Modulating the electronic configuration of Co species in MOF/MXene nanosheet derived Co-based mixed spinel oxides for an efficient oxygen evolution reaction†
Chuming
Xu‡
ab,
Xifeng
Yang‡
ab,
Shuang
Li
*ab,
Keke
Li
ab,
Benjun
Xi
b,
Qing-Wen
Han
b,
Ya-Pan
Wu
 ab,
Xue-Qian
Wu
ab,
Ru-an
Chi
b and
Dong-Sheng
Li
ab,
Xue-Qian
Wu
ab,
Ru-an
Chi
b and
Dong-Sheng
Li
 *ab
*ab
aCollege of Materials and Chemical Engineering, Key Laboratory of Inorganic Nonmetallic Crystalline and Energy Conversion Materials, China Three Gorges University, Yichang, 443002, P. R. China. E-mail: lishmail@126.com; lidongshengl@126.com
bHubei Three Gorges Laboratory, Yichang, Hubei 443007, P. R. China
First published on 16th November 2022
Abstract
The electronic configuration of Co cations at octahedral (Oct) sites plays a crucial role in the catalytic activity of Co3O4-based spinel oxides toward the oxygen evolution reaction (OER). However, there are few reports on modulating the electronic configuration of partial CoOct3+ (t62ge0g) to CoOct2+ (t62ge1g) for further promoting their OER performances. Herein, a metal–organic framework (MOF)/MXene composite material pyrolysis–reorganization strategy is developed to obtain heterogeneous Co-based mixed spinel oxides, Co3O4/Co2TiO4. By regulating the pyrolysis temperature, the mesoporous structures and the electronic configuration of CoOct cations of mixed spinel oxides can be simultaneously optimized, leading to exceptional OER performances with low overpotentials of 280 mV on a glassy carbon electrode and 260 mV on Ni foam at 10 mA cm−2 as well as good stability in alkaline solution. The synergistic catalytic effect between CoOct3+ in Co3O4 and CoOct2+ in Co2TiO4 is shown to be crucial for improving the OER activity. This finding will provide a new pathway for promoting the OER activity of Co-based spinel oxides.
Introduction
The oxygen-evolution reaction (OER) is an important half-reaction in electrolytic water splitting to hydrogen and metal–air batteries, while its slow kinetics and large reaction energy barriers greatly limit the overall efficiency of these clean energy systems.1 Exploring an excellent electrocatalytic OER catalyst is a key point to solve these problems.2–4 It is well known that noble metal catalysts such as IrO2 and RuO2 are considered to be the best OER electrocatalysts, but their high instability in acidic/alkaline media and high cost severely restrict their application.5 In virtue of low cost, environmental friendliness, and ready tunability, cobalt-based spinel oxides have been widely studied as substitutes for noble metal-based electrocatalysts for the OER.6–9 They can be described as AB2O4, where oxygen anions are arranged in a cubic close-packed lattice with metal ions located in tetrahedral (Td) and octahedral (Oct) interstices.10 A general principle demonstrates that the redox activity of Co cations in Oct sites is crucial for the OER process. This could be attributed to the high-lying eg orbital directly pointing to six adjacent O in octahedral coordination, thus creating a strong spatial overlap with an O 2p orbital.10–13 Particularly, the moderate eg occupancy (eg ≈ 1) of the metal cation at the Oct sites can balance the competition between rate-limiting steps to realize drastically improved OER activity.14–16 These findings inspire more in-depth research on Co-based spinel oxides, such as regulating the composition and valence state, to tune their eg occupancy.Co3O4, a typical Co-based spinel oxide, is highly attractive due to its competitive activity. The Td and Oct-occupied Co cations in Co3O4 would prefer the electronic configuration of CoTd2+ (e4gt32g) and CoOct3+ (t62ge0g), respectively.17 The strong orbital overlap between the octahedral Co and O is responsible for the excellent OER activity.18 To date, various synthetic strategies have been explored to modulate the electronic configuration of Co species for advanced OER activity.19,20 Among them, using metal–organic framework (MOF) materials as precursors to prepare Co3O4-based catalysts has great advantages in terms of both regulable architectures and chemical composition.21 For example, Lou's group employed Co-based zeolitic imidazolate framework-67 (ZIF-67) nanoplates as the precursors for the preparation of metal doped Co3O4 electrocatalysts with improved OER activity.22 They also synthesized Co3O4/Co–Fe oxide double-shelled nanoboxes by a novel MOF hybrid-assisted approach, achieving excellent OER performance.23 Wang et al. reported the controllable pryolysis of bimetallic Mo–Co Prussian blue analogue nanoframes to generate interconnected Co3O4–Mo2N heterostructures with superior OER activity.24 Besides, Wang's group revealed that CoOct2+ sites exhibit better activity than CoTd2+.25 However, more attention is currently focused on tuning the ratio of CoTd2+/CoOct3+ in Co3O4 or functionalizing it with other heteroatoms/metal compounds,26–31 and the perspective of introducing partial CoOct2+ (t62ge1g) in Co3O4 for further promoting the OER performance has been long ignored. Mixed Co-based spinel oxides assembled from normal and inverse spinel-structures contain different coordination environments and oxidation states of transition metals, providing them tunable geometrical configurations to optimize OER activity. Therefore, one or more components are introduced into MOFs as co-precursors to derive mixed Co3O4-based spinel oxides containing CoOct2+ and CoOct3+ species which are of great significance in regulating OER performance.
