Effect of introducing a cyclobutylmethyl group into an onium cation on the thermodynamic properties of ionic clathrate hydrates†
Sakura
Azuma
a,
Jin
Shimada
bcd,
Katsuhiko
Tsunashima
*e,
Takeshi
Sugahara
 *bc and
Takayuki
Hirai
bc
*bc and
Takayuki
Hirai
bc
aAdvanced Engineering Faculty, National Institute of Technology, Wakayama Collage, 77 Noshima, Nada, Gobo, Wakayama 644-0023, Japan
bDepartment of Materials Engineering Science, Division of Chemical Engineering, Graduate School of Engineering Science, Osaka University, 1-3 Machikaneyama, Toyonaka, Osaka 560-8531, Japan. E-mail: sugahara.takeshi.es@osaka-u.ac.jp
cDivision of Energy and Photochemical Engineering, Research Center for Solar Energy Chemistry, Graduate School of Engineering Science, Osaka University, 1-3 Machikaneyama, Toyonaka, Osaka 560-8531, Japan
dResearch Fellow of Japan Society for the Promotion of Science, 5-3-1 Kojimachi, Chiyoda-ku, Tokyo 102-0083, Japan
eDepartment of Material Science, National Institute of Technology, Wakayama Collage, 77 Noshima, Nada, Gobo, Wakayama 644-0023, Japan. E-mail: tsunashima@wakayama-nct.ac.jp
First published on 11th November 2022
Abstract
Ionic clathrate hydrates (ICHs) have been applied to thermal storage materials, which can be utilized as unusual thermal energy sources. In the present study, we developed a novel ICH including tri-n-butyl(cyclobutylmethyl)phosphonium bromide (P444(1c4)-Br) as an unprecedented guest species. The highest equilibrium temperature and the dissociation enthalpy of P444(1c4)-Br ICH were 279.5 ± 0.1 K and 202 ± 2 J g−1, respectively, at the mole fraction of 0.0255 ± 0.0008. The highest equilibrium temperature of P444(1c4)-Br ICH was slightly lower than that of the tetra-n-butylphosphonium bromide (P4444-Br) ICH. The dissociation enthalpy of P444(1c4)-Br ICH was larger than that of other ICHs with similar highest equilibrium temperatures. Cyclic hydrocarbon groups, together with normal, branched, saturated, and unsaturated hydrocarbon chains, can be one of the options for tuning the equilibrium temperature of ICHs.
Introduction
In order to achieve the efficient utilization of thermal energy, the development of a latent heat storage technology is absolutely essential. The latent heat storage technology is a method of thermal storage using the enthalpy of phase transition of the latent heat storage materials.1–3 For the latent heat storage materials, water,1–3 paraffin,3,4 and molten salt4,5 have been well known. Ionic clathrate hydrate (ICH) is also one of the promising thermal storage materials, especially, suitable for being utilized as a source of thermal energy in a temperature range of 273–300 K.6–9ICHs are crystalline inclusion compounds composed of hydrogen-bonded water molecules and quaternary alkylammonium or alkylphosphonium salts.8–12 ICHs have equilibrium temperatures around 270–300 K, with the dissociation enthalpies around 200 J g−1.6–10,13–22 Their thermodynamic properties, including the equilibrium temperature and dissociation enthalpy, can be adjusted by choosing an appropriate guest species. For example, the tetra-n-butylammonium bromide (TBAB, N4444-Br) ICH has an equilibrium temperature located around 284.8–286.1 K, and a dissociation enthalpy of about 188.8–197.3 J g−1.10,12,23–25 On the other hand, the tetra-n-butylphosphonium bromide (TBPB, P4444-Br) ICH has an equilibrium temperature located around 281.2–282.6 K,17,22,26–29 with a dissociation enthalpy of 214 J g−1.17,22 The development of ICHs with desirable thermodynamic properties should realize the efficient utilization of thermal energy. In particular, ICHs are applicable to cooling applications, such as in shipping boxes for medicine or fresh foods; they can also be used in air-conditioning systems, instead of ice or chilled water.8,30
To develop ICHs suitable for each application, it is essential to understand the relationships between the chemical structure of the guest ions and the thermodynamic properties of the ICHs. The effects of anion replacement, such as using halide anions,31–35 carboxylate anions,6,7,10,14–16,18,36,37 and inorganic anions,38–40 on the thermodynamic properties have often been investigated. In addition, several researchers have focused on the differences between the center atoms of the cations, that is, the differences between tetra-n-butylammonium and tetra-n-butylphosphonium cations.15,16,22,39–41 Recently, we reported the effects of alkyl and alkenyl groups in tri-n-butylalkylphosphonium bromide (P444R-Br)17 and tri-n-butylalkenylphosphonium bromide (P444(R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) R2)-Br)19 on the thermodynamic stabilities of ICHs. The size and shape of the alkyl and alkenyl groups significantly affect the thermodynamic properties of ICHs.
