Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Multisubstituted naphthalene synthesis from 4-hydroxy-2-pyrones through [4+2] cycloaddition with o-silylaryl triflates†
Koyo
Numata
a,
Shinya
Tabata
a,
Akihiro
Kobayashi
ab and
Suguru
Yoshida
 *a
*a
aDepartment of Biological Science and Technology, Faculty of Advanced Engineering, Tokyo University of Science, 6-3-1 Niijuku, Katsushika-ku, Tokyo 125-8585, Japan. E-mail: s-yoshida@rs.tus.ac.jp
bLaboratory of Chemical Bioscience, Institute of Biomaterials and Bioengineering, Tokyo Medical and Dental University (TMDU), 2-3-10 Kanda-Surugadai, Chiyoda-ku, Tokyo 101-0062, Japan
First published on 19th September 2023
Abstract
An efficient synthetic method for multisubstituted naphthalenes from 4-hydroxy-2-pyrones through cycloaddition with aryne intermediates is disclosed. Various highly functionalized 2-pyrones were synthesized through 4-hydroxy-2-pyrones in short steps. The resulting 2-pyrones reacted smoothly with a wide range of aryne intermediates generated from o-silylaryl triflates to provide multisubstituted naphthalenes by the Diels–Alder reaction and following decarboxylative aromatization. The aryne reaction of 2-pyrones served in the synthesis of diverse naphthalenes having 1,2,3-triazole moieties by combination with triazole formation of 4-azido-2-pyrones.
Introduction
Multisubstituted naphthalenes are a widely used class of compounds in various research areas such as pharmaceutical sciences, agrochemistry, and materials chemistry.1 Despite the significance of highly functionalized naphthalenes, it is still challenging to prepare multisubstituted naphthalenes efficiently due to the problematic reactivity and selectivity control in various transformations such as electrophilic aromatic substitutions (Fig. 1A).2 Herein, we describe an efficient method to synthesize multisubstituted naphthalenes by an aryne reaction with a broad range of 2-pyrones prepared through 4-hydroxy-2-pyrones.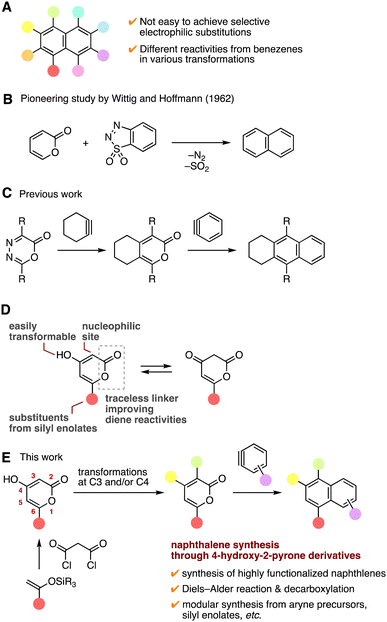 | ||
| Fig. 1 (A) Multisubstituted naphthalenes. (B) A pioneering study by Wittig and Hoffmann. (C) Our previous study. (D) Features of 4-hydroxy-2-pyrones. (E) Overview of this work. | ||
Remarkable advances in synthetic aryne chemistry have improved the accessibility of multisubstituted arenes having a variety of functional groups.3 Since a broad range of functionalized arynes served in arene synthesis and recent studies allowed us to synthesize o-silylaryl triflates bearing various functionalities in good efficiencies, we paid attention to dienes as arynophiles for naphthalene synthesis.4 In general, the Diels–Alder reactions of diverse electron-rich furans with arynes easily take place, since aryne intermediates show a highly electron-deficient nature.5 A range of naphthalenes were prepared by further deoxygenative aromatization of the cycloadducts between arynes and furans.6 Due to the poor accessibility of functionalized furans and limitations in reductive aromatizations, facile synthetic methods of multisubstituted naphthalenes are still desired.
In this study, we conceived an idea of facile naphthalene synthesis by reaction of 2-pyrones with arynes. A pioneering study on naphthalene synthesis was reported by Wittig and Hoffmann in 1962, where the Diels–Alder reaction of 2-pyrone with benzyne and subsequent retro Diels–Alder reactions removing carbon dioxide proceeded smoothly to afford naphthalene (Fig. 1B).7a We paid attention to the cyclic ester moiety as a traceless linker that would improve the diene reactivity by fixing the s-cis structure. Although various 2-pyrones served as electron-deficient dienes in the inverse electron-demand Diels–Alder reactions with electron-rich dienophiles such as vinyl ethers8 and a range of substituted 2-pyrones have served in the reaction with benzyne in recent studies,7 only limited naphthalenes have been synthesized probably due to the electronic demands as both arynes and 2-pyrones are electron-deficient classes of skeletons, in which substituted arynes were not examined and the effects of substituents on the arynes and pyrones are still unclear.7 Also, theoretical studies of the aryne reaction with 2-pyrones are sought after.
