalytic performance of Re nanoparticles (see Table S1† for comparison).
The HER kinetics of the catalysts were studied using Tafel plots obtained from the LSV curves (Fig. 3c). The Tafel slope recorded for Re@Ti3C2Tx was calculated to be 110.8 mV dec−1, which was significantly lower than that of Ti3C2Tx (186.8 mV dec−1). The lower value of Tafel slopes indicates better catalytic kinetics due to the remarkable increase in the electrocatalytic current density.40–42 Moreover, the Tafel slope value of Re@Ti3C2Tx was even comparable to that of the Pt catalyst (51.5 mV dec−1), showing great promise as low-cost catalysts. In addition, the electrocatalytic activity of commercial Re2O7 was tested to demonstrate the importance of MXene as a solid support in the catalyst. As shown in Fig. 3d, the overpotential value of Re2O7 was 365 mV to reach 10 mA cm−2. We further demonstrated the robustness and stability of our Re@Ti3C2Tx under various testing conditions. As displayed in Fig. 3e, no significant changes were observed in the LSV curves of Re@Ti3C2Tx before and after 300 continuous CV cycles. Moreover, the electrocatalytic performance of our Re@Ti3C2Tx was well maintained in water for an extended period (120 days) (Fig. 3f), revealing that our catalyst is very stable in aqueous media. In contrast, the electrocatalytic performance of the pure MXene dramatically degraded when stored in water for only 30 days (Fig. S4†).
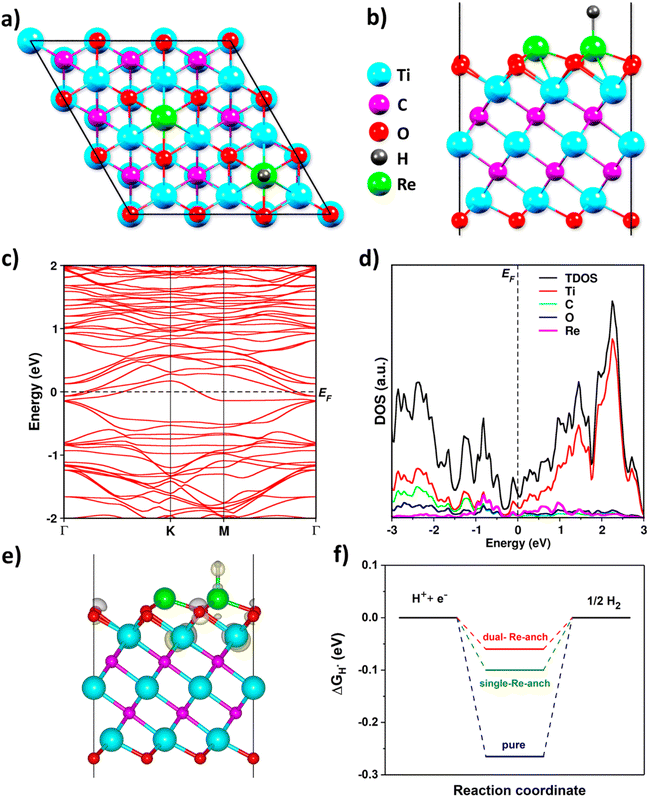
To understand the HER and the fundamental electronic properties of Ti3C2O2 (MXene) in terms of Re-defects at the atomistic level, density functional theory (DFT) calculations were performed using a Vienna Ab initio simulation package (VASP) code43 (see the DFT calculation details). Ti3C2O2 was chosen for the calculations considering the O-rich termination groups on the surface of the MXene (Fig. S5†). We considered two Re-anchored MXene structures by anchoring the surface oxygen atoms of the Ti3C2O2 slab with a single Re atom (Fig. S6a and b†) and dual Re atoms (Fig. 4a and b), respectively. We further discuss the dual Re-anchored Ti3C2O2 since it shows remarkable performance.