R2)-Br)19 on the thermodynamic stabilities of ICHs. The size and shape of the alkyl and alkenyl groups significantly affect the thermodynamic properties of ICHs.
In the case of P444R-Br ICHs (where R is a normal alkyl group),17 the equilibrium temperature of P4444-Br (R = 4) is the highest among them. The equilibrium temperatures of P4443-Br (R = 3) and P4445-Br (R = 5) are 8.2 K and 5.5 K, respectively, lower than that of P4444-Br (R = 4). With branched alkyl groups, such as isobutyl and isopentyl groups, the equilibrium temperature of the ICH is increased compared with the normal alkyl group counterparts, because the branched alkyl groups filled the inner spaces of the hydrate cages more fully than the corresponding normal alkyl groups. These results revealed how important the moderate space-filling of the hydrocarbon group in the hydrate cage is. In the present study, we investigated the thermodynamic properties of a novel ICH composed of a hydrocarbon group that is bulkier than a normal alkyl group, that is, a cycloalkylmethyl group. Here, we synthesized the tri-n-butyl(cyclobutylmethyl)phosphonium bromide (P444(1c4)-Br, as shown in Fig. 1). In the formation of ICHs, there have been no reports on onium salts with a cyclic hydrocarbon group in their onium cation. How such a bulky cyclic hydrocarbon group in the cation affects the properties of the ICH is scientifically interesting. The equilibrium phase relationship, dissociation enthalpy, and crystal structure of the P444(1c4)-Br ICH were investigated to reveal the effects of the cyclobutylmethyl group on the thermodynamic properties of the ICH.
Experimental
The chemicals used in the present study are listed in Table 1. P444(1c4)-Br was synthesized via a nucleophilic substitution reaction in toluene solvent under a nitrogen atmosphere according to the procedures described in the literature.42 The crude product was rinsed with n-hexane to remove unreacted tri-n-butylphosphine and (bromomethyl)cyclobutane. The crystalline product thus obtained was dried in vacuo at 393 K for 24 h and was stored in a glovebox filled with argon gas. P444(1c4)-Br was confirmed using 1H, 13C, and 31P NMR (Bruker, AVANCE400). The NMR results are summarized in the ESI.† From the NMR results, there were no impurity-derived signals or aberrant integration ratios. The majority of the impurities, considering the synthetic pathway, would be tri-n-butylphosphine oxide derivatives. The mass fraction purity of the P444(1c4)-Br measured via silver nitrate titration (Kyoto Electronics Manufacturing, AT-710, uncertainty: 0.004 mass fraction) was higher than 0.98.| Chemical name | Chemical formula | Molecular weight | CAS reg. no | Source | Mass fraction purity |
|---|---|---|---|---|---|
| Tri-n-butylphosphine | P(C4H9)3 | 202.32 | 998-40-3 | Nippon Chemical Industrial Co., Ltd. | >0.995 |
| (Bromomethyl) cyclobutane | C4H7CH2Br | 149.03 | 17247-58-4 | Tokyo Chemical Industry Co., Ltd. | >0.98 |
| Water | H2O | 18.02 | 7732-18-5 | Distilled | Resistivity is 0.60 MΩ cm |
Aqueous solutions were prepared at different compositions, from x1 = 0.0029 to 0.0487 (w1 = 0.053 to 0.500, respectively) using an electric balance (A&D, GF-200, uncertainty: 2 mg; and A&D, GR-300, uncertainty: 0.3 mg). The symbols x and w represent the mole and mass fractions, respectively. Approximately 1 cm3 of the aqueous solution was introduced into respective glass vials. The vials were set into a refrigerator kept at 258 K to form the ICH. After that, the vials containing the ICH were immersed into a temperature-controlled ethylene glycol bath at 270 K with a cooling antifreeze solution circulator (Taitec, CL-80R). The system temperature was elevated from 270 K with a step of 0.1 K. While the temperature was kept for at least 6 h at each temperature, the glass vials were often shaken by hand. The equilibrium temperature was determined when the hydrate crystal was completely dissolved. The temperature in the thermostatic bath was measured using a platinum resistance thermometer (an uncertainty of 0.1 K) calibrated with a thermistor thermometer (Takara, D632, reproducibility: 0.02 K, where the probe was calibrated using a Pt resistance thermometer defined by ITS90). The maximum uncertainty in the equilibrium temperature was 0.1 K.