Recently, we developed an efficient method to prepare tetrahydroanthracene derivatives by the reaction of arynes via pyrones (Fig. 1C).9a Indeed, treatment of oxadiazinones and cycloalkyne precursors with fluoride ions provided tetrasubstituted 2-pyrones. The resulting 2-pyrones reacted with arynes to furnish tetrahydroanthracene derivatives. Similar transformations of oxadiazinones with cycloalkynes and arynes were also reported by Garg and coworkers.9b,c
For the synthesis of highly functionalized naphthalenes, we focused on 4-hydroxy-2-pyrones as intermediates for di- or trisubstituted 2-pyrones because of the following three features (Fig. 1D).10 (1) A wide variety of 6-substituted 4-hydroxy-2-pyrones can be synthesized easily from silyl enolates and malonyl chloride. (2) Due to the equilibrium structures between an enol form and a keto form, electrophilic substitution easily takes place at C3 selectively. (3) Transformations at C4 involving halogenation and subsequent substitutions lead to various 4-substituted 2-pyrones. Thus, we expected that a wide variety of highly functionalized naphthalenes would be synthesized from diverse o-silylaryl triflates and 2-pyrones by the Diels–Alder reaction followed by decarboxylative aromatization (Fig. 1E). Detailed scope and limitations, applications to prepare triazoles, and theoretical studies on naphthalene synthesis have been performed.
Results and discussion
First, we prepared a wide range of di- or trisubstituted 2-pyrones from 6-substituted 4-hydroxy-2-pyrones in accordance with various reports10 with minor modifications (Fig. 2). Divergent synthetic methods using common intermediates are beneficial in preparing multisubstituted naphthalenes. For example, O-alkylation10g and dehydrative bromination10k of 4-hydroxy-6-methyl-2-pyrone (1a) smoothly took place to provide 2a and 2b in good yields (Fig. 2A). Suzuki–Miyaura cross-coupling reactions of 2b with arylboronic acids 3 in the presence of a catalytic amount of palladium acetate and JohnPhos efficiently proceeded to afford 4-arylated pyrones 2c–2e.10e We also succeeded in the synthesis of ether 2f by treatment of bromide 2b with 4-methoxyphenol (4) in the presence of potassium carbonate.10h These results clearly indicate that a wide variety of 4-substituted pyrones can be prepared easily from 4-hydroxypyrone 1a and simple starting materials such as arylboronic acids and phenols. A variety of substituents were successfully installed at nucleophilic C3 of 4-hydroxy-6-methyl-2-pyrone (1a) (Fig. 2B).10g Bromination or iodination followed by O-ethylation furnished trisubstituted 2-pyrone 2g or 2h, respectively.10g We accomplished further trifluoromethylation of 2h to give 2i in good yield according to the reported method by McGlacken and coworkers.10g Synthesis of 6-aryl-4-bromo-2-pyrones 2j–2l was realized from silyl enolates 6 and malonyl chloride (7) and subsequent dehydrative bromination (Fig. 2C).10c In addition, we achieved the preparation of 6-trifluoromethyl-substituted 2-pyrone 2m through trifluoroacetylation of acetyl acetone derivative 8 without purification of intermediates (Fig. 2D).10d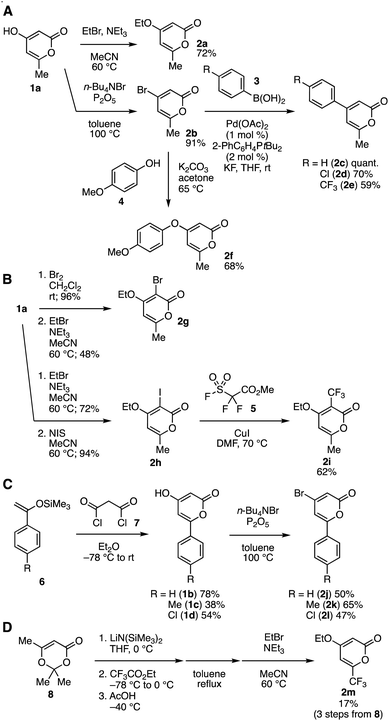 | ||
| Fig. 2 (A) Synthesis of 2a–2f. (B) Synthesis of 2g–2i. (C) Synthesis of 2j–2l. (D) Synthesis of 2m. See ESI† for details. | ||
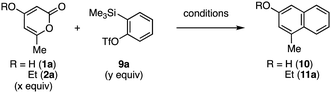
Efficient synthesis of disubstituted naphthalene 11a by the reaction of pyrone 2a with o-silylphenyl triflate 9a was accomplished (Table 1). While the desired naphthalene 10 was not obtained when using 4-hydroxy-6-methyl-2-pyrone (1a) (entry 1), we succeeded in the preparation of naphthalene 11a by the reaction of 4-ethoxy-6-methyl-2-pyrone (2a) with benzyne generated from o-silylphenyl triflate 9a with cesium fluoride in acetonitrile at room temperature (entry 2). Although the efficiency was not improved by changing the activators for generating benzyne from 9a (entries 3–5), we successfully synthesized naphthalene 11a in high yield when the reaction was conducted at 50 °C (entry 6). After modifying the ratio between pyrone 2a and o-silylphenyl triflate 9a (entries 7–9), naphthalene 11a was prepared efficiently from 2a (1.0 equiv.) and 9a (1.5 equiv.) (entry 9) and the yield was slightly decreased when using 2.0 equivalents of 9a (entry 10). We also succeeded in naphthalene synthesis on a 1 mmol scale, obviously showing good scalability of the protocol.
