Open Access Article
Open Access ArticleModified magnetic chitosan materials for heavy metal adsorption: a review
Ke Wanga,
Fanbing Zhanga,
Kexin Xua,
Yuju Chea,
Mingying Qia and
Cui Song *ab
*ab
aMarine College, Shandong University, Weihai 264209, China. E-mail: songcui@sdu.edu.cn
bShandong University-Weihai Research Institute of Industrial Technology, Weihai 264209, China
First published on 27th February 2023
Abstract
Magnetic chitosan materials have the characteristics of both chitosan and magnetic particle nuclei, showing the characteristics of easy separation and recovery, strong adsorption capacity and high mechanical strength, and have received extensive attention in adsorption, especially in the treatment of heavy metal ions. In order to further improve its performance, many studies have modified magnetic chitosan materials. This review discusses the strategies for the preparation of magnetic chitosan using coprecipitation, crosslinking, and other methods in detail. Besides, this review mainly summarizes the application of modified magnetic chitosan materials in the removal of heavy metal ions in wastewater in recent years. Finally, this review also discusses the adsorption mechanism, and puts forward the prospect of the future development of magnetic chitosan in wastewater treatment.
1. Introduction
Heavy metals are beneficial to the development of industry and the quality of human life, such as their application in electroplating, steel manufacturing and textiles,1 but excessive use has led to serious environmental and health problems. Toxic heavy metals migrate, transform, and accumulate in the biological chain, and these elements are harmful to human health.2–4 For example, humans and animals can cause nerve damage if they consume too much copper.5 High concentrations of lead ions can cause anorexia, irritability, constipation, abdominal pain, vomiting, reversible kidney damage, muscle weakness, anemia, encephalopathy, coma, and death.6,7 Consuming too many chromium ions carries a risk of causing cancer.5 Therefore, many researchers have developed effective and economical treatment technologies to reduce heavy metal ions in water sources. Common methods for reducing heavy metals in water matrices include catalytic oxidation, membrane separation, flocculation–sedimentation, adsorption, and biological methods.5 Due to its simple operation, low cost, high removal efficiency, and environmental friendliness,8 the adsorption method is considered to be a promising treatment method.In recent decades, various natural and synthetic adsorbents have been examined for their ability to remove heavy metals from contaminated solutions.9,10 Among various adsorbents, chitosan (CS) is widely used in the removal of heavy metals due to its excellent chemical properties, environmental friendliness and abundant adsorption sites.11 Chitosan itself is a promising heavy metal adsorbent, but its adsorption capacity is limited due to its pH sensitivity and weak mechanical properties.12 In addition, separating these adsorbents from water requires a complicated process and additional costs. Recently, magnetic adsorbents have attracted the interest of the scientific community because they can be easily separated from aqueous solutions.13 Chitosan has polycation chelating properties in its surface amino groups and hydroxyl groups, which makes it a very advantageous material for functionalized magnetic nanoparticles.14 In addition to the advantages of fast separation, the adsorption material modified by adding magnetic particles to chitosan also exhibits strong mechanical strength and excellent adsorption performance. Studies on magnetic chitosan adsorption have been summarized by some scholars (see Table 1). Briao et al.15 focuses on the regeneration of adsorbents, Shaumbwa et al.16 on the separation of magnetic materials, and Michailidou et al.17 on the adsorption of highly toxic elements such as uranium and mercury. We focus on the adsorption of magnetic chitosan to common polluting metal ions (Cu/Pb/Cr). This paper not only compares the preparation methods and adsorption capacity of different magnetic chitosan materials but also discusses the environmental factors affecting the adsorption capacity and the corresponding adsorption mechanism. In particular, in the comparison of adsorption methods and capabilities, we review a large number of recent studies and compare the advantages and disadvantages of modification methods innovatively. Therefore, readers can understand the latest progress of magnetic chitosan more intuitively and provide necessary knowledge reserves for follow-up research.
| Article | Aspect | |||||
|---|---|---|---|---|---|---|
| Preparation method | Adsorb object | Adsorption evaluation | Adsorption mechanism | Novelty | Emphasis | |
| The article | Coprecipitation method, crosslinking method, spray drying method, photochemical method and electrostatic drop method | Heavy metal ions (Cu, Pb, Cr) | BET surface area, adsorption capacity, pH, temp, cycles, kinetic and isotherm model | Physical adsorption, chemisorption and collaborative adsorption | Evaluate properties of magnetic chitosan prepared by different modification methods | Modification and application |
| Briao et al.15 | Co-precipitation and crosslinking method | Toxic metal ions (Pb, Cd, Hg, As) | Kinetics, equilibrium and thermodynamics | — | Adsorbent regeneration | Magnetization method |
| Shaumbwa et al.16 | Co-precipitation, crosslinking method and electrochemical crosslinking | Heavy metal ions, organic/inorganic dyes, fluorides and pesticides | Adsorption capacity, active sites and the surface area | — | Magnetic separation techniques | Application |
| Michailidou et al.17 | Hydrothermal-crosslinking and hydrothermal-precipitation method | Uranium, mercury and rare earth elements | Kinetics and thermodynamics | Interaction between the functional group and the adsorbed substance | Adsorb uranium, mercury, and rare earth elements | Absorption efficiency |
2. Magnetic chitosan
Magnetic materials generally have high saturation magnetization, which can perform rapid and effective solid–liquid separation and avoid secondary pollution.18 Magnetic chitosan mainly consists of two parts: chitosan and magnetic particles. Therefore, magnetic chitosan material has the dual characteristics of chitosan and magnetic materials. On the one hand, it has superparamagnetism and can be rapidly separated under an external magnetic field.19 On the other hand, there are special functional groups that react with heavy metals, organics and other substances, which can be used as binding sites to improve the adsorption performance.20 At present, magnetic chitosan materials have been studied and applied in many fields, such as environmental remediation,21 biomedicine,22 and petroleum exploitation.232.1 Chitosan
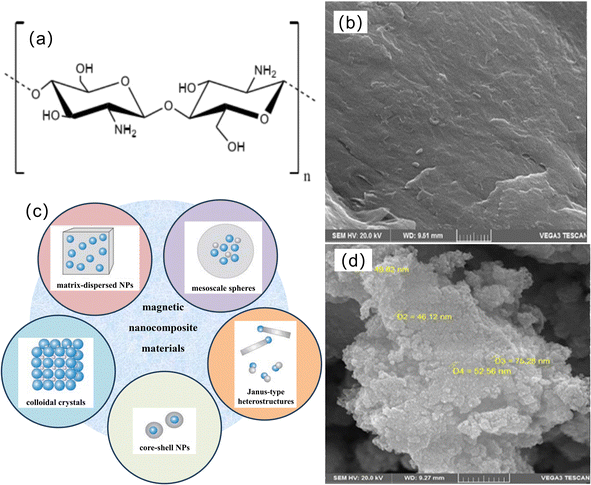
Chitosan is the only alkaline amylose in natural polysaccharides and is derived from chitin deacetylation in the cell walls of shrimp, crabs, insect shells, bacteria, and algae.24,25 The uniqueness of the molecular structure of chitosan lies in the presence of a large number of hydroxyl and amino groups, which are effective adsorption sites for removing metal ions26 (the structure and morphology are shown in Fig. 1a and b), and chitosan also has the characteristics of biodegradability and biocompatibility and does not easily cause secondary pollution, making it a better environmental functional material. However, chitosan has defects that limit its application in wastewater treatment. Therefore, people have tried to modify and improve performance through physical27 or chemical modification methods28 to improve its surface area and obtain more porosity and active sites.2.2 Magnetic particles
Magnetic particles have a large specific surface area and therefore possess high surface energies and chemical activity, which are easily oxidized in air, resulting in the loss of magnetism and dispersion.29 The addition of chitosan can maintain the stability of magnetic particles as a surface coating, reduce aggregation, prolong storage life, and improve the stability of the adsorbent.30,31 Fig. 1c shows the typical shapes of several magnetic hybrid nanocomposites.32Magnetic particles commonly include ferrite materials, such as MFe2O4 (M = Fe, Mn, Cu, Zn, Co, etc.), maghemite (γ-Fe2O3), magnetite (Fe3O4), etc. Among the various types of magnetic chitosan materials that have been reported, based on the excellent performance of Fe3O4, the most common magnetic particle used is Fe3O4, and the SEM micrographs are shown in Fig. 1d. In the study of magnetic factors affecting magnetic ions, it is found that the size of ionic magnetism is related to the band gap width of the semiconductor formed.33 The results show that the magnetic properties in materials are caused by vacancy defects caused by surface electron transitions in the environment. This surface vacancy is the reason for the ferromagnetic coupling of materials. The material exhibits stable room-temperature magnetism only when the surface vacancy distance is approximately 8 A. Among the magnetic materials mentioned above, the Fe3O4 material has the most suitable surface vacancy distance, so it is the most ideal magnetic material to be doped. Compared with Fe3O4, a material obtained by substitution of crystal sites-MFe2O4 (M = Fe, Mn, Cu, Zn, Co, etc.) have a decrease in the hyperfine magnetic field, which can be significantly detected.34 In magnetic studies, Fe3O4 shows ferromagnetism and Fe2O3 shows superparamagnetism. Compared with Fe2O3, the magnetization and coercivity fields of Fe3O4 samples are larger35 due to their biocompatibility, high magnetic susceptibility, chemical stability, harmlessness, low cost and wide application, such as biomedicine,36,37 water treatment,38 catalysis.39,40
3. Preparation of magnetic chitosan
There are many methods to synthesize magnetic chitosan, such as the coprecipitation method, crosslinking method, spray drying method,41 photochemical method,42 and electrostatic drop method. At present, two commonly used methods are the coprecipitation method and the crosslinking method.3.1 Coprecipitation method
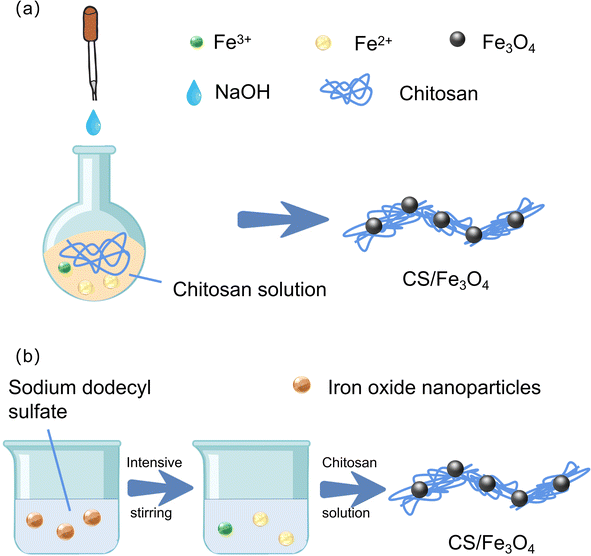
The coprecipitation method directly obtains uniform materials through chemical reactions in the solution, and the preparation process is simpler and more environmentally friendly than other methods. The coprecipitation method is divided into the in situ method and the two-step method.The important first step in the two-step method is to prepare magnetic particles. Fe3O4 nanoparticles can be easily synthesized by chemical coprecipitation,46 in addition to the hydrothermal method,47 pyrolyzis method,48 sol–gel method,49 and microemulsion method.50 The chemical coprecipitation method to synthesize Fe3O4 magnetic nanoparticles mainly involves adding alkali into the Fe2+/Fe3+ salt solution to provide an alkaline environment to generate Fe3O4. Its formation principle is formed through a nucleation and growth mechanism, as shown in Fig. 3a. The chemical coprecipitation method for synthesizing Fe3O4 has the advantages of being easy to implement and less harmful,39 and the remaining synthetic methods are less commonly used on magnetic chitosan microspheres because of their complicated processes. The average particle size of Fe3O4 produced by the chemical coprecipitation method is less than 10 nm, but the magnetic nanoparticles themselves have a hydrophobic surface, so ion agglomeration is more serious, and Fe3O4 magnetic nanoparticles are particularly easy to oxidize, which usually leads to a decrease in magnetic properties and hinders the application of magnetic chitosan microspheres. At present, Fe3O4 will be surface functionalized, and the strategies of surface functionalization can be roughly divided into surface modification of organic materials and inorganic materials. First, the functionalized magnetic nanoparticles on the surface of organic materials have a core–shell structure (Fig. 3b).29 Second, modified with inorganic materials, nontoxic silica is a very ideal surface functional coating for magnetic nanoparticles.51 Lu et al. synthesized Fe3O4@SiO2 in an oxygen-free environment.52 Jiang et al. synthesized magnetic Fe3O4@SiO2-chitosan (MFSC)53 (the synthesis process is shown in Fig. 3c). SiO2 has strong acid resistance, and the core–shell structure formed with Fe3O4 enhances the stability and dispersion of magnetic chitosan microspheres (Fig. 3d). In recent years, the green synthesis of magnetic particles using plant extracts54 such as tree bark extract,55 coconut husk,56 tekelan leaf,57 neem leaf,58 and Parkia speciosa Hassk pod extract has been widely developed. Plant extracts contain secondary metabolites such as phenolic compounds, tannins, and saponins. These compounds have hydrophobic properties and steric hindrance effects on metal surfaces, causing steric exclusion, which can control particle growth and prevent metal particle agglomeration during the synthesis process. The content of the –OH group in secondary metabolites can act as a reducing agent that maintains the balance of the Fe3+ and Fe2+ compositions in solution to produce magnetite (Fe3O4) with higher purity.59 In addition, due to the particularity of raw materials, the magnetic materials prepared have some special functions. For example, Fe3O4 that prepared from water hyacinth (WH), also known as Eichhornia crassipes extract, can inhibit bacterial growth.60 The existence of these special functions not only gives the material special properties, but also greatly improves the application range of the material.