The dissociation enthalpy of the P444(1c4)-Br ICH was investigated using a micro differential scanning calorimeter (Setaram, μDSC VII evo) at atmospheric pressure. Approximately 20 mg of the prepared P444(1c4)-Br aqueous solution was loaded into a DSC cell. The precise sample mass of the loaded aqueous solution was measured using an electric balance (A&D, BM-22) with an uncertainty of 0.02 mg. The furnace temperature was decreased to 248 K at a cooling rate of 0.5 K min−1 and then increased to the desired temperature at a heating rate of 0.1 K min−1. We calibrated the μDSC instrument using a dedicated Joule heat calibrator (Setaram, EJ3). In addition, water and naphthalene were adopted as reference compounds. The uncertainty in the dissociation enthalpy was less than 2 J g−1.
The stoichiometric composition and the hydration number were estimated from the composition at the highest equilibrium temperature value. The equilibrium temperatures around the stoichiometric composition were independent of the composition within the uncertainty (±0.1 K) of temperature. To precisely determine the stoichiometric composition and the hydration number, the apparent dissociation enthalpy (ΔdHapp) values per unit mass of aqueous solution were measured using the μDSC. The ΔdHapp value has a maximum at the stoichiometric composition because the excess amount of water or phosphonium salt remains at a composition except for the stoichiometric composition.6,19,43
The crystal structure of the P444(1c4)-Br ICH was analysed using powder X-ray diffraction (PXRD). The PXRD patterns were measured at 150 K and atmospheric pressure using a diffractometer (PANalytical, X’Pert-MPD) with a cold stage (Anton Paar, TTK450) and a Cu Kα X-ray (45 kV, 40 mA) source. The PXRD measurements were performed in step-scan mode with a scan rate of 2.7° min−1 and a step size of approximately 0.02°. PXRD pattern indexing and cell refinement were carried out using the Chekcell44 and PowderX45 programs and the initial lattice parameters41 for the refinement.
A laser Raman microprobe spectrometer with a multichannel CCD detector (Jasco, NRS-1000) was used. A diode pumped solid state (DPSS) laser (Cobolt, Fandango) was used to irradiate the samples at 77 K. The wavelength of the DPSS laser was 514.5 nm, and the output power was adjusted to 100 mW. The spectral resolution of the obtained Raman spectra was approximately 1 cm−1.