| Entry | OR | x | y | Conditions | Product, yielda (%) |
|---|---|---|---|---|---|
| a Yields based on 1H NMR analysis. Reactions were performed in 0.1 mmol scale unless otherwise noted. b Aryne precursor 9a (50%) was recovered. c Aryne precursor 9a was consumed. d Isolated yield. e The reaction was conducted using 1.0 mmol of 2a. | |||||
| 1 | OH | 2.0 | 1.0 | CsF (2.0 equiv.), | 10, 0 |
| MeCN, rt, 24 h | |||||
| 2 | OEt | 2.0 | 1.0 | CsF (2.0 equiv.), | 11a, 35b |
| MeCN, rt, 24 h | |||||
| 3 | OEt | 2.0 | 1.0 | CsF (2.0 equiv.), | 11a, 20c |
| 18-crown-6 | |||||
| (2.0 equiv.) | |||||
| MeCN, rt, 24 h | |||||
| 4 | OEt | 2.0 | 1.0 | KF (2.0 equiv.), | 11a, 39c |
| 18-crown-6 | |||||
| (2.0 equiv.) | |||||
| THF, rt, 24 h | |||||
| 5 | OEt | 2.0 | 1.0 | Cs2CO3 (2.0 equiv.), | 11a, 17c |
| 18-crown-6 | |||||
| (2.0 equiv.) | |||||
| THF, rt, 24 h | |||||
| 6 | OEt | 2.0 | 1.0 | CsF (2.0 equiv.), | 11a, 72 |
| MeCN, 50 °C, 24 h | |||||
| 7 | OEt | 2.0 | 1.0 | CsF (2.0 equiv.), | 11a, 58 |
| MeCN, 80 °C, 24 h | |||||
| 8 | OEt | 1.0 | 1.0 | CsF (2.0 equiv.), | 11a, 61 |
| MeCN, 50 °C, 24 h | |||||
| 9 | OEt | 1.0 | 1.5 | CsF (2.0 equiv.), | 11a, 76d [70]de |
| MeCN, 50 °C, 24 h | |||||
| 10 | OEt | 1.0 | 2.0 | CsF (3.0 equiv.), | 11a, 65 |
| MeCN, 50 °C, 24 h | |||||
A wide range of multisubstituted 2-pyrones 2 reacted with benzyne generated from o-silylphenyl triflate 9a providing naphthalenes having various functionalities (Fig. 3). For example, 3-bromo-1-methylnaphthalene (11b) was prepared by the reaction of 4-bromo-6-methyl-2-pyrone (2b) with benzyne in good yield. The Diels–Alder reaction and following decarboxylative aromatization of 4-aryl-6-methyl-2-pyrones 2c–2e with benzyne took place, in which lactone intermediates without decarboxylation were not observed. We also succeeded in the efficient synthesis of 1-methyl-3-(4-methyoxyphenyloxy)naphthalene (11f) from 2-pyrone 2f. These results clearly show that diverse naphthalenes can be prepared from 2-pyrones 2, aryne precursor 9a, and arylboronic acids or phenols. Furthermore, 3-substituted 2-pyrones 11g–11i participated in the naphthalene synthesis without damaging the ethoxy, methyl, bromo, iodo, and trifluoromethyl group. We also accomplished naphthalene synthesis using 6-aryl-4-bromo-2-pyrones 2j–2l prepared from the corresponding silyl enolates. It is worth noting that 6-trifluoromethyl-substituted 2-pyrone 2m smoothly reacted with benzyne to provide 1-trifluoromethyl-substituted naphthalene 11m in moderate yield. Since various naphthalenes bearing a range of functional groups were efficiently synthesized from the corresponding 2-pyrones in which further activation was not required for the decarboxylation, diverse naphthalenes would be prepared by the aryne reaction of multisubstituted 2-pyrones prepared from silyl enolates in short steps. Of note, 2-pyrones having electron-withdrawing substituents including bromo and trifluoromethyl groups participated in the naphthalene synthesis.