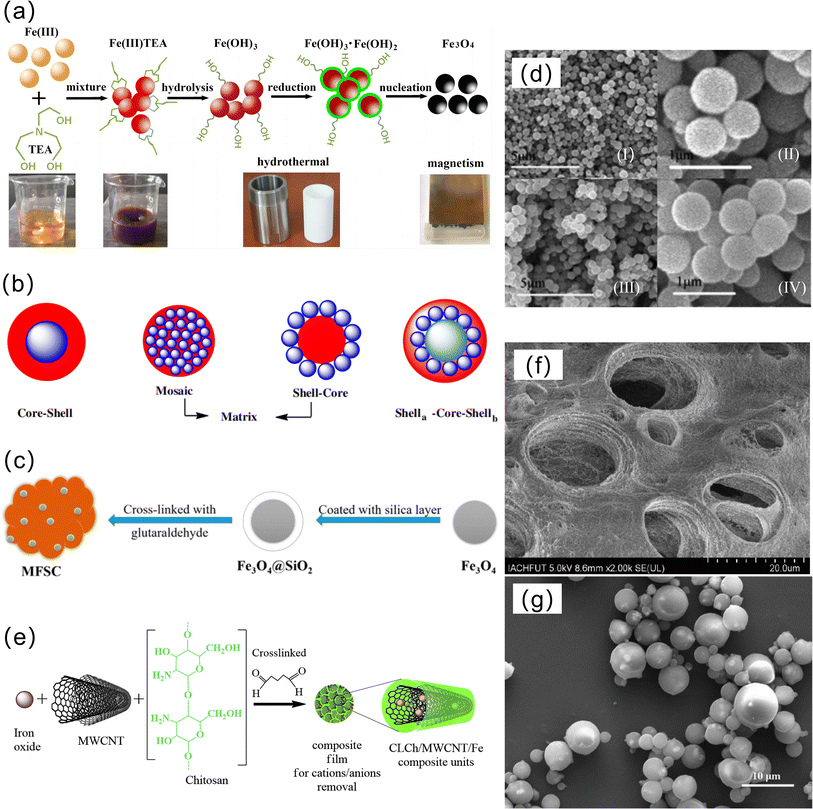 | ||
| Fig. 3 (a) Schematic diagram of the formation mechanism of Fe3O4 magnetic nanoparticles, (b) the main structure of magnetic nanoparticles modified by organic materials, (c) Fe3O4@SiO2-chitosan synthesis steps, (d) SEM images of the Fe3O4 (i and ii), Fe3O4@SiO2 (iii and iv), (e) schematic diagram of the formation of magnetic chitosan nanocomposites by two-step method,63 (f) SEM images of Fe3O4-CS beads, and (g) SEM images of magnetic chitosan microspheres. | ||
The second step of the two-step method is to mix chitosan and Fe3O4 magnetic nanoparticles and use cross-linking, microemulsion and other methods to successfully encapsulate Fe3O4 magnetic nanoparticles in chitosan microspheres, giving the chitosan microspheres good magnetism. The cross-linking method uses a cross-linking agent to connect chitosan and Fe3O4 magnetic nanoparticles (Fig. 3e). Li et al. successfully embedded magnetite nanoparticles into chitosan beads through a two-step method, forming a porous structure (Fig. 3f).61 The microemulsion rule is that the mixed solution of chitosan and Fe3O4 magnetic nanoparticles is slowly dropped into the microemulsion containing emulsifier and liquid paraffin, and the pH value is adjusted to alkaline for molding. Li et al. prepared magnetic chitosan microspheres by water-in-oil microemulsion polymerization.62 The magnetic chitosan microspheres are typically spherical with smooth surfaces and no obvious defects, as shown in Fig. 3g.
3.2 Crosslinking method
The cross-linking method involves the formation of chemical bonds by the crosslinking agent to combine polymers (Fig. 4a). The crosslinking method is divided into two kinds according to the principle: chemical crosslinking and physical crosslinking (Fig. 4b). At present, the commonly used crosslinking agents for chemical crosslinking include glutaraldehyde, epoxy cross-linking agents and silane. The physical cross-linking agents used include polyphosphate, citric acid, κ-carrageenan and alginate. Table 2 lists the properties of common crosslinkers and composites formed. Different crosslinking agents will correspondingly affect the surface area and saturation magnetization of magnetic chitosan particles.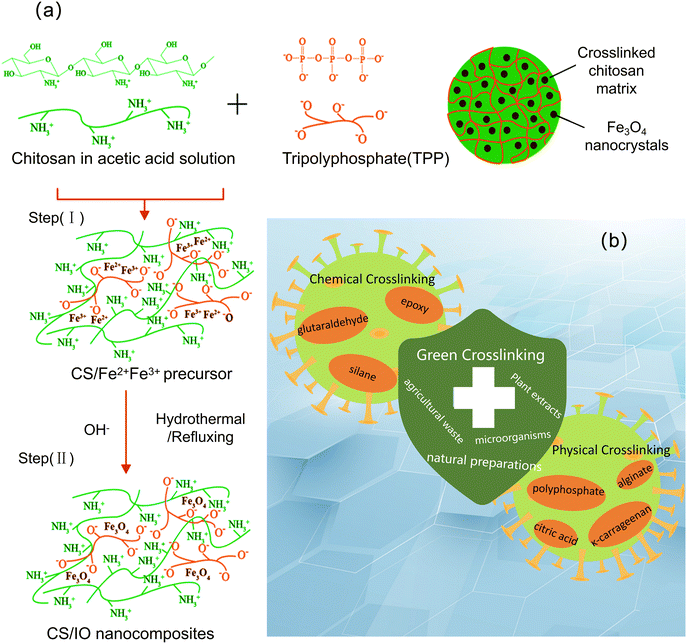 | ||
| Fig. 4 Schematic diagram of magnetic chitosan synthesized by the (a) crosslinking method and (b) related crosslinking agents. | ||
| Adsorbents | Crosslinker | Average size (nm) | BET surface area (m2 g−1) | Saturation magnetization (emu g−1) | References |
|---|---|---|---|---|---|
| a Abbreviations: ZMChi/PVA:ZnO-coated MChi/PVA (ZMChi/PVA) microspheres; CS/IO: chitosan/iron oxide nanocomposites; mLCSCar0.2: ionically crosslinked magnetic chitosan/κ-carrageenan bioadsorbents; CS-G/ZnO/Fe3O4NPs: magnetically crosslinked chitosan-glyoxal/ZnO/Fe3O4 nanoparticles; Mag@PIA-g-CS: poly(itaconic acid-g-chitosan)-coated magnetic flocculant; CCMD: carboxymethyl chitosan-modified magnetic-cored dendrimers. | |||||
| ZMChi/PVA | Glutaraldehyde | — | 75.5 | 22 | 72 |
| Bismuth ferrite/Biochar coupled magnetic material | Glutaraldehyde | — | 6.78 | 0.95 | 73 |
| CS/IO | Sodium tripolyphosphate (TPP) | 3.9 | — | 14.5 | 63 |
| mLCSCar0.2 | κ-Carrageenan | 16.7 | — | 29.1 | 74 |
| CS-G/ZnO/Fe3O4 NPs | Glyoxal | 7.3 | 10.706 | 23.37 | 75 |
| Mag@PIA-g-CS | 3-Trimethoxysilyl propylmethacrylate | — | — | 51.9 | 76 |
| CCMD | 3-Aminopropyl trimethoxysilane | 13–19 | — | — | 77 |
However, it is worth noting that most crosslinking agents are toxic and pose a certain risk to humans and organisms. In recent years, based on the development of green chemistry, the application of natural preparations in the synthesis of adsorption materials has been emphasized as an alternative method to minimize the use of toxic chemical reagents.64 Plant extracts, microorganisms and agricultural waste have a variety of active compounds and metabolites that can act as crosslinking agents in the synthesis processes, thus enabling the development of new composites with high efficiency, eco-friendliness and low cost. Queiroz et al. synthesized a composite material based on graphene oxide and magnetic chitosan using a green route with procyanidins as a crosslinking agent.65 Liu et al. synthesized a magnetic chitosan three-dimensional biosorbent for the removal of methylene blue using agricultural waste corn stalk core as a scaffold and green crosslinking.66 Some researchers have also used special crosslinking agents and methods to achieve non-toxic crosslinking. Wang et al. synthesized a nanocomposite magnetic attapulgite using ethylene diaminetetraacetic acid (EDTA) as a crosslinking agent. EDTA is safe and nontoxic, in line with the concept of a green synthetic adsorbent. Anchali et al. synthesized magnetic chitosan-magnetite gel microparticles using ethylene glycol diglycidyl ether (EDGE) as a green and safe crosslinking agent.67 Rahmi et al. used sulfuric acid as a cross-linking agent to synthesize magnetic chitosan nanocomposite beads.68 The crosslinking process using H2SO4 produces chitosan chains that form strong antiparallel structures and hydrogen bonds,69 which increases the adsorption capacity and stability of chitosan and acid resistance and reduces the crystallinity properties.70 Deniz et al. proposed a new method for preparing magnetic Fe3O4-chitosan micronanoparticles (Fe3O4-CNs) using a traditional suspension cross-linking method.71 Different from ordinary synthesis methods, they use ultrasonic treatment to control the synthesis of particles during the particle synthesis process and obtain the desired characteristics.
3.3 Other methods
In addition to the above two methods, some other methods can also be used to prepare magnetic chitosan materials, such as the spray drying method, photochemical method, and electrostatic drop method. The preparation process of the spray-drying and photochemical methods is shown in Fig. 5a. The electrostatic drop method is a combination of electrostatic drop (ESD) technology and a chemical cross-linking process for synthesis. Xu et al. used the electrostatic drop method to fabricate Fe3O4 nanoparticle-embedded porous chitosan microspheres (Fe3O4/PCSM),78 as shown in Fig. 5b. Together with the pumping action, the microscopic ferro-gels, which contain ferrous cations, ferric cations and chitosan, can be accelerated downward along the electric field strength gradient and transferred to an alkaline solution for curing. The as-prepared Fe3O4/PCSM exhibits a good spherical shape and large channels, uniform dispersion, and easy separation.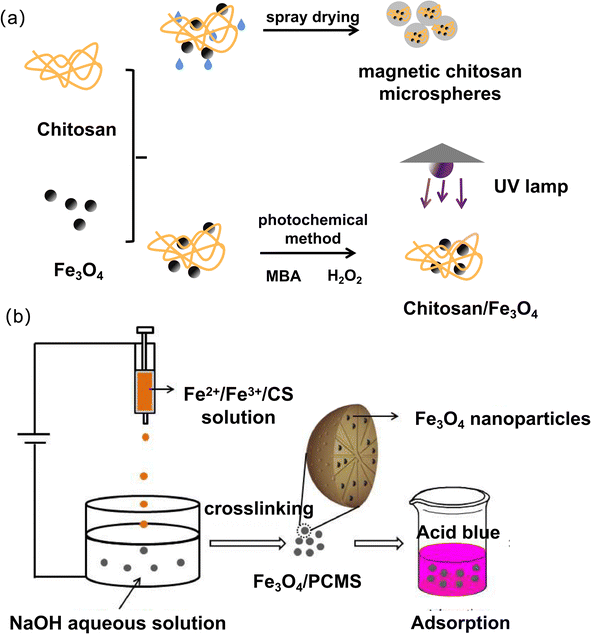 | ||
| Fig. 5 Schematic diagram of magnetic chitosan synthesized by (a) spray drying and photochemical method, (b) electrostatic drop method. | ||
4. Application of modified magnetic chitosan materials in heavy metal adsorption
Heavy metals occupy an important position in daily industrial production, but their excessive use and consumption seriously damage the environment and pose a serious threat to human health. Compared with chitosan, magnetic chitosan has more advantages in separation, recovery, mechanical strength and good adsorption. It has gradually become a promising adsorbent for heavy metals. This section summarizes the research and application of magnetic chitosan in the removal of heavy metal ions in recent years.The adsorption capability of a treatment material can be evaluated by adsorption capacity. Isotherm studies can provide information regarding the maximum adsorption capacity, which helps to compare performance between different adsorbents. Most metal ion adsorption can be fit with Langmuir or Freundlich isotherm models. The Langmuir model assumes monolayer adsorption, while the Freundlich model assumes a heterogeneous surface.79
4.1 Adsorption of Cu
Copper (Cu) and its compounds have been used as algaecides in aquaculture, as absorbents in carbon dioxide capture technology, etc.80–82. Although various cellular processes utilize copper as an enzyme cofactor,83 excessive copper ions have been found to inhibit plant growth and root development. Moreover, high copper intake in humans and animals has been linked to various forms of physiological and neurological damage.5,84,85 The World Health Organization (WHO) sets the maximum allowable limit for Cu(II) in drinking water at 2 mg L−1;86 however, in actual industrial production, a large amount of Cu will be emitted, causing inevitable damage to the environment. Therefore, the treatment of copper is necessary.We will introduce the relevant studies with great characteristics in detail as follows. Many materials with good adsorption properties are summarized in Table 3; for example, the adsorption capacities of Fe3O4-CS/EDTA and MCGON were larger, MCC and PCCM were easier to recycle, and the technology for removing copper ions in wastewater has been extensively studied. And for the adsorption process of Cu ions, most materials conform to the Langmuir model and pseudosecond-order model.
