Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Upper-rim functionalised calix[4]arenes for chemoselective Au3+ detection†
Sean P.
Bew
 *a,
Sunil V.
Sharma
*a,
Sunil V.
Sharma
 b and
William H.
Gardiner
c
b and
William H.
Gardiner
c
aSchool of Chemistry, UEA, Norwich Research Park, Norwich, NR4 7TJ, UK. E-mail: s.bew@uea.ac.uk
bSchool of Chemistry, University of St Andrews, Purdie Building, North Haugh, St Andrews, Scotland, UK
cEvotec UK Ltd, Innovation Drive, Milton Park, Abingdon, Oxfordshire, OX14 4RZ, UK
First published on 14th March 2023
Abstract
A unique, readily synthesised, upper-rim 1,3-difunctionalised calix[4]arene is reported; equipped with a fluorophore and an alkyne it mediates the efficient chemoselective detection of Au3+. Its ability to detect Au3+ is not perturbed by Au+ or excess competing and or contaminating metal e.g. platinum, cadmium, mercury, silver, sodium, magnesium or potassium cations.
Introduction
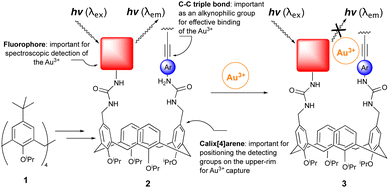
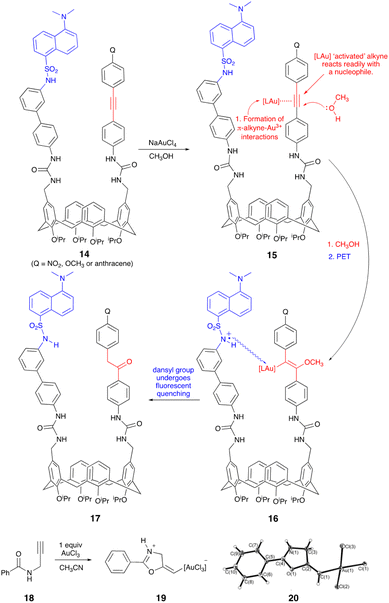
Owing to its operational simplicity and sensitivity, fluorescence spectroscopy is a powerful method for metal cation detection. Appending fluorescent and metal recognition ‘antennas’ onto easily synthesized and readily functionalized calix[4]arenes affords motifs with the potential to chemoselectively detect and differentiate between metal cations.1 A wide variety of metal detectors have previously been developed. Indeed, many are utilized in diverse applications across the breadth of science and includes chemistry, biology, biochemistry, and medicine. A typical, but non-exhaustive list of uses includes detecting polluting toxic metals2e.g., lead, mercury, cadmium, or the development of motifs for cell microscopy and the in vivo location of biologically important metal ions such as iron, calcium, zinc, sodium, potassium and copper3 as well as the non-invasive imaging of calcium in the human brain.4Recent years have witnessed a sharp increase in the use of gold in developing new methodology based on, for example, homogeneous and heterogeneous catalysis. It is, however, not the sole preserve of chemists. As noted, its potential has been recognized, explored, and unlocked in biology, medicine, and physics. For example, Au55 clusters ([Au55(Ph2PC6H4SO3)12]12−) are used to visualize cell component's5 and in medicine, Tauredon, Solganal, Sanocrycin, and Auranofin are used to fight asthma, HIV, malaria, lesions, cancer, and arthritis. Thus, although gold is in many respects ‘compatible’ it can have adverse effects on the environment,6 and in biological systems Au3+ metallodrugs can be reduced to Au+.7 For these reasons and due to its increased use, the development of new modalities and protocols for differentiating Au3+/Au+ are urgently required. Ideally, these must be of relatively low cost, easily synthesized, chemoselective and, importantly, they should not be perturbed by other metal contaminants e.g., mercury, silver, platinum, and cadmium. No reaction-based fluorescent probes for gold detection have been developed which proceed via a ‘turn off’ mechanism and use readily synthesized calix[4]arenes derived from 1 (Scheme 1).8 Exploiting our expertise and knowledge of calix[4]arenes and their upper-rim chemical functionalization9 we were intrigued by the possibility of transforming scaffold 1 into a chemospecific Au3+ metal-binder (2) which specifically presents, facilitates and, subsequently, reports its presence via adjacent alkyne and dansyl ‘antenna’ (cf.3, Scheme 1). Attracted by the chemical and operational simplicity of our system, the straightforward synthesis of entities based on 3 uses easily generated starting material 1 and proceeds via a simple, readily observable ‘turn-off’ mechanism (i.e., 2 → 3) when the metal is bound/detected (Scheme 1). With strategies to generate upper-rim functionalized calix[4]arenes in hand, key to our proposed application is the π-aurophilic alkyne which chemoselectively ‘captures’ the Au3+. Substantiating this as a mode of action, Ultimoto and Fukuda reported internal alkynes react readily with a catalytic source of Au3+ in the form of sodium tetrachloroaurate which in aqueous methanol transforms an alkyne into a ketone (17, Scheme 3).10 With the gold ‘captured’ the conformational mobility of the calix[4]arene11 helps facilitate the fluorescent dansyl group to move closer and interact with the metal whilst ‘reporting’ its presence via fluorescent quenching (Scheme 1).