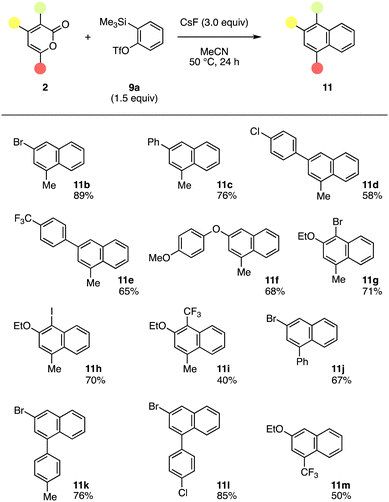 | ||
| Fig. 3 Synthesis of naphthalenes 11 using various 2-pyrones 2. Reactions were performed on a 0.1 mmol scale unless otherwise noted. | ||
Divergent o-silylaryl triflates 9 bearing electron donating or withdrawing substituents smoothly reacted with 4-bromo-6-methyl-2-pyrone (2b) furnishing tri- and tetra-substituted naphthalenes 11n–11t (Fig. 4). When using 3-methoxy-2-(trimethylsilyl)phenyl triflate (9b) as a 3-methoxybenzyne precursor, a regioisomeric mixture of naphthalenes 11n and 11n′ was obtained, where 1-methyl-3-bromo-5-methoxynaphthalene (11n) was a major product in moderate selectivity. We achieved the efficient synthesis of naphthalene 11o by the reaction of 2-pyrone 2b with 4,5-dimethylbenzyne generated from 9c triggered by cesium fluoride. The reaction of 4,5-difluorobenzyne with 2-pyrone 2b also proceeded smoothly to afford naphthalene 11p in a good yield leaving bromo and fluoro groups untouched. Dimethoxy-substituted naphthalene 11q was successfully prepared in good yields using o-silylaryl triflate 9e as a 4,5-dimethoxybenzyne precursor. Ring-fused naphthalenes 11r and 11s were also synthesized by the reaction of 2-pyrone 2b with fused arynes generated from o-silylaryl triflates 9f and 9g, respectively. In addition, 3-bromo-1-methylanthracene (11t) was prepared by the reaction of 2,3-naphthalene in moderate yield, in which overreactions of anthracene 11t also proceeded as a side reaction.
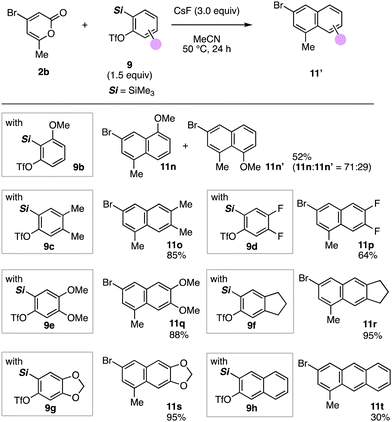 | ||
| Fig. 4 Synthesis of naphthalenes 11 using various o-silylaryl triflates 9. Reactions were performed on a 0.1 mmol scale unless otherwise noted. | ||
The naphthalene synthesis using 2-pyrones and aryne intermediates and triazole formations allowed us to prepare various triazolyl naphthalenes (Fig. 5). Indeed, we realized the synthesis of 4-azido-6-methyl-2-pyrone (2n)10a by azidation of bromide 2b with sodium azide. Treatment of azide 2n with o-silylphenyl triflate 9a with cesium fluoride in acetonitrile at 50 °C provided triazole-substituted naphthalene 12 in an excellent yield by aryne reactions at the 2-pyrone and azide moieties.11 Triazole formation of azide 2n with terminal alkyne 13 catalyzed by copper also took place efficiently to furnish 1,2,3-triazole 2o in high yield.12 Further aryne reaction of 2-pyrone 2o using o-silylaryl triflate 9f smoothly proceeded affording highly functionalized naphthalene 14. The broad scope of the click reaction using various alkynes enabled us to synthesize a wide range of multisubstituted naphthalenes having 1,2,3-triazole moieties from easily available starting materials. This result clearly demonstrates the advantage of this study: diverse pyrones and o-silylaryl triflates with a wide range of functional groups, including alkoxy, fluoro, bromo, iodo, trifluoromethyl, and triazole moieties, are applicable over conventional aryne reactions of pyrones.
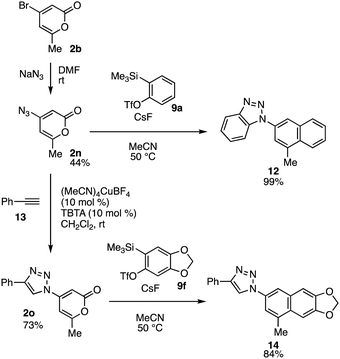 | ||
| Fig. 5 Synthesis of 1,2,3-triazoles 12 and 14. Reactions were performed on a 0.1 mmol scale unless otherwise noted. | ||
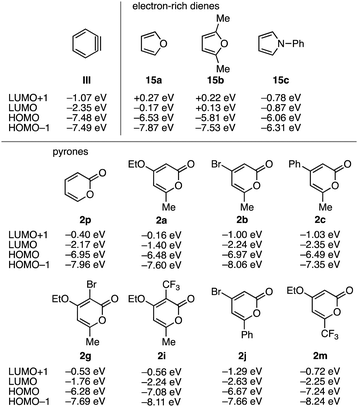
We then investigated theoretical aspects of the aryne reaction of 2-pyrones (Fig. 6 and 7). Optimized structures of benzyne (III), electron-rich dienes 15a–15c, and 2-pyrones 2 having various functionalities were obtained by DFT calculations (B3LYP, 6-311+G(d,p)) (Fig. 6). These results show that electron-deficient benzyne (III) has a low LUMO energy level, and furan (15a), 2,5-dimethylfuran (15b), and N-phenylpyrrole (15c) have high HOMO energy levels, leading to good reactivities as electron-rich dienes in Diels–Alder reactions with a benzyne intermediate. Compared to these electron-rich heteroaromatics 15a–15c, theoretical calculations of substituted 2-pyrones 2 indicate that simple 2-pyrone (2p) has low LUMO and HOMO levels, and the HOMO energy levels are significantly affected by a variety of functional groups such as ethoxy, methyl, bromo, phenyl, and trifluoromethyl groups. To our surprise, the energy gaps between the LUMO of benzyne (III) and the HOMOs of 2-pyrones 2 were significantly smaller than those between the HOMO of benzyne and the LUMOs of 2-pyrones 2. It is worth noting that 2-pyrones 2 bearing not only electron-donating groups but also electron-withdrawing groups such as a trifluoromethyl group reacted with benzyne to furnish the corresponding naphthalenes, suggesting that the efficiencies in the Diels–Alder reaction between benzyne and pyrones and subsequent decarboxylative aromatization are not significantly affected by electron donating and withdrawing functional groups.