The Ti3C2Tx nanosheet material (Tx stands for the surface F, OH and O terminations), one of the most widely studied two-dimensional early transition metal carbides (labeled MXenes), with excellent metallic conductivity, ultrathin structure and rich surface negative groups, has been considered as a promising substrate. The strategy of combining Ti3C2Tx nanosheets with various active materials such as SnO2, LDH and Co(OH)2 to improve electrochemical performances was proposed.32–34 More recently, we have also fabricated strongly coupled Ti3C2Tx/Co-MOF hybrid nanosheets by a simple solvothermal process.35 Inspired by this finding, and considering Ti3C2Tx can be pyrolysed to TiO2 under certain temperature and atmosphere, the Co3O4 derived from a Co-MOF might react with TiO2 to generate the inverse spinel cobalt-orthotitanate Co2TiO4 (CoTd2+[TiOh4+CoOh2+]O4). Therefore, the tightly coupled Co-MOF/Ti3C2Tx composite is expected to be a promising co-precursor to derive the mixed Co3O4/Co2TiO4 spinel oxide, which would be an ideal material to study the role of CoOct2+ (t62ge1g) in the OER activity of Co3O4.
Bearing these aspects in mind, herein, we for the first time report the synthesis of Co3O4/Co2TiO4 mixed spinel oxides via the pyrolysis–reorganization strategy of Co-MOF/Ti3C2Tx hybrid nanosheets. Detailed analyses revealed that the pore size and ratio of Co3O4 and Co2TiO4 can be effectively tuned by modulating the pyrolysis temperature, thereby realizing the effective regulation of OER activity through structural and composition aspects. From the viewpoint of composition, the tuning of temperature can lead to the partial conversion of CoOct3+ (t62ge0g) to CoOct2+ (t62ge1g). Benefiting from the mesoporous structures and electronic configuration regulation, efficient OER activity was achieved for the mixed spinel oxides. This work shows the promising application of Co3O4/Co2TiO4 mixed spinel oxides in the OER and also provides a new avenue for the application of MOF/MXene composites.
Experimental section
Materials
Ti3AlC2 was purchased from Laizhou Kai Kai Ceramic Materials Co. Ltd. Cobalt chloride hexahydrate (CoCl2·6H2O), terephthalic acid (1,4-BDC), N,N-dimethylformamide (DMF), Nafion solution (5 wt%) and 40% hydrofluoric acid (HF) were purchased from Macklin Chemical Reagent. Ethanol (CH3CH2OH) and potassium hydroxide (KOH) were purchased from Sinopharm Chemical Reagent Co. Ltd. All reagents were of analytical grade and were used without further purification.Preparation of CoTiOx-T catalysts
The Co–BDC/Ti3C2Tx composite was prepared according to the method recently reported by our group.35 Then, the composite was treated in a tube furnace under a normal nitrogen atmosphere at 550, 650, 750, 800 and 900 °C, respectively. Each sample was treated for 3 h, with a heating rate of 5 °C min−1, and naturally cooled to room temperature to obtain CoTiOx-550, CoTiOx-650, CoTiOx-750, CoTiOx-800 and CoTiOx-900.Electrochemical measurements
The electrochemical measurements were performed on an electrochemical workstation (CHI660E) at room temperature using a standard three-electrode in 1.0 M KOH electrolyte. Hg/HgO was used as the reference electrode. The as-prepared samples were coated on glassy carbon electrode (GC) and nickel foam (NF) substrates with different loading amounts as the working electrodes, respectively. For the GC electrode, a platinum wire was used as the counter electrode. 4 mg of sample and 30 μL Nafion solution (5 wt%) were dispersed in 1 mL of water/ethanol mixed solution (volume ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) by sonicating for 40 min to form a homogeneous ink. 5 μL ink was loaded at 0.285 mg cm−2 onto the glassy carbon electrode (3 mm diameter). For nickel foam substrates, a platinum sheet was applied as the counter electrode. The sample, acetylene black and PTFE in a mass ratio of 85
1) by sonicating for 40 min to form a homogeneous ink. 5 μL ink was loaded at 0.285 mg cm−2 onto the glassy carbon electrode (3 mm diameter). For nickel foam substrates, a platinum sheet was applied as the counter electrode. The sample, acetylene black and PTFE in a mass ratio of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 were mixed to form a homogeneous slurry with the assistance of ethanol. Then the slurry was coated on the NF to obtain a working electrode, with a loading amount of 10 mg cm−2. Cyclic voltammetry (CV) and linear sweep voltammetry (LSV) were performed at a scan rate of 30 mV s−1. Electrochemically active surface area (ECSA) was determined from double-layer capacitance using CV in the potential range between 0.15 and 0.25 V (vs. Hg/HgO) with scan speeds of 20, 40, 60, 80 and 100 mV s−1. By determining the ΔJ (Ja − Jc) at 0.2 V (vs. Hg/HgO) against the scan rate, the half of the linear slope was applied to determine the ECSA. Electrochemical impedance spectroscopy was performed in the frequency range from 0.1 to 100 kHz at 0.6 V (vs. Hg/HgO). The stability of the catalyst was determined using constant voltage–time current (I–T) data with a current density of 10 mA cm−2. All potentials in OER tests were calibrated to the reversible hydrogen electrode (RHE) based on the formula ERHE = EHg/HgO + 0.098 V + 0.0591pH (pH = 14). All potentials are given without IR correction.
5 were mixed to form a homogeneous slurry with the assistance of ethanol. Then the slurry was coated on the NF to obtain a working electrode, with a loading amount of 10 mg cm−2. Cyclic voltammetry (CV) and linear sweep voltammetry (LSV) were performed at a scan rate of 30 mV s−1. Electrochemically active surface area (ECSA) was determined from double-layer capacitance using CV in the potential range between 0.15 and 0.25 V (vs. Hg/HgO) with scan speeds of 20, 40, 60, 80 and 100 mV s−1. By determining the ΔJ (Ja − Jc) at 0.2 V (vs. Hg/HgO) against the scan rate, the half of the linear slope was applied to determine the ECSA. Electrochemical impedance spectroscopy was performed in the frequency range from 0.1 to 100 kHz at 0.6 V (vs. Hg/HgO). The stability of the catalyst was determined using constant voltage–time current (I–T) data with a current density of 10 mA cm−2. All potentials in OER tests were calibrated to the reversible hydrogen electrode (RHE) based on the formula ERHE = EHg/HgO + 0.098 V + 0.0591pH (pH = 14). All potentials are given without IR correction.
