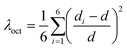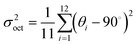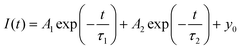Blue photoluminescence enhancement achieved by zero-dimensional organic indium halides via a metal ion doping strategy†
Fang
Lin
a,
Guicheng
Yu
a,
Shuchen
Weng
a,
Chao
Zhou
ab,
Yonglei
Han
ab,
Wei
Liu
 c,
Kang
Zhou
a,
Yongfei
Wang
c,
Kang
Zhou
a,
Yongfei
Wang
 b and
Haoran
Lin
b and
Haoran
Lin
 *a
*a
aHoffmann Institute of Advanced Materials, Shenzhen Polytechnic, 7098 Liuxian Blvd, Nanshan District, Shenzhen, 518055, China. E-mail: hlin@szpt.edu.cn
bSchool of Materials and Metallurgy, University of Science and Technology Liaoning, 185 Qianshan Zhong Road, Anshan, 114051, China
cSchool of Chemical Engineering and Technology, Sun Yat-Sen University, Zhuhai, 519000, China
First published on 8th November 2022
Abstract
Low-dimensional organic–inorganic metal halide hybrids exhibit promising optical properties for light emitting applications. However, developing lead-free blue-light emitters with high photoluminescence quantum efficiency (PLQE) remains an ongoing challenge. In our work, a novel zero-dimensional (0D) indium hybrid compound (MA)4InCl7 (MA = CH3NH3+) was developed which exhibited broadband blue emission with a PLQE of 11.2% when excited by ultraviolet (UV) light. More interestingly, upon Cs+ or Mn2+ doping, the emission of the 0D compound further blue-shifted and became narrower, while the PLQE was significantly enhanced to 18.8% (Cs+) or 20.7% (Mn2+). More prominent PLQE enhancement to 74.7% was observed after Sb3+ doping, which also altered the emission spectrum to the orange region. According to experimental characterization and theoretical calculations, we attribute the PLQE enhancement upon Cs+ and Mn2+ doping to defect passivation and the orange emission upon Sb3+ doping to altered emission centers. We have demonstrated that multiple metal ions possess the ability to improve the light emitting properties of 0D organic–inorganic metal halides, and (MA)4InCl7 could be utilized as a Sb3+ heavy metal ion sensor with high selectivity and sensitivity.
Introduction
Organic–inorganic metal halides (OIMHs) are a class of materials which are employed in a variety of optoelectronic devices such as solar cells, light-emitting diodes, detectors and lasers.1–7 Among them, low-dimensional lead-free OIMHs have received considerable research attention as stable and environmentally friendly light-emitting materials.8,9 Lowering the structural dimensionality of OIMHs from three-dimension (3D) to low-dimension significantly enhances the exciton–phonon coupling through quantum confinement and dielectric confinement effects, thus giving rise to the formation of self-trapped excitons (STEs) and representative highly efficient broadband emissions with a large Stokes shift.10Zero-dimensional (0D) OIMHs comprising isolated inorganic polyhedrons and organic cations possess high structural versatility, color tunability and high photoluminescence quantum efficiency (PLQE) due to their quantum-confined structures, making them promising materials for luminescence applications.11 For example, a series of 0D hybrid materials, such as (C5H14N3)2MnBr4,12 (C10H16N)2MnBr4,13 (TMA)2SbCl5·DMF,14 (MePPh3)2SbCl5,15 (C12H28N)2SbCl5,16 and (C4N2H14Br)4SnBr6,17 were developed with various emission colors and near-unity PLQEs. To achieve the three primary colors, blue-emissive 0D OIMHs were also reported, mostly lead-containing compounds such as (BAPrEDA)PbCl6·(H2O)2,18 (C13H19N4)2PbBr4,19 and (C9NH20)7(PbCl4)Pb3Cl11.20 However, the toxicity of lead limits their development and applications. Lead-free blue emitters were developed using all-inorganic materials in which alkali copper(I) halides such as Cs3Cu2X5,21–23 Rb2CuX3,24 and K2CuX325 have shown extraordinary PLQYs of up to 100%. On the other hand, lead-free 0D OIMHs have also been massively investigated in recent years.26,27 Several reports suggest that the substitution of lead by indium is an effective strategy to develop lead-free blue emitters. For example, the PLQE of blue-emissive (C7H8N6)InCl9 with a long lifetime of 1.2 s can be enhanced from 25.2% to 42.8% upon illumination.28 BAPP4+-associated blue emission in BAPPIn2Cl10 endows it with intriguing afterglow properties.29 TpyInClx (x = 3 or 5) emitters were tuned to be blue-emissive and with an increased PLQE of up to 47.66% by regulating the polarity of the solvent.30 [H2EP]2InCl6·Cl·H2O·C3H6O and [H3AEP]InCl6·H2O peaking at 430 nm displayed a PLQY of 13.44% and 4.12%, respectively.31 (C14H22N)InBr4, isolated tetrahedral [InBr4]− separated by organic ligands, showed a visibly bright blue emission with a PLQE of 16.36%.32 Despite the recent advances of 0D indium OIMH blue emitters, their poor stability and relatively low PLQEs still remain problematic.
For low-dimensional OIMHs, metal ion doping is considered as an attractive and effective strategy to optimize their photoluminescence properties and enhance their stability. There are also several cases of doping Sb3+ in 0D indium OIMHs to simultaneously manipulate the emission spectrum and enhance the PLQE. Upon rational control of the Sb amount, both near-unity PLQEs and a bright white-light emission were achieved in (C7H8N6)InCl9,28 BAPPIn2Cl10,29 (C8NH12)6InBr9·H2O33 and InCl7(C4H10SN4).34 However, as far as we know, there are no reports on using metal ions other than Sb3+ to dope 0D indium OIMHs. The light-emitting mechanism upon ion doping is less explored. Also, exploration of the applications of metal ion doped 0D indium compounds is limited besides Kuang's29 and Chen's34 recent works which suggest that antimony doped indium compounds could be used as anti-counterfeiting materials and solvent sensors.