The band structure of the dual Re-anchored Ti3C2O2 displays the metallic feature without a bandgap (Fig. 4c), reflecting that Re-anchoring has a good impact on the electronic properties of the material. According to the density of states (DOS), Re-anchoring creates new electronic states near the Fermi level (Fig. 4d), suggesting that Re-anchored Ti3C2O2 can to show good electronic conductivity. These features are highly supportive of the HER. Meanwhile, the charge density difference (Δρ) in the Re-anchored Ti3C2O2 is found not only on the H-atom and the Re-anchored O-atoms, but also on the next neighboring Ti-atoms (Fig. 4e), indicating that the surface atoms with the support of Re atoms tend to participate in the HER effectively. For H adsorption on the dual Re-anchored Ti3C2O2, the calculated Gibbs free energy (ΔGH*) is −0.06 eV (Fig. 4f), which was ∼5 times better value than that of the pure one. This suggests that the Re-anchoring is favorable to improve the electrocatalytic activity of MXenes for the HER.
Conclusion
In conclusion, we have successfully synthesized Ti3C2Tx (MXene) nanosheets as a solid support for hosting ultrasmall sized Re nanoparticles (Re@Ti3C2Tx) for enhanced hydrogen production. The catalytic activity of Re@Ti3C2Tx is explored using a combination of experimental analysis and DFT calculations. According to our experimental investigation and theoretical calculations, Re@Ti3C2Tx significantly improves the intermediate H* adsorption kinetics for the HER. Specifically, our newly designed Re@Ti3C2Tx showed promising catalytic activity with a low overpotential of 298 mV to achieve 10 mA cm−2, while exhibiting excellent stability over 300 continuous CV cycles and in aqueous media for 120 days. This work highlights a facile strategy for designing 2D MXene nanosheets as an efficient solid support for high performance HER electrocatalysts.Author contributions
Selengesuren Suragtkhuu: data curation, formal analysis, investigation, methodology and writing – original draft. Suvdanchimeg Sunderiya: data curation, investigation, methodology and writing – review & editing. Solongo Purevdorj: data curation, investigation, methodology and writing – review & editing. Munkhjargal Bat-Erdene: data curation, methodology and writing – review & editing. Batjargal Sainbileg: data curation, methodology, software, validation and writing – review & editing. Michitoshi Hayashi: resources, software, validation and writing – review & editing. Abdulaziz S. R. Bati: data curation, methodology and writing – review & editing. Joseph G. Shapter: funding acquisition, resources, visualization and writing – review & editing. Sarangerel Davaasambuu: conceptualization, funding acquisition, project administration, resources, supervision, validation and writing – review & editing. Munkhbayar Batmunkh: conceptualization, funding acquisition, investigation, resources, supervision, validation and writing – review & editing.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research was supported by Fellow research grant of National University of Mongolia (P2021-4197). This work was also financially supported by the Australian Research Council (DE220100521 and DP200101217). The authors thank the research group of Dr Munkhjargal Burenjargal at the National University of Mongolia for their facility support. M. H. and B. S. are thankful to the Center of Atomic Initiative for New Materials, National Taiwan University (project No. 109L4000 and 110L9008) under the Ministry of Education of Taiwan for the funding support. A. S. R. B acknowledges support from King Abdullah University of Science and Technology (KAUST) through the Ibn Rushd Postdoctoral Fellowship Award. The authors gratefully acknowledge the use of Centre for Microscopy and Microanalysis (CMM) facilities at the University of Queensland, Australia.References
- Z. Chen, X. Duan, W. Wei, S. Wang and B.-J. Ni, J. Mater. Chem. A, 2019, 7, 14971–15005 RSC.
- J. Zhu, L. Hu, P. Zhao, L. Y. S. Lee and K.-Y. Wong, Chem. Rev., 2020, 120, 851–918 CrossRef CAS PubMed.
- X. Zou and Y. Zhang, Chem. Soc. Rev., 2015, 44, 5148–5180 RSC.
- L. Yuekun, L. Li, L. Fangyan, W. Biao, G. Feng, L. Chuan, F. Jingyun, H. Feng, L. Zhang and W. Mengye, Nano Res., 2022, 15, 7986–7993 CrossRef PubMed.
- J. N. Hansen, H. Prats, K. K. Toudahl, N. Mørch Secher, K. Chan, J. Kibsgaard and I. Chorkendorff, ACS Energy Lett., 2021, 6, 1175–1180 CrossRef CAS PubMed.
