Open Access Article
Open Access ArticleAn Fe3O4 supported O-phenylenediamine based tetraaza Schiff base-Cu(II) complex as a novel nanomagnetic catalytic system for synthesis of pyrano[2,3-c]pyrazoles†
Rehab
Tahseen alhayo
a,
Ghufran Sh.
Jassim
 b,
Hasanain Amer
Naji
c,
A. H.
Shather
d,
Israa Habeeb
Naser
e,
Luay Ali
Khaleel
f and
Haider Abdulkareem
Almashhadani
b,
Hasanain Amer
Naji
c,
A. H.
Shather
d,
Israa Habeeb
Naser
e,
Luay Ali
Khaleel
f and
Haider Abdulkareem
Almashhadani
 *g
*g
aAl-Farahidi University, College of dentistry, Baghdad, Iraq
bDepartment of Chemistry, College of Science, University of Anbar, Anbar, Iraq
cFaculty of Pharmacy, Al-Turath University College, Baghdad, Iraq
dDepartment of Computer Engineering Technology Al Kitab University,Altun Kopru, Kirkuk 00964, Iraq
eMedical Laboratories Techniques Department / AL-Mustaqbal University College, 51001 Hillah, Babil, Iraq
fCollage of Dentistry, National University of Science and Technology, Dhi Qar, Iraq
gChemistry Department, College of Science, University of Baghdad, Baghdad, Iraq. E-mail: haider.200690@gmail.com; h_r200690@yahoo.com
First published on 8th November 2023
Abstract
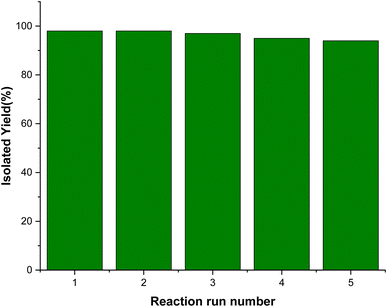
In this research, we present a post-synthetic method for synthesizing a novel nanomagnetic Cu(II) Schiff base complex and investigate its efficiency in catalytic organic conversion reactions. Various spectroscopic analyses were employed to characterize the physiochemical characteristics of the resulting nanocomposite. The experimental results successfully demonstrate the catalytic application of the prepared Cu-complex in the preparation of pyrano[2,3-c]pyrazole heterocycles. This synthesis involved a one-pot three-component condensation reaction, wherein hydrazine hydrate, ethyl acetoacetate, malononitrile, and aromatic aldehydes were combined under reflux conditions using water as the solvent. Notably, the heterogenized complex exhibited exceptional catalytic performance, achieving remarkable conversion rates and selectivity, all accomplished using only 12 mg of the catalyst. Furthermore, thorough stability assessments of this catalyst were conducted through reusability and hot filtration tests, which confirmed its non-leaching properties and demonstrated excellent results over the course of five consecutive runs.
1. Introduction
Pyrano[2,3-c]pyrazoles represent a significant group of heterocycles that have attracted significant attention within the realm of medicinal chemistry and drug discovery.1 The scaffold of these structures consists of a pyrazole ring fused with a pyran ring, forming a fused heterocyclic framework. This unique structural motif provides a versatile platform for molecular modifications and exhibits diverse biological activities, enabling the synthesis of various derivatives with enhanced biological properties.2The pyrano[2,3-c]pyrazoles synthesis involves diverse strategies, such as multicomponent reactions, cyclization reactions, or condensation reactions, which allow for the introduction of different functional groups and substitution patterns.2–5 One commonly employed strategy involves the condensation reaction between pyrazole derivatives and α,β-unsaturated carbonyl compounds, such as α,β-unsaturated ketones or aldehydes, under acidic or basic conditions.6 Another synthetic approach involves the cyclization of α,β-unsaturated ketones with hydrazine derivatives, followed by oxidative cyclization or ring-closing reactions using suitable reagents or catalysts. On the other hand, multicomponent reactions, such as the one-pot condensation of α,β-unsaturated carbonyl compounds, hydrazine derivatives, and active methylene compounds such as malononitrile, have been employed as the most efficient and simple method for this synthesis.7 In this regard, several catalytic methods have been employed for these reactions. One notable approach involves the use of Lewis acid catalysts, such as Zn, Fe and Cu complexes, which promote the generation and condensation of pyrazolone and α,β-unsaturated carbonyl intermediates, facilitating the cyclization process and resulting in the selective production of the fused pyrano[2,3-c]pyrazole rings at enhanced reaction rates.8–10 The use of catalytic methods provides several advantages, including increased reaction rates, improved yields, and enhanced control over regioselectivity and stereoselectivity, making them valuable tools in these transformations.
Heterogeneous magnetic catalysts are a promising class of materials in catalysis due to their unique combination of magnetic properties and catalytic functionality.11–15 These catalysts offer advantages such as enhanced catalyst recovery, recyclability, and improved reaction kinetics.15–18 Compared to traditional catalysts, heterogeneous magnetic catalysts simplify catalyst recovery, reduce costs, and minimize environmental impact.19,20 Additionally, their recyclability ensures long-term viability and economic feasibility, making them an attractive option in catalysis applications.21 The active regions located on the surface of the magnetic core or attached functional coatings enable a wide range of an extensive variety of catalytic reactions.22
In this regard; heterogeneous Schiff base catalysts are crucial in organic synthesis, promoting diverse and efficient chemical transformations.23,24 These catalysts consist of immobilized Schiff base ligands on solid supports, offering advantages such as enhanced catalytic activity, improved selectivity, and recyclability.25–27 They are compatible with a wide range of substrates, enabling the synthesis of various organic compounds with high yields and purity.28 The tunable and robust nature of these catalysts allows for the development of new synthetic methods and exploration of complex transformations.29,30 Overall, heterogeneous Schiff base catalysts play a vital role in advancing organic synthesis, providing efficient and environmentally friendly routes to valuable organic molecules.31
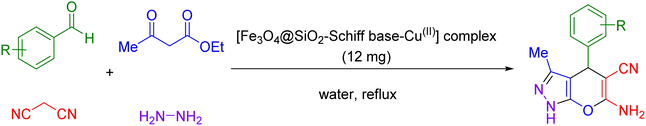
Overall, the unique combination of magnetic properties and catalytic efficiency of Schiff base complexes holds great promise for catalysis applications, opening new avenues for efficient and sustainable chemical transformations. Now, in this paper, a Schiff base complex of copper immobilized on magnetic Fe3O4 nanoparticles [Fe3O4@SiO2-Schiff base-Cu(II)] was successfully fabricated as a novel and efficient magnetically recoverable nanocatalyst for the multicomponent synthesis of pyrano[2,3-c]pyrazoles.