With this rationale in mind we were also convinced of the merits of generating entities based on 2 due to the widespread use of the calix[4]arene motif in alternate metal detection regimes which, when dovetailed with the known ability of gold salts to mediate efficient internal nonradiative relaxation decay, results in fluorescent quenching (FQ).12 Supporting our approach to gold detection are the abundant applications of the calix[4]arene motif in metal recognition but, surprisingly, the lack of any specific calixarene-based Au3+ modality. Thus, although there are many examples of cone-confined calix[4]arenes with lower-rim metal cation sensing properties,13 this fact contrasts sharply with the handful of known metal sensing upper-rim functionalized calix[4]arenes.14
Results and discussion
We wanted to synthesize upper-rim functionalized 2 with three structural components, namely an alkyne, dansyl and calix[4]arene macrocycle, and test it in a proof-of-concept study. Initiating this required an efficient and straightforward route to bis-5,17-N-arylurea derived calix[4]arene 4. Two synthetic routes were envisaged. The first used commercially available 4-iodophenyl isocyanate and the easily synthesized bis-5,17-aminomethylene derived cone-confined calix[4]arene (not shown).15 Their reaction in THF at ambient temperature afforded 4 in a 90% yield which was, after methanol trituration, pure enough to use in the next step. An alternative ionic hydrogenation route16 used triphenylsilane, TFA and two commercially available starting materials i.e., bis-5,17-diformylcalix[4]arene and 4-iodophenylurea. This afforded 4 in an unoptimised 63% yield. Installing the alkyne (4 → 5) was straightforward. A Sonogashira coupling between 4 and 1 equivalent of phenylacetylene was catalysed by tri-tert-butylphosphine (20 mol%), copper(I) iodide (20 mol%) and bis (triphenylphosphine)palladium(II) dichloride (7 mol%).The desired mono-phenylacetylene-derived 5 (Scheme 2) was used ‘as is’ in the next step [an estimated 15% of the corresponding di-phenylacetylene adduct (not shown) was also formed]. A subsequent Suzuki–Miyaura coupling with 3-(dansylamino)phenylboronic acid (6) catalysed by bis(triphenylphosphine)palladium(II) dichloride (5 mol%), tri-tert-butylphosphine (10 mol%) and potassium carbonate afforded, after chromatographic purification, the desired product 7. We propose the detection capabilities of 7 (vide infra) rely on the capacity of the dansyl group and π-aurophilic alkyne to ‘communicate’ with the gold only when all three are in close proximity (cf.3, Scheme 1). Clearly, it was important to verify the presence of the dansyl and alkyne groups, as well as the conformation of the calix[4]arene. Frustrated by its reluctance to afford crystals suitable for X-ray diffraction, we analyzed the 13C-NMR data associated with the bridging methylene's which, Mendoza et al. report, allow the conformation of a calix[4]arene to be established.17 The 13C-NMR signal at 31 ppm confirmed 7 resided in a cone-conformation. Additional physicochemical studies using FT-IR and mass spectrometry verified the dansyl and alkyne groups were present. A fundamental reason for incorporating and using the dansyl group is its strong fluorescence signal and long emission wavelength. Probing the photophysical properties of 7 a 5 μM solution had an excitation absorption maximum at 288 nm (λex) which generated a strong fluorescent emission signal (λem) with a λmax of 522.9 nm. A weaker signal was observed at 353.9 nm (Scheme 2). Confirming the signal at 522.9 nm was broadly like other λem of alternative upper- and lower-rim dansyl-derived calix[4]arenes our value is in general agreement with literature reports of λmax at 538 nm and 520–575 nm respectively.18 Recording additional spectra of 7 every two minutes for 45 minutes we established the emission (λem) intensity remained constant. This validated the fluorophore was photochemically stable.
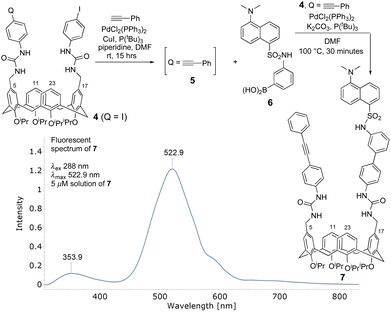 | ||
| Scheme 2 Synthesis of upper-rim bis-5,17-functionalised calix[4]arene 7 from bis-5,17-N-urea-derived calix[4]arene 4. | ||
It was important to assess if 7 was susceptible to non-chemoselective metal mediated FQ. That is, does it generate false positives? Screening individual solutions of Na+, K+, Cs+, Ca2+ and Mg2+ salts for their effect, if any, on the strong λem signal would help answer this question. Adding 1–5 equivalents of aqueous sodium chloride (2.5 μM) afforded almost identical ‘before’ and ‘after’ λem spectra, even after 3 hours (cf. Scheme 2 with Fig. 1 in the ESI†). Similarly, 1–5 equivalents of a mixture of Li+, Na+, K+, Cs+, Ca2+ and Mg2+ salts (all at 2.5 μM) had little effect. There are reports that Group 12 metal cations FQ the dansyl group. For example, Bartsch et al. described a calix[4]arene appended on the lower-rim with two dansyl groups as a fluorogenic Hg2+ selective extractant which underwent FQ.19 Screening individual solutions of Group 12 salts [cadmium acetate, mercury(II) chloride, hydrogen tetrachloroplatinate and silver tetrafluoroborate] had little effect on the photophysical properties of 7.