| Adsorbent | Ion concentration (mg L−1) | BET surface area (m2 g−1) | Adsorption capacity of Cu(II) (mg g−1) | pH; temp (° C) | Kinetic model | Isotherm model | Cycles | Advantages | Reference |
|---|---|---|---|---|---|---|---|---|---|
| a Abbreviations: MCC: chitosan matrix embedding magnetite/maghemite; CMLB: chitosan-combined magnetic biochar; PCCM: porous and shape-adjustable magnetic aerogel based on PCs, chitosan and Fe3O4;CMFO: MnFe2O4/chitosan nanoadsorbents; MCSB: magnetic chitosan/sodium alginate gel bead; CCMF: chitosan-cellulose enwrapped magnetic carbon foam; MCTS-Ag/Bi2WO6: magnetic chitosan@bismuth tungstate coated by silver; MCMAs: the magnetic chitosan microspheres immobilized on Aspergillus sydowii; ECSBNC: epichlorohydrin crosslinked chitosan Schiff base-Fe3O4 nanocomposite; MCGON: magnetic chitosan/graphene oxide nanocomposite; Fe3O4-CS/EDTA: magnetic chitosan composite adsorbent functionalized with EDTA. | |||||||||
| MCC | 2 | — | 216.8 | 6; 25 | Pseudosecond-order | Langmuir | 6 | Easy to separate, recycle and reuse | 87 |
| CMLB | 40 | 104.91 | 54.68 | 5.8; — | Pseudosecond-order | Freundlich | 3 | Good removal efficiency and environmentally friendly | 88 |
| PCCM | 100 | — | 81.3 | 5.5; — | Pseudosecond-order | Langmuir | 7 | Easily separate and use multiple cycles | 89 |
| CMFO | 200 | — | 14.86 | 4.5; 25 | Pseudosecond-order | Langmuir | — | Prepared without the use of toxic crosslinkers | 90 |
| MCSB | 60 | — | 124.53 | —; — | Pseudosecond-order | Langmuir | — | Satisfactory mechanical properties, good magnetic properties and easy to separate | 91 |
| CCMF | 100 | 68.0 | 115.65 | 6; 25 | Pseudosecond-order | Langmuir | — | High adsorption capacity | 92 |
| MCTS-Ag/Bi2WO6 | 50 | — | 68.68 | 6; — | Pseudosecond-order | Freundlich | 5 | Photo-assisted adsorption removal | 93 |
| MCMAs | 100 | — | 119.21 | —; — | Pseudosecond-order | Langmuir | 4 | Relatively high adsorption capacity, microorganisms assist in removal | 94 |
| ECSBNC | 100 | — | 90.90 | —; — | Pseudosecond-order | Langmuir | — | Nitrogen-containing functional groups improve adsorption capacity | 95 |
| MCGON | 100 | 132.9 | 217.4 | 8; 25 | Pseudosecond-order | Langmuir | 5 | Excellent adsorption capacity, high specific surface area, superparamagnetic | 96 |
| Fe3O4-CS/EDTA | 200 | 2.622 | 225.0 | —; — | Pseudosecond-order | Langmuir | 8 | Simultaneous capture of two types of pollutants | 97 |
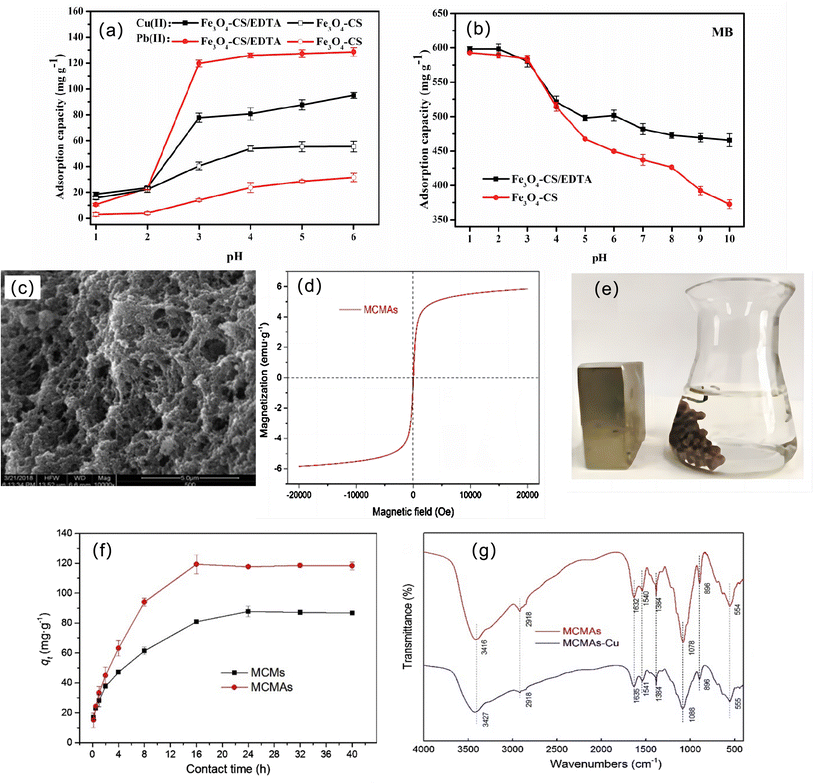
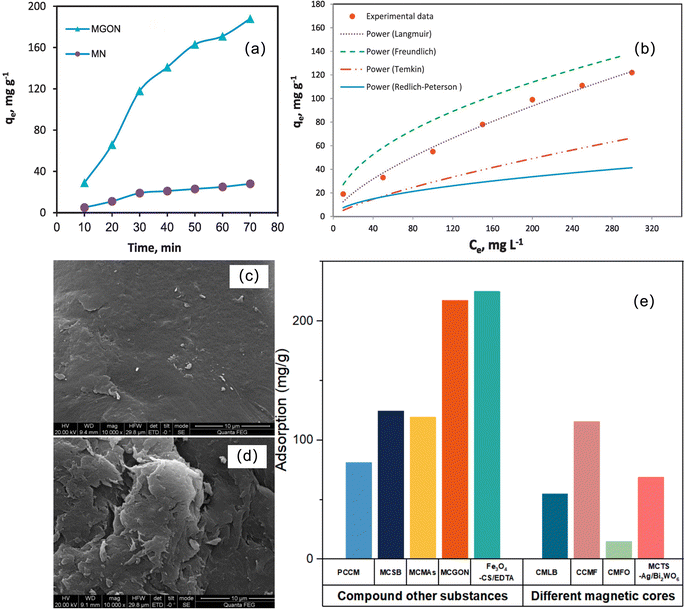
On the basis of magnetic chitosan, many studies have enhanced the adsorption capacity of magnetic chitosan by compounding other substances to improve the structure of chitosan and combining and assisting synergy. Chen et al. developed a multifunctional EDTA-functionalized magnetic chitosan composite adsorbent (Fe3O4-CS/EDTA) for the simultaneous removal of methyl blue (MB) and heavy metals (Pb(II) and Cu(II)) in complex wastewater.97 The adsorbent has synergistic removal performance for cationic metal ions and anionic dyes. The EDTA chelating group is mainly responsible for the binding of heavy metals, while the protonated amino group of CS adsorbs anionic dyes through electrostatic interactions. With increasing pH, the uptake of Cu(II) and Pb(II) increases gradually. MB is the opposite, which is related to the different adsorption mechanisms, but it can be seen that the modification of EDTA makes Fe3O4-CS have a higher adsorption performance for heavy metals and MB over a wide pH range (Fig. 6a and b). The removal of heavy metal ions from wastewater using magnetic chitosan as a microbial immobilization carrier has been reported.98,99 Magnetic chitosan microspheres immobilized on Aspergillus (MCMAs) were prepared by Zhang et al.94 MCMAs show obvious interconnected porous structures (Fig. 6c), indicating that they can provide a large number of adsorption sites for heavy metals, showing excellent immobilization properties.100 The magnetic properties of MCMA are shown in Fig. 6d and e. This indicates that MCMAs have good superparamagnetic properties and show good recycling potential.101 The addition of A. sydowii made the adsorption performance of MCMAs significantly higher than that of unadded MCMs (Fig. 6f). First, the mycelia of immobilized A. sydowii stretched to fill the voids of MCMA after incubation, resulting in a denser structure and increasing the specific surface area. Second, the cell wall of A. sydowii is composed of various functional groups, such as hydroxyl-, amino-, carboxyl-, and phosphate-102,103 and it is also confirmed that these groups are indeed involved in Cu(II) chelation (Fig. 6g). Third, microbial surfaces are often negatively charged, thereby contributing adsorption sites for binding metal ions.104 Hosseinzadeh and Ramin et al. studied the performance of magnetic CS/graphene oxide nanocomposites (MCGONs) for copper ion removal in detail.96 The introduction of graphene oxide (GO) significantly improves the adsorption performance of the material for Cu2+ ions, which is due to the presence of hydrophilic oxygenated groups such as –COOH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –OH, and –C—O—C– on the surface of GO nanosheets. Fig. 7a shows the effect of GO nanosheets on Cu removal. With the prolongation of stirring time, the adsorption capacity of the adsorbent increases, and the whole adsorption conforms to the Langmuir isotherm (Fig. 7b).
O, –OH, and –C—O—C– on the surface of GO nanosheets. Fig. 7a shows the effect of GO nanosheets on Cu removal. With the prolongation of stirring time, the adsorption capacity of the adsorbent increases, and the whole adsorption conforms to the Langmuir isotherm (Fig. 7b).
In addition, some studies have also used other magnetic particles to improve the adsorption performance of magnetic chitosan by replacing Fe3O4 magnetic particles. Taguba et al. synthesized magnetic chitosan—manganese ferrite (CMFO) by simple low-temperature combustion technology without a toxic crosslinking agent.90 The surface of CMFO is rough, and agglomerated particles appear. The maximum adsorption capacity of Cu on CMFO is 14.86 mg g−1, and the adsorption process is spontaneous and endothermic, which is consistent with the Langmuir model. Zhang et al. prepared a novel adsorbent of chitosan-cellulose enwrapped magnetic carbon foam (CCMF).92 Cellulose increases the specific surface area and porosity of the material (Fig. 7c and d), and the magnetic carbon foam makes the adsorbent lighter, making it float in water during the adsorption process, reducing the energy consumption of mechanical stirring which improves the adsorption performance and adsorption rate of metal ions.
In view of the above two kinds of modification methods, according to the comparative analysis of Fig. 7e, we found that for the modification of copper ion adsorption materials, the composite effective material to improve the material structure is better than the replacement of the magnetic core to obtain better adsorption performance, especially by introducing the material that can greatly increase the number of adsorption groups, such as Fe3O4-CS/EDTA and MCGON.
4.2 Adsorption of Pb
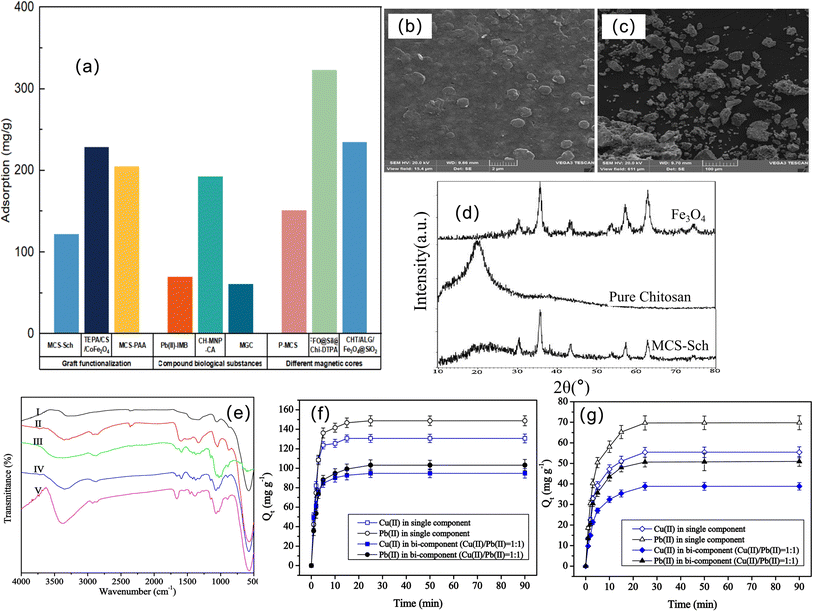
Lead (Pb) is the main component of batteries, ammunition and paint and is widely used in industrial production,105–107 but Pb is extremely toxic to humans. The International Agency for Research on Cancer has categorized lead-containing inorganic compounds under “Group 2A: probably carcinogenic to humans”.108 The United States Environmental Protection Agency has set interim drinking water health advisory levels of 0.015 mg L−1 for Pb2+ ions.109 However, lead is poisonous to living organisms when it is taken even at trace levels110,111 and lead ions in the environment are difficult to degrade, so removing Pb(II) from the environment is an attractive issue.112At present, the magnetic chitosan adsorbents for lead ions can be roughly divided into three types. One is to introduce groups through grafting and functionalization and exert a synergistic effect to enhance the adsorption of Pb ions. Another type is the preparation of biosorbent materials by combining biological substances. The last type is magnetic chitosan using other magnetic substances. We summarize these materials in Table 4. It can be seen that these adsorbents have generally better performance, among which FFO@Sil@Chi-DTPA has the largest adsorption capacity, and MCS-Sch can achieve the maximum number of cycles. For the adsorption process of Pb ions, most materials conform to the Langmuir model and pseudosecond-order model. From Fig. 8a, it can also be seen that for the adsorption of lead ions, materials modified with other magnetic substances have better adsorption effects than the other two methods, and the improvement in performance of different magnetic cores is inconsistent. The reason for the higher adsorption capacity of FFO@Sil@Chi-DTPA is related not only to the magnetic core, but also to the functional modification of DTPA. At the same time, grafting functionalization is generally superior to the means of composite biomass, which has a great relationship with the number of effective adsorption sites. However, we should also affirm the versatility of biosorbent materials, such as MGC, for cancer hyperthermia.
