Open Access Article
Open Access ArticleQuantification of the hydride donor abilities of NADH, NADPH, and BH3CN− in water†
Robert J.
Mayer
 *a and
Joseph
Moran
*a and
Joseph
Moran
 *ab
*ab
aInstitut de Science et d'Ingénierie Supramoléculaires (ISIS), CNRS UMR 7006, Université de Strasbourg, 8 Allée Gaspard Monge, 67000 Strasbourg, France. E-mail: rjmayer@unistra.fr; moran@unistra.fr
bInstitut Universitaire de France (IUF), 75005 Paris, France
First published on 6th December 2022
Abstract
The nucleophilic reactivities of the hydride donors NADH, NADPH, and BH3CN− in water were quantified using kinetic measurements with benzhydrylium ions as reference electrophiles. All three hydride donors were found to possess almost identical nucleophilic reactivities, providing a potential explanation for why they are involved in similar transformations in biochemistry and organic synthesis, respectively.
NADH, NADPH, and BH3CN− are the hydride donors of choice for reduction and reductive amination reactions in aqueous solutions in biochemistry and organic chemistry, respectively (Scheme 1A).1,2 However, despite their similar reactivity profiles, no study has yet directly compared their hydride donor abilities experimentally.
 | ||
| Scheme 1 (A) Reduction and reductive amination reactions enabled by NAD(P)H and BH3CN−. (B) Methods for quantification of hydride donor strength. | ||
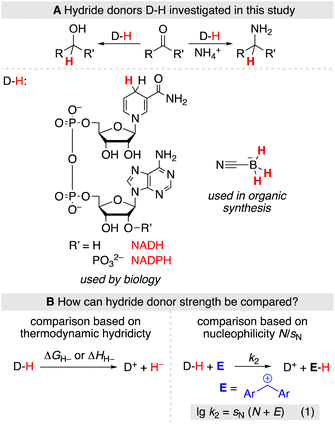
The strength of hydride donors can be compared based on thermodynamic hydricities (ΔGH− and ΔHH−), which are derived from equilibrium or calorimetric measurements and combined within thermodynamic cycles (Scheme 1B, left).3 Alternatively, hydride donor strength can be quantified based on the nucleophilicity parameters popularized by Mayr and coworkers using a linear-free energy relationship (Scheme 1B, right, eqn (1))4 which has been applied to characterize a variety of hydride donors.5 Eqn (1) allows to predict absolute rate constants k2 from the solvent-dependent nucleophilicity parameter N and susceptibility sN as well as the solvent-independent electrophilicity parameter E.
The choice of methods for an experimental comparison of the hydride donor strength of NAD(P)H and BH3CN− is delicate due to the chemically vastly different nature of both species. Thermochemical methods have been extensively applied to compare the hydride donor strength of various NADH analogues, typically in acetonitrile solutions.3,6 However, due to the instability of BH2CN,7 the oxidation product of BH3CN−, equilibrium studies are hampered. The only tentative thermochemical comparison of the hydride donor strength of BH3CN− with NADPH thus comes from quantum-chemically calculated enthalpic hydricities considering acetonitrile solvation by an implicit solvation model (ΔHH− (NADPH) = 77.1 kcal mol−1, ΔHH− (BH3CN−) = 75.3 kcal mol−1).3,8
In contrast, the application of kinetic methods for determining hydride donor strength has fewer experimental limitations. As such, they do not have specific solvent requirements, nor are they limited by the stability of the products. However, using kinetic methods, the nucleophilicity parameter N for the BH3CN− anion in water was previously only approximated from literature data of its reaction with two triarylcarbenium ions,5a,9 using an estimated sN parameter. Likewise, some dihydropyridines have previously been studied by Mayr and Richter in organic solvents and aqueous mixtures,5b but the nucleophilic reactivities of NADH and NADPH itself remain unknown. In this work, we now set out to determine the nucleophilic reactivity of NADH, NADPH, and BH3CN− in aqueous solution using a consistent set of benzhydrylium ions as reference electrophiles.10
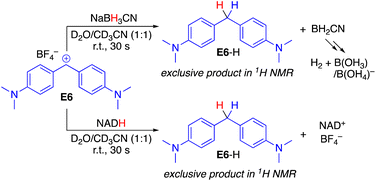
Initially, the reaction products were investigated to verify that benzhydrylium ions E react with both NADH and BH3CN− in the desired hydride transfer and not a side reaction (Scheme 2). When mixing equimolar amounts of the benzhydrylium ion E6 with NADH or analogously NaBH3CN in a D2O/CD3CN mixture, rapid decolorization of the carbocation was observed. 1H and 1H,13C-HSQC NMR analysis of the reaction products with both reductants indicated clean hydride transfer to yield the diarylmethane E6-H (see the ESI for the spectroscopic analysis†). In the case of NADH, oxidation to NAD+ occurred, whereas in the case of NaBH3CN, oxidation to boric acid and borate was observed, which are formed due to the hydrolysis of BH2CN (see the ESI pp. S4–S7†).7
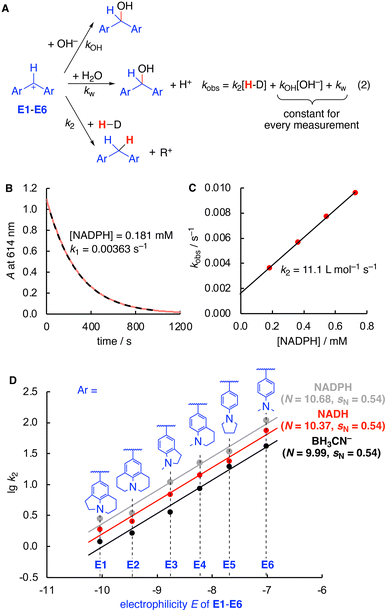