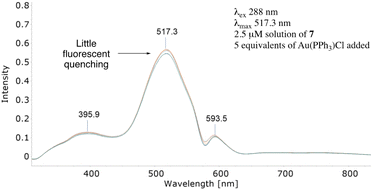
Indeed, only with >15 equivalents and extended mixing times was partial FQ observed (see ESI†). Further stress testing its photophysical properties 1–5 equivalents of a mixture of Group 2 (Mg2+ and Ca2+), Group 10 (Pt4+) and Group 12 (Cd2+ and Hg2+) salts had minimal effect on 7's λmax, even after 3 hours (Fig. 1).
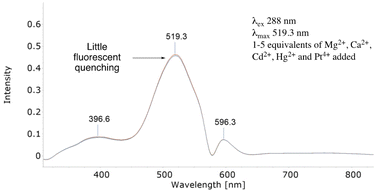 | ||
| Fig. 1 The fluorescent spectrum above of 7 outlines how a mixture of Group 2, 10 and 12 metal salts had little FQ effect on 7. | ||
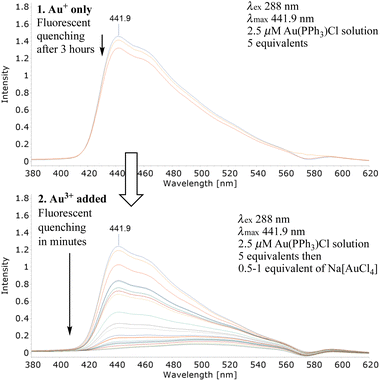
Au3+ and Au+ are strong Lewis acids, indeed both can activate alkynes to nucleophilic attack with weak nucleophiles e.g., water or simple alcohols.20 Clearly, if 7 was not able to chemoselectively differentiate Au3+ and Au+ its use would be severely limited. With this in mind we were delighted adding 5 equivalents of chloro(triphenylphosphine)gold(I) to 7 had little impact on the λmax, even after 3 hours (Fig. 2).
With a reliable synthesis in hand and robust photophysical data confirming little evidence of FQ with either single or mixtures of metal salts, it was essential to establish if 7 was able to ‘capture, sense and report’. Adding a solution of sodium tetrachloroaurate to a 5 μM solution of 7, an almost instantaneous reduction in the λmax signal was observed (Fig. 3). Increasing the equivalents from 1 to 2, 3 and 4 induced further fluorescent quenching and a reduction in the λmax intensity (Fig. 3). The lack of FQ with 7 in a protic solvent when presented with Group 10 or 12 metal salts contrasts sharply with observations using the gold salt (Group 11); this suggests key to FQ is the formation of significant and favourable interactions between the alkyne on 7 and the highly charged Au3+.
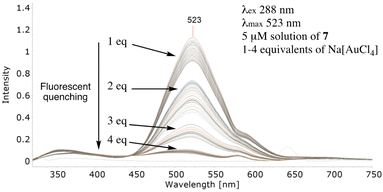 | ||
| Fig. 3 Efficient fluorescent quenching of 7 appended with an upper-rim dansyl and phenylacetylene group. | ||
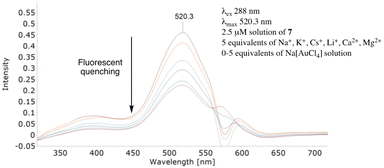
Evidently, bifunctional calix[4]arene 7 readily and efficiently detected the gold salt in the absence of, potentially, competing metal salts. Critical to its wider application however is its ability to retain this property when presented with a mixture of metal salts. So, do externally added salts inhibit or complicate the use of 7? Generating individual solutions of (2.5 μM) sodium chloride, potassium hexafluorophosphate, calcium chloride, magnesium chloride and cesium chloride (see ESI†) as well as a mixture (2.5 μM) of lithium chloride, sodium chloride, potassium hexafluorophosphate, cesium chloride, magnesium chloride and calcium chloride their addition (5 equivalents each) to 7 and, subsequently, sodium tetrachloroaurate (0–5 equivalents) allowed us to compare ‘contaminated’ (Fig. 4) with a ‘non-contaminated’ gold salt solution (Fig. 3). Fig. 4 clearly demonstrates the strong λmax signal at 520.3 nm was quenched in a manner comparable to ‘pure’ sodium tetrachloroaurate. Further probing and testing the robustness of a ‘contaminated’ system employed a mixture of 20 equivalents of cadmium acetate, mercury(II) chloride and hydrogen tetrachloroplatinate. Comparing Fig. 4 with Fig. 5 their similarities validated the efficient FQ by Au3+ to be unaffected by extraneous, potentially, competing salts.