- Y. Guo, T. Park, J. W. Yi, J. Henzie, J. Kim, Z. Wang, B. Jiang, Y. Bando, Y. Sugahara, J. Tang and Y. Yamauchi, Adv. Mater., 2019, 31, 1807134 CrossRef PubMed.
- A. S. R. Bati, M. Batmunkh and J. G. Shapter, Adv. Energy Mater., 2020, 10, 1902253 CrossRef CAS.
- Y. Li, H. Wang, L. Xie, Y. Liang, G. Hong and H. Dai, J. Am. Chem. Soc., 2011, 133, 7296–7299 CrossRef CAS PubMed.
- S. Suragtkhuu, M. Bat-Erdene, A. S. R. Bati, J. G. Shapter, S. Davaasambuu and M. Batmunkh, J. Mater. Chem. A, 2020, 8, 20446–20452 RSC.
- P. Sriram, A. Manikandan, F.-C. Chuang and Y.-L. Chueh, Small, 2020, 16, 1904271 CrossRef CAS PubMed.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- C. Wang, S. Chen and L. Song, Adv. Funct. Mater., 2020, 30, 2000869 CrossRef CAS.
- A. Lipatov, M. Alhabeb, M. R. Lukatskaya, A. Boson, Y. Gogotsi and A. Sinitskii, Adv. Electron. Mater., 2016, 2, 1600255 CrossRef.
- M. Ghidiu, M. R. Lukatskaya, M.-Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78–81 CrossRef CAS PubMed.
- X. Li, Z. Huang, C. E. Shuck, G. Liang, Y. Gogotsi and C. Zhi, Nat. Rev. Chem., 2022, 6, 389–404 CrossRef.
- L. Guojin, L. Xinliang, W. Yanbo, Y. Shuo, H. Zhaodong, Y. Qi, W. Donghong, D. Binbin, Z. Minshen and Z. Chunyi, Nano Res. Energy, 2022, 1, e9120002 CrossRef.
- J. Zhou, X. Zha, X. Zhou, F. Chen, G. Gao, S. Wang, C. Shen, T. Chen, C. Zhi, P. Eklund, S. Du, J. Xue, W. Shi, Z. Chai and Q. Huang, ACS Nano, 2017, 11, 3841–3850 CrossRef CAS PubMed.
- G. Xin, W. Changda, W. Wenjie, Z. Quan, X. Wenjie, Z. Pengjun, W. Shiqiang, C. Yuyang, Z. Kefu, L. Zhanfeng, Y. Xiya, W. Yixiu, W. Xiaojun, S. Li, C. Shuangming and L. Xiaosong, Nano Res. Energy, 2022, 1, e9120026 CrossRef.
- A. Agresti, A. Pazniak, S. Pescetelli, A. Di Vito, D. Rossi, A. Pecchia, M. Auf der Maur, A. Liedl, R. Larciprete, D. V. Kuznetsov, D. Saranin and A. Di Carlo, Nat. Mater., 2019, 18, 1228–1234 CrossRef CAS PubMed.
- M. R. Lukatskaya, O. Mashtalir, C. E. Ren, Y. Dall'Agnese, P. Rozier, P. L. Taberna, M. Naguib, P. Simon, M. W. Barsoum and Y. Gogotsi, Science, 2013, 341, 1502–1505 CrossRef CAS PubMed.
- X. Zhang, Z. Zhang, J. Li, X. Zhao, D. Wu and Z. Zhou, J. Mater. Chem. A, 2017, 5, 12899–12903 RSC.
- M. Bat-Erdene, M. Batmunkh, B. Sainbileg, M. Hayashi, A. S. R. Bati, J. Qin, H. Zhao, Y. L. Zhong and J. G. Shapter, Small, 2021, 17, 2102218 CrossRef CAS PubMed.
- Z. W. Seh, K. D. Fredrickson, B. Anasori, J. Kibsgaard, A. L. Strickler, M. R. Lukatskaya, Y. Gogotsi, T. F. Jaramillo and A. Vojvodic, ACS Energy Lett., 2016, 1, 589–594 CAS.
- S.-Y. Bae, J. Mahmood, I.-Y. Jeon and J.-B. Baek, Nanoscale Horiz., 2020, 5, 43–56 RSC.