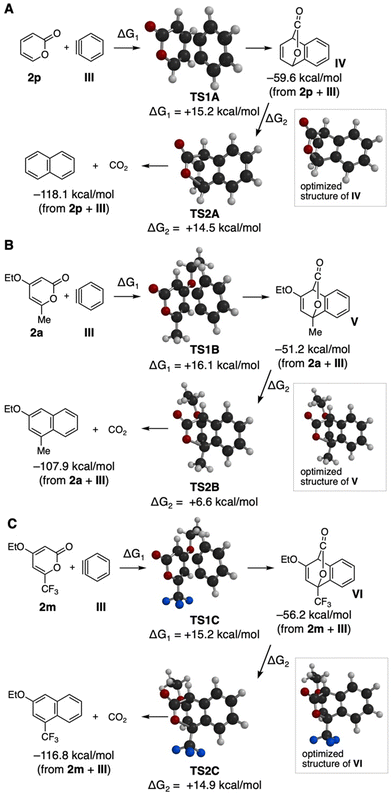 | ||
| Fig. 7 (A) Calculated reaction pathways of the reaction between 2p and III. (B) Calculated reaction pathways of the reaction between 2a and III. (C) Calculated reaction pathways of the reaction between 2m and III. Atoms in TS1A–C and TS2A–C: black = C; grey = H; red = O; blue = F. See the ESI† for details. | ||
Further DFT calculations successfully provided transition state structures TS1A–C and TS2A–C for the reactions between benzyne (III) and 2-pyrones 2p, 2a, and 2m and subsequent decarboxylation (Fig. 7). As a result, the calculated activation energies for the Diels–Alder reactions of benzyne (III) with simple 2-pyrone (2p), 4-ethoxy-6-methyl-2-pyrone (2a), and 4-ethoxy-6-trifluoromethyl-2-pyrone (2m) and subsequent decarboxylations are found to be lower than 20 kcal mol−1, which obviously supports the experimental results that the Diels–Alder reaction proceeded smoothly at room temperature. Judged from the obtained calculated activation energies, the effects of electron-donating and electron-withdrawing functional groups on Diels–Alder reactions and decarboxylations were insignificant. The low calculated activation energies for the following decarboxylative aromatization are in good accordance with the fact that intermediates without decarboxylation were not observed in the aryne reactions of 2-pyrones. These reaction pathways obtained by DFT calculations would serve to predict the efficiencies of aryne reactions providing highly functionalized naphthalenes.
Conclusions
In conclusion, we have developed an efficient method to synthesize multisubstituted naphthalenes from 2-pyrones and o-silylaryl triflates. A wide variety of 2-pyrones were synthesized using easily accessible starting materials such as silyl enolates, arylboronic acids, and phenols through 4-hydroxy-substituted 2-pyrones. Since substituent effects on the aryne reactions of 2-pyrones were not significant and further transformations including triazole formations served in the preparation of diverse 2-pyrones, a wide range of highly functionalized naphthalenes will be prepared from 2-pyrones in a modular synthetic manner. Detailed theoretical calculations supported the reaction mechanisms involving Diels–Alder reaction and decarboxylative aromatization, in which interactions between the HOMOs of 2-pyrones and the LUMO of benzyne would contribute to the [4+2] cycloaddition. Further studies such as the synthesis of diverse triazole-substituted naphthalenes from azide-substituted 2-pyrones are in progress in our group.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Central Glass Co., Ltd. for providing Tf2O. This work was supported by JSPS KAKENHI Grant Number JP22H02086 (S. Y.); The Uehara Memorial Foundation (S. Y.); Tokuyama Science Foundation (S. Y.); The Ube Foundation (S. Y.); and Inamori Research Grants (S. Y.).Notes and references
- (a) S. Makar, T. Saha and S. K. Singh, Naphthalene, a versatile platform in medicinal chemistry: Sky-high perspective, Eur. J. Med. Chem., 2019, 161, 252 CrossRef CAS PubMed; (b) S. V. Bhosale, M. A. Kobaisi, R. W. Jadhav, P. P. Morajkar, L. A. Jones and S. George, Naphthalene diimides: perspectives and promise, Chem. Soc. Rev., 2021, 50, 9845 RSC; (c) A. Saeed, S. Ashraf, M. Aziz, P. A. Channar, S. A. Ejaz, A. Fayyaz, Q. Abbas, F. A. Alasmary, A. M. Karami, A. Tehzeeb, A. Mumtaz and H. R. El-Seedi, Design, synthesis, biochemical and in silico characterization of novel naphthalene-thiourea conjugates as potential and selective inhibitors of alkaline phosphatase, Med. Chem. Res., 2023, 32, 1077 CrossRef CAS PubMed.
