Open Access Article
Open Access ArticleDES: their effect on lignin and recycling performance
Penghui Li
 ab,
Zihui Zhang
b,
Xiaoxue Zhang
ab,
Kongyan Li
ab,
Yongcan Jin
ab,
Zihui Zhang
b,
Xiaoxue Zhang
ab,
Kongyan Li
ab,
Yongcan Jin ab and
Wenjuan Wu
*ab
ab and
Wenjuan Wu
*ab
aJiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, Nanjing Forestry University, Nanjing, 210037, China. E-mail: wenjuanwu@njfu.edu.cn
bCollege of Light Industry and Food Engineering, Nanjing Forestry University, Nanjing, 210037, China
First published on 24th January 2023
Abstract
Lignocellulosic biomass raw materials are renewable resources with abundant reserves in nature, and have many advantages, such as being green, biodegradable and cheap. Lignin, one of the three significant components of lignocellulose, possesses a chemical structure rich in phenylpropane and is a primary aromatic resource for the bio-based economy. For the extraction and degradation of lignin, the most common method is the pretreatment of lignocellulose with deep eutectic solvents (DES), which have similar physicochemical properties to ionic liquids (ILs) but address the disadvantages associated with ILs (DES have the advantages of low cost, low toxicity, and non-flammability). In lignocellulose pretreatment, a large amount of solvent is generally required to achieve the desired effect. However, after treatment, a substantial volume of solvent will be wasted, and thus, the problem of the recovery and reuse of DES solution needs to be adequately solved. The methods and mechanisms of perfect DES regeneration will be discussed from the perspective of the elemental composition and features of DESs in this review, which will also outline the present DES recovery methods, such as rotary evaporation, membrane separation, freeze-drying, electrodialysis, etc. The detailed process and the advantages and disadvantages of each method since 2018 are introduced in detail. Future DES recovery methods have been prospected, and the optimization of the functional properties of DESs after recovery is discussed. It is expected to find a convenient and efficient application method for DES extraction or degradation of lignin with low energy and low cost.
Introduction
Fossil fuels, which are more than 80% derived from crude oil, natural gas, or coal, are used globally for the preparation of industrial base chemicals as well as for energy supply.1 According to the World Energy Outlook 2019, the global demand for natural energy is expected to increase at an annual rate of 1.3% over the next 20 years. If no attempts are made to improve energy efficiency or develop new sources of energy, the world's resources will be depleted.2,3 Lignocellulose biomass, such as agricultural and forestry residues or energy crops, which are readily available and abundant, can meet about 14% of the world's needs.4 Due to the above-mentioned energy shortage, lignocellulose is gaining interest as a renewable carbon resource for fuels and chemicals.5,6 Currently, research into the pretreatment of biomass is well established, and its fractionation and the processing of its components lay the foundation for biorefining.7The purpose of pretreatment is to disrupt the structure of lignocellulose. The pretreated fractionated fractions such as lignin and sugars can be used as feedstocks to produce chemicals and for conversion into energy.8 Pretreatment of lignocellulose provides the possibility of value-addition to lignin, but it is challenging to ensure that the lignin structure is not destroyed during lignin fractionation. Depending on the transformation steps applied, the physical and chemical properties of the extracted lignin can be different, and the structure of the obtained lignin is diverse. This structural complexity and diversity create resistance to the large-scale application of lignin. Since 2018, many advances have been made in the utilization of lignin, and there is an urgent need for a more efficient and economical lignin fractionation process to reduce structural complexity and open new horizons.9
Until recently, organic solvents were often used to extract bioactive compounds (e.g., acetone, benzene, toluene, cyclohexane, chloroform, dimethyl sulfoxide, N,N-dimethylformamide, tetrahydrofuran, ethyl acetate), but these conventional solvents are often toxic and volatile, causing severe environmental stress.10 Nowadays, deep eutectic solvent (DES) is widely used for lignocellulose pretreatment in the scientific community and are considered to be a “green” alternative to typical organic solvents due to their ease of preparation, low cost, low toxicity, biocompatibility, and biodegradability.11 A DES consists of two or three components that are bonded by hydrogen bonding to form a deep eutectic mixture with a melting point much lower than that of a pure single component. The most commonly used hydrogen bond acceptor (HBA) for DES preparation is choline chloride (ChCl), which can be bound to a hydrogen bond donor (HBD; e.g., urea, glycerol, carbohydrate-derived polyols or carboxylic acids, etc.).6 In a recent study, the excellent delignification ability of the acidic DES hydrogen bond acceptor ChCl and efficient lignocellulose fractionation were reported.13 In DES pretreatment with ChCl/lactic acid, a large number of β–O–4 bonds break, with partial breakage of carbon–carbon bonds (i.e., β–β′, β–5′); various monomeric phenols were also obtained in low yields, which validated the prospect of the conversion of lignin to monomeric chemicals.14 Alvarez-Vasco et al.15 used four DESs (i.e., from ChCl/acetic acid, ChCl/lactic acid, ChCl/levulinic acid, and ChCl/glycerol) for pretreatment of hardwood and cork. Large amounts of lignin could be obtained from the wood with high purity, and the obtained lignin had distinct structural characteristics. In the process of DES treatment, the ether bonds between the phenylpropane units are cleaved.
The most basic principle of green chemistry is to follow the principle of “safer solvents and additives”, in which the solvents must first ensure that the whole process is more environmentally friendly. However, lignocellulose pretreatment requires a large amount of solvent, so the solvent must be green, cheap and easy to recover.16 After the application of DES in biomass refining, the recovery and recycling of DES after treatment is inevitable.8 According to the literature, DES systems have many advantages, such as low cost and environmental friendliness. In the synthesis or recycling of DES, the essence lies in the formation or breaking of molecular bonds between the hydrogen bond donor and the hydrogen bond acceptor, and it is not easy for other side reactions to occur, so it is judged that DES is better than ionic liquids in terms of recycling.17 The recycling of DES reaction system solvents is also about to become a hot direction.18 When investigating the effect of DES recycling and reuse, attention should be paid to the performance of DES in cellulose digestion and the extraction or depolymerization of lignin in order to reduce the cost somewhat by reusing the pretreatment solvent efficiently.19 The poor recycling effect or non-recyclability of DES makes it challenging to apply most DESs for mass production.20
Definition and composition of DES
DES has been called a “modified version of ionic liquids” in terms of green sustainability because they have many similarities with ionic liquids.21 A DES is a simple mixture of hydrogen bond acceptors (mainly ammonium halides), hydrogen bond donors (e.g., amides, hydroxyl and carboxylic acid compounds). The strong hydrogen bonding between the HBA and HBD causes the electron density of the HBA and HBD to change distribution, resulting in a much lower melting point for the DES than that of the HBA or HBD alone. The resulting solvent system can be an excellent alternative solvent for green chemistry applications.22 It is also because of the hydrogen bonding that DES have very low volatility compared to conventional solvents. DESs have a wide variety of structures, as the HBA and HBD for building the DES can be arbitrarily paired, and different pairings will also have different physical and chemical properties.23 In recent years, the concept of natural deep eutectic solvents (NADESs), which are DESs composed of several natural compounds such as choline derivatives, sugars, and simple organic acids, has been proposed. NADES is a new branch of DES that still deserves to be explored in depth.24 Table 1 lists some typical DES compositions with examples.| Type | Composition | General formulaa | Typical examples |
|---|---|---|---|
| a Cat+ represents any ammonium, phosphonium or sulfonium cation; X represents a Lewis base, generally a halide anion; z: number of y molecules interacting with the anion. | |||
| I | Metal salt + organic salt | Cat+X−zMClx; M = Zn, Sn, Fe, Al, Ga, In | ZnCl2 + ChCl |
| II | Metal salt hydrate + organic salt | Cat+X−zMClx·yH2O; M = Cr, Co, Cu, Ni, Fe | CrCl2·6H2O + ChCl |
| III | HBD + organic salt | Cat+X−zRZ; Z = CONH2, COOH, OH | Urea + ChCl |
| IV | Zinc/aluminium chloride + HBD | MClx + RZ = MClx−1+·RZ + MClx+1−; M = Al, Zn and Z = CONH2, OH | AlCl3 + (CH2OH)2 |
Preparation and characterization of DES
There are many ways to formulate a DES. The most classic method is to heat a mixture of an HBA and HBD in an oil or water bath at about 80 °C and stir until a clear liquid is formed; they can also be dissolved in water and then heated until the water is removed.27 In addition to the two methods mentioned above, there are also the microwave radiation method, grinding method, ultrasonic method, freeze-drying method and other methods to prepare a DES.Some of the physical and chemical properties of deep eutectic solvents are similar to those of ionic liquids. Compared to organic solvents, DESs have the advantages of biodegradability and low vapor pressure. Some DESs are toxic, with type III (organic salts and HBD) DESs being the least toxic and type I (organic and metal salts) DESs being the most toxic.28,29 They also have high thermal stability, low volatility, and convertible polarity, and can thus be used as suitable solvents in future alternative research and industry.30 The HBD molecule interacts with the chloride ion of ChCl and induces the lower melting point of the blend caused by cationic molecular symmetry, etc.