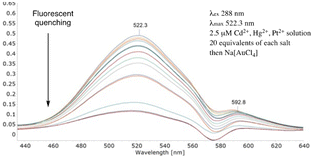 | ||
| Fig. 5 Fluorescent quenching of 7 in the presence of 20 equivalents each of Cd2+, Hg2+ and Pt4+ salts. | ||
Thus far, the alkyne was assumed to be an integral component of 7, confirming this was essential. Hydrogenation of 7 afforded the alkyne-free hydrogenated ‘diphenylethane’ adduct (not shown). Acquiring its fluorescent spectrum after 30 equivalents of sodium tetrachloroaurate had been added verified it did not undergo FQ. This result substantiates the key role the alkyne has in, presumably, ‘capturing’ and acting as an ‘aurophile’ bringing the metal into close proximity to the dansyl group generating ‘alkyne-Au3+-dansyl’ interactions (cf.3) which, ultimately, initiate FQ. Seemingly, removing its ability to form a gold-alkyne complex is the first step since, by a process of elimination, the alkyne-free system seems unable to form gold-dansyl interactions which, if formed, would still, presumably, result in FQ.
Although the exact role the macrocycle occupies is not clear, its presence is evidently important when combined with the two upper-rim substituents. Testing for a ‘macrocycle’ effect ‘linear’ macrocycle-free 8 was generated from readily available starting materials. With its urea and dansyl modalities (Fig. 6) matching those of its cyclic ‘cousin’, 8 afforded the perfect opportunity to evaluate the importance of the calix[4]arene. Adding increasing equivalents (up to 20) of sodium tetrachloroaurate a slight reduction in FQ was observed. The propensity of sulfonamide groups to bind cationic metal centers is known.21 Thus, it seems plausible the observed FQ is associated with intermolecular functional group interactions derived from two molecules of 8 combining to form a weak ‘dansyl-Au3+-dansyl’ complex like 9 (Fig. 6). The ability of upper-rim appended urea-derived calix[4]arenes to bind anions is well documented.22 Aqueous solutions of sodium tetrachloroaurate are known to undergo rapid equilibrium with water generating hydroxytrichloroaurate and chloride.23 With its two upper-rim derived urea's 7 has the potential to generate multiple hydrogen-bonds and bind chloride (10) near the alkyne and dansyl gold binding motifs. Unfortunately, quantifying this using NMR and mass spectrometry proved inconclusive. Likewise generating crystals of 10 suitable for X-ray diffraction was unsuccessful. Our thoughts focused instead on the possibility that 7 when reacted with tetraethylammonium tetrafluoroborate affords a hydrogen bonded BF4− complex (cf.11) with the tetraethylammonium cation in close proximity to the tetrafluoroborate anion, a consequence of electrostatic attraction.
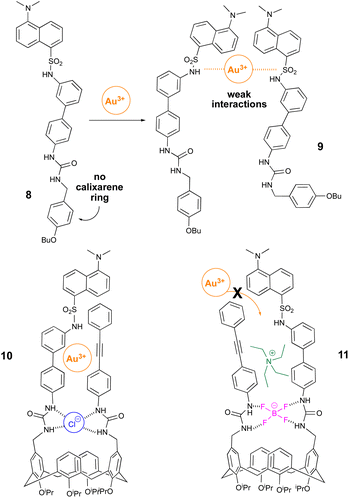 | ||
| Fig. 6 Examples of motifs generated to probe the role of the calix[4]arene (8) and the two urea's (10 and 11). | ||
We anticipated the formation of 11 would reduce gold π-alkyne co-ordination and, consequently, diminish FQ. Mindful of reports outlining N-tetraalkylammonium cations residing in, largely unsubstituted upper-rim derived calix[4]arene cavities,24 we considered it unlikely the bulky tetraethylammonium would occupy the calix[4]arene cavity of 7; a fact further compounded by its sterically encumbered upper-rim substituents. Stirring 7 with 5 equivalents of tetraethylammonium tetrafluoroborate afforded, presumably, the urea N–H bonded BF4− with its Et4N+ counterion (11, Fig. 6). Although attempts to substantiate the formation of 11via X-ray diffraction were unsuccessful when the gold salt was added sequentially (>30 equivalents), a relatively small drop in the λmax was observed (ESI†). This indicates the metal is largely blocked from interacting with the triple bond and dansyl group of 11. Accordingly, we surmise from 8 and 11 that two urea groups are key parts of the architecture of 7. These preliminary results establish the importance of the alkyne, with this in mind we wanted to probe this further exploring the stereoelectronic effect associated with reducing or increasing alkyne electron-density and, consequently, its ability to interact with the Au3+. Our assumption focused on the premise that reducing alkyne electron density (12) would reduce its interaction with the metal and, as such, afford reduced fluorescent quenching. Of course, on this basis, incorporating electron-rich13 the opposite would be expected; that is, increased fluorescent quenching. The synthesis of para-nitrophenyl-derived 12 and para-methoxyphenyl 13 followed the same general procedure outlined in Scheme 2 in 56% and 68% yields respectively.