- (a) C. B. de Koning, A. L. Rousseau and W. A. L. van Otterlo, Modern methods for the synthesis of substituted naphthalenes, Tetrahedron, 2003, 59, 7 CrossRef CAS; (b) S. Prévost, Regioselective C−H Functionalization of Naphthalenes: Reactivity and Mechanistic Insights, ChemPlusChem, 2020, 85, 476 CrossRef PubMed.
- Modern Aryne Chemistry, ed. A. T. Biju, Wiley-VCH, Weinheim, 2021 Search PubMed.
- (a) P. M. Tadross and B. M. Stoltz, A Comprehensive History of Arynes in Natural Product Total Synthesis, Chem. Rev., 2012, 112, 3550 CrossRef CAS PubMed; (b) A. Bhunia, S. R. Yetra and A. T. Biju, Recent advances in transition-metal-free carbon–carbon and carbon–heteroatom bond-forming reactions using arynes, Chem. Soc. Rev., 2012, 41, 3140 RSC; (c) S. Yoshida and T. Hosoya, The Renaissance and Bright Future of Synthetic Aryne Chemistry, Chem. Lett., 2015, 44, 1450 CrossRef CAS; (d) A. E. Goetz, T. K. Shah and N. K. Garg, Pyridynes and indolynes as building blocks for functionalized heterocycles and natural products, Chem. Commun., 2015, 51, 34 RSC; (e) S. S. Bhojgude, A. Bhunia and A. T. Biju, Employing Arynes in Diels–Alder Reactions and Transition-Metal-Free Multicomponent Coupling and Arylation Reactions, Acc. Chem. Res., 2016, 49, 1658 CrossRef CAS PubMed; (f) J.-A. García-López and M. F. Greaney, Synthesis of biaryls using aryne intermediates, Chem. Soc. Rev., 2016, 45, 6766 RSC; (g) J. Shi, Y. Li and Y. Li, Aryne multifunctionalization with benzdiyne and benztriyne equivalents, Chem. Soc. Rev., 2017, 46, 1707 RSC; (h) F. I. M. Idiris and C. R. Jones, Recent advances in fluoride-free aryne generation from arene precursors, Org. Biomol. Chem., 2017, 15, 9044 RSC; (i) T. Roy and A. T. Biju, Recent advances in molecular rearrangements involving aryne intermediates, Chem. Commun., 2018, 54, 2580 RSC; (j) S. Yoshida, Controlled Reactive Intermediates Enabling Facile Molecular Conjugation, Bull. Chem. Soc. Jpn., 2018, 91, 1293 CrossRef CAS; (k) T. Matsuzawa, S. Yoshida and T. Hosoya, Recent advances in reactions between arynes and organosulfur compounds, Tetrahedron Lett., 2018, 59, 4197 CrossRef CAS; (l) H. Takikawa, A. Nishii, T. Sakai and K. Suzuki, Aryne-based strategy in the total synthesis of naturally occurring polycyclic compounds, Chem. Soc. Rev., 2018, 47, 8030 RSC; (m) Y. Nakamura, S. Yoshida and T. Hosoya, Recent Advances in Synthetic Hetaryne Chemistry, Heterocycles, 2019, 98, 1623 CrossRef; (n) D. B. Werz and A. T. Biju, Uncovering the Neglected Similarities of Arynes and Donor–Acceptor Cyclopropanes, Angew. Chem., Int. Ed., 2020, 59, 3385 CrossRef CAS PubMed.
- (a) M.-X. Zhang, W. Shan, Z. Chen, J. Yin, G.-A. Yu and S. H. Liu, Diels–Alder reactions of arynes in situ generated from DA reaction between bis-1,3-diynes and alkynes, Tetrahedron Lett., 2015, 56, 6833 CrossRef CAS; (b) S. S. Bhojgude, A. Bhunia and A. T. Biju, Employing Arynes in Diels–Alder Reactions and Transition-Metal-Free Multicomponent Coupling and Arylation Reactions, Acc. Chem. Res., 2016, 49, 1658 CrossRef CAS PubMed; (c) F. García, D. Peña, D. Pérez and E. Guitián, Aryne Cycloadditions for the Synthesis of Functional Polyarenes, in Modern Aryne Chemistry, ed. A. T. Biju, Wiley-VCH, Weinheim, 2021, pp. 27–68 Search PubMed.