The charge delocalization process arising from intermolecular hydrogen bonding is generally applicable to salt–HBD mixtures. Based on DFT, the relationship between the molecular structure type of a DES and its Tf can be studied31.
Table 2 summarizes most of the more classical DES species and their physical properties. Interestingly, the physical properties of DESs, such as density, viscosity, freezing point, and even electrical conductivity, vary widely. Density can also reflect some essential characteristics of DESs, with most of them having a higher density than water. Electrical conductivity is affected by temperature; the kinetic energy of the molecules increases and the frequency of intermolecular collisions becomes faster the DES warms up, and thus, the electrical conductivity of DES is proportional to temperature.32 Viscosity, similar to electrical conductivity, is also an important property of deep eutectic solvents. Most of deep eutectic solvents have a high viscosity (η > 100 mPa s, 25 °C) as well as a wide range of viscosity due to hydrogen bonding. Abbott et al.33 tested the solubility of more than a dozen metal oxides commonly used in ChCl-based DESs and found that there are active metal oxides (e.g., ZnO) that are almost entirely soluble; in contrast, many covalent oxides (e.g., TiO2) are practically insoluble. The temperature has some influence on solubility, for example, the solubilities of metal oxides (Cu2O and ZnO) are very much influenced by temperature.
| DES composition | HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD mole ratio HBD mole ratio |
Freezing point/°C | Density/(g cm−3) | Viscosity/(mPa s) | Surface tension/(mN m−1) (25 °C) | Conductivity/(mS cm−1) | Ref. |
|---|---|---|---|---|---|---|---|
| Choline chloride/1,3-dimethyl urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
70 | — | — | — | — | 12 |
| Choline chloride/1,1-dimethyl urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
149 | — | — | — | — | 12 |
| Choline chloride/benzamide | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
92 | — | — | — | — | 12 |
| Choline chloride/urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
12.0 | 1.25 | 750 (25 °C) | 52.00 | 0.75 (25 °C) | 25 |
| Choline chloride/glycerol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
17.8 | 1.18 | 259 (25 °C) | 55.80 | 1.05 (25 °C) | 25 |
| Choline chloride/glycerol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
— | 1.20 | 450 (20 °C) | — | — | 34 |
| Choline chloride/ethylene glycol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
−12.9 | 1.12 | 37 (25 °C) | 49.00 | 7.61 (25 °C) | 25 |
| Choline chloride/butylene glycol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
−32.0 | 1.06 | 140 (20 °C) | 47.17 | 1.64 (25 °C) | 25 |
| Choline chloride/propanedioic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10.0 | — | 721 (25 °C) | 65.70 | 0.55 (25 °C) | 26 |
| Choline chloride/trifluoroacetic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
— | 1.34 | 77 (40 °C) | 35.90 | 0.29 (40 °C) | 34 |
| Choline chloride/trifluoroacetamide | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
51.0 | 1.34 | 77 (40 °C) | — | — | 25 |
| Choline chloride/zinc chloride | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
— | — | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (25 °C) 000 (25 °C) |
— | 0.06 (42 °C) | 25 |
| Choline chloride/chromium chloride crystals | — | — | — | 2346 (25 °C) | 77.30 | 0.37 (25 °C) | 26 |
| Choline chloride/imidazole | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
56.0 | — | 15 (70 °C) | — | 12.00 (60 °C) | 25 |
| Tetrabutylammonium bromide/imidazole | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
— | — | 810 (20 °C) | — | 0.24 (20 °C) | 25 |
| Methyltriphenylphosphonium bromide/glycerol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
−5.55 | 1.30 | — | 58.94 | 0.06 (25 °C) | 25 |
| Methyltriphenylphosphonium bromide/ethylene glycol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
−49.3 | 1.23 | — | 51.29 | 1.09 (25 °C) | 25 |
| Methyltriphenylphosphonium bromide/triglyceride | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
−21.0 | 1.19 | — | 49.58 | — | 25 |
| Zinc chloride/glycol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
— | 1.45 | — | — | — | 25 |
| Zinc chloride/urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
9 | 1.63 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340 (25 °C) 340 (25 °C) |
— | 0.18 (42 °C) | 25 |
| Ethylammonium chloride/urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
— | 1.14 | 128 (40 °C) | 52.90 | 0.35 (40 °C) | 25 |
| Ethylammonium chloride/acetamide | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
— | 1.04 | 64 (40 °C) | — | 0.69 (40 °C) | 25 |
| Choline chloride/adipic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
85 | — | — | — | — | 35 |
| Choline chloride/malonic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 | — | — | — | — | 35 |
| Choline chloride/benzoic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95 | — | — | — | — | 35 |
| Choline chloride/phenylacetic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 | — | — | — | — | 35 |
| Choline chloride/phenylpropionic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 | — | — | — | — | 35 |
| Choline chloride/oxalic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
34 | — | — | — | — | 35 |
| Choline chloride/citric acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
69 | — | — | — | — | 35 |
Role of DES in lignocellulose processing
Extraction of lignin
The processing of lignocellulose is complex and difficult, and the efficient extraction of lignin is a challenging problem.36 Malaeke et al.37 solubilized lignin up to 50% w/w (the most impressive lignin extraction in most solvents) in a DES consisting of resorcinol–ChCl coupled with ultrasonic irradiation, and the solubility of cellulose in the prepared DES is very low; thus, the complete isolation of lignin is no longer a dream. Often, the detachment of hemicellulose prior to lignin extraction from lignocellulose has an impact on DES treatment. Lou et al.38 studied the effect of hemicellulose removal on the later DES extraction of wheat straw lignin with ChCl and lactic acid, and they found that DES pre-soaking facilitated lignin extraction by breaking the ether bond between lignin and hemicellulose and causing cell wall rupture and swelling. In fact, DES is required to break a variety of aryl ether and ester bonds in lignin for complete lignin extraction. The two-stage DES treatment method, with prepreg at room temperature followed by extraction at high temperature, can produce high yields and purity of lignin. A schematic diagram of the pretreatment process is shown in Fig. 1. The DES consisting of choline chloride with lactic acid can trigger H-bonding, polarity, π–π and ionic/charge interactions with lignin during the pretreatment. In addition, lactic acid can promote hemicellulose hydrolysis and thus lignin extraction. The mechanism of lignin solubilization in DES is the cleavage of many types of aryl ether and ester bonds in lignin, while in an acidic DES, protons catalyze the cleavage of the ethers and esters present in the lignin–carbohydrate complex (LCC), leading to lignin extraction and depolymerization.39 During DES pretreatment, the H-bonds between OH⋯Cl in DES are stronger than the OH⋯O bonds in lignin. The presence of large amounts of Cl− allows the DES to cleave the LCC in the biomass as well as some ether bonds in lignin. In addition, the ChCl–LA DES is able to slightly cleave the ether bonds even at ambient temperature.40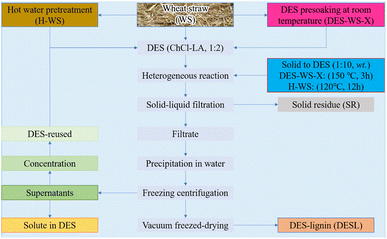 | ||
Fig. 1 Schematic diagram of two-step wheat straw fractionation and DES (ChCl–LA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) recovery.38 2) recovery.38 | ||