Having established the identity of the reactions of E with both hydride donors, kinetic studies were next performed. Nucleophilicity parameters were determined using a consistent set of amino-substituted benzhydrylium ions E as reference electrophiles (Fig. 1).10 UV/Vis spectroscopy was used to study the kinetics of the reactions of hydride donors with the colored benzhydrylium ions E1–E6 in an aqueous buffered solution at pH 7 and 20 °C. The disappearance of the color of the benzhydrylium ions E is due to three competing reactions (Fig. 1A): the reaction with either hydroxide or water, as well as the actual reaction of interest with the hydride donor. Due to the large excess of the hydride donor over the electrophiles (>10 equiv.), pseudo-first order conditions resulted, from which the pseudo-first order rate constants kobs were determined by non-linear fitting (Fig. 1B). As the kinetics were measured in 0.05 M phosphate buffer at pH 7, the concentration of hydroxide and water are identical for all measurements.11
Accordingly, a correlation of kobs with the nucleophile concentration was found to be linear with the slope corresponding to k2 and the intercept with the ordinate to kOH[OH−] + kw (Fig. 1A, eqn (2) and Fig. 1C). Finally, the correlations of lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k2 (Table 1) and the electrophilicity parameters E of E1–E6 afforded linear relationships, the slope of which corresponds to sN. The nucleophilicity N is obtained from the intercept with the abscissa, as according to eqn (1) at lg
k2 (Table 1) and the electrophilicity parameters E of E1–E6 afforded linear relationships, the slope of which corresponds to sN. The nucleophilicity N is obtained from the intercept with the abscissa, as according to eqn (1) at lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k2 = 0 the electrophilicity E equals the −N (Fig. 1D).
k2 = 0 the electrophilicity E equals the −N (Fig. 1D).
As evident from both the comparison of the absolute rate constants in Table 1 as well as the nucleophilicity parameters in Fig. 1D, NADH, NADPH, and BH3CN− possess a very similar nucleophilic reactivity for hydride transfer toward carbenium ions with rate constants between BH3CN− and NADPH differing by factors of about 2.5 in all cases, except E3 where the difference is around a factor of 3. Despite large methodological differences (experimental vs. computational, water vs. MeCN, kinetic vs. thermochemical values), the observed similarity in kinetic hydride donor strength in water is in line with the similar enthalpic hydricities obtained computationally in MeCN.3 Notably, NADPH is kinetically a slightly better hydride donor compared to NADH by approx. a factor of 1.5 in rate constants. This small but significant difference might be attributed to conformational differences of both species in solution due to the presence or absence of the phosphate group that can be involved in intramolecular hydrogen bonding.12
Having established the nucleophilic reactivity of NADH, NADPH and BH3CN−, a comparison with other previously investigated hydride donors now becomes possible (Fig. 2). The nucleophilic reactivities of NADH and NADPH are approximately one N-unit lower than that of the frequently employed analogue N-benzyl-1,4-dihydronicotineamide (BDNA, N = 11.36, sN = 0.66), which was previously studied in a water-acetonitrile mixture due to its limited solubility in pure water.5b The close similarity of the nucleophilic reactivities of BDNA and NADH, which differ only by the presence of a benzyl group versus an adenine dinucleotide, illustrates that substitution at nitrogen does not largely alter the reactivity of the dihydronicotinamide. Due to their rapid hydrolysis, a comparison of the reactivity of BH3CN− with other borohydrides is difficult. However, previously the nucleophilicity of BH3CN− was reported in DMSO, where it was found to be around 1.5 N units higher.
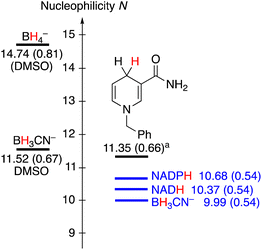 | ||
Fig. 2 Comparison of the nucleophilicity parameters N of different hydride donors (sN parameters in brackets). a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Studied in 90% water/10% acetonitrile. Studied in 90% water/10% acetonitrile. | ||
On the one hand, the very similar nucleophilicity of NAD(P)H and BH3CN− that we have observed in our experiments is surprising given the large reactivity range of hydride donors previously characterized in organic solvents.5a,13 On the other hand, this observation might quantitatively rationalize why both NAD(P)H and BH3CN− display similar reactivity in the contexts of biochemistry and organic synthesis, respectively, enabling reductions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N functionalities.1,2,14 Future research will show if the close similarity in the nucleophilicity of NADH and BH3CN− found toward reference carbenium ions like E still holds with neutral electrophiles like carbonyl compounds, where solvation effects on the electrophile as well as acid/base catalysis might become relevant.