2. Experimental
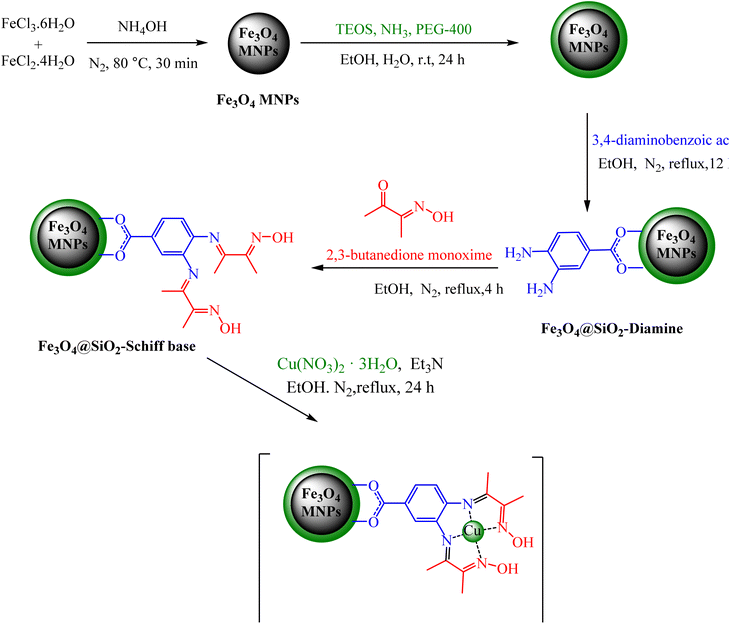
2.1. Typical procedure for the synthesis of [Fe3O4@SiO2-Schiff base-Cu(II)] complex
In the first step, silica-modified Fe3O4 MNPs were prepared using a previously established procedure.32 Simultaneously, 2 g of Fe3O4@SiO2 MNPs were dispersed in 100 mL of 95% ethanol, and a 20 mL solution of 0.25 M 3,4-diaminobenzoic acid in ethanol was added to the well-dispersed suspension and then refluxed for 12 hours under N2 atmosphere. Afterward, the resulting Fe3O4@SiO2-diamine powder was magnetically collected, washed several times with ethanol and dried under vacuum. Afterwards, 1 g of Fe3O4@SiO2-diamine was dispersed in ethanol for 30 minutes. Then, a 20 mL solution of 0.3 M 2,3-butanedione monoxime in ethanol was added dropwise to the reaction mixture, which was then refluxed for 4 hours under a N2 atmosphere. The synthesized Fe3O4@SiO2-Schiff base MNPs were accumulated, washed with ethanol and dried under vacuum. Next, 1 g of Fe3O4@SiO2-Schiff base MNPs was dispersed in 100 mL of ethanol, and 3 drops of triethylamine and 25 mL of a 0.2 M ethanolic solution of Cu(NO3)2·3H2O were added dropwise to the prepared suspension and refluxed for 24 hours under a N2 atmosphere. Finally, the obtained [Fe3O4@SiO2-Schiff base-Cu(II)] complex was collected, purified through washing with water and dried under vacuum (Scheme 1).2.2. General procedure to produce 2 pyrano[2,3-c]pyrazoles catalyzed via [Fe3O4@SiO2-Schiff base-Cu(II)] complex
A mixture of ethyl acetoacetate (1 mmol) and hydrazine hydrate (1 mmol), [Fe3O4@SiO2-Schiff base-Cu(II)] complex (12 mg), and 3 mL of water were mixed in a round-bottomed until 3-methyl-1H-pyrazol-5(4H)-one precipitate was formed. Following that, malononitrile (1 mmol), and aldehyde (1 mmol) were introduced into the flask and the reaction was agitated under reflux conditions. Once the reaction was deemed complete, which was monitored using thin-layer chromatography (TLC), a simple work-up procedure was performed. This involved using a magnetic field to separate the catalyst from the mixture (diluted with hot ethanol). The resulting crude products were then purified through recrystallization using a mixture of ethanol and diethyl ether and well-characterized by 1H and 13C NMR spectroscopy (ESI†).3. Results and discussions
3.1. Catalyst characterization
FT-IR analysis was conducted to examine the formation of catalytic moieties on the surface. As depicted in Fig. 1, the FT-IR spectra of Fe3O4 and Fe3O4@SiO2 align with previous reports regarding the characteristic features of these samples.33 The peaks observed at 575 cm−1 and 619 cm−1 correspond to the mode of stretching vibrations in metal–oxygen bonds.34 Furthermore, the faint peak at 810 cm−1 and the broad absorption peak ranging from 993 cm−1 to 1318 cm−1 can be ascribed to the stretching of Si–O–Si bonds.35 The bands in the range of 2870 cm−1 to 2940 cm−1 and above 3000 cm−1 indicate the C–H stretching vibrations of aliphatic (methyl moieties) and aromatic C–H groups (present in the 3,4-diaminobenzoic acid) on the surface.35 The peak appearing in the region of 3400 cm−1 is related to the N–H groups, which overlap with the O–H stretching vibrations. Additionally, the peaks in the 1200–1500 cm−1 range are related to C–N and C–O bonds. In the case of Fe3O4@SiO2-Schiff base MNPs, the strong peak at 1627 cm−1 represents the stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds and confirms the formation of imine groups. This indicates the attachment of the [Schiff base-Cu(II)] complex onto the nanomagnetic support.