Subjecting separate solutions of metal-free 12 and 13 to fluorescent analysis (2.5 μM, λex 288 nm, λmax 520.7 nm) established, as anticipated, the λem of 12 to have reduced fluorescence intensity (Fig. 8) whilst electron-rich 13 exhibited increased intensity (Fig. 9). Sequential addition of 1, 2, 3, 4 and 5 equivalents of aqueous sodium tetrachloroaurate afforded, under otherwise identical conditions, a greater reduction in the fluorescence intensity of electron-rich13. Accordingly, we surmise increasing electron-density on the alkyne resulted, as anticipated, in augmented Au3+ sensitivity.
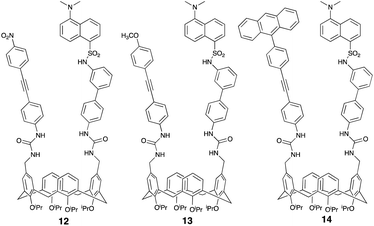 | ||
| Fig. 7 Dansyl- and urea-derived cone-calix[4]arenes synthesised and appended with an electron-poor (12), electron-rich (13) or an anthracene-derived alkyne (14). | ||
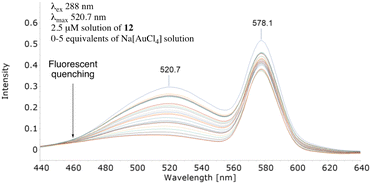 | ||
| Fig. 8 Effect on the metal-mediated fluorescent quenching properties of 12 when a conjugated electron-withdrawing para-nitrophenyl group is appended. | ||
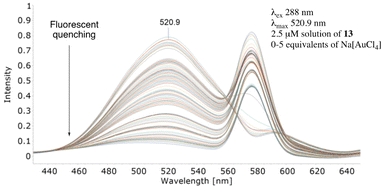 | ||
| Fig. 9 Effect on the metal-mediated fluorescent quenching properties of 13 when a conjugated electron-donating para-methoxyphenyl group is appended. | ||
Preliminary work established 7 did not detect Au+ (Fig. 2). However, it is known that alkyne activation using Au+ or Au3+ co-ordination (π → σ*) is facilitated if the electrophilic, cationic intermediate is stabilised via extensive delocalisation.25 Having synthesised extensively conjugated 9-(4-ethynylphenyl)anthracene-derived 14 (Fig. 7) from 4 in 2 steps (58% overall yield) we wanted to probe its ability to display enhanced sensitivity to Au3+ in the presence of Au+. Mixing solutions of 14 (2.5 μM) and 5 equivalents of chloro(triphenylphosphine)gold(I), the emission spectrum was acquired over 3 hours; little change was observed viz. Fig. 10. To the same solution containing the Au+ was added 1 equivalent of sodium tetrachloroaurate (cf.7). An almost immediate reduction in the λem and rapid, fluorescent quenching was observed (Fig. 10).
Mechanism of fluorescent quenching
Our preliminary results using 7–8 and 12–14 substantiate the importance of the ‘triad’ comprising the calix[4]arene, the alkyne and the fluorophore (cf.3). Focusing on the two key properties outlined below, we wanted to rationalize the observed efficient fluorescent quenching of the fluorophore based on:(i) The ability of a calix[4]arene bearing an N-sulphonylcarboxamide e.g., 7, 12–14 to interact with Au3+.
(ii) The propensity of the alkyne to act as an aurophile inducing electron-charge transfer from the alkyne to the metal which stimulates alkyne polarization and subsequent reaction with a nucleophile.
Interestingly, although alkyne coordinated linear Au+ complexes have been known since the 1970′s, alkyne π-systems comprising square-planar Au3+ have, only recently, been verified.26 Nevertheless, invoking the findings of Belanzoni et al.27 we propose Au3+-alkyne coordination proceeds via a classically described dominant σ-component coupled with smaller, but significant, π-back-donation (Dewar–Chatt–Duncanson model). Resulting in significant polarization of the π-alkyne electrons the Au3+ induces strong triple bond polarization and increased alkyne reactivity; this facilitates nucleophilic attack by, for example, a protic solvent ( 15 → 16, Scheme 3).28 Supporting the proposed formation of (E)-vinylgold(III) species 16 we note, with interest, the identification via X-ray diffraction of 19, see crystal structure 20 (Scheme 3). Oxyauration of N-(propargyl)benzamide 18 using Au3+ activates the C–C triple bond (Scheme 3) which initiates a 5-exo-trig cyclisation and the formation of 19 with the Au3+ in a square-planar complex.29 As noted earlier, support for ketone formation (17, Scheme 3) was from a 1991 report by Ultimoto and Fukuda who described how a wide variety of structure and function diverse alkynes readily react in the presence of 2 mol% sodium tetrachloroaurate and aqueous methanol affording ketones in excellent yield. Importantly, and with reference to our observed lack of FQ with chloro(triphenylphosphine)gold(I), when Ultimoto incorporated gold(I) potassium dicyanoaurate [KAu(CN)2] the reaction completely failed to mediate any alkyne hydration.10 As the dansyl group on 7 is slightly acidic (pKa 3.8 and 9.9) partial sulphonamide dissociation in methanol would afford a dansyl anion which we propose has the potential to undergo complex formation with the closely located, upper-rim bound Au3+. Thus, overall and consistent with known mechanisms of transition-metal ion-induced fluorescent quenching the ligand-immobilized excited dansyl fluorophore is quenched via a photoinduced electron transfer (PET) mechanism from the excited dansyl group which is in close proximity to the π-[LAu]-bound complex (15 → 16, Scheme 3).
