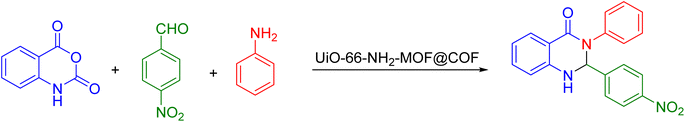Open Access Article
Open Access ArticleRetracted Article: Unique and outstanding catalytic behavior of a novel MOF@COF composite as an emerging and powerful catalyst in the preparation of 2,3-dihydroquinazolin-4(1H)-one derivatives†
Mohammad Ali
Ghasemzadeh
 * and
Boshra
Mirhosseini-Eshkevari
* and
Boshra
Mirhosseini-Eshkevari
Department of Chemistry, Qom Branch, Islamic Azad University, Post Box: 37491-13191, Qom, I. R. Iran. E-mail: qasemzade.a@gmail.com; ma.ghasemzadeh@iau.ac.ir
First published on 6th November 2023
Abstract
The creation of an emerging porous structure using the hybridization of UiO-66-NH2-MOF, a zirconium-based metal–organic framework (MOF), with a covalent organic framework (COF) based on terephthaldehyde and melamine (UiO-66-NH2-MOF@COF), was assessed using SEM, XRD, EDX/mapping, FT-IR, BET, and TGA analyses. Using the obtained composite as a potential recoverable heterogeneous nanocatalyst, different aldehydes were condensed with isatoic anhydride and anilines or ammonium acetate under solvent-free conditions to create derivatives of 2,3-dihydroquinazolin-4(1H)-one. Examining the catalytic capabilities of the designed UiO-66-NH2-MOF@COF to efficiently produce 2,3-dihydroquinazolin-4(1H)-ones was a standout activity. Low catalyst loading, simple set-up, outstanding yields, and catalyst recoverability are all benefits of this research.
1. Introduction
Metal–organic frameworks are a remarkable class of porous substances with outstanding attributes such as excellent surface area, high thermal stability, chemical stability, and ultrahigh porosity.1–3 Thus, they are greatly used in sensors,4 separation,5 medicinal,6 storage,7 and catalysis.8,9Likewise, covalent organic frameworks (COFs) are a significant group of 2D or 3D-ordered porous materials composed of organic building blocks linked by reversible covalent bonds.10,11
Outstanding features are exhibited by these porous materials such as good to excellent porosity, large surface area, excellent adsorption abilities, adjustable frameworks, great thermal and chemical stability, highly ordered structures, and low density.12,13 COFs based on network chemistry are made from light elements (O, C, B, N, etc.) via excellent covalent bonding. They have represented superior potential in different usages.14,15 New MOF/COF hybrids integrate the extremely good capabilities of MOF and COF structures, inclusive of excessive crystallinities, excessive porosities, large floor areas, the potential to enhance the structures with purposeful groups, and improved chemical and mechanical stabilities.16,17 Therefore, it is expected that the development of MOF-COF hybrid mesoporous materials could enhance their inherent weak characters and have a synergistic effect to originate multifunctional properties for unique programs. Currently, MOF-COF hybrid resources have been applied to related synthetic approaches using amino-functionalized MOFs to graft imine-based COFs.18,19 Significant interest has been attracted by multi-component reactions (MCRs) in organic syntheses since they can create target products in a single operation with no isolation of intermediates. Thus, energy and reaction times are reduced.20,21 Organic reactions under circumstances without solvent have highly encouraged chemists' attention mainly from the green chemistry aspect. Green chemistry methods are considerable for reducing byproducts, produced waste, and energy costs. Potential MCRs in the absence of solvent conditions using nanostructures as catalysts could improve their efficacy economically and ecologically.22,23
Quinazolin-4-ones are significant bicyclic heterocycles with significant pharmacological and biological properties such as antifungal,24 analgesic,25 antidiabetic,26 antitumor,27 antibacterial,28 anticonvulsant,29 and antihypertensive.30
Because of considerable attention to 2,3-dihydroquinazolin-4(1H)-ones, various approaches were established to produce substituted dihydroquinazolin-4(1H)-ones.31–33 Among the important methods are substances such as isatoic anhydride, and aldehydes exposed to primary amine or ammonium acetate using various catalysts or reagents such as amberlyst-15 microwave-assisted,34 Zn(PFO)2,35 silica-bonded N-propylsulfamic acid (SBNPSA),36p-toluenesulfonic acid,37 silica sulfuric acid,38 ceric ammonium nitrate,39 montmorillonite K-10,40 Ga(OTf)3,41 and ionic liquids.42
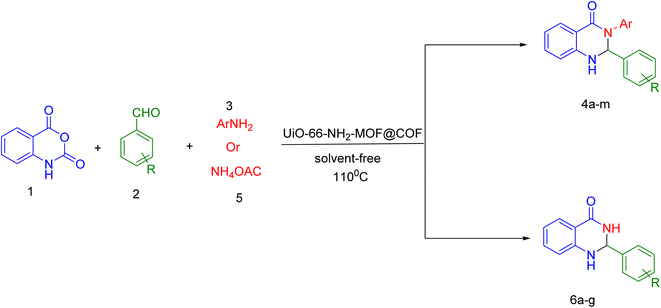
In the following of our studies43–47 on the synthesis of novel heterogeneous catalysts, in this research we report how to prepare and use UiO-66-NH2-MOF@COF as a bifunctional acid–base catalyst for the production of 2,3-dihydroquinazolin-4(1H)-ones by ternary condensation between isatoic anhydride, aldehydes, and ammonium acetate or primary amines under solvent-free conditions (Scheme 1).
2. Experimental
2.1. Synthesis of UiO-66-NH2-MOF
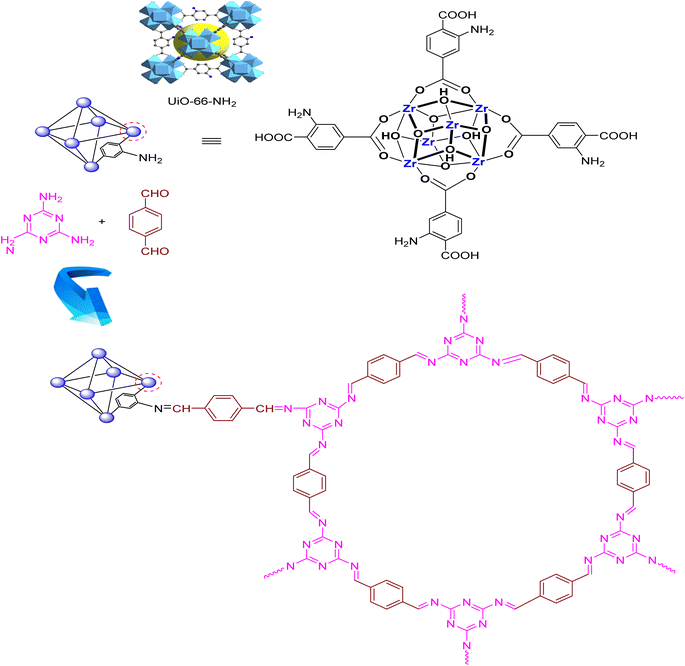
To prepare UiO-66-NH2, a reported technique was applied with a slight modification.48 A solution of 2-aminoterephthalic acid (1.56 g, 6.4 mmol) and zirconium chloride (1.05 g, 4.5 mmol) in DMF (40 mL) was exposed to ultrasonic irradiation at room temperature for 15 min. Then, 17 mL of acetic acid was added to the solution and the mixture was sonicated again for 15 min. The mixture was then placed into an autoclave for heating at 135 °C for 24 h. The resultant solid was filtered and washed with acetone and dimethylformamide. Ultimately, the resultant residue was dried at 80 °C for 6 h.2.2. Synthesis of the UiO-66-NH2-MOF@COF nanocomposite
0.2 g of UiO-66-NH2, 0.5 g of terephthaldehyde (3.73 mmol), 0.5 g of melamine (3.96 mmol), distilled water (5 mL), and DMSO (25 mL) were placed in a Teflon-lined stainless-steel autoclave and kept for 12 h at 180 °C. The resulting precipitate was achieved after cooling the reaction media and then was filtrated, rinsed with EtOH, and finally dried overnight at room temperature (Scheme 2).2.3. General preparation of 2,3-dihydroquinazolin-4(1H)-ones (4a–m) and (6a–g) using the UiO-66-NH2-MOF@COF nanocomposite
A mixture of isatoic anhydride, aldehydes, and anilines or ammonium acetate with a molar ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mmol) in UiO-66-NH2-MOF@COF (0.007 g) was mixed in the absence of any solvent at 110 °C for 10–25 min. Thin layer chromatography (TLC) was used to monitor the reaction progress. After completion of the reaction as determined by TLC, the solid obtained was dissolved in dichloromethane, and the catalyst was insoluble in CH2Cl2 and separated by centrifugation. Then, the solvent was evaporated and the residue was recrystallized from ethanol to afford the pure product.
1 mmol) in UiO-66-NH2-MOF@COF (0.007 g) was mixed in the absence of any solvent at 110 °C for 10–25 min. Thin layer chromatography (TLC) was used to monitor the reaction progress. After completion of the reaction as determined by TLC, the solid obtained was dissolved in dichloromethane, and the catalyst was insoluble in CH2Cl2 and separated by centrifugation. Then, the solvent was evaporated and the residue was recrystallized from ethanol to afford the pure product.
Spectral data of the new products are given below.
3. Results and discussion
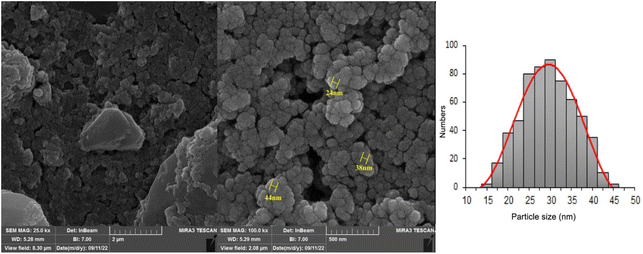
The procedure displayed in Scheme 2 is used to synthesize the target catalyst including UiO-66-NH2-MOF@COF. To characterize and confirm the structure, spectroscopic techniques including SEM, XRD, EDX/mapping, FT-IR, BET, and TGA were used.The morphology and particle size of UiO-66-NH2-MOF@COF were evaluated by assessing the prepared sample by FE-SEM (Fig. 1). A spherical shape and nano-scale dimension were represented by FE-SEM images of UiO-66-NH2-MOF@COF. As can be seen from Fig. 1, the particle size of UiO-66-NH2-MOF@COF are approximately in the range of 15 to 45 nm with an average particle size of about 25–35 nm, which is in good agreement with the results calculated using the Scherrer equation.
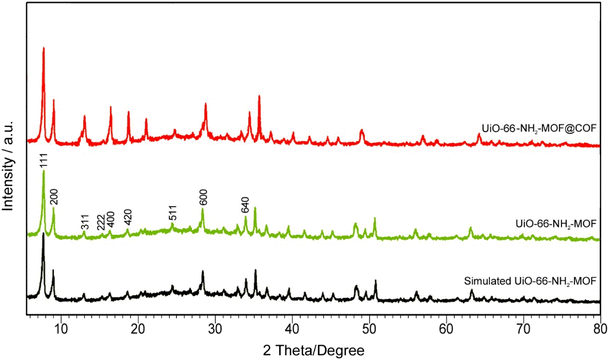
XRD analysis was utilized to prove the as-synthesized sample crystalline structures. Unambiguous evidence was represented by the coexistence of characteristic diffraction peaks for the COF and UiO-66-NH2 while successfully preparing UiO-66-NH2-MOF@COF. According to Fig. 2, the composite represented robust diffraction peaks allocated to UiO-66-NH2. There were relatively weak characteristic peaks of the COF layer. The obvious diffraction peaks at (111), (200), (311), (222), (400), (420), (511), (600) and (640) correspond to the construction of UiO-66-NH2 consistent with the reported values (Fig. 2).49 It is indicated that the catalyst contains pure phases with no distinctive peaks associated with the impurities. The particle size of UiO-66-NH2-MOF@COF nanoparticles is determined to be about 28 nm using Debye–Scherrer's equation (K = 0.90) which has good agreement with SEM analysis.
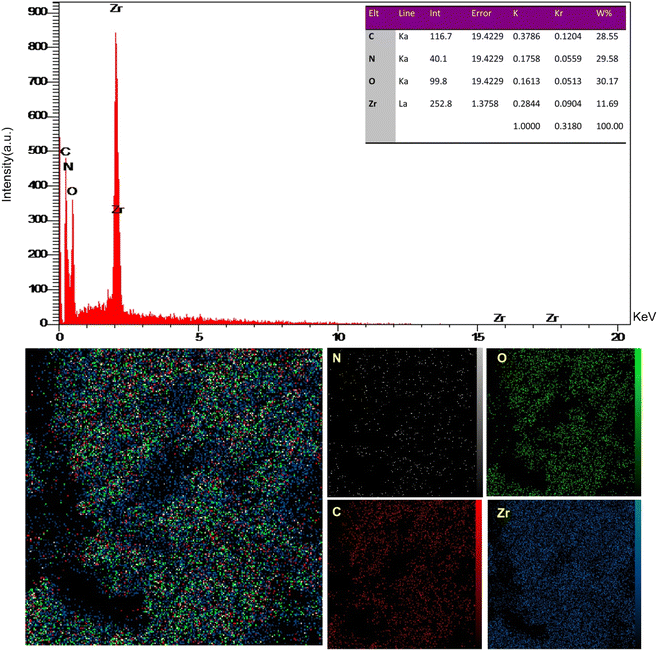
The EDX analysis and X-ray mapping of UiO-66-NH2-MOF@COF are illustrated in Fig. 3. The percentage of index elements is represented by the EDX spectrum in UiO-66-NH2-MOF@COF (N = 29.58%, C = 28.55%, Zr = 11.69%, and O = 30.17%) confirming the resultant UiO-66-NH2-MOF/COF nanostructure. There was no impurity in the prepared nanocomposite based on the elemental distribution of C, N, O, and Zr in Fig. 3. Besides, within UiO-66-NH2-MOF@COF, the uniform dispersion of components was revealed. Fig. 3 displays the uniform outline of the elements in the prepared nanostructure.
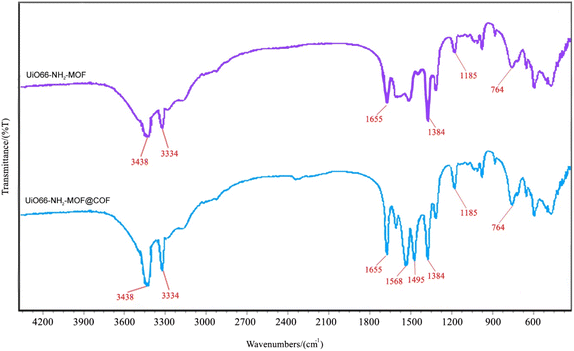
Fig. 4 indicates the FT-IR spectra of the UiO-66-NH2-MOF@COF and UiO-66-NH2 nanostructure. The peak at around 764 cm−1 originated from Zr–O bonds in UiO-66-NH2 (refs. 50 and 51). Two peaks at 1185 and 1655 cm−1 correspond to symmetric and asymmetric stretching vibrations of the carboxylate COO− ion.52 The peak at 1384 cm−1 might denote the C–N tensile vibrations. There are two characteristic peaks at 3334 and 3438 cm−1, which show the existence of NH2 and 2-aminoterephthalic acid moieties in UiO-66-NH2. The construction of the triazine cycle presents robust stretching vibrations at 1495 cm−1 and 1568 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) in the spectrum of UiO-66-NH2-MOF@COF reflecting that melamine was successfully incorporated into the framework.
N) in the spectrum of UiO-66-NH2-MOF@COF reflecting that melamine was successfully incorporated into the framework.
To calculate the pore volume and BET surface area, N2 adsorption was used along with the pore size distribution patterns determined from the desorption branch of the N2 isotherm using the Barrett–Joyner–Halenda (BJH) model (Fig. 5). The available surface area was 1028 m2 g−1 according to the BET plots. Besides, the pore volume of the cavities in UiO-66-NH2-MOF@COF was 0.325 cm3 g−1 (Fig. 5a). As seen in Fig. 5b, the type IV adsorption–desorption isotherm with a hysteresis loop denotes the designating of its mesoporous features.53 As shown in Fig. 5c, the pore-size distribution of the UiO-66-NH2-MOF@COF nanocomposite is 1.85 nm from the N2 isotherm desorption branch through the BJH model, which signifies the coexistence of structural pores along with inter-particle pores.
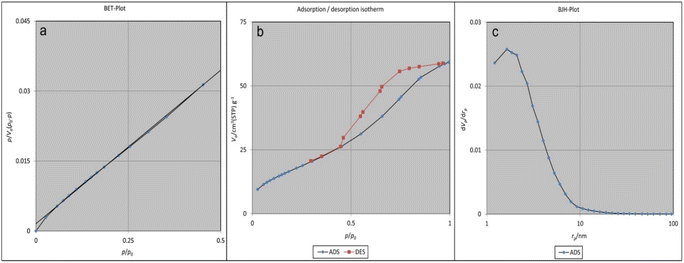 | ||
| Fig. 5 BET-plot of UiO-66-NH2-MOF@COF (a), adsorption/desorption of UiO-66-NH2-MOF@COF (b), and BJH-plot of UiO-66-NH2-MOF@COF (c). | ||
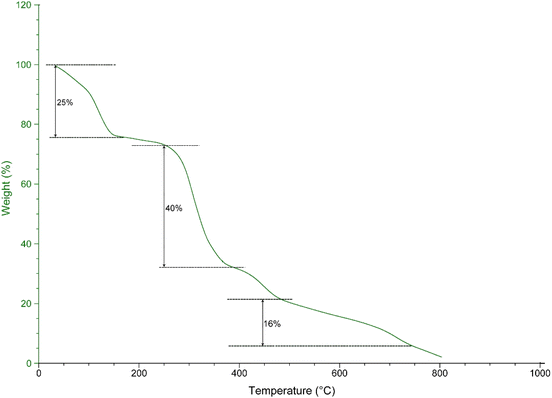
The TGA analyses were utilized to assess the stability and resistance of the nanocomposite against thermal decomposition. The loss of weight of UiO-66-NH2-MOF@COF followed by increasing the temperature is shown in Fig. 6. UiO-66-NH2-MOF@COF revealed three-stage weight losses in the TGA curve. The first weight loss at 0–150 °C (25%) represents the loss of solvents and adsorbed water from the framework while the second one at 250–350 °C (40%) denotes the degradation of UiO-66-NH2 in the structure of UiO-66-NH2-MOF@COF.54 Ultimately, the third weight loss step at >400 °C (16%) shows the COF structure collapse.55
Subsequently, we assessed the effectiveness of the catalyst in synthesizing some heterocyclic compounds including 2,3-dihydroquinazolin-4(1H)-ones. We initially chose the reaction of aniline, isatoic anhydride, and 4-nitrobenzaldehyde as a model study evaluating the optimized reaction conditions (Scheme 3). We explored the influences of various catalysts, solvents, and temperatures.
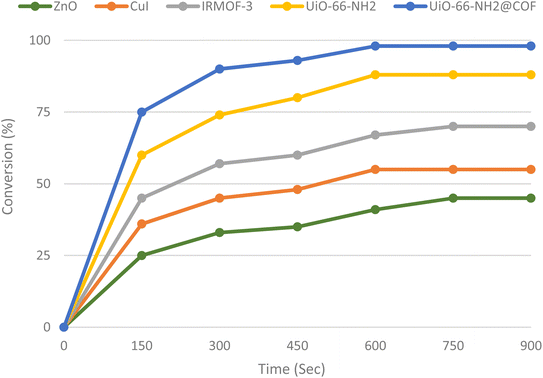
The present research is significant because of the astonishingly catalytic performance of our catalyst compared to other catalysts such as ZnO, CuI, IRMOF-3, and UiO-66-NH2, which were investigated in the model reaction using each catalyst (0.01 g) separately. The results in Fig. 7 show that the best outcomes were achieved (98% yield within 10 min) using UiO-66-NH2-MOF@COF in the absence of any solvent (Fig. 7).
In continuation, we examined the influence of different quantities of UiO-66-NH2-MOF@COF in the model study. Table 1 shows the results obtained from the model study for determining the best amount of the catalyst in the range of 80–120 °C. The best outcomes were achieved using 0.007 g of UiO-66-NH2-MOF@COF at 110 °C.
| Entry | Catalyst amount (g) | Temp. (oC) | Time (min) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: UiO-66-NH2-MOF@COF, isatoic anhydride (1 mmol), 4-nitrobenzaldehyde (1 mmol), and aniline (1 mmol). b Isolated yield. | ||||
| 1 | 0.007 | 80 | 100 | 72 |
| 2 | 0.007 | 90 | 100 | 86 |
| 3 | 0.007 | 100 | 40 | 90 |
| 4 | 0.007 | 110 | 10 | 98 |
| 5 | 0.007 | 120 | 10 | 98 |
| 6 | 0.005 | 110 | 15 | 94 |
| 7 | 0.01 | 110 | 10 | 98 |
Various solvents such as dichloromethane, acetonitrile, n-hexane, toluene, ethanol, and water and also solvent-free conditions were examined in this model reaction (Fig. 8). According to the results, more effectiveness was found in the absence of solvent compared to using solvents in the model study (98% yield within 10 min).
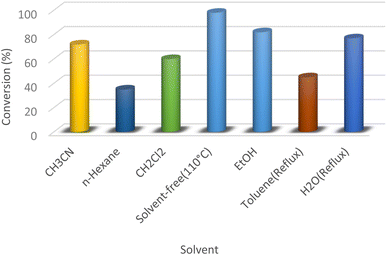 | ||
| Fig. 8 The preparation of dihydroquinazolin-4(1H)-one in the presence of different solvents and under solvent-free conditions. | ||
We used diverse anilines, aldehydes with isatoic anhydride, when we applied the optimal protocol to assess the efficiency and scope of this reaction. It should be noted that the reaction efficiently continues to provide the correspondent product in outstanding yields. This technique presented a decent tolerance for different substitutions (Table 2). Moreover, the effects of position and the electronic nature of groups on the phenyl rings displayed no robust influence on the synthesis. Moreover, dihydroquinazolinones were achieved in excellent yields within a short time (85–98%).
| Entry | R | Amine (ArNH2 or NH4OAC) | Product | Time (min) | Yieldb (%) | M.p. °C | Lit. M.p. oC |
|---|---|---|---|---|---|---|---|
a Reaction conditions: UiO-66-NH2-MOF@COF (0.007 g), isatoic anhydride, aldehyde, aryl amine or NH4OAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) under free solvent conditions.
b Isolated yield.
c New products. 1 molar ratio) under free solvent conditions.
b Isolated yield.
c New products.
|
|||||||
| 1 | H | Ph | 4a | 20 | 91 | 213–215 | 214–216 (ref. 56) |
| 2 | 4-Me | Ph | 4b | 14 | 85 | 211–213 | 213–214 (ref. 57) |
| 3 | 4-OMe | Ph | 4c | 15 | 89 | 202–204 | 205–207 (ref. 33) |
| 4 | 4-Cl | Ph | 4d | 16 | 94 | 220–223 | 222–224 (ref. 33) |
| 5 | 3-NO2 | Ph | 4e | 12 | 92 | 183–185 | 186–188 (ref. 57) |
| 6 | 4-NO2 | Ph | 4f | 10 | 98 | 192–194 | 195–197 (ref. 57) |
| 7 | 4-Me | 4-MeC6H4 | 4g | 25 | 90 | 244–246 | 243–247 (ref. 58) |
| 8 | 4-OMe | 4-ClC6H4 | 4h | 18 | 87 | 243–245 | 244–247 (ref. 59) |
| 9 | 4-F | 4-Me | 4i | 17 | 93 | 240–242 | 241–243 (ref. 59) |
| 10 | 4-Cl | 5-Cl, 2-OHC6H3 | 4j | 10 | 89 | 233–236 | 235–237 (ref. 58) |
| 11 | 2,6-Cl2 | Ph | 4k | 14 | 93 | 231–232 | 234–236 (ref. 57) |
| 12 | 4-Br | Ph | 4l | 16 | 96 | 218–220 | 221–223 (ref. 57) |
| 13 | 4-SMe | 4-BrC6H4 | 4m | 25 | 94 | 246–248 | —c |
| 14 | 3-NO2 | NH4OAC | 6a | 20 | 91 | 184–186 | 186–188 (ref. 33) |
| 15 | 2-Cl | NH4OAC | 6b | 15 | 96 | 202–204 | 203–205 (ref. 56) |
| 16 | 4-OH, 3-OMe | NH4OAC | 6c | 25 | 87 | 223–225 | 226–227 (ref. 33) |
| 17 | H | NH4OAC | 6d | 20 | 91 | 222–224 | 225–226 (ref. 57) |
| 18 | 4-OMe | NH4OAC | 6e | 17 | 93 | 187–189 | 192–193 (ref. 57) |
| 19 | 4-Me | NH4OAC | 6f | 19 | 89 | 230–232 | 233–234 (ref. 57) |
| 20 | 4-SMe | NH4OAC | 6g | 20 | 87 | 235–237 | —c |
Ultimately, the merits of this method are demonstrated by comparing our catalyst with some of the other reported catalysts. Some representative examples are the reaction of 4-nitrobenzaldehyde, isatoic anhydride, and aniline. As seen from Table 3, our method in this paper presented superior results to most of the methods in the literature.
| Entry | Catalyst | Conditions | Time/yield (%) | References |
|---|---|---|---|---|
| a Based on the reaction between isatoic anhydride, aniline and 4-nitrobenzaldehyde. | ||||
| 1 | Zn(PFO)2 | EtOH/reflux | 6 h/77–82 | 35 |
| 2 | P-TSA | EtOH/reflux | 3 h/68–85 | 37 |
| 3 | Silica sulfuric acid | Solvent-free, 80 °C | 5 h/70–80 | 38 |
| 4 | Montmorillonite K-10 | EtOH/reflux | 6.5 h/73–92 | 40 |
| 5 | Ga(OTf)3 | EtOH/70 °C/stir | 35 min/71–91 | 41 |
| 6 | UiO-66-NH2-MOF@COF | Solvent-free, 110 °C | 10 min/98 | This work |
We proposed a mechanism for synthesizing dihydroquinazolines through the UiO-66-NH2-MOF@COF nanocomposite based on our studies and also according to the previous literature which is presented in Scheme 4.41 The catalyst has both Lewis acidic sites (Zr2+) and basic sites (imine groups of the COF) on its surfaces while abstracting the acidic protons of the reactants and activating the carbonyl groups and double bonds of the substrates and intermediates.60 Mechanistically, the reaction can proceed through the primary activation of the isatoic anhydride. Next, an intermediate I is produced, by attacks of the N-nucleophilic amine on the carbonyl moiety, presenting intermediate IIvia decarboxylation reaction. 2-Amino-N-substituted-amide III is formed via the proton transfer of intermediate II. The proton capture of intermediate II by an intramolecular reaction leads to the creation of intermediate III which undertakes nucleophilic attack on aryl aldehyde for the formation of the imine intermediate IV. In intermediate IV, the catalyst activates the imine moiety associated with intramolecular cyclization by imine to form intermediate Vvia a cyclization reaction. Then, the corresponding products are obtained by a 1,5-proton shift.
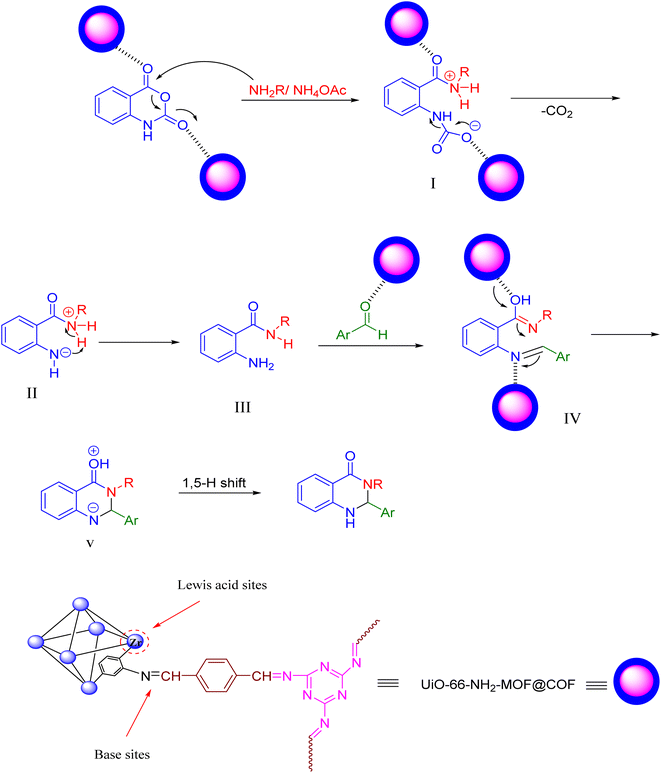 | ||
| Scheme 4 Proposed mechanism pathway for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones using UiO-66-NH2-MOF@COF. | ||
4. Recycling and reusing of the catalyst
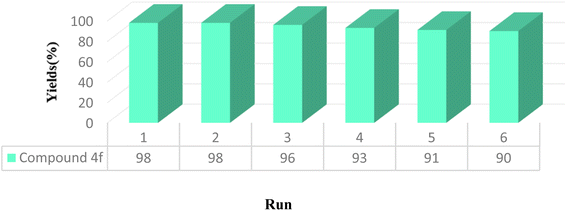
The half-life and recoverability of UiO-66-NH2-MOF@COF are the most important features, that should be considered in the practical application of heterogeneous systems. An investigation was performed on the likelihood of recycling the catalyst in the model study. The model reaction was carried out under the optimized conditions. After completion of the reaction (as determined by TLC), the residue was dissolved in dichloromethane and the catalyst was insoluble in CH2Cl2 and separated by centrifugation. The recovered catalyst was then washed several times with water to remove any impurities and then was dried for further use at 50 °C for 12 h. The recovered catalyst was used in subsequent uses (six times) under the same conditions without significant loss of its catalytic activity (Fig. 9).In addition, the FT-IR spectrum, XRD pattern, and SEM image of the catalyst after six cycles were recorded. As seen from Fig. 10, the intensity of some peaks was reduced but the crystalline structure of the catalyst remained. Comparison of the SEM image of the reused catalyst with that of the fresh catalyst shows more agglomeration of the particles in the recovered catalyst. These facts prove that the efficiency, appearance, and structure of the UiO-66-NH2-MOF@COF catalyst remained intact in the recycled catalyst and there was no considerable deformation.
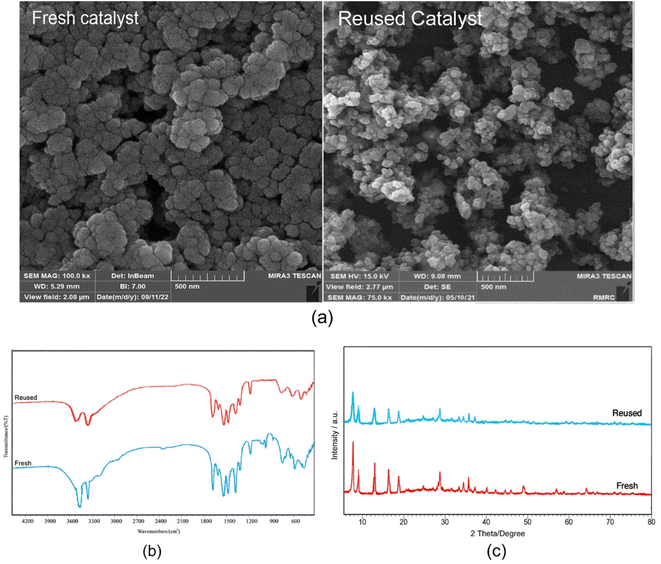 | ||
| Fig. 10 SEM images (a), FT-IR spectrum (b), and XRD pattern (c) of the recovered UiO-66-NH2-MOF@COF after 6 runs. | ||
5. Conclusion
In this report we successfully prepared UiO-66-NH2-MOF@COF as a novel, effective and low-cost catalyst for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones through the three-component condensation of aldehydes, isatoic anhydride, and ammonium acetate (or primary amines). SEM, XRD, EDX/mapping, FT-IR, BET, and TGA methods proved the as-synthesized catalyst. This environmentally friendly technology has advantages such as being solvent-free, catalyst reusability, easy product isolation, short reaction times, and excellent product yields with short reaction time.Conflicts of interest
There are no conflicts to declare.References
- K. Farha and J. T. Hupp, Acc. Chem. Res., 2010, 43, 1166–1175 CrossRef.
- M. Bosch, S. Yuan, W. Rutledge and H.-C. Zhou, Acc. Chem. Res., 2017, 50, 857–865 CrossRef CAS.
- L. He, W. Li, Z. W. Jiang, T. T. Zhao, Y. Li, C. M. Li, C. Z. Huang and Y. F. Li, Chem. Eng. J., 2019, 374, 1231–1240 CrossRef CAS.
- X. Fang, B. Zong and S. Mao, Nano-Micro Lett., 2018, 10, 64–82 CrossRef.
- X. Zhao, Y. Wang, D.-S. Li, X. Bu and P. Feng, Adv. Mater., 2018, 30, 1705189–1705220 CrossRef.
- A. C. McKinlay, R. E. Morris, P. Horcajada, G. Ferey, R. Gref, P. Couvreur and C. Serre, Angew. Chem., Int. Ed., 2010, 49, 6260–6266 CrossRef CAS.
- X. Li, X. Yang, H. Xue, H. Pang and Q. Xu, EnergyChem, 2020, 2, 100027–100056 CrossRef.
- D. Yang and B. C. Gates, ACS Catal., 2019, 9, 1779–1798 CrossRef CAS.
- H. Zhou, M. Zheng, H. Tang, B. Xu, Y. Tang and H. Pang, Small, 2020, 16, 1904252–1904260 CrossRef CAS.
- P. Albacete, J. I. Martinez, X. Li, A. Lopez-Moreno, S. Mena-Hernando, A. E. Platero-Prats, C. Montoro, K. P. Loh, E. M. Perez and F. Zamora, J. Am. Chem. Soc., 2018, 140, 12922–12929 CrossRef CAS.
- Y. Zhao, L. Guo, F. Gandara, Y. Ma, Z. Liu, C. Zhu, H. Lyu, C. A. Trickett, E. A. Kapustin, O. Terasaki and O. M. Yagh, J. Am. Chem. Soc., 2017, 139, 13166–13172 CrossRef CAS.
- W. Zhao, L. Xia and X. Liu, CrystEngComm, 2018, 20, 1613–1634 RSC.
- Z. D. Li, H. Q. Zhang, X. H. Xiong and F. Luoc, J. Solid State Chem., 2019, 277, 484–492 CrossRef CAS.
- L. Zhu and Y.-B. Zhang, Molecules, 2017, 22, 1149–1177 CrossRef.
- S. Cao, B. Li, R. Zhu and H. Pang, Chem. Eng. J., 2019, 355, 602–623 CrossRef CAS.
- C. Altintas, I. Erucar and S. Keskin, CrystEngComm, 2022, 24, 7360–7371 RSC.
- Y. Peng, M. Zhao, B. Chen, Z. Zhang, Y. Huang, F. Dai, Z. Lai, X. Cui, C. Tan and H. Zhang, Adv. Mater., 2018, 30, 17054–1717058 Search PubMed.
- F. M. Zhang, J. L. Sheng, Z. D. Yang, X. J. Sun, H. L. Tang, M. Lu, H. Dong, F. C. Shen, J. Liu and Y. Q. Lan, Angew. Chem., Int. Ed., 2018, 57, 12106–12110 CrossRef CAS.
- Y. Yan, T. He, B. Zhao, K. Qi, H. Liu and B. Y. Xia, J. Mater. Chem. A, 2018, 6, 15905–15926 RSC.
- A. Shaabani, A. Maleki, A. H. Rezayan and A. Sarvary, Mol. Diversity, 2011, 15, 41–68 CrossRef CAS.
- M. J. Climent, A. Corma and S. Iborra, RSC Adv., 2012, 2, 16–58 RSC.
- A. Kumar and R. A. Maurya, Tetrahedron, 2007, 63, 1946–1952 CrossRef CAS.
- L. F. Tietze, T. Kinzel and C. C. Brazel, Acc. Chem. Res., 2009, 42, 367–378 CrossRef CAS.
- J. Bartroli, E. Turmo, M. Alguero, E. Boncompte, M. L. Vericat, L. Conte, J. Ramis, M. Merlos, J. Garcia-Rafanell and J. Forn, J. Med. Chem., 1998, 41, 1869–1882 CrossRef CAS.
- O. I. El-Sabbagh, S. M. Ibrahim, M. M. Baraka and H. Kothayer, Arch. Pharm., 2010, 343, 274–281 CrossRef CAS.
- J. Rudolph, W. P. Esler, S. O'Connor, P. D. Coish, P. L. Wickens, M. Brands, D. E. Bierer and B. T. Bloomquist, et al. , J. Med. Chem., 2007, 50, 5202–5216 CrossRef CAS PubMed.
- M.-J. Hour, L.-J. Huang, S.-C. Kuo, Y. Xia, K. Bastow, Y. Nakanishi, E. Hamel and K.-H. Lee, J. Med. Chem., 2000, 43, 4479–4487 CrossRef CAS.
- R. J. Alaimo and H. E. Russell, J. Med. Chem., 1972, 15, 335–336 CrossRef CAS.
- D. C. White, T. D. Greenwood, A. L. Downey, J. R. Bloomquist and J. F. Wolfe, Bioorg. Med. Chem., 2004, 12, 5711–5717 CrossRef CAS.
- R. J. Abdel-Jalil, W. Volter and M. Saeed, Tetrahedron Lett., 2004, 45, 3475–3476 CrossRef CAS.
- T. Magyar, F. Miklós, L. Lázár and F. Fülöp, Chem. Heterocycl. Compd., 2015, 50, 1464–1470 CrossRef CAS.
- S. Fozooni and H. Firoozi, Chem. Heterocycl. Compd., 2015, 51, 340–345 CrossRef CAS.
- M. Wang, T. T. Zhang, J. J. Gao and Y. Liang, Chem. Heterocycl. Compd., 2012, 48, 897–902 CrossRef CAS.
- M. P. Surpur, P. R. Singh, S. B. Patil and S. D. Samant, Synth. Commun., 2007, 37, 1965–1970 CrossRef CAS.
- L. M. Wang, L. Hu and J. H. Shao, J. Fluorine Chem., 2008, 129, 1139–1145 CrossRef CAS.
- K. Niknam, N. Jafarpour and E. Niknam, Chin. Chem. Lett., 2011, 22, 69–72 CrossRef CAS.
- M. Baghbanzadeh, P. Salehi, M. Dabiri and G. Kozehgary, Synthesis, 2006, 2, 344–348 Search PubMed.
- (a) M. Dabiri, P. Salehi and M. Baghbanzadeh, Catal. Commun., 2008, 9, 785 CrossRef CAS; (b) P. Salehi, M. Dabiri and M. A. Zolfigol, Tetrahedron Lett., 2005, 46, 7051–7053 CrossRef CAS.
- M. Baghbanzadeh, M. Dabiri and P. Salehi, Heterocycles, 2008, 75, 2809–2815 CrossRef CAS.
- P. Salehi, M. Dabiri, M. Baghbanzadeh and M. Bahramnejad, Synth. Commun., 2006, 36, 2287 CrossRef CAS.
- J. X. Chen, D. Z. Wu, F. He, M. C. Liu, H. Y. Wu, J. C. Ding and W. K. Su, Tetrahedron Lett., 2008, 49, 3814–3818 CrossRef CAS.
- M. Dabiri, P. Salehi and M. Baghbanzadeh, Monatsh. Chem., 2007, 138, 1191–1194 CrossRef CAS.
- J. Safaei-Ghomi and M. A. Ghasemzadeh, Arabian J. Chem., 2017, 10, S1774–S1780 CrossRef CAS.
- J. Safari, M. Tavakoli and M. A. Ghasemzadeh, Appl. Organomet. Chem., 2018, 33, e4748–e4759 CrossRef.
- M. A. Ghasemzadeh, Acta Chim. Slov., 2015, 62, 977–985 CrossRef CAS.
- M. A. Ghasemzadeh and J. Safaei-Ghomi, J. Chem. Res., 2014, 38, 313–316 CrossRef CAS.
- J. Safaei-Ghomi, M. A. Ghasemzadeh and A. Kakavand-Qalenoei, J. Saudi Chem. Soc., 2016, 20, 502–509 CrossRef CAS.
- X.-Y. Xu, C. Chu, H. Fu, X.-D. Du, P. Wang, W. Zheng and C.-C. Wang, Chem. Eng. J., 2018, 350, 436 CrossRef CAS.
- H. Fotovat, M. Khajeh, A. Oveisi, M. Ghaffari-Moghaddam and S. Daliran, Microchim. Acta, 2018, 10, 185–192 Search PubMed.
- B. He and X. Dong, Sens. Actuators, B, 2019, 294, 192–198 CrossRef CAS.
- D. Song, Q. Chen, C. Zhai, H. Tao, L. Zhang, T. Jia, Z. Lu, W. Sun, P. Yuan and B. Zhu, Chemosensors, 2022, 10, 10158–10169 Search PubMed.
- T. Loiseau, C. Serre, C. Huguenard, G. Fink, F. Taulelle, M. Henry, T. Bataille and G. Ferey, Chem. Eur. J., 2004, 10, 1373–1382 CrossRef CAS.
- L. Zhu and Y.-B. Zhang, Molecules, 2017, 22, 1149–1177 CrossRef.
- B. M. Eshkevari, M. A. Ghasemzadeh and M. Esnaashari, Appl. Organomet. Chem., 2019, 33, e5027–e5739 CrossRef.
- Z. Rafiee, J. Iran. Chem. Soc., 2021, 18, 2657–2664 CrossRef CAS.
- Z. Song, L. Liu, Y. Wang and X. Sun, Res. Chem. Intermed., 2012, 38, 1091–1092 CrossRef CAS.
- A. Olyaei, F. Rahbarian and M. Sadeghpour, Chem. Heterocycl. Compd., 2015, 51, 899–902 CrossRef CAS.
- K. Niknam, M. R. Mohammadizadeh and S. Mirzaee, Chin. J. Chem., 2011, 29, 1417–1422 CrossRef CAS.
- Z. H. Zhang, H. Y. Lu, S. H. Yang and J. W. Gao, J. Comb. Chem., 2010, 12, 643–646 CrossRef CAS.
- M. Aguirre-Díaz, L. Gándara, F. Iglesias, M. Snejko, N. Gutiérrez-Puebla and E. Ángeles Monge, J. Am. Chem. Soc., 2015, 137, 6132–6135 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3na00805c |
| This journal is © The Royal Society of Chemistry 2023 |