Characterization
X-ray diffraction (XRD) was studied on an Ultima IV with Cu Kα radiation (λ = 1.5406 Å). The microstructure was analysed by transmission electron microscopy (TEM, JEM-2100Plus) and scanning electron microscopy (SEM, JSM-7500F). Thermogravimetric analysis was performed on a Synchronous thermal analyzer (STA 449 F5). The specific surface areas of the samples were measured using N2 sorption–desorption isotherms, based on the Brunauer–Emmett–Teller model (BET; ASAP 2020). X-ray photoelectron spectra (XPS) were acquired on an ESCALAB250XI. Raman spectroscopy was performed on a Thermo Scientific DXR.Results and discussion
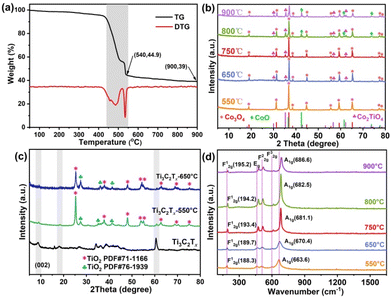
The preparation process of the mixed Co-based spinel oxides is illustrated in Fig. S1.† First, the Co–BDC/Ti3C2Tx hybrid nanosheets were fabricated using a solvothermal strategy recently reported by our group.35 The obtained precursor was then pyrolyzed into mixed Co-based spinel oxides under an ordinary nitrogen atmosphere. In order to understand the pyrolysis process of the Co–BDC/Ti3C2Tx precursor, the TG–DTG curve was first obtained. As shown in Fig. 1a, a slight weight loss is observed before 420 °C, corresponding to the escape of physically adsorbed water molecules. Next, a massive dissociation is found in the range of 420–540 °C, which could be ascribed to the decomposition and reorganization of Co–BDC and Ti3C2Tx, as shown by the corresponding DTG curve. Based on the result, we explored the composition and structure of the pyrolysis products at 550–900 °C. Moreover, all of the as-obtained products are named CoTiOx-T for convenience, where T denotes the pyrolysis temperature in °C.Fig. 1b shows the XRD patterns of the products at different pyrolysis temperatures including 550, 650, 750, 800 and 900 °C. It can be seen that when the pyrolysis temperature is 550 °C, Co3O4 (JCPDS card no. 42-1467) and very weak diffraction peaks at 35.36° and 62.2° that belong to the (311) and (440) planes of Co2TiO4 (JCPDS card no. 39-1410) are detected in the resulting product. Interestingly, more and stronger Co2TiO4 diffraction peaks appeared as the pyrolysis temperature increased. Of note, the (311) plane of both phases is the strongest peak. Based on the quantitative equation reported in previous literature about Co3O4 and Co2TiO4,36,37 the weight ratio of Co2TiO4/Co3O4 is calculated to be 5.4, 10.9, 16.5, 26.1 and 37.8% for CoTiOx-T (550–900 °C). Additionally, when the pyrolysis temperatures of the composite are 800 and 900 °C, a new phase of CoO (JCPDS card no. 43-1004) appears in the XRD patterns, in which the peaks at 36.5, 42.4, 61.5 and 73.7° are ascribed to (111), (200), (220) and (311). This result could be ascribed to the partial decomposition of Co3O4 to CoO at higher temperature, which can be confirmed by the significant decrease in the intensity of the (311) plane of Co3O4. Besides, the formation process of Co2TiO4 was also explored by annealing Ti3C2Tx at 550 and 650 °C. As shown in Fig. 1c, the XRD pattern of Ti3C2Tx annealed at 550 °C displayed that except for the generation of mixed phase TiO2, the characteristic diffraction peak located at small angle of MXene still exists, which indicates that Ti3C2Tx is not completely transformed into TiO2 at this temperature. Since a small amount of Ti3C2Tx with weak crystallization (Fig. 1c) was added during the preparation process of the Co–BDC/Ti3C2Tx precursor, its diffraction peaks are not detected in CoTiOx-550. However, when Ti3C2Tx was annealed at 650 °C, almost all Ti3C2Tx was decomposed into TiO2. Co3O4 is the pyrolysis product of single Co–BDC annealed at 650 °C, as confirmed by XRD (Fig. S2†). Thus, the formation process of Co2TiO4 above 650 °C could be attributed to the chemical reaction of Co3O4 and TiO2. The diffraction peaks of excess unreacted TiO2 are not detected in the XRD pattern of CoTiOx-T (650–900 °C), which is mainly caused by the small yield of TiO2 and the strong diffraction peak of Co3O4.
The structure information of the products was further evidenced by Raman spectroscopy. As shown in Fig. 1d, all samples have the same five Raman peaks, which located at 170–700 cm−1 corresponding to the F12g, Eg, F22g, F32g and A1g modes of Co-based spinel oxides, respectively.36 Among them, the F12g mode is attributed to the translational movement of the whole tetrahedron. The two vibrational F22g and F32g modes belong to the asymmetric stretching and bending of the oxygen anion in the octahedral group (BO6). The Eg corresponds to symmetric bending of oxygen with respect to cations in a tetrahedral surrounding. The strongest A1g mode relates to the symmetric stretching of oxygen in the tetrahedral group (AO4). It can be seen that all the five Raman modes shift to higher wavenumber with increasing temperature which is caused by the substitution of more Ti ions in the octahedral sites, and it increases the bond length between the octahedral cation and the oxygen (B–O).36 This phenomenon is in good agreement with the XRD result. Noted that no characteristic peaks at 1360 cm−1 and 1580 cm−1 of carbon were observed, which indicates that the synthesized products have no carbon material. This phenomenon may be due to the fact that ordinary nitrogen contains oxygen and oxygen is generated in Co–BDC/Ti3C2Tx cracking, which promotes the conversion of all C into gaseous substances.