Here, we have synthesized a lead-free 0D indium-based OIMH (MA)4InCl7 (MA = CH3NH3+), which exhibits a bright broadband blue emission peaked at 455 nm with a PLQE of 11.2%. The metal ion doping strategy was applied to this material and a series of compounds including (MA)4InCl7:Cs+, (MA)4InCl7:Mn2+ and (MA)4InCl7:Sb3+ were obtained. Reduced full width at half maxima (FWHM) and increased PLQE (from 11.2% to 20.7%) have been achieved through introducing Cs+ or Mn2+ into the pristine material. Upon Sb3+ doping, (MA)4InCl7:Sb3+ exhibits significantly shifted orange STE emission and a greatly enhanced PLQE of up to 74.7%, which makes it a perfect sensor for Sb3+ ion sensing.
Results and discussion
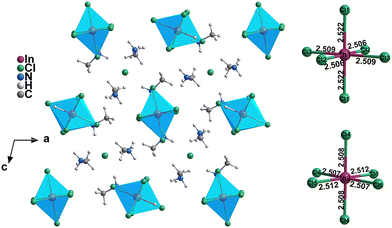
Single crystals of (MA)4InCl7 and the doped compounds were grown via a solvothermal method (see the ESI† for details). The feeding ratios were selected to achieve the maximum PLQEs (Fig. S1 and Table S5, ESI†) and the following characterization is based on these feeding ratios. The single crystal structures of (MA)4InCl7 and the doped ones were determined by single crystal X-ray diffraction (SCXRD) and the crystallographic details are listed in Table S1 (ESI†). As shown in Fig. 1, the 0D crystallographic structure of (MA)4InCl7 adopts a P2/n space group, which can be regarded as individual InCl63− octahedra embedded periodically in the matrix comprising CH3NH3+ and Cl− ions. Interestingly, there are two geometrically different inorganic octahedra, named [In(1)Cl6]3−, and [In(2)Cl6]3−, respectively. We calculated the extent of distortion of two [InCl6]3− octahedra based on In–Cl bond lengths and Cl–In–Cl bond angles by the following equations:35where d is the average In–Cl bond length, di is the length of each of the six In–Cl bonds, and θi is the angle of each of the twelve bonds of the octahedron in Tables S2 and S3 (ESI†).
The calculated extent of distortion (λoct1 = 7.21 × 10−6, λoct2 = 6.82 × 10−7; σ2oct1 = 0.271, σ2oct2 = 0.264) is much smaller than those of the reported Sn2+-, Bi3+-, and Sb3+-based OIMHs because of the strongly antibonding nature of the ns orbitals of the later ones.36,37 In addition, the lattice parameters (Table S1, ESI†) and the extent of distortion of InCl63− for all doped compounds (Table S4, ESI†) are similar to those of the pristine compound, which indicate that a small amount of metal doping do not alter the crystal structure significantly. It is worth mentioning that Cs+ is observable in the experimental crystal structure of (MA)4InCl7:Cs+ in which Cs+ ions have substituted a small amount of MA+ (8% occupancy in each MA+), which improves the crystal symmetry leading to different space groups compared to the pristine compound. Nevertheless, the two crystals are still almost the same with similar atom positions, distances, compositions, etc., which is evidenced by the similarity of the area in the red dash line and unit cell of (MA)4InCl7:Cs+ in Fig. S2 (ESI†). Mn2+ and Sb3+ ions were not observed in the crystal structures of (MA)4InCl7:Mn2+ and (MA)4InCl7:Sb3+, probably due to the small doping ratio of Mn2+ and similar sizes between Sb3+ and In3+. The experimentally measured powder X-ray diffraction (PXRD) patterns of the pristine and doped compounds display high similarity to their simulated patterns as shown in Fig. S3 (ESI†), not only suggesting the reliability of the crystal structures, but also further evidencing the unchanged crystal structures upon metal ion doping.
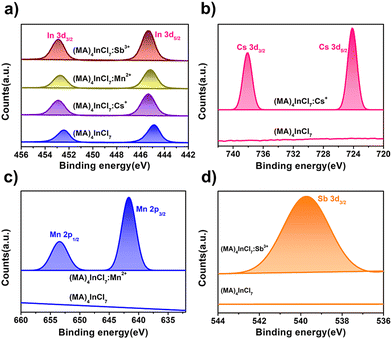
To determine the actual atom ratios of the doped compounds, inductively coupled plasma optical emission spectrometry (ICP-OES) was utilized and the experimental ratios are summarized in Table S5 (ESI†). The amount of Sb3+ is close to the actual experimental amount (∼10%), while the experimental ratios of Cs+ or Mn2+ (∼2.5% and ∼0.2% respectively) are much less than the feeding ratios (∼5.8% and ∼3.0% respectively), indicating that Sb3+ can be more efficiently incorporated into the crystal with a homogeneous distribution than the other dopants (Cs+ and Mn2+). Effective doping was also confirmed by X-ray photoelectron spectroscopy (XPS). As shown in Fig. 2a and Fig. S4 (ESI†), In 3d3/2 and In 3d5/2 peaks (at 452.43 and 444.88 eV) in pristine (MA)4InCl7 are shifted to higher binding energy by 0.50, 0.40 and 0.45 eV upon Cs+, Mn2+ and Sb3+ doping, respectively. Accordingly, the peaks of Cs 3d3/2 and Cs 3d5/2 (738.1 and 724.2 eV), Mn 2p1/2 and Mn 2p3/2 (653.5 and 641.6 eV) and Sb 3d3/2 (539.9 eV) are observed in (MA)4InCl7:Cs+, (MA)4InCl7:Mn2+ and (MA)4InCl7:Sb3+ respectively, verifying the presence of doped ions in the crystals (Fig. 2b–d).
The PL properties of all compounds were characterized by steady state and time-resolved emission spectroscopy, which are summarized in Table 1. We compare the photophysical properties of (MA)4InCl7 with (MA)4InCl7:Cs+ and (MA)4InCl7:Mn2+ first. (MA)4InCl7 exhibits a broadband blue emission peaked at 455 nm with a PLQE of 11.2% when excited by 365 nm UV light as shown in Fig. 3a. The large Stokes shift and broadband emission suggest a self-trapped exciton mechanism, in which the generated excitons go through exciton–phonon interactions into self-trapped states (STE) with lower energy as commonly observed in other 0D OIMHs.38–41 Cs+ and Mn2+ doping slightly blue-shifts and narrows the blue emission peak. This phenomenon could have possibly originated from either the less extent of exciton–phonon coupling strength or more homogenous crystal structure. Given that the experimental crystal structures remain almost unchanged upon doping, the latter reason is more likely. Interestingly, the PLQE is significantly enhanced to 18.8% and 20.7% for Cs+ doping and Mn2+ doping, respectively, which is not reported for other 0D indium OIMHs before.
| Materials | Optical bandgap (eV) | λ exc (nm) | λ em (nm) | FWHM (nm) | PLQE (%) | τ (ns) |
|---|---|---|---|---|---|---|
| (MA)4InCl7 | 3.34 | 273, 366 | 455 | 152 | 11.2 | 6.72 |
| (MA)4InCl7:Cs+ | 3.36 | 298, 359 | 445 | 139 | 18.8 | 6.53 |
| (MA)4InCl7:Mn2+ | 3.00 | 278, 363 | 445 | 114 | 20.7 | 7.94 |
| (MA)4InCl7:Sb3+ | 3.23 | 283, 323 | 611 | 164 | 74.7 | 5420 |
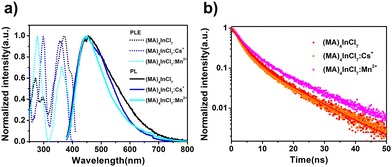 | ||
| Fig. 3 (a) Excitation and emission spectra of (MA)4InCl7, (MA)4InCl7:Cs+ and (MA)4InCl7:Mn2+. (b) Time-resolved PL decays of (MA)4InCl7, (MA)4InCl7:Cs+ and (MA)4InCl7:Mn2+. | ||
Furthermore, the PL decay dynamics of (MA)4InCl7, (MA)4InCl7:Cs+ and (MA)4InCl7:Mn2+ are characterized by time-resolved PL (TRPL) spectroscopy at room temperature (Fig. 3b). The PL decay curves can be well fitted using a bi-exponential fitting function as follows:
As shown in Table S6 (ESI†), the short lifetime τ1 and the long lifetime τ2 for all three compounds lie in the range of 2–3 ns (81–84%) and 11–12 ns (19–16%) respectively, resulting in an average PL decay lifetime of 6–7 ns. We attribute the fast decay and the slow decay components to the non-radiative and radiative recombination processes of the excitons, respectively. The faster decay through the non-radiative channel with a larger weighted amplitude could well explain the relatively low PLQE of the three compounds. Also, the radiative recombination rate an a nanosecond scale implies that the emission may be classified as fluorescence originating from singlet excitons. Together with the possible STE emission mechanism, the blue emission of (MA)4InCl7, (MA)4InCl7:Cs+ and (MA)4InCl7:Mn2+ could be attributed to the 1STE to 1S0 transition of In3+ (Fig. 4e), which could be confirmed from the density functional theory (DFT) calculations in the following section.
When it comes to the Sb3+ doped compound (MA)4InCl7:Sb3+, the emission mechanism is a totally different story. Upon Sb3+ doping, partial substitution of InCl63− to SbCl63− was anticipated, due to the similar size and chemical reactivity of the two elements. Compared with that of the pristine (MA)4InCl7, the emission peak of (MA)4InCl7:Sb3+ significantly red-shifted to 611 nm, with the PLQE greatly enhanced to 74.7%. This orange emission is likely to originate from the STE emission from the SbCl63− moiety suggested by previously reported 0D antimony OIMHs and antimony doped 0D indium OIMHs.15,42,43 To prove that, the excitation wavelength–dependent PL spectra of (MA)4InCl7:Sb3+ were first collected at room temperature. There is not much variation in the PL spectra excited at different wavelengths (from 250 to 360 nm) at room temperature, which confirms that the orange emission stems from the same excited states (Fig. S5, ESI†). The large Stokes shift of 170 nm and the broadband emission are typical characteristics of STE emissions.
Temperature-dependent PL spectra were further obtained to confirm the photoluminescence mechanism by monitoring the electron dynamics of the excited states. As shown in Fig. 4a, there emerges another emission peak located at 436 nm when the temperature was lowered to 77 K (excited by 280 nm UV light), which is not observed at room temperature. According to previous studies, the first excited state of OIMHs with 5s2Sb3+ could split into a singlet state (1P1) and triplet states (3Pn, n = 0, 1, and 2) and then go through lattice distortion to form self-trapped excitons.44,45 The 1STE to 3STE transition of Sb3+ was suspended when the temperature was lowered to 77 K due to the energy barrier between the two states, resulting in dual emission at 77 K. The lifetimes of 4.65 ns for the 436 nm emission peak and 6.11 μs for the 578 nm emission peak clearly evidence the fast and slow transitions of 1STE → 1S0 and 3STE → 1S0 respectively (Fig. 4b and c). Therefore, the orange emission of (MA)4InCl7:Sb3+ has originated from the 3STE of SbCl63+ as a result of metal ion substitution and fast 1STE to 3STE transition at room temperature (Fig. 4f). In addition, the absence of emission from InCl63− in (MA)4InCl7:Sb3+ implies efficient energy transfer from excited InCl63− to SbCl63−.
A negative thermal quenching effect was observed for the 578 nm peak of (MA)4InCl7:Sb3+ in the temperature-dependent PL spectra. As shown in Fig. 4d, the peak intensity first increases and then decreases when the temperature increases from 77 K to 297 K. This can be explained by the existence of shallow traps (STs) in 0D OIMHs as suggested by Sun et al.46 The excitons in the shallow traps could be thermally activated to detrap into the STE of (MA)4InCl7:Sb3+ to increase the emission intensity (Fig. 4f). It's intriguing that the negative thermal quenching effect was not observed for (MA)4InCl7, (MA)4InCl7:Cs+ and (MA)4InCl7:Mn2+. This may imply that they possess deeper traps that excitons could not detrap at room temperature. The shallow/deep traps in these 0D indium OIMHs could explain the dramatic enhancement of PLQE when (MA)4InCl7 was doped with Sb3+.
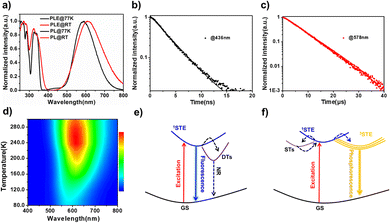
We found a big difference in the crystal morphologies between the pristine and doped ones using a UV-microscope. The photographs of the four compounds under ambient and UV light are shown in Fig. 5a–h. We observed that the doped crystals are more regular-shaped and transparent with fewer bulk defects compared with the parental (MA)4InCl7. This indicates that metal ion doping can improve the crystal quality and reduce bulk defects. The defect passivation effect introduced by metal ion doping may be an imperative reason for the PLQE enhancement in these 0D OIMHs due to the reduced trap-assisted non-radiative recombination centers. Better crystal quality also results in a more homogenous crystal structure, which is supported by the UV absorption spectra of the four compounds. As shown in Fig. S6 (ESI†), (MA)4InCl7, (MA)4InCl7:Cs+ and (MA)4InCl7:Mn2+ exhibit various degrees of Urbach tails which indicate that there are a certain number of sub-band-gap states underneath the conduction band of each structure upon photoexcitation, which could be the result of defects, structural disorders or inhomogeneity of crystals.47–51 When passivated by Cs+ and Mn2+, the sub-band-gap states of (MA)4InCl7 should partially diminished, explaining the reduced FWHM and increased PLQE. A very small Urbach tail was observed for (MA)4InCl7:Sb3+, suggesting minimized sub-band-gap states and homogenous crystals with less defects. Less defect-mediated degradation may explain the high stability of (MA)4InCl7:Sb3+, which maintained a high PLQE when stored under ambient or under continuous UV irradiation for 4 days (Fig. S7, ESI†).
To validate the origin of the emission from the four compounds, we calculated the electronic band structure and partial density of states (PDOS) of (MA)4InCl7, (MA)4InCl7:Cs+, (MA)4InCl7:Mn2+ and (MA)4InCl7:Sb3+ using density functional theory as shown in Fig. 6. Models of (MA)4InCl7:Cs+ and (MA)4InCl7:Mn2+ for DFT calculations were built by partially replacing MA+ by Cs+ and Mn2+ (two MA+ were replaced by one Mn2+ for charge balance) respectively, which could be regarded as the doped Cs+ and Mn2+ occupying the defect sites of MA+ vacancy. The model of (MA)4InCl7:Sb3+ for DFT calculations was based on partial substitution of In3+ by Sb3+. The single crystal structures of these models are listed in Fig. S8 (ESI†) and the calculated band structures of these four compounds are shown in Fig. S9 (ESI†). The calculated band gaps of (MA)4InCl7 and (MA)4InCl7:Cs+ are consistent with their experimental values, while the calculated band gaps of (MA)4InCl7:Mn2+ and (MA)4InCl7:Sb3+ are expected to be underestimated due to the well-known PBE band gap error. Nevertheless, the decreased band gaps upon Mn2+ and Sb3+ doping follow the trend of the calculation results. (MA)4InCl7 exhibits a slightly indirect bandgap of 3.36 eV. The flat conduction band and valence band indicate large effective mass of charges and localized electronic states commonly observed in other 0D OIMHs. The calculated partial density of states suggest that the valence band maximum (VBM) is comprised mostly of Cl 3p, while the conduction band minimum (CBM) is the hybrid of In 5s and Cl 3p orbitals. The contribution of MA+ organic moieties to the band structure is negligible. Therefore, the excitation of (MA)4InCl7 should lead to a self-trapped exciton localized on the InCl63− inorganic moiety, which in turn distorts its structural conformation. The band structure of (MA)4InCl7:Cs+ is almost identical to that of (MA)4InCl7, suggesting that Cs+ was not involved in the electron transition of the blue emission. In contrast, Mn2+ doping introduced mid-gap states, lowering the theoretical bandgap to 2.07 eV. It was reported that doped Mn2+could exhibit red emission arising from the d–d electron transition,12,52–55 which could be the origin of the PL intensity increase at ∼650 nm in Fig. 3a and the absorption increase at 330 nm in Fig. S6 (ESI†). However, these changes of photoluminescent properties upon Mn2+ doping are limited due to the small doping ratio of Mn2+. The blue emission of (MA)4InCl7:Mn2+ should have the same origin as (MA)4InCl7 and the dramatic PLQE enhancement upon Mn2+ doping should come from defect passivation and better crystal quality as analyzed above. Combined with the TRPL results, we conclude that (MA)4InCl7, (MA)4InCl7:Cs+ and (MA)4InCl7:Mn2+ share the same light-emitting mechanism, in which the broadband blue emission with large Stokes shift is the manifestation of the radiative recombination of the singlet excitons from the self-trapped excited states of In3+ (Fig. 4e). On the other hand, Sb3+ doping also introduced mid-gap states which lowers the bandgap to 2.31 eV. Evidenced by the PL properties and negative thermal quenching effect, the high PLQE of (MA)4InCl7:Sb3+ could be attributed to the highly efficient radiative recombination of 3STE on SbCl63− and the absence of deep trap states (Fig. 4f).
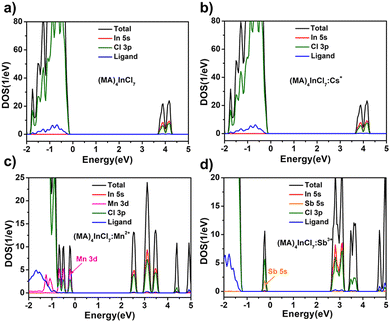 | ||
| Fig. 6 Partial density of states of (a) (MA)4InCl7, (b) (MA)4InCl7:Cs+, (c) (MA)4InCl7:Mn2+ and (d) (MA)4InCl7:Sb3+. | ||
As mentioned above, the pristine (MA)4InCl7 exhibits intense blue emission but quickly turned to orange after the reaction with Sb3+, which makes it a promising antimony ion sensor. To prove this concept, a demo sensor was simply made by mixing (MA)4InCl7 powder with ethanol which do not dissolve the compound. As shown in Fig. 7a, when a small amount of dilute Sb3+ ethanol solution was added to the sensor, the emission under UV rapidly switched to orange accompanied by the PL intensity increase. The working curve of the sensor was measured by correlating the PL intensity of the 610 nm emission with the concentration of Sb3+, which shows a near-linear relationship beneficial for the quantitative analysis. This sensor also exhibits an ultra-low detection limit of 0.1 mM (22.8 ppm) for antimony metal ions, and a fast responsive time of up to 15 s in the working region. We also tested its selectivity by adding solution of other metal ions (Pb2+ and Bi3+), which introduces little changes to its PL spectrum as shown in Fig. S11 (ESI†), suggesting that the sensor is highly selective for Sb3+ among other heavy metal ions.
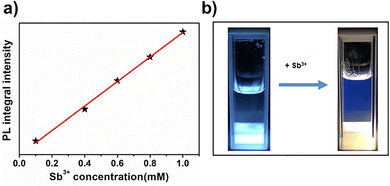 | ||
| Fig. 7 (a) The sensor demo and the PL change after adding Sb3+ ethanol solution (excited by 365 nm UV light). (b) Working curve of the Sb3+ sensor. | ||
Conclusions
In summary, we have developed a series of lead-free 0D indium OIMHs including pristine (MA)4InCl7 and its doped compounds (MA)4InCl7:Cs+, (MA)4InCl7:Mn2+, and (MA)4InCl7:Sb3+. The blue emission of (MA)4InCl7, (MA)4InCl7:Cs+ and (MA)4InCl7:Mn2+ originates from 1STE of its inorganic moiety InCl63−, as determined from experimental characterization and theoretical calculations. By using a metal ion doping strategy, both narrower blue emission and a significantly increased PLQE of up to 18.8% (Cs+ doping) or 20.7% (Mn2+ doping) have been achieved, which are attributed to the defect passivation effect and homogenous crystal growth. Moreover, 7-fold PLQE enhancement and shifted orange emission were realized through Sb3+ doping. The divergent emission originates from 3STE of the altered emission center SbCl63− which makes it a promising candidate for antimony ion sensing. Our work not only proposes several efficient lead-free blue light-emitting materials, but also shows the potential of the metal ion doping strategy in achieving PLQE enhancement and emission spectrum manipulation. We also unprecedently demonstrated a new application of organic indium halide as a metal ion sensor.Author contributions
Lin F, Yu G and Weng S designed and synthesized the samples, performed characterization and investigated the references; Zhou C and Han Y helped to analyze the data; Zhou K collected and analyzed the single crystal X-ray diffraction data. Lin F wrote the paper with support from Lin H, Liu W and Wang Y. Lin H supervised the project and revised the article. All authors contributed to the general discussion.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation of China (22005202) and the Scientific Research Startup Fund for Shenzhen High-Caliber Personnel of SZPT (6022310054k) for financial support.References
- C. Wang, Y. Zhang, F. Gu, Z. Zhao, H. Li, H. Jiang, Z. Bian and Z. Liu, Illumination Durability and High-Efficiency Sn-Based Perovskite Solar Cell under Coordinated Control of Phenylhydrazine and Halogen Ions, Matter, 2021, 4, 709–721 CrossRef CAS.
- R. Gao, M. S. Kodaimati and D. Yan, Recent Advances in Persistent Luminescence Based on Molecular Hybrid Materials, Chem. Soc. Rev., 2021, 50, 5564–5589 RSC.
- Y. Wang, L. Song, Y. Chen and W. Huang, Emerging New-Generation Photodetectors Based on Low-Dimensional Halide Perovskites, ACS Photonics, 2020, 7, 10–28 CrossRef CAS.
- T. Wu, Y. Wang, X. Li, Y. Wu, X. Meng, D. Cui, X. Yang and L. Han, Efficient Defect Passivation for Perovskite Solar Cells by Controlling the Electron Density Distribution of Donor-pi-Acceptor Molecules, Adv. Energy Mater., 2019, 9, 1803766 CrossRef.
- G. Weng, J. Tian, S. Chen, J. Xue, J. Yan, X. Hu, S. Chen, Z. Zhu and J. Chu, Giant Reduction of the Random Lasing Threshold in CH3NH3PbBr3 Perovskite Thin Films by Using a Patterned Sapphire Substrate, Nanoscale, 2019, 11, 10636–10645 RSC.
- M. H. Park, J. S. Kim, J. M. Heo, S. Ahn, S. H. Jeong and T. W. Lee, Boosting Efficiency in Polycrystalline Metal Halide Perovskite Light-Emitting Diodes, ACS Energy Lett., 2019, 4, 1134–1149 CrossRef CAS.
- D. B. Velusamy, M. A. Haque, M. R. Parida, F. Zhang and T. Wu, O. F. Mohammed and H. N. Alshareef, 2D Organic-Inorganic Hybrid Thin Films for Flexible UV-Visible Photodetectors, Adv. Funct. Mater., 2017, 27, 1605554 CrossRef.
- T. Xu, Y. Li, M. Nikl, R. Kucerkova, Z. Zhou, J. Chen, Y.-Y. Sun, G. Niu, J. Tang, Q. Wang, G. Ren and Y. Wu, Lead-Free Zero-Dimensional Organic-Copper(I) Halides as Stable and Sensitive X-ray Scintillators, ACS Appl. Mater. Interfaces, 2022, 14, 14157–14164 CrossRef CAS.
- G. M. Song, Z. Y. Li, P. F. Gong, R. J. Xie and Z. S. Lin, Tunable White Light Emission in a Zero-Dimensional Organic-Inorganic Metal Halide Hybrid with Ultra-High Color Rendering Index, Adv. Opt. Mater., 2021, 9, 7 Search PubMed.
- M. D. Smith, B. A. Connor and H. I. Karunadasa, Tuning the Luminescence of Layered Halide Perovskites, Chem. Rev., 2019, 119, 3104–3139 CrossRef CAS PubMed.
- S. Sun, M. Lu, X. Gao, Z. Shi, X. Bai, W. W. Yu and Y. Zhang, 0D Perovskites: Unique Properties, Synthesis, and Their Applications, Adv. Sci., 2021, 8, 2102689 CrossRef.
- G. Zhou, J. Ding, X. Jiang, J. Zhang, M. S. Molokeev, Q. Ren, J. Zhou, S. Li and X.-M. Zhang, Coordination Units of Mn2+ Modulation Toward Tunable Emission in Zero-Dimensional Bromides for White Light-Emitting Diodes, J. Mater. Chem. C, 2022, 10, 2095–2102 RSC.
- G. J. Zhou, Z. Y. Liu, J. L. Huang, M. S. Molokeev, Z. W. Xiao, C. G. Ma and Z. G. Xia, Unraveling the Near-Unity Narrow-Band Green Emission in Zero-Dimensional Mn2+-Based Metal Halides: A Case Study of (C10H16N)2Zn1-xMnxBr4 Solid Solutions, J. Phys. Chem. Lett., 2020, 11, 5956–5962 CrossRef CAS PubMed.
- Q. Wei, T. Chang, R. Zeng, S. Cao, J. Zhao, X. Han, L. Wang and B. Zou, Self-Trapped Exciton Emission in a Zero-Dimensional (TMA)2SbCl5·DMF Single Crystal and Molecular Dynamics Simulation of Structural Stability, J. Phys. Chem. Lett., 2021, 12, 7091–7099 CrossRef CAS.
- J. L. Li, Y. F. Sang, L. J. Xu, H. Y. Lu, J. Y. Wang and Z. N. Chen, Highly Efficient Light-Emitting Diodes Based on an Organic Antimony(III) Halide Hybrid, Angew. Chem., Int. Ed., 2022, 61, e202113450 CAS.
- L. Lian, P. Zhang, X. Zhang, Q. Ye, W. Qi, L. Zhao, J. Gao, D. Zhang and J. Zhang, Realizing Near-Unity Quantum Efficiency of Zero-Dimensional Antimony Halides through Metal Halide Structural Modulation, ACS Appl. Mater. Interfaces, 2021, 13, 58908–58915 CrossRef CAS PubMed.
- C. Zhou, H. Lin, Y. Tian, Z. Yuan, R. Clark, B. Chen, L. J. van de Burgt, J. C. Wang, Y. Zhou, K. Hanson, Q. J. Meisner, J. Neu, T. Besara, T. Siegrist, E. Lambers, P. Djurovich and B. Ma, Luminescent Zero-Dimensional Organic Metal Halide Hybrids with Near-Unity Quantum Efficiency, Chem. Sci., 2018, 9, 586–593 RSC.
- C. Sun, K. Jiang, M. F. Han, M. J. Liu, X. K. Lian, Y. X. Jiang, H. S. Shi, C. Y. Yue and X. W. Lei, A Zero-Dimensional Hybrid Lead Perovskite with Highly Efficient Blue-Violet Light Emission, J. Mater. Chem. C, 2020, 8, 11890–11895 RSC.
- H. Lin, C. Zhou, M. Chaaban, L. J. Xu, Y. Zhou, J. Neu, M. Worku, E. Berkwits, Q. He, S. Lee, X. Lin, T. Siegrist, M. H. Du and B. Ma, Bulk Assembly of Zero-Dimensional Organic Lead Bromide Hybrid with Efficient Blue Emission, ACS Mater. Lett., 2019, 1, 594–598 CrossRef CAS.
- C. Zhou, H. Lin, M. Worku, J. Neu, Y. Zhou, Y. Tian, S. Lee, P. Djurovich, T. Siegrist and B. Ma, Blue Emitting Single Crystalline Assembly of Metal Halide Clusters, J. Am. Chem. Soc., 2018, 140, 13181–13184 CrossRef CAS PubMed.
- R. Roccanova, A. Yangui, H. Nhalil, H. Shi, M.-H. Du and B. Saparov, Near-Unity Photoluminescence Quantum Yield in Blue-Emitting Cs3Cu2Br5–xIx (0 ≤ x ≤ 5), ACS Appl. Electron. Mater., 2019, 1, 269–274 CrossRef CAS.
- T. Jun, K. Sim, S. Iimura, M. Sasase, H. Kamioka, J. Kim and H. Hosono, Lead-Free Highly Efficient Blue-Emitting Cs3Cu2 I5 with 0D Electronic Structure, Adv. Mater., 2018, 30, e1804547 CrossRef PubMed.
- J. Li, T. Inoshita, T. Ying, A. Ooishi, J. Kim and H. Hosono, A Highly Efficient and Stable Blue-Emitting Cs5Cu3Cl6 I2 with a 1D Chain Structure, Adv. Mater., 2020, 32, e2002945 CrossRef PubMed.
- Z. Zhou, Y. Li, Z. Xing, Z. Li, K. S. Wong and J. E. Halpert, Potassium and Rubidium Copper Halide A2CuX3 (A = K, Rb, X = Cl, Br) Micro- and Nanocrystals with Near Unity Quantum Yields for White Light Applications, ACS Appl. Nano Mater., 2021, 4, 14188–14196 CrossRef CAS.
- T. D. Creason, T. M. McWhorter, Z. Bell, M.-H. Du and B. Saparov, K2CuX3 (X = Cl, Br): All-Inorganic Lead-Free Blue Emitters with Near-Unity Photoluminescence Quantum Yield, Chem. Mater., 2020, 32, 6197–6205 CrossRef CAS.
- P. Li, X. Liu, Y. Zhang, C. Liang, G. Chen, F. Li, M. Su, G. Xing, X. Tao and Y. Song, Low-Dimensional Dion-Jacobson-Phase Lead-Free Perovskites for High-Performance Photovoltaics with Improved Stability, Angew. Chem., Int. Ed., 2020, 59, 6909–6914 CrossRef CAS PubMed.
- C. Ni, G. Hedley, J. Payne, V. Svrcek, C. McDonald, L. K. Jagadamma, P. Edwards, R. Martin, G. Jain, D. Carolan, D. Mariotti, P. Maguire, I. Samuel and J. Irvine, Charge Carrier Localised in Zero-Dimensional (CH3NH3)3Bi2I9 Clusters, Nat. Commun., 2017, 8, 170 CrossRef.
- S. Feng, Y. Ma, S. Wang, S. Gao, Q. Huang, H. Zhen, D. Yan, Q. Ling and Z. Lin, Light/Force-Sensitive 0D Lead-Free Perovskites: From Highly Efficient Blue Afterglow to White Phosphorescence with Near-Unity Quantum Efficiency, Angew. Chem., Int. Ed., 2022, e202116511, DOI:10.1002/anie.202116511.
- J. H. Wei, J. F. Liao, L. Zhou, J. B. Luo, X. D. Wang and D. B. Kuang, Indium-Antimony-Halide Single Crystals for High-Efficiency White-Light Emission and Anti-counterfeiting, Sci. Adv., 2021, 7, 9 Search PubMed.
- Y. Zhang, S. Yuan, Y. Yuan, Y. Bao, Q. Ran, E. Liu, J. Fan and W. Li, Alleviation of π–π* Transition Enabling Enhanced Luminescence in Emerging TpyInClx (x = 3, 5) Perovskite Single Crystals, Adv. Opt. Mater., 2021, 10, 2102041 CrossRef.
- Y. Y. Ma, H. Q. Fu, X. L. Liu, Y. M. Sun, Q. Q. Zhong, W. J. Xu, X. W. Lei, G. D. Liu and C. Y. Yue, Zero-Dimensional Organic–Inorganic Hybrid Indium Chlorides with Intrinsic Blue Light Emissions, Inorg. Chem., 2022, 61, 8977–8981 CrossRef CAS.
- D. Chen, S. Hao, G. Zhou, C. Deng, Q. Liu, S. Ma, C. Wolverton, J. Zhao and Z. Xia, Lead-Free Broadband Orange-Emitting Zero-Dimensional Hybrid (PMA)3InBr6 with Direct Band Gap, Inorg. Chem., 2019, 58, 15602–15609 CrossRef CAS PubMed.
- Z. Li, G. Song, Y. Li, L. Wang, T. Zhou, Z. Lin and R. J. Xie, Realizing Tunable White Light Emission in Lead-Free Indium(III) Bromine Hybrid Single Crystals through Antimony(III) Cation Doping, J. Phys. Chem. Lett., 2020, 11, 10164–10172 CrossRef CAS PubMed.
- Y. Wu, C. M. Shi, L. J. Xu, M. Yang and Z. N. Chen, Reversible Luminescent Vapochromism of a Zero-Dimensional Sb3+-Doped Organic-Inorganic Hybrid, J. Phys. Chem. Lett., 2021, 12, 3288–3294 CrossRef CAS PubMed.
- D. Cortecchia, S. Neutzner, A. R. Srimath Kandada, E. Mosconi, D. Meggiolaro, F. De Angelis, C. Soci and A. Petrozza, Broadband Emission in Two-Dimensional Hybrid Perovskites: The Role of Structural Deformation, J. Am. Chem. Soc., 2017, 139, 39–42 CrossRef CAS PubMed.
- R. Zhang, X. Mao, Y. Yang, S. Yang, W. Zhao, T. Wumaier, D. Wei, W. Deng and K. Han, Air-Stable, Lead-Free Zero-Dimensional Mixed Bismuth-Antimony Perovskite Single Crystals with Ultra-broadband Emission, Angew. Chem., Int. Ed., 2019, 58, 2725–2729 CrossRef CAS PubMed.
- B. M. Benin, D. N. Dirin, V. Morad, M. Woerle, S. Yakunin, G. Raino, O. Nazarenko, M. Fischer, I. Infante and M. V. Kovalenko, Highly Emissive Self-Trapped Excitons in Fully Inorganic Zero-Dimensional Tin Halides, Angew. Chem., Int. Ed., 2018, 57, 11329–11333 CrossRef CAS PubMed.
- M. Li and Z. Xia, Recent progress of zero-dimensional luminescent metal halides, Chem. Soc. Rev., 2021, 50, 2626–2662 RSC.
- D. Chen, S. Hao, L. Fan, Y. Guo, J. Yao, C. Wolverton, M. G. Kanatzidis, J. Zhao and Q. Liu, Broad Photoluminescence and Second-Harmonic Generation in the Noncentrosymmetric Organic-Inorganic Hybrid Halide (C6H5(CH2)4NH3)4MX7 center dot H2O (M = Bi, In, X = Br or I), Chem. Mater., 2021, 33, 8106–8111 CrossRef CAS.