N bonds and confirms the formation of imine groups. This indicates the attachment of the [Schiff base-Cu(II)] complex onto the nanomagnetic support.
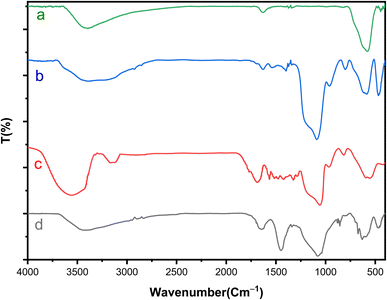 | ||
| Fig. 1 FTIR spectrum of (a) Fe3O4, (b) Fe3O4@SiO2, (c) Fe3O4@SiO2-diamine and (d) [Fe3O4@SiO2-Schiff base-Cu(II)] complex. | ||
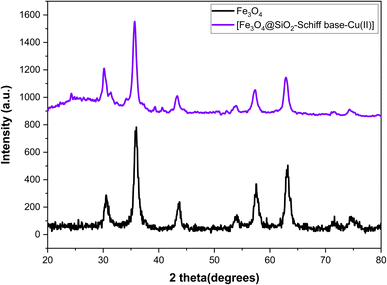
The X-ray diffraction (XRD) analysis was conducted to examine the structural properties of the [Fe3O4@SiO2-Schiff base-Cu(II)] complex (Fig. 2). The XRD pattern displayed the characteristic peaks of crystalline structure of Fe3O4 (JCPDS file no 19-0629) at 2θ = 30.15°, 35.65°, 43.33, 53.89°, 57.31°, 62.89° and 74.33° that are related to planes (220), (311), (400), (422), (511), and (440), (533), respectively.36 The results show that the post synthetic modification process did not change the crystalline structure of the final magnetic nanoparticles and it was remained intact. The presence of an amorphous SiO2 layer can be confirmed by a broad peak at 2θ = 24.29°. The prepared catalyst exhibited some additional diffraction peaks at 2θ = 24.29°, 31.37°, 39.33° and 40.61 which could be due to the addition of [Schiff base-Cu(II)] complex on Fe3O4@SiO2 surface.
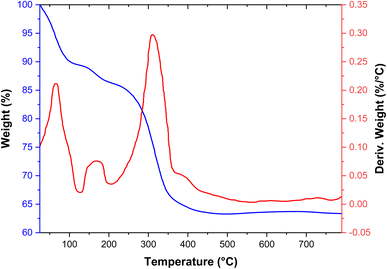
The thermal stability of the [Fe3O4@SiO2-Schiff base-Cu(II)] complex was investigated using TGA and DSC analysis (Fig. 3). The weight loss observed below 200 °C is due to the removal of organic solvents and moisture that were adsorbed.37 Furthermore, a weight loss of approximately 23% was occurred at 200–470 °C, indicating the separation and breakdown of the Schiff base organic groups from the nanocomposite surface through pyrolysis. This process was characterized by a noticeable endothermic peak in the DSC analysis. The nanocomposite demonstrated stability up to around 200 °C, thanks to the inherent thermal stability of the Schiff base design. Consequently, it can be easily employed in organic reactions and high-temperature conditions.
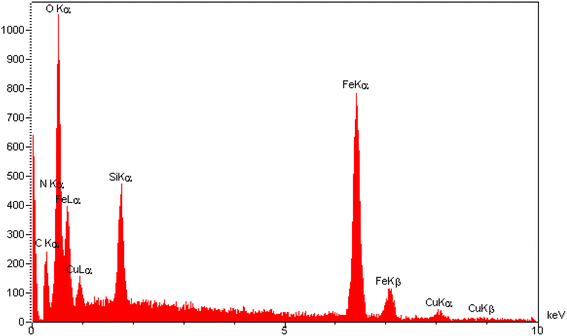
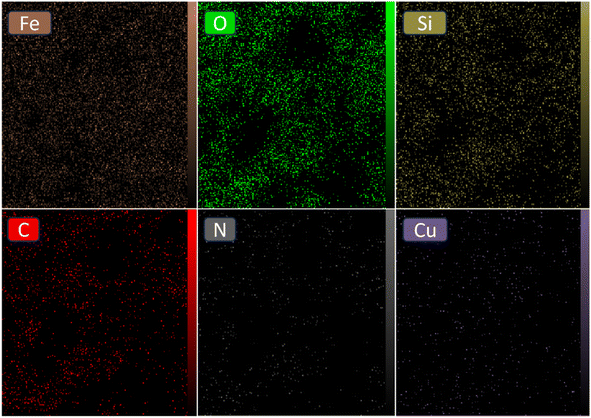
The elemental composition of the [Fe3O4@SiO2-Schiff base-Cu(II)] complex was examined through EDAX analysis (Fig. 4). This analysis confirmed the presence of distinct peaks corresponding to the Fe, Si, and O elements, which is consistent with the TGA results indicating that over 60% of the sample consists of inorganic materials, including the Fe3O4 support and SiO2 shell surrounding its surface. Furthermore, the observation of peaks for C and N provides further confirmation of the synthesis of the Schiff base layer derived from the prepared ligand. Lastly, the presence of Cu in the sample confirms the successful bonding of electron-donor atoms to copper ions and the formation of the desired catalyst. The results clearly demonstrate the successful preparation of the designed composite using the post-synthetic modification method.
Furthermore, the EDS mapping images clearly illustrate the even distribution of the [Schiff base-Cu(II)] complex across the Fe3O4@SiO2 surface (Fig. 5). The significant percentages of Fe, O, and Si elements indicate their prominent presence in the composite material. On the other hand, the presence of carbon and nitrogen elements can be attributed to the successful incorporation of the Schiff base ligand, which shows a homogeneous distribution within the composite structure. The close alignment between the distribution patterns of copper and nitrogen reinforces the evidence of effective coordination between N atoms and copper ions within the catalyst. Collectively, the EDS mapping analysis provides comprehensive visual evidence of the uniform distribution and elemental composition of the [Fe3O4@SiO2-Schiff base-Cu(II)] complex.