| Adsorbent | Ion concentration (mg L−1) | Adsorption capacity of Pb(II) (mg g−1) | pH; temp. (°C) | Kinetic model | Isotherm model | Cycles | Advantages | Reference |
|---|---|---|---|---|---|---|---|---|
| a Abbreviations: MCS-Sch: magnetic chitosan-(D-glucosamine methyl)benzaldehyde; MCS-PAA: polyacrylic acid grafted magnetic chitosan nanocomposite; (Fe3O4/CS-Se)2: diphenyl diselenide onto chitosan carrying magnetite (Fe3O4) nanoparticles; HA-MG-CH: grafted hydrazinyl amine magnetite-chitosan; Pb(II)-IMB: lead-ion-imprinted magnetic biosorbent; CH-MNP-CA: chitosan-conjugated magnetite nanobiocomposite; MGC: magnetic nanobiocomposite based on activated chitosan-magnetic guanidinylated chitosan nanobiocomposite; P-MCS: phosphorylated magnetic chitosan composite; FFO@Sil@Chi-DTPA: DTPA-modified chitosan-coated magnetic silica nanoparticle; CHT/ALG/Fe3O4@SiO2: magnetic chitosan/alginate/Fe3O4@SiO2 hydrogel composites; MCF3DG: novel magnetic three-dimensional graphene/chitosan/nickel ferrite nanocomposite. | ||||||||
| MCS-Sch | 10 | 121.95 | 5; — | — | Freundlich | 7 | The simplicity and minimum usage of the toxic compounds | 113 |
| TEPA/CS/CoFe2O4 | 200 | 228.3 | 5; 30 | Pseudosecond-order | Langmuir | — | High magnetization, efficient adsorption performance | 114 |
| MCS-PAA | 100 | 204.89 | 6; — | Pseudosecond-order | Langmuir | 5 | Excellent acid resistance | 115 |
| (Fe3O4/CS-Se)2 | 100 | 100.3 | 6; — | — | — | 4 | Easy to separate, short extraction times and less adsorbent | 116 |
| HA-MG-CH | 150 | 144.41 | 5; 25 | Pseudosecond-order | Sips | 5 | Effective reduction of metal ions below allowable levels | 117 |
| Pb(II)-IMB | 200 | 69.48 | 5; — | Pseudosecond-order | Langmuir | 5 | Efficient adsorption, good selectivity, and convenient magnetic separation | 118 |
| CH-MNP-CA | — | 192.308 | 6.1; — | Pseudosecond-order | Langmuir | 5 | No secondary waste production, high saturation magnetization | 119 |
| MGC | 25 | 60.60 | 7; 60 | Pseudosecond-order | Langmuir | — | Applied to two fields with good adsorption performance | 120 |
| P-MCS | 40 | 151.06 | 6; 45 | Pseudosecond-order | Langmuir | 5 | Excellent magnetic separation and regeneration performance, cost savings | 121 |
| FFO@Sil@Chi-DTPA | — | 322.58 | 6; — | Pseudosecond-order | Langmuir | — | Excellent selective adsorption of multiple pollutants | 122 |
| CHT/ALG/Fe3O4@SiO2 | 180 | 234.77 | 4.2; 20 | Elovich | Langmuir | Simple and innovative preparation, stable in acid water system | 123 | |
| MCF3DG | 16 | — | 8.5; — | — | — | 5 | Lightweight with good strength and weight resistance, good magnetic response, non-toxic properties | 124 |
The first type is graft functionalization. Graft functionalization can greatly improve the adsorption properties of magnetic chitosan,125 and many different reactive groups have been used to decorate polymers, including EDTA and analogs,126 amino acids,127 amidoxime,128 and aminophosphonate.129 Shahraki et al. synthesized a magnetic chitosan nanocomposite material based on Schiff base functionalization: MCS-Sch.113 The surface of MCS-Sch is rough, magnetic Fe3O4 nanoparticles are embedded or mounted inside CS-Sch (Fig. 8b and c), and the crystal structure of Fe3O4 is not destroyed after MCS-Sch formation (Fig. 8d). As the pH value increases, the removal percentage of Pb(II) increases. The advantage of this material is that the presence of azomethine and aromatic rings can enhance the ability of MCS-Sch to interact with metal ions. Fan et al. used tetraethylenepentamine (TEPA) to further modify magnetic CS/CoFe2O4 for the adsorption of Pb(II) (or Cu(II)) in one- and two-component solutions.114 The spectrum of chitosan/CoFe2O4(IV) was compared with that of TEPA-modified chitosan/CoFe2O4(V), as shown in Fig. 8e. It can be seen that a strong peak appears at 1655 cm−1, indicating that TEPA modification will generate more amino groups that can bind metal ions, resulting in better adsorption performance. Fig. 8f and g shows the effect of contact time on the adsorption capacity of Cu(II) (or Pb(II)) in single and bicomponent (Cu(II)/Pb(II) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solutions on chitosan/CoFe2O4 and TEPA-modified chitosan/CoFe2O4. It was obvious that the uptakes of Cu(II) and Pb(II) on chitosan/CoFe2O4 were evidently improved after TEPA modification.
1) solutions on chitosan/CoFe2O4 and TEPA-modified chitosan/CoFe2O4. It was obvious that the uptakes of Cu(II) and Pb(II) on chitosan/CoFe2O4 were evidently improved after TEPA modification.
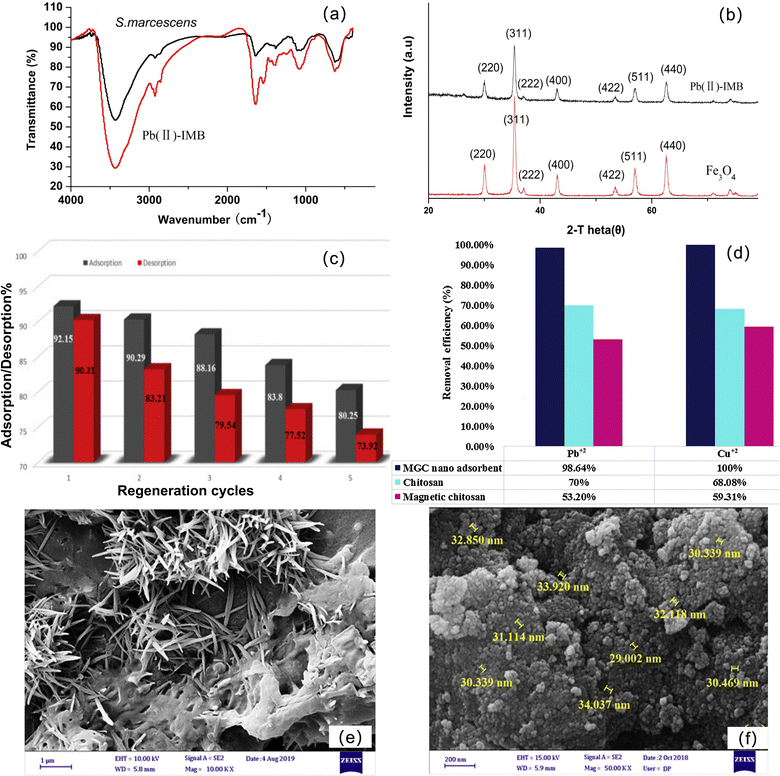
The second is combining biological substances to make magnetic chitosan bioadsorbent materials. He et al. synthesized a novel biological material with high adsorption capacity and good selectivity for Pb2+: lead-ion-imprinted magnetic biosorbent (Pb(II)-IMB).118 FT-IR shows that Pb(II)-IMB is rich in adsorbable groups or sites, which are favorable for the adsorption of metal ions (Fig. 9a). In addition, XRD showed that the prepared adsorbent was magnetic, which was beneficial to the separation of substances in water (Fig. 9b). Cheraghipour et al. synthesized chitosan-conjugated magnetite nanobiocomposites (CH-MNP-CA).119 One of the notable advantages of this nanobiocomposite is its chemical conjugation, which does not have the weakness of the ultimate chitosan detachment of a physical bond and makes it easy for magnetic separation and does not produce secondary waste. At the same time, the nanoadsorbent also exhibited excellent renewability and reusability up to at least five cycles (Fig. 9c). Eivazzadeh-Keihan et al. designed a magnetic guanidinylated chitosan nanobiocomposite (MGC).120 Compared with chitosan and magnetic chitosan, the material showed a better adsorption effect of heavy metal ions (the adsorption rates of Cu2+ and Pb2+ were 100% and 98.64%, respectively) (Fig. 9d) and can also be used for extracorporeal hyperthermia of cancer. After the in situ preparation of Fe3O4 magnetic cores to form MGC, the morphology of guanidyl chitosan changed greatly. The FE-SEM image of guanidyl chitosan is bar-shaped (Fig. 9e), while MGC has uniform and almost identical spherical morphological features, and the average size is between 30 nm and 34 nm (Fig. 9f).
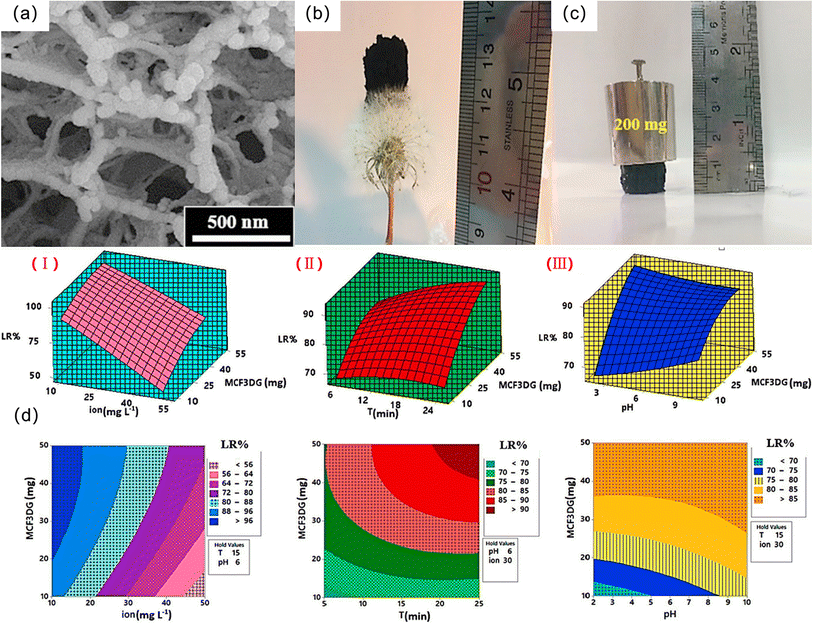
In addition to the two types mentioned above, there are also studies that choose to use chitosan and other magnetic substances (such as CoFe2O4, NiFe2O4, etc.) to synthesize magnetic chitosan adsorption materials. Wu et al. synthesized a phosphorylated magnetic chitosan composite material (P-MCS).121 With the increase in pH value, the adsorption of metal ions increases. The high adsorption capacity of Pb(II) on P-MCS is mainly due to coordination with the phosphonate group.130 Nasiri et al. synthesized a novel magnetic three-dimensional graphene/chitosan/nickel ferrite nanocomposite (MCF3DG).124 MCF3DG has a honeycomb-like 3D porous structure, and the NiFe2O4 nanoparticles are spherical due to magnetic attraction and attach to the surface of the material (Fig. 10a). The as-prepared nanocomposites are light in weight and exhibit good strength and weight resistance (Fig. 10b and c). MCF3DG has a good magnetic response with a saturation magnetization of approximately 11 emu g−1. Based on plotting two-dimensional (2D) contour maps and three-dimensional (3D) response surface maps (Fig. 10d), the effects of experimental variables (amount of absorbent, lead ion concentration, contact time, and pH) on lead ion removal efficiency were obtained. With increasing MCF3DG dosage, the initial Pb(II) ion concentration decreased, the contact time increased, the pH increased, and the removal efficiency of Pb(II) increased, which could exceed 98%, showing excellent adsorption capacity.