Degradation of lignin
DES also has the ability to degrade lignin. However, this ability has not been widely exploited. Li et al.18 used ChCl–p-TsOH (ChCl–p-toluenesulfonic acid) to degrade lignin under mild reaction conditions; the ether bonds in lignin were broken, and regenerated lignin (some phenols and ketones, and a few aldehydes) with a low molecular weight and narrow polydispersity index was obtained.In addition, the depolymerization of lignin in DES can be achieved electrochemically. Di Marino et al.41,42 attempted the electrochemical depolymerization of industrial lignin in DES, and the depolymerization yielded lignin oligomers and some aromatic monomers (e.g., vanillin, guaiacol, etc.). A complete set of processes for lignin extraction and depolymerization was achieved in the same DES, reducing the number of steps and energy consumption. The good solubilization ability of the DES itself for lignin led to an enhanced yield in terms of depolymerization. In addition, DESs can cleave the ether bond through two pathways. The first degradation route is the removal of Cα alcohols and the formation of highly reactive benzyl carbons in the lignin side chain, with the disruption of the Cα double bond and the conjugated structure, which is the classical mechanism of lignin depolymerization in an acidic DES.43 The second degradation route is the oxidation of the Cα position and the acylation of the Cγ position, during which the OH group at the Cα position is oxidized. The Cα ketone is essential to facilitate the cleavage of the β-O-4′ linkage, and the mechanism of C–O cleavage involves formylation, elimination and hydrolysis.44 In addition to cleavage of the β-O-4′ bond, other possible reactions such as dehydration, acylation, deethoxylation, and condensation were also observed during DES pretreatment, as shown in Fig. 2.13
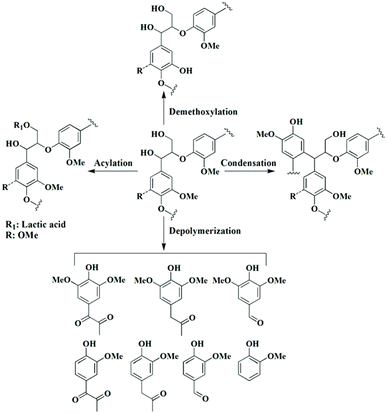 | ||
| Fig. 2 Possible pathways of lignin degradation during DES treatment.13 | ||
DES recovery and utilization in lignocellulose pretreatment
The role played by DES in the extraction or depolymerization of lignin from numerous lignocelluloses cannot be underestimated, and its recovery and recycling are critical due to the large volume of DES consumed. There are several methods to recover DES efficiently. Rotary evaporation is the simplest and most common one;45–47 other recovery methods are membrane separation (nanofiltration and reverse osmosis),46 freeze-drying47 and electrodialysis.48 In the following sections, each method will be described in detail and the performance of the recovered DES reapplied to the lignin extraction and depolymerization process will be evaluated. In fact, there are only two methods of solvent recovery: one is to extract the treated solute product from the solvent, e.g., membrane filtration and extraction; the other is to transfer the solvent out of the reaction, e.g., rotary evaporation and electrodialysis.Rotary evaporation (vacuum evaporation)
The rotary evaporation method is based on a rotary vacuum evaporator, which is based on the principle of evaporating the solution to be extracted by heating and diffusion in a rotary flask under negative pressure. The solution is collected by liquefaction in a cooler. Li et al.45 used vacuum evaporation for DES recovery, and after five cycles at 90 °C, the hydrogen bonding interactions in DES solutions decrease and the acidity decreases, and the delignification capacity of aqueous DES solutions decreases by about 20%, with a lactic acid/ChCl aqueous solution recovery of 69%. Xie et al.46 pretreated radiata pine using a deep eutectic solvent consisting of benzyltrimethylammonium chloride/formic acid. The recovered DES may have impurities in the product, which caused the lignin selectivity to become low and the yield to decrease after five recovery cycles, probably around 10%. The yield after the decrease still managed to reach 66.6%, which was still better than that of the alkaline ethanol extraction. These recovery and utilization measures strongly reflect the promising prospects of DES application in industry. Thulluri et al.48 subjected rice straw to DES–THF treatment. After separating the lignin, the DES solvent mixture at the end of the reaction was subjected to rotary evaporation. The DES was paired once again into a new solution and then treatment was continued with the fresh rice straw. According to the experimental results, the continuously reconstituted DES–THF solvent could go through 10 cycles, and the solvent recovery rate was consistently above 74%. After 10 cycles, the cellulose yield was still 91% and the hemicellulose yield was 68%, and the lignin concentration of 46% could be maintained to be removed. This study fully reflects the recoverability of DES, and the purity of the DES obtained is still considerable. Not coincidentally, Kumar et al.49 added tetrahydrofuran/sodium chloride aqueous solution to the ChCl/lactic acid system to achieve liquid surface separation, and the obtained DES also contained sodium chloride, which had to be evaporated off using a rotary evaporator. Ethanol was added to dissolve the DES to separate it from the salt, and finally, the ethanol simply had to be removed to obtain pure DES. Kumar et al.50 used lactic acid/betaine and lactic acid/ChCl to form a NADES and used it to pretreat rice straw lignocellulose, after which the NADES reagent was recovered from the aqueous solution by rotary evaporation at 60 °C. The recovered NADES was utilized in place of the new pretreatment chemical, with no noticeable difference in the results.Phadtare et al.51 prepared a ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio) DES system for degradation and explored the recovery properties of the DES at the end of the reaction and its efficacy when reused. The recovery of DES was performed by stirring extraction with water, filtering under a vacuum with a funnel, and then evaporating the water in a rotary evaporator, after which the DES was purified and the yield did not decrease much in the evaluation of the degradation performance of the recovered DES. Similarly, Li et al.18 used a DES composed of p-toluenesulfonic acid with higher acidity and ChCl to degrade alkaline lignin and recovered the DES at the end of the reaction (Fig. 3). First, alkaline lignin was dissolved in DES (1
2 molar ratio) DES system for degradation and explored the recovery properties of the DES at the end of the reaction and its efficacy when reused. The recovery of DES was performed by stirring extraction with water, filtering under a vacuum with a funnel, and then evaporating the water in a rotary evaporator, after which the DES was purified and the yield did not decrease much in the evaluation of the degradation performance of the recovered DES. Similarly, Li et al.18 used a DES composed of p-toluenesulfonic acid with higher acidity and ChCl to degrade alkaline lignin and recovered the DES at the end of the reaction (Fig. 3). First, alkaline lignin was dissolved in DES (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 mass ratio), and then 250 µL distilled water was added. The degradation reaction was allowed to complete. 100 mL of distilled water was added to precipitate the lignin; the mixture was then centrifuged, and the solid residue was washed repeatedly to obtain the regenerated lignin. The supernatant was collected, the water was evaporated via vacuum evaporation, and the purified DES was then recovered. DES recovery is complex and diverse, and Yan et al.52 evaluated the recoverability and recovery rate of a DES in three different corncob pretreatment procedures, as shown in Fig. 4. Repeated use of the DES ten times revealed no significant decrease in pretreatment efficiency for the recovered DES.