- J. Zhang, Y. Zhao, X. Guo, C. Chen, C.-L. Dong, R.-S. Liu, C.-P. Han, Y. Li, Y. Gogotsi and G. Wang, Nat. Catal., 2018, 1, 985–992 CrossRef CAS.
- M. Cabán-Acevedo, M. L. Stone, J. R. Schmidt, J. G. Thomas, Q. Ding, H. C. Chang, M. L. Tsai, J. H. He and S. Jin, Nat. Mater., 2015, 14, 1245–1251 CrossRef PubMed.
- J. Mahmood, F. Li, S. M. Jung, M. S. Okyay, I. Ahmad, S. J. Kim, N. Park, H. Y. Jeong and J. B. Baek, Nat. Nanotechnol., 2017, 12, 441–446 CrossRef CAS PubMed.
- T. T. Yang, R. B. Patil, J. R. McKone and W. A. Saidi, Catal. Sci. Technol., 2021, 11, 6832–6838 RSC.
- M. Kim, Z. Yang, J. H. Park, S. M. Yoon and B. A. Grzybowski, ACS Appl. Nano Mater., 2019, 2, 2725–2733 CrossRef CAS.
- M. Alhabeb, K. Maleski, B. Anasori, P. Lelyukh, L. Clark, S. Sin and Y. Gogotsi, Chem. Mater., 2017, 29, 7633–7644 CrossRef CAS.
- L. Yu, A. S. R. Bati, T. S. L. Grace, M. Batmunkh and J. G. Shapter, Adv. Energy Mater., 2019, 9, 1901063 CrossRef.
- M. Ghidiu, J. Halim, S. Kota, D. Bish, Y. Gogotsi and M. W. Barsoum, Chem. Mater., 2016, 28, 3507–3514 CrossRef CAS.
- S. Ghosh, H.-C. Lu, S. H. Cho, T. Maruvada, M. C. Price and D. J. Milliron, J. Am. Chem. Soc., 2019, 141, 16331–16343 CrossRef CAS PubMed.
- V. Urbanová, N. Antonatos, J. Plutnar, P. Lazar, J. Michalička, M. Otyepka, Z. Sofer and M. Pumera, ACS Nano, 2021, 15, 2374–2385 CrossRef PubMed.
- M. Zhong, S. Yan, J. Xu, C. Wang and X. Lu, Inorg. Chem. Front., 2022, 9, 4881–4891 RSC.
- K. Zhang, M. Di, L. Fu, Y. Deng, Y. Du and N. Tang, Carbon, 2020, 157, 90–96 CrossRef CAS.
- C. T. Sims, C. M. Craighead and R. I. Jaffee, JOM, 1955, 7, 168–179 CrossRef CAS.
- Y. Zou, S. A. Kazemi, G. Shi, J. Liu, Y. Yang, N. M. Bedford, K. Fan, Y. Xu, H. Fu, M. Dong, M. Al-Mamun, Y. L. Zhong, H. Yin, Y. Wang, P. Liu and H. Zhao, EcoMat, 2022, e12274 Search PubMed.
- C. C. L. McCrory, S. Jung, I. M. Ferrer, S. M. Chatman, J. C. Peters and T. F. Jaramillo, J. Am. Chem. Soc., 2015, 137, 4347–4357 CrossRef CAS PubMed.
- M. Cabán-Acevedo, M. L. Stone, J. R. Schmidt, J. G. Thomas, Q. Ding, H.-C. Chang, M.-L. Tsai, J.-H. He and S. Jin, Nat. Mater., 2015, 14, 1245–1251 CrossRef PubMed.
- M.-R. Gao, J.-X. Liang, Y.-R. Zheng, Y.-F. Xu, J. Jiang, Q. Gao, J. Li and S.-H. Yu, Nat. Commun., 2015, 6, 5982 CrossRef CAS PubMed.
- D. H. Kweon, M. S. Okyay, S.-J. Kim, J.-P. Jeon, H.-J. Noh, N. Park, J. Mahmood and J.-B. Baek, Nat. Commun., 2020, 11, 1278 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2na00782g |
| This journal is © The Royal Society of Chemistry 2023 |