- (a) C. Bozzo and M. D. Pujol, Deoxygenation of 5,12-Epoxy-5,12-dihydro-5,12-dimethyl-1,4-benzodioxino[2,3-g]isoquinoline with Iron Compounds. Synthesis of Antitumour Agents, Synlett, 2000, 550 CAS; (b) J. L. Marshall, D. Lehnherr, B. D. Lindner and R. R. Tykwinski, Reductive Aromatization/Dearomatization and Elimination Reactions to Access Conjugated Polycyclic Hydrocarbons, Heteroacenes, and Cumulenes, ChemPlusChem, 2017, 82, 967 CrossRef CAS PubMed; (c) E. M. Serum, S. Selvakumar, N. Zimmermann and M. P. Sibi, Valorization of 2,5-furandicarboxylic acid. Diels–Alder reactions with benzyne, Green Chem., 2018, 20, 1448 RSC.
- (a) G. Wittig and R. W. Hoffmann, Dehydrobenzol aus 1.2.3-Benzothiadiazol-1.1-dioxyd, Chem. Ber., 1962, 95, 2718 CrossRef CAS; (b) C. May and C. J. Moody, A concise synthesis of the antitumour alkaloid ellipticine, J. Chem. Soc., Chem. Commun., 1984, 926 RSC; (c) C. J. Moody, Diels–Alder reactivity of pyrano[3,4-b]indol-3-ones, stable analogues of indole-2,3-quinodimethanes, J. Chem. Soc., Perkin Trans. 1, 1985, 2505 RSC; (d) J. F. P. Andrews, P. M. Jackson and C. J. Moody, Pyrrole-2,3-quinodimethane analogues in the synthesis of indoles. Part 2.1 Synthesis and Diels–Alder reactions of 1,6-dihydropyrano[4,3-b]pyrrol-6(1H)-ones, Tetrahedron, 1993, 49, 7353 CrossRef CAS; (e) S. Escudero, D. Pérez, E. Guitián and L. Castedo, A New Convergent Approach to the Polycyclic Framework of Dynemicin A, J. Org. Chem., 1997, 62, 3028 CrossRef CAS PubMed; (f) S. Escudero, D. Pérez, E. Guitián and L. Castedo, [4+2] Cycloadditions between 2-pyrones and benzyne. Application to the synthesis of binaphthyls, Tetrahedron Lett., 1997, 38, 5375 CrossRef CAS; (g) Y. Kuninobu, H. Takata, A. Kawata and K. Takai, Rhenium-Catalyzed Synthesis of Multisubstituted Aromatic Compounds via C−C Single-Bond Cleavage, Org. Lett., 2008, 10, 3133 CrossRef CAS PubMed; (h) P.-P. Yeh, D. S. B. Daniels, D. B. Cordes, A. M. Z. Slawin and A. D. Smith, Isothiourea-Mediated One-Pot Synthesis of Trifluoromethyl Substituted 2-Pyrones, Org. Lett., 2014, 16, 964 CrossRef CAS PubMed; (i) T. K. Shah, J. M. Medina and N. K. Garg, Expanding the Strained Alkyne Toolbox: Generation and Utility of Oxygen-Containing Strained Alkynes, J. Am. Chem. Soc., 2016, 138, 4948 CrossRef CAS PubMed; (j) J. Huang, L. Li, H. Chen, T. Xiao, Y. He and L. Zhou, Synthesis of 3-Aryl-2-pyrones by Palladium-Catalyzed Cross-Coupling of Aryl Iodides with Cyclic Vinyldiazo Ester, J. Org. Chem., 2017, 82, 9204 CrossRef CAS PubMed; (k) A. Szlapa-Kula, S. Kula, M. Filapek, A. Fabianczyk, K. Bujak, M. Siwy, S. Kotowicz, H. Janeczek, K. Smolarek, S. Maćkowski, S. Krompiec and E. Schab-Balcerzak, Synthesis, electrochemistry and optical properties with electroluminescence ability of new multisubstituted naphthalene derivatives with thiophene and carbazole motifs, J. Lumin., 2018, 196, 244 CrossRef CAS; (l) B. S. Chinta, D. Lee and T. R. Hoye, Coumarin (5,6-Benzo-2-pyrone) Trapping of an HDDA-Benzyne, Org. Lett., 2021, 23, 2189 CrossRef CAS PubMed.
- (a) K. Afarinkia, V. Vinader, T. D. Nelson and G. H. Posner, Diels–Alder cycloadditions of 2-pyrones and 2-pyridones, Tetrahedron, 1992, 48, 9111 CrossRef CAS; (b) G. Huang, C. Kouklovsky and A. de la Torre, Inverse-Electron-Demand Diels–Alder Reactions of 2-Pyrones: Bridged Lactones and Beyond, Chem. – Eur. J., 2021, 27, 4760 CrossRef CAS PubMed.
- (a) T. Meguro, S. Chen, K. Kanemoto, S. Yoshida and T. Hosoya, Modular Synthesis of Unsymmetrical Doubly-ring-fused Benzene Derivatives Based on a Sequential Ring Construction Strategy Using Oxadiazinones as a Platform Molecule, Chem. Lett., 2019, 48, 582 CrossRef CAS; (b) E. R. Darzi, J. S. Barber and N. K. Garg, Cyclic Alkyne Approach to Heteroatom-Containing Polycyclic Aromatic Hydrocarbon Scaffolds, Angew. Chem., Int. Ed., 2019, 58, 9419 CrossRef CAS PubMed; (c) M. Ramirez, E. R. Darzi, J. S. Donaldson, K. N. Houk and N. K. Garg, Cycloaddition Cascades of Strained Alkynes and Oxadiazinones, Angew. Chem., Int. Ed., 2021, 60, 18201 CrossRef CAS PubMed.
- (a) M. Cervera, M. Moreno-Manas and R. Pleixats, 4-amino-6-methyl-2H-pyran-2-one. Preparation and reactions with aromatic aldehyde, Tetrahedron, 1990, 46, 7885 CrossRef CAS; (b) M. Sato, J.-i Sakaki, Y. Sugita, S. Yasuda, H. Sakoda and C. Kaneko, Two lactone formation reactions from 1,3-dioxin-4-ones having hydroxyalkyl group at the 6-position: Difference in ring opening and closure, Tetrahedron, 1991, 47, 5689 CrossRef CAS; (c) J. V. N. V. Prasad, K. S. Para, P. J. Tummino, D. Ferguson, T. J. McQuade, E. A. Lunney, S. T. Rapundalo, B. L. Batley, G. Hingorani, J. M. Domagala, S. J. Gracheck, T. N. Bhat, B. Liu, E. T. Baldwin, J. W. Erickson and T. K. Sawyer, Nonpeptidic Potent HIV-1 Protease Inhibitors: (4-Hydroxy-6-phenyl-2-oxo-2H-pyran-3-yl)thiomethanes That Span P1-P2′ Subsites in a Unique Mode of Binding, J. Med. Chem., 1995, 38, 898 CrossRef CAS PubMed; (d) S. Gatti McArthur, E. Goetschi, W. S. Palmer, J. Wichmann and T. J. Woltering, Acetylenyl-pyrazolo-pyrimidine Derivatives as mGluR2 Antagonists, WO2006099972A1, 2006; (e) I. J. S. Fairlamb, L. R. Marrison, J. M. Dickinson, F.-J. Lu and J. P. Schmidt, 2-Pyrones possessing antimicrobial and cytotoxic activities, Bioorg. Med. Chem., 2004, 12, 4285 CrossRef CAS PubMed; (f) T. Shinozuka, K. Shimada, S. Matsui, T. Yamane, M. Ama, T. Fukuda, M. Taki, Y. Takeda, E. Otsuka, M. Yamato and S. Naito, Arylamine based cathepsin K inhibitors: Investigating P3 heterocyclic substituents, Bioorg. Med. Chem., 2006, 14, 6807 CrossRef CAS PubMed; (g) S. L. Clarke and G. P. McGlacken, Access to trifluoromethylated 4-alkoxy-2-pyrones, pyridones and quinolones, Tetrahedron, 2015, 71, 2906 CrossRef CAS; (h) K. Mackey, L. M. Pardo, A. M. Prendergast, M.-T. Nolan, L. M. Bateman and G. P. McGlacken, Cyclization of 4-Phenoxy-2-coumarins and 2-Pyrones via a Double C–H Activation, Org. Lett., 2016, 18, 2540 CrossRef CAS PubMed; (i) Y. Zheng, S. Dong, K. Xu, D. Liu and W. Zhang, Pd-Catalyzed Asymmetric Allylic Substitution Cascade of Substituted 4-Hydroxy-2H-pyrones with meso-Allyl Dicarbonates, Org. Lett., 2022, 24, 3440 CrossRef CAS PubMed; (j) R. Samala, M. K. Basu and K. Mukkanti, Regioselective functionalization of pyrones: Facile synthesis of 6-styrylpyrones via KHMDS-mediated aldol condensation, Tetrahedron Lett., 2022, 88, 153574 CrossRef CAS; (k) M. Baak, B. Jaun, F. Belaj and R. Neier, Conformations of 4-tert-Butyloxy-, 4-(Trimethylsilyl)oxy- and 4-(Trimethylstannyl)oxy-6-methyl-2H-pyran-2-ones in the Crystalline State and in Solution, Helv. Chim. Acta, 2022, 105, e202200029 CrossRef CAS.
- F. Shi, J. P. Waldo, Y. Chen and R. C. Larock, Benzyne Click Chemistry: Synthesis of Benzotriazoles from Benzynes and Azides, Org. Lett., 2008, 10, 2409 CrossRef CAS PubMed.
-
(a) C. W. Tornøe, C. Christensen and M. Meldal, Peptidotriazoles on Solid Phase:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides, J. Org. Chem., 2002, 67, 3057 CrossRef PubMed;
(b) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS;
(c) T. R. Chan, R. Hilgraf, K. B. Sharpless and V. V. Fokin, Polytriazoles as Copper(I)-Stabilizing Ligands in Catalysis, Org. Lett., 2004, 6, 2853 CrossRef CAS PubMed.
[1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides, J. Org. Chem., 2002, 67, 3057 CrossRef PubMed;
(b) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS;
(c) T. R. Chan, R. Hilgraf, K. B. Sharpless and V. V. Fokin, Polytriazoles as Copper(I)-Stabilizing Ligands in Catalysis, Org. Lett., 2004, 6, 2853 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nj03831a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2023 |