4.3 Adsorption of Cr
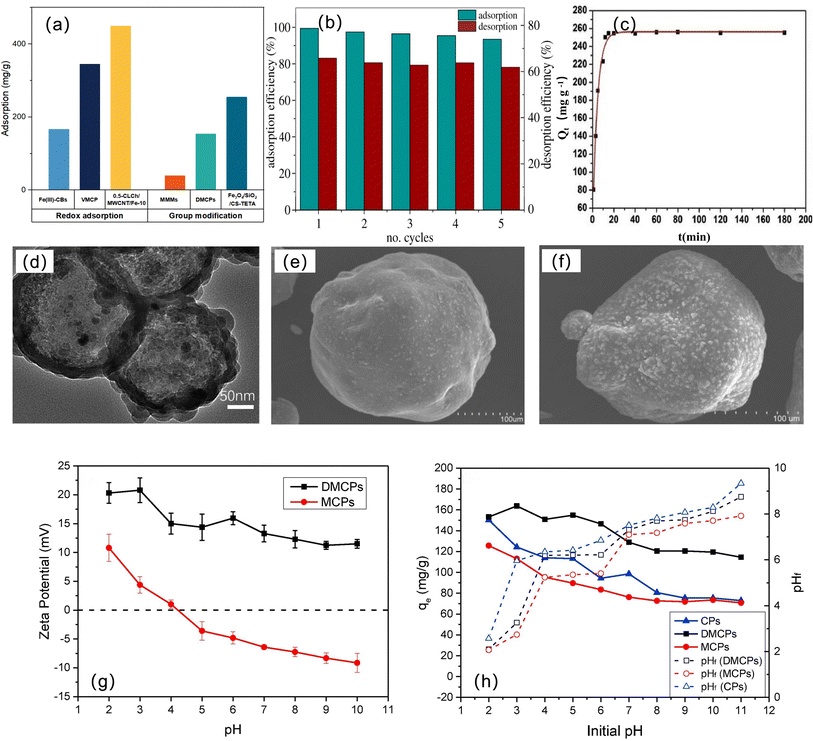
Cr exists in the form of trivalent chromium (Cr(III)) and hexavalent chromium (Cr(VI)) under natural conditions.131 Chromium is one of the trace elements necessary for life, but chromium is toxic, and excessive use will pollute the environment.132 Cr(VI) is more toxic than Cr(III), and it is a carcinogen that is easily absorbed by the body and accumulates in the body.133 The World Health Organization stipulates that the maximum allowable concentration of Cr6+ in drinking water is 50 ppb,134 but chromium exists in actual industrial wastewater and natural water, so chromium ion treatment is required before water supply.Table 5 summarizes many examples of magnetic chitosan materials for Cr(VI) adsorption. It can be seen that MC and 0.5-CLCh/MWCNT/Fe-10 have better recyclability and the best adsorption performance, and Fe3O4/SiO2/CS-TETA has a larger specific surface area. For the adsorption process of Cr, most of them conform to the Langmuir isothermal adsorption line. In terms of adsorption kinetics, some of the materials conform to pseudofirst-order kinetics, and the rest conform to pseudosecond-order kinetics, with no large bias. It can be seen from Fig. 11a that for the modification of chromium ion adsorption materials, the effect of reduction adsorption is indeed better than that of group modification, which may be related to the immobilization of Cr(III) after reduction. Most of the studies on Cr on magnetic chitosan materials are aimed at the adsorption of Cr6+, and we believe that more efficient Cr(III) adsorption of magnetic chitosan and its derivatives remains to be developed.
| Adsorbent | Ion concentration (mg L−1) | BET surface area (m2 g−1) | Adsorption capacity of Cr(VI) (mg g−1) | pH; temp (°C) | Kinetic model | Isotherm model | Cycles | Advantages | Reference |
|---|---|---|---|---|---|---|---|---|---|
| a Abbreviations: MC: chitosan (90% deacetylation) coated with magnetic adsorbent; Fe(III)-CBs: Fe(III)-crosslinked chitosan beads; ECSBNC: epichlorohydrin crosslinked chitosan Schiff base-Fe3O4 nanocomposite; MMMs: mixed matrix membranes; DMCPs: poly([2-(methacryloxy)ethyl]trimethylammonium chloride)-modified magnetic chitosan particles; Fe3O4/SiO2/CS-TETA: triethylenetetramine-modified hollow Fe3O4/SiO2/chitosan magnetic nanocomposites; VMCP: poly(4-vinylpyridine)-decorated magnetic chitosan biopolymer; 0.5-CLCh/MWCNT/Fe-10: multiwalled carbon nanotubes (MWCNTs) doped with magnetic iron oxide and deposited in crosslinked chitosan (CLCh). | |||||||||
| MC | — | — | — | 5.32; 30 | Pseudofirst-order | Langmuir | 10 | Nanoscale, large surface area | 135 |
| Fe(III)-CBs | 5.0 | — | 166.3 | 6; 25 | Pseudofirst-order | Langmuir-Freundlich | 5 | Reductive adsorption, small average diameter, easy separation and regeneration | 136 |
| ECSBNC | 100 | — | 83.33 | 3; — | Pseudosecond-order | Langmuir | — | Spontaneous and endothermic adsorption process | 95 |
| MMMs | 20 | — | 38.91 | 2; 30 | Pseudofirst-order | Langmuir | 5 | High recovery and excellent reusability | 137 |
| DMCPs | 200 | — | 153.85 | 3; 30 | Pseudosecond-order | Langmuir | 5 | Wide pH range adsorption, easy separation and good reusability | 138 |
| Fe3O4/SiO2/CS-TETA | 150 | 131.4 | 254.6 | 2.5; 25 | Pseudosecond-order | Langmuir | 5 | High adsorption capacity, fast adsorption rate and good reuse characteristics | 139 |
| VMCP | 500 | — | 344.83 | —; 25 | Pseudosecond-order | Langmuir | 5 | Excellent removal performance | 140 |
| 0.5-CLCh/MWCNT/Fe-10 | 100 | 70.90 | 449.30 | 4; 25 | Pseudosecond-order | Langmuir | 10 | High reuse rate, wide removal pH range, good removal effect | 141 |
Due to the high mobility of Cr(VI), Cr(VI) adsorbed on the adsorbent surface can be released into the solution again and cannot reduce the toxicity caused by Cr(VI).142 Therefore, many studies on the reduction adsorption of Cr(VI) by magnetic chitosan not only have the advantages of adsorption but also realize the detoxification of Cr(VI) and the immobilization of Cr(III).143 Wu et al. synthesized Fe(III)-crosslinked chitosan beads (Fe(III)-CBs).136 The adsorption process fit the Langmuir–Freundlich isotherm best, the adsorption capacity was determined to be 166.3 mg g−1, and Cr(VI) adsorbed on the beads was reduced to Cr(III). After 5 adsorption/desorption/regeneration cycles, the initial adsorption capacity remained above 93% (Fig. 11b). Zheng et al. prepared a poly(4-vinylpyridine)-decorated magnetic chitosan biopolymer (VMCP) as an absorbent and reductant to remove Cr(VI).140 The kinetic and isothermal experiments show that the Cr(VI) removal system is well described by the pseudosecond-order and Langmuir models, and VMCP has rapid separation and satisfactory regeneration ability. After 5 adsorption–desorption cycles, the adsorption capacity was still as high as 246.0 mg g−1.
However, magnetic chitosan materials will have magnetic particles occupying the surface of chitosan, resulting in a decrease in effective adsorption sites, such as when –NH2 adsorption is weakened. Therefore, some studies have been modified by group modification, such as the introduction of amino group-rich substances, and obtained a better adsorption effect. Wang et al. prepared a triethylenetetramine ()-modified hollow Fe3O4/SiO2/chitosan magnetic nanocomposites (Fe3O4/SiO2/CS-TETA).139 The material reached adsorption equilibrium within 15 minutes, and the adsorption capacity reached 254.6 mg g−1 (Fig. 11c), showing the characteristics of fast adsorption speed and high adsorption capacity, due to the hollow structure of the prepared material (Fig. 11d), increasing the adsorption sites of metal ions on the material.144 Zheng et al. synthesized a new type of adsorbent: poly([2-(methacryloxy)ethyl]trimethylammonium chloride) (PDMC)-modified magnetic chitosan particles (DMCPs).138 PDMC contains abundant quaternary ammonium salt groups, and the introduction of PDMC significantly improves the adsorption performance of magnetic chitosan.145 The surface of DMCPs is rougher than that of magnetic chitosan particles (MCPs) and possesses more active sites (Fig. 11e and f). In addition, it can be seen from Fig. 11g and h that the zeta potential of DMCPs is positive in the entire pH range tested, while the zeta potential of MCPs will decrease to below 0, so DMCPs have a wider pH range and better adsorption performance for anionic pollutants than MCPs.
4.4 Adsorption of other metal ions
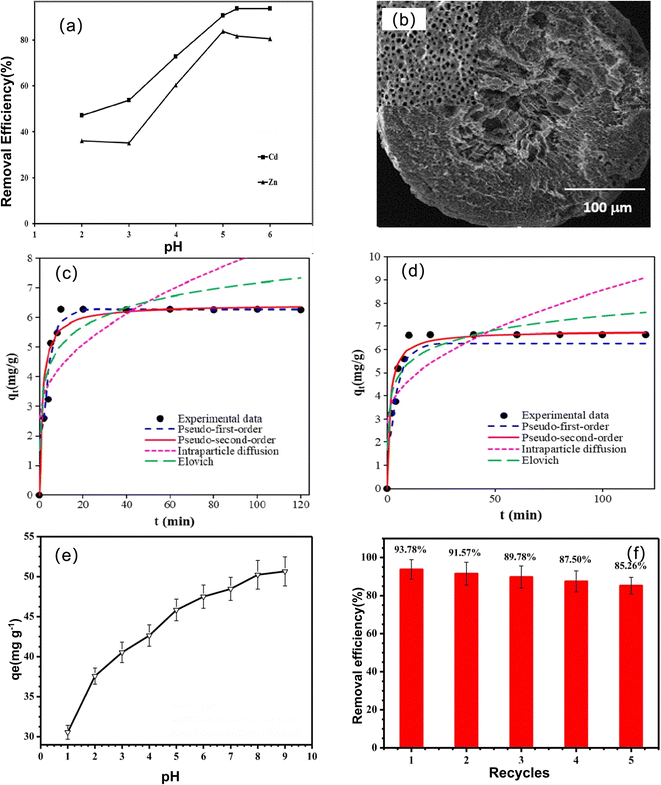
In addition to the abovementioned metal ions, magnetic chitosan materials have also been studied and applied in the adsorption of other metal ions, such as Zn2+, Pd2+, Cd2+, Hg2+, Mn2+, and Fe3+.Fatemeh et al. developed a magnetic chitosan/Al2O3/Fe3O4 nanocomposite (EDTA-M-Cs) to remove Cd(II) and Zn(II) metal ions.146 EDTA functionalization increases the adsorption capacity of Cd and Zn ions by ∼5.6 and ∼14.3 times, and the removal rates are 99.98 and 83.81%, respectively. The adsorption of Cd(II) and Zn(II) ions by EDTA-M-Cs is highly pH-dependent (Fig. 12a). Mubarak et al. prepared a chitosan/grafted halloysitenanotubes@Znγmagnetite quaternary nanocomposite (Ch/g-HNTs@ZnγM).147 Magnetic iron exists in the internal structure of the Ch/g-HNTs@ZnγM material, and gaps and holes with high electron density are clearly exhibited on its surface (Fig. 12b). The adsorption of Mn(II) decreased with increasing ion concentration, while Fe showed the opposite trend, and the maximum adsorption and removal rates of Fe(III) and Mn(II) ions were 99.06% and 87.1%, respectively. Rahmi et al. prepared magnetic chitosan beads and applied them to remove Hg(II) and Cd(II).148 The adsorption capacities of magnetic chitosan beads for Hg(II) and Cd(II) are 0.4 and 3.04 mg g−1, respectively, which are higher than those of pure chitosan. Maryam et al. synthesized magnetic chitosan nanoparticles (Fe3O4-CSN)149 for the removal of vanadium (V(V)) and palladium (Pd(II)) ions. The removal efficiencies of V(V) and Pd(II) ions are 99.9% and 92.3%, respectively, and both ions conform to pseudosecond-order kinetics (Fig. 12c and d), which is a combination of physical adsorption and chemical adsorption. Thermodynamic studies show that the adsorption process is exothermic and spontaneous.
Co, Ni, rare earth elements, etc., are strategic and scarce resources with important industrial applications. Therefore, the adsorption and recovery of these metal ions has been extensively explored by researchers. Li et al. prepared a new type of amidoxime-based magnetic adsorbent.150 Under the optimal conditions, the maximum adsorption efficiency for Ni(II) ions is 32.68 mg g−1. Dong et al. synthesized magnetic phosphorylated chitosan composite (P-MCS).151 The amount of Co(II) adsorption increases significantly with increasing pH value(Fig. 12e). The maximum adsorption capacity of Co(II) is 46.1 mg g−1, and it still retains 85.26% of the adsorption capacity after five cycles (Fig. 12f).
Based on the above content, we can conclude that magnetic chitosan materials have been widely used in the field of metal ions in wastewater, and there have been many studies on further modification by introducing groups, strengthening the structure of chitosan, and synergistic effects of composite substances to further enhance the adsorption performance of magnetic chitosan materials. At the same time, its adsorption performance varies with the initial concentration of metal ions, the pH value of the solution, the reaction temperature, the contact time and the chemical properties of different metal ions. It is necessary to consider its reusability and desorption reagents in practical applications.
