Enhanced photoelectrochemical water oxidation over a surface-hydroxylated BiVO4 photoanode: advantageous charge separation and water dissociation†
Yaru
Li
 a,
Fangxia
Xie
a,
Zijun
Sun
a,
Zhuobin
Yu
a,
Fangxia
Xie
a,
Zijun
Sun
a,
Zhuobin
Yu
 b,
Jianxin
Liu
b,
Jianxin
Liu
 a,
Xiaochao
Zhang
a,
Xiaochao
Zhang
 a,
Yawen
Wang
a,
Yawen
Wang
 a,
Yunfang
Wang
a,
Rui
Li
*ac and
Caimei
Fan
a,
Yunfang
Wang
a,
Rui
Li
*ac and
Caimei
Fan
 *a
*a
aCollege of Chemical Engineering and Technology, Taiyuan University of Technology, Taiyuan, 030024, P. R. China. E-mail: fancm@163.com; lirui13233699182@163.com
bInstrumental Analysis Center, Taiyuan University of Technology, Taiyuan, 030024, P. R. China
cCollege of Environmental Science and Engineering, Taiyuan University of Technology, Taiyuan, 030024, P. R. China
First published on 21st November 2022
Abstract
Bismuth vanadate (BiVO4) is one of the most effective photoanode materials for photoelectrochemical water splitting, but its reaction rate is greatly limited by the poor separation efficiency of photo-generated charges and sluggish kinetics. In this work, a surface hydroxylation strategy was carried out to overcome the above-mentioned shortcomings. A simple post-synthetic NaOH immersion method was used to successfully hydroxylate the BiVO4 photoanode surface without affecting its bulk properties. The modified surface hydroxyl group not only promotes the photo-generated charge separation efficiency of hydroxylated BiVO4 but also tailors its local electronic structure to form high-valence Bi(3+x)+ states. The Bi(3+x)+ species serving as the active site is advantageous for water dissociation, thus decreasing the free energy barrier of the potential-determining step for the boosted overall water oxidation kinetics. As a result, the best hydroxylated BiVO4 photoanode achieves a higher photocurrent density of 1.14 mA cm−2 at 1.23 V vs. RHE under visible light irradiation, which is 4.75 times that of bare BiVO4 (0.24 mA cm−2), and moreover, a cathodic shift of 190 mV at 1 mA cm−2 is obtained. Our work affords a practical method with strong operability to improve the intrinsic photoelectrochemical performance of photoanodes by the efficient surface hydroxylation strategy.
Introduction
The environmental pollution and the impending energy shortage caused by massive consumption of fossil fuels have serious impacts on human survival.1,2 It is an urgent requirement to develop renewable sources of green energy. Since Fujishima and Honda described the process of photoelectrochemical (PEC) water splitting to generate hydrogen (H2) and oxygen (O2) in 1972,3 harnessing solar energy in the form of green chemical fuel H2via PEC water splitting has been recognized to be one of the most promising methods.4,5 Generally, the PEC performance for H2 generation is determined by a water oxidation half reaction due to its four-charge process.6 Therefore, the search for efficient and cheap photoanode materials has led scientists to explore various kinds of semiconductors.7–10 Among them, monoclinic bismuth vanadate (BiVO4) has been studied as a promising visible-light-driven photoanode for solar energy conversion, because of its low cost, narrow band gap (∼2.4 eV) and suitable band-gap structure for water oxidation.11,12 However, its severe photo-generated charge recombination and sluggish oxidation kinetics have hampered its further application in water splitting.13It is well known that PEC water oxidation is a multi-step and four-charge surface reaction process as follows:
| H2O + * + h+ → OH* + H+ | (1) |
| OH* + h+ → O* + H+ | (2) |
| O* + H2O + h+ → OOH* + H+ | (3) |
| OOH* + h+ → *O2 + H+ | (4) |
| *O2 → * + O2 | (5) |
Recently, surface hydroxylation has been put forward as an efficient surface modifying strategy for enhancing the water oxidation activity of catalysts. For example, Li et al. reported that hole transfer can be facilitated by enriching the α-Fe2O3 surface with hydroxyl groups via a sonication step, which had no change in the bulk properties of photoanode materials.18 Pei et al. found that with the hydroxyl group modification, the electrocatalytic activity of graphene-supported single atomic catalysts (Co and Fe) can be improved by the stable adsorption of intermediates (OH*, O* and OOH*) via a density functional theory study.19 Furthermore, this modification strategy avoids the problems of blocking light or creating additional interfacial traps that exist in an overcoated layer.20,21 More importantly, as for BiVO4 semiconductors, the hydroxyl group engineering can directly oxidize [Bi–O]− or [V–O]− to [Bi–O] or [V–O]˙, which is beneficial for the initiation step for water photooxidation.22 In addition, as a changeable valence metal element, Bi has an electronic structure that could be easily tailored under the action of surface groups and then enhance the reactivity towards water adsorption and oxygen-related reactions.23 Therefore, surface hydroxylation is a suitable strategy for the promotion of the water oxidation property of BiVO4, and it is necessary to expound the function of surface hydroxylation. However, there is no relevant report.