Conclusions
We report preliminary, first examples of simple, effectively designed macrocycles which when appended with a fluorophore and an alkyne react efficiently via a chemoselective aurophilic process. Our study demonstrates the synergistic behaviour of an alkyne which, when dovetailed with a dansyl fluorophore and a calix[4]arene, co-operatively and chemoselectively differentiates Au3+ in the presence of Au+. Of note, the presence of sodium, potassium, cesium, magnesium or calcium salts as well as potentially alkyne-activating Group 12 metals such as cadmium, mercury and platinum salts do not interfere with the aurophilic properties of 7. Given the generality of our synthetic approach, the utility/high demand for the compounds described it is our opinion this work has significant potential for the development of new sensing platforms with applications in the environment and or biology. Furthermore, due to the overarching simplicity and straightforward synthetic methodology outlined in this proof-of-concept study it is our hope this work will pave the way for future advances in supramolecular-based modalities with real-time target applications. In summary, we consider the work outlined herein to be of general interest to, not only, macrocyclic, organic and synthetic chemistry-based academics but will also garner the attention of industrial and analytical chemists working at the frontiers of biological, pharmaceutical, agrochemical, analytical and materials chemistry.Author contributions
SPB undertook project conceptualization, supervision and project administration. SVS and WHG undertook the methodology and reaction investigation studies.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the financial assistance of the UEA (CHE43.3.60), the EU Interreg Manche/Channel cross-border projects Innovative Synthesis: Chemistry and Entrepreneurship (IS:CE-chem: ref. 4061) and ‘Academy-Industry chemistry channel’ (AIcc: ref. 4196), Interreg, Ministry of Defence (R19648) and the EPSRC UK National Mass Spectrometry Facility at Swansea University.References
- J. S. Kim and D. T. Quang, Calixarene-Derived Fluorescent Probes, Chem. Rev., 2007, 107, 3780 CrossRef CAS PubMed.
- B. N. Ahamed, I. Ravikumar and P. Ghosh, A new chemosensor that signals Hg(II), Cu(II) and Zn(II) at different emission wavelengths: selectivity toward Hg(II) in acetonitrile, New J. Chem., 2009, 33, 1825 RSC; W. Lin, X. Cao, Y. Ding, L. Yuan and L. Long, A highly selective and sensitive fluorescent probe for Hg2+ imaging in live cells based on a rhodamine-thioamide-alkyne scaffold, Chem. Commun., 2010, 46, 3529 RSC; T. Cheng, Y. Xu, S. Zhang, W. Zhu, X. Qian and L. Duan, A highly sensitive and selective OFF-ON fluorescent sensor for cadmium in aqueous solution and living cell, J. Am. Chem. Soc., 2008, 130, 16160 CrossRef CAS PubMed; H. Mu, R. Gong, Q. Ma, Y. Sun and E. Fu, A novel colorimetric and fluorescent chemosensor: synthesis and selective detection for Cu2+ and Hg2+, Tetrahedron Lett., 2007, 48, 5525 CrossRef; E. M. Nolan and S. J. Lippard, A “turn on” fluorescent sensor for the selective detection of mercuric ion in aqueous media, J. Am. Chem. Soc., 2003, 125, 14270 CrossRef PubMed.
- C. Yu, L. Cheng, J. Zhang, J. Li, P. Liu, W. Wang and B. Yan, “Off-On” based fluorescent chemosensor for Cu2+ in aqueous media and living cells, Talanta, 2011, 85, 1627 CrossRef CAS PubMed; W.-Y. Liu, H.-Y. Li, B.-X. Zhao and J.-Y. Miao, A new fluorescent and colourimetric probe for Cu2+ in live cells, Analyst, 2012, 137, 3466 RSC; L.-J. Fan and W. E. Jones, A highly selective and sensitive inorganic/organic hybrid polymer fluorescence “turn-on” chemosensory system for iron cations, J. Am. Chem. Soc., 2006, 128, 6784 CrossRef PubMed.
- K. L. Rogers, S. Picaud, E. Roncali, R. Boisgard, C. Colasante, J. Stinnakre, B. Tavitian and P. Brûlet, Non-invasive in vivo imaging of calcium signalling in mice, PLoS One, 2007, 10, e974 CrossRef PubMed.
- W. Zi and F. D. Toste, Recent advances in enantioselective gold catalysis, Chem. Soc. Rev., 2016, 45, 4567 RSC; R. Ciriminna, E. Falletta, C. D. Pina, J. H. Teles and M. Pagliaro, Industrial applications of gold catalysis, Angew. Chem., Int. Ed., 2016, 55, 14210 CrossRef CAS PubMed.
- M.-C. Daniel and D. Astruc, Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications towards biology, catalysis, and nanotechnology, Chem. Rev., 2004, 104, 293 CrossRef CAS PubMed.
- I. Tolbatov, A. Marrone, C. Coletti and N. Re, Computational studies of Au(I) and Au(III) anticancer metallodrugs: A survey, Molecules, 2021, 26, 7600 CrossRef CAS PubMed.
- S. Singha, D. Kim, H. Seo, S. W. Cho and K. H. Ahn, Fluorescence sensing systems for gold and silver species, Chem. Soc. Rev., 2015, 44, 4367 RSC; J. F. Zhang, Y. Zhou, J. Yoon and J. S. Kim, Recent progress in fluorescent and colourimetric chemosensors for detection of precious metal ions (silver, gold and platinum ions), Chem. Soc. Rev., 2011, 40, 3416 RSC.
- R. A. Brimage, S. P. Bew, S. V. Sharma and N. L'Hermite, Upper-rim appended hybrid calixarenes via click chemistry, Org. Lett., 2007, 9, 3713 CrossRef PubMed.
- Y. Fukuda and K. Ultimoto, Effective transformation of unactivated alkynes into ketones or acetals with a gold(III) catalyst, J. Org. Chem., 1991, 56, 3729 CrossRef CAS.
- Comprehensive Supramolecular Chemistry II, ed. G. W. Gokel and L. Barbour, Elsevier, 2017 Search PubMed.
- C.-C. Huang, Z. Yang, K.-H. Lee and H.-T. Chang, Synthesis of highly fluorescent gold nanoparticles for sensing mercury(II), Angew. Chem., Int. Ed., 2007, 46, 6824 CrossRef CAS PubMed.
- V. S. Talanov, E. D. Roper, N. M. Buie and G. G. Talanova, A new fluorogenic mono-ionizable calix[4]arene dansylcarboxamide as as selective chemosensor of soft metal ions, Tl+ and Hg2+, Tetrahedron Lett., 2007, 48, 8022 CrossRef CAS; S. K. Kim, S. H. Lee, J. Y. Lee, R. A. Barsch and J. S. Kim, An excimer-based, binuclear, on-off switchable calix[4]crown chemosensor, J. Am. Chem. Soc., 2004, 126, 16499 CrossRef PubMed; E. D. Roper, G. G. Talanova, N. M. Buie, M. G. Gorbunova, R. A. Bartsch and V. S. Talanova, Novel fluorogenic calix[4]arene- bis (crown ether) for selective recognition of thallium(I), Chem. Commun., 2005, 5673 Search PubMed; T. Nabeshisma, T. Saiki, J. Iwabuchi and S. Akine, Stepwise and dramatic enhancement of anion recognition with a triple-site receptor based on the calix[4]arene framework using two different cationic effectors, J. Am. Chem. Soc., 2005, 127, 5507 CrossRef PubMed.
- Z. Liang, Z. Liu, L. Jiang and Y. Gao, A new fluorescent chemosensor for copper(II) and molecular switch controlled by light, Tetrahedron Lett., 2007, 48, 1629 CrossRef CAS; X. H. Sun, W. Li, P. F. Xia, H.-B. Luo, Y. Wie, M. S. Wong, Y.-K. Cheng and S. Shuang, Phenyl-calix[4]arene-based fluorescent sensors: cooperative binding for carboxylates, J. Org. Chem., 2007, 72, 2419 CrossRef PubMed; G.-K. Li, Z.-X. Xu, C.-F. Chen and Z.-T. Huang, A highly efficient and selective, colorimetric and near-infrared fluorescent turn-on chemosensor for Cu2+ based on BODIPY, Chem. Commun., 2008, 1774 RSC; T.-L. Kao, C.-C. Wang, Y.-T. Pan, Y.-J. Shaio, J.-Y. Yen, C.-M. Shu, G.-H. Lee, S.-M. Peng and W.-S. Chung, Upper-rim allyl- and arylazo-coupled calix[4]arenes as highly sensitive chromogenic sensors for Hg2+ ion, J. Org. Chem., 2005, 70, 2912 CrossRef PubMed; I.-T. Ho, G.-H. Lee and W.-S. Chung, Synthesis of upper-rim allyl and p-methoxyphenylazocalix[4]arenes and their efficiencies in chromogenic sensing of Hg2+ ion, J. Org. Chem., 2007, 72, 2434 CrossRef PubMed.
- M. Rezankova, J. Budka, J. Miksatko, V. Eigner, I. Cisarova, P. Curinova and P. Lhotak, Anion receptors based on intramolecularly bridged calix[4]arenes bearing ureido functions, Tetrahedron, 2017, 73, 742 CrossRef CAS.
- R. A. Brimage, G.-H. Gipson, S. P. Bew, S. V. Sharma and S. Thurston, Hybrid calix[4]arenes via ionic hydrogenation and transition-metal-mediated processes, Org. Lett., 2009, 11, 2483 CrossRef PubMed.
- C. Jaime, J. de Mendoza, P. Prados, P. M. Nieto and C. Sánchez, Carbon-13 NMR chemical shifts. A single rule to determine the conformation of calix[4]arenes, J. Org. Chem., 1991, 56, 3372 CrossRef CAS.
- R. Métivier, I. Leray and B. Valeur, Lead and mercury sensing by calixarene-based fluoroionophores bearing two or four dansyl fluorophores, Chem. – Eur. J., 2004, 10, 4480 CrossRef PubMed; Calixarenes and beyond, ed. P. Neri, J. L. Sessler and M.-X. Wang, Springer, 2016 Search PubMed.
- G. G. Talanova, N. S. A. Elkarim, V. S. Talanov and R. A. Bartsch, A calixarene-based fluorogenic reagent for the selective mercury(II) recognition, Anal. Chem., 1999, 71, 3106 CrossRef CAS PubMed.
- R. Casado, M. Contel, M. Laguna, P. Romero and S. Sanz, Organometallic gold(III) compounds as catalysts for the addition of water and methanol to terminal alkynes, J. Am. Chem. Soc., 2003, 125, 11925 CrossRef CAS PubMed.
- A. Ashraf, W. A. Siddiqui, J. Akbar, G. Mustafa, H. Krautscheid, N. Ullah, B. Mirza, F. Sher, M. Hanif and C. G. Hartinger, Metal complexes of benzoimidazole derived sulfonamide: Synthesis, molecular structures and antimicrobial activity, Inorg. Chim. Acta, 2016, 443, 179 CrossRef CAS; S. Hamura, T. Oda, Y. Shimizu, K. Matsubara and H. Nagashima, “Bidentate” and “tridentate” sulfonamide ligands for titanium complexes: crystal structures and solution dynamics elucidating an η2- or η3-coordination mode, J. Chem. Soc., Dalton Trans., 2002, 1521 RSC; T. Topala, A. Pascual-Álvarez, M. Á. Moldes-Tolosa, A. Pascual-Álvarez, M. Á. Moldes-Tolosa, A. Bodoki, A. Castiñeiras, J. Torres, C. Del Pozo, J. Borrás and G. Alzuet-Piña, New sulfonamide complexes with essential metal ions [Cu(II), Co(II), Ni(II), and Zn(II)], Effect of the geometry and the metal ion on DNA binding and nuclease activity, BSA protein interaction, Inorg. Biochem., 2020, 202, 110823 CrossRef PubMed.
- B. Schazmann, N. Alhashimy and D. Diamond, Chloride selective calix[4]arene optical sensor combining urea functionality with pyrene excimer transduction, J. Am. Chem. Soc., 2006, 128, 8607 CrossRef CAS PubMed.
- V. Amendola, L. Fabbrizzi and L. Mosca, Anion recognition by hydrogen bonding: anion-based receptors, Chem. Soc. Rev., 2010, 39, 3889 RSC.
- P. Thuery, Z. Asfari, N. Zouhair and J. Vicens, Two triethylammonium ion complexes of monoanionic calix[4]arene, Acta Crystallogr., 2002, C58, o223–o225 CAS.
- Gold Catalysis: A Homogenous Approach, ed. M. Veronique and D. F. Toste, World Scientific, 2014 Search PubMed.
- L. Rocchigiani, J. Fernandez-Cestau, G. Agonigi, I. Chambrier, P. H. M. Budzelaar and M. Bochmann, Gold(III) alkyne complexes: Bonding and reaction pathways, Angew. Chem., Int. Ed., 2017, 56, 13861 CrossRef CAS PubMed.
- L. Gregori, D. Sorbelli, L. Belpassi, F. Tarantelli and P. Belanzoni, Alkyne activation with gold(III) complexes: A quantitative assessment of the ligand effect by charge-displacement analysis, Inorg. Chem., 2019, 58, 3115 CrossRef CAS PubMed.
- C. H. Leung, M. Baron and A. Biffs, Gold-catalyzed intermolecular alkyne hydrofunctionalizations, Catalysts, 2020, 10, 1210 CrossRef CAS.
- O. A. Egorova, H. Seo, Y. Kim, D. Moon, Y. M. Rhee and K. H. Ahn, Characterization of vinylgold intermediates: Gold-mediated cyclization of acetylenic amides, Angew., Chem. Int. Ed., 2011, 50, 11446 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3qo00021d |
| This journal is © the Partner Organisations 2023 |