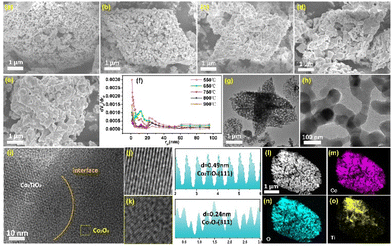
The morphology of the as-prepared products is observed from scanning electron microscopy (SEM) images. As displayed in Fig. 2a–c, 2D porous interconnected network structures composed of tiny nanoparticles were generated at 550–750 °C, which could be attributed to the nanosheet morphology of the Co–BDC/Ti3C2Tx precursor. Moreover, the size of nanoparticles increases with elevating temperature. When the temperature rises to 800 and 900 °C, the agglomeration of CoTiOx nanoparticles is obvious and it directly leads to the collapse of the network structure (Fig. 2d and e), which can result in the degradation of electrochemical performance. Nitrogen adsorption/desorption measurement was performed to study the surface area and pore size distribution of the products. As shown in Fig. S3,† all samples display type-IV isotherms. The CoTiOx-650 sample exhibits the largest Brunauer–Emmett–Teller (BET) specific surface area of 12.32 m2 g−1 among the five composites. The pore size distribution of the samples was analyzed by the Barrett–Joyner–Halenda (BJH) method. Obviously, mesoporous structures with pore diameter in the range of 10–30 nm are produced in CoTiOx composites (Fig. 2f). Moreover, it can be seen that there is a decrease in pore volume at 20–30 nm with increasing temperature and this is mainly ascribed to the nanoparticles that gradually sinter and agglomerate together, agreeing well with the results of SEM images. Compared with other samples, CoTiOx-650 has a larger specific surface area and more abundant mesoporous structure, which would be beneficial for exposing rich active sites and facilitating electron/ion transfer at the electrode/electrolyte interface.
Furthermore, the low and high magnification transmission electron microscopy (TEM) images of CoTiOx-650 also show its porous structure with a 2D interconnected network based on uniform nanoparticles (Fig. 2g and h). As can be seen, the direct pyrolysis–reorganization of Co–BDC/Ti3C2Tx nanosheets into CoTiOx can avoid interparticle sintering and effectively separate the nanoparticles, resulting in the formation of monodisperse nanocrystals with uniform size. Moreover, the corresponding high-resolution TEM (HRTEM) image in Fig. 2i clearly reveals the existence of hetero-interfaces where atom arrangement is quite distinct along the two sides of interfaces. The magnified HRTEM images (Fig. 2j and k) give two different interplanar distances of 0.24 nm and 0.49 nm, which can be well indexed to the (311) and (111) planes of the Co3O4 and Co2TiO4 phases, respectively, further confirming the co-existence of the two phases in CoTiOx-650. Of note, in contrast to the well-ordered lattice atoms of Co2TiO4(111) (Fig. 2j), the obvious lattice distortions occur in the Co3O4(311) plane (Fig. 2k). Thereby, CoTiOx-650 possesses a heterostructure at the atomic level and lattice distortions, which are expected to provide more active sites with high electrochemical reactivity for oxygen-containing intermediates, leading to enhanced OER performance.38,39 The selected area electron diffraction (SAED) image in other areas of CoTiOx-650 (Fig. S4†) also shows two sets of diffraction spots, which originate from the characteristic faces of Co3O4(311) and Co2TiO4(311). Notably, the TiO2(110) lattice plane was also observed. This is because excess TiO2 is produced by the pyrolysis of Ti3C2Tx at 650 °C, which almost has no OER activity (Fig. S5†). Besides, the HAADF (Fig. 2l) and elemental mapping images (Fig. 2m–o) reveal that the Ti distribution is different from that of Co and O in the 2D porous structure, further confirming the formation of a heterojunction in CoTiOx-650. In a word, the tightly coupled Co–BDC/Ti3C2Tx precursor pyrolysis–reorganization strategy is successfully developed to obtain heterogeneous Co-based mixed spinel oxides with a porous interconnected network structure.
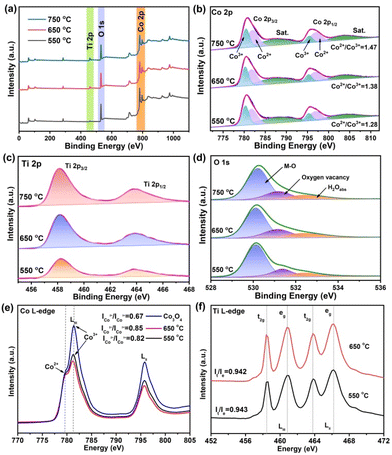
X-ray photoelectron spectroscopy (XPS) was further conducted to investigate the composition and valence state of CoTiOx-650. To better reveal the change of the phase composition, the XPS spectra of CoTiOx-550 and CoTiOx-750 were also studied for comparison. As shown in Fig. 3a, the survey spectra demonstrate the presence of Ti, O and Co in the three samples. The fine-scanned Co 2p XPS spectra with fitted peaks are presented in Fig. 3b, in which the characteristic peaks at 780.3 and 795.3 eV belong to Co3+ species, while the two peaks at 781.8 and 796.9 eV are ascribed to Co2+ species, along with satellite peaks at 787.9 and 804.1 eV.26 The relative atomic ratio of Co2+/Co3+ on the surface of the samples could be obtained by comparing the area that the fitted curve covered. It could be clearly seen that the atomic ratio of Co2+/Co3+ (1.47) in CoTiOx-750 is higher than that in CoTiOx-650 (1.38) and CoTiOx-550 (1.28), revealing that more Co2+ species are generated in CoTiOx-750, which agrees well with the XRD result. Since the Co ions of Co2TiO4 are composed of 1/2 CoOh2+ and 1/2 CoTd2+, the amount of CoOh2+ increases in CoTiOx-750. The fine-scanned Ti 2p XPS spectra of these samples in Fig. 3c exhibit the same two peaks at 458.1 eV (2p3/2) and 463.8 eV (2p1/2) with a separation value of 5.7 eV, confirming the same chemical