Hence, we first in situ synthesized a BiVO4 film on a metal Bi substrate and then directly soaked it in a NaOH solution to form the surface-hydroxylated BiVO4 photoanode. The hydroxylated BiVO4 photoanode obtained by controlling the appropriate reaction conditions exhibited superior PEC water oxidation activity and stability under visible light irradiation, indicating that this facile treatment method is practically feasible. Via the structural, morphological and optical characterizations, photoelectrochemical measurements and density functional theory (DFT) calculations, the connotation of the surface hydroxylation strategy to improve the reaction rate of BiVO4 was further clarified. Based on the explored essential relationship between surface behaviour and water oxidation activity, the PEC reaction mechanism of the hydroxylated BiVO4 photoanode was finally proposed, which would provide valuable reference for the subsequent research on water oxidation.
Experimental
Reagents and chemicals
Ammonium metavanadate (NH4VO3) was purchased from Aladdin Company. Sodium hydroxide (NaOH) and nitric acid (HNO3) were purchased from Tianjin Kermel Chemical Reagent Co., Ltd. All reagents were of analytical grade and used without further purification. Bi substrates were purchased from Wochang Metal Products Co., Ltd and used after further treatment.Preparation of surface-hydroxylated BiVO4 photoanodes
Characterizations and photoelectrochemical tests
X-Ray diffraction (XRD) images of the samples were recorded using a Rigaku D/max 2700 diffractometer with Cu Kα (λ = 1.5418 Å) radiation operating at 40 kV and 30 mA. Scanning electron microscopic (SEM) images were acquired using a JEOL JSM-7001F at an accelerating voltage of 10 kV. Fourier transform infrared (FTIR) spectra were recorded using a Shimadzu IRAffinity-1 FTIR spectrometer in the frequency range of 4000–500 cm−1. Raman spectra were recorded from 50 to 1200 cm−1 using a Renishaw inVia microscopic spectrometer. X-Ray photoemission spectroscopy (XPS) and valence band (VB)-XPS were performed using a VG MultiLab 2000 system with an Mg Kα X-ray source operating at 20 kV. Photoluminescence (PL) spectra were recorded using a FLS980 Series of Fluorescence Spectrometer.Photoelectrochemical tests were performed using an Admiral Squidstat Plus electrochemical system (America) with a three-electrode glass cell in a 0.1 M Na2SO4 electrolyte solution (pH ≈ 6.5). A 300 W Xe lamp (PLS-SXE 300 +, Perfect Light, China) equipped with a 420 nm filter (λ > 420 nm) was used as the simulated visible light source with a light intensity of 120 mW cm−2. The reference electrode was a standard calomel electrode (SCE), the counter electrode was a Pt wire and the working electrode was the prepared BiVO4 photoanode. All the potential vs. RHE data presented in this study were calculated according to the Nernst equation: ERHE = ESCE + 0.0591 pH + 0.24 V. Linear scan voltammetries (LSVs) were performed at a scan rate of 10 mV s−1. Cyclic voltammetric (CV) curves were recorded from 0.45 to 0.8 V vs. RHE at scan rates of 10, 20, 30, 40 and 50 mV s−1. Chrono-amperometric (I–t) measurements were conducted at 1.23 V vs. RHE, and the generated H2 and O2 gases were analyzed using a gas chromatograph (Panna A91 Plus, 5 Å molecular sieve column and Ar carrier). Mott–Schottky curves were obtained at a fixed frequency of 2 kHz. Electrochemical impedance spectroscopy (EIS) measurements were performed in the frequency range of 0.1 Hz to 105 Hz at an amplitude of 10 mV.
DFT calculations
All calculations were based on DFT (density functional theory) using the CASTEP (Cambridge sequential total energy package) module in Materials Studio.25 The generalized gradient approximation (GGA) with the Perdew–Burke–Ernzerhof (PBE) functional was used for the exchange–correlation interaction.26 In all calculations, a DFT+D empirical correction was taken to describe the van der Waals interaction.27 The plane wave cut-off energy was set to 450 eV. The Monkhorst-Pack k-point grids (2 × 2 × 1) were utilized to approximate the Brillouin-zone integrations. Total energy, force convergence threshold and maximum displacement were less than 10−5 eV per atom, 0.02 eV Å−1 and 5.0 × 10−4 Å, respectively. The calculation model of bare BiVO4 was built based on the BiVO4 (010) surface. The BiVO4 (010) slab was modeled by a 2 × 2 × 1 supercell, and its size is as follows: a = 10.2869 Å, b = 10.2777 Å. For surface-hydroxylated BiVO4, the one hydroxy group was bonded to a Bi atom on the BiVO4 (010) slab. A vacuum layer thickness of 12 Å was set to avoid the influence between neighbouring layers in the slab model. The reaction Gibbs free energy (ΔG) for each step was calculated by the expression: ΔG = ΔE + ΔEZPE − TΔS, where ΔE, ΔEZPE, and ΔS represent the total energy, zero-point energy, and entropy, respectively.Results and discussion
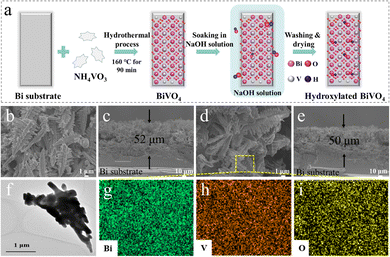
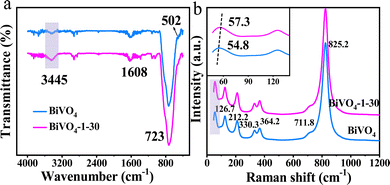
In this research, the surface-hydroxylated BiVO4 film was successfully developed on the metal Bi substrate (Fig. S1, ESI†) by a facile hydrothermal-NaOH immersion technique. The schematic diagram of its preparation process is shown in Fig. 1a. The morphology, structure and composition of the as-prepared samples were studied by various characterizations. The XRD analysis characterizes the crystalline structure of the prepared BiVO4 samples. The detectable patterns of bare BiVO4 and hydroxylated BiVO4-X–Y samples are both well consistent with that of monoclinic BiVO4 (JCPDS No. 14-0688), proving the successful synthesis of BiVO4 photoanodes (Fig. S2, ESI†).28 The SEM and TEM images show that BiVO4 and BiVO4-1–30 photoanodes have the same dendritic morphology with a thickness of 50–52 μm (Fig. 1b–f). The EDS mapping of BiVO4-1–30 verifies the uniformly distributed Bi, V and O spots (Fig. 1g–i). In addition, via N2 adsorption–desorption isotherms test, the similar specific surface area and pore average diameter are found for BiVO4 and BiVO4-1–30 (Fig. S3, ESI†). These phenomena manifest that the post-synthetic NaOH immersion treatment is effective without affecting the bulk properties of the BiVO4 photoanode.The FTIR characterization was used to investigate the surface composition of BiVO4 and BiVO4-1–30 samples. As shown in Fig. 2a, the broad regions peaked at 723 and 502 cm−1 could be attributed to the vibrations of V–O and Bi–O bonds, respectively. The peak at 1608 cm−1 is assigned to H–O–H of the adsorbed H2O in samples.29 Specially, it is clearly found that BiVO4-1–30 has a broader and stronger spectrum than that of the bare BiVO4 sample around the 3445 cm−1 region (purple labeled in Fig. 2a), which is ascribed to the stretching vibrations of O–H groups chemisorbed on the surface of catalyst.30,31 This result provides evidence for the BiVO4-X–Y photoanode with a hydroxylated surface obtained by the treatment of the NaOH solution.
The Raman spectroscopy further offered an intuitive observation of the chemical bonding vibrations in BiVO4 and BiVO4-1–30 samples. Fig. 2b displays that the two presented samples have the same vibrational bands around 126.7 cm−1, 212.2 cm−1, 330.3 cm−1, 364.2 cm−1, 711.8 cm−1 and 825.2 cm−1.32,33 However, the peak associated with the vibrational mode of the Bi–O bond shows difference, where it is at 54.8 cm−1 for BiVO4 but shifts to 57.3 cm−1 for BiVO4-1–30 (inset of Fig. 2b, purple labeled).34 One possible explanation for this change is that OH− anions are inclined to adsorb at Bi3+ cations with the electrostatic adsorption effect to form a hydroxylated surface; hence, the vibration of the Bi–O bond in BiVO4-1–30 is influenced.35,36
Furthermore, the detailed information on the elemental composition and chemical valence states of the samples are obtained by XPS. As shown in Fig. 3a, the full-scale XPS spectra reveal the presence of Bi 4f, O 1s and V 2p. The C 1s signal peak at a binding energy of 284.6 eV is applied to calibrate the relevant XPS peak position. In Fig. 3b, the peak at around 524.13 eV is the characteristic peak of Na Auger,37 which is due to the residual Na element in the preparation process of the BiVO4 and surface-hydroxylated BiVO4 photoanodes.31 The identical V 2p high resolution spectra were found for BiVO4 and BiVO4-1–30 photoanodes, indicating the unchanged bonding environment of the V5+ element with a hydroxylated surface.38 For the high-resolution Bi 4f spectra, the binding energies of Bi 4f7/2 and Bi 4f5/2 in bare BiVO4 are located at 159 eV and 164.3 eV, respectively; however, the Bi 4f peaks of BiVO4-1–30 exhibit higher binding energies shifting to 159.31 eV and 164.61 eV, respectively (Fig. 3c).39 It means that the higher valence Bi(3+x)+ state was obtained due to the strong electronic interaction between the strongly electronegative OH− group and Bi3+ site.40 Concomitantly, a negative shift for O 1s peaks in BiVO4-1–30 is revealed (Fig. 3d).32 These demonstrations obviously confirm that the surface-hydroxylated BiVO4 photoanode was successfully prepared, and the modified hydroxyl groups could effectively regulate the surface states of BiVO4 photoanodes for a beneficial water oxidation reaction.23
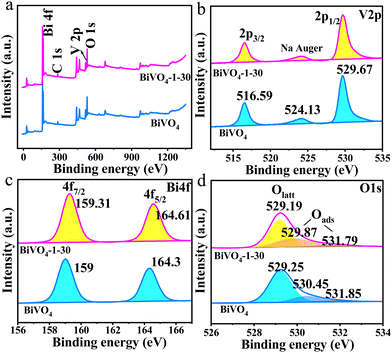 | ||
| Fig. 3 (a) XPS spectra, high-resolution XPS spectra for (b) V 2p, (c) Bi 4f, and (d) O 1s of bare BiVO4 and BiVO4-1–30 photoanodes. | ||
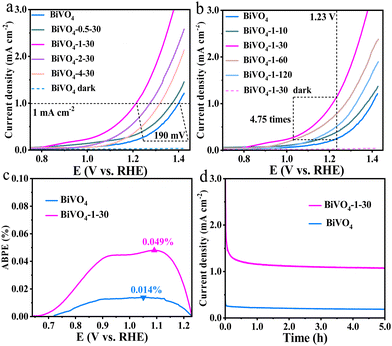
The linear sweep voltammetry (LSV) method was employed to investigate the PEC water oxidation performance of the prepared BiVO4 photoanodes in the three-electrode cell. As shown in Fig. 4a and b, the current densities of both bare BiVO4 and BiVO4-1–30 photoanodes are ignorable in the darkness, illustrating that the surface hydroxyl group has no obvious effect without the photo-generated charges. Under the visible light illumination, all of the prepared BiVO4 photoanodes display water oxidation activity, and the activities are greatly increased with the treatment of NaOH. The minimum overpotential (at 1 mA cm−2) was obtained with 1 M NaOH treatment for 30 min (BiVO4-1–30 sample), which decreases by ∼190 mV with respect to bare BiVO4 (Fig. 4a). The current density of BiVO4-1–30 attains 1.14 mA cm−2 at 1.23 V vs. RHE, being ∼4.75 times than that of bare BiVO4 achieving 0.24 mA cm−2 (Fig. 4b). For the applied bias photon-to-current efficiency (ABPE) estimation, eqn (E1) (ESI†) was applied. As shown in Fig. 4c, the maximum ABPE of the BiVO4-1–30 photoanode is ∼0.049% at 1.09 V vs. RHE; however, the maximum ABPE of bare BiVO4 is only ∼0.014% at 1.05 V vs. RHE. Furthermore, Fig. S4 (ESI†) exhibits that the maximum ABPE of the BiVO4-1–30 photoanode (∼0.045% at 1.09 V) is also higher than that of bare BiVO4 (∼0.009% at 1.12 V) in the two-electrode configuration, which reflects that the system with the BiVO4-1–30 photoanode is with better overall water splitting performance.
The PEC water oxidation stability of the samples was evaluated by chronoamperometry at 1.23 V vs. RHE. It shows that BiVO4-1–30 keeps around 1.08 mA cm−2 after testing for 5 h, demonstrating its excellent PEC stability (Fig. 4d). Importantly, the photocurrent of BiVO4-1–30 is always higher than that of the bare BiVO4 sample. Meanwhile, the H2 and O2 gases generated under the same conditions were also measured. As shown in Fig. S5 (ESI†), the total amount of H2 and O2 gases after 5 h was measured to be 117.69 and 58.85 μmol, respectively, by using the BiVO4-1–30 photoanode, which are higher than that measured by the bare BiVO4 one as 27.66 and 13.83 μmol, respectively,. Taken together, the results reveal that the surface hydroxylation strategy by a simple NaOH treatment could greatly improve the PEC water oxidation property of BiVO4 photoanodes, hence enhancing the overall water splitting performance.
The UV-vis diffuse reflectance spectra (DRS) display that both bare BiVO4 and BiVO4-1–30 photoanodes have excellent absorption in the visible light region (Fig. S6, ESI†). To evaluate the solar energy conversion capability of prepared photoanodes, the incident photon-to-current conversion efficiency (IPCE) was performed from the region of 420–700 nm excitation wavelengths at 1.23 V vs. RHE. The IPCE value was figured out using eqn (E2) (ESI†). The BiVO4-1–30 reveals the highest IPCE of 16% at 420 nm, whereas the IPCE value of bare BiVO4 is only around 3.69% at 420 nm (Fig. 5a). The factors of light harvesting efficiency (LHE), bulk charge separation (ηsep) and surface injection (ηinj) efficiencies are closely linked to IPCE.41 According to the DRS data, the LHE was calculated using eqn (E3) (ESI†). It can be found the two samples display similar light harvesting ability (Fig. 5b), stating that the surface hydroxylation has no effect on LHE. Fig. S7 (ESI†) shows the LSV curves, which were measured in the electrolytes of 0.1 M Na2SO4 with/without 0.1 M Na2SO3 (hole scavenger). Based on the LSV curves, the ηsep and ηinj were estimated using eqn (E6) and (E7) (ESI†), respectively. It is well known that ηsep is to test the migration capacity of holes in the photoanode phase to the photoanode/electrolyte interface, and ηinj reveals the efficacy of holes on the photoanode/electrolyte interface to participate in the water oxidation process.42 As Fig. 5c shows, ηsep of bare BiVO4 at 1.23 V vs. RHE is only 16.45%, while ηsep of BiVO4-1–30 increases to 28.7%. Moreover, compared with bare BiVO4, ηinj of BiVO4-1–30 is greatly improved. The ηinj value of BiVO4-1–30 at 1.23 V vs. RHE has reached 53.16%, which is much higher than that of bare BiVO4 which is 19.22% (Fig. 5d). The enhanced ηsep and ηinj values confirm the significantly promoted separation and utilization efficiency of photo-generated holes by surface hydroxylation, hence enhancing the PEC water oxidation activity of the BiVO4-1–30 photoanode.
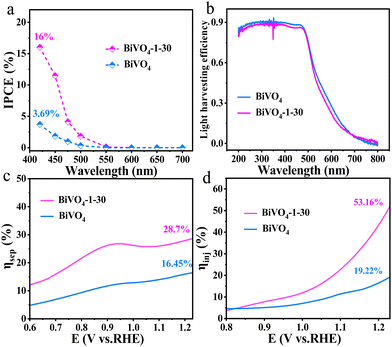 | ||
| Fig. 5 (a) IPCE plots, (b) light harvesting efficiency (LHE), (c) the charge separation efficiency (ηsep) and (d) charge injection efficiency (ηinj) of bare BiVO4 and BiVO4-1–30 photoanodes. | ||
In order to further explore the reason for the increased PEC water oxidation properties of the hydroxylated BiVO4 photoanode, the electrochemical impedance spectroscopy (EIS) was investigated, in which the arc radius reflects the charge transfer resistance.43 Compared with bare BiVO4 photoanode, the arc radius of BiVO4-1–30 is smaller, which indicates that the hydroxylated surface reduces the charge transfer resistance for the improved water oxidation kinetics (Fig. 6a).38 Furthermore, the transient photocurrent response (PC) and photoluminescence (PL) curves were applied to investigate the separation condition of photo-generated carrier. The relatively higher PC spikes (Fig. 6b) and lower PL curve (Fig. 6c) of BiVO4-1–30 than those of bare BiVO4 indicate that the hydroxylated surface can assist faster charge separation, effectively reduce the recombination of carriers, thus making more holes to participate in the water oxidation reaction.44 At last, the cyclic voltammograms (CV) (Fig. S8, ESI†) were applied to investigate the electrochemically active surface area plots (ECSA). As Fig. 6d shows, the BiVO4-1–30 sample displays a bigger linear slope of 3.59 mF cm−2 than that of bare BiVO4 of 1.6 mF cm−2, which reflects the capacitance and is proportional to the ECSA.45 In other words, BiVO4-1–30 possesses an increased ECSA, so that it could provide more active sites for PEC water oxidation to improve the activity.
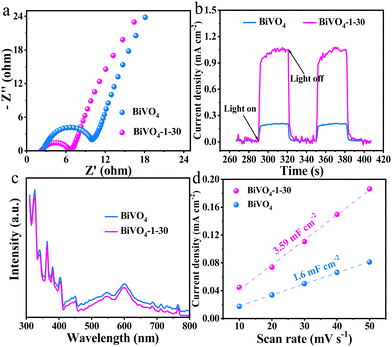 | ||
| Fig. 6 (a) EIS, (b) photocurrent response, (c) PL spectra and (d) charging current densities recorded at 0.625 VRHE at different scan rates of bare BiVO4 and BiVO4-1–30 photoanodes. | ||
The performance of photoanodes is closely related to its energy band structure. Therefore, it was studied to explore the effect of the hydroxylated surface on the photo-induced carrier mechanism. The DRS, valence band XPS (VB-XPS) and Mott–Schottky test were performed to determine the band gaps, valence band (VB) and conduction band (CB) positions. Plotted by the DRS spectra, Fig. 7a exhibits the band gap energy values of 2.38 and 2.4 eV for bare BiVO4 and BiVO4-1–30 photoanodes, respectively. The VB-XPS spectra (Fig. 7b) show that the energy gaps are 1.56 and 1.72 eV for bare BiVO4 and BiVO4-1–30, respectively, which represent the difference in values between the VB and Fermi level (Ef).46 The value of flat band potential (EFB) is nearly equal to that of Ef.47 Calculated by Mott–Schottky plots, the EFB values of two samples are 0.20 and 0.15 V vs. SCE (Fig. 7c), i.e. 0.42 and 0.37 V vs. NHE (normal hydrogen electrode), respectively.46 Consequently, the VB positions are equal to 1.98 and 2.09 V, respectively. Furthermore, based on the band gap energies, the CB positions are −0.40 and −0.31 V of bare BiVO4 and BiVO4-1–30 photoanodes, respectively. The diagram of the band energy structure is shown in Fig. 7d. It is directly observed that BiVO4-1–30 with the hydroxylated surface possesses a positive shift of VB position. The more positive position of VB is advantageous for the generation of photo-induced holes with more powerful oxidizing ability.48 Moreover, the flat potential value decreases from 0.44 V to 0.39 V vs. NHE after the surface hydroxylation. The negative shift of the flat potential is regarded to increase the band bending, thus promote the interfacial hole transfer of the hydroxylated BiVO4 photoanodes.49
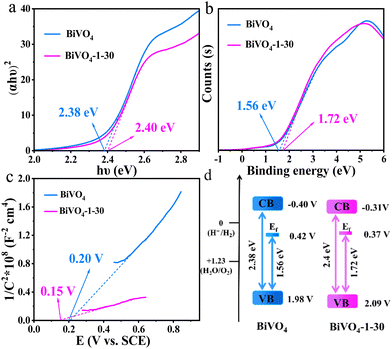 | ||
| Fig. 7 (a) The optical band gap estimation, (b) VB-XPS spectra, (c) Mott–Schottky plots, and (d) schematic band structure diagram of bare BiVO4 and BiVO4-1–30 photoanodes. | ||
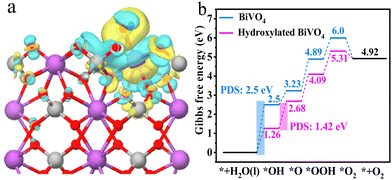
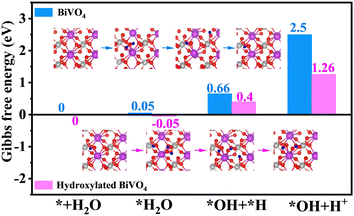
Density functional theory (DFT) calculations were carried out to deeply study the reason for the enhanced water oxidation activity of hydroxylated BiVO4 photoanodes. The optimized models are shown in Fig. S9 (ESI†). It can be seen that the hydroxyl group tends to adsorb at the Bi cation site, which is consistent with the change of Bi in Raman and XPS results. The charge density difference of hydroxylated BiVO4 with regard to BiVO4 was simulated. It clearly reveals that the Bi atom donates its electrons mainly to nearby O atoms and the hydroxyl group via orbital hybridization (Fig. 8a). Namely, the electrons have an active tendency to transfer from BiVO4 to the surface hydroxyl group, and thus, holes could efficaciously separate from them on the hydroxylated BiVO4 photoanode surface for water oxidation reactions.50 Meanwhile, electron-deficient Bi, i.e. the high-valence Bi(3+x)+ state, can be obtained, which is reported to be beneficial for water adsorption and oxygen-relevant reactions towards the boosted water oxidation kinetics.23,51 Therefore, the relevant Gibbs free energy for each step in the overall water oxidation progress of bare BiVO4 and hydroxylated BiVO4 photoanodes was calculated (Fig. 8b). It was obviously that the first step (* + H2O + h+ → *OH + H+) is the potential-determining step (PDS) for bare BiVO4 with the biggest overpotential of 2.5 eV. However, with the modification of the hydroxyl group for hydroxylated BiVO4, the energy barrier of this step is decreased to 1.26 eV, and the PDS changes to the second step (*OH + h+ → *O + H+) with an overpotential of 1.42 eV. Notably, the free energy change in each step for hydroxylated BiVO4 is close to the ideal 1.23 eV, being more beneficial for proceeding the water oxidation process.52
In detail, the first step (PDS: * + H2O + h+ → *OH + H+) is the catalytic dissociation of the H2O molecule with the generation of *OH. Hence, the reactant H2O behavior on the photoanode surface was considered. As shown in Fig. 9, the H2O molecule has a lower energy barrier of adsorption on hydroxylated BiVO4 (−0.05 eV) than that on bare BiVO4 (0.05 eV), indicating more favoured H2O adsorption (* + H2O → *H2O). Hydroxylated BiVO4 also exhibits a quite low barrier of 0.45 eV for *H2O dissociation (*H2O → *OH + *H); in contrast, a higher energy barrier is 0.61 eV on bare BiVO4. These show the much easier dissociation of H2O at the high-valence Bi(3+x)+ active site, leading to a faster *OH supply. Meanwhile, the *H desorption (*OH + *H → *OH + H+) plays a very important role in the water dissociation process, in which the rapid *H desorption would shift the equilibrium toward water dissociation, thus the reaction rate can be increased.22 It can be found that hydroxylated BiVO4 model exhibits a much lower energy barrier of 0.86 eV for *H desorption than that of bare BiVO4 (1.84 eV), further proceeding the water dissociation kinetics.
Based on the above-mentioned findings, a possible PEC water oxidation mechanism of the prepared BiVO4 photoanode is proposed, as displayed in Fig. 10. When the BiVO4 photoanode is excited by visible light, the photo-generated electrons (e−) in bulk migrate to the Pt cathode through the external circuit to reduce H+ to H2, and the holes (h+) move to the photoanode/electrolyte reaction interface to oxidize water to O2. Due to the sluggish kinetics of PDS step (* + H2O + h+ → *OH + H+) and the massive recombination of e−–h+ on bare BiVO4, the overall water oxidation process is hard to take place, while for the surface-hydroxylated BiVO4 photoanode, the water oxidation process becomes easy. Combined with various characterization studies, the great change is ascribed to the hydroxylated surface modification. The hydroxyl group is considered as a modification ligand on the surface of BiVO4, which plays the key roles in (i) modifying the electronic configuration of Bi sites to attain a high-valence Bi(3+x)+ state toward boosted water dissociation, thus decreasing the PDS energy barrier for the enhanced overall water oxidation kinetics and (ii) promoting efficient e− separation to itself, so more h+ could take part in the water oxidation process rather than recombining with e−. Taken together, the surface-hydroxylated BiVO4 photoanode displays the enhanced PEC water oxidation activity, which even attains the level of most reported BiVO4-based composite photoanodes (Table S1, ESI†). Meanwhile, the applied post-synthetic NaOH immersion way for surface hydroxylation is more practically feasible and cost-effective than other methods (Table S2, ESI†). These advantages will be more meaningful for practical PEC water oxidation applications.
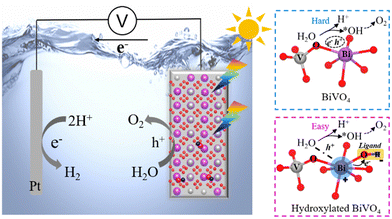 | ||
| Fig. 10 The illustration of proposed PEC water oxidation reaction mechanism of bare BiVO4 and hydroxylated BiVO4 photoanodes. | ||
Conclusions
A surface-hydroxylated BiVO4 photoanode has been successfully prepared by a hydrothermal and NaOH post-treatment method. The optimal hydroxylated BiVO4 photoanode displayed a 4.75 times higher photocurrent density and a cathodic shift of 190 mV than those of the bare BiVO4 photoanode under visible light irradiation. The greatly enhanced activity verified the high efficiency of surface hydroxylation strategy in improving the PEC water oxidation property of BiVO4. The modified hydroxyl group not only plays a role in enhancing the electron–hole separation efficiency, but also leads to high-valence Bi(3+x)+ states for promoting the water oxidation kinetics. This work affords a practical surface hydroxylation method with strong operability and high efficiency, which would provide a valuable reference for the subsequent research on PEC water oxidation.Author contributions
Yaru Li: methodology, data curation, writing – original draft; Fangxia Xie: validation; Zijun Sun: software; Zhuobin Yu: investigation, funding acquisition; Jianxin Liu: resources, funding acquisition; Xiaochao Zhang: supervision, funding acquisition; Yawen Wang: conceptualization; Yunfang Wang: formal analysis; Rui Li: writing – review & editing, funding acquisition; Caimei Fan: project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (No. 22008167, 21978187, 21978196), Natural Science Foundation for Young Scientists of Shanxi Province (No. 201901D211100, 20210302124336, 201901D211058).Notes and references
- L. Jiang, Q. Qin, Y. Wang, Y. Su, L. Xia, S. Lin, W. Yao, Q. Wu, Y. Min and Q. Xu, J. Colloid Interface Sci., 2022, 619, 257–266 CrossRef CAS.
- N. Mughal, A. Arif, V. Jain, S. Chupradit, M. Shabbir, C. Ramos-Meza and R. Zhanbayev, Energy Strategy Rev., 2022, 39, 100745 CrossRef.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS.
- B. Trześniewski, I. Digdaya, T. Nagaki, S. Ravishankar, I. Herraiz-Cardona, D. Vermaas, A. Longo, S. Gimenez and W. Smith, Energy Environ. Sci., 2017, 10, 1517–1529 RSC.
- Y. Yang, C. Zhang, C. Lai, G. Zeng, D. Huang, M. Cheng, J. Wang, F. Chen, C. Zhou and W. Xiong, Adv. Colloid Interface Sci., 2018, 254, 76–93 CrossRef CAS PubMed.
- B. Kang, M. Hussain, X. Cheng, C. Peng and Z. Wang, J. Colloid Interface Sci., 2022, 626, 146–155 CrossRef CAS.
- C. Lhermitte and B. Bartlett, Acc. Chem. Res., 2016, 49, 1121–1129 CrossRef CAS.
- L. Xia, J. Bai, J. Li, Q. Zeng, L. Li and B. Zhou, Appl. Catal., B, 2017, 204, 127–133 CrossRef CAS.
- G. Zheng, J. Wang, H. Liu, V. Murugadoss, G. Zu, H. Che, C. Lai, H. Li, T. Ding, Q. Gao and Z. Guo, Nanoscale, 2019, 11, 18968–18994 RSC.
- X. Li, J. Wang, J. Xia, Y. Fang, Y. Hou, X. Fu, M. Shalom and X. Wang, ChemSusChem, 2022, 15, e202200330 CAS.
- Y. Park, K. McDonald and K. Choi, Chem. Soc. Rev., 2013, 42, 2321–2337 RSC.
- T. Soltani, A. Tayyebi and B. Lee, Sol. Energy Mater. Sol. Cells, 2018, 185, 325–332 CrossRef CAS.
- P. Hajra, S. Kundu, A. Maityc and C. Bhattacharya, Chem. Eng. J., 2019, 374, 1221–1230 CrossRef CAS.
- S. Anantharaj and S. Noda, Small, 2020, 16, 1905779 CrossRef CAS.
- Y. Li, S. Li, Y. Wu, J. Zhang, Y. Yang, H. Mao, Y. Zhang and X. Song, Appl. Surf. Sci., 2022, 578, 151914 CrossRef CAS.
- K. Song, F. He, E. Zhou, L. Wang, H. Hou and W. Yang, J. Energy Chem., 2022, 68, 49–59 CrossRef CAS.
- J. Zhang, Q. Zhang and X. Feng, Adv. Mater., 2019, 31, 1808167 CrossRef.
- C. Tang, B. Sun, M. Li, J. Zhang, X. Fan, F. Gao, Y. Tong, L. Dong and Y. Li, J. Mater. Chem. A, 2019, 7, 8050 RSC.
- X. Zhao, X. Liu, B. Huang, P. Wang and Y. Pei, J. Mater. Chem. A, 2019, 7, 24583–24593 RSC.
- J. Kim, D. Youn, K. Kang and J. Lee, Angew. Chem., Int. Ed., 2016, 55, 10854–10858 CrossRef CAS PubMed.
- W. Kim, T. Tachikawa, D. Satoca, H. Kim, T. Majima and W. Choi, Energy Environ. Sci., 2013, 6, 3732–3739 RSC.
- M. Long and W. Cai, Chin. J. Catal., 2008, 29, 881–883 CrossRef CAS.
- L. Cao, Q. Luo, W. Liu, Y. Lin, X. Liu, Y. Cao, W. Zhang, Y. Wu, J. Yang, T. Yao and S. Wei, Nat. Catal., 2019, 2, 134–141 CrossRef CAS.
- Y. Li, X. Zhang, J. Liu, Y. Wang, Y. Wang, R. Li and C. Fan, Catal. Commun., 2020, 144, 106071 CrossRef CAS.
- D. Zhong, T. Li, D. Wang, L. Li, J. Wang, G. Hao, G. Liu, Q. Zhao and J. Li, Nano Res., 2022, 15, 162–169 CrossRef CAS.
- X. Gao, Y. Zhou, S. Liu, Z. Cheng, Y. Tan and Z. Shen, Appl. Surf. Sci., 2020, 502, 144155 CrossRef CAS.
- A. Tkatchenko and M. Scheffler, Phys. Rev. Lett., 2009, 102, 073005 CrossRef.
- S. Hernández, S. Thalluri, A. Sacco, S. Bensaid, G. Saracco and N. Russo, Appl. Catal., A, 2015, 504, 266–271 CrossRef.
- S. Chen, M. Yuan, W. Feng, W. Liu, W. Zhang, H. Xu, X. Zheng, G. Shen, C. Guo and L. Wang, Water Res., 2020, 185, 116220 CrossRef CAS.
- M. Janus, M. Inagaki, B. Tryba, M. Toyoda and A. Morawski, Appl. Catal., B, 2006, 63, 272–276 CrossRef CAS.
- L. Nie, J. Yu, X. Li, B. Cheng, G. Liu and M. Jaroniec, Environ. Sci. Technol., 2013, 47, 2777–2783 CrossRef CAS.
- R. Mirabal-Rojas, O. Depablos-Rivera, S. Thalluri, J. Medina, M. Bizarro, J. Perez-Alvarez, S. Rodil and A. Zeinert, Appl. Phys. A: Mater. Sci. Process., 2016, 122, 325 CrossRef.
- T. Dabodiya, P. Selvarasu and A. Murugan, Inorg. Chem., 2019, 58, 5096–5110 CrossRef CAS.
- G. Zhu, S. Li, J. Gao, F. Zhang, C. Liu, Q. Wang and M. Hojamberdiev, Appl. Surf. Sci., 2019, 493, 913–925 CrossRef CAS.
- X. Ren, G. Cui, L. Chen, F. Xie, Q. Wei, Z. Tian and X. Sun, Chem. Commun., 2018, 54, 8474–8477 RSC.
- N. Kharche, M. Hybertsen and J. Muckerman, Phys. Chem. Chem. Phys., 2014, 16, 12057–12066 RSC.
- H. Nesbitt, G. Bancroft, G. Henderson, R. Ho, K. Dalby, Y. Huang and Z. Yan, J. Non-Cryst. Solids, 2011, 357, 170–180 CrossRef CAS.
- X. Zhong, H. He, J. Du, Q. Ren, J. Huang, Y. Tang, J. Wang, L. Yang, F. Dong, L. Bian and Y. Zhou, Electrochim. Acta, 2019, 304, 301–311 CrossRef CAS.
- R. Li, F. Xie, J. Liu, Y. Wang, Y. Wang, X. Zhang and C. Fan, Dalton Trans., 2016, 45, 9182–9186 RSC.
- Y. Bi, Z. Zhang, X. Huang and B. Zhang, Energy Environ. Sci., 2022, 15, 2867–2873 RSC.
- S. Majumder, M. Gu and K. Kim, Appl. Surf. Sci., 2022, 574, 151562 CrossRef CAS.
- H. Bai, X. Li, Y. Zhao, W. Fan, Y. Liu, Y. Gao, D. Xu, J. Ding and W. Shi, Appl. Surf. Sci., 2021, 538, 148150 CrossRef CAS.
- H. Wu, L. Wu, S. Shen, Y. Liu, G. Cai, X. Wang, Y. Qiu, H. Zhong, Z. Xing, J. Tang, Z. Dai, C. Jiang and F. Ren, Int. J. Hydrogen Energy, 2020, 45, 9408–9415 CrossRef CAS.
- Z. Jia, R. Lv, L. Guo, J. Zhang, R. Li, J. Liu and C. Fan, Sep. Purif. Technol., 2021, 257, 117872 CrossRef CAS.
- G. Liu, K. Wang, X. Gao, D. He and J. Li, Electrochim. Acta, 2016, 211, 871–878 CrossRef CAS.
- N. Tian, Y. Zhang, X. Li, K. Xiao, X. Du, F. Dong, G. Waterhouse, T. Zhang and H. Huang, Nano Energy, 2017, 38, 72–81 CrossRef CAS.
- H. Li, X. Quan, S. Chen and H. Yu, Appl. Catal., B, 2017, 209, 591–599 CrossRef CAS.
- H. Zhang, W. Wei, F. Hou, W. Guo, Q. Zhang, T. Wang and A. Wei, Sep. Purif. Technol., 2021, 265, 118510 CrossRef CAS.
- X. Xiong, C. Zhang, X. Zhang, L. Fan, L. Zhou, Y. Chu, W. Huang, C. Wu, J. Li, X. Yang and D. Han, Electrochim. Acta, 2021, 389, 138795 CrossRef CAS.
- G. Zuo, Y. Wang, W. Teo, A. Xie, Y. Guo, Y. Dai, W. Zhou, D. Jana, Q. Xian, W. Dong and Y. Zhao, Angew. Chem., Int. Ed., 2020, 59, 11287–11292 CrossRef CAS.
- Y. Liu, X. Liu, X. Wang, H. Ning, T. Yang, J. Yu, A. Kumar, Y. Luo, H. Wang, L. Wang, J. Lee, A. Jadhav, H. Hu, M. Wu, M. Kim and H. Lee, ACS Nano, 2021, 15, 15017–15026 CrossRef CAS.
- J. Nørskov, J. Rossmeisl, A. Logadottir and L. Lindqvist, J. Phys. Chem. B, 2004, 108, 17886–17892 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2nj04470f |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2023 |