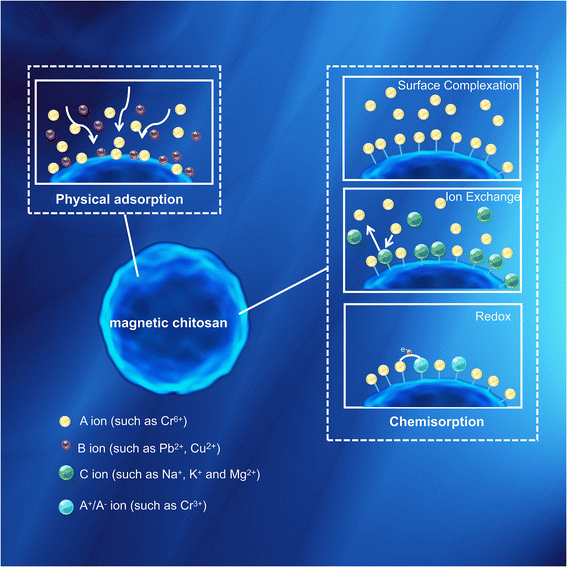
5. Adsorption mechanism
The type of surface functional groups and the adsorption environment will affect the adsorption effect, so the main adsorption mechanism of heavy metals on different magnetic chitosan adsorption materials will be different. According to the existing research results, the adsorption mechanism of heavy metal ions on magnetic chitosan can be divided into two categories, including physical adsorption and chemical adsorption (Fig. 13).5.1 Physical adsorption
Physical adsorption is a mechanism that occurs through weak electrostatic interactions such as van der Waals forces.152 Since physical adsorption is mainly caused by intermolecular forces, the adsorption affinity is often weak. Therefore, the adsorption process may be reversible. At the same time, the strength of electrostatic interactions is closely related to the pH value of the solution, the valence state of heavy metals, the ionic radius and other factors. For example, heavy metal ions exist in different forms under different pH conditions. Hachem et al.153 studied the adsorption of Pb(II). At low pH, the Pb species are mainly Pb2+ and PbNO3+, and there is competition between H+ and Pb2+. As the pH value increases, the positive charge density at the surface position of the adsorbent decreases, so the ability of the adsorbent to adsorb Pb2+ ions increases, but when the pH is higher than 6, the Pb species will become Pb(OH)2, which reduces the adsorption ability.154 Zheng et al. studied the adsorption of Cr(VI).155 At pH 2.0–6.4, the main Cr(VI) substances are HCrO4− and Cr2O72−, while at pH 6.4 or higher, the main ionic form of Cr(VI) is CrO42− and the high adsorption capacity of Cr(VI) ions occurs at lower pH. Therefore, it can be concluded that the adsorption properties of Pb(II) and Cr(VI) show an opposite trend with the change in pH (Fig. 14a), which is due to the electrostatic interaction between the charges of the ions and the protonated amino and hydroxyl groups.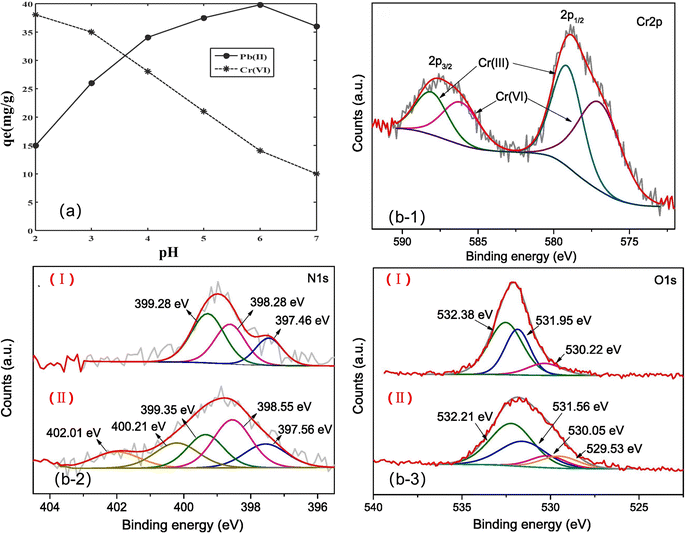 | ||
| Fig. 14 (a) Effect of pH on the metal ions removal using the PVA/chitosan/a-Fe3O4-2 nanofibrous membrane,137, (b-1) Cr 2p XPS spectra of Cr-loaded VMCP; (b-2) N 1s spectra before (i) and after adsorption (ii); (b-3) O 1s spectra before (i) and after adsorption (ii). | ||
5.2 Chemisorption
Chemisorption often occurs through three separate forms, including ion exchange, surface complexation, redox, or a combination of these steps.In the case of ion exchange, when there are exchangeable ions (such as Na+, K+ and Mg2+) on the magnetic chitosan surface through direct or indirect coupling, the heavy metal ions in the aqueous solution are exchanged with these exchangeable ions to remove heavy metal ions. Zhang et al.156 reported that ion exchange is one of the adsorption mechanisms for Cr(VI) removal by Fe3O4-CS/PDAC.
Surface complexation depends on the interaction between surface functional groups and heavy metal ions. Positively or negatively charged groups on the surface of magnetic chitosan, such as hydroxyl, carboxyl, ester or amine groups, attract oppositely charged ions in solution and form complexes through chemical bonding on the surface. For example, Ping et al.157 reported that –NH/–NH2, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O– and C–O–H functional groups in Fe3O4-ATP/EDTA/CS play important roles in the adsorption process of heavy metal ions.
C–O– and C–O–H functional groups in Fe3O4-ATP/EDTA/CS play important roles in the adsorption process of heavy metal ions.
Redox affects the chemical behavior of elements by changing their existing forms, mainly occurring in heavy metals with variable valence. Cai et al.158 studied the adsorption mechanism and found that under acidic conditions, strongly oxidizing Cr(VI) can be reduced to Cr(III) through the reducing –OH group in the adsorbent. According to FTIR and XPS spectra, Zheng et al.140 explained that the main adsorption mechanism of the prepared magnetic chitosan biopolymer (VMCP) was that Cr(VI) was adsorbed on the positively charged VMCP surface and Cr(VI) was reduced to Cr(III), followed by the coordination between Cr(III) and N atoms (Fig. 14b).
5.3 Collaborative adsorption
At the same time, there is a process of physical adsorption and chemical adsorption. Song et al.159 reported the abundant –NH, –OH, and –SH groups in Cyshtcc-Fe3O4, which can perform ion exchange and surface complexation of various heavy metal ions. The physical adsorption electrostatic interaction also improves the adsorption capacity of the composites. Huang et al. investigated the adsorption mechanism for the simultaneous capture of methylene blue (MB) and Pb(II) by the FFO@Sil@Chi-DTPA material in a binary pollution system122 (Fig. 15). Pb(II) binds to the carboxyl group by electrostatic attraction, binds to the hydroxyl group by hydrogen bonding, and coordinates with amino groups through complexation; the MB adsorbed onto the adsorbent surface can create new specific sites (the sulfonic acid groups in MB molecules) to enhance Pb(II) adsorption. Additionally, the Pb(II) adsorbed onto the adsorbent surface can act as the cation bridge and enhance the adsorption of MB via electrostatic attraction.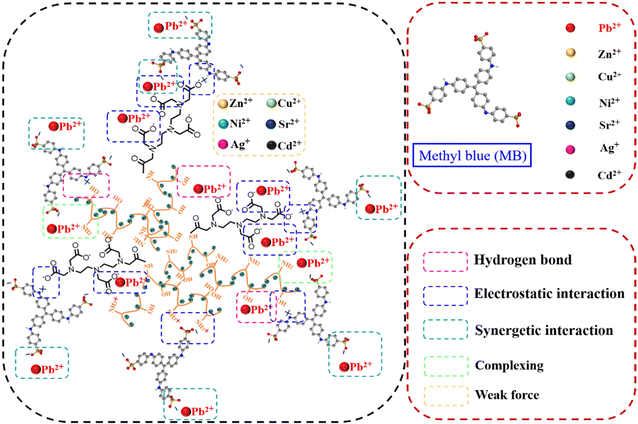 | ||
| Fig. 15 Proposed adsorption mechanism using FFO@Sil@Chi-DTPA to capture MB and Pb(II) simultaneously. | ||
Based on Tables 3–5, most of the research indicates that the adsorption behavior of Cu(II), Pb(II) and Cr(VI) can be well described by the Langmuir model, and a few fit the Freundlich model. Therefore, the results indicate that the magnetic chitosan has a uniform distribution of surface sites active for metal adsorption. At the same time, it is also very suitable for the pseudosecondary model, indicating that chemical adsorption is the rate determining step of the adsorption process.
6. Conclusions and future perspectives
In recent years, the problem of water pollution has become increasingly serious. It is urgent to develop green and sustainable water treatment technology. Magnetic chitosan microspheres have the advantages of low cost, high adsorption rate, good biological activity, and easy separation and regeneration. This is undoubtedly one of the important directions of water treatment in the future. Although magnetic chitosan has been widely studied and modified to enhance its stability and adsorption performance, the adsorption performance of magnetic chitosan for some ions is still not ideal, and the use of toxic chemical reagents in the preparation process is not green enough. Therefore, in future research, it is necessary to develop greener ways to prepare materials and increase the recycling of materials to synthesize green and environmentally friendly adsorbents, such as replacing toxic crosslinking agents with natural products and green synthesis of magnetic cores. To further improve the adsorption performance, we believe that it may be possible to combine chitosan with biological substances, such as the immobilization of biological enzymes on the surface of magnetic chitosan to accelerate the decomposition of pollutants by catalytic degradation of enzymes.At the same time, in order to realize the practical application of adsorbents, it should be noted that there are many kinds of pollutants in the actual sewage treatment. At present, most studies are aimed at the adsorption of single-component heavy metal ions. Therefore, in the future, it is possible to explore and improve the highly selective adsorption of adsorption materials on multi-component mixed pollutants.
Author contributions
All authors listed have significantly contributed to the development and the writing of this article. Ke Wang and Fanbing Zhang contributed to data collection, analysis and drafting the manuscript. Kexin Xu and Yuju Che contributed to pictures collection and typesetting. Mingying Qi and Cui Song contributed to the conception of the study and helped perform the analysis with constructive discussions. After the first draft is completed, all authors read, discuss, revise and finalize the manuscript.Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.Acknowledgements
The authors acknowledge supports from the National Natural Science Foundation of China (4217030124), Natural Science Foundation of Shandong Province (ZR2021MB052, ZR2020MB140), and Shandong University-Weihai Research Institute of Industry Technology Research Projects (0004202107020002).References
- J. Ma, S. Zuo-Jiang, Y. He, Q. Sun, Y. Wang, W. Liu, S. Sun and K. Chen, R. Soc. Open Sci., 2016, 3, 160524 CrossRef PubMed.
- N. Vatanpour, J. Feizy, H. H. Talouki, Z. Es’haghi, L. Scesi and A. M. Malvandi, Chemosphere, 2020, 245, 125639 CrossRef CAS PubMed.
- K. Rana, M. Kumar and A. Kumar, Water, Air, Soil Pollut., 2020, 231, 450 CrossRef CAS.
- N. Adimalla, J. Chen and H. Qian, Ecotoxicol. Environ. Saf., 2020, 194, 110406 CrossRef CAS PubMed.
- A. Kumar, M. Cabral-Pinto, M. Kumar and P. A. Dinis, Appl. Sci., 2020, 10, 7078 CrossRef CAS.
- L. M. Liao, M. C. Friesen, Y.-B. Xiang, H. Cai, D.-H. Koh, B.-T. Ji, G. Yang, H.-L. Li, S. J. Locke, N. Rothman, W. Zheng, Y.-T. Gao, X.-O. Shu and M. P. Purdue, Environ. Health Perspect., 2016, 124, 97–103 CrossRef CAS PubMed.
- P. Mitra, S. Sharma, P. Purohit and P. Sharma, Crit. Rev. Clin. Lab. Sci., 2017, 54, 506–528 CrossRef CAS PubMed.
- D. C. Ong, M. D. G. de Luna, S. M. B. Pingul-Ong and C. C. Kan, J. Environ. Manage., 2018, 223, 723–730 CrossRef CAS PubMed.
- M. L. F. A. De Castro, M. L. B. Abad, D. A. G. Sumalinog, R. R. M. Abarca, P. Paoprasert and M. D. G. de Luna, Sustainable Environ. Res., 2018, 28, 197–205 CrossRef CAS.
- S. B. Tumampos, B. M. B. Ensano, S. M. B. Pingul-Ong, D. C. Ong, C. C. Kan, J. J. Yee and M. D. G. de Luna, Int. J. Environ. Res. Public Health, 2021, 18, 3050 CrossRef CAS PubMed.
- I. O. Saheed, W. D. Oh and F. B. M. Suah, J. Hazard. Mater., 2021, 408, 124889 CrossRef CAS PubMed.
- U. Habiba, T. C. Joo, T. A. Siddique, A. Salleh, B. C. Ang and A. M. Afifi, Int. J. Biol. Macromol., 2017, 104, 1133–1142 CrossRef CAS PubMed.
- T. V. J. Charpentier, A. Neville, J. L. Lanigan, R. Barker, M. J. Smith and T. Richardson, ACS Omega, 2016, 1, 77–83 CrossRef CAS PubMed.
- Y. Z. Xiao, H. F. Liang and Z. C. Wang, Mater. Res. Bull., 2013, 48, 3910–3915 CrossRef CAS.
- G. D. Briao, J. R. de Andrade, M. G. C. da Silva and M. G. A. Vieira, Environ. Chem. Lett., 2020, 18, 1145–1168 CrossRef CAS.
- V. R. Shaumbwa, D. G. Liu, B. Archer, J. L. Li and F. Su, J. Appl. Polym. Sci., 2021, 138, e51241 CrossRef.
- G. Michailidou, I. Koumentakou, E. V. Liakos, M. Lazaridou, D. A. Lambropoulou, D. N. Bikiaris and G. Z. Kyzas, Polymers, 2021, 13, 3137 CrossRef CAS PubMed.
- S. X. Liu, B. Yu, S. Wang, Y. Q. Shen and H. L. Cong, Adv. Colloid Interface Sci., 2020, 281, 102165 CrossRef CAS PubMed.
- L. S. Ganapathe, M. A. Mohamed, R. M. Yunus and D. D. Berhanuddin, Magnetochemistry, 2020, 6, 68 CrossRef CAS.
- J. Tong and L. G. Chen, Anal. Lett., 2013, 46, 2635–2656 CrossRef CAS.
- V. R. Shaumbwa, D. G. Liu, B. Archer, J. L. Li and F. Su, J. Appl. Polym. Sci., 2021, 138, e51241 CrossRef.
- A. G. Perez, E. Gonzalez-Martinez, C. R. D. Aguila, D. A. Gonzalez-Martinez, G. G. Ruiz, A. G. Artalejo and H. Yee-Madeira, Colloids Surf., A, 2020, 591, 124500 CrossRef.
- G.-T. Zhu, X.-L. Hu, S. He, X.-M. He, S.-K. Zhu and Y.-Q. Feng, J. Chromatogr. A, 2018, 1564, 42–50 CrossRef CAS PubMed.
- F. Zhao, B. Y. Yu, Z. G. Yue, T. Wang, M. Wen, Z. B. Liu and C. S. Zhao, J. Hazard. Mater., 2007, 147, 67–73 CrossRef CAS PubMed.
- N. H. Shalaby, E. M. M. Ewais, R. M. Elsaadany and A. Ahmed, Egypt. J. Pet., 2017, 26, 661–668 CrossRef.
- H. El Knidri, R. Belaabed, A. Addaou, A. Laajeb and A. Lahsini, Int. J. Biol. Macromol., 2018, 120, 1181–1189 CrossRef CAS PubMed.
- Y. L. Nunes, F. L. de Menezes, I. G. de Sousa, A. L. Gama Cavalcante, F. T. Tavares Cavalcante, K. d. S. Moreira, A. L. Barros de Oliveira, G. F. Mota, J. E. da Silva Souza, I. R. de Aguiar Falcao, T. G. Rocha, R. B. Rodrigues Valerio, P. B. Almeida Fechine, M. C. Martins de Souza and J. C. S. dos Santos, Int. J. Biol. Macromol., 2021, 181, 1124–1170 CrossRef CAS PubMed.
- A. Popa, A. Visa, B. Maranescu, I. Hulka and L. Lupa, Materials, 2021, 14, 7894 CrossRef CAS PubMed.
- W. Wu, Q. He and C. Jiang, Nanoscale Res. Lett., 2008, 3, 397–415 CrossRef CAS PubMed.
- A. Hiroki, H. T. Tran, N. Nagasawa, T. Yagi and M. Tamada, Radiat. Phys. Chem., 2009, 78, 1076–1080 CrossRef CAS.
- B. H. Hameed and M. I. El-Khaiary, J. Hazard. Mater., 2008, 157, 344–351 CrossRef CAS PubMed.
- S. Liu, B. Yu, S. Wang, Y. Shen and H. Cong, Adv. Colloid Interface Sci., 2020, 281, 102165 CrossRef CAS PubMed.
- P. G. Krishnan, S. Muthukumaran and V. Raja, J. Lumin., 2021, 238, 118258 CrossRef CAS.
- U. A. Agu, M. I. Oliva, S. G. Marchetti, A. C. Heredia, S. G. Casuscelli and M. E. Crivello, J. Magn. Magn. Mater., 2014, 369, 249–259 CrossRef CAS.
- J. Sahadevan, R. Sojiya, N. Padmanathan, K. Kulathuraan, M. G. Shalini, P. Sivaprakash and S. E. Muthu, Magnetic property of Fe2O3 and Fe3O4 nanoparticle prepared by solvothermal process, Tiruchirappalli, India, 2021 Search PubMed.
- M. Imran, A. M. Affandi, M. M. Alam, A. Khan and A. I. Khan, Nanotechnology, 2021, 32, 422001 CrossRef CAS PubMed.
- J. A. Flood-Garibay and M. A. Mendez-Rojas, Colloids Surf., A, 2021, 615, 126236 CrossRef CAS.
- Z. Chen, X. Song, W. W. M. Soh, Y. Wen, J. Zhu, M. Zhang and J. Li, Gels, 2021, 7, 201 CrossRef CAS PubMed.
- L. Shen, B. Li and Y. Qiao, Materials, 2018, 11, 324 CrossRef PubMed.
- S. Rezayati, F. Kalantari, A. Ramazani, S. Sajjadifar, H. Aghahosseini and A. Rezaei, Inorg. Chem., 2022, 61, 992–1010 CrossRef CAS PubMed.
- S. J. Olusegun, G. L. S. Rodrigues, E. T. F. Freitas, L. R. S. Lara, W. R. Rocha and N. D. S. Mohallem, J. Hazard. Mater., 2019, 380, 120872 CrossRef CAS PubMed.
- L. Zhang, X. Zhu, S. Zheng and H. Sun, Biochem. Eng. J., 2009, 46, 83–87 CrossRef CAS.
- D.-M. Liu, C. Dong, J. Zhong, S. Ren, Y. Chen and T. Qiu, Carbohydr. Polym., 2020, 245, 116572 CrossRef CAS PubMed.
- J. Jumadi, A. Kamari, N. A. Rahim, S. T. S. Wong, S. N. M. Yusoff, S. Ishak, M. M. Abdulrasool and S. Kumaran, J. Phys.: Conf. Ser., 2019, 1397, 012027–012028 CrossRef CAS.
- H. Zhao, J. Xu, W. Lan, T. Wang and G. Luo, Chem. Eng. J., 2013, 229, 82–89 CrossRef CAS.
- S. Ben Ayed, H. M. Sbihi, M. Azam, S. I. Al-Resayes, M. T. Ayadi and F. Ayari, Desalin. Water Treat., 2021, 220, 446–458 CrossRef CAS.
- Y. Xu, Y. Zhang, X. Song and H. Liu, Funct. Mater. Lett., 2019, 12, 1950019 CrossRef CAS.
- R. Hufschmid, H. Arami, R. M. Ferguson, M. Gonzales, E. Teeman, L. N. Brush, N. D. Browning and K. M. Krishnan, Nanoscale, 2015, 7, 11142–11154 RSC.
- M. Liu, X. Sun, Z. Liao, Y. Li, X. Qi, Y. Qian, H. Fenniri, P. Zhao and J. Shen, Drug Delivery, 2019, 26, 732–743 CrossRef CAS PubMed.
- K. Zhang, X. Zhang, X. Zhao, X. Gai, W. An, G. Fang, A. Zhang and X. Chen, J. Mater. Sci.: Mater. Electron., 2019, 30, 17333–17341 CrossRef CAS.
- G. H. Du, Z. L. Liu, X. Xia, Q. Chu and S. M. Zhang, J. Sol-Gel Sci. Technol., 2006, 39, 285–291 CrossRef CAS.
- C.-H. Lu, G.-H. Chen, B. Yu, H.-L. Cong, L.-M. Kong and L. Guo, Integr. Ferroelectr., 2017, 182, 46–52 CrossRef CAS.
- Y. Jiang, W. Cai, W. Tu and M. Zhu, J. Chem. Eng. Data, 2019, 64, 226–233 CrossRef CAS.
- I. Fatimah, E. Z. Pratiwi and W. P. Wicaksono, Egypt. J. Aquat. Res., 2020, 46, 35–40 CrossRef.
- A. V. Ramesh, D. R. Devi, S. M. Botsa and K. Basavaiah, J. Asian Ceram. Soc., 2018, 6, 145–155 CrossRef.
- A. Sebastian, A. Nangia and M. N. V. Prasad, J. Cleaner Prod., 2018, 174, 355–366 CrossRef CAS.
- E. C. Nnadozie and P. A. Ajibade, Mater. Lett., 2020, 263, 127145 CrossRef CAS.
- Y. C. Lopez and M. Antuch, Curr. Opin. Green Sustainable Chem., 2020, 24, 32–37 CrossRef.
- M. Rahmayanti, A. N. Syakina, I. Fatimah and T. Sulistyaningsih, Chem. Phys. Lett., 2022, 803, 139834 CrossRef CAS.
- P. Senthilkumar, L. Surendran, B. Sudhagar, R. S. D. S. Kumar and G. Bupesh, Mater. Res. Express, 2019, 6, 095405 CrossRef CAS.
- X. Li, K. Cui, Z. Guo, T. Yang, Y. Cao, Y. Xiang, H. Chen and M. Xi, Chem. Eng. J., 2020, 379, 122324 CrossRef CAS.
- X. Li, D. Zeng, P. Ke, G. Wang and D. Zhang, RSC Adv., 2020, 10, 7163–7169 RSC.
- S. Vaewbundit and P. Siriphannon, Soft Matter, 2021, 17, 6238–6247 RSC.
- R. N. Queiroz, P. Prediger and M. G. A. Vieira, J. Hazard. Mater., 2022, 422, 126904 CrossRef CAS PubMed.
- R. N. Queiroz, T. d. F. Neves, M. G. C. da Silva, V. R. Mastelaro, M. G. A. Vieira and P. Prediger, J. Cleaner Prod., 2022, 361, 132244 CrossRef CAS.
- S. Liu, H. Ge, S. Cheng and Y. Zou, Environ. Technol., 2020, 41, 2109–2121 CrossRef CAS PubMed.
- A. Kalidason and T. Kuroiwa, React. Funct. Polym., 2022, 173, 105220 CrossRef CAS.
- I. Rahmi and I. Mustafa, Microchem. J., 2019, 144, 397–402 CrossRef CAS.
- S. Y. Nam and Y. M. Lee, J. Membr. Sci., 1999, 157, 63–71 CrossRef.
- R. Huang, Q. Liu, J. Huo and B. Yang, Arabian J. Chem., 2017, 10, 24–32 CrossRef CAS.
- D. A. Sahbaz, A. Yakar and U. Gunduz, Part. Sci. Technol., 2019, 37, 728–736 Search PubMed.
- N. A. Abdelwahab and A. M. Ghoneim, Mater. Sci. Eng., B, 2018, 228, 7–17 CrossRef CAS.
- X. Cai, J. Li, Y. Liu, X. Hu, X. Tan, S. Liu, H. Wang, Y. Gu and L. Luo, Int. J. Environ. Res. Public Health, 2020, 17, 6 CrossRef CAS PubMed.
- M. H. Karimi, G. R. Mandavinia, B. Massoumi, A. Baghban and M. Saraei, Int. J. Biol. Macromol., 2018, 113, 361–375 CrossRef CAS PubMed.
- A. Reghioua, D. Barkat, A. H. Jawad, A. S. Abdulhameed and M. R. Khan, Sustainable Chem. Pharm., 2021, 20, 100379 CrossRef CAS.
- B. Liu, X. Chen, H. Zheng, Y. Wang, Y. Sun, C. Zhao and S. Zhang, Carbohydr. Polym., 2018, 181, 327–336 CrossRef CAS PubMed.
- H.-R. Kim, J.-W. Jang and J.-W. Park, J. Hazard. Mater., 2016, 317, 608–616 CrossRef CAS PubMed.
- P. Xu, M. Zheng, N. Chen, Z. Wu, N. Xu, J. Tang and Z. Teng, J. Taiwan Inst. Chem. Eng., 2019, 104, 210–218 CrossRef CAS.
- N. Ayawei, A. N. Ebelegi and D. Wankasi, J. Chem., 2017, 2017, 3039817 Search PubMed.
- M. Gholami, A. Jonidi-Jafari, M. Farzadkia, A. Esrafili, K. Godini and M. Shirzad-Siboni, J. Environ. Manage., 2021, 294, 112962 CrossRef CAS PubMed.
- D. A. Islam, A. Chakraborty, A. Roy, S. Das and H. Acharya, ChemistrySelect, 2018, 3, 11816–11823 CrossRef CAS.
- Y. Zhao, X. Yang, P. Pan, J. Liu, Z. Yang, J. Wei, W. Xu, Q. Bao, H. Zhang and Z. Liao, J. Electron. Mater., 2020, 49, 6695–6705 CrossRef CAS.
- J. Rihel, Nat. Chem. Biol., 2018, 14, 638–639 CrossRef CAS PubMed.
- J. Kumar, K. B. Sathua and S. J. S. Flora, Cell. Mol. Biol., 2019, 65, 27–35 CrossRef.
- D. M. Marques, V. Veroneze Junior, A. B. da Silva, J. R. Mantovani, P. C. Magalhaes and T. C. de Souza, Water, Air, Soil Pollut., 2018, 229, 138 CrossRef.
- R. Viriyatum and C. E. Boyd, J. World Aquacult. Soc., 2016, 47, 667–675 CrossRef CAS.
- M. E. Peralta, R. Nistico, F. Franzoso, G. Magnacca, L. Fernandez, M. E. Parolo, E. Garcia Leon and L. Carlos, Adsorption, 2019, 25, 1337–1347 CrossRef CAS.
- F. Xiao, J. Cheng, W. Cao, C. Yang, J. Chen and Z. Luo, J. Colloid Interface Sci., 2019, 540, 579–584 CrossRef CAS PubMed.
- H. Li, Z. Wang, X. Liu, F. Cui, C. Chen, Z. Zhang, J. Li, L. Song and R. Bai, Chem. Phys. Lett., 2020, 755, 137805 CrossRef CAS.
- M. A. M. Taguba, D. C. Ong, B. M. B. Ensano, C.-C. Kan, N. Grisdanurak, J.-J. Yee and M. D. G. de Luna, Water, 2021, 13, 1662 CrossRef CAS.
- H.-C. Tao, S. Li, L.-J. Zhang, Y.-Z. Chen and L.-P. Deng, Environ. Geochem. Health, 2019, 41, 297–308 CrossRef CAS PubMed.
- Z. Zhang, H. Li, J. Li, X. Li, Z. Wang, X. Liu and L. Zhang, Chem. Phys. Lett., 2019, 731, 136573 CrossRef CAS.
- L. Yang, Y. Peng, C. Qian, G. Xing, J. He, C. Zhao and B. Lai, Chemosphere, 2021, 263, 128120 CrossRef CAS PubMed.
- C. Zhang, S. Liu, S. Li, Y. Tao, P. Wang, X. Ma and L. Chen, Colloids Surf., A, 2019, 581, 123813 CrossRef CAS.
- S. M. Anush and B. Vishalakshi, Int. J. Biol. Macromol., 2019, 133, 1051–1062 CrossRef CAS PubMed.
- H. Hosseinzadeh and S. Ramin, Int. J. Biol. Macromol., 2018, 113, 859–868 CrossRef CAS PubMed.
- B. Chen, H. Zhao, S. Chen, F. Long, B. Huang, B. Yang and X. Pan, Chem. Eng. J., 2019, 356, 69–80 CrossRef CAS.
- X. Shen, J. Zhao, N. Bonet-Garcia, E. Villagrasa, A. Sole, X. Liao and C. Palet, Int. J. Environ. Sci. Technol., 2022, 20, 801–814 CrossRef.
- M. R. Afrooz, B. K. Moghadas and S. Tamjidi, J. Alloys Compd., 2022, 893, 162321 CrossRef CAS.
- Z. Yu, X. Zhang and Y. Huang, Ind. Eng. Chem. Res., 2013, 52, 11956–11966 CrossRef CAS.
- H. Y. Zhu, R. Jiang, L. Xiao and G. M. Zeng, Bioresour. Technol., 2010, 101, 5063–5069 CrossRef CAS PubMed.
- A. Aisopou, I. Stoianov and N. J. D. Graham, Water Res., 2012, 46, 235–246 CrossRef CAS PubMed.
- J. Wang and C. Chen, Biotechnol. Adv., 2006, 24, 427–451 CrossRef CAS PubMed.
- Y. Wu, X. Qiu, S. Cao, J. Chen, X. Shi, Y. Du and H. Deng, J. Colloid Interface Sci., 2019, 539, 533–544 CrossRef CAS PubMed.
- A. Kumar, K. Amit, M. M. S. Cabral-Pinto, A. K. Chaturvedi, A. A. Shabnam, G. Subrahmanyam, R. Mondal, D. K. Gupta, S. K. Malyan, S. S. Kumar, S. A. Khan and K. K. Yadav, Int. J. Environ. Res. Public Health, 2020, 17, 2179 CrossRef CAS PubMed.
- M. D. G. de Luna, L. M. Bellotindos, R. N. Asiao and M.-C. Lu, Hydrometallurgy, 2015, 155, 6–12 CrossRef CAS.
- J. Cela, W. Bonilla and R. Urrutia-Goyes, IOP Conference Series: Earth and Environmental Science, 2021, 728, 012006–012007 Search PubMed.
- J. Garcia-Leston, J. Mendez, E. Pasaro and B. Laffon, Environ. Int., 2010, 36, 623–636 CrossRef CAS PubMed.
- L. Lv, N. Chen, C. Feng, J. Zhang and M. Li, RSC Adv., 2017, 7, 27992–28000 RSC.
- F. Bouhamed, Z. Elouear and J. Bouzid, J. Taiwan Inst. Chem. Eng., 2012, 43, 741–749 CrossRef CAS.
- S. Huang, C. Ma, Y. Liao, C. Min, P. Du, Y. Zhu and Y. Jiang, React. Funct. Polym., 2016, 106, 76–85 CrossRef CAS.
- S. Gunduz and S. Akman, Food Chem., 2013, 141, 2634–2638 CrossRef CAS PubMed.
- S. Shahraki and H. S. Delarami, Carbohydr. Polym., 2018, 200, 211–220 CrossRef CAS PubMed.
- C. Fan, K. Li, J. Li, D. Ying, Y. Wang and J. Jia, J. Hazard. Mater., 2017, 326, 211–220 CrossRef CAS PubMed.
- D. Hu, Z. Lian, H. Xian, R. Jiang, N. Wang, Y. Weng, X. Peng, S. Wang and X.-K. Ouyang, Int. J. Biol. Macromol., 2020, 154, 1537–1547 CrossRef CAS PubMed.
- B. Fahimirad, Y. Rangraz, A. Elhampour and F. Nemati, Microchim. Acta, 2018, 185, 560 CrossRef PubMed.
- M. F. Hamza, Y. Wei, H. I. Mira, A. A. H. Abdel-Rahman and E. Guibal, Chem. Eng. J., 2019, 362, 310–324 CrossRef CAS.
- Y. He, W. Xiao, G. Li, F. Yang, P. Wu, T. Yang, C. Chen and P. Ding, Environ. Technol., 2019, 40, 499–507 CrossRef CAS PubMed.
- E. Cheraghipour and M. Pakshir, Chemosphere, 2020, 260, 127560 CrossRef CAS PubMed.
- R. Eivazzadeh-Keihan, F. Radinekiyan, S. Asgharnasl, A. Maleki and H. Bahreinizad, J. Mater. Res. Technol., 2020, 9, 12244–12259 CrossRef CAS.
- D. Wu, Y. Wang, Y. Li, Q. Wei, L. Hu, T. Yan, R. Feng, L. Yan and B. Du, J. Mol. Liq., 2019, 277, 181–188 CrossRef CAS.
- Y. Huang, H. Zheng, X. Hu, Y. Wu, X. Tang, Q. He and S. Peng, J. Hazard. Mater., 2022, 422, 126856 CrossRef CAS PubMed.
- D. P. Facchi, A. L. Cazetta, E. A. Canesin, V. C. Almeida, E. G. Bonafe, M. J. Kipper and A. F. Martins, Chem. Eng. J., 2018, 337, 595–608 CrossRef CAS.
- R. Nasiri, N. Arsalani and Y. Panahian, J. Cleaner Prod., 2018, 201, 507–515 CrossRef CAS.
- M. F. Hamza, M. M. Aly, A. A. H. Abdel-Rahman, S. Ramadan, H. Raslan, S. Wang, T. Vincent and E. Guibal, Materials, 2017, 10, 539 CrossRef PubMed.
- F. Zhao, E. Repo, M. Sillanpaa, Y. Meng, D. Yin and W. Z. Tang, Ind. Eng. Chem. Res., 2015, 54, 1271–1281 CrossRef CAS.
- A. A. Galhoum, A. A. Atia, M. G. Mahfouz, S. T. Abdel-Rehem, N. A. Gomaa, T. Vincent and E. Guibal, J. Mater. Sci., 2015, 50, 2832–2848 CrossRef CAS.
- M. F. Hamza, J.-C. Roux and E. Guibal, Chem. Eng. J., 2018, 344, 124–137 CrossRef CAS.
- E. A. Imam, I. E.-T. El-Sayed, M. G. Mahfouz, A. A. Tolba, T. Akashi, A. A. Galhoum and E. Guibal, Chem. Eng. J., 2018, 352, 1022–1034 CrossRef CAS.
- Y. Cai, C. Wu, Z. Liu, L. Zhang, L. Chen, J. Wang, X. Wang, S. Yang and S. Wang, Environ. Sci.: Nano, 2017, 4, 1876–1886 RSC.
- Y. Ma, W.-J. Liu, N. Zhang, Y.-S. Li, H. Jiang and G.-P. Sheng, Bioresour. Technol., 2014, 169, 403–408 CrossRef CAS PubMed.
- M. Imran, Z. U. H. Khan, M. M. Iqbal, J. Iqbal, N. S. Shah, S. Munawar, S. Ali, B. Murtaza, M. A. Naeem and M. Rizwan, Environ. Pollut., 2020, 261, 114231 CrossRef CAS PubMed.
- R. T. Kapoor, M. F. Bani Mfarrej, P. Alam, J. Rinklebe and P. Ahmad, Environ. Pollut., 2022, 301, 119044 CrossRef CAS PubMed.
- S. Azizi, I. Kamika and M. Tekere, PLoS One, 2016, 11, e0155462 CrossRef PubMed.
- T. Ravi and S. A. Jabasingh, J. Appl. Polym. Sci., 2018, 135, 45878 CrossRef.
- Y. Wu, Y. Zhang, J. Qian, X. Xin, S. Hu, S. Zhang and J. Wei, R. Soc. Open Sci., 2017, 4, 170905 CrossRef PubMed.
- S. Koushkbaghi, A. Zakialamdari, M. Pishnamazi, H. F. Ramandi, M. Aliabadi and M. Irani, Chem. Eng. J., 2018, 337, 169–182 CrossRef CAS.
- C. Zheng, H. Zheng, Y. Wang, Y. Wang, W. Qu, Q. An and Y. Liu, Bioresour. Technol., 2018, 267, 1–8 CrossRef CAS PubMed.
- X. Wang, X. Liu, C. Xiao, H. Zhao, M. Zhang, N. Zheng, W. Kong, L. Zhang, H. Yuan, L. Zhang and J. Lu, Microporous Mesoporous Mater., 2020, 297, 110041 CrossRef.
- C. Zheng, H. Zheng, Y. Sun, B. Xu, Y. Wang, X. Zheng and Y. Wang, Bioresour. Technol., 2019, 293, 122038 CrossRef CAS PubMed.
- J. d. O. Marques Neto, C. R. Bellato and D. d. C. Silva, Chemosphere, 2019, 218, 391–401 CrossRef CAS PubMed.
- Y. Mu, Z. Ai, L. Zhang and F. Song, ACS Appl. Mater. Interfaces, 2015, 7, 1997–2005 CrossRef CAS PubMed.
- S. Shi, J. Yang, S. Liang, M. Li, Q. Gan, K. Xiao and J. Hu, Sci. Total Environ., 2018, 628–629, 499–508 CrossRef CAS PubMed.
- S. Ji, C. Miao, H. Liu, L. Feng, X. Yang and H. Guo, Nanoscale Res. Lett., 2018, 13, 178 CrossRef PubMed.
- L. V. Alves Gurgel, J. C. Perin de Melo, J. C. de Lena and L. F. Gil, Bioresour. Technol., 2009, 100, 3214–3220 CrossRef PubMed.
- F. Karimi, A. Ayati, B. Tanhaei, A. L. Sanati, S. Afshar, A. Kardan, Z. Dabirifar and C. Karaman, Environ. Res., 2022, 203, 111753 CrossRef CAS PubMed.
- M. F. Mubarak, A. H. Ragab, R. Hosny, I. A. Ahmed, H. A. Ahmed, S. M. El-Bahy and A. El Shahawy, Polymers, 2021, 13, 2714 CrossRef CAS PubMed.
- F. Rahmi, F. P. Lelifajri and R. Sembiring, IOP Conf. Ser.: Mater. Sci. Eng., 2019, 276, 012004–012007 CrossRef.
- M. Omidinasab, N. Rahbar, M. Ahmadi, B. Kakavandi, F. Ghanbari, G. Z. Kyzas, S. Silva Martinez and N. Jaafarzadeh, Environ. Sci. Pollut. Res., 2018, 25, 34262–34276 CrossRef CAS PubMed.
- H. Li, F.-X. Zhou, B.-H. He, G.-X. Wang, W.-Y. Xie and E. Liang, ChemistrySelect, 2020, 5, 8033–8039 CrossRef CAS.
- D. Yuan, W. Zhang, J. Cui, L. He, J. Wang, C. Yan, Y. Kou and J. Li, Environ. Sci. Pollut. Res., 2020, 27, 2588–2598 CrossRef CAS PubMed.
- F. Qi, Z. Dong, D. Lamb, R. Naidu, N. S. Bolan, Y. S. Ok, C. Liu, N. Khan, M. A. H. Johir and K. T. Semple, Chemosphere, 2017, 180, 564–573 CrossRef CAS PubMed.
- K. Hachem, S. A. Jasim, M. E. Al-Gazally, Y. Riadi, G. Yasin, A. T. Jalil, M. M. Abdulkadhm, M. M. Saleh, M. N. Fenjan, Y. F. Mustafa and A. D. Khalaji, J. Chin. Chem. Soc., 2022, 69, 512–521 CrossRef CAS.
- H. H. Najafabadi, M. Irani, L. R. Rad, A. H. Haratameh and I. Haririan, RSC Adv., 2015, 5, 16532–16539 RSC.
- L. Zhou, Y. Liu, S. Liu, Y. Yin, G. Zeng, X. Tan, X. Hu, X. Hu, L. Jiang, Y. Ding, S. Liu and X. Huang, Bioresour. Technol., 2016, 218, 351–359 CrossRef CAS PubMed.
- M. Zhang, Z. Zhang, Y. Peng, L. Feng, X. Li, C. Zhao and K. Sarfaraz, Int. J. Biol. Macromol., 2020, 156, 289–301 CrossRef CAS PubMed.
- P. Sun, W. Zhang, B. Zou, L. Zhou, Z. Ye and Q. Zhao, Int. J. Biol. Macromol., 2021, 182, 1138–1149 CrossRef CAS PubMed.
- W. Cai, F. Zhu, H. Liang, Y. Jiang, W. Tu, Z. Cai, J. Wu and J. Zhou, Chem. Eng. Res. Des., 2019, 144, 150–158 CrossRef CAS.
- X. Song, L. Li, L. Zhou and P. Chen, Chem. Eng. Res. Des., 2018, 136, 581–592 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |