Open Access Article
Open Access ArticleBioinks adapted for in situ bioprinting scenarios of defect sites: a review
Ruojing Lia,
Yeying Zhaoa,
Zhiqiang Zhenga,
Yangyang Liua,
Shurui Songa,
Lei Songa,
Jianan Ren*ab,
Jing Dong*ac and
Peige Wang *a
*a
aDepartment of Emergency Surgery, The Affiliated Hospital of Qingdao University, 16 Jiangsu Road, Qingdao 266000, China. E-mail: wpgzyz@163.com
bDepartment of General Surgery, The Affiliated General Hospital of Nanjing Military Region, 305 Zhongshan East Road, Nanjing 210016, China. E-mail: jiananr@nju.edu.cn
cSpecial Medicine Department, Medical College, Qingdao University, Qingdao 266071, China. E-mail: dongjing6@hotmail.com
First published on 3rd March 2023
Abstract
In situ bioprinting provides a reliable solution to the problem of in vitro tissue culture and vascularization by printing tissue directly at the site of injury or defect and maturing the printed tissue using the natural cell microenvironment in vivo. As an emerging field, in situ bioprinting is based on computer-assisted scanning results of the defect site and is able to print cells directly at this site with biomaterials, bioactive factors, and other materials without the need to transfer prefabricated grafts as with traditional in vitro 3D bioprinting methods, and the resulting grafts can accurately adapt to the target defect site. However, one of the important reasons hindering the development of in situ bioprinting is the absence of suitable bioinks. In this review, we will summarize bioinks developed in recent years that can adapt to in situ printing scenarios at the defect site, considering three aspects: the in situ design strategy of bioink, the selection of commonly used biomaterials, and the application of bioprinting to different treatment scenarios.
1. Introduction
The repair of tissue and organ defects caused by severe trauma or tumor resection is a major challenge for surgeons worldwide. When tissues and organs are extensively damaged, the existing traditional surgical repair methods include the inability to provide more donor tissues, the possibility of causing additional damage or postoperative infection, and high cost.1 Therefore, it is expected that 3D bioprinting will eventually replace traditional repair for organ defects. Three-dimensional bioprinting in the modern sense mainly refers to the utilization of cells/cell clusters, bioactive factors and biomaterials as raw materials, which are printed layer by layer by 3D printing to construct bionic tissues or organ transplants with three-dimensional structures and biological functions.2–4 However, the irregular shape of many defect sites, the difficulty of perfectly matching the printed graft to the shape of the defect, and the potential for the graft to fail to adjust to the extremely complex and delicate internal environment of the human body after implantation are obstacles to the progress of in vitro bioprinting techniques.5–7On the basis of inkjet bioprinting, Campbell8 initially proposed the idea of “in situ bioprinting,” which has since gained much attention in the areas of clinical medicine, regenerative medicine, and tissue engineering. In situ bioprinting is based on the scan results of the defect site through computed tomography (CT), magnetic resonance imaging (MRI), or optical scanning. Instead of transferring a prefabricated graft, in situ bioprinting can print cells from biological materials or bioactive factors directly on the defect site, and the graft can precisely adapt to the target defect site, further realizing precise control of cell distribution and arrangement in spatial location, organic combination of cells and biological materials, and precise simulation of tissue microenvironment.7,9,10 In situ bioprinting is mostly intraoperatively performed and is often called intraoperative bioprinting (IOB).11,12 IOB refers to a bioprinting process performed on living subjects in a surgical setting, which makes it possible to deliver gene-activated substrates directly to the defect site.13,14 Unfortunately, the industry has not clearly defined whether the two are equivalent.
Although promising, in situ bioprinting is an emerging field. In addition to the lack of reliable in situ bioprinters, another major obstacle to the advancement of this field is the absence of suitable bioinks. Over the last five years, research results on in situ bioprinting relevant to various clinical disciplines have emerged, but few articles have detailed the selection of bioinks suitable for different in situ printing scenarios. In this review, we discuss bioinks that have emerged in recent years that can be adapted to in situ printing scenarios of defect sites, considering the in situ design strategies of bioinks, the selection of commonly used biomaterials, and the application of bioprinting to different treatment scenarios.
2. In situ design strategies for bioinks
2.1 The role of hydrogels in bioinks for in situ 3D bioprinting
Bioink is defined as a bioprintable medium that includes biological materials (e.g., alginate and gelatin), cells, and functional factors.15,16 These living cells or factors cannot grow without a “biological microenvironment” consisting of sufficient water, oxygen, nutrients, and suitable pH. As a unique “soft material”, hydrogel can meet the needs of this complex microenvironment and is the basis for almost all 3D bioprinting bioinks.17,18Hydrogels are a class of hydrophilic, three-dimensional network structured substances that swell rapidly in water without dissolving and have good biocompatibility and water retention, so they have attracted widespread attention in the past decade.19–21 Because of their structural similarity to the extracellular matrix (ECM), hydrogels have been widely studied and applied in biomedical fields, including tissue engineering and drug delivery.22–26 Three-dimensional bioprinting technology enables high-precision and rapid printing using bioinks prepared from hydrogels for regeneration and repair of various tissues and organs. The selection of hydrogels should take into account cell construction, proliferation, long-term survival, mechanical strength, porosity, degradability, biocompatibility, and print suitability.27–29 In addition, attention needs to be paid to the interaction of bioinks with key cells, including cell settlement in the cartridge, cell viability during extrusion, and cell viability after ink curing.30–32
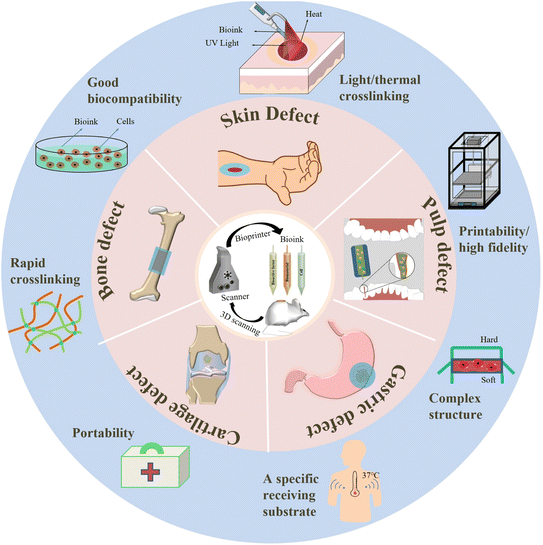
2.2 Design considerations of bioinks for in situ 3D bioprinting
With in situ printing, bioink can be directly deposited into organ defects, which enables in situ bioprinting to have the advantages of avoiding defects during transplantation and reducing the treatment time and pain of patients compared with in vitro bioprinting and transplantation. It is important to stress that the following points need to be fully considered at the design stage of bioink33–37 (Fig. 1). (i) Good biocompatibility is essential for the application of biomaterials in vivo, so bioink must be non-toxic and non-immunogenic. (ii) Rapid cross-linking is necessary for in situ bioprinting due to the inevitable movement of patients during clinical operations. (iii) When a certain link in the microenvironment fluctuates, it will affect the concentration of the bioink and the crosslinking concentration of the reagent at the printing site. Therefore, non-chemical crosslinking, especially light-crosslinking and thermal crosslinking, which do not cause secondary toxicity, are the main crosslinking methods for in situ 3D bioprinting bioinks. (iv) Unlike the temperature-controlled receiving substrates on in vitro bioprinters, in situ bioprinting has a specific receiving substrate whose temperature is usually fixed around body temperature (37 °C), so the rheological properties of bioinks cannot be controlled by changing the temperature, which may affect the thermal curing of heat-sensitive bioinks and ultimately affect the print quality. (v) The cross-linked bioink should have a low mechanical modulus to encapsulate cells to exert a therapeutic effect, but this contradicts the high mechanical properties of the bioink structure required for the defect site. This paradox can be resolved by building a composite structure that prints a strong scaffold with a soft hydrogel inside. (vi) Support structures cannot always be used for in situ bioprinting, which requires the bioinks to have high fidelity and structural stability. (vii) The in situ bioink is preferably portable, providing fast and effective assistance for rescue in emergencies such as wars, disasters, and acute trauma.To avoid infection during clinical use, bioink must be sterilized prior to handling and binding to cellular components.38,39 It should be noted that sterilization methods can adversely affect bioink. For example, the free radicals produced by radiation sterilization and ultraviolet sterilization can affect the hydrodynamics and shapeability of bioinks and, in turn, damage the microstructure of bioinks. High-energy water vapor generated by high-pressure steam sterilization and free radicals generated by radiation sterilization and ultraviolet sterilization can cause the loss of activity of biological macromolecules such as proteins and enzymes and destroy the activity of biological macromolecules contained in bioinks. Residues from ethylene oxide sterilization are carcinogenic and affect the survival of encapsulated cells in bioinks, so the use of this sterilization method should be reduced for cell-loaded bioinks.40 In conclusion, we strongly urge that when selecting materials for in situ 3D printing bioink, much attention should be paid to the potential effects of different sterilization methods on the selected materials in clinical scenarios.
In addition, the requirements for bioink imposed by different forms of printing technology are quite different, so the design of bioink is also closely related to the printing process selected. At present, the printing methods mainly used for in situ 3D bioprinting are droplet/inkjet-based bioprinting and extrusion-based bioprinting. Droplet/inkjet-based bioprinting, which involves thermally or acoustically spraying a bioink onto a tissue defect to construct a graft, requires the bioink to be in a liquid state and have an appropriate viscosity to be ejected from a nozzle orifice. At the same time, the method can print on a non-horizontal surface, which is of great significance for applying the in situ 3D biological printing technology to repair complex tissue damage.7,41 Extrusion-based bioprinting technology continuously extrudes bioink from a nozzle using a pneumatic or mechanical extrusion system and prints it into a designed three-dimensional structure. However, the interference of various enzymes and ions at the defect site may affect the cross-linking process of the bioink, leading to premature cross-linking or nozzle tip blockage.6,42
Laser-assisted bioprinting and reduction polymerization-based bioprinting are also beginning to emerge in in situ printing. Laser-assisted bioprinting uses a high-energy laser pulse to produce high-pressure bubbles in a thin layer of bioink that are then ejected to specified locations. In the sterile environment of the operating room, doctors can guide the fully automatic robot printer to realize micrometer to millimeter cell implantation, and the bioink can print to the defect site more accurately.43 Bioprinting based on reduction polymerization involves selectively crosslinking a photocurable polymer solution loaded with cells using a light source, which has the advantages of high printing speed and high resolution. When applied to in situ printing, stronger penetration of ultraviolet and visible light is required to achieve curing of the printed structure deep in the tissue.44–46 However, these two printing methods expose cells to ultraviolet light during the printing process, which is harmful to the encapsulated cells.47,48 Therefore, how to achieve in situ UV-curing of bioink on the premise of protecting cells from damage is a problem that we should think about in the design stage.
3. Materials available for in situ 3D bioprinting
Different bioinks can induce different degrees of cell responses. Alginate, collagen, gelatin, hyaluronic acid, silk fibroin, chitosan, and peptides are the seven most commonly used materials for in situ bioprinting. Here, we will introduce these common materials in detail, and show some representative research results (Table 1).| Biomaterial | Crosslinking mode | Active ingredient/animal model | Result |
|---|---|---|---|
| a “—”indicates that this part cannot be found in the article. | |||
| Phenyl propionic acid-conjugated gelatin (GHPA)/graphene oxide (GO)64 | Dual enzyme-mediated cross-linking reaction | C2C12 myoblasts | Provides a cell-suitable cellular microenvironment that supports adhesion, spreading, and growth. And promotes the myogenic differentiation of C2C12 cells |
| Collagen type I (COL)/agarose (AG)/sodium alginate (SA)58 | — | Chondrocytes | Suppresses dedifferentiation of chondrocytes and preserves the phenotype. And promotes proliferation and survival of chondrocytes |
| GelMA/hyaluronic acid76 | — | hADSCs/Articular cartilage regeneration and repair model | High biocompatibility and adequate mechanical strength that can facilitates the regeneration and repair of articular cartilage |
| Catechol-functionalized, gelatin methacrylate (GelMA/C)77 | Oxidative crosslinking | HCASMCs/bioprinted vascular construct model | Improves vascular remodeling of both smooth muscle and endothelium |
| Tyramine-functionalized/alginate hydrogel (ALG-TYR)/collagen (COL)51 | Enzymatic crosslinking and thermo-responsive crosslinking | Printable, retains high fidelity after printing, and has high cell survivability | |
| Methacrylate alginate/Ca2+/chitosan52 | — | — | Achieves double contraction and deformation of the sodium alginate hydrogel structure |
| Microalgae/alginate/GelMA54 | — | Oxygenic photosynthesis unicellular microalga (chlorella pyrenoidosa)/the diabetic chronic wounds | Accelerates wound healing |
| Thiol-modified hyaluronic acid (HA-S)/polyethylene glycol diacrylate (PEGDA)80 | Michael-type nucleophilic addition reaction and a prolonged maturation of disulfide crosslinks | — | Has tunable mechanical and bio-adhesive ligand properties |
| Chitosan (CH)/oxidized hyaluronic acid (HAD)81 | Schiff base reaction | — | Maintains cellular phenotypic integrity and promotes extracellular matrix production |
| Chitosan/2-hydroxy-4-(2-hydroxyethoxy)-2-methylpropiophenone/fish skin collagen92 | Thermo/photo dual cure crosslinks | — | Has tunable mechanical properties, proper microstructure, and biodegradability for 3D cell culture, and improves cytocompatibility |
| The newly designed peptide sequences Ac-Ile-Val-Phe-Lys-NH2 (IVFK) and Ac-Ile-Val-Cha-Lys-NH2 (IVZK)96 | Self-assembly | — | The hydrogel proves to be durable, easily printable and offers excellent biocompatibility |
3.1 Alginate
Alginate is a biocompatible anionic polymer derived from brown algae.49 It has been used in a variety of biomedical scenarios, such as promoting wound healing, drug delivery, and tissue engineering. Because of its low viscosity and zero shear viscosity, pure alginate has a poor ability to maintain its shape. Alginate oxidized by periodate is easy to hydrolyze.50 To overcome such common obstacles, alginate is often modified or blended with other materials to optimize its performance for in situ 3D printing.For example, Kim51 presented 3D bioprinting with tunable gelation kinetics by controlling the covalent crosslinking density and gelation time of a tyramine-functionalized alginate hydrogel (ALG-TYR) via enzymatic reactions with horseradish peroxidase and hydrogen peroxide. Then Kim introduced collagen into the ALG-TYR hydrogel network to increase the mechanical modulus and cytocompatibility. Finally, Kim printed a vascular ECM-mimicking scaffold with this hybrid hydrogel and demonstrated that the scaffold was capable of supporting tissue growth for clinical translation in regenerative and personalized medicine. In a seminal work, Cao et al.52 applied visible light-cured methacrylate alginate bioink to 3D-bioprinted cell-loaded biofilms. The researchers prepared sodium alginate structures with high structural accuracy using a direct-writing printer and immersed them in Ca2+ solution and chitosan solution, respectively, to achieve double contraction and deformation of the sodium alginate hydrogel structure. With the continuous soaking of sodium alginate structures in both solutions, the mechanical properties of sodium alginate hydrogels were continuously enhanced. Hakimi et al.53 proposed a handheld skin printer, and consistent sheet formation was achieved by coordinating the flow rates at which bioink and cross-linker solution were delivered with the speed at which a pair of rollers actively translated the cartridge along the surface. This printer enables the in situ formation of biomaterial and skin tissue sheets of different homogeneous and architected compositions, so it can be used for wound healing in situ.
To overcome the non-biological activity, uncontrollable biodegradability, and unstable structural/mechanical stability of alginate, various advanced strategies have been proposed in recent years, either relying on reformulation of bioink formulations (e.g., physical mixing and chemical modification) or relying on innovations of bioprocessing processes (e.g., aerosol-assisted, microgel bioink, collaborative printing, micro/nanoscale printing, and 4D bioprinting), allowing alginate-based bioink applications to expand widely.
Besides, Wang et al.54 inspired by the natural symbiotic relationship between salamanders and algae, presented novel living photosynthetic scaffolds using an in situ microfluidic-assisted 3D bioprinting strategy for adapting irregular-shaped wounds and promoting their healing. Photosynthetically viable unicellular microalgae were introduced directly during 3D printing, and the generated scaffolds could produce sustainable oxygen under light. Thus, the scaffolds could significantly accelerate the chronic wound closure by alleviating local hypoxia and increasing angiogenesis and ECM synthesis. These results indicate that the in situ bioprinting of living photosynthetic microalgae offers an effective autotrophic biosystem for promoting wound healing, suggesting a promising therapeutic strategy for diverse tissue engineering applications.
3.2 Collagen
Collagen is a major component of the ECM in the skin and accounts for approximately 30% of the total protein in mammals.55,56 It consists of different numbers of triple helices and different α polypeptide chains, which can form 28 different cell-binding sequences. As the most common protein in mammals, collagen does not elicit significant immune responses and can promote cell adhesion and growth.35 However, the immunogenicity of collagen is susceptible to other proteins, cross-linking reagents, and residual cells, and may also contribute to inflammation and disease transmission.57 In addition, the mechanical properties of collagen at physiological temperature are unstable and its gelation rate is slow, which limits its application scope as a bioink when used alone. Therefore, collagen is often combined with other biomaterials to create bioinks with improved structural integrity, printability, and bioactivity.A significant number of studies have begun to focus on optimizing collagen as part of a multi-component bioink and applying it to 3D bioprinting. Yang et al.,58 for example, used collagen type I or agarose mixed with sodium alginate to serve as 3D bioprinting bioinks and incorporated chondrocytes to construct in vitro 3D-printed cartilage tissue. This approach improved the printed tissue's mechanical strength and effectively inhibited chondrocyte dedifferentiation. Moreover, the combination of collagen type I and sodium alginate effectively suppressed the dedifferentiation of chondrocytes and preserved the phenotype, so this combination integrated good mechanical properties with biological properties. Similarly, a study by Liu et al.59 demonstrated the applicability of collagen–alginate composite bioink in cartilage bioprinting, showing that printed collagen–alginate saline gels could support sustained drug release from incorporated poly(ε-caprolactone) microspheres. Heidenreich60 investigated the rheological properties of collagen–chitosan composite bioink with different components and showed that it had stable mechanical properties and almost negligible cytotoxic effects on NIH-3T3 fibroblasts. Hence, this bioink should be suitable for in situ bioprinting.
3.3 Gelatin
Gelatin is a natural polymer produced by hydrolysis of collagen,61 which can be formed after cooling at low temperatures (20–30 °C). Gelatin possesses thermosensitive properties that allow its molecular bonds to be easily destroyed by high temperatures, enabling printing and stacking in a temperature-controlled manner.62 For example, the rapid gelation of gelatin at moderate temperatures allows the printed structure to have strong initial stability even when other unstable materials are added.63 Due to their thermosensitive qualities, biocompatibility, and other benefits, gelatin and its derivatives have generally become more popular natural bioinks. These materials hold significant promise as candidates for in situ 3D printing bioinks and have strong potential as candidates for in situ 3D printing bioinks. Gelatin as a type of bioink has good biocompatibility, solubility, and degradability.61 Gelatin-based bioink viscosity can be easily altered by adjusting the temperature or increasing the concentration of gelatin in bioink. In addition, gelatin has several side chains that allow it to be chemically cross-linked and modified and enable it to be successfully applied to in situ 3D printing. To more accurately replicate the ECM and simulate its intrinsic characteristics in loaded cells, Kang et al.64 used 3D-printed bioink composed of phenol-rich gelatin and graphene oxide as a component of myogenic-inducing materials to form a hydrogel network in situ through a double-enzyme-mediated cross-linking reaction to provide an appropriate cellular microenvironment and promote myogenic differentiation of C2C12 skeletal myoblasts, which showed good application prospects in tissue engineering and regenerative medicine.Gelatin in the form of methacryloylated gelatin (GelMA) is widely used in tissue engineering, particularly in the generation of bone, cartilage, skin, and vascular networks.65,66 GelMA is a modified gel with photosensitive functional groups introduced into the gelatin side chain,67,68 which retains the good biocompatibility and degradation properties of gelatin, forms covalent cross-linked hydrogels with good thermal stability under the action of UV light and photo-initiators, and shows good printing adaptability and biocompatibility in the field of 3D bioprinting.69,70 However, it is difficult to form biological scaffolds by extrusion 3D printing due to the poor mechanical properties and structural maintenance of GelMA crosslinked by light. In addition, GelMA hydrogel is beneficial for cell adhesion and remodeling because of its arginine–glycine–aspartate (RGD) peptide sequence and matrix metalloproteinase (MMP) sequence.71 It should be noted that the porosity of GelMA hydrogels plays an important role in the transport of oxygen and nutrients required for cell growth.72 Studies have shown that GelMA hydrogels with relatively low concentrations (i.e., ≤ 5% w/v) are more conducive to cell growth,73,74 but the decrease of concentration will lead to the decrease in the compression modulus, which deteriorates the mechanical properties of GelMA hydrogel.75 High-concentration gels have good shear-thinning behavior and high mechanical properties, but often smaller pore size and lower swelling rate, which is detrimental to the diffusion of nutrients and oxygen needed for cell survival. Therefore, it is important to find a balance between supporting cell growth and obtaining adequate mechanical properties. The preparation of GelMA-related bioinks with appropriate pore size, biological properties, and mechanical properties that are suitable for various tissue engineering is a difficult challenge, and it seems to be a good option to overcome these problems by adjusting gel concentration or by mixing with other components.
Duchi et al.76 described an in situ approach that allows 3D bioprinting of human adipose-derived stem cells laden in 10% GelMa/2% HAMa hydrogel. They used coaxial extrusion to obtain a core/shell bioscaffold with high cell viability and adequate mechanical properties for articular cartilage regeneration and repair. Cui77 developed a catechol-functionalized gelatin methacrylate that undergoes rapid oxidative crosslinking in situ to form an elastic hydrogel, which can be engineered with controllable mechanical strength, high cell/tissue adhesion, and excellent bio-functionalization. At the same time, they also demonstrated that in situ bioprinted vascular structures have appropriate biomechanical properties, higher tissue affinity, excellent perfusion, and permeability, and show significant potential in creating biomimetic, functional vascular systems.
3.4 Hyaluronic acid
Hyaluronic acid is present in the ECM and is abundant in the skin, connective tissues, and eyes, and it has good biocompatibility, biodegradability, and bioabsorbability.78,79 However, due to its high water solubility and low stability, hyaluronic acid is not suitable as a stand-alone bioink because of its lack of robustness as a supporting structure. These shortcomings need to be addressed by crosslinking hyaluronic acid or combining it with other components.Our ideal bioink should have suitable properties that facilitate scaffold expansion, differentiation, and remodeling into suitable tissue, and pay more attention to the firmness of the gel and scaffold binding site and allow cells to attach to this scaffold. For example, Godesky80 investigated a hydrogel system based on thiol-modified hyaluronic acid and polyethylene glycol diacrylate. This gel scaffold can form appropriate support structures, has adjustable mechanical properties, and has good bioadhesive ligand properties to support the growth of tissue cells.
Thomas et al.81 aimed to study the effects of the stiffness composition of a two-component injectable hydrogel based on chitosan and oxidized hyaluronic acid on the growth and functionality of encapsulated chondrocytes. Gel stiffness was found to have a great impact on the chondrocyte microenvironment, such as maintaining cell phenotypic integrity and promoting ECM production. This study is of great reference value for the practical application of biomaterials.
At present, research designs for in situ printed bioinks based on hyaluronic acid are still lacking. However, with in situ printing as an emerging field and hyaluronic acid as a candidate bioprinting ink, their combination may hold unexpected potential.
3.5 Silk fibroin
Silk fibroin from Bombyx mori is easy to process, abundant in sources, and can form strong materials through physicochemical reactions, which have certain textile properties, biodegradability, cytocompatibility, and other valuable characteristics.82,83 By adjusting the β-sheet content, crosslinking degree and morphological structure of silk fibroin bioink, its mechanical properties and its degradation rate in vivo can be adjusted.84,85 In addition, silk fibroin may help to avoid cell-specific effects in some cases because silk fibroin lacks the RGD sequence as a cell adhesion epitope, making it a viable option for quality bioinks.86 It has been shown that the structure and function of cartilage pairing can be optimized by integrating fibroin with gelatin loaded growth factors into bioink for 3D printing.83,87 Silk fibroin and glycidyl methacrylate can be mixed to form a bioink that has excellent mechanical and rheological properties, and is suitable for constructing blood vessels in the hydrogel state. This provides many possibilities for the remodeling of tissue structures such as blood vessels and highly complex organ structures such as the brain.88Compared with ordinary 3D printing, in situ printing can more ideally adapt to target defects and promote tissue repair and regeneration. McGill et al.86 created a method for using silk fibroin bioink to make constructs composed of bioink with encapsulated cell function, and they applied this method to manufacturing patient-specific memory-shaped implants. In addition, they demonstrated the attachment of peptides to silk fibroin hydrogels through crosslinking of tyrosine with horseradish peroxidase and hydrogen peroxide. This cross-linking mechanism has non-negligible potential for extrusion 3D printing in the clinical setting because it is capable of extracting patient-specific anatomical data and designing corresponding shape memory implants.
In summary, as a bioink that can be used for in situ printing, silk fibroin should be designed and processed comprehensively, especially in terms of viscosity, rheology, encapsulation, and biocompatibility. The performance of silk fibroin bioinks for in situ printing can be improved by changing the concentration of fibroin solutions or incorporating other biopolymers to compensate for the limitations of individual components.
3.6 Chitosan
Chitosan is a product of the deacetylation of chitin and contains –NH2 and –OH active moieties that can be easily combined with other polymers. The special molecular structure and physicochemical properties of chitosan cause it to have good biocompatibility, biodegradability, adhesion, and antibacterial and anti-inflammatory properties.89,90 In addition, chitosan can be slowly degraded by lysozyme in vivo to form monosaccharides or oligosaccharides that can be absorbed by the human body, and its degradation performance, mechanical properties, and biological properties can be improved by modification or the addition of components.91 Therefore, chitosan has been widely used in medical tissue engineering as a natural biomaterial.Bioinks for in situ printing should have the advantages of rapid solidification in addition to good mechanical properties, degradability, and other essential properties. Therefore, Liu et al.92 proposed a facile design for a thermo/photo dual-cure composite hydrogel made of methacrylated HBC (MHBC) and soluble collagen. The composite hydrogel exhibited rapid thermally induced sol–gel transition and contraction, adjustable mechanical properties, appropriate microarchitecture, biodegradability suitable for 3D cell culture, and improved cytocompatibility by modulating the methacrylation and chitosan/collagen (M/C) ratio of MHBC. Both desirable printability and cytocompatibility enable the M/C composite hydrogel to be a potential candidate as a bioink for in situ 3D bioprinting.
Puertas-Bartolomé et al.93 presented a novel bio-printing methodology based on a dual-syringe system with a static mixing tool that allows in situ crosslinking of a two-component hydrogel-based ink in the presence of living cells. The reactive hydrogel system consists of carboxymethyl chitosan and partially oxidized hyaluronic acid that undergo fast self-covalent crosslinking via Schiff base formation. This allows better structural integrity, precise adaptation to the defect site, and promotion of soft tissue regeneration.
On the premise of ensuring rapid curing, all the above-mentioned studies minimize damage to the organism caused by UV light curing or chemical cross-linker curing, and the effect on the tissue structure is almost negligible. From these studies, we can see that chitosan has a bright application prospect as a bioink for in situ printing, but it is often necessary to enhance the mechanical strength of chitosan by combining it with additional components.
3.7 Peptides
Peptides, compounds with two or more amino acids connected by peptide bonds, are intermediate substances between amino acids and proteins. Each peptide has a unique composition structure, and the structure of a peptide determines its function. Peptides were discovered in 1990 when a self-assembled peptide as a repeat fragment was found in yeast protein.94 Ultrashort amphiphilic peptides form β-fibrils through α-helical intermediates,95 which have been shown to self-assemble into nanofibrous hydrogels that resemble native ECMs, provide an environment conducive to cell survival and maintain the basic physiological functions of cells. In addition, the self-assembly of peptides can be modulated by adjusting their internal factors (e.g., amino acid sequence, repeat unit number of assembled motifs, and peptide concentration) and external stimuli (e.g., temperature, pH, and salt concentration) to exhibit stimulus–responsive properties and adjustable mechanical properties.96,97 Another advantage of self-assembled peptides is their inherent biocompatibility and biodegradability, which enable them to stimulate the extracellular environment, support cell growth, and be used in biomedical research in vitro and in vivo, heralding their good application prospects as bioinks.98–100 Moreover, their short length and ease of functionalization are conducive to synthesis and customization.Currently, there are research teams working to explore self-assembled peptide bioinks for in situ 3D printing. For example, Rauf et al.101 reported a unique in situ 3D bioprinting method. In their research, two novel ultra-short tetramer peptides, AC-Ile-Val-Cha-lys-NH2 (IVZK) and AC-Ile-Val-Ph-lys-NH2 (IVFK), were developed at ambient temperature. Their results demonstrate that the finished structures are highly durable and biocompatible when printed using the newly developed peptides IVZK and IVFK as bioinks. This shows the great potential of ultra-short tetramer peptides as bioinks for in situ printing. In all, self-assembled peptides have the advantages of excellent biomimicry, stimulus responsiveness, biocompatibility, biodegradability, ease of synthesis, and functionalization, which makes them ideal choices for bioinks. The application of self-assembled peptides to bioprinting can reproduce the dynamic complexity of biological tissues, thereby advancing the biomedical applications of current peptide hydrogel scaffolds.102
4. In situ 3D bioprinting in different printing scenarios
In situ 3D bioprinting adds many demanding requirements compared with conventional 3D bioprinting because of the changing application scenarios. First, the in situ printing environment could be a battlefield, disaster relief scenario, or other sunpredictable environment, which requires bioink to have rheological stability, meaning the printing performance will be unaffected if the printing environment changes drastically from low to high temperature. Second, bioinks should be able to keep the printed structure from collapsing under high body temperature and blood-filled infiltration environment and enable the printed cells to survive efficiently and quickly functionalize to start the damage repair as soon as possible. In addition, the printed structures need to adhere to the defective tissues strongly enough so that they will not detach from the defect during in vivo repair and cause secondary damage. Finally, the rapid functionalization of printed tissues and the portability for acute treatment are urgent issues that need to be addressed.34Different printing scenarios have different needs for bioinks. For scenarios such as printing tissues or organs, bioinks need to meet the anatomical structure and physiological needs of the site;103 for scenarios such as specific external environments, the functionalization of bioinks becomes more important. Hence, the following section will introduce the latest advances in in situ 3D bioprinting in several specific scenarios common in clinical settings.
4.1 Bone/cartilage defects
Bone/cartilage grafts are mainly limited due to their scarcity, donor site complications of autologous transplantation, and immune rejection of allogeneic transplantation, so there are still many challenges in clinical treatment. However, natural bone/cartilage is structurally and functionally heterogeneous and anisotropic, and different regions have unique material composition and mechanical and biological properties, so current tissue engineering strategies cannot perfectly reconstruct the anatomy of natural osteochondral tissue.104 As an emerging tissue engineering technology, in situ 3D bioprinting technology creates highly ordered complex structures out of bioactive materials and implants them into host tissues for repair, which can be used as an alternative to bone/cartilage transplantation.105Keriquel et al.43,109 used a laser-assisted bioprinting system to repair mouse calvaria defects in a minimally invasive manner by in situ printing of nano-hydroxyapatite lasers. Subsequently, the group used mesenchymal stem cells, nanohydroxyapatite, and type I collagen as bioinks in the repair of critical-sized cranial defects in mice and successfully induced in situ hemodynamic reconstruction and subsequent tissue regeneration of the bone defects.110 Vidal et al.111 used biphasic calcium phosphate to repair 15 mm critical-sized rabbit ulnar defects by in situ printing of prevascularized synthetic bone grafts, and micro-CT and histological examination showed that the bone regeneration rate of prevascularized synthetic bone grafts was significantly higher than that of nonvascularized artificial bone after 8 weeks.
In a recent study, Xie et al.34 proposed a novel idea of “bio-concrete” ink, in which pre-functionalized cell-laden microspheres were used as “stones” and highly concentrated GelMA hydrogel pre-polymerization solution was used as “cement”. (Fig. 2). Moreover, they developed a robotic in situ 3D bioprinting system to achieve in situ repair of irregular wounds. They believe the advantage of in situ printing with bioconcrete bioink is its 100% biological components, which can promote the self-repair of skull defects at the histological level, rather than simply repairing the skull with prostheses.
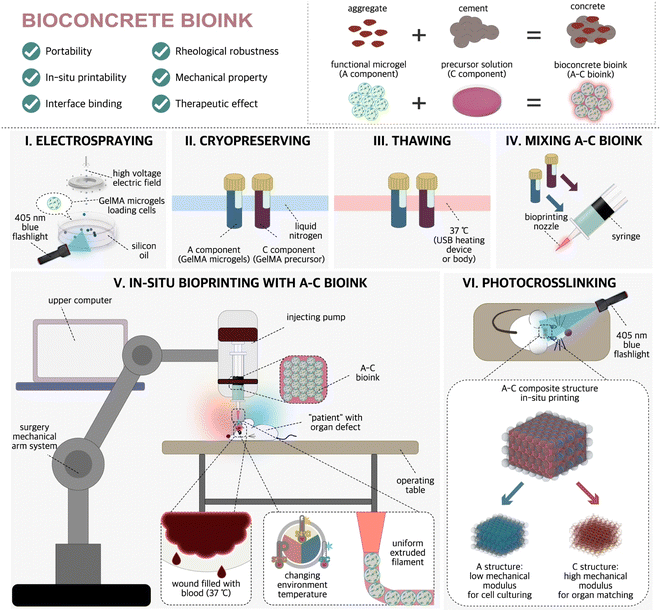 | ||
| Fig. 2 Train of thought of A–C bioink designing and sketch of the preparing/using method.34 Reproduced from ref. 34 with permission from the Springer Nature Limited, Copyright© 2023. | ||
In addition, the multilayer bionic scaffold exhibited good osteo–inductive activity and facilitated cell survival in the scaffold, making it a promising scaffold biomaterial for clinical applications. Zhang et al.112 used low-temperature in situ 3D bioprinting to construct a novel bioactive poly (lactic-co-glycolic acid)/β-tricalcium phosphate composite scaffold loaded with graphene oxide and bone morphogenetic protein-2-like peptide to repair critical-size bone defects. In vitro experiments and in vivo animal experiments have shown that hierarchical porous structural interfaces are important regulators of cellular activity and differentiation. However, there are still many bone tissue injuries with multiple pathological changes in clinical practice, which require stents loaded with therapeutic drugs. Feng et al.113 developed a unique cell-infiltrating and injectable gelatin hydrogel that effectively prevented bone mineral density reduction and promoted bone formation in an animal model of steroid-related osteonecrosis in mice by in situ injection of an injectable hydrogel encapsulating bone marrow mesenchymal stem cells and icaritin. This study demonstrates the feasibility of using injectable hydrogels as therapeutic drug carriers and provides a new direction for subsequent clinical applications.
An important challenge of in situ 3D bioprinting technology is to maintain the viability of living cells, the sensitivity of growth factors, and the activity of bioactive substances. Recently, a handheld bioink extrusion device was developed for cartilage repair. O'Connell et al.119 developed a handheld bioprinting device called “Biopen” to manually control the deposition of GelMA/methacrylate-hyaluronic acid hydrogels and repair cartilage defects in situ by UV cross-linking. In vitro studies have shown that human adipose-derived stem cells remain highly active in hydrogels one week of after printing.47 Subsequently, the research group used this device to conduct sheep animal experiments, and the results showed that at 8 weeks after in situ printing, the scaffolds showed good cartilage regeneration effects at both the macroscopic shape and microscopic protein gene levels.120–122
A 3D-bioprinted difunctional scaffold based on aptamer HM69-mediated mesenchymal stem cell-specific recruitment and factor-enhanced cell chondrogenesis developed in a recent study may be a promising strategy for articular cartilage regeneration in situ.123 In this study, aptamers that could specifically recognize and recruit autologous mesenchymal stem cells were chemically conjugated to the ECM of acellular cartilage and then mixed with GelMA to form a photocrosslinkable bioink for 3D bioprinting, and the biodegradable polymer poly(ε-caprolactone) was selected to provide mechanical strength for 3D bioprinted constructs. This bifunctional scaffold provides a favorable microenvironment for cell adhesion and proliferation and promotes chondrogenesis, thus greatly improving cartilage repair in rabbit full-thickness defects. Chen et al.44 used digital near-infrared photopolymerization printing technology to non-invasively print subcutaneous bioink into customized tissue structures in situ by in vitro irradiation with near-infrared light. In further experiments, the researchers printed auricular structures containing chondrocytes non-invasively and subcutaneously in mice based on digital near-infrared photopolymerization. The scaffolds maintained good cosmetic structure after one month, and type II collagen secretion by chondrocytes was observed.
4.2 Skin defects
The normal wound healing process is very precise and includes a series of processes including hemostasis, inflammation, proliferation, and ECM remodeling. In pathophysiological conditions such as trauma, burns, and chronic wounds (e.g.,wounds resulting from diabetes and pressure ulcers), this normal healing process can be severely dysregulated, resulting in the loss of most skin tissue and failure to heal.124,125 Traditional repair methods, such as autologous skin grafting, have poor timeliness of treatment due to limited skin sources and long preparation times. By contrast, in situ skin bioprinting is an on-site printing strategy that scans wound morphological characteristics after debridement and directly deposits cells and biomaterials on the defect,11,126,127 which can solve the problem of poor timeliness.Bioinks, as delivery media for encapsulated cells, need to provide a microenvironment for the maturation of skin bioprinting in addition to minimizing cell damage during the printing process.128 Alongside the biomechanical and structural characteristics of the skin, shape fidelity and printing resolution should also be taken into account. Bioinks need to be easily printed with good resolution and able to maintain their structure to accommodate the skin maturation process after printing. Another important factor to consider is the rate at which materials degrade in vivo; scaffolds should degrade at rates that match ECM production and remodeling activities.129,130
As the largest and most superficial organ of the human body, the skin is the most suitable organ for in situ bioprinting therapy. Many in situ bioprinting studies have focused on repairing skin defects, and some progress has been made in animal experiments.53,131 Zhao et al.132 integrated platelet-rich plasma (PRP) at a concentration of 5% into an alginate–gelatin (AG) composite hydrogel for in situ extrusion bioprinting of full-thickness rat skin defects and found that the addition of PRP improved the cellular behavior of seed cells, regulated the tube formation and macrophage polarization of vascular endothelial cells in a paracrine manner, accelerated high-quality wound closure, regulated inflammation and initiated angiogenesis compared with AG bioink alone (Fig. 3).
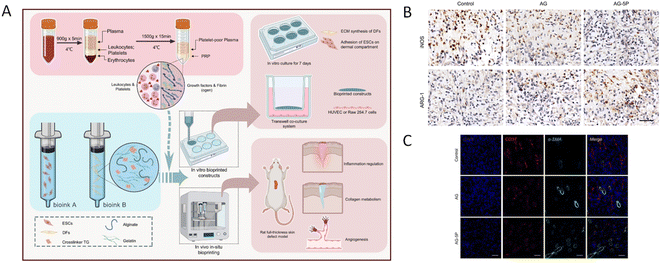 | ||
| Fig. 3 (A) Schematic illustration of bioprinting process using PRP containing multi-component bioink. (B) Immunohistochemical staining of iNOS and ARG-1 to determine the polarization of macrophages on day 3. Scale bar: 50 μm. (C) Evaluation of in vivo angiogenesis in the in situ bioprinted constructs on day 7. Immunofluorescent staining of CD31 and α-SMA for mature blood vessels. Scale bar: 50 μm.132 Reproduced from ref. 132 with permission from the Elsevier, Copyright© 2022. | ||
4.3 Other defects
To meet the demand of minimally invasive and precise treatment in clinical practice, 3D bioprinting is being transformed from in vitro printing to non-invasive in situ printing and other forms of in vivo printing. Zhao et al.133 developed a miniature bioprinting platform that can be installed on endoscopes. This printing platform has Delta robots that can be miniaturized in combination with microelectromechanical systems, so it has the advantages of reducing in vivo invasiveness, small size, and fast response speed. The printing platform enters the human body through the endoscope and performs tissue repair after reaching the injury site, realizing in situ printing in the body. To simulate the anatomical structure of the stomach, the team used a gelatin–alginate hydrogel with human gastric epithelial cells and human gastric smooth muscle cells as bioink to print a layered tissue scaffold in a stomach model (Fig. 4). Follow-up cell culture results showed that the printed cells maintained a high survival rate and stable proliferation ability in the tissue scaffold, which indicated that the cells in the printed tissue scaffold had good biological functions. Gastric wall injury is a common gastrointestinal problem, and about 12% of the world's population suffers from varying degrees of injury. If left untreated, open wounds in the stomach wall can lead to serious consequences, even requiring surgical intervention. Therefore, this work has been called an innovative advance in the fields of bioprinting and clinical science, presenting a major step toward a new approach to treating gastric wall injuries and establishing a proof of concept for the field of bioprinting.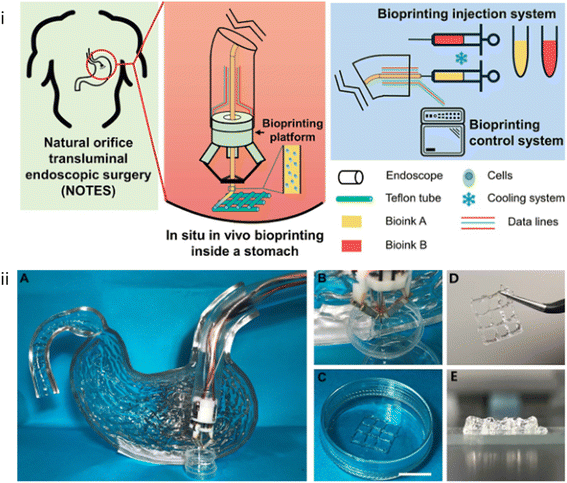 | ||
| Fig. 4 (i) Schematic of in situ in vivo bioprinting taking the case of treatment for gastric wall injuries. (ii) Bioprinting experiment equipment. (A) Bioprinting platform installed to a curved pipe mimicked an endoscope to process bioprinting inside a model of stomach. (B) The process of in situ in vivo bioprinting. (C) The printed 2-layer tissue scaffolds consisting of GES-1 cells and HGSMCs before cross-linking. (Scale bar: 1 cm). (D and E) The printed 8-layer scaffold with favorable mechanical properties.133 Reproduced from ref. 133 with permission from the IOP Publishing Ltd, Copyright© 2020. | ||
The dental pulp is a soft tissue rich in nerves and blood vessels, which has the function of nutrition, sensation, and defense against various pathogens. In addition, it produces dentin and maintains the biological and physiological viability of dentin.134,135 Pulpitis is one of the most common diseases related to the dental pulp and is usually caused by caries and trauma. Some studies have shown that periodontal disease is also associated with a variety of systemic diseases, including diabetes, cardiovascular disease, neurodegenerative diseases, and cancer.136,137 Because traditional root canal therapy cannot regenerate pulp tissue,138,139 at present, some scholars have performed pulp-dentin regeneration through 3D bioprinting, and many scientific research teams have also achieved some research results in exploring bioinks suitable for tooth regeneration, mainly including fibrin, collagen, sodium alginate, gelatin, GelMA, and some new bioinks.140,141 Duarte Campos et al.142 designed a handheld-based in situ bioprinting strategy that ultimately enabled angiogenesis within the root canal using collagen bioinks with appropriate biological properties for bioprinting. Due to the simplicity and convenience of the technique, it is feasible for clinical use.
5. Future and challenges
To date, relevant studies have validated the feasibility and practicality of in situ 3D bioprinting technology through animal experiments, and graft constructs made with this technology are anticipated to address the shortage of transplanted tissues and organs while also meeting the specific needs of patients for new tissues and organs designed in real time. However, more research is required for clinical translation. To further validate their biocompatibility, safety, and sterility, as well as to reduce printing parameters that require operator control to ensure printing precision and surgical quality, future development and optimization of bioinks and bioprinters suitable to in situ bioprinting are required. In addition, with the development of artificial intelligence technology, surgical robots can obtain the three-dimensional structure of defects and quickly deposit bioink in real-time visual analysis, which greatly shortens the operation time and reduces the pain of patients.107,143“Four-dimensional bioprinting” has developed recently since 3D bioprinting only takes into account the biological structure's starting state and pays little attention to post-printing dynamics.144 The fourth dimension in 4D bioprinting is time, which stresses the capability of printing multiple materials with time or the creation of a customized-material system that can transform from one shape to another. A more comprehensive definition of 4D printing is that a 3D-printed structure is exposed to a predetermined stimulus (e.g., temperature, water, light, pH, etc.), and its function, shape, and properties can change over time.145,146 Excitingly, stimulus-responsive bioinks—which undergo conformational changes under specific trigger conditions (e.g., temperature, pH, humidity, electric current, magnetic field, light, acoustics, or a combination of these stimuli) and may reproduce the natural morphological and structural changes of tissues—show great potential in 4D bioprinting.147
Bioprinting technology has been rapidly maturing over the past 20 years of development. We believe that the biggest bottleneck to further development is insufficient research on the development process of tissues and organs, making it difficult to print structures with both the desired appearance and functions. Moreover, ethical and clinical regulatory issues pose significant obstacles, as the production of in vivo tissues/organs may lead to biosafety and liability issues, and regulators are unsure of how to respond to the potentially uncertain risks (e.g., immune reactions) of this technology.
6. Conclusion
In conclusion, as an emerging tissue engineering technology, in situ printing technology can simplify surgical procedures and reduce the dependence of surgical outcomes on the surgeon's skill level, thereby reducing postoperative complications and achieving early recovery. Although some studies have verified the feasibility and practicability of in situ 3D bioprinting technology at the level of animal experiments, in situ bioprinting still requires more improvement and validation. Before the clinical adoption of in situ bioprinting, major breakthroughs need to be made in bioink, printing accuracy, and other aspects of the procedure. However, we believe that the advantages of in situ bioprinting make it an important development direction for bioprinting.Author contributions
Conceptualization, Peige Wang and Jianan Ren; writing—original draft preparation, Ruojing Li and Yeying Zhao; writing—review and editing, Ruojing Li, Zhiqiang Zheng and Shurui Song; drafting, Ruojing Li, Yangyang Liu and Lei Song; project administration, Peige Wang and Jing Dong. All authors read and approved the final manuscript.Conflicts of interest
There is no conflict of interest regarding the publication of this paper.Acknowledgements
We thank LetPub (http://www.letpub.com) for its linguistic assistance during the preparation of this manuscript. And we sincerely thank Li Ze of Nanjing University for his guidance in writing this review. This study is funded by the Taishan Scholar Foundation of Shandong Province (Grant No. 2018092901).References
- P. Datta, V. Ozbolat, B. Ayan, A. Dhawan and I. T. Ozbolat, Biotechnol. Bioeng., 2017, 114, 2424–2431 CrossRef CAS PubMed
.
- A. Zennifer, S. Manivannan, S. Sethuraman, S. G. Kumbar and D. Sundaramurthi, Biomater Adv., 2022, 134, 112576 CrossRef PubMed
.
- P. Prabhakaran, T. Palaniyandi, B. Kanagavalli, V. Ram Kumar, R. Hari, V. Sandhiya, G. Baskar, B. K. Rajendran and A. Sivaji, Acta Histochem., 2022, 124, 151932 CrossRef CAS PubMed
.
- L. van der Elst, C. F. de Lima, M. G. Kurtoglu, V. N. Koraganji, M. X. Zheng and A. Gumennik, Adv. Fiber Mater., 2021, 3, 59–75 CrossRef CAS
.
- P. Jain, H. Kathuria and N. Dubey, Biomaterials, 2022, 287, 121639 CrossRef CAS PubMed
.
- N. Ashammakhi, S. Ahadian, I. Pountos, S. K. Hu, N. Tellisi, P. Bandaru, S. Ostrovidov, M. R. Dokmeci and A. Khademhosseini, Biomed. Microdevices, 2019, 21, 42 CrossRef PubMed
.
- N. Hong, G. H. Yang, J. Lee and G. Kim, J. Biomed. Mater. Res., Part B, 2018, 106, 444–459 CrossRef CAS PubMed
.
- P. G. Campbell and L. E. Weiss, Expert Opin. Biol. Ther., 2007, 7, 1123–1127 CrossRef CAS PubMed
.
- N. A. Elkasabgy and A. A. Mahmoud, AAPS PharmSciTech, 2019, 20, 256 CrossRef PubMed
.
- S. Singh, D. Choudhury, F. Yu, V. Mironov and M. W. Naing, Acta Biomater., 2020, 101, 14–25 CrossRef CAS PubMed
.
- G. Ying, J. Manríquez, D. Wu, J. Zhang, N. Jiang, S. Maharjan, D. H. Hernández Medina and Y. S. Zhang, Mater. Today Bio, 2020, 8, 100074 CrossRef CAS PubMed
.
- M. Albouy, A. Desanlis, S. Brosset, C. Auxenfans, E. J. Courtial, K. Eli, S. Cambron, J. Palmer, L. Vidal, A. Thépot, M. Dos Santos and C. A. Marquette, Plast. Reconstr. Surg. Glob. Open, 2022, 10, e4056 CrossRef PubMed
.
- Y. Wu, D. J. Ravnic and I. T. Ozbolat, Trends Biotechnol., 2020, 38, 594–605 CrossRef CAS PubMed
.
- K. K. Moncal, R. S. Tigli Aydın, K. P. Godzik, T. M. Acri, D. N. Heo, E. Rizk, H. Wee, G. S. Lewis, A. K. Salem and I. T. Ozbolat, Biomaterials, 2022, 281, 121333 CrossRef CAS PubMed
.
- J. Groll, J. A. Burdick, D. W. Cho, B. Derby, M. Gelinsky, S. C. Heilshorn, T. Jüngst, J. Malda, V. A. Mironov, K. Nakayama, A. Ovsianikov, W. Sun, S. Takeuchi, J. J. Yoo and T. B. F. Woodfield, Biofabrication, 2018, 11, 013001 CrossRef CAS PubMed
.
- S. Heid and A. R. Boccaccini, Acta Biomater., 2020, 113, 1–22 CrossRef CAS PubMed
.
- J. Malda, J. Visser, F. P. Melchels, T. Jüngst, W. E. Hennink, W. J. Dhert, J. Groll and D. W. Hutmacher, Adv. Mater., 2013, 25, 5011–5028 CrossRef CAS PubMed
.
- A. Fatimi, O. V. Okoro, D. Podstawczyk, J. Siminska-Stanny and A. Shavandi, Gels, 2022, 8, 179 CrossRef PubMed
.
- Y. Jiang, J. Huang, X. Wu, Y. Ren, Z. Li and J. Ren, Int. J. Biol. Macromol., 2020, 149, 148–157 CrossRef CAS PubMed
.
- J. Huang, Y. Jiang, Y. Liu, Y. Ren, Z. Xu, Z. Li, Y. Zhao, X. Wu and J. Ren, Bioact. Mater., 2020, 6, 770–782 CrossRef PubMed
.
- G. Chen, J. Ren, Y. Deng, X. Wu, J. Huang, G. Wang, Y. Zhao and J. Li, J. Biomed. Nanotechnol., 2017, 13, 1660–1672 CrossRef CAS PubMed
.
- C. Ghobril and M. W. Grinstaff, Chem. Soc. Rev., 2015, 44, 1820–1835 RSC
.
- J. Huang, Y. Deng, J. Ren, G. Chen, G. Wang, F. Wang and X. Wu, Carbohydr. Polym., 2018, 186, 54–63 CrossRef CAS PubMed
.
- Z. Li, C. Wu, Z. Liu, Z. Li, X. Peng, J. Huang, J. Ren and P. Wang, RSC Adv., 2020, 10, 1331–1340 RSC
.
- H. Wang, H. Yu, X. Zhou, J. Zhang, H. Zhou, H. Hao, L. Ding, H. Li, Y. Gu, J. Ma, J. Qiu and D. Ma, Front. Bioeng. Biotechnol, 2022, 10, 905438 CrossRef PubMed
.
- Y. J. Chen, X. T. Dong, M. Shafiq, G. Myles, N. Radacsi and X. M. Mo, Adv. Fiber Mater., 2022, 4, 959–986 CrossRef CAS
.
- C. B. Highley, C. B. Rodell and J. A. Burdick, Adv. Mater., 2015, 27, 5075–5079 CrossRef CAS PubMed
.
- H. Hong, Y. B. Seo, D. Y. Kim, J. S. Lee, Y. J. Lee, H. Lee, O. Ajiteru, M. T. Sultan, O. J. Lee, S. H. Kim and C. H. Park, Biomaterials, 2020, 232, 119679 CrossRef CAS PubMed
.
- S. Ostrovidov, S. Salehi, M. Costantini, K. Suthiwanich, M. Ebrahimi, R. B. Sadeghian, T. Fujie, X. Shi, S. Cannata, C. Gargioli, A. Tamayol, M. R. Dokmeci, G. Orive, W. Swieszkowski and A. Khademhosseini, Small, 2019, 15, e1805530 CrossRef PubMed
.
- N. Chen, K. Zhu, Y. S. Zhang, S. Yan, T. Pan, M. Abudupataer, G. Yu, M. F. Alam, L. Wang, X. Sun, Y. Yu, C. Wang and W. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 30585–30595 CrossRef CAS PubMed
.
- B. S. Kim, Y. W. Kwon, J. S. Kong, G. T. Park, G. Gao, W. Han, M. B. Kim, H. Lee, J. H. Kim and D. W. Cho, Biomaterials, 2018, 168, 38–53 CrossRef CAS PubMed
.
- J. Liu, M. Shahriar, H. Xu and C. Xu, Biofabrication, 2022, 14 CAS
.
- M. Samandari, A. Mostafavi, J. Quint, A. Memić and A. Tamayol, Trends Biotechnol., 2022, 40, 1229–1247 CrossRef CAS PubMed
.
- M. Xie, Y. Shi, C. Zhang, M. Ge, J. Zhang, Z. Chen, J. Fu, Z. Xie and Y. He, Nat. Commun., 2022, 13, 3597 CrossRef CAS PubMed
.
- M. Hospodiuk, M. Dey, D. Sosnoski and I. T. Ozbolat, Biotechnol. Adv., 2017, 35, 217–239 CrossRef CAS PubMed
.
- M. Samandari, J. Quint, A. Rodríguez-delaRosa, I. Sinha, O. Pourquié and A. Tamayol, Adv. Mater., 2022, 34, e2105883 CrossRef PubMed
.
- P. S. Gungor-Ozkerim, I. Inci, Y. S. Zhang, A. Khademhosseini and M. R. Dokmeci, Biomater. Sci., 2018, 6, 915–946 RSC
.
- R. Galante, T. J. A. Pinto, R. Colaço and A. P. Serro, J. Biomed. Mater. Res., Part B, 2018, 106, 2472–2492 CrossRef CAS PubMed
.
- M. Di Foggia, U. Corda, E. Plescia, P. Taddei and A. Torreggiani, J. Mater. Sci.: Mater. Med., 2010, 21, 1789–1797 CrossRef CAS PubMed
.
- Z. Dai, J. Ronholm, Y. Tian, B. Sethi and X. Cao, J. Tissue Eng., 2016, 7 DOI:10.1177/2041731416648
.
- J. R. Dias, N. Ribeiro, S. Baptista-Silva, A. R. Costa-Pinto, N. Alves and A. L. Oliveira, Front. Bioeng. Biotechnol, 2020, 8, 85 CrossRef PubMed
.
- L. Vidal, C. Kampleitner, M. Brennan, A. Hoornaert and P. Layrolle, Front. Bioeng. Biotechnol, 2020, 8, 61 CrossRef PubMed
.
- V. Keriquel, H. Oliveira, M. Rémy, S. Ziane, S. Delmond, B. Rousseau, S. Rey, S. Catros, J. Amédée, F. Guillemot and J. C. Fricain, Sci. Rep., 2017, 7, 1778 CrossRef PubMed
.
- Y. Chen, J. Zhang, X. Liu, S. Wang, J. Tao, Y. Huang, W. Wu, Y. Li, K. Zhou, X. Wei, S. Chen, X. Li, X. Xu, L. Cardon, Z. Qian and M. Gou, Sci. Adv., 2020, 6, eaba7406 CrossRef CAS PubMed
.
- S. Ji and M. Guvendiren, APL Bioeng., 2021, 5, 011508 CrossRef PubMed
.
- E. A. Aisenbrey, A. Tomaschke, E. Kleinjan, A. Muralidharan, C. Pascual-Garrido, R. R. McLeod, V. L. Ferguson and S. J. Bryant, Macromol. Biosci., 2018, 18 DOI:10.1002/mabi.201700267
.
- S. Duchi, C. Onofrillo, C. D. O'Connell, R. Blanchard, C. Augustine, A. F. Quigley, R. M. I. Kapsa, P. Pivonka, G. Wallace, C. Di Bella and P. F. M. Choong, Sci. Rep., 2017, 7, 5837 CrossRef PubMed
.
- F. Agostinacchio, X. Mu, S. Dirè, A. Motta and D. L. Kaplan, Trends Biotechnol., 2021, 39, 719–730 CrossRef CAS PubMed
.
- Z. Emami, M. Ehsani, M. Zandi and R. Foudazi, Carbohydr. Polym., 2018, 198, 509–517 CrossRef CAS PubMed
.
- J. Lee, J. Hong, W. Kim and G. H. Kim, Carbohydr. Polym., 2020, 250, 116914 CrossRef CAS PubMed
.
- S. D. Kim, S. Jin, S. Kim, D. Son and M. Shin, Polymers, 2022, 14 Search PubMed
.
- P. R. Cao, L. M. Tao, J. H. Gong, T. M. Wang, Q. H. Wang, J. P. Ju and Y. M. Zhang, ACS Appl. Polym. Mater., 2021, 3, 6167–6175 CrossRef CAS
.
- N. Hakimi, R. Cheng, L. Leng, M. Sotoudehfar, P. Q. Ba, N. Bakhtyar, S. Amini-Nik, M. G. Jeschke and A. Günther, Lab Chip, 2018, 18, 1440–1451 RSC
.
- X. Wang, C. Yang, Y. Yu and Y. Zhao, Research, 2022, 2022, 9794745 CAS
.
- S. Ricard-Blum, Cold Spring Harb. Perspect. Biol., 2011, 3, a004978 Search PubMed
.
- J. Stepanovska, M. Otahal, K. Hanzalek, M. Supova and R. Matejka, Gels, 2021, 7, 252 CrossRef CAS PubMed
.
- V. A. Kumar, N. L. Taylor, A. A. Jalan, L. K. Hwang, B. K. Wang and J. D. Hartgerink, Biomacromolecules, 2014, 15, 1484–1490 CrossRef CAS PubMed
.
- X. Yang, Z. Lu, H. Wu, W. Li, L. Zheng and J. Zhao, Mater. Sci. Eng. C, 2018, 83, 195–201 CrossRef CAS PubMed
.
- S. Liu, D. Huang, Y. Hu, J. Zhang, B. Chen, H. Zhang, X. Dong, R. Tong, Y. Li and W. Zhou, RSC Adv., 2020, 10, 39241–39250 RSC
.
- A. C. Heidenreich, M. Perez-Recalde, A. G. Wusener and E. B. Hermida, Polym. Test., 2020, 82, 9 CrossRef
.
- M. Sun, X. Sun, Z. Wang, S. Guo, G. Yu and H. Yang, Polymers, 2018, 10, 1290 CrossRef PubMed
.
- X. Wang, Q. Ao, X. Tian, J. Fan, H. Tong, W. Hou and S. Bai, Polymers, 2017, 9, 401 CrossRef PubMed
.
- J. Berg, T. Hiller, M. S. Kissner, T. H. Qazi, G. N. Duda, A. C. Hocke, S. Hippenstiel, L. Elomaa, M. Weinhart, C. Fahrenson and J. Kurreck, Sci. Rep., 2018, 8, 13877 CrossRef PubMed
.
- M. S. Kang, J. I. Kang, P. Le Thi, K. M. Park, S. W. Hong, Y. S. Choi, D. W. Han and K. D. Park, ACS Macro Lett., 2021, 10, 426–432 CrossRef CAS PubMed
.
- F. B. Albrecht, F. F. Schmidt, A. C. Volz and P. J. Kluger, Gels, 2022, 8, 611 CrossRef CAS PubMed
.
- S. Z. Zou, S. N. Fan, A. L. Oliveira, X. Yao, Y. P. Zhang and H. L. Shao, Adv. Fiber Mater., 2022, 4, 758–773 CrossRef CAS
.
- S. Young, M. Wong, Y. Tabata and A. G. Mikos, J. Control Release, 2005, 109, 256–274 CrossRef CAS PubMed
.
- A. I. Van Den Bulcke, B. Bogdanov, N. De Rooze, E. H. Schacht, M. Cornelissen and H. Berghmans, Biomacromolecules, 2000, 1, 31–38 CrossRef CAS PubMed
.
- H. Stratesteffen, M. Köpf, F. Kreimendahl, A. Blaeser, S. Jockenhoevel and H. Fischer, Biofabrication, 2017, 9, 045002 CrossRef PubMed
.
- C. McBeth, J. Lauer, M. Ottersbach, J. Campbell, A. Sharon and A. F. Sauer-Budge, Biofabrication, 2017, 9, 015009 CrossRef PubMed
.
- K. Ma, T. Zhao, L. Yang, P. Wang, J. Jin, H. Teng, D. Xia, L. Zhu, L. Li, Q. Jiang and X. Wang, J. Adv. Res., 2020, 23, 123–132 CrossRef CAS PubMed
.
- P. E. Ludwig, T. J. Huff and J. M. Zuniga, J. Tissue Eng., 2018, 9 DOI:10.1177/2041731418769863
.
- C. Colosi, S. R. Shin, V. Manoharan, S. Massa, M. Costantini, A. Barbetta, M. R. Dokmeci, M. Dentini and A. Khademhosseini, Adv. Mater., 2016, 28, 677–684 CrossRef CAS PubMed
.
- T. Billiet, E. Gevaert, T. De Schryver, M. Cornelissen and P. Dubruel, Biomaterials, 2014, 35, 49–62 CrossRef CAS PubMed
.
- N. E. Fedorovich, M. H. Oudshoorn, D. van Geemen, W. E. Hennink, J. Alblas and W. J. Dhert, Biomaterials, 2009, 30, 344–353 CrossRef CAS PubMed
.
- S. Duchi, C. Onofrillo, C. O'Connell, G. G. Wallace, P. Choong and C. Di Bella, Methods Mol. Biol., 2020, 2140, 145–157 CrossRef CAS PubMed
.
- H. Cui, W. Zhu, Y. Huang, C. Liu, Z. X. Yu, M. Nowicki, S. Miao, Y. Cheng, X. Zhou, S. J. Lee, Y. Zhou, S. Wang, M. Mohiuddin, K. Horvath and L. G. Zhang, Biofabrication, 2019, 12, 015004 CrossRef PubMed
.
- M. N. Collins and C. Birkinshaw, Carbohydr. Polym., 2013, 92, 1262–1279 CrossRef CAS PubMed
.
- S. H. Park, J. Y. Seo, J. Y. Park, Y. B. Ji, K. Kim, H. S. Choi, S. Choi, J. H. Kim, B. H. Min and M. S. Kim, NPG Asia Mater., 2019, 11, 16 CrossRef
.
- M. D. Godesky and D. I. Shreiber, Biointerphases, 2020, 14, 061005 CrossRef PubMed
.
- L. V. Thomas, R. Vg and P. D. Nair, Int. J. Biol. Macromol., 2017, 104, 1925–1935 CrossRef CAS PubMed
.
- S. D. Aznar-Cervantes, A. Pagan, B. Monteagudo Santesteban and J. L. Cenis, Sci. Rep., 2019, 9, 6703 CrossRef PubMed
.
- S. Chameettachal, S. Midha and S. Ghosh, ACS Biomater. Sci. Eng., 2016, 2, 1450–1463 CrossRef CAS PubMed
.
- S. Midha, S. Murab and S. Ghosh, Biomaterials, 2016, 97, 133–153 CrossRef CAS PubMed
.
- S. Chawla, S. Midha, A. Sharma and S. Ghosh, Adv. Healthc. Mater., 2018, 7, e1701204 CrossRef PubMed
.
- M. McGill, J. M. Grant and D. L. Kaplan, Ann. Biomed. Eng., 2020, 48, 1905–1915 CrossRef PubMed
.
- W. Shi, M. Sun, X. Hu, B. Ren, J. Cheng, C. Li, X. Duan, X. Fu, J. Zhang, H. Chen and Y. Ao, Adv. Mater., 2017, 29 DOI:10.1002/adma.201701089
.
- S. H. Kim, Y. K. Yeon, J. M. Lee, J. R. Chao, Y. J. Lee, Y. B. Seo, M. T. Sultan, O. J. Lee, J. S. Lee, S. I. Yoon, I. S. Hong, G. Khang, S. J. Lee, J. J. Yoo and C. H. Park, Nat. Commun., 2018, 9, 1620 CrossRef PubMed
.
- Q. Wu, D. Therriault and M. C. Heuzey, ACS Biomater. Sci. Eng., 2018, 4, 2643–2652 CrossRef CAS PubMed
.
- Y. Yang, Z. Wang, Y. Xu, J. Xia, Z. Xu, S. Zhu and M. Jin, Gels, 2022, 8, 314 CrossRef CAS PubMed
.
- Y. Sun, C. Yang, X. Zhu, J. J. Wang, X. Y. Liu, X. P. Yang, X. W. An, J. Liang, H. J. Dong, W. Jiang, C. Chen, Z. G. Wang, H. T. Sun, Y. Tu, S. Zhang, F. Chen and X. H. Li, J. Biomed. Mater. Res. A, 2019, 107, 1898–1908 CrossRef CAS PubMed
.
- Y. Liu, X. Luo, W. Wu, A. Zhang, B. Lu, T. Zhang and M. Kong, Int. J. Biol. Macromol., 2021, 182, 689–700 CrossRef CAS PubMed
.
- M. Puertas-Bartolomé, M. K. Włodarczyk-Biegun, A. Del Campo, B. Vázquez-Lasa and J. San Román, Polymers, 2020, 12, 1986 CrossRef PubMed
.
- S. Zhang, T. Holmes, C. Lockshin and A. Rich, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 3334–3338 CrossRef CAS PubMed
.
- C. A. Hauser, R. Deng, A. Mishra, Y. Loo, U. Khoe, F. Zhuang, D. W. Cheong, A. Accardo, M. B. Sullivan, C. Riekel, J. Y. Ying and U. A. Hauser, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 1361–1366 CrossRef CAS PubMed
.
- E. Gazit, Chem. Soc. Rev., 2007, 36, 1263–1269 RSC
.
- S. Zhang, Nat. Biotechnol., 2003, 21, 1171–1178 CrossRef CAS PubMed
.
- Y. Chau, Y. Luo, A. C. Cheung, Y. Nagai, S. Zhang, J. B. Kobler, S. M. Zeitels and R. Langer, Biomaterials, 2008, 29, 1713–1719 CrossRef PubMed
.
- Y. Nagai, L. D. Unsworth, S. Koutsopoulos and S. Zhang, J. Control Release, 2006, 115, 18–25 CrossRef CAS PubMed
.
- E. L. Bakota, Y. Wang, F. R. Danesh and J. D. Hartgerink, Biomacromolecules, 2011, 12, 1651–1657 CrossRef CAS PubMed
.
- S. Rauf, H. H. Susapto, K. Kahin, S. Alshehri, S. Abdelrahman, J. H. Lam, S. Asad, S. Jadhav, D. Sundaramurthi, X. Gao and C. A. E. Hauser, J. Mater. Chem. B, 2021, 9, 1069–1081 RSC
.
- Y. Loo and C. A. Hauser, J. Mater. Chem. B, 2015, 11, 014103 Search PubMed
.
- D. J. Ravnic, A. N. Leberfinger, S. V. Koduru, M. Hospodiuk, K. K. Moncal, P. Datta, M. Dey, E. Rizk and I. T. Ozbolat, Ann. Surg., 2017, 266, 48–58 CrossRef PubMed
.
- Y. Wu, P. Kennedy, N. Bonazza, Y. Yu, A. Dhawan and I. Ozbolat, Cartilage, 2021, 12, 76–92 CrossRef PubMed
.
- L. Li, F. Yu, J. Shi, S. Shen, H. Teng, J. Yang, X. Wang and Q. Jiang, Sci. Rep., 2017, 7, 9416 CrossRef PubMed
.
- Y. L. Park, K. Park and J. M. Cha, Micromachines, 2021, 12 Search PubMed
.
- L. Li, J. Shi, K. Ma, J. Jin, P. Wang, H. Liang, Y. Cao, X. Wang and Q. Jiang, J. Adv. Res., 2021, 30, 75–84 CrossRef PubMed
.
- Y. He, W. Wang, S. Lin, Y. Yang, L. Song, Y. Jing, L. Chen, Z. He, W. Li, A. Xiong, K. W. K. Yeung, Q. Zhao, Y. Jiang, Z. Li, G. Pei and Z. Y. Zhang, Bioact. Mater., 2022, 9, 491–507 CrossRef CAS PubMed
.
- V. Keriquel, F. Guillemot, I. Arnault, B. Guillotin, S. Miraux, J. Amédée, J. C. Fricain and S. Catros, Biofabrication, 2010, 2, 014101 CrossRef PubMed
.
- O. Kérourédan, D. Hakobyan, M. Rémy, S. Ziane, N. Dusserre, J. C. Fricain, S. Delmond, N. B. Thébaud and R. Devillard, Biofabrication, 2019, 11, 045002 CrossRef PubMed
.
- L. Vidal, M. Brennan, S. Krissian, J. De Lima, A. Hoornaert, P. Rosset, B. H. Fellah and P. Layrolle, Acta Biomater., 2020, 114, 384–394 CrossRef CAS PubMed
.
- Y. Zhang, C. Wang, L. Fu, S. Ye, M. Wang and Y. Zhou, Molecules, 2019, 24, 1669 CrossRef CAS PubMed
.
- Q. Feng, J. Xu, K. Zhang, H. Yao, N. Zheng, L. Zheng, J. Wang, K. Wei, X. Xiao, L. Qin and L. Bian, ACS Cent. Sci., 2019, 5, 440–450 CrossRef CAS PubMed
.
- T. Shen, Y. Dai, X. Li, S. Xu, Z. Gou and C. Gao, ACS Biomater. Sci. Eng., 2018, 4, 1942–1953 CrossRef CAS PubMed
.
- J. H. Galarraga, M. Y. Kwon and J. A. Burdick, Sci. Rep., 2019, 9, 19987 CrossRef CAS PubMed
.
- K. S. Lim, F. Abinzano, P. N. Bernal, A. Albillos Sanchez, P. Atienza-Roca, I. A. Otto, Q. C. Peiffer, M. Matsusaki, T. B. F. Woodfield, J. Malda and R. Levato, Adv. Healthc. Mater., 2020, 9, e1901792 CrossRef PubMed
.
- A. J. Sophia Fox, A. Bedi and S. A. Rodeo, Sports Health, 2009, 1, 461–468 CrossRef PubMed
.
- J. Huang, J. Xiong, D. Wang, J. Zhang, L. Yang, S. Sun and Y. Liang, Gels, 2021, 7, 144 CrossRef CAS PubMed
.
- C. D. O’Connell, C. Di Bella, F. Thompson, C. Augustine, S. Beirne, R. Cornock, C. J. Richards, J. Chung, S. Gambhir, Z. Yue, J. Bourke, B. Zhang, A. Taylor, A. Quigley, R. Kapsa, P. Choong and G. G. Wallace, Biofabrication, 2016, 8, 015019 CrossRef PubMed
.
- C. Di Bella, S. Duchi, C. D. O'Connell, R. Blanchard, C. Augustine, Z. Yue, F. Thompson, C. Richards, S. Beirne, C. Onofrillo, S. H. Bauquier, S. D. Ryan, P. Pivonka, G. G. Wallace and P. F. Choong, J. Tissue Eng. Regen. Med., 2018, 12, 611–621 CrossRef CAS PubMed
.
- C. Onofrillo, S. Duchi, C. D. O'Connell, R. Blanchard, A. J. O'Connor, M. Scott, G. G. Wallace, P. F. M. Choong and C. Di Bella, Biofabrication, 2018, 10, 045006 CrossRef PubMed
.
- S. Duchi, C. Onofrillo, C. O'Connell, G. G. Wallace, P. Choong and C. Di Bella, Methods Mol. Biol., 2020, 2140, 145–157 CrossRef CAS PubMed
.
- Z. Yang, T. Zhao, C. Gao, F. Cao, H. Li, Z. Liao, L. Fu, P. Li, W. Chen, Z. Sun, S. Jiang, Z. Tian, G. Tian, K. Zha, T. Pan, X. Li, X. Sui, Z. Yuan, S. Liu and Q. Guo, ACS Appl. Mater. Interfaces, 2021, 13, 23369–23383 CrossRef CAS PubMed
.
- D. Chouhan, N. Dey, N. Bhardwaj and B. B. Mandal, Biomaterials, 2019, 216, 119267 CrossRef CAS PubMed
.
- J. Wu, Z. Xiao, A. Chen, H. He, C. He, X. Shuai, X. Li, S. Chen, Y. Zhang, B. Ren, J. Zheng and J. Xiao, Acta Biomater., 2018, 71, 293–305 CrossRef CAS PubMed
.
- C. Zhou, Y. Yang, J. Wang, Q. Wu, Z. Gu, Y. Zhou, X. Liu, Y. Yang, H. Tang, Q. Ling, L. Wang and J. Zang, Nat. Commun., 2021, 12, 5072 CrossRef CAS PubMed
.
- H. Li, F. Cheng, D. P. Orgill, J. Yao and Y. S. Zhang, Essays Biochem., 2021, 65, 533–543 CrossRef PubMed
.
- M. Varkey, D. O. Visscher, P. P. M. van Zuijlen, A. Atala and J. J. Yoo, Burns Trauma, 2019, 7, 4 Search PubMed
.
- J. Gopinathan and I. Noh, Biomater. Res., 2018, 22, 11 CrossRef PubMed
.
- A. Parak, P. Pradeep, L. C. du Toit, P. Kumar, Y. E. Choonara and V. Pillay, Drug Discov. Today, 2019, 24, 198–205 CrossRef CAS PubMed
.
- M. Albanna, K. W. Binder, S. V. Murphy, J. Kim, S. A. Qasem, W. Zhao, J. Tan, I. B. El-Amin, D. D. Dice, J. Marco, J. Green, T. Xu, A. Skardal, J. H. Holmes, J. D. Jackson, A. Atala and J. J. Yoo, Sci. Rep., 2019, 9, 1856 CrossRef PubMed
.
- M. Zhao, J. Wang, J. Zhang, J. Huang, L. Luo, Y. Yang, K. Shen, T. Jiao, Y. Jia, W. Lian, J. Li, Y. Wang, Q. Lian and D. Hu, Mater. Today Bio, 2022, 16, 100334 CrossRef CAS PubMed
.
- W. Zhao and T. Xu, Biofabrication, 2020, 12, 045020 CrossRef CAS PubMed
.
- K. M. Galler, M. Weber, Y. Korkmaz, M. Widbiller and M. Feuerer, Int. J. Mol. Sci., 2021, 22 Search PubMed
.
- Y. C. Bae and A. Yoshida, J. Oral Sci., 2020, 62, 126–130 CrossRef CAS PubMed
.
- G. Hajishengallis and T. Chavakis, Nat. Rev. Immunol., 2021, 21, 426–440 CrossRef CAS PubMed
.
- P. M. Preshaw and S. M. Bissett, Br. Dent. J., 2019, 227, 577–584 CrossRef PubMed
.
- T. Connert, R. Weiger and G. Krastl, Int. Endod. J., 2022, 55, 995–1002 CrossRef PubMed
.
- Z. Xie, Z. Shen, P. Zhan, J. Yang, Q. Huang, S. Huang, L. Chen and Z. Lin, Int. J. Mol. Sci., 2021, 22 Search PubMed
.
- J. Han, D. S. Kim, H. Jang, H. R. Kim and H. W. Kang, J. Tissue Eng., 2019, 10 DOI:10.1177/2041731419845849
.
- H. Yu, X. Zhang, W. Song, T. Pan, H. Wang, T. Ning, Q. Wei, H. H. K. Xu, B. Wu and D. Ma, J. Endod., 2019, 45, 706–715 CrossRef PubMed
.
- D. F. Duarte Campos, S. Zhang, F. Kreimendahl, M. Köpf, H. Fischer, M. Vogt, A. Blaeser, C. Apel and M. Esteves-Oliveira, Connect Tissue Res., 2020, 61, 205–215 CrossRef CAS PubMed
.
- J. Lipskas, K. Deep and W. Yao, Sci. Rep., 2019, 9, 3746 CrossRef PubMed
.
- G. Qu, J. Huang, Z. Li, Y. Jiang, Y. Liu, K. Chen, Z. Xu, Y. Zhao, G. Gu, X. Wu and J. Ren, Mater. Today Bio, 2022, 16, 100363 CrossRef CAS PubMed
.
- B. Gao, Q. Yang, X. Zhao, G. Jin, Y. Ma and F. Xu, Trends Biotechnol., 2016, 34, 746–756 CrossRef CAS PubMed
.
- Q. Yang, B. Gao and F. Xu, Biotechnol. J., 2020, 15, e1900086 CrossRef PubMed
.
- N. Ashammakhi, S. Ahadian, F. Zengjie, K. Suthiwanich, F. Lorestani, G. Orive, S. Ostrovidov and A. Khademhosseini, Biotechnol. J., 2018, 13, e1800148 CrossRef PubMed
.
| This journal is © The Royal Society of Chemistry 2023 |