20 mass ratio), and then 250 µL distilled water was added. The degradation reaction was allowed to complete. 100 mL of distilled water was added to precipitate the lignin; the mixture was then centrifuged, and the solid residue was washed repeatedly to obtain the regenerated lignin. The supernatant was collected, the water was evaporated via vacuum evaporation, and the purified DES was then recovered. DES recovery is complex and diverse, and Yan et al.52 evaluated the recoverability and recovery rate of a DES in three different corncob pretreatment procedures, as shown in Fig. 4. Repeated use of the DES ten times revealed no significant decrease in pretreatment efficiency for the recovered DES.
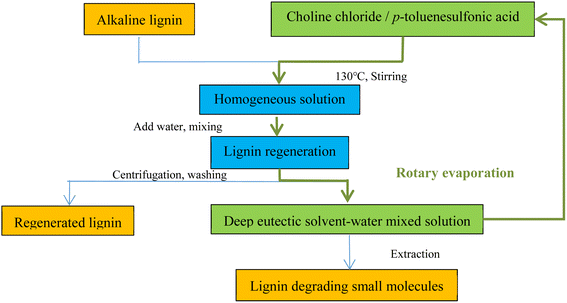 | ||
| Fig. 3 Flow chart of DES (choline chloride and p-toluenesulfonic acid) degradation of lignin and solvent recycling.18 | ||
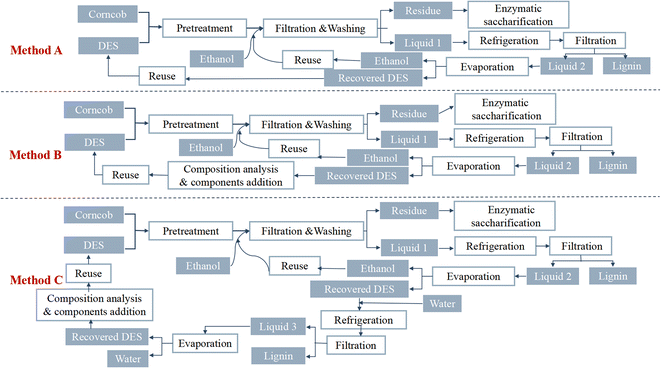 | ||
| Fig. 4 Schematic diagram of three corncob pretreatment procedures using recovered DES (choline chloride and oxalic acid).52 | ||
Extraction or recrystallization
Extraction is based on the principle of different solubilities, so that one of the components is completely soluble or insoluble in the extractant for the purpose of recovery. Mamilla et al.53 pretreated beech wood in a DES composed of ChCl and oxalic acid, added water to the centrifuge to separate the solid matter, and then added acetone to recover and purify the DES. The DES was finally precipitated as a solid in 75% yield. Maugeri et al.54 used a DES to separate an alcohol–ester mixture. After the extraction and separation of the product, the remaining residue-specific solvent was removed from the DES, and the residue could be extracted from the DES using another solvent. Finally, the recycled DES product could be obtained. Li et al.55 extracted a desulfurized DES with ether. After four cycles, the solvent recovery performance was good, and the extraction efficiency was considerable. However, with repeated use, the extraction efficiency of the DES will decrease, and after the sixth recycling, the extraction capacity will drop significantly. Mulia et al.56 extracted α-mangosteen from mangosteen peel powder, studied the recovery of DES from the extraction stage, and used an ethyl acetate three-step stripping method to extract the waste DES. The results indicated that the DES can be recovered and reused. Yang et al.57 studied the separation of reused FeCl3–DES. As shown in Fig. 5, oxalic acid dihydrate could be obtained by a recrystallization process with a recovery of 60%. The pH of the filtrate was then adjusted to alkaline to convert the iron complex to Fe(OH)3 precipitate and the residual oxalic acid to sodium oxalate. The Fe(OH)3 was filtered off, and the filtrate was dried by reduced-pressure distillation, which was followed by ethanol washing to obtain purified sodium oxalate, and the ethanol phase was dried using reduced-pressure distillation. In the final step, choline chloride is dissolved in hot isopropanol and the sugars can be precipitated. In this process, all chemicals can be recovered.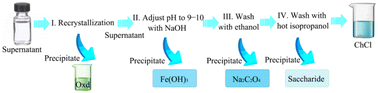 | ||
Fig. 5 Flow chart of the separation of recovered FeCl3-catalyzed DES (the DES was composed of oxalic acid dihydrate, choline chloride, and FeCl3·6H2O in a mass ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2).57 0.2).57 | ||
Nanofiltration or reverse osmosis
Nanofiltration can separate ionic liquids from solvents. Haerens et al.58 recovered the ChCl/ethylene glycol (EG) DES system from a synthetic waste stream and used nanofiltration, reverse osmosis and osmotic evaporation to remove water. It was found that the osmotic evaporation method was less effective in DES mixtures with too much water. However, for the concentrated DES, the osmotic pressure is still the main limiting factor for the membrane separation and purification process of the DES. Ultrafiltration is an efficient and cost-effective method for DES recovery. Ippolitov et al.59 used RC70PP commercial regenerated cellulose membranes and Ultracel 5 kDa UF membranes to recover solvents of different lignin fractions (DES, a mixture of choline chloride and lactic acid, respectively, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), as shown in Fig. 6. The addition of ethanol to the used DES reduces the viscosity of the solvent, thus enabling filtration. A two-pass ultrafiltration process using 10 kDa and 5 kDa membranes removed approximately 95% of the lignin and hemicellulose from the used DES. The membranes used also showed the ability to fractionate polymer compounds into fractions above and below 10 kDa.
10), as shown in Fig. 6. The addition of ethanol to the used DES reduces the viscosity of the solvent, thus enabling filtration. A two-pass ultrafiltration process using 10 kDa and 5 kDa membranes removed approximately 95% of the lignin and hemicellulose from the used DES. The membranes used also showed the ability to fractionate polymer compounds into fractions above and below 10 kDa.
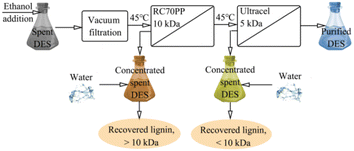 | ||
Fig. 6 Two-pass ultrafiltration membrane process (DES composition: choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactic acid = 1 lactic acid = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10).59 10).59 | ||
In addition to rotary evaporation, there is a general and simple method—evaporation. However, this method is very energy-intensive, requires higher temperatures, and may lead to a loss of material at high evaporation temperatures. Chen et al.60 explored the recyclability and reusability of a (ChCl/EG) DES. They removed impurities using an adsorption resin and membrane filtration and found the lignin in the first three cycles of lignocellulose could be treated with it. The removal rate was about 70%, but decreased in the fourth cycle. It can be seen that the aqueous ChCl and EG were successfully recovered and reused for at least three cycles with good performance.