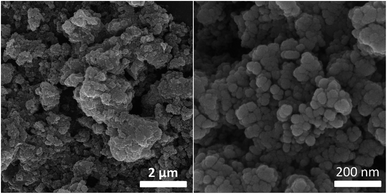
The morphology of the [Fe3O4@SiO2-Schiff base-Cu(II)] complex was examined using scanning electron microscopy (SEM). The SEM analysis, as depicted in Fig. 6, clearly demonstrated the uniform spherical shape of the resulting nanocomposite, characterized by a rough surface texture. A notable observation was made when comparing these particles to the uncoated Fe3O4 counterparts, revealing a noticeable increase in size. This size increment strongly indicates the presence of SiO2 and [Schiff base-Cu(II)] shells that envelop the Fe3O4 particles. Remarkably, the SEM analysis revealed no significant agglomeration in the sample, signifying the successful distribution and stability of the nanocomposite.
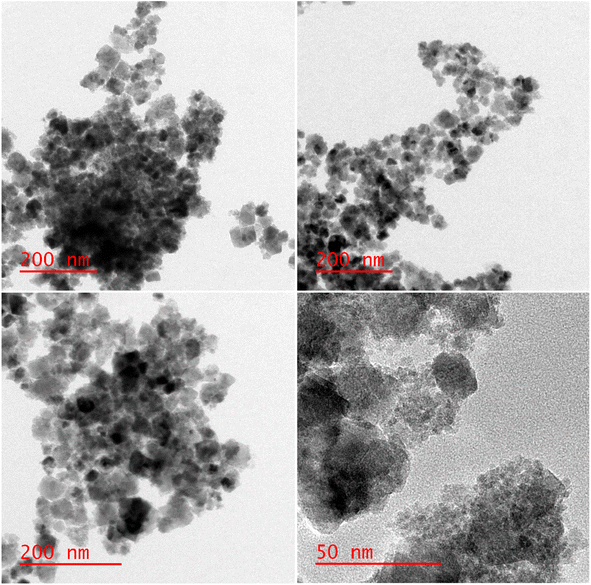
The structure and size of the [Fe3O4@SiO2-Schiff base-Cu(II)] complex were analyzed using HR-TEM (Fig. 7). The particles exhibited a nearly spherical shape and demonstrated a high level of crystallinity. Their sizes ranged from 25 to 32 nm. They were surrounded by an amorphous layer, which contributed to their irregular shape. This shape suggested that the Fe3O4 nanoparticles were well-dispersed, likely due to their small size and interaction with the grafted layers. In certain areas, some aggregation was observed, possibly attributed to the magnetic property of nanoparticles. These results confirm the successful formation of the desired complex on the surface of the modified magnetic nanoparticles.
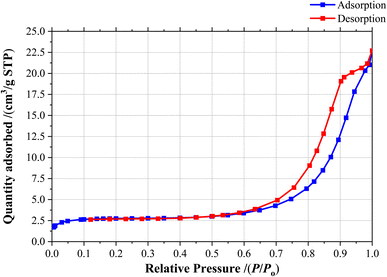
Through BET analysis, the [Fe3O4@SiO2-Schiff base-Cu(II)] complex was examined to determine its isotherm type, surface area and pore characteristics (Fig. 8). The measured values were 27.2 nm, 39.59 m2 g−1, and 0.041 cm3 g−1 for mean pore diameter, surface area, and total pore volume, respectively. The nanocomposite displayed a type IV isotherm and exhibited a mesoporous structure, evident from its average pore volume. Comparing these results with previous research, it can be inferred that the modification with [Schiff base-Cu(II)] complex led to a reduction in the BET characteristics of Fe3O4@SiO2. These surface characteristics of the [Fe3O4@SiO2-Schiff base-Cu(II)] complex provide ample reaction sites for direct interaction with organic reactants, resulting in an improved catalytic capacity.
The magnetic characteristics of the synthesized magnetic nanoparticles were evaluated using a vibration sample magnetometer (VSM). Fig. 9 provides a clear depiction of the saturation magnetization (Ms) values obtained for (a) Fe3O4, (b) Fe3O4@SiO2, (c) Fe3O4@SiO2-diamine, and (d) [Fe3O4@SiO2-Schiff base-Cu(II)] complex, which were determined as 74, 53, 33, and 24 emu g−1, respectively. The introduction of an amorphous SiO2 layer on the surface resulted in a notable decrease in the Ms value of Fe3O4 due to the non-magnetic nature silica. The difference in Ms values between Fe3O4@SiO2 and Fe3O4@SiO2-diamine indicated the bonding of a significant quantity of 3,4-diaminobenzoic acid groups to the surface of the Fe3O4 nanoparticles modified with silica. Moreover, the subsequent formation of organic functional groups (Schiff base) could diminish the surface moments of individual particles, leading to an overall reduction in magnetism. This decline in magnetic properties verifies the binding of iron nanoparticles to the [SiO2-Schiff base-Cu(II)] complex and validates its successful synthesis. Despite the lower Ms value of [Fe3O4@SiO2-Schiff base-Cu(II)] compared to pure Fe3O4, it remains adequate for effective magnetic separation, facilitating quicker removal from crude solutions.
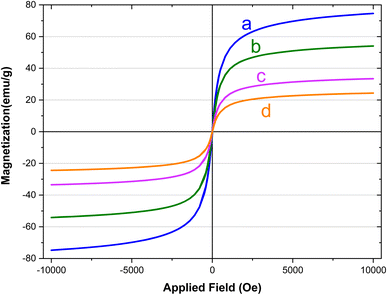 | ||
| Fig. 9 The VSM analyses of (a) Fe3O4, (b) Fe3O4@SiO2, (c) Fe3O4@SiO2-diamine and (d) [Fe3O4@SiO2-Schiff base-Cu(II)] complex. | ||
3.2. Catalytic performance
We examined the performance of the magnetic [Fe3O4@SiO2-Schiff base-Cu(II)] complex as a catalyst in the one-pot reaction involving hydrazine hydrate, ethyl acetoacetate, benzaldehyde, and malononitrile, which serves as a model reaction for producing the desired pyrano[2,3-c]pyrazoles (Table 1). Initially, we investigated the model reaction under reflux conditions, using different green solvents such as water, alcohols, and solvent-free conditions. Fortunately, the results indicated that the highest yield was achieved when water was used as the solvent. Next, we examined the impact of catalyst loading and found that the best yield was obtained using 12 mg of the catalyst that was chosen as the minimum amount of the catalyst required for the transformation. Increasing the catalyst amount and extending the reaction duration did not enhance the reaction efficiency. Additionally, we studied the effect of temperature as another significant factor and observed a decrease in reaction efficiency when the temperature was lowered to room temperature. In the final optimization step, we compared the reaction under catalyst-free conditions and with catalyst intermediates to evaluate the desired Cu complex. The best outcome was obtained when the [Fe3O4@SiO2-Schiff base-Cu(II)] complex was used as the catalyst. Based on these findings, we determined that the optimal reaction conditions involved using 12 mg of [Fe3O4@SiO2-Schiff base-Cu(II)] complex in water under reflux conditions.| Entry | Catalyst | Catalyst amount (mg) | Solvent | Temperature (°C) | Time (min) | Yielda,b (%) |
|---|---|---|---|---|---|---|
| a Conditions: ethyl acetoacetate (1 mmol), hydrazine hydrate (1 mmol), benzaldehyde (1 mmol), malononitrile (1 mmol) and (1 mmol), catalyst (mg) and solvent (3 mL). b Isolated yield. | ||||||
| 1 | [Fe3O4@SiO2-Schiff base-Cu(II)] complex | 5 | EtOH | Reflux | 10 | 68 |
| 2 | [Fe3O4@SiO2-Schiff base-Cu(II)] complex | 5 | MeOH | Reflux | 10 | 65 |
| 3 | [Fe3O4@SiO2-Schiff base-Cu(II)] complex | 5 | Water | Reflux | 10 | 73 |
| 4 | [Fe3O4@SiO2-Schiff base-Cu(II)] complex | 5 | PEG-400 | 100 | 10 | 70 |
| 5 | [Fe3O4@SiO2-Schiff base-Cu(II)] complex | 5 | Solvent-free | 100 | 10 | 58 |
| 6 | [Fe3O4@SiO2-Schiff base-Cu(II)] complex | 7 | EtOH | Reflux | 10 | 81 |
| 7 | [Fe3O4@SiO2-Schiff base-Cu(II)] complex | 9 | EtOH | Reflux | 10 | 93 |
| 8 | [Fe3O4@SiO2-Schiff base-Cu(II)] complex | 12 | EtOH | Reflux | 10 | 98 |
| 9 | [Fe3O4@SiO2-Schiff base-Cu(II)] complex | 14 | EtOH | Reflux | 10 | 98 |
| 10 | [Fe3O4@SiO2-Schiff base-Cu(II)] complex | 12 | EtOH | Reflux | 30 | 98 |
| 11 | [Fe3O4@SiO2-Schiff base-Cu(II)] complex | 12 | EtOH | 45 | 10 | 47 |
| 12 | [Fe3O4@SiO2-Schiff base-Cu(II)] complex | 12 | EtOH | 25 | 10 | Trace |
| 13 | Catalyst free | — | EtOH | Reflux | 12 h | Trace |
| 14 | Fe3O4 | 12 | EtOH | Reflux | 60 | 25 |
| 15 | Fe3O4@SiO2 | 12 | EtOH | Reflux | 60 | 21 |
| 16 | Fe3O4@SiO2-diamine | 12 | EtOH | Reflux | 60 | 33 |
| 17 | Fe3O4@SiO2-Schiff base | 12 | EtOH | Reflux | 60 | 47 |
In next step, we explored the wide applicability and versatility of this method by utilizing the prescribed reaction conditions to examine a diverse range of aromatic and heteroaromatic aldehydes. This comprehensive set of aldehydes included various electron-donating and electron-withdrawing groups, such as halogens, nitro, methoxy, hydroxyl, and amino functionalities. The experimental results showcased exceptional yields of the desired pyrano[2,3-c]pyrazoles products, demonstrating the effectiveness of the approach (Table 2). Interestingly, we observed that the electron-withdrawing groups significantly influenced both the reaction rate and yield, leading to the most favorable outcomes. This finding suggests that electronic and hindrance effects play a decisive role in reaction progress. Additionally, we successfully transformed heterocyclic carbaldehydes into the target products, achieving impressive yields within remarkably short reaction times. This remarkable efficiency highlights the potential of this method for synthesizing pyrano[2,3-c]pyrazoles from a wide range of substrates, including those with complex and heterocyclic structures. Finally, an investigation into scaling up reactions was ultimately conducted using 15 mmol of benzaldehyde as the model substrate. This endeavor yielded the corresponding product in an excellent yield, effectively confirming the method's scalability and applicability.
| Entry | Aldehyde | Product | Time (min) | Yielda,b (%) | Melting point (°C) | |
|---|---|---|---|---|---|---|
| Measured | Literature | |||||
| a Isolated yield. b Conditions: ethyl acetoacetate (1 mmol), hydrazine hydrate (1 mmol) arylaldehyde (1 mmol), malononitrile (1 mmol), [Fe3O4@SiO2-Schiff base-Cu(II)] (12 mg) in water solvent (3 mL) at reflux conditions. | ||||||
| 1 |
|
|
10 | 98 | 243–245 | 243–-245 (ref. 38) |
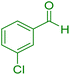
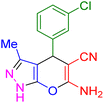
| 2 |
|
|
12 | 98 | 234–236 | 234–236 (ref. 38) |
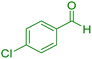
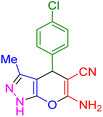
| 3 |
|
|
15 | 96 | 230–231 | 229–230 (ref. 39) |
| 4 |
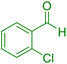
|
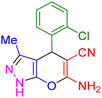
|
20 | 94 | 238–240 | 237–240 (ref. 40) |
| 5 |
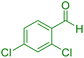
|
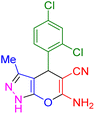
|
30 | 93 | 195–197 | 196–198 (ref. 40) |
| 6 |
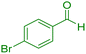
|
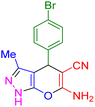
|
15 | 97 | 181–183 | 180–182 (ref. 38) |
| 7 |
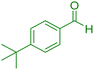
|
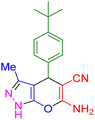
|
25 | 96 | 221–222 | 221–222 (ref. 41) |
| 8 |
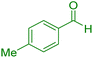
|
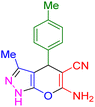
|
20 | 95 | 207–209 | 207–209 (ref. 38) |
| 9 |
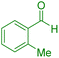
|
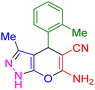
|
35 | 94 | 172–174 | 172–174 (ref. 42) |
| 10 |
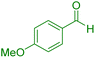
|
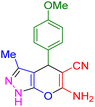
|
30 | 91 | 209–211 | 209–211 (ref. 38) |
| 11 |
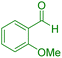
|
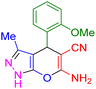
|
45 | 88 | 246–248 | 246–248 (ref. 43) |
| 12 |
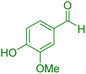
|
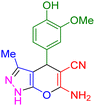
|
55 | 90 | 235–237 | 235–237 (ref. 41) |
| 13 |
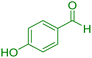
|
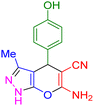
|
35 | 92 | 221–223 | 222–224 (ref. 38) |
| 14 |
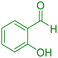
|
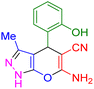
|
70 | 87 | 209–211 | 209–211 (ref. 44) |
| 15 |
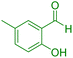
|
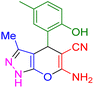
|
70 | 87 | 227–228 | 227–228 (ref. 41) |
| 16 |
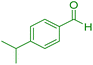
|
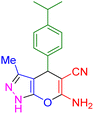
|
20 | 87 | 182–184 | 182–183 (ref. 45) |
| 17 |
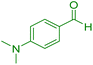
|
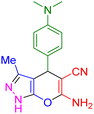
|
75 | 88 | 218–220 | 218–219 (ref. 41) |
| 18 |
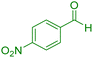
|
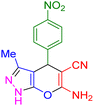
|
10 | 99 | 250–251 | 250–251 (ref. 46) |
| 19 |
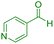
|
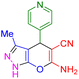
|
45 | 90 | 229–231 | 228–230 (ref. 47) |
| 20 |
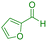
|
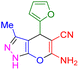
|
75 | 89 | 214–215 | 215–216 (ref. 41) |
| 21 |
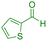
|
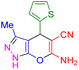
|
65 | 86 | 223–224 | 222–224 (ref. 47) |
Scheme 2 presents a proposed mechanism for [Fe3O4@SiO2-Schiff base-Cu(II)] complex catalyzed the synthesis of pyrano[2,3-c]pyrazoles.48 The initial step involves the Cu-catalyzed cyclocondensation of a beta-keto ester and hydrazine hydrate, resulting in the formation of a five-membered N-heterocycle referred to as a pyrazalone intermediate. This pyrazalone intermediate demonstrates the presence of two resonance structures (II, III). Concurrently, Cu also facilitates the Knoevenagel condensation of aryl malononitrile and aldehydes. Subsequently, the pyrazalone intermediate engages in a Michael reaction with the β-aryl-α-cyanoacrylate compound generated from the Knoevenagel condensation. Subsequent to this, a Thorpe–Ziegler type cyclization takes place, result in the production of the corresponding pyrano[2,3-c]pyrazoles heterocycles.
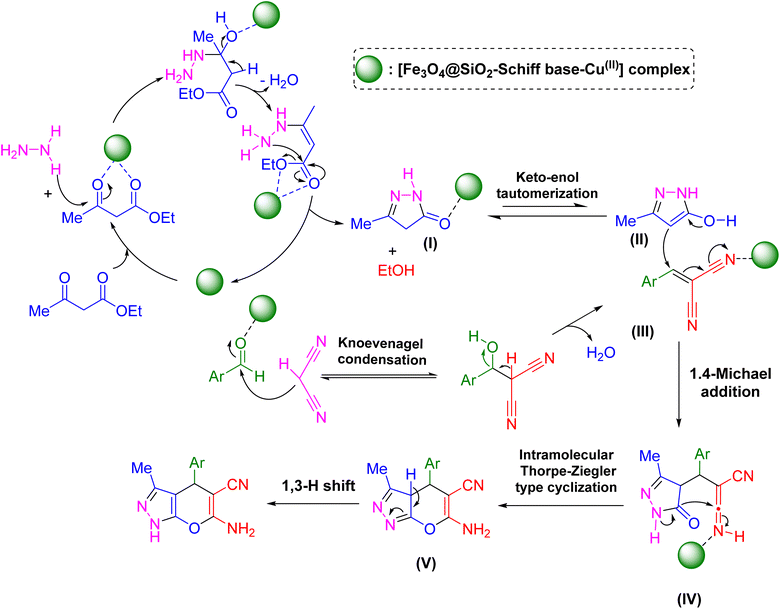 | ||
| Scheme 2 Suggested reaction pathway for the synthesis of pyrano[2,3-c]pyrazoles facilitated by the catalytic properties of the [Fe3O4@SiO2-Schiff base-Cu(II)] complex. | ||
| Entry | Catalyst | Time (min) | Yield (%) | Refa |
|---|---|---|---|---|
| a Isolated yield. | ||||
| 1 | Mg–Al hydrotalcite | 60 | 87 | 49 |
| 2 | ZnO NPs | 60 | 94 | 50 |
| 3 | Thiamine hydrochloride | 15 | 91 | 51 |
| 4 | ZnS NPs | 8 | 92 | 52 |
| 5 | RuIII@CMC/Fe3O4 | 30 | 93 | 39 |
| 6 | Fe3O4@SiO2/Si(OEt)(CH2)3NH/CC/EDA/Cu(OAc)2 | 6 | 86 | 53 |
| 7 | Fe3O4@SiO2/Si(OEt)(CH2)3@melamine@TC@Cu(OAc)2 | 7 | 92 | 54 |
| 8 | [Fe3O4@SiO2-Schiff base-Cu(II)] complex | 10 | 98 | This work |
Conclusion
In conclusion, our study successfully synthesized a heterogeneous Schiff base complex of copper-modified magnetic Fe3O4 catalyst, which was thoroughly characterized. This complex proved to be highly efficient in catalyzing the synthesis of pyrano[2,3-c]pyrazole derivatives. The structural and morphological analysis provided confirmation of the successful synthesis of the magnetic [Fe3O4@SiO2-Schiff base-Cu(II)] complex. Through the direct cyclization of in situ generated pyrazalone and substituted β-aryl-α-cyanoacrylate intermediates, we were able to synthesize a series of pyrazole compounds fused with 4-substituted pyran derivatives. This study offered notable advantages, including the simplicity of the experimental procedures and the ease of recovering the [Fe3O4@SiO2-Schiff base-Cu(II)] complex through magnetic separation, which could be repeated for at least five cycles. These findings contribute to the development of efficient and practical catalytic systems for producing derivatives of pyrano[2,3-c]pyrazole. Further research in this area may explore the potential applications of these compounds in various fields such as pharmaceuticals, agrochemicals, and materials science.Ethical statement
There are no human or animal studies in this work.Data availability
All data generated or analysed during this study are included in this published article and its ESI† files.Author contributions
Rehab Tahseen alhayo and Ghufran Sh. Jassim: writing -original draft, review/editing, synthesis, characterization, and analysis of catalyst. Hasanain Amer Naji: catalysis studies and analysis. A. H. Shather: software and review/editing. Israa Habeeb Naser: analysis, acquiring research funding. Luay Ali Khaleel: analysis, review/editing, acquiring research funding. Haider Abdulkareem Almashhadani: conceptualization, analysis, review draft, acquiring research funding and supervision.Conflicts of interest
The authors declare no competing interests.References
- H. T. Nguyen, M. N. H. Truong, T. Van Le, N. T. Vo, H. D. Nguyen and P. H. Tran, ACS Omega, 2022, 7, 17432–17443 CrossRef CAS PubMed.
- S. Sikandar and A. F. Zahoor, J. Heterocycl. Chem., 2021, 58, 685–705 CrossRef CAS.
- T. E. Ali, D. A. Bakhotmah and M. A. Assiri, Synth. Commun., 2020, 50, 3314–3325 CrossRef CAS.
- F. Ghorbanipour, S. M. Nezhad, S. A. Pourmousavi, E. N. Zare and G. Heidari, Inorg. Chem. Commun., 2023, 147, 110271 CrossRef CAS.
- S. A. El-Assaly, A. E. H. A. Ismail, H. A. Bary and M. G. Abouelenein, Curr. Chem. Lett., 2021, 10, 309–328 CrossRef.
- M. E. A. Zaki, H. A. Soliman, O. A. Hiekal and A. E. Rashad, Z. Naturforsch. Sect. C J. Biosci., 2006, 61, 1–5 CrossRef CAS PubMed.
- M. Mohammadi, A. Ghorbani-Choghamarani and N. Hussain-Khil, J. Phys. Chem. Solids, 2023, 177, 111300 CrossRef CAS.
- R. Eivazzadeh-Keihan, R. Taheri-Ledari, N. Khosropour, S. Dalvand, A. Maleki, S. M. Mousavi-Khoshdel and H. Sohrabi, Colloids Surf., A, 2020, 587, 124335 CrossRef CAS.
- K. Pradhan, S. Paul and A. R. Das, Catal. Sci. Technol., 2014, 4, 822 RSC.
- M. A. Ghasemzadeh, B. Mirhosseini-Eshkevari and J. Dadashi, J. Mol. Struct., 2022, 1261, 132843 CrossRef CAS.
- M. A. Ghasemzadeh, B. Mirhosseini-Eshkevari and J. Dadashi, Sci. Rep., 2023, 13, 9089 CrossRef CAS PubMed.
- Y. Guo, S. A. Delbari, A. Sabahi Namini, Q. Van Le, J. Y. Park, D. Kim, R. S. Varma, H. W. Jang, A. T. Raissi, M. Shokouhimehr and C. Li, Mol. Catal., 2023, 547, 113362 CrossRef CAS.
- L. Zare Fekri, M. Nateghi-Sabet and M. Nikpassand, Org. Prep. Proced. Int., 2022, 54, 449–456 CrossRef CAS.
- F. Casti, F. Basoccu, R. Mocci, L. De Luca, A. Porcheddu and F. Cuccu, Molecules, 2022, 27, 1988 CrossRef CAS PubMed.
- M. Nikpassand, L. Z. Fekri and A. Pourahmad, Lett. Org. Chem., 2020, 17, 360–365 CrossRef CAS.
- P. Shakib, M. G. Dekamin, E. Valiey, S. Karami and M. Dohendou, Sci. Rep., 2023, 13, 8016 CrossRef CAS PubMed.
- A. Simon and S. Mathai, J. Organomet. Chem., 2023, 996, 122768 CrossRef CAS.
- M. Nikpassand, A. Keyhani, L. Z. Fekri and R. S. Varma, J. Mol. Struct., 2022, 1251, 132065 CrossRef CAS.
- M. Liu, Y. Ye, J. Ye, T. Gao, D. Wang, G. Chen and Z. Song, Magnetochemistry, 2023, 9, 110 CrossRef CAS.
- M. Torabi, L. Z. Fekri and M. Nikpassand, J. Mol. Struct., 2022, 1250, 131761 CrossRef CAS.
- W. Xie and J. Li, Renewable Sustainable Energy Rev., 2023, 171, 113017 CrossRef CAS.
- M. Sajjadi, M. Nasrollahzadeh, H. Ghafuri, T. Baran, Y. Orooji, N. Y. Baran and M. Shokouhimehr, Int. J. Biol. Macromol., 2022, 209, 1573–1585 CrossRef CAS PubMed.
- R. Malav and S. Ray, Inorg. Chim. Acta, 2023, 551, 121478 CrossRef CAS.
- D. I. Ugwu and J. Conradie, Inorg. Chim. Acta, 2023, 553, 121518 CrossRef CAS.
- H. Keypour, J. Kouhdareh, S. Alavinia, K. Rabiei, M. Mohammadi, A. Maryamabadi and S. Babaei, J. Organomet. Chem., 2023, 989, 122646 CrossRef CAS.
- K. Rabiei, Z. Mohammadkhani, H. Keypour and J. Kouhdareh, RSC Adv., 2023, 13, 8114–8129 RSC.
- V. D. Manvatkar, R. Y. Patle, P. H. Meshram and R. S. Dongre, Chem. Pap., 2023, 77(10), 5641–5662 CrossRef CAS.
- A. Tombesi and C. Pettinari, Inorganics, 2021, 9, 81 CrossRef CAS.
- M. K. Patil, V. H. Masand and A. K. Maldhure, Curr. Nanosci., 2020, 17, 634–645 CrossRef.
- H. Ahmad and M. K. Hossain, Mater. Adv., 2022, 3, 859–887 RSC.
- C. Liu, Synth. Commun., 2021, 51, 2237–2264 CrossRef CAS.
- M. Kazemnejadi, S. A. Alavi G., Z. Rezazadeh, M. A. Nasseri, A. Allahresani and M. Esmaeilpour, Appl. Organomet. Chem., 2020, 34, e5388 CrossRef CAS.
- X. Tan, P. Sudarsanam, J. Tan, A. Wang, H. Zhang, H. Li and S. Yang, J. Environ. Chem. Eng., 2021, 9, 104719 CrossRef CAS.
- F. Ghobakhloo, D. Azarifar, M. Mohammadi and M. Ghaemi, Appl. Organomet. Chem., 2022, 36, e6823 CrossRef CAS.
- S. Ghorbani, D. Habibi, S. Heydari, M. Mohammadi and M. Ariannezhad, Environ. Sci. Pollut. Res., 2022, 30, 32762–32775 CrossRef PubMed.
- R. H. Althomali, M. K. Abbood, F. M. A. Altalbawy, E. A. M. Saleh, S. S. Abdullaev, A. jaber Ibrahim, S. A. Ansari and R. M. Romero-Parra, J. Mol. Struct., 2023, 1290, 135911 CrossRef CAS.
- F. Ghobakhloo, D. Azarifar and M. Mohammadi, J. Phys. Chem. Solids, 2023, 175, 111222 CrossRef CAS.
- F. Mohamadpour, J. Chem. Sci., 2020, 132, 72 CrossRef CAS.
- Y. Chen, Z. Zhang, W. Jiang, M. Zhang and Y. Li, Mol. Diversity, 2019, 23, 421–442 CrossRef CAS.
- F. Hassanzadeh-Afruzi, H. Dogari, F. Esmailzadeh and A. Maleki, Appl. Organomet. Chem., 2021, 35, e6363 CrossRef CAS.
- H. T. Nguyen, T. Van Le and P. H. Tran, J. Environ. Chem. Eng., 2021, 9, 105228 CrossRef CAS.
- N. Hosseini Mohtasham and M. Gholizadeh, Res. Chem. Intermed., 2020, 46, 3037–3066 CrossRef CAS.
- M. M. Heravi, R. Malakooti, K. Kafshdarzadeh, Z. Amiri, V. Zadsirjan and H. Atashin, Res. Chem. Intermed., 2022, 48, 203–234 CrossRef CAS.
- K. G. Patel, N. M. Misra, R. H. Vekariya and R. R. Shettigar, Res. Chem. Intermed., 2018, 44, 289–304 CrossRef CAS.
- A. B. Atar, J. T. Kim, K. T. Lim and Y. T. Jeong, Synth. Commun., 2014, 44, 2679–2691 CrossRef CAS.
- R. Kumari, A. Varghese, L. George and K. B. Akshaya, J. Mol. Liq., 2016, 222, 828–835 CrossRef CAS.
- M. Bakherad, A. Keivanloo, M. Gholizadeh, R. Doosti and M. Javanmardi, Res. Chem. Intermed., 2017, 43, 1013–1029 CrossRef CAS.
- B. Halder, H. S. Maity, F. Banerjee, A. B. Kachave and A. Nag, Polycyclic Aromat. Compd., 2022, 42, 3302–3317 CrossRef CAS.
- S. W. Kshirsagar, N. R. Patil and S. D. Samant, Synth. Commun., 2011, 41, 1320–1325 CrossRef CAS.
- S. U. Tekale, S. S. Kauthale, K. M. Jadhav and R. P. Pawar, J. Chem., 2013, 2013, 1–8 CrossRef.
- M. D. Nikam, P. S. Mahajan, A. V. Chate, S. K. Dabhade and C. H. Gill, J. Chil. Chem. Soc., 2015, 60, 2847–2850 CrossRef CAS.
- A. V. Borhade and B. K. Uphade, J. Iran. Chem. Soc., 2015, 12, 1107–1113 CrossRef CAS.
- A. Ghanbarpour, A. Khazaei, A. R. Moosavi-Zare, T. Akbarpour, M. Mohammadi and N. Sarmasti, Polycycl. Aromat. Compd., 2023, 43, 3192–3215 CrossRef CAS.
- M. Soleimani, T. Akbarpour and A. Khazaei, Polycycl. Aromat. Compd., 2023, 1–27 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3na00906h |
| This journal is © The Royal Society of Chemistry 2023 |