valence of the Ti4+ valence state.40 As for O 1s XPS spectra (Fig. 3d), the samples display similar spectra. Specifically, the peak at 530.1 eV is typical for metal–oxygen bonds (M–O), and the peak located at 532.5 eV is assigned to the surface-adsorbed water molecules (H2Oads). The peak at 531.2 eV indicates the presence of a small amount of oxygen vacancies in samples, which is beneficial for the electrocatalytic OER process.9 Besides, the electronic structures of the CoTiOx-650 and CoTiOx-550 samples were also analyzed by soft X-ray adsorption near-edge structure (XANES). Furthermore, the XANES spectra of single Co3O4 derived from the Co-MOF at 650 °C were also studied for comparison. As displayed in Fig. 3e, the Co L-edge of Co3O4 shows the main peak for Co3+ both in LIII and LII, and a shoulder peak with lower intensity for Co2+ in LIII.41 For CoTiOx-650, the intensity ratio of Co2+/Co3+ is 0.85, which is larger than that of CoTiOx-550 (0.82) and Co3O4 (0.67), further showing the existence of more CoOh2+ in CoTiOx-650. As for the Ti L-edge, the peak positions of the two samples show the same feature and two bands (LIII and LII) with 2p spin–orbit coupling. The two peaks are split into t2g and eg characteristic of the TiO6 ligand field (Oh).41 The two samples exhibit similar intensity ratios of t2g/eg, implying almost no change in the surface Ti valence state. As is known, the occupancy of eg orbitals in 3d transition-metal-based electrocatalysts determines the strength of the metal–oxygen bond, which strongly affects the OER overpotential.14 Electrocatalysts with too few or too much eg filling (0 or 2) would make the oxygen binding too weak or too strong, respectively, resulting in poor OER performance. The OER activity can be optimized with moderate eg filling (eg ≈ 1) of the octahedral sites, which could balance the competition between rate-limiting steps.16 Compared with the rich CoOct3+ (t62ge0g) electronic configuration in CoTiOx-550, the presence of more CoOct2+ (t62ge1g) in CoTiOx-650 would bring efficient OER activity.
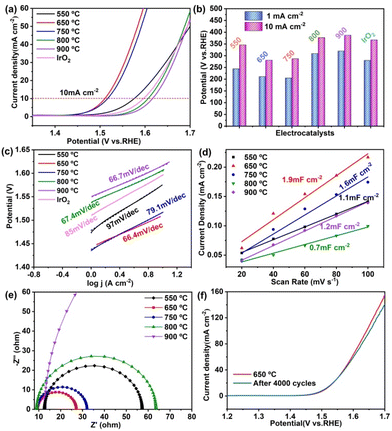
To verify our speculation, the OER performances of the as-prepared CoTiOx samples were evaluated in a three-electrode cell with an electrochemical workstation (CHI660E) at room temperature in 1.0 M KOH solution. The Pt electrode and HgO/Hg electrode were used as the counter electrode and reference electrode, respectively. First, the catalysts with 0.285 mg cm−2 uniform loading on the glassy carbon electrode were used as working electrodes for electrochemical tests. Fig. 4a shows the polarization curves of five CoTiOx samples and commercial IrO2, in which CoTiOx-650 obviously exhibits the best OER activity among the catalysts, and the relative OER performance of these catalysts is 650 > 750 > 550 > 800 > 900. Specifically, as displayed in Fig. 4b, CoTiOx-650 gives a small onset overpotential of 250 mV at a current density of 1 mA cm−2, revealing the good OER activity. It's well known that the overpotential requirement for achieving a current density of 10 mA cm−2 is an important parameter to estimate OER activity. Herein, CoTiOx-650 shows a remarkably low overpotential (η10) of 280 mV, which is smaller than that of CoTiOx-550 (346 mV), CoTiOx-750 (287 mV), CoTiOx-800 (377 mV), CoTiOx-900 (387 mV) and IrO2 (367 mV). Besides, its superior catalytic activity is further reflected by comparing the slopes of Tafel plots (Fig. 4c) for CoTiOx-650 (66.4 mV dec−1) with those of CoTiOx-550 (97 mV dec−1), CoTiOx-750 (79.1 mV dec−1), CoTiOx-800 (67.4 mV dec−1), CoTiOx-900 (66.7 mV dec−1) and IrO2 (85 mV dec−1), revealing that CoTiOx-650 is more beneficial for practical application.
To reveal the reason for the excellent OER performance of CoTiOx-650, the electrochemical double-layer capacitance (Cdl), which is linearly proportional to the electrochemically effective surface area, was measured by the cyclic voltammetry (CV) method. As displayed in Fig. S6† and Fig. 4d, CoTiOx-650 shows the largest Cdl of 1.9 mF cm−2 among these CoTiOx samples, indicating the highest electrochemically active surface area, which can expose more active sites, and it greatly contributes to the excellent OER performance. Moreover, the current density further normalized with the electrochemically active surface area was captured to estimate the intrinsic OER activity of the CoTiOx samples. As shown in Fig. S7,† the normalized LSV curves indicate that the OER activity of CoTiOx-750 is slightly higher than that of CoTiOx-650, and both are greater than that of CoTiOx-550. This result can be ascribed to the generation of more Co2TiO4 with higher CoOh2+ in CoTiOx-750, revealing the important positive effect of CoOh2+ on the intrinsic OER activity. However, due to the higher specific surface area and much richer porous structure of CoTiOx-650 than CoTiOx-750, its overall OER activity is better. As for CoTiOx-800 and CoTiOx-900, despite more Co2TiO4 generation, their OER activity still drops sharply, indicating that the decomposition of Co3O4 to CoO is unfavorable for OER activity, meanwhile showing the important role of CoOh3+ in the OER process. In a word, the synergistic catalytic effect between CoOct3+ in Co3O4 and CoOct2+ in Co2TiO4 is shown to be crucial for improving the OER activity.
More insights into the structure–performance relationship of the as-obtained mixed spinel oxides were revealed by electrochemical impedance spectra (EIS). As displayed in Fig. 4e, the Nyquist plots reveal a significant decrease of the charge-transfer resistance (Rct) from 250 Ω (CoTiOx-900) to 18 Ω (CoTiOx-650), indicating the fastest charge transport kinetics of CoTiOx-650. This result could be ascribed to the highly porous structure and the synergistic catalytic effect between CoOct3+ in Co3O4 and CoOct2+ in Co2TiO4 of CoTiOx-650. Specifically, the former is favourable for exposing rich active sites and facilitating electron/ion transfer at the electrode/electrolyte interface,42 while the latter can directly promote more electron transfer between surface octahedral cations (CoO6) and adsorbed-OOH intermediates,25 thereby leading to the fast charge transport process of CoTiOx-650, which is responsible for the superior OER performance. Besides, stability is another important parameter for advanced electrocatalysts. Therefore, a long-term CV cycling test was conducted to measure the stability of CoTiOx-650. As displayed in Fig. 4f, the polarization curves measured before and after 4000 CV cycles were compared, which shows negligible difference between the initial polarization curve and the final one, indicating the excellent durability of CoTiOx-650.
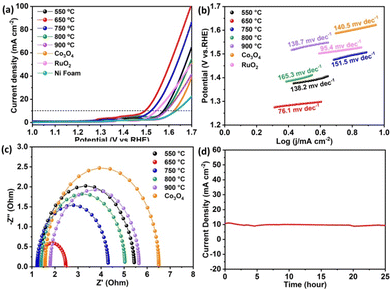
To further confirm the excellent OER performance of the CoTiOx-650 catalyst, the electrocatalytic performances of the obtained samples coated on nickel (Ni) foam with a high loading amount of 10 mg cm−2 were also estimated, as they are beneficial for industrial application. Meanwhile, the electrochemical performance of individual Co3O4 obtained by pyrolysis of the Co-MOF at 650 °C was also tested for comparison. As displayed in Fig. 5a–c, the trends of CoTiOx-T coated on NF are similar to that in the case of using a GCE as the substrate, proving that CoTiOx-650 has excellent substrate-independent OER activity.
The deposition of CoTiOx-650 onto Ni foam results in a slightly enhanced OER performance with an η10 overpotential of 260 mV, which could be attributed to the excellent conductivity of Ni foam. It is worth noting that the performance of CoTiOx-650 is far superior to that of Co3O4, strongly confirming the crucial role of Co2TiO4 with abundant CoOh2+ in the OER activity of CoTiOx-650. Besides, to test the stability of the modified Ni foam electrode, the chronoamperometry curve of CoTiOx-650 at a potential of 1.49 V vs. RHE was obtained. As shown in Fig. 5d, CoTiOx-650 exhibits a current density of 10 mA cm−2 with a negligible loss even after the OER process for 25 h, further showing its superior stability for the OER. The above analyses demonstrate that CoTiOx-650 has excellent OER activity, which is also remarkable compared with previously reported values for advanced catalysts (Table S1†). Finally, the XRD and XPS of CoTiOx-650 after the stability test were investigated, as shown in Fig. S8.† It can be seen that except for some crystalline CoTiOx-650 being oxidized to form amorphous CoOOH, the overall phase remains as Co3O4 and Co2TiO4 after the long-term OER test. In a word, CoTiOx-650 is an efficient and stable OER catalyst.
Conclusions
In summary, we have successfully synthesized mixed Co-based spinel oxides as efficient catalysts for the OER via a simple MOF/MXene pyrolysis–reorganization strategy. Detailed electrochemical measurements demonstrate that their OER performances can be remarkably tuned by modulating the electronic configuration of Co species in the mixed spinel oxides. By changing the pyrolysis temperature, CoOct3+ (t62ge0g) is partially converted into CoOct2+ (t62ge1g), which is beneficial to promote the OER activity. Meanwhile, a controllable mesoporous structure is also achieved in the catalyst, providing the 2D porous interconnected network morphology with maximum exposure of active sites. As a result, the optimal Co3O4/Co2TiO4 catalyst at 650 °C exhibits superior OER activity and excellent stability. Our study will provide valuable guidelines for designing advanced electrocatalysts from the pyrolysis–reorganization strategy of MOF/MXene-based composites.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the NSF of China (no. 21971143, 21805165), the 111 Project (D20015), the Major Research and Development Project of Hubei Three Gorges Laboratory (2022-3), and ITOYMR in the Higher Education Institutions of Hubei Province (T201904). The authors also thank Prof. W. S. Yan (National Synchrotron Radiation Laboratory, University of Science and Technology of China) for soft X-ray absorption spectrometry testing.Notes and references
- M. Koper, Thermodynamic theory of multi-electron transfer reactions: Implications for electrocatalysis, J. Electroanal. Chem., 2011, 660, 254–260 CrossRef CAS.
- Y. Liu, C. Xiao, P. Huang, M. Cheng and Y. Xie, Regulating the Charge and Spin Ordering of Two-Dimensional Ultrathin Solids for Electrocatalytic Water Splitting, Chem, 2018, 4, 1263–1283 CAS.
- Z.-J. Chen, T. Zhang, X.-Y. Gao, Y.-J. Huang, X.-H. Qin, Y.-F. Wang, K. Zhao, X. Peng, C. Zhang, L. Liu, M.-H. Zeng and H.-B. Yu, Engineering Microdomains of Oxides in High-Entropy Alloy Electrodes toward Efficient Oxygen Evolution, Adv. Mater., 2021, 33, 2101845 CrossRef CAS.
- Q. Wen, Y. Zhao, Y. Liu, H. Li and T. Zhai, Ultrahigh-Current-Density and Long-Term-Durability Electrocatalysts for Water Splitting, Small, 2022, 18, 2104513 CrossRef CAS.
- T. Nguyen, G. Scherer and Z. Xu, A facile synthesis of size-controllable IrO2 and RuO2 nanoparticles for the oxygen evolution reaction, Electrocatalysis, 2016, 7, 420–427 CrossRef CAS.
- J. Li, D. Chu, H. Dong, D. Baker and R. Jiang, Boosted oxygen evolution reactivity by igniting double exchange interaction in spinel oxides, J. Am. Chem. Soc., 2019, 142, 50–54 CrossRef PubMed.
- D. He, X. Song, W. Li, C. Tang, J. Liu, Z. Ke, C. Jiang and X. Xiao, Active Electron Density Modulation of Co3O4-Based Catalysts Enhances their Oxygen Evolution Performance, Angew. Chem., Int. Ed., 2020, 59, 6929–6935 CrossRef CAS.
- J. Xie, J. Li, L. Kang, J. Li, Z. Wei, F. Lei, P. Hao and B. Tang, Molten-Salt-Protected Pyrolytic Approach for Fabricating Borate-Modified Cobalt–Iron Spinel Oxide with Robust Oxygen-Evolving Performance, ACS Sustainable Chem. Eng., 2021, 9, 14596–14604 CrossRef.
- J. Bao, X. Zhang, B. Fan, J. Zhang, M. Zhou, W. Yang, X. Hu, H. Wang, B. Pan and Y. Xie, Ultrathin Spinel-Structured Nanosheets Rich in Oxygen Deficiencies for Enhanced Electrocatalytic Water Oxidation, Angew. Chem., 2015, 127, 7507–7512 CrossRef.
- Y. Zhou, S. Sun, C. Wei, Y. Sun, P. Xi, Z. Feng and Z. Xu, Significance of Engineering the Octahedral Units to Promote the Oxygen Evolution Reaction of Spinel Oxides, Adv. Mater., 2019, 31, 1902509 CrossRef CAS PubMed.
- S. Sun, Y. Sun, Y. Zhou, S. Xi, X. Ren, B. Huang, H. Liao, L. Wang, Y. Du and Z. Xu, Shifting Oxygen Charge Towards Octahedral Metal: A Way to Promote Water Oxidation on Cobalt Spinel Oxides, Angew. Chem., 2019, 131, 6103–6108 CrossRef.
- Y. Zhou, S. Sun, J. Song, S. Xi, B. Chen, Y. Du, A. Fisher, F. Cheng, X. Wang, H. Zhang and Z. Xu, Enlarged Co-O Covalency in Octahedral Sites Leading to Highly Efficient Spinel Oxides for Oxygen Evolution Reaction, Adv. Mater., 2018, 30, 1802912 CrossRef PubMed.
- Y. Sun, X. Ren, S. Sun, Z. Liu, S. Xi and Z. Xu, Engineering High-Spin State Cobalt Cations in Spinel Zinc Cobalt Oxide for Spin Channel Propagation and Active Site Enhancement in Water Oxidation, Angew. Chem., 2021, 133, 14657–14665 CrossRef.
- Y. Sun, G. Chen, S. Xi and Z. Xu, Catalytically Influential Features in Transition Metal Oxides, ACS Catal., 2021, 11, 13947–13954 CrossRef CAS.
- J. Suntivich, K. May, H. Gasteiger, J. Goodenough and Y. Shao-Horn, A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles, Science, 2011, 334, 1383–1385 CrossRef CAS PubMed.
- C. Wei, Z. Feng, G. Scherer, J. Barber, Y. Shao-Horn and Z. Xu, Cations in Octahedral Sites: A Descriptor for Oxygen Electrocatalysis on Transition-Metal Spinels, Adv. Mater., 2017, 29, 1606800 CrossRef PubMed.
- H.-Y. Wang, S.-F. Hung, H.-Y. Chen, T.-S. Chan, H.-M. Chen and B. Liu, In Operando Identification of Geometrical-Site-Dependent Water Oxidation Activity of Spinel Co3O4, J. Am. Chem. Soc., 2016, 138, 36–39 CrossRef CAS PubMed.
- Y. Xu, F. Zhang, T. Sheng, T. Ye, D. Yi, Y. Yang, S. Liu, X. Wang and J. Yao, Clarifying the controversial catalytic active sites of Co3O4 for the oxygen evolution reaction, J. Mater. Chem. A, 2019, 7, 23191–23198 RSC.
- J. Zhang, J. Qian, J. Ran, P. Xi, L. Yang and D. Gao, Engineering Lower Coordination Atoms onto NiO/Co3O4 Heterointerfaces for Boosting Oxygen Evolution Reactions, ACS Catal., 2020, 10, 12376–12384 CrossRef CAS.
- B. Sidhureddy, J. Dondapati and A. Chen, Shape-controlled synthesis of Co3O4 for enhanced electrocatalysis of the oxygen evolution reaction, ChemComm, 2019, 55, 3626–3629 RSC.
- X. Yang, J. Chen, Y. Chen, P. Feng, H. Lai, J. Li and X. Luo, Novel Co3O4 Nanoparticles/Nitrogen-Doped Carbon Composites with Extraordinary Catalytic Activity for Oxygen Evolution Reaction (OER), Nano-Micro Lett., 2018, 10, 1–11 CrossRef PubMed.
- S. Zhang, B. Guan, X. Lu, S. Xi, Y. Du and X. Lou, Metal Atom-Doped Co3O4 Hierarchical Nanoplates for Electrocatalytic Oxygen Evolution, Adv. Mater., 2020, 32, 2002235 CrossRef CAS.
- X. Wang, L. Yu, B. Guan, S. Song and X. Lou, Metal–Organic Framework Hybrid-Assisted Formation of Co3O4/Co-Fe Oxide Double-Shelled Nanoboxes for Enhanced Oxygen Evolution, Adv. Mater., 2018, 30, 1801211 CrossRef PubMed.
- T. Wang, P. Wang, W. Zang, X. Li, D. Chen, Z. Kou, S. Mu and J. Wang, Nanoframes of Co3O4-Mo2N Heterointerfaces Enable High-Performance Bifunctionality toward Both Electrocatalytic HER and OER, Adv. Funct. Mater., 2022, 32, 2107382 CrossRef CAS.
- Z. Liu, G. Wang, X. Zhu, Y. Wang, Y. Zou, S. Zang and S. Wang, Optimal Geometrical Configuration of Cobalt Cations in Spinel Oxides to Promote Oxygen Evolution Reaction, Angew. Chem., Int. Ed., 2020, 59, 4736–4742 CrossRef CAS.
- L. Xu, Q. Jiang, Z. Xiao, X. Li, J. Huo, S. Wang and L. Dai, Plasma-Engraved Co3O4 Nanosheets with Oxygen Vacancies and High Surface Area for the Oxygen Evolution Reaction, Angew. Chem., 2016, 128, 5363–5367 CrossRef.
- Z. Xiao, Y.-C. Huang, C.-L. Dong, C. Xie, Z. Liu, S. Du, W. Chen, D. Yan, L. Tao, Z. Shu, G. Zhang, H. Duan, Y. Wang, Y. Zou, R. Chen and S. Wang, Operando Identification of the Dynamic Behavior of Oxygen Vacancy-Rich Co3O4 for Oxygen Evolution Reaction, J. Am. Chem. Soc., 2020, 142, 12087–12095 CrossRef CAS PubMed.
- D. Yan, R. Chen, Z. Xiao and S. Wang, Engineering the electronic structure of Co3O4 by carbon-doping for efficient overall water splitting, Electrochim. Acta, 2019, 303, 316–322 CrossRef CAS.
- K.-L. Yan, J.-F. Qin, J.-H. Lin, B. Dong, J.-Q. Chi, Z.-Z. Liu, F.-N. Dai, Y.-M. Chai and C.-G. Liu, Probing the active sites of Co3O4 for the acidic oxygen evolution reaction by modulating the Co2+/Co3+ ratio, J. Mater. Chem. A, 2018, 6, 5678–5686 RSC.
- Y. Peng, H. Hajiyani and R. Pentcheva, Influence of Fe and Ni Doping on the OER Performance at the Co3O4(001) Surface: Insights from DFT+U Calculations, ACS Catal., 2021, 11, 5601–5613 CrossRef CAS.
- P. Menezes, A. Indra, V. Gutkin and M. Driess, Boosting electrochemical water oxidation through replacement of Oh Co sites in cobalt oxide spinel with manganese, ChemComm, 2017, 53, 8018–8021 RSC.
- R. Zheng, C. Shu, Z. Hou, A. Hu, J. Zhao, Y. Guo, M. He, Y. Yan and J. Long, Interfacial electronic structure design of MXene-based electrocatalyst via vacancy modulation for lithium-oxygen battery, Carbon, 2020, 166, 273–283 CrossRef CAS.
- L. Hu, M. Li, X. Wei, H. Wang, Y. Wu, J. Wen, W. Gu and C. Zhu, Modulating interfacial electronic structure of CoNi LDH nanosheets with Ti3C2Tx MXene for enhancing water oxidation catalysis, Chem. Eng. J., 2020, 398, 125605 CrossRef CAS.
- P. Liu, W. Yang, F. Xiao, Y. Qi, S. Jamil and M. Xu, Efficient Anchoring of Polysulfides Based on Self-Assembled Ti3C2Tx Nanosheet-Connected Hollow Co(OH)2 Nanotubes for Lithium–Sulfur Batteries, ACS Appl. Mater. Interfaces, 2021, 13, 57285–57293 CrossRef CAS.
- X. Yang, C. Xu, S. Li, Y.-P. Wu, X.-Q. Wu, Y.-M. Yin and D. Li, Thermal treatment for promoting interfacial interaction in Co-BDC/Ti3C2Tx hybrid nanosheets for hybrid supercapacitors, J. Colloid Interface Sci., 2022, 617, 633–640 CrossRef CAS.
- S. Nayak, K. Dasari, D. Joshi, P. Pramanik, R. Palai, V. Sathe, N. Tiwari and S. Thota, Spectroscopic studies of Co2TiO4 and Co3O4 two-phase composites, Phys. Status Solidi B, 2016, 253, 2270–2282 CrossRef CAS.
- R. Spurr and H. Myers, Quantitative Analysis of Anatase-Rutile Mixtures with an X-Ray Diffractometer, Anal. Chem., 1957, 29, 760–762 CrossRef CAS.
- C. Li, Y. Liu, Z. Zhuo, H. Ju, D. Li, Y. Guo, X. Wu, H. Li and T. Zhai, Local Charge Distribution Engineered by Schottky Heterojunctions toward Urea Electrolysis, Adv. Energy Mater., 2018, 8, 1801775 CrossRef.
- Y. Tian, L. Xu, M. Li, D. Yuan, X. Liu, J. Qian, Y. Dou, J. Qiu and S. Zhang, Interface Engineering of CoS/CoO@N-Doped Graphene Nanocomposite for High-Performance Rechargeable Zn-Air Batteries, Nano-Micro Lett., 2021, 13, 1–15 CrossRef CAS.
- H. Li, H. Wang, Q. Gao, B. Han, K. Xia and C. Zhou, Hierarchical flower-like Co2TiO4 nanosheets with unique structural and compositional advantages to boost peroxymonosulfate activation for degradation of organic pollutants, J. Mater. Chem. A, 2020, 8, 20953–20962 RSC.
- Y. Yan, C. Liu, H. Jian, X. Cheng, T. Hu, D. Wang, L. Shang, G. Chen, P. Schaaf, X. Wang, E. Kan and T. Zhang, Substitutionally Dispersed High-Oxidation CoOx Clusters in the Lattice of Rutile TiO2 Triggering Efficient Co-Ti Cooperative Catalytic Centers for Oxygen Evolution Reactions, Adv. Funct. Mater., 2021, 31, 2009610 CrossRef CAS.
- X. Yang, Y. Tian, S. Li, Y.-P. Wu, Q. Zhang, D.-S. Li and S. Zhang, Heterogeneous Ni-MOF/V2CTx-MXene hierarchically-porous nanorods for robust and high energy density hybrid supercapacitors, J. Mater. Chem. A, 2022, 10, 12225–12234 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2qi02098j |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2023 |