Freeze-drying
Freeze-drying of a DES can also be performed for DES solvent extraction. Jeong et al.47 extracted saponins via ternary DES extraction consisting of glycerol/L-proline/sucrose, and this method has a high recovery rate. After the end of the reaction, the recovery DES step involved washing the solution out from the solid phase extractor and freeze-drying it to complete the recovery of the DES. After three cycles, the DES extraction efficiency was 83% relative to that of the newly synthesized solvent, with promising performance. If this method is applied to lignocellulose pretreatment, it may have to be removed and purified to obtain a pure DES aqueous solution. Grillo et al.61 used a DES as a solvent for extracting anthocyanins, and water was added to the obtained product so that the DES and anthocyanins could be separated. The anthocyanins were removed using a resin, and the remaining components were DES and water. Finally, the water in the DES aqueous solution was removed by freeze-drying to obtain ChCl and lactic acid with a yield of about 79.5%.Membrane-based electrodialysis
Comprehensively comparing all separation and recovery technologies, the membrane separation method is relatively environmentally friendly. It does not require solvents and has low energy consumption.Electrodialysis ultrafiltration technology also has efficient applications in DES recycling. Electrodialysis is voltage-driven and selectively migrates anions and cations through an ion-exchange membranes to recover the desired products. Liang et al.62 studied the case of electrodialysis applied to the recovery of ionic liquids and found that for the recovery of hydrophilic ILs, the membrane method is still mainly utilized because of its low energy consumption and simplicity. According to the working principle of electrodialysis, the electrolyte component of the DES (e.g., ChCl) can be transferred through the ion-exchange membrane, while the other components of the DES (e.g., organic acids) are separated because non-electrolytes cannot pass through the membrane. Liang et al.8 applied electrodialysis to DES recovery and selected a (ChCl/EG) DES system, in which lignin could be quickly removed with the help of the ultrafiltration method. The remaining DES was diluted using the electrodialysis method, in which an electric field was applied to the system to separate ChCl from EG by ion-exchange membranes, as a way to recover the DES components (Fig. 7). The recoveries were 92% for ChCl and 96% for EG, and their purity was above 98%. The DES fraction recovered by coupled electrodialysis-ultrafiltration reconstitutes the DES system with a more minor difference in treatment effect than the newly synthesized DES system. The generation of this technology is necessary for the recovery of actual biomass refining involving DES and for high-volume industrial applications.
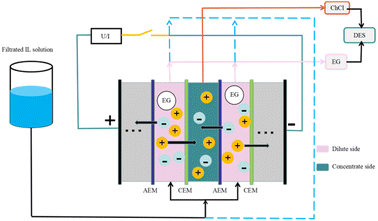 | ||
| Fig. 7 Process for the recovery of a DES (ChCl/EG) by electrodialysis treatment.8 | ||
Dewaxed eucalyptus wood underwent hydrothermal treatment (8 g g−1 liquid to solid ratio of blue eucalyptus wood to water, 170 °C, 4 h), was washed with hot ethanol and water and was then treated with DES (hydrothermal residue![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DES = 1
DES = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 90 °C, 24 h) to sequentially fractionate hemicellulose and lignin. The resulting mixture was filtered through a funnel and washed with water, and lignin was precipitated by the addition of water. The mass balance diagram is shown in Fig. 8.
20, 90 °C, 24 h) to sequentially fractionate hemicellulose and lignin. The resulting mixture was filtered through a funnel and washed with water, and lignin was precipitated by the addition of water. The mass balance diagram is shown in Fig. 8.
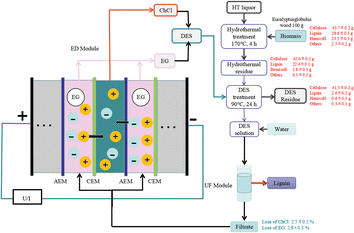 | ||
| Fig. 8 Summary of the mass balance and DES (ChCl/EG) recovery of major components during membrane-based ultrafiltration and electrodialysis methods for the pretreatment of Eucalyptus globulus wood.8 | ||
The recycling of DES is of great significance for the development of biomass refining. The currently reported DES recycling technologies include rotary evaporation, membrane and electrodialysis, etc. Derivatives of the separated biomass can also be used to make new DES, which in turn enables closed-loop biomass refining and improves production efficiency, e.g., Table 3. From Table 3, it can be seen that rotary evaporation is a popular method among researchers and is widely used due to the relatively simple operation, high durability of the equipment and low cost. However, the purity of the DES recovered by electrodialysis and ultrafiltration is higher. The actual selection of the recovery method should be combined with the specific parameters required for the biomass refining process using the solvent, and the equipment cost, energy consumption size, and durability required for different recovery methods should be considered to select the appropriate method.
| Raw material | Types of DES (ratio is molar ratio) | Recycling method | The effect after reuse | References |
|---|---|---|---|---|
| Radiata pine | BTMAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FA = 1 FA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Rotary evaporation | After five recoveries, the pretreatment efficiency still maintained its lignin yield (78.1–66.6%) and enzymatic saccharification (92.4–76.6%) | 46 |
| Corncob | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OA = 1 OA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Rotary evaporation | After 10 cycles, the recovery process using supplemental oxalic acid was not significantly reduced, with significant dextran digestion and glucose recovery (66.23% and 64.43%, respectively) | 52 |
| Substrate (sawdust) | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA = 1 LA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Rotary evaporation | DES treatment in combination with a biphasic separation strategy resulted in a DES recovery of >95% and a lignin yield of 74% (90% purity) | 49 |
| Rice straw | 90% LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ChCl (3 ChCl (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) aqueous solution 1) aqueous solution |
Rotary evaporation | The recovery yield of DESs reached about 90%, with 69% recovery after 5 cycles of reuse at 90 °C. The recovered DES maintained good pretreatment capacity with 60–70% glucose yield | 45 |
| Switchgrass | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol = 1 glycerol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Rotary evaporation | The DES can be successfully recovered and reused for at least four pre-processing cycles while maintaining its pre-processing capacity | 19 |
| Rice straw | TBAB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-aminoethanol = 1 2-aminoethanol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Rotary evaporation | A solvent system consisting of DES and THF allows selective delignification of rice straw for up to 10 cycles under milder conditions | 48 |
| Corncob | BTMAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA LA |
Rotary evaporation | 80–95% of DESs were recovered in each recovery. A slight decrease in enzymatic digestion was observed as the number of DESs recovered increased, but was above 80% | 63 |
| Oil palm empty fruit bunch | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea = 1 urea = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, ChCl 2, ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol = 1 glycerol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Rotary evaporation | Compared to untreated OPEFB, OPEFB pretreated with first and second recycled DES showed consistently damaged surface structure, and increasing the number of solvent reuses will reduce structural damage | 64 |
| Wheat straw | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA = 1 LA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Rotary evaporation | In the recovery experiments, the hemicellulose and lignin content of pretreated wheat straw gradually increased with the number of cycles. There was no significant decrease in the enzymatic efficiency of wheat straw (over 85%) after three DES replicates | 65 |
| Eucalyptus | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA = 1 LA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
Filtration and rotary evaporation | After four cycles, the enzymatic digestibility of cellulose decreased from 94.3% to 73.8% due to the effect of hemicellulose and lignin degradation compounds during DES pretreatment | 66 |
| Corn straw | 40 mL ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG (1 EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) DES with 2 g corn straw and 0.5 g phosphotungstic acid 2) DES with 2 g corn straw and 0.5 g phosphotungstic acid |
Rotary evaporation | The recovery was significantly increased to 62.7% within 4 cycles. Due to the decrease in hydrogen ion concentration and hydrogen bonding interactions in the PTA/DES system during the cycle, the lignin content of the treated corn stover increased to 13.44% and 60.3% of the lignin remained dissolved in the 4th cycle, respectively | 67 |
| Poplar sawdust | MA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ChCl ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG = 1 EG = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
Rotary evaporation | After three cycles, the solid recovery was 79.50%, 82.16% and 82.59% due to the increase of hemicellulose and lignin degradation products, respectively | 68 |
| Beached eucalyptus kraft pulp | Oxalic acid dihydrate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ChCl ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FeCl3·6H2O = 4.43 FeCl3·6H2O = 4.43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
Recrystallization and precipitation | The separated FeCl3–DES can be directly reused at least three times for CNC production, and all components of the reused solvent can be separated by a simple separation process | 57 |
| Eucalyptus globulus wood | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG = 1 EG = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Ultrafiltration and electrodialysis | The recoveries of ChCl and EG after electrodialysis treatment were close to 92% and 96%, respectively, and the purity reached 98–99% | 8 |
| Betula pendula | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA = 1 LA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
Ultrafiltration | Ultrafiltration recovers both the high molar mass lignin fraction and the spent DES | 60 |
| Alkaline lignin | [Bmim]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDTA = 1 EDTA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, ChCl 1, ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SA = 1 SA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Phase separation at low temperature, catalyst phase separation by decanting operation, followed by extractant catalyst phase, separation and drying of catalyst from product for recycling | The lignin degradation rate of [Bmim]Cl–EDTA was 82.98% after 4 cycles. When ChCl–SA was used as the catalyst, the degradation rate of lignin was 79.67% after 4 cycles of catalyst use. The catalytic effect of temperature-responsive DES did not change significantly after 4 cycles of use, and the catalyst could be easily separated from the reaction system after decreasing the temperature, while maintaining relatively stable catalytic performance | 69 |
| Alkaline lignin | ChCl was used as the HBA, and urea, EG, glycerol, acetic acid, formic and acetic mixed acid, OA, and p-toluenesulfonic acid were used as the HBD respectively | Rotary evaporation | Seven DES solutions were found that the recovery of DES still reached more than 80% at the first recovery | 70 |
Recovery of DES reactions in non-lignin systems
The recovery of DES is essentially the same for reactions in which non-lignin substrates are involved. For example, Zhang et al.71 recovered millet polyphenols with a DES (octano-linalool), which was a hydrophilic DES at the time of extraction of the product and a hydrophobic DES at the time of the recovery cycle. After 5 repetitions, the treatment effect remained basically the same. The recovery step of DES was as follows: a hydrochloric acid solution added to the extract converted the hydrophilic DES to a hydrophobic DES for the purpose of recovery (Fig. 9). When a DES is used for lignin-related reactions, the conventional method is rotary evaporation. The method of recovery will be different for different DES applications.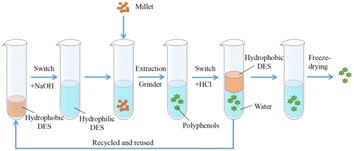 | ||
| Fig. 9 Extraction strategy of millet polyphenols with switchable DES (octano-linalool).71 | ||
Zhang et al.72 used a DES (betaine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid = 1
acetic acid = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 with 30% water content) to extract flavonoids from Polygonatum sibiricum. The DES was effectively recovered using ultra-high capacitance porous activated carbon (the recovery of DES after ten reuses was about 92% of the original DES). The DES recovery procedure was as follows (Fig. 10): the DES extract was mixed with a carbon adsorbent material, vortexed, centrifuged, and filtered using a membrane filter, and the recovery of the DES was 95%. The flavonoids were easily adsorbed on the carbon material and separated, and the DES was purified.
4 with 30% water content) to extract flavonoids from Polygonatum sibiricum. The DES was effectively recovered using ultra-high capacitance porous activated carbon (the recovery of DES after ten reuses was about 92% of the original DES). The DES recovery procedure was as follows (Fig. 10): the DES extract was mixed with a carbon adsorbent material, vortexed, centrifuged, and filtered using a membrane filter, and the recovery of the DES was 95%. The flavonoids were easily adsorbed on the carbon material and separated, and the DES was purified.
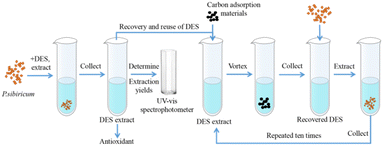 | ||
| Fig. 10 Schematic diagram of DES (betaine–acetic acid) extraction of flavonoids and solvent recovery.72 | ||
Alhassan et al.73 evaluated the efficiency of a DES synthesized from a mixture of choline chloride and para-toluenesulfonic acid along with a silica carrier (So-DES) and no carrier (Un-DES) as non-homogeneous and homogeneous catalysts to catalyze oil esterification reactions using methanol and methyl tert-butyl ether as solvents. The catalysts Un-DES and So-DES were reused four and seven times, respectively. The catalysts were regenerated using a high-speed centrifuge and then further pretreated with ether and allowed to stand in an oven for 24 hours. The oven temperature was maintained slightly above the boiling point of water to ensure complete water and solvent removal. Fig. 11 shows the performance of So-DES and Un-DES during the recovery experiments, with the yields obtained for the first four experiments using the Un-DES catalyst showing a slight decrease in the activity of the catalysts studied. When So-DES was reused, a decrease in catalyst activity was observed after about eight consecutive runs; the deactivation could be the result of reduced acidic reference density and catalyst strength.
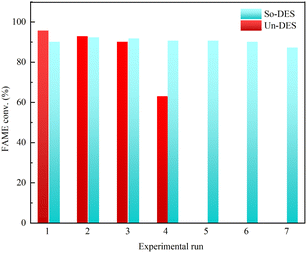 | ||
| Fig. 11 Catalyst recycling and efficiency (DES composed of choline chloride and para-toluenesulfonic acid with So-DES and Un-DES).73 | ||
Renewable DESs can be recovered and reused in biomass processing, and also have the potential to reduce costs and chemical use, enabling cost-competitive biorefineries in the future. However, the following issues and bottlenecks are still pending for DES recovery methods.
● Rotary evaporation is a standard method for recovering DESs. When recovering a DES, the removal of water is faster, a single sample with a large volume can be processed at one time, the operation is more straightforward, and the cost is low, except for the purchase cost of the equipment. A single sample size is processed and cannot be used efficiently. The addition of ethanol and water to the DES mixture will result in the loss of DES if the solution boils during rotary evaporation. A condition of using the rotary evaporation method for lignocellulose after DES treatment is that the residue should be completely separated together with the product first, after which rotary evaporation can be used to obtain a high-purity DES recovery solution.
● The permeate evaporation reported so far can only remove trace amounts of water. If more significant results are to be obtained, large membrane areas are required, and improvements to the membranes are needed to maintain high water fluxes. Nanofiltration and reverse osmosis can completely filter out the aqueous DES solution and are more effective than osmotic evaporation. However, they lack simplicity, and the cost of preparation and installation is slightly more complicated. The replacement and maintenance of the membrane also consume time and money. Adsorption filtration uses evaporation to directly remove water, which is straightforward but too energy consuming.
● The organic solvents (e.g., water, ether, acetone) are selected according to the solubility of the DES, and the extraction temperature is optimized to achieve efficient extraction. Co-extraction can also be used (a mixture of two or more extractants is used to extract the DES). Reverse extraction can reverse-extract organic alcohols and organic acids in a DES from the organic phase to the aqueous phase one-by-one to separate the components that have been separated. There should also be no limitations for extraction solvents, such as supercritical CO2 extraction techniques.
● Ultrafiltration and electrodialysis methods using membranes are undoubtedly disadvantageous. Ion-exchange membranes do not require regeneration, but they do not seem to be a good choice in terms of cost and ease of operation. Anodes are prone to corrosion, and cathodes easily undergo scaling. Electrodialysis is relatively low cost compared with reverse osmosis membrane separation technology, but the ion removal performance is poor, and the extraction of the DES is slightly less effective.
Conclusions
The study of the recovery, utilization and environmental impact of reaction solvents and catalysts is a popular and novel topic to promote green chemistry goals. Compared to ILs, DES are emerging as green solvents with the advantages of low price, simple preparation, low biotoxicity and biodegradability, making them more promising for lignin extraction and degradation in lignocellulose pretreatment. This review article provides a comprehensive and specific overview of the basics and properties of deep eutectic solvents. Their definition and composition are clarified. Next, their preparation methods are summarized, along with their main physicochemical properties, especially their density, viscosity, conductivity and surface tension. In this section, it is emphasized that DESs can affect the physicochemical properties of solvents depending on the type and ratio of components, as a way to meet specific requirements for solvents. In the subsequent sections, we explore the role of DESs in lignocellulose pretreatment and the role of DESs in lignin degradation. Finally, we focus on the recovery performance of DESs using different methods, such as rotary evaporation, for DES recovery and purification, and their performance when reused. In the future, we would like to optimize each DES recovery method, for example, to improve its recovery rate for a given energy consumption and to seek purification methods to increase its purity rate with a view toward otherwise unchanged function. The recycling of DESs is an integral part of conducting chemical reactions; whether a DES can be recycled or not is often an important criterion to evaluate the goodness of solvents for chemical reaction systems. Recyclable DESs reflect the potential value of such reaction solvents as low cost and environmentally friendly, making high-value products based on DES degradation of biomass an excellent development space.Abbreviations
| DES | Deep eutectic solvents |
| ChCl | Choline chloride |
| HBA | Hydrogen bond acceptor |
| HBD | Hydrogen bond donor |
| ILs | Ionic liquid |
| EG | Ethylene glycol |
| BTMAC | Benzyltrimethylammonium chloride |
| FA | Formic acid |
| LA | Lactic acid |
| SA | Salicylic acid |
| MA | Maleic acid |
| OA | Oxalic acid |
| TBAB | Tetra-n-butylammonium bromide |
| DFT | Density functional theory |
| NADES | Natural deep eutectic solvents |
| THF | Tetrahydrofuran |
| LCC | Lignin–carbohydrate composite |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Natural Science Foundation of China (31730106, 32271797).Notes and references
- T. K. F. Dier, D. Rauber, D. Durneata, R. Hempelmann and D. A. Volmer, Sci. Rep., 2017, 7, 5041 CrossRef PubMed.
- International Energy Agency, World Energy Outlook 2019, 2019, https://www.oecd-ilibrary.org/content/publication/weo-2018-en, accessed 20th Feb 2020 Search PubMed.
- E. K. New, S. K. Tnah, K. S. Voon, K. J. Yong, A. Procentese, K. P. Y. Shak, W. Subramonian, C. K. Cheng and T. Y. Wu, J. Environ. Manage., 2022, 307, 114385 CrossRef CAS PubMed.
- M. Brebu, T. Tamminen and I. Spiridon, J. Anal. Appl. Pyrolysis, 2013, 104, 531–539 CrossRef CAS.
- J. Liu, Y. Zhu, Y. Liao, W. Lv, L. Ma and C. Wang, Top. Curr. Chem., 2018, 376, 29 CrossRef PubMed.
- W. Li, K. Amos, M. Li, Y. Pu, S. Debolt, A. J. Ragauskas and J. Shi, Biotechnol. Biofuels, 2018, 11, 304 CrossRef CAS PubMed.
- B. Soares, D. J. P. Tavares, J. L. Amaral, A. J. D. Silvestre, C. S. R. Freire and J. A. P. Coutinho, ACS Sustainable Chem. Eng., 2017, 5(5), 4056–4065 CrossRef CAS.
- X. Liang, Y. Fu and J. Chang, Sep. Purif. Technol., 2019, 210, 409–416 CrossRef CAS.
- L. Das, M. Li, J. Stevens, W. Li, Y. Pu, A. J. Ragauskas and J. Shi, ACS Sustainable Chem. Eng., 2018, 6(8), 10408–10420 CrossRef CAS.
- J. Wang, W. Jing, H. Tian, M. Liu, H. Yan, W. Bi and D. D. Y. Chen, ACS Sustainable Chem. Eng., 2020, 8(32), 12080–12088 CrossRef CAS.
- X. J. Shen, T. Chen, H. M. Wang, Q. Mei, F. Yue, S. Sun, J. L. Wen, T. Q. Yuan and R. C. Sun, ACS Sustainable Chem. Eng., 2020, 8(5), 2130–2137 CrossRef CAS.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 1, 70–71 RSC.
- S. Hong, X. J. Shen, B. Pang, Z. Xue, X. F. Cao, J. L. Wen, Z. H. Sun, S. S. Lam, T. Q. Yuan and R. C. Sun, Green Chem., 2020, 22, 1851–1858 RSC.
- S. Hong, X. J. Shen, Z. Sun and T. Q. Yuan, Front. Energy Res., 2020, 8, 573198 CrossRef.
- C. Alvarez-Vasco, R. Ma, M. Quintero, M. Guo, S. Geleynse, K. K. Ramasamy, M. Wolcottc and X. Zhang, Front. Energy Res., 2016, 18, 5133–5141 RSC.
- J. L. K. Mamilla, U. Novak, M. Grilc and B. Likozar, Biomass Bioenergy, 2019, 120, 417–425 CrossRef CAS.
- P. H. Li, J. P. Ren and W. J. Wu, China Pulp Pap., 2022, 41, 78–85 Search PubMed.
- L. Li, Z. Wu, X. Xi, B. Liu, Y. Cao, H. Xu and Y. Hu, J. Renewable Mater., 2021, 9(2), 219–235 CAS.
- Z. Chen, W. D. Reznicek and C. Wan, Bioresour. Technol., 2018, 263, 40–48 CrossRef CAS PubMed.
- A. Isci and M. Kaltschmitt, Biomass Convers. Biorefin., 2021, 12(Suppl 1), 197–226 Search PubMed.
- W. Pitacco, C. Samorì, L. Pezzolesi, V. Gori, A. Grillo, M. Tiecco, M. Vagnoni and P. Galletti, Food Chem., 2022, 379, 132156 CrossRef CAS PubMed.
- S. Soltani and H. Sereshti, Food Chem., 2022, 380, 132181 CrossRef CAS PubMed.
- C. Li, Z. Cai, Y. Ma, Y. Cao, K. Huang and L. Jiang, Bioresour. Technol., 2022, 167, 106713 CAS.
- A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2014, 2(5), 1063–1071 CrossRef CAS.
- Y. L. Loow, E. K. New, G. H. Yang, L. Y. Ang, L. Y. W. Foo and T. Y. Wu, Potential use of deep eutectic solvents to facilitate lignocellulosic biomass utilization and conversion, Cellulose, 2017, 24(9), 3591–3618 CrossRef CAS.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114(21), 11060–11082 CrossRef CAS PubMed.
- E. K. New, S. K. Tnah, K. S. Voon, K. J. Yong, A. Procentese, K. P. Y. Shak, W. Subramonian, C. K. Cheng and T. Y. Wu, J. Environ. Manage., 2022, 307, 114385 CrossRef CAS PubMed.
- I. Juneidi, M. Hayyan and M. A. Hashim, RSC Adv., 2015, 5, 83636–83647 RSC.
- I. Juneidi, M. Hayyan and O. M. Ali, Environ. Sci. Pollut. Res., 2016, 23, 7648–7659 CrossRef CAS PubMed.
- N. Mgxadeni, O. Mmelesi, B. Kabane and I. Bahadur, J. Mol. Liq., 2022, 351, 118596 CrossRef CAS.