Results and discussion
Equilibrium phase relationship (temperature–composition (T–x)) data obtained via direct observation are summarized in Table 2 and shown in Fig. 2. The equilibrium temperature values in the composition range from x1 = 0.0219 to 0.0286 were located at 279.5 K and were independent of the composition within the uncertainty of 0.1 K. To precisely determine the stoichiometric composition of the P444(1c4)-Br ICH, the apparent dissociation enthalpy values per unit mass of aqueous solution, ΔdHapp, were measured via μDSC. The ΔdHapp value at each composition is shown in Fig. 3, where the quadratic curve in the lower figure is for visualization of the trend. From the vertex of the curve, we determined that the stoichiometric composition of the P444(1c4)-Br ICH was x1 = 0.0255 ± 0.0008 (w1 = 0.338 ± 0.005, n = 38 ± 1). The eutectic temperature of the P444(1c4)-Br ICH was also determined from the onset of the eutectic peak of the DSC thermograms at low compositions. The eutectic temperature of the P444(1c4)-Br ICH was 272.1 ± 0.1 K.| x 1 | w 1 | T/K |
|---|---|---|
| a Standard uncertainties u are u(x) = 0.0008, u(w) = 0.005, and u(T) = 0.1 K. b Stoichiometric composition, which was estimated from the apparent dissociation enthalpy values via DSC measurements. The highest equilibrium temperature was measured from the visual observation. | ||
| 0.0029 | 0.053 | 273.1 |
| 0.0056 | 0.099 | 275.6 |
| 0.0087 | 0.146 | 277.6 |
| 0.0126 | 0.199 | 278.9 |
| 0.0142 | 0.219 | 279.0 |
| 0.0158 | 0.239 | 279.1 |
| 0.0179 | 0.262 | 279.3 |
| 0.0198 | 0.283 | 279.4 |
| 0.0219 | 0.304 | 279.5 |
| 0.0238 | 0.322 | 279.5 |
| 0.0255b | 0.338b | 279.5b |
| 0.0256 | 0.339 | 279.5 |
| 0.0286 | 0.365 | 279.5 |
| 0.0300 | 0.377 | 279.4 |
| 0.0329 | 0.399 | 279.4 |
| 0.0358 | 0.420 | 279.4 |
| 0.0388 | 0.441 | 279.3 |
| 0.0436 | 0.471 | 279.0 |
| 0.0487 | 0.500 | 279.0 |
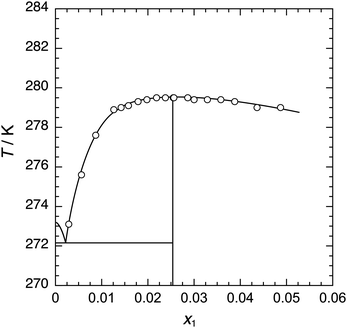 | ||
| Fig. 2 Equilibrium temperature (T)–composition (x) diagram for the P444(1c4)-Br + water system, where x1 denotes the proportion of P444(1c4)-Br in the aqueous solution. | ||
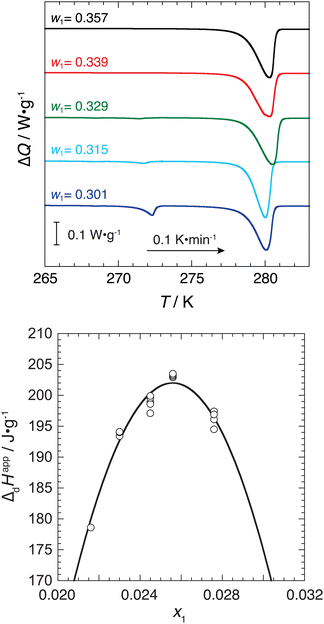 | ||
| Fig. 3 DSC thermograms (upper) and apparent dissociation enthalpy ΔdHapp (lower) for the aqueous P444(1c4)-Br system. The curve in the lower figure is for visualization of the trend. | ||
The highest equilibrium temperature of the P444(1c4)-Br ICH was 2.8 K lower than that of the P4444-Br ICH17 (the highest equilibrium temperature was 282.32 K at x1 = 0.026). On the other hand, the highest equilibrium temperature for the tri-n-butylpropylphosphonium bromide (P4443-Br) ICH was reported to be 274.1 K at x1 = 0.026,17 which is 5.4 K lower than that of the P444(1c4)-Br ICH. The largest diameter of the cyclobutylmethyl group is lower than that of the n-butyl group because the C–C–C angle in the cyclobutyl group is 90°, whereas the C–C–C angle in the n-butyl group is almost 113°. Generally, it is well known that the thermodynamic stability of ICHs is mainly determined by the interactions between guest ions and water molecules.10,15,41 The guest ions, which fill the inner-space of the hydrate cages efficiently (whose size is similar to that of the inner-space of the hydrate cage), increase the thermodynamic stability of ICHs. In comparison with the cyclobutylmethyl group, the butyl group can stabilize the hydrate cages more efficiently. The largest diameter of the cyclobutylmethyl group is closer to that of the n-butyl group than that of the n-propyl group. Therefore, the equilibrium temperature of the P444(1c4)-Br ICH was close to that of the P4444-Br ICH. In comparison with the P444(i-5)-Br ICH,17 which has a branched alkyl group, the equilibrium temperature of the P444(1c4)-Br ICH was 9.3 K lower than that of the P444(i-5)-Br ICH. The space-filling of the cyclobutylmethyl group may not be as efficient as that of the isopentyl group, although both alkyl groups have a similar largest-diameter. This is due to the planar configuration of the cyclobutylmethyl group.