- V. Morad, S. Yakunin and M. V. Kovalenko, Supramolecular Approach for Fine-Tuning o the Bright Luminescence from Zero-Dimensional Antimony(III) Halides, ACS Mater. Lett., 2020, 2, 845–852 CrossRef CAS PubMed.
- C. Zhou, Y. Tian, Z. Yuan, H. Lin, B. Chen, R. Clark, T. Dilbeck, Y. Zhou, J. Hurley, J. Neu, T. Besara, T. Siegrist, P. Djurovich and B. Ma, Highly Efficient Broadband Yellow Phosphor Based on Zero-Dimensional Tin Mixed-Halide Perovskite, ACS Appl. Mater. Interfaces, 2017, 9, 44579–44583 CrossRef CAS.
- J. Lin, K. Liu, H. Ruan, N. Sun, X. Chen, J. Zhao, Z. Guo, Q. Liu and W. Yuan, Zero-Dimensional Lead-Free Halide with Indirect Optical Gap and Enhanced Photoluminescence by Sb Doping, J. Phys. Chem. Lett., 2022, 13, 198–207 CrossRef CAS PubMed.
- V. Morad, S. Yakunin and M. V. Kovalenko, Supramolecular Approach for Fine-Tuning of the Bright Luminescence from Zero-Dimensional Antimony(III) Halides, ACS Mater. Lett., 2020, 2, 845–852 CrossRef CAS PubMed.
- J. Xu, S. Li, C. Qin, Z. Feng and Y. Du, Identification of Singlet Self-Trapped Excitons in a New Family of White-Light-Emitting Zero-Dimensional Compounds, J. Mater. Chem. C, 2020, 124, 11625–11630 CAS.
- K. M. McCall, V. Morad, B. M. Benin and M. V. Kovalenko, Efficient Lone-Pair-Driven Luminescence: Structure-Property Relationships in Emissive 5s2 Metal Halides, ACS Mater. Lett., 2020, 2, 1218–1232 CrossRef CAS.
- B. B. Zhang, J. K. Chen, J. P. Ma, X. F. Jia, Q. Zhao, S. Q. Guo, Y. M. Chen, Q. Liu, Y. Kuroiwa, C. Moriyoshi, J. Zhang and H. T. Sun, Antithermal Quenching of Luminescence in Zero-Dimensional Hybrid Metal Halide Solids, J. Phys. Chem. Lett., 2020, 11, 2902–2909 CrossRef CAS PubMed.
- X. Meng, S. Ji, Q. Wang, X. Wang, T. Bai, R. Zhang, B. Yang, Y. Li, Z. Shao, J. Jiang, K. L. Han and F. Liu, Organic-Inorganic Hybrid Cuprous-Based Metal Halides for Warm White Light-Emitting Diodes, Adv. Sci., 2022, e2203596, DOI:10.1002/advs.202203596.
- V. Diez-Cabanes, J. Even, D. Beljonne and C. Quarti, Electronic Structure and Optical Properties of Mixed Iodine/Bromine Lead Perovskites, Adv. Opt. Mater., 2021, 9, 2001832 CrossRef CAS.
- F. Liu, Y. Zhang, C. Ding, S. Kobayashi, T. Izuishi, N. Nakazawa, T. Toyoda, T. Ohta, S. Hayase, T. Minemoto, K. Yoshino, S. Dai and Q. Shen, Highly Luminescent Phase-Stable CsPbI3 Perovskite Quantum Dots Achieving Near 100% Absolute Photoluminescence Quantum Yield, ACS Nano, 2017, 11, 10373–10383 CrossRef CAS.
- S. De Wolf, J. Holovsky, S. J. Moon, P. Loper, B. Niesen, M. Ledinsky, F. J. Haug, J. H. Yum and C. Ballif, Organometallic Halide Perovskites: Sharp Optical Absorption Edge and Its Relation to Photovoltaic Performance, J. Phys. Chem. Lett., 2014, 5, 1035–1039 CrossRef CAS.
- F. Urbach, The Long-Wavelength Edge of Photographic Sensitivity and Electronic Absorption of Solids, Phys. Rev., 1953, 92, 1324–1326 CrossRef CAS.
- Z. Yu, H. Peng, Q. Wei, T. Huang, S. Yao, Y. Tian, C. Peng and B. Zou, The Magnetic Polaron Modulated Luminescence Bands of Organic-Inorganic Hybrid Ferroelectric Anti-Perovskite (C3H9N)3Cd2Cl7 Doped with Mn2+, Mater. Today Chem., 2022, 24, 100781 CrossRef CAS.
- S. Yan, W. Tian, H. Chen, K. Tang, T. Lin, G. Zhong, L. Qiu, X. Pan and W. Wang, Synthesis of 0D Manganese-Based Organic–Inorganic Hybrid Perovskite and Its Application in Lead-Free Red Light-Emitting Diode, Adv. Funct. Mater., 2021, 31, 2100855 CrossRef CAS.
- B. Su, G. Zhou, J. Huang, E. Song, A. Nag and Z. Xia, Mn2+-Doped Metal Halide Perovskites: Structure, Photoluminescence, and Application, Laser Photonics Rev., 2021, 15, 2000334 CrossRef CAS.
- H. Yuan, F. Massuyeau, N. Gautier, A. B. Kama, E. Faulques, F. Chen, Q. Shen, L. Zhang, M. Paris and R. Gautier, Doped Lead Halide White Phosphors for Very High Efficiency and Ultra-High Color Rendering, Angew. Chem., Int. Ed., 2020, 59, 2802–2807 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, crystallographic data, PXRD patterns, TGA data, UV-vis absorption spectra and PL spectra. CCDC 2195355–2195358. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qm00866a |
| This journal is © the Partner Organisations 2023 |