- M. Atilhan, G. García and S. Aparicio, Chem. Phys. Lett., 2015, 634, 151–155 CrossRef.
- H. Qin, X. Hu, J. Wang, H. Cheng, L. Chen and Z. Qi, Green Energy Environ., 2020, 5(1), 8–21 CrossRef.
- A. P. Abbott, G. Capper, D. L. Davies, K. J. McKenzie and S. U. Obi, J. Chem. Eng. Data, 2006, 51(4), 1280–1282 CrossRef CAS.
- B. Tang and K. H. Row, Monatsh. Chem., 2013, 144(10), 1427–1454 CrossRef CAS.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126(29), 9142–9147 CrossRef CAS PubMed.
- Q. Xiao, M. Dai, H. Zhou, M. Huang, L. T. Lim and C. Zeng, Carbohydr. Polym., 2022, 282, 119105 CrossRef CAS PubMed.
- H. Malaeke, M. R. Housaindokht, H. Monhemi and M. Izadyar, J. Mol. Liq., 2018, 263, 193–199 CrossRef CAS.
- R. Lou and X. Zhang, Bioresour. Technol., 2022, 344, 126174 CrossRef CAS PubMed.
- H. Xu, J. Peng, Y. Kong, Y. Liu, Z. Su, B. Li, X. Song, S. Liu and W. Tian, Bioresour. Technol., 2020, 310, 123416–123428 CrossRef CAS PubMed.
- Y. Liu, W. Chen, Q. Xia, B. Guo, Q. Wang, S. Liu, Y. Liu, J. Li and H. Yu, ChemSusChem, 2017, 10, 1692–1700 CrossRef CAS PubMed.
- D. Di Marino, D. Stöckmann, S. Kriescher, S. Stiefel and M. Wessling, Green Chem., 2016, 18, 6021–6028 RSC.
- D. Di Marino, V. Aniko, A. Stocco, S. Kriescher and M. Wessling, Green Chem., 2017, 19, 4778–4784 RSC.
- K. Lundquist and R. Lundgren, Acta Chem. Scand., 1972, 26, 2005–2023 CrossRef CAS.
- A. Rahimi, A. Ulbrich, J. J. Coon and S. S. Stahl, Nature, 2014, 515, 249–252 CrossRef CAS PubMed.
- A. L. Li, X. D. Hou, K. P. Lin, X. Zhang and M. H. Fu, J. Biosci. Bioeng., 2018, 126(3), 346–354 CrossRef CAS PubMed.
- J. Xie, J. Chen, Z. Cheng, S. Zhu and J. Xu, Carbohydr. Polym., 2021, 269, 118321 CrossRef CAS PubMed.
- K. M. Jeong, M. S. Lee, M. W. Nam, J. Zhao, Y. Jin, D. K. Lee, S. W. Kwon, J. H. Jeong and J. Lee, J. Chromatogr. A, 2015, 1424, 10–17 CrossRef CAS PubMed.
- C. Thulluri, R. Balasubramaniam and H. R. Velankar, Sci. Rep., 2021, 11, 1591 CrossRef CAS PubMed.
- S. Kumar, S. Sharma, S. M. Arumugam, C. Miglani and S. Elumalai, ACS Sustainable Chem. Eng., 2020, 8(51), 19140–19154 CrossRef CAS.
- A. K. Kumar, B. S. Parikh and M. Pravakar, Environ. Sci. Pollut. Res., 2016, 23(10), 9265–9275 CrossRef CAS PubMed.
- S. B. Phadtare and G. S. Shankarling, Green Chem., 2010, 12, 458–462 RSC.
- G. Yan, Y. Zhou, L. Zhao, W. Wang, Y. Yang, X. Zhao, Y. Chen and X. Yao, Ind. Crops Prod., 2022, 183, 115005 CrossRef CAS.
- J. L. K. Mamilla, U. Novak, M. Grilc and B. Likozar, Biomass Bioenergy, 2019, 120, 417–425 CrossRef CAS.
- Z. Maugeri, W. Leitner and P. D. de María, Tetrahedron Lett., 2012, 53(51), 6968–6971 CrossRef CAS.
- C. Li, D. Li, S. Zou, Z. Li, J. Yin, A. Wang, Y. Cui, Z. Yao and Q. Zhao, Green Chem., 2013, 15, 2793–2799 RSC.
- K. Mulia, Y. Yoksandi, N. Kurniawan, I. F. Pane and E. A. Krisant, J. Phys.: Conf. Ser., 2019, 1198, 062003 CrossRef CAS.
- X. Yang, H. Xie, H. Du, X. Zhang, Z. Zou, Y. Zou, W. Liu, H. Lan, X. Zhang and C. Si, ACS Sustainable Chem. Eng., 2019, 7, 7200–7208 CrossRef CAS.
- K. Haerens, S. V. Deuren, E. Matthijs and B. V. der Bruggen, Green Chem., 2010, 12, 2182–2188 RSC.
- V. Ippolitov, I. Anugwom, R. van Deun, M. Mänttäri and M. Kallioinen-Mänttäri, Membranes, 2022, 12(1), 86 CrossRef CAS PubMed.
- Z. Chen, X. Bai, A. Lusi and C. Wan, ACS Sustainable Chem. Eng., 2018, 6(9), 12205–12216 CrossRef CAS.
- G. Grillo, V. Gunjević, K. Radošević, I. R. Redovniković and G. Cravotto, Antioxidants, 2020, 9(11), 1069 CrossRef CAS PubMed.
- X. Liang, Y. Fu and J. Chang, Bioresour. Technol., 2016, 220, 289–296 CrossRef CAS PubMed.
- Z. Guo, Q. Zhang, T. You, X. Zhang, F. Xu and Y. Wu, Green Chem., 2019, 21, 3099–3108 RSC.
- K. M. Lee, J. D. Quek, W. Y. Tey, S. Lim, H. S. Kang, L. K. Quen, W. A. W. Mahmood, S. I. S. Jamaludin, K. H. Teng and K. S. Khoo, Biochem. Eng. J., 2022, 187, 108587 CrossRef CAS.
- M. Hu, L. Yuan, Z. Cai, W. Zhang, Q. Fu and D. Ji, Bioresour. Technol., 2023, 367, 128242 CrossRef CAS PubMed.
- X. Shen, J. Wen, Q. Mei, X. Chen, D. Sun, T. Yuan and R. Sun, Green Chem., 2019, 21, 275–283 RSC.
- J. Xie, J. Xu, Z. Cheng, S. Zhu and B. Wang, Ind. Crops Prod., 2021, 172, 114058 CrossRef CAS.
- S. Han, R. Wang, K. Wang, J. Jiang and J. Xu, Bioresour. Technol., 2022, 363, 127905 CrossRef CAS PubMed.
- S. Wang, Y. Li, X. Wen, Z. Fang, X. Zheng, J. Di, H. Li, C. Li and J. Fang, Ind. Crops Prod., 2022, 177, 114430 CrossRef CAS.
- P. H. Li, Y. Lu, X. Y. Li, J. P. Ren, Z. W. Jiang, B. Jiang and W. J. Wu, Polymers, 2022, 14(23), 5100 CrossRef CAS PubMed.
- H. Zhang, W. Zhao, T. Bai, L. Fu, Z. Chen, X. Jing and X. Wang, LWT-Food Sci. Technol., 2022, 170, 114082 CrossRef CAS.
- H. Zhang, F. Hao, Z. Yao, J. Zhu, X. Jing and X. Wang, Microchem. J., 2022, 175, 107168 CrossRef CAS.
- Y. Alhassan and N. Kumar, Waste Biomass Valorization, 2016, 7, 1055–1065 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |