Open Access Article
Open Access ArticleResearch progress related to thermosensitive hydrogel dressings in wound healing: a review
Ruting
Gu
 a,
Haiqing
Zhou
a,
Zirui
Zhang
b,
Yun
Lv
c,
Yueshuai
Pan
d,
Qianqian
Li
e,
Changfang
Shi
f,
Yanhui
Wang
*g and
Lili
Wei
*h
a,
Haiqing
Zhou
a,
Zirui
Zhang
b,
Yun
Lv
c,
Yueshuai
Pan
d,
Qianqian
Li
e,
Changfang
Shi
f,
Yanhui
Wang
*g and
Lili
Wei
*h
aDepartment of Thoracic Surgery, The Affiliated Hospital of Qingdao University, Qingdao 266000, China. E-mail: 13805424386@163.com
bEmergency Departments, The Affiliated Hospital of Qingdao University, Qingdao 266000, China
cSchool of Nursing, Qingdao University, Qingdao 266000, China
dDepartment of Critical Care Medicine, The Affiliated Hospital of Qingdao University, Qingdao 266000, China
eOphthalmology Department, The Affiliated Hospital of Qingdao University, Qingdao 266000, China
fDepartment of Spinal Surgery, The Affiliated Hospital of Qingdao University, Qingdao 266000, China
gDepartment of Oral Implantology, The Affiliated Hospital of Qingdao University, Qingdao 266000, China. E-mail: qdwyh06@163.com
hOffice of the Dean, The Affiliated Hospital of Qingdao University, Qingdao, 266000, China. E-mail: weilili@qduhospital.cn
First published on 26th October 2023
Abstract
Wound healing is a dynamic and complex process in which the microenvironment at the wound site plays an important role. As a common material for wound healing, dressings accelerate wound healing and prevent external wound infections. Hydrogels have become a hot topic in wound-dressing research because of their high water content, good biocompatibility, and adjustable physical and chemical properties. Intelligent hydrogel dressings have attracted considerable attention because of their excellent environmental responsiveness. As smart polymer hydrogels, thermosensitive hydrogels can respond to small temperature changes in the environment, and their special properties make them superior to other hydrogels. This review mainly focuses on the research progress in thermosensitive intelligent hydrogel dressings for wound healing. Polymers suitable for hydrogel formation and the appropriate molecular design of the hydrogel network to achieve thermosensitive hydrogel properties are discussed, followed by the application of thermosensitive hydrogels as wound dressings. We also discuss the future perspectives of thermosensitive hydrogels as wound dressings and provide systematic theoretical support for wound healing.
Introduction
The skin is the first line of defense that protects the human body from external bacteria and viruses, and its integrity is extremely important for human safety.1 When the integrity of the skin is compromised, patients experience discomfort, including pain, bleeding, inflammation, delayed healing, scarring, and even death.2,3 Wound healing is the basic stage of skin injury reconstruction. It is a dynamic and overlapping process, usually divided into four continuous and possibly overlapping processes: hemostasis, inflammation, proliferation, and remodeling (Fig. 1).4,5 These processes are regulated by complex networks comprising various cells and biological mediators.6,7 Studies have confirmed that temperature, pH, and other factors affect wound healing.8–12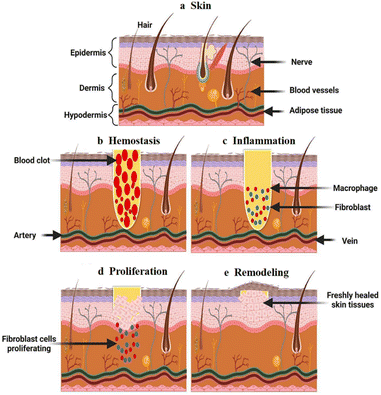 | ||
| Fig. 1 Schematic diagram of the different wound healing phases: (a) normal skin; (b) hemostasis involves the promotion of platelets and formation of clots; (c) inflammation recruits leukocytes to the wound site to release cytokines; (d) the proliferation phase involves angiogenesis and extracellular matrix production; (e) the remodeling phase involves the organization and contraction of the extracellular matrix. This figure has been reproduced from ref. 4 with permission from Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., copyright 2022. | ||
Wound dressings protect wounds from infection by absorbing osmotic fluid and isolating the external environment.13,14 Traditional dressings, such as gauze, cotton wool, and bandages can stop bleeding, absorb wound exudate, and protect the wound from bacterial infection; however, they do not have antibacterial or anti-inflammatory functions15,16 and may cause adhesion scabs and even hinder the wound healing process.17,18 Compared to traditional medical dressings, hydrogels have the advantages of a three-dimensional network structure, strong water absorption, and the maintenance of a moist environment in the wound to reduce the infection rate and accelerate wound healing;19 as such, they have become a research hotspot in the field of wound dressing. More importantly, hydrogel dressings can be endowed with excellent properties through structural design and functional integration,20 thus playing an important role in the wound healing process.
Thermosensitive hydrogels are smart polymer hydrogels that undergo phase transitions under external temperature stimulation and have good biocompatibility and mechanical properties.21 The gelation of thermosensitive hydrogels is triggered by temperature, and they exhibit a phase transition at the critical solution temperature. Once the desired temperature is reached, known as the sol–gel transition, hydrogels form immediately.22 The temperature-induced sol–gel transition is safer in vivo and is more suitable for injection systems because it does not require denaturing crosslinkers,23 leading to an easier and more economical process.24,25 They have excellent temperature sensitivity, drug delivery,26–28 cell loading,29,30 protein delivery,31,32 and gene therapy.33,34 As a free-flowing liquid before low-temperature gelation, the hydrogel precursor solution can be injected into the wound area to quickly fill irregular defects under physiological conditions, form a gel, and fill any irregular wound site or deep wound.35 In addition, encapsulation in the flow state allows the therapeutic agent to be evenly dispersed in the hydrogel, whereas the rapid sol–gel transition at body temperature prevents the initial burst release of the therapeutic agent, thus controlling the release behavior.36 These features are key to their application in human medicine,37 particularly in the field of wound dressing. However, a systematic review of thermosensitive hydrogel wound dressings has not been conducted.
Herein, we summarize the progress in thermosensitive hydrogel wound dressings and discuss the polymers used in these dressings. The mechanisms of temperature sensitivity are reviewed and we discuss the different types of thermosensitive hydrogels and their applications in different wounds. Finally, we look ahead to the future development of thermosensitive hydrogels for wound-healing applications and make recommendations.
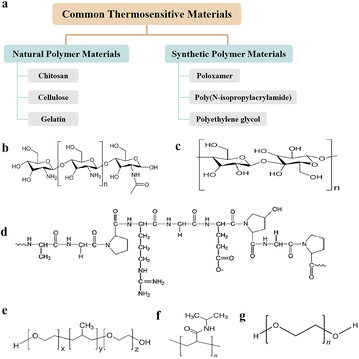
Common thermosensitive materials
An ideal wound dressing needs to be biocompatible and non-sensitive, with appropriate adhesion and mechanical properties, able to effectively cover the entire irregular wound, absorb the exudate tissue fluid in time, ensure the environmental humidity of the wound, allow gas exchange, and prevent bacterial infection of the wound, so that it can be easily replaced to alleviate the pain of patients in the process of wound management.38,39Thermosensitive biomaterials are novel smart materials for preparing thermosensitive hydrogels. These sources can be divided into natural and synthetic polymer materials (Fig. 2a).40
Natural bioactive polymers
Natural bioactive polymers are often used as wound dressings due to their biocompatibility and biodegradability.41,42 The most commonly used natural bioactive polymers for thermosensitive hydrogels are chitosan,43,44 cellulose45,46 and gelatin.47,48Chitosan (CS) is a polycation biodimer that has a wide range of sources, and its structure consists of an N-acetylglucosamine group linked by a β-1,4-glycosidic bond and glucosamine residues (Fig. 2b).49 CS exhibits good histocompatibility, biodegradability, adhesiveness, antimicrobial activity, and low cytotoxicity.50–53 Because of these valuable features, CS has been widely used in the fields of medicine and biology.52,54 However, because of the slow temperature response and low mechanical strength of chitosan, it usually needs to be improved by combining it with other substances.36 Common chitosan thermosensitive hydrogels include chitosan–sodium glycerophosphate thermosensitive gels, chitosan graft gels, sulfhydryl chitosan hydrogels, and chitosan/collagen gels.55 Previous studies have shown that CS activates platelets and macrophages, resulting in excellent blood characteristics.56 It can also promote the release of vascular endothelial growth factors, thereby promoting the formation of blood vessels and accelerating repair.57 In addition, chitosan exhibits excellent antibacterial properties that can reduce the probability of wound infection and accelerate wound healing.58 The CS structure is easy to modify, easy to process, and can be made into a hydrogel. The CS hydrogel dressing has the characteristics of high repair efficiency, simple design and preparation, and has potential value in the field of wound dressing.54
Cellulose, which has been used for a long time and has a long history, is the main material used in traditional gauzes. Cellulose derivatives are biological macromolecules containing glucose molecules connected by β-1,4-glycosidic bonds (Fig. 2c). Hydroxypropyl cellulose and methyl cellulose are representative polymers used in cellulose-based thermosensitive hydrogels. Cellulose-based thermosensitive hydrogels are widely used as biomedical materials because of their hydrophilicity, biocompatibility, biodegradability, and nontoxicity.21 At the structural level, cellulose is an ideal material for synthesizing biocompatible hydrogels because it easily modifies functional groups.59–61 The high biocompatibility and functional structure of cellulose polymer allows cellulose nanocomposites to be combined with various antibacterial agents.62,63 Oscar et al. developed a multifaceted formulation based on CNT-containing cellulose–chalcone, which can sustain the release of therapeutic substances with antimicrobial and wound-healing properties.64 Hydrogels can potentially be utilized to treat complex wounds owing to their improved wound-healing properties and prevention of potential infections.
Gelatin is a natural protein derived from the hydrolysis of collagen; its molecular chain contains a large number of active functional groups (Fig. 2d). Gelatin is highly biocompatible and biodegradable in a physiological environment.47,65 Although mainly derived from animals, the digestive process confers gelatin very low antigenicity, with the formation of harmless metabolic products upon degradation while in body.66,67 When the temperature is below 25 °C, an aqueous gelatin solution solidifies due to the formation of triple helices and a rigid three-dimensional network.68 At the physiological temperature, the physically formed hydrogels are dissolved.69 Therefore, gelatin is often chemically crosslinked or combined with other polymers resulting in hydrogels that are stable at 37 °C. Studies have reported thermoresponsive hydrogel systems for drug delivery by combining gelatin with a chitosan/Pluronic mixture70,71 to promote wound healing.
Synthetic polymers
Synthetic materials include poloxamers,72,73 poly(N-isopropylacrylamides)74,75 and polyethylene glycol.76,77 Poloxamer is a synthetic amphiphilic copolymer78,79 consisting of a poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) (PEO–PPO–PEO) triblock copolymer (Fig. 2e),80 and the number of molecules can be adjusted to affect their hydrophilicity or hydrophobicity. Poloxamer, copolymers with good amphipathicity, double responsiveness, and biocompatibility, have important applications in the field of synthetic dressings.81,82 Poloxamer hydrogel dressings have good elasticity and plasticity and can closely fit wounds to isolate bacteria. Their transparent texture can also be used to observe the condition of the wound while simultaneously keeping the wound moist, which is especially suitable for dry wounds, such as burns.83,84 Poloxamer hydrogels have good dual responsiveness (pH and temperature) and biocompatibility and can bind to the phospholipid bilayer of cells to protect the cell membrane and promote the expression of growth factors and the proliferation of fibroblasts, thereby accelerating wound healing.85–87Poly(N-isopropylacrylamide) (PNIPAM) is a temperature-sensitive polymer polymerized from the monomer N-isopropyl acrylamide (NIPAM) via free-radical solution polymerization.88 The molecular chain of PNIPAM contains both hydrophobic isopropyl (–CH(CH3)2) groups and hydrophilic amide (–CONH–) groups in its structure (Fig. 2f).89 Polymer molecules form hydrogen bonds with each other because of hydrogen bonding with water molecules. PNIPAM dissolves in water to form a solution at room temperature and precipitates after increasing the temperature.90,91 This property makes PNIPAM suitable for biomedical applications, such as controlled wound dressings, drug delivery systems, and engineering scaffolds.92–94 Jiang et al. developed a new hydrogel, acryloyl-lysine (A-Lys), which was incorporated into poly(ethylene glycol)-crosslinked PNIPAM.95 It exhibits good biological activity while maintaining its swelling properties and thermal reaction behavior. Polyhexamethylene biguanide, an antimicrobial agent, was loaded onto hydrogels. These materials can promote wound healing and provide active antimicrobial agents that inhibit infections.
Poly(ethylene glycol) (PEG)-based polymers are water-swellable hydrophilic cross-linked polymers with high elasticity (Fig. 2g). Their hydrophilic and nonionic characteristics make them highly biocompatible and relatively resistant to protein adsorption;96 thus, they are suitable for use in medical products, such as drug delivery and tissue engineering. The copolymerization of biocompatible polyesters and PEG forms hydrogel systems. By appropriately adjusting the lengths of the hydrophobic polyester and PEG blocks, a gel system with thermally responsive properties can be obtained, and its thermally responsive properties can be improved. Poly((lactic acid)-co-(glycolic acid)) and poly(ethylene glycol) (PLGA–PEG–PLGA) have the features of biocompatibility, thermal sensitivity and non-toxicity. The temperature sensitivity of the copolymer was controlled by hydrophobic (PLGA) and hydrophilic (PEG) groups in the polymer. Chen et al. prepared flexible synthetic hydrogel sealants using PEG. This study showed that PEG-based hydrogels have good biocompatibility and safety.97 The effectiveness and convenience of using PEG-based hydrogels for wound closure and bleeding control in animal studies are discussed.
Mechanism of thermosensitive hydrogels
Thermosensitive hydrogels are water-soluble polymer systems mainly composed of a macromolecular backbone with hydrophilic and hydrophobic groups. They can change hydrophilic and hydrophobic properties and the size and volume of the gel network with a change in ambient temperature and undergo sol–gel or gel–sol phase transition.98 At the molecular level, the temperature sensitivity of thermosensitive hydrogels is due to hydrogen bonding between hydrophilic groups and water molecules and hydrophobic interactions between hydrophobic groups in the structure.99 With a change in temperature, the hydrophilic and hydrophobic interactions of the hydrogel are affected, leading to a change in the internal network structure and overall volume of the gel.Thermosensitive hydrogels are classified as thermal shrinkage- and thermal expansion-type thermosensitive hydrogels according to their swelling mechanism.100 A heat-shrinkable thermosensitive hydrogel is defined as being in a state of contraction when the temperature is higher than the phase transition temperature, and expansion when the temperature is lower than the phase transition temperature. The opposite was observed for the thermally expanding thermosensitive hydrogel: it shrank at temperatures below the phase transition temperature and expanded at temperatures above the phase transition temperature. The corresponding critical temperature is called Volume Phase Transition Temperature (VPTT). These features are key to applications in drug delivery, cell culture, tissue engineering, protein delivery, gene therapy, wound healing etc.36
The LCST represents the low critical solution temperature during phase separation.101 When the temperature is lower than the LCST, the molecular chain is mainly formed by hydrogen bonding between the hydrophilic group and the water molecule. The molecular chain stretches and opens, allowing the gel to fully absorb water and swell into a liquid shape. When heated above the LCST, intermolecular hydrogen bond interactions were destroyed, and hydrophobic interactions became dominant (Fig. 3a). At this time, the molecular chain contracted, and the gel was dehydrated and contracted. Around the upper critical solution temperature (UCST), thermally responsive polymers undergo phase transitions. When the environment is below the UCST, the material does not dissolve. When heated above the UCST, the material dissolves (Fig. 3b). The swelling and contraction of the thermosensitive hydrogel are shown in Fig. 3c.
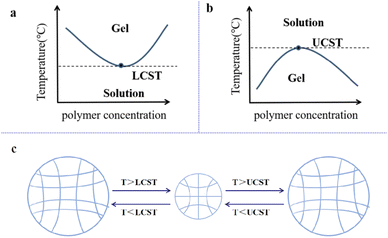 | ||
| Fig. 3 (a) The LCST-type formulation undergoes sol–gel transition with an increase in temperature. (b) UCST-type formulation undergoes sol–gel transition as the temperature decreases. These figures have been reproduced from ref. 21 with permission from MDPI, copyright 2022. (c) Schematic of the swelling/contraction of the temperature-sensitive hydrogel. | ||
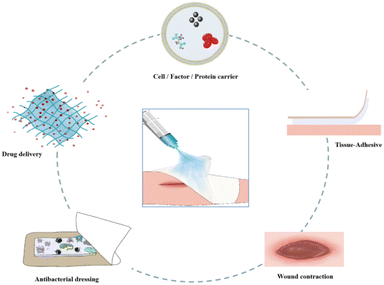
Various types of thermosensitive hydrogel dressings
Thermosensitive hydrogels are of great interest in the biomedical field owing to their biodegradability, non-toxicity, inherent biocompatibility, wide availability, and embracing functionality. The excellent temperature sensitivity and injection characteristics of thermosensitive hydrogels make them suitable for drug, cell, protein, or factor delivery,102–104 and they can be used to fill any irregular wound site,105 showing their characteristics and advantages for wound healing (Fig. 4). A summary of the applications, name of dressing composition, main features and other relevant information about thermosensitive hydrogel dressings is presented in Table 1.| Application | Name of dressing | Composition | Main features | T sol–gel | Ref. |
|---|---|---|---|---|---|
| Antibacterial dressing | HPCH/TA/Fe hydrogel | Chitin/tannic acid/ferric ion (HPCH/TA/Fe) | Thermosensitive, injectable, pH-sensitivity and good antibacterial properties | 37 °C | 106 |
| PLELnBG–QCS-C hydrogel | The catechol-modified quaternized chitosan (QCS-C), poly(D,L-lactide)–poly(ethylene glycol)–poly(D,L-lactide) (PLEL), nano-scaled bioactive glass (nBG) | Thermosensitive, injectable and antibacterial properties | 32.6 °C | 107 | |
| CS hybrid hydrogels | Chitosan (CS) and poly(D,L-lactide)–poly(ethylene glycol)–poly(D,L-lactide) (PPP) | Thermosensitive, applied conveniently, good fluidity, hemostatic and antibacterial barrier | 37 °C | 108 | |
| HA–POL hydrogel | Hyaluronic acid (HA) –poloxamer 407(HA–POL) | Thermosensitive, bio-degradable, bacterial invasion, greater air permeability and effective moisturizing | 30.0 °C | 109 | |
| Drug delivery | IB–Hep–PNIPAM hydrogel | Poly(N-isopropylacrylamide), N-acetylated heparin | Load and release of ibuprofen | 37 °C | 110 |
| Thermosensitive and injectable | |||||
| Curcumin–Alg–pNIPAM hydrogel | Poly(N-isopropylacrylamide), sodium alginate (Alg-g-pNIPAM) | Delivery of curcumin | 27–42 °C | 111 | |
| Thermosensitive, injectable, anti-oxidant and anti-inflammatory properties | |||||
| Cur-M-H | Curcumin (Cur), poly(ethylene glycol), PEG–PCL copolymer | Load and release of Cur | 37 °C | 112 | |
| Thermosensitive, biodegradable, antioxidant and anti-inflammatory, well tissue adhesiveness | |||||
| Dihydromyricetin-loaded hydrogel | Poloxamer, chitosan, hyaluronic acid | Load and release of dihydromyricetin | 37 °C | 113 | |
| Thermosensitive, good biocompatibility, non-toxic, antioxidant and anti-inflammatory | |||||
| AuNPs hydrogel | Poloxamer 407, gold nanoparticles (AuNPs), polyethylene glycol (PEG), poly allyl amine hydrochloride (PAH) | Load and release of AuNPs | ∼35.5 °C | 114 | |
| Thermosensitive, excellent colloidal stability and antibacterial properties | |||||
| MSC&FMZC hydrogel | F127 hydrogel, MgO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnO/chitosan hybrid particles (FMZC) ZnO/chitosan hybrid particles (FMZC) |
3D mesenchymal stem cells (MSCs) delivery vehicle | 37 °C | 115 | |
| Thermosensitive, suitable, nontoxic, higher antibacterial and antibiofilm activity | |||||
| Ni4Cu2/F127 hydrogel | F127 hydrogel, Ni4Cu2 hollow nanospheres | Load and release of therapeutic nanozymes | 24 °C | 116 | |
| Thermosensitive, sprayable, nontoxic, phase-change, porous, anti-inflammatory properties, angiogenesis | |||||
| CP-nCur/gelatin hydrogel | Chitosan-P123 (CP), gelatin | Load and release of curcumin (nCur) | 36.7 °C | 70 | |
| Thermosensitive, injectable, biocompatible, biodegradable | |||||
| CH/GP/Cur hydrogel | Chitosan/β-glycerophosphate (CH/GP) | Load and release of curcumin (Cur) | 29 °C | 117 | |
| Thermosensitive, distinct anti-oxidative, antimicrobial and anti-nuclear factor-κB-signaling capacities | |||||
| Icariin + PEG hydrogels | DL-Lactide, glycolide and polyethylene glycol | Load and release of icariin | 30 °C | 118 | |
| Thermosensitive, good mechanical properties and biocompatibility | |||||
| PDA/P2–4@Ag gel | Poly(ε-caprolactone-co-glycolide)-b-poly(ethylene glycol)-b-poly(ε-caprolactone-co-glycolide) (PDA/P2) triblock copolymer | Load and release of Ag | 34–32 °C | 119 | |
| Thermosensitive, reactive oxygen species (ROS)-scavenging and antibacterial properties | |||||
| PNI/RA-Amps/E hydrogel | RA-Amps (RADA16-Acp-RRWRVIVKW), antibacterial peptide (Amps), PNIPAM | Load and release of MGF E peptide | ∼32 °C | 120 | |
| Thermosensitive, injectable, good biocompatibility, good mechanical properties, and antibacterial and carrier functions | |||||
| PNIPAAm–CNC hybrid hydrogels | Poly(N-isopropylacrylamide) (PNIPAAm), cellulose nanocrystals (CNC) | Load and release of metronidazole | 36–39 °C | 121 | |
| Thermosensitive, injectable, antimicrobial properties | |||||
| Cell/factor/protein carrier | Hydrogel-BMSCs | N,N′-Bis(acryloyl)cystamine, N-isopropylacrylamide (NIPAM) | Load with bone marrow-derived mesenchymal stem cells (BMSCs) | 37 °C | 122 |
| Thermosensitive, biocompatible, can provide a relatively aseptic and stable environment for the wounds and the transplanted BMSCs | |||||
| SA/bFGF@pNIPAM/DS@p(NIPAM-co-AA) hydrogel | Sodium alginate (SA), poly(N-isopropylacrylamide) nanogels (pNIPAM NGs), p(N-isopropylacrylamide-co-acrylic acid) nanogels [p(NIPAM-co-AA) NGs] | Delivery of diclofenac sodium (DS) and basic fibroblast growth factor (bFGF) | 33 °C/40 °C | 92 | |
| Thermosensitive, good mechanical properties, high fluid uptake ability, appropriate water vapor transmission rate, and no cytotoxicity | |||||
| Chitosan/collagen/β-GP hydrogels | Chitosan/collagen/β-glycerophosphate (β-GP) | Delivery of 3D Mesenchymal Stem Cell (3D MSCs) | 37 °C | 123 | |
| Thermosensitive, injectable and provides an environment conductive to the attachment and proliferation of encapsulated 3D MSCs | |||||
| Hydrogel-hUCMSCs | Chitosan/glycerol phosphate sodium/cellulose nanocrystals CS/GP/CNC | Load with human umbilical cord-mesenchymal stem cells (hUCMSCs) | 32.5 °C | 124 | |
| Thermosensitive, injectable and low toxicity | |||||
| PU/hydrogel | N-Isopropyl acrylamide (NIPAAm), acrylic acid (AAc) | Load and release of fibroblast growth factor-2 (FGF-2) | 32 °C | 125 | |
| Thermosensitive, injectable, easy to strip off and absorb exudates | |||||
| PF–MC hybrid hydrogel | Gly-TETA (GT), Pluronic F-127 (PF) and methylcellulose (MC) | Load and release of siMMP-9 | 37 °C | 126 | |
| Thermosensitive, excellent biocompatibility and no toxicity | |||||
| PF-127/hADSCs–Exos complexes | Pluronic F-127 (PF-127) | Release of hADSCs–Exos | 37 °C | 127 | |
| Thermosensitive, injectable and biocompatible | |||||
| β-GP hydrogel | Amniotic membrane (AM), chitosan/β-glycerophosphate (β-GP), polylactic acid (PLA) microparticles | Load with H2O2 | 32.4 ± 2 °C | 128 | |
| Thermosensitive, injectable, nontoxic and oxygen-generating capacity | |||||
| CSMNA patch | Chitosan (CS), microneedless (MN), N-isopropylacrylamide (NIPAM) | Load and release of VEGF | 37 °C | 129 | |
| Thermosensitive, microneedle structure and antibacterial property | |||||
| PLEL hydrogel | Poly(D,L-lactide)–poly(ethylene glycol)–poly(D, L-lactide) (PDLLA–PEG–PDLLA: PLEL), platelet-rich plasma (PRP) and growth factors (GFs) | Load and release of PRP GFs | 37 °C | 130 | |
| Thermosensitive, injectable and nontoxic | |||||
| EGF-Cur-NP/H hydrogel | Nanoparticle/hydrogel (NP/H), growth factor (EGF) and curcumin (Cur) | Load and release of EGF and Cur | 37 °C | 131 | |
| Thermosensitive, injectable, low cytotoxicity, safety, biodegradable | |||||
| CS-C/OPM/β-GP hydrogel | Catechol-functionalized chitosan (CS–C), β-sodium glycerophosphate (β-GP) and oyster peptide microspheres (OPM) | Load and release of oyster peptide | 36 °C | 132 | |
| Thermosensitive, no cytotoxicity, good cytocompatibility and blood compatibility | |||||
| eLHBC hydrogel | L-DOPA and ε-poly-L-lysine, hydroxybutyl chitosan (HBC) | Delivery of mesenchymal stem cells (MSCs) | 17.68 °C | 133 | |
| Thermosensitive, antimicrobial properties and prevention of wound infection and inflammation response | |||||
| sECM–MC hydrogels | Soluble extracellular matrix and methylcellulose (sECM), methylcellulose (MC) | Delivery of stem cells | 34 °C | 134 | |
| Thermosensitive, injectable and soluble | |||||
| HBC/hPL hydrogel | Hydroxybutyl chitosan (HBC), human platelet lysate (hPL) | Load and release of hPL | 14 °C | 135 | |
| Thermosensitive and soluble | |||||
| PF127-PT-b1 gel | Pluronic F-127 (PF127), antimicrobial peptidomimetics (AMPMs) | Release of PT-b1 | 22 °C | 136 | |
| Thermosensitive, antibacterial properties and absorbable | |||||
| Tissue-adhesive hydrogels | Gel–PCLA hydrogel | Gelatins (Gs), b-poly(caprolactone-co-lactide) (PCLA) | Thermosensitive, injectable, tissue-adhesive, porous structure, biocompatibility and biodegradability | 37 °C | 137 |
| NIPAAm–DAA–HA hydrogel | N-Isopropyl acrylamide, dopamine acrylate (DAA) and stearyl methacrylate (C18) | Thermosensitive, adhesive, self-healing, antibacterial properties and dual injection system | 25∼32 °C | 138 | |
| DN gel hydrogel | Catechol–Fe3+ and NIPAAm-methacryloyl | Thermosensitive, mechanical flexibility, good adhesive strength, injectable, self-healing, antibacterial, and hemostatic properties | 37 °C | 139 | |
| DG-loaded HP hydrogel | Hydroxypropyl chitosan (HPCS) and poly(N-isopropylacrylamide) (PNIPAM) | Load and release of dipotassium glycyrrhizinate (DG) | 37 °C | 140 | |
| Thermosensitive, injectable, highly ductile, self-healable, biocompatible and antimicrobial activity | |||||
| GA–HGC hydrogels | Hexanoyl glycol chitosan (HGC), gallic acid (GA) | Thermosensitive, self-healing properties, high compressive strength, strong tissue adhesive, strength biodegradability, excellent biocompatibility | 35 °C | 141 | |
| Wound contraction | QCS/rGO–PDA/PNIPAm hydrogel | Quaternized chitosan (QCS), polydopamine-coated reduced graphene oxide (rGO–PDA), and poly(N-isopropylacrylamide) (PNIPAm) | Thermosensitive, biomechanically active, good self-healing property, self-contraction and tissue adhesion properties, drug release ability, anti-infection, antioxidation, and conductivity | 33 °C | 142 |
| PNIPAm–AA/QCS–CD/PPY hydrogels | Quaternized chitosan-graft-cyclodextrin, adenine, and polypyrrole nanotubes | Thermosensitive, suitable mechanical properties, self-healing, on-demand removal, antioxidant, hemostasis, and photothermal/intrinsic antibacterial activity | 32 °C | 143 | |
| Rg3-gel | Ginsenoside Rg3, poloxamer 407 | Thermosensitive, injectable, good network structures, swelling water retention capacity, excellent biocompatibility, good antibacterial, antioxidant properties and wound contraction | 22 °C | 144 | |
| Reticular supramolecular hydrogels | Poly(N-isopropylacrylamide) (PNIPAM) | Thermosensitive, self-healing, contraction, low cytotoxicity and good biocompatibility | 31 °C | 145 | |
| GNA hydrogel | Methacrylic anhydride modified gelatin (GelMA), acrylic acid (AA) and N-isopropylacrylamide (NIPAM) | Thermosensitive, mechanically active and wound contraction | 35 °C | 146 |
Antibacterial dressing
Infection is a common complication of wounds, and its prevention is very important in wound repair. If the wound is infected, it will cause wound suppuration, prolong healing time, and even cause sepsis in severe cases, endangering life and health. Therefore, important requirements have been proposed for the antibacterial properties of wound dressings. Hydrogels with antibacterial activity, such as chitosan, contain antibacterial structures in their natural polymers and related derivatives. Ma et al. developed a novel in situ injectable hydroxypropyl chitin/TA/ferric ion (HPCH/TA/Fe) composite hydrogel.106 The incorporation of TA provided hydrogen bonding and hydrophobic forces between the TA and HPCH, which improved the mechanical strength of the HPCH hydrogel. When the temperature is below the temperature of gelation (Tgel), the pre-cooled hydrogel precursor solution can be injected into an irregular wound area and the solution rapidly gels at a physiological temperature. Also, the TA in hydrogels can be used as a crosslinker to improve the mechanical properties of hydrogels. In addition, it can be used as an antibacterial agent and is released continuously for up to seven days in acidic environments (Fig. 5a). Similarly, a hybrid hydrogel was prepared using CS and poly(D,L-lactide)–poly(ethylene glycol)–poly(D,L-lactide) (PPP) through a simple process for convenient application.108 PPP molecules established interaction with CS through H-bonds and formed complexes as loose long chains, in contact with each other, responding to body temperature and rapidly forming hydrogels within approximately 5 s. The optimal formula included a 7% CS PPP micellar solution, exhibiting excellent liquidity and allowing optional application as a spray. The formed hydrogels showed tight adherence to the human skin, rapidly ceased bleeding, effectively inhibited bacterial growth (Fig. 5b), and accelerated wound healing. Moreover, this simple hybrid hydrogel can be easily prepared and applied and has potential applicability in clinical wound treatment. Although hydrogel dressings can protect wounds from infection, they can be deformed by external forces, leading to infection. Thermosensitive hydrogel materials offer a solution to this problem; when the external environment changes, a phase transition occurs, which can quickly fill irregular wound areas and form gels under physiological conditions. Therefore, the use of thermosensitive hydrogels with antibacterial properties for wound healing is highly desirable.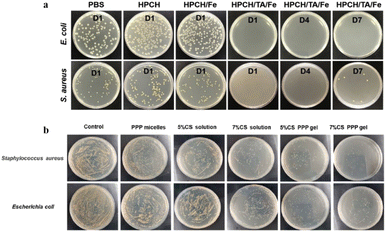 | ||
| Fig. 5 (a) Bacterial colonies of E. coli and S. aureus after coculture with PBS, HPCH, HPCH/Fe (day 1), and HPCH/TA/Fe (days 1, 4, 7) hydrogels, respectively. This figure has been reproduced from ref. 106 with permission from Elsevier, copyright 2020. (b) Inhibition of bacterial growth in a rat liver hemorrhage model. This figure has been reproduced from ref. 108 with permission from Elsevier, copyright 2021. | ||
Drug delivery
Drugs play an important role in wound healing.147 Thermosensitive hydrogels have been used as drug carriers in the biomedical field148–150 because of their injectability, ability to carry therapeutic agents for specific site delivery, prolonged drug effects, improved patient compliance, and reduced systemic toxicity. Thermosensitive hydrogels can be used as drug carriers for local delivery and activate immune cells to promote wound healing.Heparin was coupled to poly(N-isopropyl acrylamide) by developing an injectable in situ gel-forming polymer, and ibuprofen was encapsulated in the hydrogel.110 Hep–PNIPAM hydrogel had the highest swelling ratio and formed a hydrogel at 37 °C. Ibuprofen is a non-steroidal anti-inflammatory drug (NSAID) that is critical during the inflammatory phase of healing; however, it has a short half-life. Ibuprofen was encapsulated in the Hep–PNIPAM hydrogel by simple mixing and was formulated as an injectable solution. When the temperature was up to 37 °C, ibuprofen was released mainly by diffusion and was followed by hydrogel erosion. The ibuprofen release profiles from the Hep–PNIPAM hydrogels are shown in Fig. 6a. The drug release curve revealed that the cumulative release was ∼70% and sustained ibuprofen release lasted for 1 week. Hydrogels create a moist environment on wounds, absorb exudates, and prevent dehydration. In combination with anti-pain drugs, they create an environment conducive to wound healing.
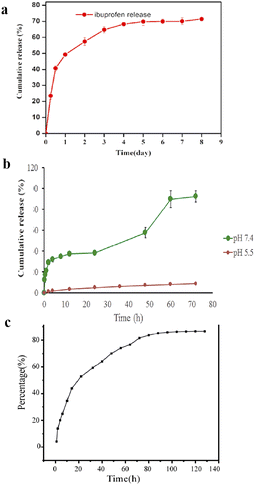 | ||
| Fig. 6 (a) In vitro drug release profiles for the ibuprofen-loaded Hep–PNIPAM hydrogel at pH 7.4 and 37 °C. This figure has been reproduced from ref. 110 with permission from MDPI, copyright 2020. (b) The cumulative in vitro release of curcumin from the formulation as a function of time and pH. This figure has been reproduced from ref. 111 with permission from Elsevier, copyright 2021. (c) In vitro release of dihydromyricetin. This figure has been reproduced from ref. 113 with permission from Elsevier, copyright 2022. | ||
Curcumin is one of the most potent natural antioxidants.151 Therefore, a thermosensitive graft copolymer of alginate pNIPAM was synthesized for curcumin in the wound area.111
The polymer showed excellent thermal gelation properties at different pH and concentration ranges, with good rheological properties. These characteristics allowed the formulation of an injectable dressing of curcumin with easy application to all types of wounds and lesions with different surface geometries and depths. The optimum copolymer showing the gelling temperature at 35 °C, Alg-g-NIPAM(15) was selected for curcumin loading. The cumulative release percentage of curcumin from Cur-F is presented in Fig. 6b. The formulation showed a triphasic release pattern at PBS pH 7.4, the curcumin release time increased up to 72 h (92.4% ± 5.4). This prolonged release of curcumin can be due to both a lag time being needed for the system to absorb water and a slow process of polymer erosion leading to the release of curcumin molecules encapsulated within more internal parts of the gel. This extended release is beneficial for wound healing.
Zhao et al. designed a 37 °C-sensitive hydrogels system using poloxamer 407 as a thermosensitive matrix with chitosan and hyaluronic acid, loaded with dihydromyricetin as a wound dressing.113 They took the gelation time and temperature as response values to optimize the proportion of various materials and obtained the formula that can be used as a gel at the temperature closest to the human body. Then, 10% dihydromyricetin was added to the gel for structural characterization. Scanning electron microscopy showed that the dihydromyricetin hydrogels performed better in terms of homogeneous distribution and structural quantity, enabling dihydromyricetin to enter cells and be released. The hydrogel was able to release myricetin dihydrate at 37 °C for up to 100 h, and the cumulative release rate was 86% according to the in vitro drug release test (Fig. 6c); therefore, it can reduce the frequency of repeated dressing changes in clinical practice.
Thermosensitive hydrogels for drug delivery were developed by mixing thermosensitive polymers with other natural polymers. The critical gelation transition temperature is close to the physiological environmental temperature of the human body and can be regulated, playing the role of continuous drug release at the right time and target site, thereby improving its suitability and sustainability as a drug carrier. As a physically cross-linked temperature-sensitive hydrogel, there are no organic solvents or nontoxic initiators in the preparation and use processes, which makes it safer. Therefore, thermosensitive hydrogel materials are ideal for sustained drug-release systems.
Cell/factor/protein carrier
Dressings loaded with cells, factors, or proteins can accelerate wound healing. Hydrogels have a high water content and a structure similar to that of the natural extracellular matrix, which can be used as a carrier for cell delivery.152 In addition, thermosensitive hydrogels can effectively control and continuously release proteins, avoiding adverse side effects that may occur due to the instability of proteins and the need for repeated dosing over time.153The treatment of local wounds with mesenchymal stem cells (MSC) is widely used as a promising approach to enhance tissue regeneration.154–156 A new strategy was proposed in which three-dimensional mesenchymal stem cell (3D MSC) spheroids were combined with an injectable thermosensitive chitosan/collagen/β-glycerol phosphate (β-GP) hydrogel to accelerate chronic wound healing by enhanced vascularization and paracrine effects.123 (Fig. 7a). Chitosan/collagen/β-GP solution mixed with 3D MSC spheroids was rapidly turned into a gel at body temperature by physical cross-linking, completely covering the wound. The hydrogel provided an environment conducive to the attachment and proliferation of encapsulated MSCs, particularly by accelerating the proliferation and paracrine factor secretion of 3D MSC spheroids, thereby promoting wound healing in mice.
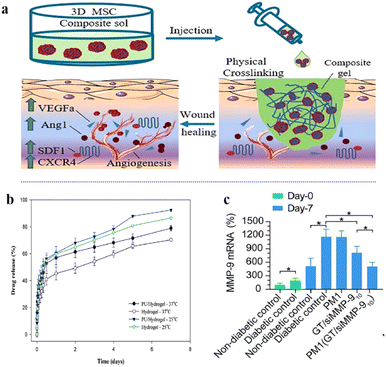 | ||
| Fig. 7 (a) Schematic illustration of the formation of injectable material. The hydrogel-3D MSCs combination. This figure has been reproduced from ref. 123 with permission from Elsevier, copyright 2022. (b) The release profile of FGF-2 from the hydrogel and PU/hydrogel composite at 25 °C and 37 °C. This figure has been reproduced from ref. 125 with permission from the Royal Society of Chemistry, copyright 2015. (c) MMP-9 protein levels in skin tissues of rats by western blot at day-0 and day-7 after respective treatments. This figure has been reproduced from ref. 126 with permission from BMC, copyright 2021. | ||
Lin et al. developed a polyurethane (PU)/hydrogel, a copolymer of the thermosensitive N-isopropyl acrylamide (NIPAAm) and acrylic acid (Aac).125 NIPAAm was simultaneously copolymerized with Aac, the hydrogel was grafted onto porous PU using-ray irradiation, and fibroblast growth factor-2 (FGF-2) was loaded into the composite. The release of FGF-2 can last for at least 7 days (Fig. 7b), because the interactions between the negative charge of Aac (with COO–) and the positive charge of FGF-2 maintain its slow release. This prevents the fast release of FGF-2 due to its high diffusibility. Therefore, the temperature-sensitive feature of the hydrogel can be used to control the release of the active ingredient, as well as to absorb the wound exudate and to keep the wound in a moist environment. The PU/hydrogel composite strips are easier to use than commercial wound dressings due to their thermal sensitivity properties.
The process of wound healing is closely related to the dynamic balance of extracellular matrix (ECM).157 Matrix metalloproteinases (MMPs) play an important role in regulating the metabolic balance between ECM synthesis and degradation.158 A hybrid thermosensitive hydrogel dressing was developed for the localized and prolonged delivery of siMMP-9.126 The thermosensitive hydrogel consisted of Pluronic F-127 (PF) and methylcellulose (MC), and the GT/siMMP9 complex was loaded onto the hydrogel. The siMMP-9 was first complexed with GT via electrostatic interaction, and then GT/siMMP-9 was encapsulated in PM hydrogel. The thermosensitive hybrid hydrogel could be formed in situ and then form a strong interface with arbitrarily shaped wounds. In addition, the hybrid hydrogel released GT/siMMP9 continuously through temperature-sensitive control for up to seven days, resulting in significant MMP-9 silencing (Fig. 7c) and accelerated wound healing in diabetic rats.
Traditional thermal gels often exhibit poor physical properties and low binding affinities for cells or proteins, which limit their practical application. Modified thermosensitive hydrogels can be used as carriers of cells or proteins for wound healing owing to their good biocompatibility, biodegradability, and physical stability.
Tissue-adhesive hydrogels
Although various types of hydrogel dressings have been used in soft tissue regeneration, the high water content of the hydrogel polymer network makes them brittle.159 Hydrogels with superior mechanical properties meet the requirements for tissue regeneration. Therefore, it is necessary to develop adhesive, elastic, and biodegradable hydrogels. Many researchers have developed synthetic hydrogels that mimic biological tissues. However, the damaged covalent bonds in the hydrogel network cannot be easily recombined when the hydrogels are subjected to cyclic loading or deformation,160–162 and exhibit poor adhesion or self-healing abilities. Biocompatible and biodegradable polymer materials can be used in composite hydrogel wound dressings for rapid tissue adhesion and closure.b-Poly(caprolactone-co-lactide) (PCLa-b-PEG-b-PCLA, referred to as PCLA), a bioabsorbable temperature-sensitive block copolymer, is conjugated to the backbone of injectable gelatins (IGs) to form a dynamic coordination network.137 The investigator adhered an aqueous solution of PCLA copolymer hydrogel and IG conjugate hydrogel to the rat skin (Fig. 8a). The higher number of PCLA block polymer in the conjugates creates a hydrophobic balance to maintain the gel state at body temperature (37 °C). In addition, the existence of functional groups in the Gel–PCLA conjugate can make hydrogen bonding that contributes to the improvement of gel strength. The PCLA copolymers showed temperature-sensitivity and the freely flowing PCLA copolymer sols at low temperature were transformed into stable hydrogels. Therefore, the in situ formation of IGs can be considered as promising wound dressing materials for the treatment of multiple wounds.
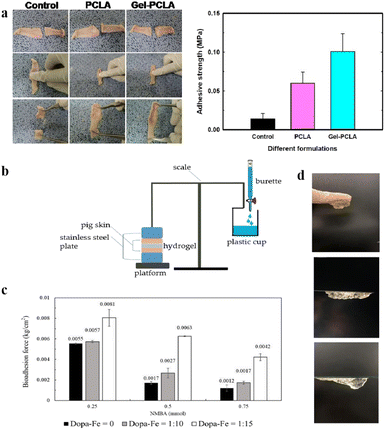 | ||
| Fig. 8 (a) The adhesive strength of PCLA and IG hydrogels was estimated through a universal testing machine. This figure has been reproduced from ref. 137 with permission from the American Chemical Society, copyright 2019. (b) A bioadhesion testing device by gravimetric measurement. (c) Comparison of hydrogel bioadhesion with DAA–Fe3+ ratios and different concentrations of crosslinking agent (NMBA). (d) Image of adhesive hydrogels on the glove, polystyrene, and glass surfaces. These figures have been reproduced from ref. 138 with permission from MDPI, copyright 2022. | ||
An injectable multifunctional hydrogel of a mussel-inspired adhesive and self-healing hydrogel was shown to be suitable as a wound-healing dressing for treating deep wounds.138 This was inspired by ocean mussels, which can use catechol groups and metal ions to attach to the surfaces of wet objects in fluid environments and self-heal after taking damage.163,164 Using a thermally sensitive material as the hydrogel base, the sol–gel transformation can be controlled according to temperature changes in the human body. Methacrylate (C18) was added to improve the mechanical properties. Furthermore, chelation was conducted between dopamine acrylate with a catechol group and iron ions (Fe3+) in a ferric chloride solution. A sol–gel transition can be quickly achieved through the thermosensitive properties and action of catechol–iron ions, which produces adhesion and self-healing effects. The bioadhesion experimental setup is shown in Fig. 8b. The results showed that as the DAA–Fe3+ ratio increased, the adhesiveness of the hydrogel increased (Fig. 8c). The actual adhesion properties of the gel are presented, including its successful adhesion to a glove, polystyrene plate, and glass slide (Fig. 8d).
The hydrogel covered the wound while absorbing exudate and blood from the wound, creating a protective barrier against bacterial infection. However, owing to the high water content of the hydrogel, the wet wound environment, and continuous exudation, it is difficult for hydrogel dressings to adhere to the wound tissue. Tissue adhesion in healing dressings is critical for deep-tissue wounds. Thus, injectable hydrogels that keep a wound moist while maintaining high adhesion can be used for further applications in wound healing.
Wound contraction
Wound closure is a key step in surgery and trauma handling and it allows for the biological event of healing through the joining of wound edges.165 The hydrogels' thermoresponsive self-contraction ability assists wound closure in the early stages of wound healing. When the hydrogel adheres tightly to the surrounding skin, the contraction of the hydrogel triggers the wound closure. Therefore, a kind of novel multifunctional wound dressing that has biochemical self-contraction ability while promoting wound closure is desired for wound-healing application.A recent study reported a two-pronged strategy of biomechanically active and biochemically functional hydrogel wound dressing.142 They used a series of biomechanically active injectable self-healing hydrogels based on quaternized chitosan (QCS), polydopamine-coated reduced graphene oxide (rGO–PDA), and poly(N-isopropylacrylamide) (PNIPAm) as multifunctional wound dressings. QCS, rGO–PDA, and NIPAm monomers were fully mixed, and the QCS/rGO–PDA/PNIPAm hydrogels were synthesized with the addition of initiator APS/TEMED and cross-linker BIS via free radical polymerization. The researchers applied a hydrogel with outstanding tissue adhesion properties on the wound. Subsequently, because of the thermoresponsive self-contraction ability, when the temperature increased above 33 °C (LCST of PNIPAm), the volume of the hydrogel decreased rapidly, which was beneficial for the initial stage of wound healing through the contraction force-assisted closure of cracked wounds (Fig. 9a). Hydrogels adhere strongly to the skin and assist wound closure by actively contracting wounds through self-contraction.
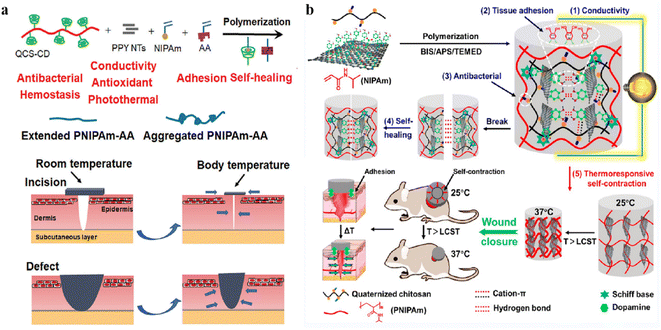 | ||
| Fig. 9 (a) Schematic diagram of the hydrogels assisting wound closure by thermoresponsive self-contraction. This figure has been reproduced from ref. 142 with permission from the American Chemical Society, copyright 2022. (b) Schematic representation of the PNIPAm–AA/QCS–CD/PPY hydrogel via a dual physical crosslinking strategy and the illustration of simultaneous wound closure and dressing assisted by PNIPAm–AA/QCS–CD/PPY hydrogel. This figure has been reproduced from ref. 143 with permission from Wiley-VCH GmbH, copyright 2022. | ||
Yu et al.143 developed a novel supramolecular poly(N-isopropylacrylamide) hybrid hydrogel dressing composed of quaternized chitosan-graft-cyclodextrin, adenine, and polypyrrole nanotubes via host–guest interactions and hydrogen bonds. Adenine was introduced into the PNIPAm polymer backbone via free-radical polymerization forming PNIPAm–AA. The researchers added QCS for its biocompatibility, high solubility, inherent antibacterial activities, and synergistic tissue adhesion. At the same time, PPY NTs were introduced to further impart antioxidant properties, antibacterial capacity, and conductivity to the hydrogels. Physical crosslinking, including host–guest interactions and hydrogel bonding, was employed to fabricate the multifunctional hydrogels in this work (Fig. 9b). The hydrogel demonstrated a thermal contraction of 47% of the remaining area after 2 h at 7 °C and tissue adhesion of 5.74 kPa, which are essential for noninvasive wound closure. PNIPAm–AA/QCS–CD/PPY hydrogels adhere strongly to the skin and assist wound closure by actively contracting wounds through self-contraction to promote wound closure and wound healing.
Thermosensitive hydrogel for diverse wounds
Skin wound healing results from a series of complex biological and molecular events, including angiogenesis, cell adhesion, migration, proliferation, differentiation, and extracellular matrix (ECM) deposition.166 Several factors affect the process and duration of wound healing. Appropriate wound dressings play important roles in promoting wound healing. Thermosensitive hydrogels are characterized by their excellent temperature sensitivity and injection characteristics and can be used to fill irregular wound sites. Simultaneously, they can be loaded with bioactive molecules or drugs to make functional complementation dressings. Thermosensitive hydrogels can be used to treat chronic diabetic, burn, and surgical wounds (Fig. 10). A summary of thermosensitive hydrogel dressings for diverse wounds is presented in Table 2.| Application | Name of dressing | Composition | Main features | T sol–gel | Ref. |
|---|---|---|---|---|---|
| Chronic diabetic wounds | SOD–PP hydrogels | Poly(N-isopropyl-acrylamide)/poly(γ-glutamic acid) (PP) | Load and release of superoxide dismutase (SOD) | 28 °C | 75 |
| Thermosensitive, injectable, antioxidant and good biocompatibility | |||||
| PM (GT/siMMP-9) hydrogel | Pluronic F-127 (PF) and methylcellulose (MC) | Load and release of Gly-TETA (GT)/matrix metalloproteinase 9 siRNA (siMMP-9) | 37 °C | 126 | |
| Thermosensitive, excellent biocompatibility, no toxicity and provided a moist environment | |||||
| BMSCs + hydrogel | N-Isopropylacrylamide (NIPAM), poly(amidoamine) (PAA) and bone marrow mesenchymal stem cells (BMSCs) | Delivery of BMSCs | 33 °C | 171 | |
| Thermosensitive, injectable, anti-inflammatory and biocompatible | |||||
| L. lactis thermo-sensitive hydrogel | Poloxamer | Delivery lactic acid | 37 °C | 172 | |
| Thermosensitive, anti-inflammatory, good biocompatibility | |||||
| CS + LG hydrogel | L-Glutamate acid (LG) and chitosan (CS) | Thermosensitive, good swelling, thermal stability, smooth surface topography, and controlled biodegradability | 37 °C | 199 | |
| ADSCs–PF127 hydrogel | Pluronic F127 (PF127) | Delivery of adipose-derived stem cells (ADSCs) | 37 °C | 173 | |
| Thermosensitive, injectable and good biocompatibility | |||||
| PBNPs@PLEL | Poly(D,L-lactide)–poly(ethylene glycol)–poly(D,L-lactide) (PDLLA–PEG–PDLLA) hydrogel (PLEL) and Prussian blue nanoparticles (PBNPs) | Release of PBNPs | ∼28.2 °C | 176 | |
| Thermosensitive, injectable, anti-inflammatory, ROS scavenging and mitochondrial function restoration | |||||
| PPCN + SDF-1 hydrogel | Poly(polyethylene glycol citrate-n-isopropyl acrylamide) (PPCN) | Load and release of stromal cell-derived factor-1 (SDF-1) | 37 °C | 177 | |
| Thermosensitive, injectable and antioxidant | |||||
| Poxin hydrogel | Poloxamox 407 and insulin (Poxin) | Delivery of insulin | 25 °C | 181 | |
| Thermosensitive, injectable, nontoxic and good biocompatibility | |||||
| ROS-sensitive hydrogel | N-Isopropylacrylamide (NIPAAm), 2-hydroxyethyl methacrylate (HEMA) and benzoyl peroxide (BPO) | Delivery of recombinant human MG53 (rhMG53) protein | 19 °C | 200 | |
| Thermosensitive, injectable and ROS-scavenging | |||||
| CRMs-hydrogel | Pluronic F127, Pluronic F68 | Load and release of curcumin and rifampicin | 33 °C | 201 | |
| Thermosensitive, excellent intracellular and extracellular bacterial | |||||
| CNPs@GMs/hydrogel | Gelatin microspheres (GMs), cur nanoparticles (CNPs) and Pluronic F127 and F68 | Load and release of curcumin (Cur), MMP9-responsive | 32.97 ± 0.52 °C | 71 | |
| Thermosensitive, efficient, safe, anti-oxidants | |||||
| CBP/GMs@Cel&INS hydrogel | Matrix metalloproteinase-9 (MMP-9), polyvinyl alcohol (PVA) and chitosan, phenylboric acid | Load and release of insulin (INS) and celecoxib | 25 °C | 202 | |
| Thermosensitive, injectable, self-adaptive, remodelling and self-healing properties | |||||
| hUCMSC–exos-PF-127 hydrogel | Pluronic F-127 (PF-127), human umbilical cord MSC (hUCMSC)-derived exosomes (hUCMSC-exos) | Delivery of hUCMSC-exos | 12.4–17.8 °C | 203 | |
| Thermosensitive and injectable | |||||
| hASCs-hydrogel | Human decellularized adipose matrix (hDAM) | Delivery of human adipose-derived stem cells (hASCs) | 31 °C | 204 | |
| Thermosensitive and provide a favorable microenvironment for hASCs | |||||
| Nr-CWS hydrogel | Nocardia rubra cell wall skeleton (Nr-CWS), poloxamers 407 and 188 | Load and release of Nr-CWS | 20 °C | 205 | |
| Thermosensitive and good affinity | |||||
| Burn wounds | CLD–hFGF2 hydrogel | Camelina lipid droplets (CLD), poloxamer P188 and P407 | Load and release of human fibroblast growth factor 2 (hFGF2) | 24–25 °C | 190 |
| Thermosensitive, injectable, high bioactivity | |||||
| Chitosan–PDEAAm hydrogel | Chitosan, poly(N,N-diethylacrylamide) (PDEAAm) | Load and release of incorporated antibiotics | 37 °C | 191 | |
| Thermosensitive, easy to remove and antibacterial properties | |||||
| ZnMet–PF127 hydrogel | Pluronic F127 (PF127) | Load and release of ZnMet | 37 °C | 192 | |
| Thermosensitive, sprayable, anti-inflammatory and antibacterial effects | |||||
| AgNPs–MC hydrogel | Methylcellulose (MC) hydrogel, silver oxide nanoparticles (NPs) and silver acetate (CH3COOAg) | Release of Ag ions | 33.6–37 °C | 206 | |
| Thermosensitive, injectable, excellent antimicrobial activity | |||||
| AgSD/NS hydrogel | Silver sulfadiazine/nanosuspensions (AgSD/NSs) and poloxamer 407 | Load and release of AgSD/NS | 29.8 °C | 207 | |
| Thermosensitive, non-toxic and antibacterial | |||||
| CAT–Gel–Alg hydrogel | Gelatin (Gel)–alginate (Alg) biocompatible hydrogel (Gel–Alg) | Delivery of catalase (CAT) and ROS scavenging | 20 °C | 208 | |
| Thermosensitive, antioxidant-biocompatible, safe and sustainable | |||||
| PLX/CS/HA-VM hydrogel | Poloxamer 407 (PLX), chitosan (CS), hyaluronic acid (HA), melatonin (MLT), vitamin A, D3 and E | Delivery of MLT, vitamins A, D3 and E | 30 ± 0.2 °C | 209 | |
| Thermosensitive, antibacterial and antioxidant | |||||
| FA-loaded hydrogels | Ferulic acid (FA), chitosan/gelatin/glycerol phosphate | Load and release of FA | 37 °C | 210 | |
| Thermosensitive, excellent antioxidant and biocompatible | |||||
| MSC–CM/hydrogel | Conditioned medium (CM), chitosan, collagen, β-glycerophosphate (β-GP) and mesenchymal stem cells (MSCs) | Delivery of MSCs | 37 °C | 211 | |
| Thermosensitive, good biocompatibility and low cytotoxicity | |||||
| CEAllo hydrogel | Poloxamer 407 | Thermosensitive, safe and allows for the application of the cells thinly | 20 °C | 212 | |
| RHC–chitosan hydrogels | Chitosan, recombinant human collagen-peptide (RHC) | Release of RHC | 15/17/24 °C | 213 | |
| Thermosensitive, injectable, superior mechanical strength and excellent attachment substrate for cells | |||||
| Surgical wounds | AgSPHA hydrogel | Hyaluronic acid (HA), corn silk extract (CSE) and silver nanoparticles (Ag NPs) | Thermosensitive, injectable, good mechanical properties, antibacterial properties and biocompatibility | 32.5 °C | 214 |
| GT–SA–TPFX hydrogel | Sodium alginate (SA), gelatin (GT), ferric ions and protocatechualdehyde | Thermosensitive, injectable, sufficient mechanical, adhesive strength, good biocompatibility, self-healing capacity and antibacterial and antioxidation properties | ∼43 °C | 197 | |
| TRS hydrogel | N-Isopropylacrylamide, butylacrylate | Thermosensitive, injectable, shape adaptability, safety, effectiveness and easy to remove | 32 °C | 198 | |
| mXG/HBC hydrogel | Galactose-modified xyloglucan (mXG) and hydroxybutyl chitosan (HBC) | Thermosensitive, injectable, excellent cytocompatibility, hemocompatibility and antibacterial properties | mXG/HBC ratio | 215 | |
| ZnG/rhEGF@Chit/Polo hydrogels | Chitosan/poloxamer-based thermosensitive hydrogels, zinc gluconate/recombinant human epidermal growth factor (ZnG/rhEGF) | Load and release of ZnG/rhEGF | 34.85 °C | 216 | |
| Thermosensitive, good cytocompatibility, mild non-irritating and biocompatible | |||||
| PTX-loaded PECE hydrogel | Poly(ethylene glycol)–poly(3-caprolactone)–poly(ethylene glycol) (PEG–PCL–PEG, PECE) | Release of paclitaxel | 37 °C | 217 | |
| Thermosensitive, injectable and biocompatible | |||||
| HBC–PSB hydrogel | Hydroxybutyl chitosan (HBC) and poly(sulfobetaine methacrylate) (PSBMA) | Thermosensitive, injectable, non-toxic, good synergistic antibacterial, excellent self-healing behaviors and anti-adhesion | PSBMA content | 218 |
Chronic diabetic wounds
Diabetes is a common clinical disease. According to the findings of the International Diabetes Federation (IDF), approximately 537 million adults worldwide will be living with diabetes in 2021.167 Diabetic wounds are stimulated by high blood glucose, often accompanied by a persistent inflammatory response, oxidative stress injury, angiogenesis disorders and a high risk of bacterial infection, resulting in skin ulcer, tissue necrosis, wound infection and other problems, and its treatment is complex and expensive, which is a worldwide problem.168 Therefore, in addition to the original properties of hydrogel dressings, an ideal diabetic wound hydrogel should have anti-inflammatory, antibacterial, antioxidant, pro-angiogenic, and hypoglycemic effects.In chronic wounds, repeated tissue damage causes an excessive release of cytokines, continuously stimulating and recruiting immune cells to the injured site, resulting in an overactive inflammatory response and the prevention of wound healing.169,170 Chen et al. developed a biodegradable, multifunctional crosslinker and an N-isopropylacrylamide (NIPAM)-based, thermosensitive hydrogel to carry BMSCs to treat diabetic ulcers.171 The crosslinker contained an RGD-like motif that promoted the attachment and differentiation of BMSCs. The polymer was in the sol state at room temperature; after immersion in a 33 °C water bath, it experienced sol–gel transition in minutes. Researchers seeded BMSCs on the surface of hydrogels, which can adhere to and proliferate on the hydrogel. The results showed that the expression of pro-inflammatory M1-type macrophages in the experimental group was lower than that in the control group, and the expression of M2-type macrophages was higher (Fig. 11a and b). A hydrogel loaded with BMSCs can promote the adhesion and differentiation of BMSC, which is conducive to wound healing and improves the inflammatory microenvironment of diabetic wounds. In another study, a thermosensitive hydrogel dressing loaded with Lactobacillus lactis was prepared.172 The hydrogel material produced and delivered lactic acid in situ, both in vitro and in vivo, promoted the transformation of macrophages from M1 to M2, and accelerated the healing of chronic diabetic wounds.
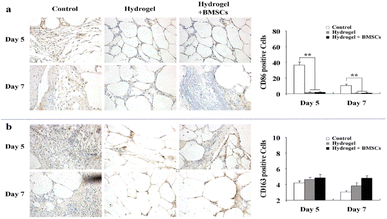 | ||
| Fig. 11 (a) The expression of M1 macrophages. (b) The expression of M2 macrophages. These figures have been reproduced from ref. 171 with permission from Nature, copyright 2015. | ||
Neovascularization plays a crucial role in wound healing. However, microvascular formation in the wounds of patients with diabetic feet is impaired, resulting in wounds being unable to obtain sufficient oxygen, nutrients, and growth factors.171 In a recent study, rat adipose-derived mesenchymal stem cells (ADSCs) were loaded into Pluronic F127 (PF127) to prepare a novel thermosensitive hydrogel material, and the effect of PF127 hydrogel-loaded ADSCs on angiogenesis in large diabetic skin wounds was observed.173 Immunohistochemistry, micro-vessel counting, wound area measurement, and VEGF measurement showed that the experimental group had more new capillaries and faster wound healing than the control group (P < 0.05). PF127 hydrogel-loaded ADSCs accelerated wound healing by promoting the formation of new capillaries in diabetic rat wounds.
High-reactive oxygen species (ROS) concentrations are one of the reasons for chronic wound healing caused by diabetes.174 Dressings that scavenge ROS can effectively reduce the concentration of reactive oxygen species in a wound, improve its inflammatory microenvironment, and promote wound healing.175 Xu et al. prepared a thermosensitive poly(D,L-lactide)–poly(ethylene glycol)–poly(D,L-lactide) (PDLLA–PEG–PDLLA) hydrogel (PLEL)-based wound dressing in which PBNPs were homogeneously incorporated. The system at 37 °C was in a gel state. This injectable PBNPs@PLEL system can adapt to various wound types and form a gel within 30 s in vivo.176 Once placed, PBNPs@PLEL exhibited sustained and controlled release behavior. PBNPs were slowly released and exerted sustained antioxidant activity after the injection of PBNPs@PLEL into the wound site (Fig. 12a). Zhu et al. synthesized poly(polyethylene glycol citrate-n-isopropyl acrylamide) (PPCN) via sequential polycondensation and free radical polymerization reactions.177 PPCN has intrinsic antioxidant properties due to the diol-citrate esters in its backbone that may reduce oxidative stress at the wound after gelatinization above the LCST (37 °C). A thermosensitive hydrogel material with sustained release of SDF-1 was prepared, which not only played an antioxidant role but also accelerated the regeneration of wound granulation tissue and promoted rapid wound healing (Fig. 12b). SDF-1 was introduced to promote angiogenesis and cell migration. When the temperature dropped to 37 °C, there was sustained release of SDF-1 from the hydrogel.
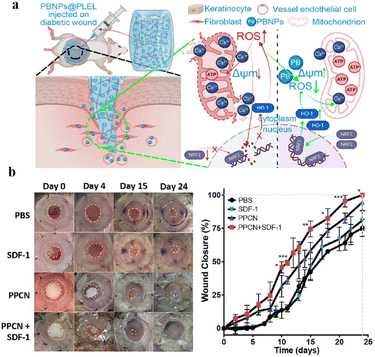 | ||
| Fig. 12 (a) Schematic overview of the use of PBNPs@PLEL for the enhancement of diabetic wound healing due to its ability to scavenge ROS and to promote the functional recovery of mitochondria. This figure has been reproduced from ref. 176 with permission from ACS Publications, copyright 2022. (b) PPCN + SDF-1 promotes the healing of diabetic wounds. This figure has been reproduced from ref. 177 with permission from Elsevier, copyright 2016. | ||
The application of topical insulin has also been shown to be of great help in improving wound healing.178,179 However, due to molecular instability and sustained delivery effects, local insulin administration still faces great challenges.180 A previous study reported that a thermosensitive hydrogel combined with insulin injection improved skin wound healing in diabetic mice.181 Poloxamer 407 is widely employed for drug delivery because it is reported to be nontoxic and can form gels at 25 °C. Poloxamer 407 and insulin were injected into the wounds of diabetic mice and compared with those of other controls to measure the rate of wound healing at 28 days intervals (Fig. 13a). The results showed that the expression of both transforming growth factor beta 1 (TGF-β1) and α-smooth muscle actin (α-SMA) increased significantly in the early stages of diabetic wounds after insulin injection (Fig. 13b). Poloxamer + insulin-treated mice displayed enhanced wound healing.
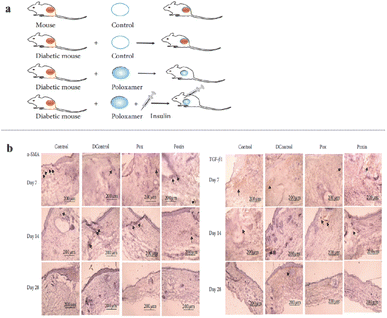 | ||
| Fig. 13 (a) Experimental mice model: control, diabetic mouse (dControl), poloxamer (Pox), and poloxamer plus insulin (Poxin). (b) α-SMA and TGF-β1 antigen expression in the wound bed on days 7, 14, and 28 after wounding: samples, dControl, Pox, and Poxin groups. Black arrows show examples of secretion sites of the antigens. These figures have been reproduced from ref. 181 with permission from Hindawi, copyright 2022. | ||
The emergence of thermosensitive hydrogels has provided a new approach to the management of diabetic wounds. Thermosensitive hydrogels can be attached to different materials to adapt to different types of wounds. The sustained release of drugs or bioactive molecules from thermosensitive hydrogels is important for promoting chronic wound healing. The generation of chronic diabetic wounds is a multifaceted and multilinked process. Based on the different stages of diabetic wound healing, more targeted and individualized thermosensitive hydrogel dressings with additional functions can be designed.
Burn wounds
Burns are a common type of injury in humans and are generally caused by exposure to hot liquids, vapors, or gases.182,183 Different classifications were performed according to the depth of the burn wounds.184 Burn wound dressings need to be replaced frequently, which aggravates pain and discomfort.185 Traditional burn wound dressings easily adhere to the wound after absorbing the wound exudate. When dressings are removed, the wound is injured again, and new tissue tears easily.186,187 Therefore, an ideal dressing for the treatment of burn wounds should provide a local moist wound-healing environment for the body, adapt to different wound shapes, and improve the therapeutic effect of wound-healing drugs.Human fibroblast growth factor 2 (hFGF2) can promote fibroblast proliferation, migration, and differentiation; the local administration of hFGF2 in burn wounds can promote granulation tissue regeneration and angiogenesis,188 but it can easily lose its biological activity.189 In order to provide a supporting environment for hFGF2 and controls its release in a stable manner. Zhang et al. encapsulated camelina lipid droplet (CLD)-hFGF2 in thermosensitive hydrogels.190 The poloxamer composite hydrogels were synthesized from P188 and P407. The shell of these spherical micelles consists of a polyoxyethylene nucleus and an expanded polyoxyethylene chain. With the increase in temperature, the hydrophobic polyoxyethylene segment in the hydrogel loses water and polymerizes into micelles. As the temperature continues to rise, the formation arrangement of micelles forms hydrogels. They can quickly gel on the surface of burn wounds, reduce drug flow loss and improve the drug utilization rate. The prepared CLD-hFGF2 hydrogel can gel with changes in temperature (Fig. 14a) and undergo in vitro degradation. CLD-hFGF2 exhibited an initial burst release from the hydrogels after 12 h (approximately 30%), followed by a slow release over 72 h (almost 60%) after drug delivery, CLD-hFGF2 promoted the proliferation and migration of NIH3T3 cells. In vivo experiments also showed that the CLD-hFGF2 hydrogel accelerated extracellular matrix (ECM) regeneration and angiogenesis, significantly improved burn wound healing rates, and reduced the number of dressing changes. In another study, a heat-responsive wound dressing was synthesized using chitosan and poly(N,N-diethylacrylamide) (PDEAAm).191 It has temperature-dependent and swelling properties and is easy to separate at lower temperatures. This ability to modulate adhesion will facilitate a non-traumatic manner of removal from wound sites, especially burn wounds. Antimicrobial activity studies have demonstrated the ability of the dressing to control the release of incorporated antibiotics and enhance wound healing by inhibiting bacterial growth.
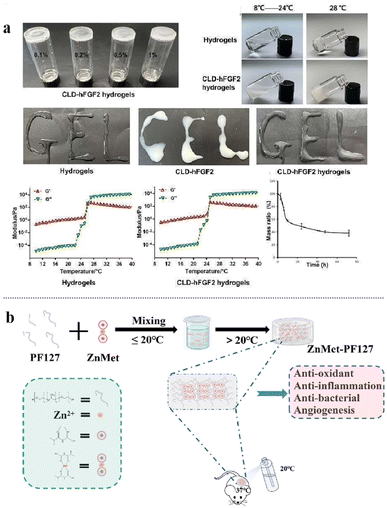 | ||
| Fig. 14 (a) The thermosensitive property of CLD-hFGF2 hydrogels and in vitro degradability. This figure has been reproduced from ref. 190 with permission from MDPI, copyright 2022. (b) Schematic diagram of the ZnMet-PF127 preparation process and application. This figure has been reproduced from ref. 192 with permission from Elsevier, copyright 2022. | ||
Burn wounds vary in depth and area and their shapes are usually irregular. Moreover, owing to the complexity and severity of burn wounds, traditional dressings cannot meet the requirements for burn wound healing. In a recent study, the thermosensitive hydrogel Pluronic F127 (PF127) was used for the first time as a sprayer for skin wound healing (Fig. 14b).192 A coordination complex of zinc and metformin (ZnMet) was loaded onto PF127. The polymer did not change the properties of PF127, and the porous structure allowed the uniform release of the drug in the tissue. Sprayable ZnMet-PF127 evenly filled irregular skin defects in the liquid state. After ZnMet-PF127 was sprayed on the skin wound, it immediately changed from liquid to semi-solid due to its thermosensitive characteristics, isolating the skin wound from the external environment and reducing wound infection. It can also provide a wet environment for burn wounds as well as anti-inflammatory and antibacterial effects to promote wound healing.
Thermosensitive hydrogels can quickly gel at body temperature. When dressings are changed, a gel-solution thermosensitive conversion can be used to facilitate fading and reduce unnecessary pain. At the same time, it is not limited by the shape and depth of the wound and can completely cover the wound due to its injectable features. In addition, thermosensitive hydrogels loaded with drugs or bioactive substances exhibit good biocompatibility, which can promote burn wound healing and reduce the frequency of dressing changes through the sustained release of drugs or factors. These properties make thermosensitive hydrogels promising materials for a wide range of applications in burn wounds.
Surgical wounds
Traditional closure methods for surgical wounds include the use of sutures or staples, which are usually invasive and may cause unsatisfactory tissue integration, trauma, and leakage of content.193–196 Thermosensitive hydrogels exhibit good biocompatibility, tissue adhesion, and temperature-sensitivity. They can avoid secondary injury to the surgical incision, reduce the frequency of dressing changes, and promote wound healing, providing a new choice for surgical wound healing. Recently, a novel bio-inspired injectable wound dressing with superior mechanical properties, adhesion strength, temperature adhesion, and self-healing ability was developed.197 Ferric ions were added to a multifunctional adhesive composed of sodium alginate (SA), gelatin (GT) and protocatechualdehyde for suture-less post-wound closure. The thermal-response phase transition of GT, the Schiff base bond, and the catechol–Fe-coordinate bond in the network give the hydrogel injectable, shape-adaptable, and strong adhesion and self-healing abilities at body temperature. Rat skin incisions were created, and then sutures, biomedical glue, and adhesive hydrogel were used to heal the incision; one without treatment was set as the control group. Compared with the other control groups, the mechanical strength of the skin tissue treated with the hydrogel group was stronger, the incision was smoother, and the incision closure effect was better than that of the other groups (Fig. 15a). This is primarily because the temperature-dependent adhesion of the hydrogel makes the sealant easy to operate. The hydrogel can separate fault-tolerant adhesion from bad adhesion on the instrument by sensing the local temperature. In addition, its good self-healing ability allows the reopened incision to be closed repeatedly.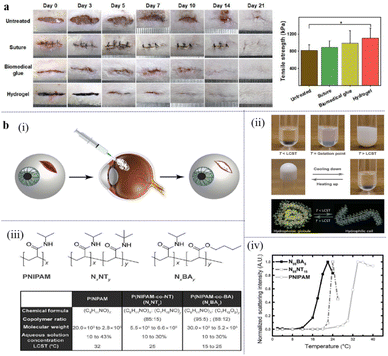 | ||
| Fig. 15 (a) Images of the incisions closed by suturing and the tensile strength of the healed skin tissues on day 21. This figure has been reproduced from ref. 197 with permission from Nano-Micro Lett., copyright 2022. (b) The design of a thermoresponsive hydrogel to seal scleral perforation. (i) A schematic depicting the implementation of a temperature-mediated adhesive hydrogel that adapts to wound shape and a tool for its deployment at a perforation site in the sclera. (ii) Images of the changes in physical properties as the sealing hydrogel transitions from hydrophilic coils to hydrophobic globules at its lower critical solution temperature (LCST). (iii) Molecular structures of formulations of poly(N-isopropylacrylamide) (PNIPAM) with butylacrylate (BA) or N-tert-butylacrylamide (NT) and a table of resulting alterations of molecular properties and LCST values. (iv) Normalized scattering intensity as a function of temperature for PNIPAM, poly(NIPAM-co-NT) (N85NT15), and poly(NIPAM-co-BA) (N95BA5). A.U., arbitrary units. These figures have been reproduced from ref. 198 with permission from the American Association for the Advancement of Science, copyright 2017. | ||
Good adhesive strength and injection performance can effectively close the incision and promote healing. This multifunctional hydrogel may contribute to the healing of surgical incisions, and the fast and effective closure of surgical wounds is critical. If not treated promptly, injuries can rapidly escalate and produce adverse outcomes. Bayat et al. designed a system for temporarily closing open eye lesions that restored the intraocular pressure (IOP) and was easily removed (Fig. 15b).198 Through the reversible and thermal response properties of PNIPAM, its thermal response behavior and mechanical strength were adjusted to form a hydrogel that could fill irregular edges and seal the wound. Their thermal-sensitive behavior allows thermal sealants (TRS) to be easily removed by applying cold water without additional trauma to the surrounding tissue during placement or removal. Hydrogels have the advantages of rapid reversible wound occlusion, shape adaptability, safety, and effectiveness, as well as translational potential as intelligent wound sealants, which can be used as intelligent wound dressings for the temporary occlusion of surgical incisions or wounds. Thermosensitive hydrogels can be thermally responsive through biodynamic bonding with rapid injectable filling, good adhesive strength, responsive adhesion ability, and easy placement or removal properties, making them promising candidates for surgical incision dressings.
Conclusions and further perspectives
Wound healing is a complex dynamic biological process. With the development and integration of biomedicine and materials science, the research and development of new hydrogels with high efficiency, intelligence, and microenvironmental adaptability provide new ideas and opportunities for wound repair. Thermosensitive hydrogels make use of the specificity of polymer materials in response to external temperatures so that the polymer undergoes reversible conformational changes under external temperature changes and realizes the transformation process from solution to gel. It is an excellent carrier for loading various bioactive substances or drugs in liquid form and can promote the transdermal absorption of drugs after being turned into a gel. Simultaneously, good mechanical ability and tissue adhesion of thermosensitive hydrogels, easy removal, and other characteristics are crucial for wound management. A few points should be emphasized when designing new thermosensitive hydrogels for wound healing as follows. (a) The mechanical stiffness of the thermosensitive hydrogels formed in situ has a great influence on their practical application. (b) The selection of the thermosensitive polymer types will affect the biocompatibility and biodegradability of gel systems. (c) Appropriate temperature-sensitive materials should be selected according to different wound types. (d) The duration of gelation time should be controlled reasonably according to the therapeutic needs to avoid the sudden release of drugs, factors, proteins, etc.Although thermosensitive hydrogels have achieved some success in wound treatment as wound-healing dressings, some problems remain. For example, most studies are based on animal and tissue models, which are different from those of the human body. Conducting research in a clinic requires further research and testing. In addition, the shortcomings of thermosensitive hydrogels include slow temperature response, poor biocompatibility and low mechanical properties, and will affect the expansion in the field of wound healing application. Therefore, the development of a successful thermosensitive hydrogel for wound healing depends on the development of sophisticated material engineering. The future direction of thermosensitive hydrogels for wound healing is to optimize and improve the characteristics of hydrogels to satisfy the treatment needs, and to prepare smart hydrogels with lower cost and lower toxicity, which is expected to realize personalized and refined treatment of different wounds and have broad development potential and clinical application prospects. Therefore, this study can provide a reference for the design and development of smart hydrogel wound dressings.
Author contributions
Ruting Gu: conceptualization, data curation, methodology, writing – original draft. Haiqing Zhou: data curation, methodology, validation. Zirui Zhang: methodology, formal analysis. Yun Lv: supervision, validation. Yueshuai Pan: data curation. Qianqian Li: data curation. Changfang Shi: visualization. Yanhui Wang: supervision, writing – review & editing. Lili Wei: project administration, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank for the financial support of Shandong Provincial Natural Science Foundation (ZR2022QE273).References
- E. S. Chambers and M. Vukmanovic-Stejic, Immunol., 2020, 160, 116–125 CrossRef CAS PubMed.
- J. Larouche, S. Sheoran and K. Maruyama, Adv. Wound Care, 2018, 7, 209–231 CrossRef PubMed.
- K. Singh, E. Camera and L. Krug, Nat. Commun., 2018, 9, 3425 CrossRef PubMed.
- E. Kolanthai, Y. Fu and U. Kumar, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2022, 14, e1741 CAS.
- J. Koehler, F. P. Brandl and A. M. Goepferich, Eur. Polym. J., 2018, 100, 1–11 CrossRef CAS.
- P. Martin, Science, 1997, 276, 75–81 CrossRef CAS PubMed.
- G. Luo, Y. Lu and C. Huang, Zhonghua Shaoshang Zazhi, 2023, 39, 9–14 CAS.
- R. Derwin, D. Patton and P. Avsar, Int. Wound J., 2022, 19, 1397–1408 CrossRef PubMed.
- G. Gethin, J. D. Ivory and D. Sezgin, Wound Repair Regen., 2021, 29, 843–847 CrossRef PubMed.
- T. M. Honnegowda, U. E. Padmanabha and P. Rao, World J. Plast. Surg., 2016, 5, 265–273 Search PubMed.
- L. Y. Zhu, S. X. Guo and P. Wu, Zhonghua Shaoshang Zazhi, 2018, 34, 829–832 CAS.
- Y. Liang, M. Li and Y. Yang, ACS Nano, 2022, 16, 3194–3207 CrossRef CAS PubMed.
- J. Rojas, M. Bedoya and Y. Ciro, Cellulose, 2015, 32, 193–228 Search PubMed.
- Y. Li, X. Jiang and Z. Qin, J. Xi'an Jiaotong Univ., Med. Sci., 2022, 43, 943–951 Search PubMed.
- C. Canal, F. Gaboriau and S. Villeger, Int. J. Pharm., 2009, 367, 155–161 CrossRef CAS PubMed.
- K. Chen, F. Wang and S. Liu, Int. J. Biol. Macromol., 2020, 148, 501–509 CrossRef CAS PubMed.
- S. Hu, S. Bi and D. Yan, Carbohydr. Polym., 2018, 184, 154–163 CrossRef CAS PubMed.
- H. Peng, X. Ning and G. Wei, Carbohydr. Polym., 2018, 195, 349–355 CrossRef CAS PubMed.
- A. R. Abbasi, M. Sohail and M. U. Minhas, Int. J. Biol. Macromol., 2020, 155, 751–765 CrossRef CAS PubMed.
- Y. Liang, J. He and B. Guo, ACS Nano, 2021, 15, 12687–12722 CrossRef CAS PubMed.
- R. Fan, Y. Cheng and R. Wang, Polymers, 2022, 14, 2379 CrossRef CAS PubMed.
- P. Patel, A. Mandal and V. Gote, J. Polym. Res., 2019, 26, 1–11 CrossRef CAS.
- Y. K. Du, U. P. Shinde and B. Yeon, Prog. Polym. Sci., 2013, 38, 672–701 CrossRef.
- M. Calderón, M. A. Quadir and M. Strumia, Biochimie, 2010, 92, 1242–1251 CrossRef PubMed.
- C. Kojima, Expert Opin. Drug Delivery, 2010, 7, 307–319 CrossRef CAS PubMed.
- W. Zhang, X. Jin and H. Li, Carbohydr. Polym., 2018, 186, 82–90 CrossRef CAS PubMed.
- M. F. Jéssica, F. M. Simone, A. Gizelda Maria, S. Danilo Martins Dos, C. F. Sérgio Paulo and S. Amilton Martins Dos, Colloids Surf., B, 2018, 175, 73–83 Search PubMed.
- L. Pham, L. H. Dang and M. D. Truong, Biomed. Pharmacother., 2019, 117, 109183 CrossRef CAS PubMed.
- L. Zhang, S. Gurankit, M. Zhang, S. X. Chen, K. G. Xu, P. C. Xu, X. Wang, Y. H. Chen, L. Zhang and L. Zhang, Mater. Sci. Eng., C, 2018, 90, 159–167 CrossRef PubMed.
- C. Qu, Z. Bao and X. Zhang, Int. J. Biol. Macromol., 2019, 125, 78–86 CrossRef CAS PubMed.
- B. Tang, J. shan and T. Yuan, Carbohydr. Polym., 2019, 209, 198–206 CrossRef CAS PubMed.
- M. Dhrubojyoti, A. Md and S. Santhosh, J. Drug Delivery Sci. Technol., 2018, 46, 498–510 CrossRef.
- J. I. Lee, H. S. Kim and H. S. Yoo, Int. J. Pharm., 2009, 373, 93–99 CrossRef CAS PubMed.
- A. Paul, A. Hasan and H. A. Kindi, Acs Nano, 2014, 8, 8050–8062 CrossRef CAS PubMed.
- J. Qu, X. Zhao and Y. Liang, Biomaterials, 2018, 183, 185–199 CrossRef CAS PubMed.
- H. Huang, X. Qi and Y. Chen, Saudi Pharm. J., 2019, 27, 990–999 CrossRef CAS PubMed.
- M. Wang, M. Chen and W. Niu, Biomaterials, 2020, 261, 120301 CrossRef CAS PubMed.
- D. Ma, Preparation and Properties of Multifunctional Composite Hydrogel-based Wound Dressings, Jilin University, 2022 Search PubMed.
- J. S. Boateng, K. H. Matthews and H. N. Stevens, J. Pharm. Sci., 2008, 97, 2892–2923 CrossRef CAS PubMed.
- S. Han, M. Li and X. Liu, Colloids Surf., B, 2013, 102, 833–841 CrossRef CAS PubMed.
- S. Alven, S. Peter and Z. Mbese, Polymers, 2022, 14, 724 CrossRef CAS PubMed.
- M. Prasathkumar and S. Sadhasivam, Int. J. Biol. Macromol., 2021, 186, 656–685 CrossRef CAS PubMed.
- A. M. Mohammed, S. K. Osman and K. I. Saleh, AAPS PharmSciTech, 2020, 21, 131 CrossRef CAS PubMed.
- W. Zheng, J. Wang and L. Xie, J. Mater. Sci.: Mater. Med., 2019, 30, 106 CrossRef PubMed.
- R. Nigmatullin, V. Gabrielli and C. Juan, Cellulose, 2019, 26, 529–542 CrossRef CAS.
- E. Dashtimoghadam, H. Salimi-Kenari and R. Nasseri, Colloids Surf., A, 2020, 590, 124489 CrossRef CAS.
- S. H. Nezhadi, P. F. Choong and F. Lotfipour, J. Drug Targeting, 2009, 17, 731–738 CrossRef CAS PubMed.
- L. Klouda, Eur. J. Pharm. Biopharm., 2015, 97, 338–349 CrossRef CAS PubMed.
- C. N. Schnell, M. V. Galván and M. A. Zanuttini, Cellulose, 2019, 1465–1481 Search PubMed.
- Z. Lu, J. Gao and Q. He, Carbohydr. Polym., 2017, 156, 460–469 CrossRef CAS PubMed.
- N. Sayari, A. Sila and B. E. Abdelmalek, Int. J. Biol. Macromol., 2016, 87, 163–171 CrossRef CAS PubMed.
- L. Pighinelli and M. Kucharska, Carbohydr. Polym., 2013, 93, 256–262 CrossRef CAS PubMed.
- M. A. Matica, F. L. Aachmann and A. Tøndervik, Int. J. Mol. Sci., 2019, 20, 5889 CrossRef CAS PubMed.
- D. Zhang, S. Lu and Z. Hu, Appl. Chem. Ind., 2019, 48, 1734–1739 Search PubMed.
- B. Zhang, Z. Zou and X. Nie, Shandong Chem. Ind., 2020, 49, 49–51 CAS.
- H. Ueno, F. Nakamura and M. Murakami, Biomaterials, 2001, 22, 2125–2130 CrossRef CAS PubMed.
- Z. Mao, H. Shi and R. Guo, Acta Biomater., 2009, 5, 2983–2994 CrossRef CAS PubMed.
- O. M. Dragostin, S. K. Samal and M. Dash, Carbohydr. Polym., 2016, 141, 28–40 CrossRef CAS PubMed.
- L. H. Fu, C. Qi and M. G. Ma, J. Mater. Chem. B, 2019, 7, 1541–1562 RSC.
- Y. Pan, X. Zhao and X. Li, Polymers, 2019, 11, 1846 CrossRef CAS PubMed.
- F. Feizabadi, M. Minaiyan and A. Taheri, Curr. Drug Delivery, 2018, 15, 840–849 CrossRef CAS PubMed.
- M. Alavi, e-Polym., 2019, 19, 103–119 CAS.
- F. Wang, Y. Pan and P. Cai, Bioresour. Technol., 2017, 241, 482–490 CrossRef CAS PubMed.
- O. Forero-Doria, E. Polo and A. Marican, Carbohydr. Polym., 2020, 242, 116383 CrossRef CAS PubMed.
- Y. Huang, L. Bai and Y. Yang, J. Colloid Interface Sci., 2022, 608, 2278–2289 CrossRef CAS PubMed.
- M. Santoro, A. M. Tatara and A. G. Mikos, J. Controlled Release, 2014, 190, 210–218 CrossRef CAS PubMed.
- A. O. Elzoghby, J. Controlled Release, 2013, 172, 1075–1091 CrossRef CAS PubMed.
- L. Klouda and A. G. Mikos, Eur. J. Pharm. Biopharm., 2008, 68, 34–45 CrossRef CAS PubMed.
- C. Joly-Duhamel, D. Hellio and M. Djabourov, Langmuir, 2002, 18, 7208–7217 CrossRef CAS.
- L. Pham, L. H. Dang and M. D. Truong, Biomed. Pharmacother., 2019, 117, 109183 CrossRef CAS PubMed.
- J. Liu, Z. Chen and J. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 16315–16326 CrossRef CAS PubMed.
- W. Boonlai, V. Tantishaiyakul and N. Hirun, AAPS PharmSciTech, 2018, 19, 2103–2117 CrossRef CAS PubMed.
- B. Shriky, A. Kelly and M. Isreb, J. Colloid Interface Sci., 2020, 565, 119–130 CrossRef CAS PubMed.
- X. Zhai, Z. Cui and Y. Li, Heliyon, 2022, 8, e12063 CrossRef CAS PubMed.
- Y. Dong, H. Zhuang and Y. Hao, Int. J. Nanomed., 2020, 15, 1939–1950 CrossRef CAS PubMed.
- B. Darabian, H. Bagheri and S. Mohammadi, Prog. Biomater., 2020, 9, 45–64 CrossRef CAS PubMed.
- L. Huang, Z. Zhu and D. Wu, Carbohydr. Polym., 2019, 225, 115110 CrossRef CAS PubMed.
- L. Du, L. Tong and Y. Jin, Wound Repair Regen., 2012, 20, 904–910 CrossRef PubMed.
- E. Gioffredi, M. Boffito and S. Calzone, Procedia CIRP, 2016, 49, 125–132 CrossRef.
- C. Fu, F. Ren and Q. Zhang, Colloid Polym. Sci., 2015, 293, 2191–2200 CrossRef CAS.
- T. Li, Q. Bao and J. Shen, Int. J. Pharm., 2020, 580, 119238 CrossRef CAS PubMed.
- P. S. Sundaran, A. Bhaskaran and S. T. Alex, J. Mater. Sci.: Mater. Med., 2017, 28, 88 CrossRef PubMed.
- J. Wasiak, H. Cleland and F. Campbell, Cochrane Database Syst. Rev., 2013, 2013, CD002106 Search PubMed.
- J. L. Soriano-Ruiz, A. C. Calpena-Campmany and M. Silva-Abreu, Int. J. Biol. Macromol., 2020, 142, 412–422 CrossRef CAS PubMed.
- M. Kim, F. Heinrich and G. Haugstad, Langmuir, 2020, 36, 3393–3403 CrossRef CAS PubMed.
- V. Kant, A. Gopal and D. Kumar, Acta Histochem., 2014, 116, 5–13 CrossRef CAS PubMed.
- T. A. Kwiatkowski, A. L. Rose and R. Jung, Am. J. Physiol.: Cell Physiol., 2019, 318, C253–C262 CrossRef PubMed.
- J. Zhou, G. Chen and D. Chen, Acta Mater. Compositae Sin., 2022, 1–9 Search PubMed.
- M. J. Ansari, R. R. Rajendran and S. Mohanto, Gels, 2022, 8, 454 CrossRef CAS PubMed.
- L. Tang, L. Wang and X. Yang, Prog. Mater. Sci., 2020, 115, 100702 CrossRef.
- W. J. Chuang and W. Y. Chiu, Polymer, 2012, 53, 2829–2838 CrossRef CAS.
- X. Lin, X. Guan and Y. Wu, Mater. Sci. Eng., C, 2020, 115, 111123 CrossRef CAS PubMed.
- Y. Hu, Y. Shin and S. Park, Polymers, 2022, 15, 122 CrossRef PubMed.
- S. Ziane, S. Schlaubitz and S. Miraux, Eur. Cells Mater., 2012, 23, 147–160 CrossRef CAS PubMed , discussion 160.
- B. Jiang, J. C. Larson and P. W. Drapala, J. Biomed. Mater. Res., Part B, 2012, 100, 668–676 CrossRef PubMed.
- T. Kardan, R. Mohammadi and S. Taghavifar, Int. J. Lower Extremity Wounds, 2021, 20, 263–271 CrossRef CAS PubMed.
- S. L. Chen, R. H. Fu and S. F. Liao, Cell Transplant., 2018, 27, 275–284 Search PubMed.
- M. H. Park, M. K. Joo and B. G. Choi, Acc. Chem. Res., 2012, 45, 424–433 CrossRef CAS PubMed.
- T. Thambi, Y. Li and D. S. Lee, J. Controlled Release, 2017, 267, 57–66 CrossRef CAS PubMed.
- Z. Li and J. Guan, Expert Opin. Drug Delivery, 2011, 8, 991–1007 CrossRef CAS PubMed.
- S. Chatterjee and P. C. Hui, Polymers, 2021, 13, 2086 CrossRef CAS PubMed.
- R. Yang, V. Sabharwal and N. Shlykova, JCI Insight, 2018, 3, e123415 CrossRef PubMed.
- R. Censi, T. Vermonden and M. J. van Steenbergen, J. Controlled Release, 2009, 140, 230–236 CrossRef CAS PubMed.
- H. Li, Q. Ji and X. Chen, J. Biomed. Mater. Res., Part A, 2017, 105, 265–273 CrossRef CAS PubMed.
- Y. J. Dong, J. F. Liao and Z. C. Kong, Appl. Catal., B, 2018, 237, 9–17 CrossRef CAS.
- M. Ma, Y. Zhong and X. Jiang, Carbohydr. Polym., 2020, 236, 116096 CrossRef CAS PubMed.
- Z. Zheng, S. Bian and Z. Li, Carbohydr. Polym., 2020, 249, 116826 CrossRef CAS PubMed.
- X. Jin, Q. Fu and Z. Gu, Int. J. Biol. Macromol., 2021, 184, 787–796 CrossRef CAS PubMed.
- X. Li, A. Li and F. Feng, Anim. Models Exp. Med., 2019, 2, 107–113 CrossRef PubMed.
- Y. H. Chang, Polymers, 2020, 12, 2619 CrossRef PubMed.
- M. Zakerikhoob, S. Abbasi and G. Yousefi, Carbohydr. Polym., 2021, 271, 118434 CrossRef CAS PubMed.
- C. Gong, Q. Wu and Y. Wang, Biomaterials, 2013, 34, 6377–6387 CrossRef CAS PubMed.
- Y. Zhao, X. Liu and X. Peng, Int. J. Biol. Macromol., 2022, 216, 475–486 CrossRef CAS PubMed.
- N. N. Mahmoud, S. Hikmat and G. D. Abu, Int. J. Pharm., 2019, 565, 174–186 CrossRef CAS PubMed.
- M. Rafie and A. Meshkini, Eur. J. Pharm. Sci., 2021, 167, 106031 CrossRef CAS PubMed.
- X. Jin, W. Zhang and J. Shan, ACS Appl. Mater. Interfaces, 2022, 14, 50677–50691 CrossRef CAS PubMed.
- Y. Zhao, J. G. Liu and W. M. Chen, Exp. Ther. Med., 2018, 15, 1304–1313 CAS.
- Y. Y. Teng, M. L. Zou and S. Y. Liu, Front. Bioeng. Biotechnol., 2022, 10, 902894 CrossRef PubMed.
- P. Ge, S. Chang and T. Wang, Nanoscale, 2023, 15, 644–656 RSC.
- T. Feng, H. Wu and W. Ma, J. Mater. Chem. B, 2022, 10, 6143–6157 RSC.
- K. Zubik, P. Singhsa and Y. Wang, Polymers, 2017, 9, 119 CrossRef PubMed.
- Z. Lei, G. Singh and Z. Min, Mater. Sci. Eng., C, 2018, 90, 159–167 CrossRef CAS PubMed.
- M. Yang, S. He and Z. Su, ACS Omega, 2020, 5, 21015–21023 CrossRef CAS PubMed.
- H. Xu, S. Huang and J. Wang, Int. J. Biol. Macromol., 2019, 137, 433–441 CrossRef CAS PubMed.
- Y. J. Lin, G. H. Lee and C. W. Chou, J. Mater. Chem. B, 2015, 3, 1931–1941 RSC.
- B. Lan, L. Zhang and L. Yang, J. Nanobiotechnol., 2021, 19, 130 CrossRef CAS PubMed.
- Y. Zhou, X. L. Zhang and S. T. Lu, Stem Cell Res. Ther., 2022, 13, 407 CrossRef CAS PubMed.
- T. F. Dadkhah, I. Shabani and A. Shabani, Carbohydr. Polym., 2022, 281, 119020 CrossRef PubMed.
- J. Chi, X. Zhang and C. Chen, Bioact. Mater., 2020, 5, 253–259 Search PubMed.
- M. Qiu, D. Chen and C. Shen, Int. J. Mol. Sci., 2016, 17, 1001 CrossRef PubMed.
- X. Li, X. Ye and J. Qi, Int. J. Nanomed., 2016, 11, 3993–4009 CrossRef PubMed.
- D. Zhang, Q. Ouyang and Z. Hu, Int. J. Biol. Macromol., 2021, 173, 591–606 CrossRef CAS PubMed.
- M. P. Tian, A. D. Zhang and Y. X. Yao, Carbohydr. Polym., 2021, 261, 117878 CrossRef CAS PubMed.
- E. J. Kim, J. S. Choi and J. S. Kim, Biomacromolecules, 2016, 17, 4–11 CrossRef CAS PubMed.
- T. Lim, Q. Tang and Z. Zhu, J. Biomed. Mater. Res., Part A, 2020, 108, 2111–2122 CrossRef CAS PubMed.
- X. Chen, C. Zhou and J. Wang, Adv. Healthcare Mater., 2022, 11, e2200546 CrossRef PubMed.
- M. H. Turabee, T. Thambi and D. S. Lee, ACS Appl. Bio Mater., 2019, 2, 2500–2510 CrossRef CAS PubMed.
- K. Y. Chang, Y. N. Chou and W. Y. Chen, Polymers, 2022, 14, 3346 CrossRef CAS PubMed.
- M. Hou, X. Wang and O. Yue, Biomater. Adv., 2022, 134, 112556 CrossRef PubMed.
- D. Y. Zhu, Z. P. Chen and Z. P. Hong, Acta Biomater., 2022, 143, 203–215 CrossRef CAS PubMed.
- S. G. Park, M. X. Li and W. K. Cho, Carbohydr. Polym., 2021, 260, 117808 CrossRef CAS PubMed.
- M. Li, Y. Liang and J. He, Chem. Mater., 2020, 32, 9937–9953 CrossRef CAS.
- R. Yu, M. Li and Z. Li, Adv. Healthcare Mater., 2022, 11, e2102749 CrossRef PubMed.
- X. Peng, C. Ding and Y. Zhao, Front. Bioeng. Biotechnol., 2022, 10, 831007 CrossRef PubMed.
- D. Y. Zhu, X. J. Chen and Z. P. Hong, ACS Appl. Mater. Interfaces, 2020, 12, 22534–22542 CrossRef CAS PubMed.
- Z. Li, J. Huang and Y. Jiang, Adv. Healthcare Mater., 2022, 11, e2201878 CrossRef PubMed.
- T. Maver, S. Hribernik, T. Mohan and K. Stana-Kleinschek, RSC Adv., 2015, 5, 77873–77884 RSC.
- M. T. Cook, P. Haddow and S. B. Kirton, Adv. Funct. Mater., 2020, 31, 2008123 CrossRef.
- T. Sarwan, P. Kumar and Y. E. Choonara, Front. Mater., 2020, 7, 1–17 CrossRef.
- W. Treesuppharat, P. Rojanapanthu and C. Siangsanoh, Biotechnol. Rep., 2017, 15, 84–91 CrossRef CAS PubMed.
- T. Ak and I. Gülçin, Chem.-Biol. Interact., 2008, 174, 27–37 CrossRef CAS PubMed.
- H. Liu, J. Liu, C. Qi, Y. P. Fang, L. Zhang, R. X. Zhuo and X. L. Jiang, Acta Biomater., 2016, 35, 228–237 CrossRef CAS PubMed.
- E. Redwan, Hum. Antibodies, 2007, 16, 137–158 CAS.
- J. Guo, H. Hu and J. Gorecka, Am. J. Physiol.: Cell Physiol., 2018, 315, C885–C896 CrossRef CAS PubMed.
- J. Y. Li, K. K. Ren and W. J. Zhang, Stem Cell Res. Ther., 2019, 10, 247 CrossRef PubMed.
- C. Yue, Z. Guo and Y. Luo, Stem Cells Int., 2020, 2020, 7430968 Search PubMed.
- S. A. Eming, P. Martin and M. Tomic-Canic, Sci. Transl. Med., 2014, 6, 265sr6 Search PubMed.
- P. Zhu, Dynamic Changes of MMP-9 and TIMP-1 in Skin Wound Healing of Diabetic Rats, Sun Yat-sen University, 2006 Search PubMed.
- P. Calvert, Adv. Mater., 2009, 21, 743–756 CrossRef CAS.
- Y. Yang and M. W. Urban, Chem. Soc. Rev., 2013, 42, 7446–7467 RSC.
- M. H. Turabee, T. Thambi and J. S. Lym, Biomater. Sci., 2017, 5, 837–848 RSC.
- L. Han, L. Yan and K. Wang, NPG Asia Mater., 2017, e372 CrossRef CAS.
- H. W. Chien, H. Y. Lin and C. Y. Tsai, Polymers, 2020, 12, 2008 CrossRef CAS PubMed.
- H. W. Chien and T. H. Chiu, Eur. Polym. J., 2020, 130, 109654 CrossRef CAS.
- C. K. Sen, G. M. Gordillo and S. Roy, Wound Repair Regen., 2009, 17, 763–771 CrossRef PubMed.
- H. Yaseen and M. Khamaisi, Cell. Immunol., 2020, 356, 104154 CrossRef CAS PubMed.
- D. J. Magliano and E. J. Boyko, IDF Diabetes Atlas, International Diabetes Federation, Brussels, 2021 Search PubMed.
- R. M. Stoekenbroek, J. Lokin and M. M. Nielen, Diabetologia, 2017, 60, 1271–1275 CrossRef PubMed.
- R. Frykberg and J. Banks, Adv. Wound Care, 2015, 4, 560–582 CrossRef PubMed.
- M. Phillipson and P. Kubes, Trends Immunol., 2019, 40, 635–647 CrossRef CAS PubMed.
- S. Chen, J. Shi and M. Zhang, Sci. Rep., 2015, 5, 18104 CrossRef CAS PubMed.
- Y. Lu, J. Deng and J. Wang, Chin. J. Burns, 2020, 36, 13 Search PubMed.
- K. Lin, L. Fan and S. Wang, Chin. J. Diabetes, 2017, 25, 449–453 Search PubMed.
- J. H. Kim, B. Yang and A. Tedesco, Sci. Rep., 2019, 9, 19318 CrossRef CAS PubMed.
- W. Cao and C. Gao, Chin. J. Burns, 2021, 37, 9 Search PubMed.
- Z. Xu, Y. Liu and R. Ma, ACS Appl. Mater. Interfaces, 2022, 14, 14059–14071 CrossRef CAS PubMed.
- Y. Zhu, R. Hoshi and S. Chen, J. Controlled Release, 2016, 238, 114–122 CrossRef CAS PubMed.
- O. Rezvani, E. Shabbak and A. Aslani, Ostomy/Wound Manag., 2009, 55, 22–28 Search PubMed.
- M. C. Ribeiro, V. Correa and F. Silva, Eur. J. Pharm. Sci., 2020, 150, 105330 CrossRef CAS PubMed.
- L. I. Moura, A. M. Dias and E. C. Leal, Acta Biomater., 2014, 10, 843–857 CrossRef CAS PubMed.
- L. Fang, H. Wu and X. Li, Int. J. Endocrinol., 2022, 2022, 7847011 Search PubMed.
- N. Tian, J. Wei and Y. Li, J. Colloid Interface Sci., 2020, 566, 69–78 CrossRef CAS PubMed.
- Y. Dong, M. Cui and J. Qu, Acta Biomater., 2020, 108, 56–66 CrossRef CAS PubMed.
- A. Markiewicz-Gospodarek, M. Kozioł and M. Tobiasz, Int. J. Environ. Res. Public Health, 2022, 19, 1338 CrossRef CAS PubMed.
- K. S. Romanowski, J. Carson and K. Pape, J. Burn Care Res., 2020, 41, 1129–1151 CrossRef PubMed.
- Y. Huangfu, S. Li and L. Deng, ACS Appl. Mater. Interfaces, 2021, 13, 59695–59707 CrossRef CAS PubMed.
- S. Chakrabarti, B. Mazumder and J. Rajkonwar, Sci. Rep., 2021, 11, 3357 CrossRef CAS PubMed.
- X. Fu, Z. Shen and Y. Chen, Lancet, 1998, 352, 1661–1664 CrossRef CAS PubMed.
- Z. Xiao, X. Zheng and Y. An, Biomater. Sci., 2021, 9, 882–891 RSC.
- Y. Zhang, W. He and S. Zhang, Int. J. Mol. Sci., 2022, 23, 12716 CrossRef CAS PubMed.
- L. M. Geever, K. E. Mcevoy and J. I. Ngadaonye, Int. J. Polym. Mater., 2014, 63, 873–883 CrossRef.
- Z. Liu, W. Tang and J. Liu, Bioact. Mater., 2023, 20, 610–626 CAS.
- S. D. Li, N. Chen, X. P. Li, Z. P. Xie, Z. Y. Ma, J. Zhao, X. Hou and X. B. Yuan, Adv. Funct. Mater., 2020, 30, 2000130 CrossRef CAS.
- X. Zhao, Y. Liang and Y. Huang, Adv. Funct. Mater., 2020, 30, 1910748 CrossRef CAS.
- Y. Liang, Z. Li and Y. Huang, ACS Nano, 2021, 15, 7078–7093 CrossRef CAS PubMed.
- Y. Ma, J. Yao and Q. Liu, Adv. Funct. Mater., 2020, 30, 1001820 Search PubMed.
- Y. Liang, H. Xu and Z. Li, Nano-Micro Lett., 2022, 14, 185 CrossRef CAS PubMed.
- N. Bayat, Y. Zhang and P. Falabella, Sci. Transl. Med., 2017, 9, eaan3879 CrossRef PubMed.
- P. Thangavel, B. Ramachandran and S. Chakraborty, Sci. Rep., 2017, 7, 10701 CrossRef PubMed.
- H. Niu, H. Li and Y. Guan, Bioact. Mater., 2022, 18, 104–115 CAS.
- Q. Zhao, J. Liu and S. Liu, ACS Appl. Mater. Interfaces, 2022, 14, 46224–46238 CrossRef CAS PubMed.
- W. Zhou, Z. Duan and J. Zhao, Bioact. Mater., 2022, 17, 1–17 CAS.
- J. Yang, Z. Chen and D. Pan, Int. J. Nanomed., 2020, 15, 5911–5926 CrossRef CAS PubMed.
- Z. Chen, B. Zhang and J. Shu, J. Biomed. Mater. Res., Part A, 2021, 109, 1418–1428 CrossRef CAS PubMed.
- J. Wang, B. Zhao and L. Sun, PLoS One, 2022, 17, e0279727 CrossRef CAS PubMed.
- M. H. Kim, H. Park and H. C. Nam, Carbohydr. Polym., 2018, 181, 579–586 CrossRef CAS PubMed.
- X. Liu, H. Gan and C. Hu, Int. J. Nanomed., 2019, 14, 289–300 CrossRef CAS PubMed.
- H. M. Abdel-Mageed, E. A. A. Abd and R. B. Abdel, 3 Biotech, 2022, 12, 73 CrossRef PubMed.
- J. L. Soriano, A. C. Calpena and M. J. Rodríguez-Lagunas, Pharmaceutics, 2020, 13, 8 CrossRef PubMed.
- C. Y. Tsai, L. C. Woung and J. C. Yen, Carbohydr. Polym., 2016, 135, 308–315 CrossRef CAS PubMed.
- P. Zhou, X. Li and B. Zhang, BioMed Res. Int., 2019, 2019, 5768285 Search PubMed.
- H. Yim, H. T. Yang and Y. S. Cho, Burns, 2014, 40, 1642–1649 CrossRef PubMed.
- A. Deng, Y. Yang and S. Du, Mater. Sci. Eng., C, 2021, 119, 111555 CrossRef CAS PubMed.
- P. Makvandi, G. W. Ali and S. F. Della, Carbohydr. Polym., 2019, 223, 115023 CrossRef CAS PubMed.
- E. Zhang, Q. Guo and F. Ji, Acta Biomater., 2018, 74, 439–453 CrossRef CAS PubMed.
- S. Lin, L. Pei and W. Zhang, Mater. Sci. Eng., C, 2021, 130, 112450 CrossRef CAS PubMed.
- N. Lei, C. Gong and Z. Qian, Nanoscale, 2012, 4, 5686–5693 RSC.
- X. Dong, F. Yao and L. Jiang, J. Mater. Chem. B, 2022, 10, 2215–2229 RSC.
| This journal is © The Royal Society of Chemistry 2023 |