N functionalities.1,2,14 Future research will show if the close similarity in the nucleophilicity of NADH and BH3CN− found toward reference carbenium ions like E still holds with neutral electrophiles like carbonyl compounds, where solvation effects on the electrophile as well as acid/base catalysis might become relevant.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
R. J. M. thanks the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) for a fellowship (MA 9687/1-1). This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement no. 101001752). The authors thank Prof. Herbert Mayr for helpful discussion.References
- J. E. McMurry and T. P. Begley, The Organic Chemistry of Biological Pathways, Roberts and Company, Greenwood Village, CO, ed. 2nd, 2016 Search PubMed.
- R. F. Borch, M. D. Bernstein and H. D. Durst, J. Am. Chem. Soc., 1971, 93, 2897–2904 CrossRef CAS.
- For a review of methods including a compilation of thermodynamic values, see: S. Ilic, A. Alherz, C. B. Musgrave and K. D. Glusac, Chem. Soc. Rev., 2018, 47, 2809–2836 RSC.
- (a) H. Mayr and M. Patz, Angew. Chem., Int. Ed. Engl., 1994, 33, 938–957 CrossRef; (b) H. Mayr, T. Bug, M. F. Gotta, N. Hering, B. Irrgang, B. Janker, B. Kempf, R. Loos, A. R. Ofial, G. Remennikov and H. Schimmel, J. Am. Chem. Soc., 2001, 123, 9500–9512 CrossRef CAS.
- (a) M. Horn, L. H. Schappele, G. Lang-Wittkowski, H. Mayr and A. R. Ofial, Chem. – Eur. J., 2013, 19, 249–263 CrossRef CAS; (b) D. Richter and H. Mayr, Angew. Chem., Int. Ed., 2009, 48, 1958–1961 CrossRef CAS PubMed; (c) A. Alherz, C.-H. Lim, J. T. Hynes and C. B. Musgrave, J. Phys. Chem. B, 2017, 122, 1278–1288 CrossRef PubMed; (d) J. Zhang, J.-D. Yang and J.-P. Cheng, Angew. Chem., Int. Ed., 2019, 58, 5983–5987 CrossRef CAS PubMed.
- (a) S. Yasui and A. Ohno, Bioorg. Chem., 1986, 14, 70–96 CrossRef CAS; (b) A. Anne and J. Moiroux, J. Org. Chem., 1990, 55, 4608–4614 CrossRef CAS; (c) M. M. Kreevoy, D. Ostovic, I. S. H. Lee, D. A. Binder and G. W. King, J. Am. Chem. Soc., 1988, 110, 524–530 CrossRef CAS; (d) X.-Q. Zhu, Y.-Y. Mu and X.-T. Li, J. Phys. Chem. B, 2011, 115, 14794–14811 CrossRef CAS.
- E. C. Evers, W. O. Freitag, J. N. Keith, W. A. Kriner, A. G. MacDiarmid and S. Sujishi, J. Am. Chem. Soc., 1959, 81, 4493–4496 CrossRef CAS.
- J. Shi, X.-Y. Huang, H.-J. Wang and Y. Fu, J. Chem. Inf. Model., 2011, 52, 63–75 CrossRef PubMed.
- C. A. Bunton, S. K. Huang and C. H. Paik, Tetrahedron Lett., 1976, 18, 1445–1448 CrossRef.
- R. J. Mayer, N. Hampel, P. Mayer, A. R. Ofial and H. Mayr, Eur. J. Org. Chem., 2019, 412–421 CrossRef CAS.
- The pH-dependency of the reaction was tested on the reaction of E1 with BH3CN–. Identical rate constants k2 were obtained at pH 7 and pH 4, the latter pH value corresponding to that relevant for synthetic use of BH3CN– in the reduction of carbonyl groups (ref. 2). Similar studies with NAD(P)H at lower pH are hampered by their rapid hydrolysis at acidic pH.
- X. Cao, L. Wu, J. Zhang and M. Dolg, J. Comput. Chem., 2020, 41, 305–316 CrossRef CAS.
- There is a freely accessible database of reactivity parameters (E, N, and sN) available under: https://www.cup.lmu.de/oc/mayr/reaktionsdatenbank2/.
- We have recently used BH3CN– as an analog of NADH to investigate reduction and reductive aminations of biological keto acids: R. J. Mayer and J. Moran, Angew. Chem., Int. Ed., 2022, 61, e202212237 ( Angew. Chem. , 2022 , 134 , e202212237 ) CAS.
Footnote |
| † Electronic supplementary information (ESI) available: experimental procedures, NMR spectra, kinetic data and correlations. See DOI: https://doi.org/10.1039/d2ob02041f |
| This journal is © The Royal Society of Chemistry 2023 |