The dissociation enthalpy of the P444(1c4)-Br ICH, as shown in Fig. 3, was determined to be 202 ± 2 J g−1, which is larger than other ICHs, such as the P4444-Br, tetra-n-butylphosphonium formate, and tetra-n-butylphosphonium succinate ICHs, where the dissociation enthalpies for these ICHs were reported to be 214 ± 3,17,22 187 ± 3,15 and 192 ± 3 J g−1,18 respectively. The large dissociation enthalpy of the P444(1c4)-Br ICH is attributed to the large hydration number of 38, since ICHs containing a large number of water molecules (hydrogen bonds) have a larger enthalpy.
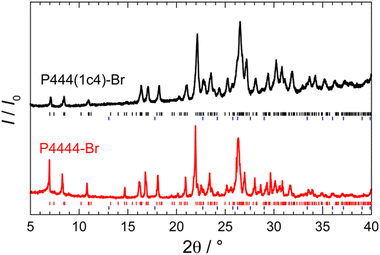
We conducted the PXRD measurements at 150 K. The obtained PXRD pattern of the P444(1c4)-Br ICH is shown in Fig. 4, alongside the PXRD pattern of the P4444-Br ICH. The crystal structure of the P4444-Br ICH has been reported as orthorhombic with the Pmma space group.41 The PXRD pattern of the P4444-Br ICH obtained in the present study agrees well (91% matched) with previously reported patterns.41 The PXRD pattern of the P444(1c4)-Br ICH, which is very similar to that of the P4444-Br ICH, can be mostly described (94% matched) as reflections (as shown by the tickmarks) from the orthorhombic structure with the space group of Pmma. Assuming that the crystal structure of the P444(1c4)-Br ICH is the same as that of the P4444-Br ICH, the lattice parameters of the P444(1c4)-Br and P4444-Br ICHs are summarized in Table 3. The lattice parameters resemble each other closely.
The hydration number, which is the number of water molecules coordinated to a guest ion pair, was calculated from the stoichiometric composition. The hydration number n of the P444(1c4)-Br ICH was 38, which also supports the similarity to the P4444-Br ICH, because the hydration number of this ICH is around 38 from the results of single crystal structural analysis and other data.17,41
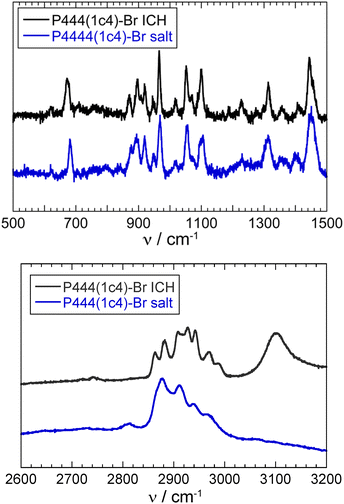
The Raman spectra of the P444(1c4)-Br and P4444-Br ICHs measured at 77 K are shown in Fig. 5. Significant differences between them exist at 944, 966, 1016, and 2917 cm−1, which correspond to the ring-breathing, ring-deformation, and C–H stretching vibration modes of the cyclobutyl group.46 Almost all of the other peaks were similar to those of the P4444-Br ICH. Fig. 6 shows the Raman spectra of the P444(1c4)-Br ICH and the P444(1c4)-Br salt. The peak at 673 cm−1 in the P444(1c4)-Br ICH, which is derived from the symmetric C–P stretching mode,47 was shifted to a wavenumber lower than that (685 cm−1) in the P444(1c4)-Br salt. In the high-wavenumber region, the peaks corresponding to the C–H stretching vibration modes became sharp. Such behavior has been seen in typical ICHs.48,49
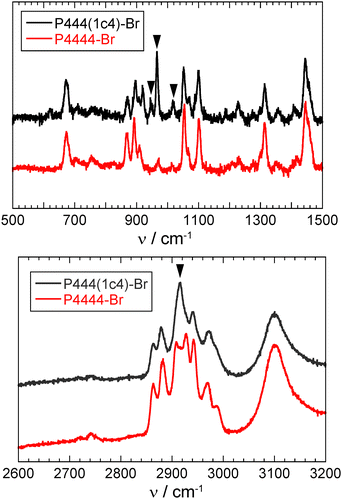 | ||
| Fig. 5 Raman spectra of the P444(1c4)-Br and P4444-Br ICHs recorded at 77 K. The marked peaks correspond to the vibration modes of the cyclobutyl group. | ||
Conclusions
We established a novel guest species with a cyclobutylmethyl group for ICH formation. The equilibrium phase relationship and dissociation enthalpy of the P444(1c4)-Br ICH were investigated. The highest equilibrium temperature and dissociation enthalpy of the P444(1c4)-Br ICH were 279.5 ± 0.1 K and 202 ± 2 J g−1, respectively. The equilibrium temperature of the P444(1c4)-Br ICH was lower than that of the P4444-Br ICH, because the largest diameter of the cyclobutylmethyl group was shorter than the diameter that is best fitted for the cage. In comparison with branched alkyl groups, the equilibrium temperature of the P444(1c4)-Br ICH was also lower than that of the P444(i-5)-Br ICH. The space-filling of the cyclobutylmethyl group may not be as efficient as that of the isopentyl group. The hydration number n of the P444(1c4)-Br ICH was determined as 38 ± 1. This is the same as that of the P4444-Br ICH. Introduction of the cyclobutylmethyl group instead of a normal butyl group decreases the equilibrium temperature of the ICH slightly, while the crystal structure is not changed. Therefore, cyclic hydrocarbon groups are also options for tuning the equilibrium temperature of ICHs. Our findings for the P444(1c4)-Br ICH should help us to understand the science of ICHs, and how such a bulky group in the onium cation affects the properties of ICHs.Author contributions
Sakura Azuma: conceptualization, methodology, validation, formal analysis, investigation, data curation, writing – original draft, writing – review and editing, visualization. Jin Shimada: conceptualization, methodology, validation, formal analysis, investigation, data curation, writing – original draft, writing – review and editing, visualization, funding acquisition. Katsuhiko Tsunashima: conceptualization, methodology, validation, resources, writing – review and editing, supervision, project administration, funding acquisition. Takeshi Sugahara: conceptualization, methodology, validation, resources, writing – review and editing, supervision, project administration, funding acquisition. Takayuki Hirai: writing – review and editing, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI Grant-in-Aid for JSPS Fellows (JP21J20788 for J.S.) and Grant-in-Aid for Scientific Research (JP19K05412 for K.T. and JP22K05050 for T.S.). The authors acknowledge scientific support from the Gas-Hydrate Analysing System (GHAS) of the Division of Chemical Engineering, Graduate School of Engineering Science, Osaka University. The Nippon Chemical Industrial Co. Ltd. is also acknowledged for the supply of the tri-n-butylphosphine.Notes and references
- A. de Gracia and L. F. Cabeza, Energy Build., 2015, 103, 414 CrossRef.
- J. Heier, C. Bales and V. Martin, Renew. Sustain. Energy Rev., 2015, 42, 1305 CrossRef.
- S. M. Hasnain, Energy Convers. Manag., 1998, 39, 1127 CrossRef CAS.
- B. He and F. Setterwall, Energy Convers. Manag., 2002, 43, 1709 CrossRef CAS.
- P. Zhang, F. Ma and X. Xiao, Appl. Energy, 2016, 173, 255 CrossRef CAS.
- T. Sugahara, H. Machida, S. Muromachi and N. Tenma, Int. J. Refrig., 2019, 106, 113 CrossRef CAS.
- H. Machida, T. Sugahara and I. Hirasawa, Commun. Mater., 2021, 2, 66 CrossRef CAS.
- Z. Yin, J. Zheng, H. Kim, Y. Seo and P. Linga, Adv. Appl. Energy, 2021, 26, 100022 CrossRef.
- T. V. Rodionova, I. S. Terekhova and A. Y. Manakov, Energy Fuels, 2022, 36, 10458 CrossRef CAS.
- Y. A. Dyadin and K. A. Udachin, J. Struct. Chem., 1987, 28, 394 CrossRef.
- W. Shimada, M. Shiro, H. Kondo, S. Takeya, H. Oyama, T. Ebinuma and H. Narita, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2005, 61, o65 CrossRef PubMed.
- T. V. Rodionova, V. Yu. Komarov, G. V. Villevald, T. D. Karpova, N. V. Kuratieva and A. Yu. Manakov, J. Phys. Chem. B, 2013, 117, 10677 CrossRef CAS PubMed.
- W. Lin, D. Dalmazzone, W. Fürst, A. Delahaye, L. Fornaison and P. Clain, J. Chem. Thermodyn., 2013, 61, 132 CrossRef CAS.
- T. Miyamoto, R. Koyama, N. Kurokawa, A. Hotta, S. Alavi and R. Ohmura, Front. Chem., 2020, 8, 547 CrossRef CAS.
- J. Shimada, M. Shimada, T. Sugahara, K. Tsunashima, A. Tani, Y. Tsuchida and M. Matsumiya, J. Chem. Eng. Data, 2018, 63, 3615 CrossRef CAS.
- J. Shimada, M. Shimada, T. Sugahara and K. Tsunashima, Fluid Phase Equilib., 2019, 485, 61 CrossRef CAS.
- J. Shimada, M. Shimada, T. Sugahara, K. Tsunashima, Y. Takaoka and A. Tani, Chem. Eng. Sci., 2021, 236, 116514 CrossRef CAS.
- J. Shimada, M. Yamada, A. Tani, T. Sugahara, K. Tsunashima, Y. Tsuchida and T. Hirai, J. Chem. Eng. Data, 2022, 67, 67 CrossRef CAS.
- S. Azuma, J. Shimada, K. Tsunashima, T. Sugahara, A. Tani and T. Hirai, J. Chem. Eng. Data, 2022, 67, 1415 CrossRef CAS.
- H. Machida and T. Sugahara, CrystEngComm., 2018, 20, 3328 RSC.
- T. Sugahara and H. Machida, J. Chem. Eng. Data, 2017, 62, 2721 CrossRef CAS.
- T. Suginaka, H. Sakamoto, K. Iino, S. Takeya, M. Nakajima and R. Ohmura, Fluid Phase Equilib., 2012, 317, 25 CrossRef CAS.
- X. Zhou and D. Liang, Chem. Eng. J., 2019, 378, 122128 CrossRef CAS.
- H. Oyama, W. Shimada, T. Ebinuma, Y. Kamata, S. Takeya, T. Uchida, J. Nagao and H. Narita, Fluid Phase Equilib., 2005, 234, 131 CrossRef CAS.
- J. Deschamps and D. D. Dalmazzone, J. Therm. Anal. Calorim., 2009, 98, 113 CrossRef CAS.
- N. Mayoufi, D. Dalmazzone, W. Fürst, A. Delahaye and L. Fournaison, J. Chem. Eng. Data, 2010, 55, 1271 CrossRef CAS.
- N. Mayoufi, D. Dalmazzone, A. Delahaye, P. Clain, L. Fournaison and W. Fürst, J. Chem. Eng. Data, 2011, 56, 2987 CrossRef CAS.
- W. Lin, D. Dalmazzone, W. Fürst, A. Delahaye, L. Fournaison and P. Clain, J. Chem. Thermodyn., 2013, 61, 132 CrossRef CAS.
- P. Zhang, N. Ye, H. Zhu and X. Xiao, J. Chem. Eng. Data, 2013, 58, 1781 CrossRef CAS.
- X. Zhang, Q. Shi, L. Luo, Y. Fan, Q. Wang and G. Jia, Energies, 2021, 14, 8233 CrossRef CAS.
- M. Oshima, M. Kida and J. Nagao, J. Chem. Eng. Data, 2016, 61, 3334 CrossRef CAS.
- H. Sakamoto, K. Sato, K. Shiraiwa, S. Takeya, M. Nakajima and R. Ohmura, RSC Adv., 2011, 1, 315 RSC.
- K. Sato, H. Tokutomi and R. Ohmura, Fluid Phase Equilib., 2013, 337, 115 CrossRef CAS.
- M. Siddiq and R. Wigent, J. Chem. Eng. Data, 2019, 64, 303 CrossRef CAS.
- C. Henriques and R. Wigent, J. Chem. Eng. Data, 2020, 65, 3079 CrossRef.
- H. Nakayama and S. Torigata, Bull. Chem. Soc. Jpn, 1984, 57, 171 CrossRef CAS.
- H. Nakayama and K. Watanabe, Bull. Chem. Soc. Jpn, 1978, 51, 2518 CrossRef CAS.
- H. Nakayama and Bull Chem, Soc. Jpn, 1983, 56, 877 CrossRef CAS.
- Y. Arai, R. Koyama, F. Endo, A. Hotta and R. Ohmura, J. Chem. Thermodyn, 2019, 131, 330 CrossRef CAS.
- T. Kobori, S. Muromachi, T. Yamasaki, S. Takeya, Y. Yamamoto and R. Ohmura, Cryst. Growth Des., 2015, 15, 3862 CrossRef CAS.
- S. Muromachi, S. Takeya, Y. Yamamoto and R. Ohmura, CrystEngComm, 2014, 16, 2056 RSC.
- K. Yoshii, K. Yamaji, T. Tsuda, K. Tsunashima, H. Yoshida, M. Ozaki and S. Kuwabata, J. Phys. Chem. B, 2013, 117, 15051 CrossRef CAS PubMed.
- R. Koyama, A. Hotta and R. Ohmura, J. Chem. Thermodyn., 2020, 144, 106088 CrossRef CAS.
- Chekcell; https://www.ccp14.ac.uk. Chekcell developed by L. Laugier and B. Bochu, Laboratoire des Materiaux et du Genie Physique, Ecole Superieure de Physique de Grenoble: Grenoble, France (accessed April 28, 2011).
- C. Dong, PowderX: Windows-95-based Program for Powder X-ray Diffraction Data Processing, J. Appl. Crystallogr, 1999, 32, 838 CrossRef CAS.
- F. A. Miller, R. J. Capwell, R. C. Lord and D. G. Rea, Spectrochim. Acta, Part A, 1972, 28, 603 CrossRef CAS.
- L. W. Daasch and D. C. Smith, J. Chem. Phys., 1951, 19, 22 CrossRef CAS.
- J. Sakamoto, S. Hashimoto, T. Tsuda, T. Sugahara, Y. Inoue and K. Ohgaki, Chem. Eng. Sci., 2008, 63, 5789 CrossRef CAS.
- M. Oshima, W. Shimada, S. Hashimoto, A. Tani and K. Ohgaki, Chem. Eng. Sci., 2010, 65, 5442 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: NMR results of synthesized P444(1c4)-Br, and Raman results of P444(1c4)-Br and P4444-Br salts. See DOI: https://doi.org/10.1039/d2nj04361k |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2023 |